Summary
Background
The contamination of ecosystem compartments by microplastics (MPs) is an ubiquitous problem. MPs have been observed in mice tissues, and recently in human blood, stool and placenta. However, two aspects remain unclear: whether MPs accumulate in peripheral organs, specifically in the liver, and if liver cirrhosis favours this process. We aimed to examine human liver tissue samples to determine whether MPs accumulate in the liver.
Methods
This proof-of-concept case series, conducted in Germany, Europe, analyzed tissue samples of 6 patients with liver cirrhosis and 5 individuals without underlying liver disease. A total of 17 samples (11 liver, 3 kidney and 3 spleen samples) were analyzed according to the final protocol. A reliable method for detection of MP particles from 4 to 30 µm in human tissue was developed. Chemical digestion of tissue samples, staining with Nile red, subsequent fluorescent microscopy and Raman spectroscopy were performed. Morphology, size and composition of MP polymers were assessed.
Findings
Considering the limit of detection, all liver, kidney and spleen samples from patients without underlying liver disease tested negative for MPs. In contrast, MP concentrations in cirrhotic liver tissues tested positive and showed significantly higher concentrations compared to liver samples of individuals without underlying liver disease. Six different microplastic polymers ranging from 4 to 30 µm in size were detected.
Interpretation
This proof-of-concept case series assessed the presence of MPs in human liver tissue and found six different MP polymers in the liver of individuals with liver cirrhosis, but not in those without underlying liver disease. Future studies are needed to evaluate whether hepatic MP accumulation represents a potential cause in the pathogenesis of fibrosis, or a consequence of cirrhosis and portal hypertension.
Funding
No funding was received for conducting this investigator driven study.
Keywords: Microplastics, Human tissue, Liver cirrhosis, Raman spectroscopy
Abbreviations: MPs, microplastics; PS, polystyrene; SOP, standard operating procedure; MELD, model for end-stage liver disease; PET, polyethylene terephthalate; PVC, polyvinyl chloride; PMMA, polymethyl methacrylate; POM, polyoxymethylene; PP, polypropylene; SD, standard deviation; SBP, spontaneous bacterial peritonitis
Research in context.
Evidence before this study
Microplastics (MPs) are found in all ecosystems. Tissue accumulation of microplastics has been observed in mice, and recently MPs have been detected in human blood stream, stool and placenta. However, it remained unclear whether MPs can deposit in human tissues of peripheral organs, in particular in the liver.
Added value of this study
This proof-of-concept case series assessed the presence of MPs in human liver tissue and found six different MP polymers in the liver of individuals with liver cirrhosis, but not in those without underlying liver disease.
Implications of all the available evidence
Our results indicate that chronic liver disease seems to be a key driver in MP accumulation in human liver and that there is a need to evaluate whether hepatic MP accumulation represents a potential cause in the pathogenesis of fibrosis, or a consequence of cirrhosis and portal hypertension.
Alt-text: Unlabelled box
Introduction
Microplastics (MPs) have been recognized as a global environmental problem, as there are reports of contamination of all ecosystem compartments by microplastics.1, 2, 3 In addition, the observation that the occurring concentrations of plastic particles are increasing exponentially with decreasing size poses a new challenge.4 MPs can be classified into primary (manufactured particles, such as pre-production pellets, abrasion particles, such as microbeads in cosmetics) and secondary particles derived from predominantly UV-induced and mechanical degradation of larger plastic pieces.1,5,6 In addition to environmental pollution of marine and freshwaters, sediments and the atmosphere, there is a lot of evidence of MP ingestion by various marine species.6, 7, 8, 9, 10, 11, 12 Furthermore, MPs have been detected in human food, such as seafood, sugar, salt and honey, but also in drinking and ground water, and have therefore without a doubt, arrived in the human food chain.6,13, 14, 15, 16, 17 Other possible exposure routes for MPs towards the human body are inhalation and dermal uptake.2,16,18,19 Although a lifetime MP exposure model (simulating an average human lifetime) estimated that MPs may accumulate in the liver, real accumulation and biodistribution of MPs in the human body remain unstudied.20
There is ongoing discussion regarding the impact of MPs on human health. Peyer's patches, lymphoid follicles of the ileum, have been reported to be major sites of uptake and transport of nano- and microparticles in the gastrointestinal tract, whereas respiratory endothelial cells have been reported to take up inhaled nanoparticles via endocytic or phagocytic processes, as known from animal studies.10,21,22 MP-induced oxidative stress and pro-inflammatory effects have been suggested by various studies.10,23, 24, 25, 26 Accumulation of polystyrene (PS) microspheres in the gut, liver and kidney associated with disturbances in energy, lipid metabolism, mucus secretion and the gut microbiota in mammals have been reported.25,27,28 However, others could not reproduce these findings, as they observed MP accumulation in the gut, but not in parenchymatous organs in reporter gene mice being treated with PS.29 Data are conflicting and controversially discussed.30,31 Furthermore, it remains unclear how much a dose or size-dependent effect may be expected. Despite the controversy, studies are required to determine how and to what extent, MPs impact human health.
Very recently, MPs have been detected in human blood of healthy donors.32 Others, detected MPs in human stool samples, pointing to involuntary ingestion.33 Although this study was critically discussed within the community, a group from Beijing has very recently validated these findings, as they also found different types of MPs in human stool samples.34 Accordingly, MPs were reported in human colectomy samples obtained due to colorectal cancer, bleeding arterio-venous malformation, colonic perforation, and trauma.35 However, collected MP particles were comparably large (800–1600 µm) and were thus not capable of crossing the gastrointestinal epithelium.36 It is assumed that particles <150 µm are theoretically capable of crossing the gastrointestinal barrier due to corresponding evidence in mammalian bodies.36, 37, 38, 39 The size to which MP particles could migrate through the intestinal wall, is still unclear.40,41 Whether presence of MP in the bloodstream or in the stool also indicates abundance in the peripheral organs remains to be investigated.
Ragusa and colleagues reported proof of MPs in human placenta.42 Recently, Braun et al. investigated MPs (>50 µm) in placental tissue, meconium, and maternal stool in a clinical setting of caesarean deliveries.43 Despite the fact that all analyzed matrices were tested positive for MPs, the authors suggest interpreting their results with caution due to background contamination issues.
To shed further light, we performed this proof-of-concept case series, aiming (i) to analyze whether MPs can be found in peripheral organs, specifically in the liver, and (ii) to study the morphology, size and composition of MP polymers ranging from 4-30 µm in patients with and without underlying liver disease.
Methods
Sample material
Liver specimens of patients with chronic liver disease were derived from liver explants in 6 liver transplant recipients. Liver, kidney, and spleen specimens of 5 individuals without underlying chronic liver disease were obtained from complete autopsies performed in Hamburg between June and August 2020.
Ethics
The study was approved by the local ethics committee (WF-129/20) and performed in accordance the Declaration of Helsinki. Patients with liver cirrhosis provided written informed consent. Specimen collection and analysis were performed according to the local law - Hamburgisches Krankenhaus Gesetz §12.1
Sample processing
Samples of about 1–2 cm³ were dissected using stainless steel scalpels and tweezers and stored in labeled glass jars covered with aluminum caps at −20 °C prior to treatment and analysis. The weight of sampled tissue ranged from 0.7 to 7.1 g.
Within initial screening, 3 samples of human liver tissue were analyzed, 5 human liver and 3 calf samples were used for method development and another 6 samples (2 each of human liver, kidney, and spleen tissue) for method improvement (details see S.I.). A total of 17 human tissue samples were analyzed according to the final Standard Operating Procedure (Suppl. Figure 1).
Standard Operating Procedure (SOP human tissue)
Based on the pre-tests and method development, a standard operating procedure (SOP) for human tissue was set up and applied to human samples consisting of tissue from liver (n = 11), spleen (n = 3) and kidney (n = 3).
Throughout the SOP, anti-contamination and quality assurance/quality control measurements were followed including a clean working environment and used material, pre-cleaning of beakers and devices, covering of samples, and pre-filtration of any solutions (<0.45 µm) used for digestion, rinsing and solution (refer to Suppl. material, S.I for details). Blank samples (which did not contain tissue) were analyzed simultaneously to ensure that samples have not been contaminated during the analytic process. Reference samples were not included in this study since no certified material in the respective size category is commercially available, so far.
Sample digestion (see detailed sample digestion steps and information within S.I)
Tissue samples of approximately 2 cm³ were weighed, rinsed with filtered MilliQ water and transferred to glass beakers.
Digestion solution consisting of potassium hydroxide (10 M, 40.5%) and sodium hypochlorite (6–14%) in a 2:1 ratio was added (5 ml per g wet weight of tissue). Sample suspensions were digested at 40 °C for 72 h.
Sample suspensions were filtered via silver membrane filters (Millipore membrane filters, silver, 0.45 µm, diameter 25 mm) and re-transferred to beakers. The resulting suspensions were subjected to a second digestion step applying hydrogen peroxide (H2O2, 30%, 30 ml) and defatting with acetone. Following a second filtration step filter residues and the filter were transferred to beakers with ethanol (30 ml).
Quantification of microplastic particles via fluorescence microscopy
For quantification via the staining protocol, a volume of 6 ml of Nile Red solution (1 mg/ml chloroform) was added to the sample suspension in ethanol. Stained suspensions were left to stand for 24 h and subsequently filtered onto pre-cleaned Anodisc filters (Sartorius Anodisc filters, AlO, 0.2 µm, diameter 25 mm). This also includes the rinsing solutions from the silver filter (three times with ethanol). The filtration funnel was rinsed two times with ethanol, one time with MilliQ water and finally, one time with ethanol in order to rinse off the dying solution residues from the Anodisc filter.
Anodisc and silver filter were placed onto glass microscopic slides and were further investigated under the fluorescence microscope (microscope AxioScope 5/7 KMAT, filter set HE43, AxioCam 503 color, ZEN Software). Particles that were significantly distinguishable from the background by the emitted fluorescence were counted, dimensions were measured and shapes were recorded. Concentrations were recalculated to the initial tissue weight of the sample.
Qualification of microplastic particles via µRaman spectroscopy
Two to six particles per sample that were significantly identified were located with the µRaman microscope (DXR2xi, Thermofisher) at the filter using the digital image derived from the fluorescence microscope. Chemical composition was measured via recording spectra with the µRaman spectroscope (532 and 785 nm, laser intensity and exposure adjusted from 0.1 to 10.0 mW and 30 to 1 Hz respectively according to particle size, composition and laser setup, 25 µm or 50 µm pinhole, 1000 spectra integrated) and evaluated against 10 relevant external spectral libraries including SLoPP and SLoPP-E44 and 2 self-generated libraries on synthetic polymers. The threshold value of the Raman spectra was set at >70% agreement. This could not be achieved with 10 particles (n = 8 60–70%, n = 2 <60%) which were nevertheless integrated based on expert-based decision due to the strong evidence of agreement.
Statistical evaluation
Statistical analyses were performed using R (R Core Team 2020, version 3.6.3) in an R Studio environment (RStudio Team 2019, version 1.2.5033) and IBM SPSS Statistics (IBM Corp. 2019, version 26.0). The Shapiro-Wilk test was applied to test for normal distribution of microplastic concentrations. Depending on the distribution of the parameters studied, tests for differences were performed using the Kruskal-Wallis test (non-parametric rank sum test) followed by a post-hoc Dunn's test. For tests of the differences between the organs of the same patients (patient 1 to 3), a Wilcoxon test was also performed. All P values reported are two-sided and p<0.05 was considered as significant.
Limit of detection (LOD) and limit of quantitation (LOQ) were calculated. LOD is the lowest analyte concentration, where detection is feasible, likely to be reliably distinguished from the highest apparent analyte concentration when testing replicates of a blank sample containing no analyte (blank samples). LOD was calculated as the mean +3 SD. LOQ is the lowest concentration at which the analyte can not only be reliably detected, but at which predefined targets for further measurement accuracy are achieved. LOQ was calculated as the mean +10 SD.45
Role of Funders
No external funding was received for conducting this academic study. Funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Characteristics of studied individuals
Liver specimens of 2 female and 4 male patients with end-stage liver disease, 4 with alcoholic liver disease, 1 with autoimmune hepatitis, and 1 with alcoholic liver disease combined with HCV-cirrhosis were assessed. Median age was 56 years, median model for end-stage liver disease (MELD) score was 23. BMI of these patients was median 23.9 (range 18.2–43.8). All of them were Caucasians. None of the cirrhosis patients underwent previous liver surgery, liver specific or embolization treatments, such as selective internal radiation therapy (SIRT), transarterial chemoembolization (TACE), or transjugular intrahepatic portosystemic shunt (TIPS) implementation, nor suffered from malignant disease. Specimens of 5 individuals (1 female and 4 male) without underlying liver disease (median age 67 years) served as control group.
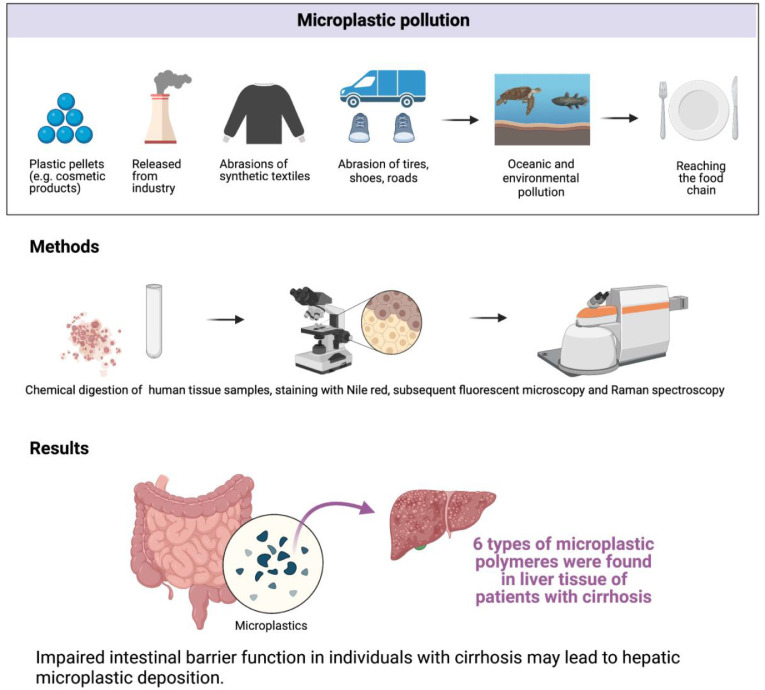
Figure 1 (study flow chart) summarizes the sources of MP pollution, methods as well as main findings of this study.
Figure 1.
Flow chart summarizing the relevance of MP pollution, methods as well as main findings of this study (created with BioRender).
MP concentrations in blank and human organ samples
MP concentrations in blank samples ranged from 1 to 2 particles per sample within the analyses. In total, 102 MP particles were detected in all 17 human tissue samples. MP concentrations in human tissues showed median particle numbers of 6 particles per sample and 1.4 particles per gram tissue, respectively. After the blank value correction, this corresponds to 1.2 particles per gram tissue, respectively. Lowest concentrations in human tissue with a median value of 0.0 particles per gram tissue were detected in kidney samples (0 particles per sample) (see Table 1, Figure 2). The concentrations in spleen samples were in median 1.1 per g tissue (5 particles per sample, after blank value correction: 0.9 particles per g tissue / 4 particles per sample). A median value of 4.6 particles per g tissue was detected in liver tissue of all patients (1 to 14 particles per sample, after blank value correction: 3.2 particles per g tissue/0 to 13 particles per sample). As shown in Figure 2, blank values were at a constant low level, such as concentrations determined in kidney tissue. In contrast, concentrations in spleen tissue were lower concerning Patients no. 1 and 3, but reveal a more distinct signal for Patient no. 2 (2.2 particles per g tissue, after blank value correction 1.9 particles per g tissue). A more heterogeneous distribution is also given concerning liver tissue where in total, 11 patients were included. Concentrations ranged from 0.3 to 11.9 particles per gram tissue in patients no. 1 to 11 (1 to 14 particles per sample, after blank value correction: 0.0 to 9.9 particles per g tissue, 0 to 13 particles per sample). Highest concentrations were detected in liver tissue samples of patients with cirrhosis (Patient no. 6 to 11) with values ranging from 4.6 to 11.9 particles per g tissue (6 to 14 particles per sample, after blank value correction: 3.2 to 9.9 particles per g tissue/4 to 13 particles per sample) compared to patients without underlying hepatic disease (Patient no. 1 to 5) with values ranging from 0.3 to 1.9 particles per g tissue (1 to 8 particles per sample, after blank value correction: 0.0 to 1.5 particles per g tissue/0 to 6 particles per sample).
Table 1.
Descriptive statistics - patient 1 to 11.
| n | Mean | Standard deviation (SD) |
Median | Minimum | Maximum | 25th percentile |
75th percentile |
|
|---|---|---|---|---|---|---|---|---|
| Overall samples | 17 | 3.4 (2.8) |
4.1 (3.5) |
1.4 (1.2) |
0.0 (0.0) |
11.9 (9.9) |
0.5 (0.2) |
6.2 (5.4) |
| Blank | 7 | 0.9 | 0.7 | 0.6 | 0.2 | 2.2 | 0.3 | 1.6 |
| Kidney | 3 | 0.2 (0.0) |
0.3 (0.3) |
0.0 (0.0) |
0.0 (0.0) |
0.5 (0.3) |
0.0 (0.0) |
n.d. (n.d.) |
| liver (patients without cirrhosis) |
5 | 1.0 (0.7) |
0.7 (0.7) |
0.7 (0.5) |
0.3 (0.0) |
1.9 (1.5) |
0.4 (0.1) |
1.7 (1.4) |
| liver (patients with cirrhosis) |
6 | 8.3 (6.9) |
3.2 (2.9) |
8.4 (7.4) |
4.6 (3.2) |
11.9 (9.9) |
4.7 (3.5) |
11.6 (9.8) |
| Spleen | 3 | 1.2 (1.0) |
0.9 (0.9) |
1.1 (0.9) |
0.4 (0.2) |
2.2 (1.9) |
0.4 (0.2) |
n.d. (n.d.) |
MP concentrations are reported in particles per gram tissue (basing on the mean weight of the tissue samples); blank corrected values are given in brackets.
Figure 2.
MP concentrations per patient, organ and associated blank (number per weight of tissue). Limit of Detection LOD = 3.0282 (mean + 3*SD); Limit of Quantification QOD = 7.9982 (mean + 10*SD).
MP concentrations in liver samples derived from patients with cirrhosis were significantly higher than those from blank samples (which underwent the same processing procedures) (n = 6, p = 0.009, α = 0.05, Table 2). Concerning samples of liver, kidney and spleen derived from healthy patients (without underlying cirrhosis), no significant difference between MP concentrations could be detected.
Table 2.
Test on significant differences between blank corrected MP-concentrations in kidney, spleen and liver from patients with and without cirrhosis.
| Sample matrix | Kidney | Spleen | Liver (patients without cirrhosis) |
Liver (patients with cirrhosis) |
|
|---|---|---|---|---|---|
| Kidney | significance | 0.258 | 0.303 | 0.001 | |
| significance (Bonferroni corrected) |
1.000 | 1.000 | 0.008 | ||
| Spleen | significance | 0.258 | 0.814 | 0.056 | |
| significance (Bonferroni corrected) |
1.000 | 1.000 | 0.334 | ||
| liver (patients without cirrhosis) | significance | 0.303 | 0.814 | 0.012 | |
| significance (Bonferroni corrected) |
1.000 | 1.000 | 0.071 | ||
| liver (patients with cirrhosis) | significance | 0.001 | 0.056 | 0.012 | |
| significance (Bonferroni corrected) |
0.008 | 0.334 | 0.071 |
P-values refer to the respective direct comparison of the samples (see column and row). Statistically significant values are printed in bold. Bonferroni correction based on n=6 tests).
Size distribution of MP particles in blank and human organ samples
Particle sizes of detected MPs did not differ significantly within different tissues. Particles in blank samples ranged from 3.3 to 20.1 µm (median 9.9 µm), particles detected in human tissue ranged from 3.0 to 29.5 µm (median 9.8 µm). See Suppl. Figures 2 and 3 for size distribution according to different organs.
Chemical composition and surface characteristics of MP particles in blank and human organ samples
Five MP particles originating from blank samples were spectroscopically analyzed. Two of these were identified as polyethylene terephthalate (PET) and one particle each was identified as PS and silicone. One particle did not give a conclusive signal. Particles from human tissue were composed of PS, polyvinyl chloride (PVC), PET, polymethyl methacrylate (PMMA), polyoxymethylene (POM), and polypropylene (PP) (Figure 3). Nine particles could not be identified due to immediate thermal degradation.
Figure 3.
Polymeric composition of particles found in human tissue samples (n = 56).
The quality of the obtained spectra depended on the presence of pigments, thermal degradation due to the laser excitation, and the general state of degradation of the selected particles (Figure 4). Mean (±standard deviation, SD) signal to noise ratio was 24±13 and mean match quality was 76±12%. Most particles had rough, uneven surfaces and showed signs of degradation.
Figure 4.
Examples of MP particles found in human tissue samples displayed as fluorescence images (left column) and true color microscope images (mid column) as well as related Raman-spectra. White scale bar indicates 10 µm, library reference spectra are displayed in red, particle spectra are given in green. S/N: signal to noise ratio.
The morphology of all MP particles was examined and classified as fragments despite 1 particle that was identified as microbead made from PS.
Discussion
MPs are found in all ecosystems and MP pollution represents a major challenge for the near future. However, although observations have been made of MP accumulation in different organs and tissues of rodents and there is proof of MP in human stool, and in the blood stream, it is unclear as to whether MPs can accumulate in humans, under which conditions and in which tissues.
Liver cirrhosis is a clinically relevant disease is the 11th leading cause of death. The absolute number of chronic liver disease, independently of stage and/or severity, is estimated up to 1.5 billion cases worldwide. The most common causes of liver cirrhosis worldwide are viral hepatitis (HBV/HCV), alcoholic liver disease and, increasingly, non-alcoholic fatty liver disease (NAFLD).46
In this proof-of-concept case series, we aimed to assess the presence of MPs in human tissue and to analyze the morphology, size and composition of MP polymers ranging from 4 to 30 µm. To this end, we analyzed cirrhotic liver samples and liver, spleen and kidney samples of patients without underlying liver disease.
We report presence of MPs in human liver tissue samples of individuals with liver cirrhosis. Notably, no significant proof of MPs was observed in sample groups of kidney, spleen and liver tissue derived from patients without underlying liver disease. We reveal that MP concentrations are elevated in cirrhotic hepatic tissue samples in contrast to non-cirrhotic liver samples, indicating that chronic liver disease seems to be a key driver in MP accumulation in human liver.
Furthermore, our analysis showed that various polymer types of MP can be found in cirrhotic liver tissue. In addition to the commonly observed plastic polymers PS, PVC and PET, we also found PMMA, POM and PP. Surprisingly, some MP particles identified had altered surfaces, in terms of presumed degradation. This suggests that particles had been deposited in the organ for a long time and had been exposed to possible biochemical processes. However, this observation requires further investigation.
Considering the accumulation in cirrhotic liver samples, the exact role of MPs in liver disease remains to be fully explored, i.e. whether they are a cause or a consequence. The role of MPs in the liver regarding hepatic fibrogenesis and cirrhosis development remains unclear. Very recently, it was reported that MP exposure led to a significant expression of fibrosis markers, such as transforming growth factor-β, fibronectin and α-smooth muscle actin in rats.47 Another group, not long ago, observed that polystyrene MPs induce oxidative stress and apoptosis of the myocardium in a rat model and are ultimately able to induce cardiac fibrosis via the Wnt/β-catenin pathway.48 We know that this pathway also plays an important role in pathogenesis of liver fibrosis.49 Therefore, it cannot be excluded that MPs represent a potential co-factor or cause of hepatic fibrogenesis.
On the other hand, MP accumulation in the liver could be considered as a consequence of chronic liver disease. For example, it is hypothesized that portal hypertension (the main cause of the clinical complications of liver cirrhosis), leading to impaired intestinal barrier function (also known as “leaky gut”), allows MP particles to migrate through the intestinal wall, and be transported to the liver.50 It could be speculated that MPs enter the portal venous circulation, possibly in a similar way as microorganisms penetrate the intestinal wall in the context of bacterial translocation in patients with spontaneous bacterial peritonitis (SBP), a common complication of liver cirrhosis with portal hypertension. Bacterial translocation in cirrhosis is assumed to be caused by an increased gastrointestinal permeability as a result of an impairment of the cell-cell junction, in the intestinal wall.51 Bacteria that can pass through the intestinal wall in the context of SBP are relatively small, such as E. coli with a diameter of about 1.5 µm and a length of 2 to 6 µm.52 Larger immune cells, such as macrophages (with a diameter of up to 21 µm), are also found in the intestinal wall, and are assumed to be able to migrate through it.53,54 Besides the actual size, other factors, such as the functional integrity of the intestinal wall itself, the polarity, water solubility or fat solubility of a substance, or whether it is taken up actively (e.g. transporter controlled) or passively (e.g. along a gradient), determine which substance may pass the intestinal wall and which cannot.55 Inflamed mucosal areas could contribute to MP uptake and/or translocation. Schmidt et al. investigated the potential of MP uptake in patients with Crohn's disease and colitis ulcerosa, after rectal MP application. In biopsies, they observed a significantly enhanced accumulation of MPs in inflamed mucosal areas and ulcerous lesions, in comparison to healthy controls.41 Plastic polymers have been detected in the blood stream in 22 healthy volunteers. These findings are very interesting because we see that MPs can be absorbed and circulate systemically in the body. However, no conclusions can be drawn from this study about a possible deposition in peripheral organs or about an association with disease.32 How and where (small intestine or large intestine) MPs are presumably taken up systemically needs to be investigated in future studies.
It needs to be kept in mind that MP concentrations detected in human tissue are generally very low and close to or even below the detection limit of currently available approaches. We therefore developed a reliable and sensitive method to detect MP particles from 4 to 30 µm. Further comments on methodology and considerations regarding future approaches are given in the supplement.
This study had several limitations. First, because the methodology requires chemical digestion, we cannot determine exactly where in the liver MPs accumulated and whether MPs were deposited intracellularly, for example in Kupffer cells. However, we can still assume potential sites either in hepatocytes, cholangiocytes, or immune cells, such as the Kupffer cells or hepatic macrophages. If the MP particles were merely in the bloodstream, without any parenchymatous accumulation in the liver, such particles would also be expected in spleen and kidney samples. Future studies, possibly using electron microscopy, should clarify the specific accumulation sites. Second, this study sets a lower MP detection limit at 4 µm. Future approaches should consider increasing optical resolution of fluorescent microscopy to address particle sizes down to 1 µm. Third, the sample size was rather small. However, this is a proof-of-concept case series, to analyze different human tissue samples for presence of MPs. Next, exact data on the dietary habits and living conditions are not available. Unfortunately, therefore, we cannot draw any conclusions regarding the possible primary alimentary cause of MP ingestion. MPs could also be enriched in the body through surgical or interventional measures or examinations. However, none of the studied cirrhosis patients underwent liver surgery or radiological embolization procedures (such as SIRT, TACE), which may have left MP residues. Adjustment for potential confounders, such as age or sex was not possible due to the small sample size. Lastly, a unified methodology to detect MPs in human tissue has not yet been established, making comparison with other scientific work difficult. Nevertheless, it should be noted that after intensive preliminary work, and improvement of the existing methodology, we were able to establish a high quality, and credible method for MP detection in human tissue. However, future studies are needed to reproduce these findings in larger cohorts.
Taken together, this proof-of-concept case series assessed the presence of MPs in human liver tissue. We observed that (i) MPs were found in the liver of individuals with liver cirrhosis, but not in those without underlying liver disease; and that (ii) six different microplastic polymers ranging from 4 to 30 µm in size could be identified. Our results indicate that chronic liver disease seems to be a key driver in MP accumulation in human liver and that there is a need to evaluate whether hepatic MP accumulation represents a potential cause in the pathogenesis of fibrosis, or a consequence of cirrhosis and portal hypertension.
Contributors
All authors read and approved the final version of the manuscript. T.H., E.F. concept and design, have accessed and verified the data, responsible for decision to submit the manuscript; M.T., E.F. experiments and procedures, accessed and verified the data; T.H., B.L., M.S., K.P. sample collection and data acquisition; T.H., M.T., E.F: drafting the manuscript; A.C., L.F., K.P., S.H., critical revision for important intellectual content.
Data sharing statement
Data are available on request from the authors.
Declaration of interests
All authors declare that they have no conflict of interests regarding this manuscript.
Acknowledgement
No external funding was received for conducting this academic study. We want to thank Jenny Wigger, for her assistance with sample collection, and Elaine Hussey for linguistic proofreading and revision.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104147.
Contributor Information
Thomas Horvatits, Email: t.horvatits@uke.de.
Elke Kerstin Fischer, Email: elke.fischer@uni-hamburg.de.
Appendix. Supplementary materials
References
- 1.de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biol. 2018;24(4):1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702 doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 3.van Raamsdonk LWD, van der Zande M, Koelmans AA, et al. Current insights into monitoring, bioaccumulation, and potential health effects of microplastics present in the food chain. Foods. 2020;9(1):72. doi: 10.3390/foods9010072. Published online 9 Jan 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooi M, Koelmans AA. Simplifying microplastic via continuous probability distributions for size, shape, and density. Environ Sci Technol Lett. 2019;6(9):551–557. [Google Scholar]
- 5.Arthur C, Baker J, Bamford H, editors. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Micro-plastic Marine Debris. Vol. 2009. NOAA Technical Memorandum NOS-OR&R-30; 2009. [Google Scholar]
- 6.van Raamsdonk LWD, van der Zande M, Koelmans AA, et al. Current insights into monitoring, bioaccumulation, and potential health effects of microplastics present in the food chain. Foods. 2020;9(1):72. doi: 10.3390/foods9010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barboza LGA, Gimenez BCG. Microplastics in the marine environment: current trends and future perspectives. Mar Pollut Bull. 2015;97(1-2):5–12. doi: 10.1016/j.marpolbul.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Klein M, Fischer EK. Microplastic abundance in atmospheric deposition within the metropolitan area of Hamburg, Germany. Sci Total Environ. 2019;685:96–103. doi: 10.1016/j.scitotenv.2019.05.405. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Busquets R, Campos LC. Assessment of microplastics in freshwater systems: a review. Sci Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135578. [DOI] [PubMed] [Google Scholar]
- 10.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 11.Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136 doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Zhang Y, Kang S, Wang Z, Wu C. Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.141948. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson TM, Vethaak AD, Almroth BC, et al. Screening for microplastics in sediment, water, marine invertebrates and fish: method development and microplastic accumulation. Mar Pollut Bull. 2017;122(1-2):403–408. doi: 10.1016/j.marpolbul.2017.06.081. [DOI] [PubMed] [Google Scholar]
- 14.Karbalaei S, Hanachi P, Walker TR, Cole M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ Sci Pollut Res. 2018;25(36):36046–36063. doi: 10.1007/s11356-018-3508-7. [DOI] [PubMed] [Google Scholar]
- 15.Koelmans AA, Mohamed Nor NH, Hermsen E, et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–422. doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Xu EG, Li J, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54(7):3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang S, Olga V, et al. The potential effects of microplastic pollution on human digestive tract cells. Chemosphere. 2022;291(Pt 1) doi: 10.1016/j.chemosphere.2021.132714. [DOI] [PubMed] [Google Scholar]
- 18.Cho YM, Choi K-H. The current status of studies of human exposure assessment of microplastics and their health effects: a rapid systematic review. Environ Anal Health Toxicol. 2021;36(1) doi: 10.5620/eaht.2021004. Epub 4 Feb 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman A, Sarkar A, Yadav OP, Achari G, Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. Lifetime accumulation of microplastic in children and adults. Environ Sci Technol. 2021;55(8):5084–5096. doi: 10.1021/acs.est.0c07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deville S, Penjweini R, Smisdom N, et al. Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim Biophys Acta (BBA) - Mol Cell Res. 2015;1853(10):2411–2419. doi: 10.1016/j.bbamcr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34(3):J226–JJ33. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175(3):191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 24.Jeong CB, Kang HM, Lee MC, et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci Rep. 2017;7:41323. doi: 10.1038/srep41323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong C, Valiyaveettil S, Tang B. Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health. 2020;17(5):1509. doi: 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371(6530):672–674. doi: 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- 27.Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7(1):46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631-632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Stock V, Bohmert L, Lisicki E, et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol. 2019;93(7):1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- 30.Bohmert L, Stock V, Braeuning A. Plausibility of microplastic uptake in a paper by Deng et al. Scientific reports 7:46687, 2017. Arch Toxicol. 2019;93(1):217–218. doi: 10.1007/s00204-018-2383-9. [DOI] [PubMed] [Google Scholar]
- 31.Deng Y, Zhang Y. Response to uptake of microplastics and related health effects: a critical discussion of Deng et al. Scientific reports 7: 46687, 2017. Arch Toxicol. 2019;93(1):213–215. doi: 10.1007/s00204-018-2384-8. [DOI] [PubMed] [Google Scholar]
- 32.Leslie HA, van Velzen M, Brandsma SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163 doi: 10.1016/j.envint.2022.107199. Epub 24 Mar 2022. [DOI] [PubMed] [Google Scholar]
- 33.Schwabl P, Koppel S, Konigshofer P, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171(7):453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Li YB, He HR, Zhang JF, Ma GS. You are what you eat: microplastics in the feces of young men living in Beijing. Sci Total Environ. 2021;767 doi: 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim YS, Tuan Anuar S, Azmi AA, et al. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5(1):116–121. doi: 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health. 2020;17(4) doi: 10.3390/ijerph17041212. Published online 13 Feb 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain N. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50(1-2):107–142. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 38.Volkheimer G. Hematogenous dissemination of ingested polyvinyl chloride particles. Ann N Y Acad Sci. 1975;246(1 Toxicity of V):164–171. doi: 10.1111/j.1749-6632.1975.tb51092.x. [DOI] [PubMed] [Google Scholar]
- 39.Lusher A, Hollman PCH, Mendoza-Hill J. Vol. 2017. Food and Agriculture Organization of the United Nations; Rome: 2017. p. 126. (Microplastics in Fisheries and Aquaculture: Status of Knowledge on their Occurrence and Implications for Aquatic Organisms and Food Safety). [Google Scholar]
- 40.Carr KE, Smyth SH, McCullough MT, Morris JF, Moyes SM. Morphological aspects of interactions between microparticles and mammalian cells: intestinal uptake and onward movement. Progr Histochem Cytochem. 2012;46(4):185–252. doi: 10.1016/j.proghi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt C, Lautenschlaeger C, Collnot E-M, et al. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa—a first in vivo study in human patients. J Control Rel. 2013;165(2):139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 43.Braun T, Ehrlich L, Henrich W, et al. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics. 2021;13(7):921. doi: 10.3390/pharmaceutics13070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munno K, De Frond H, O'Donnell B, Rochman CM. Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E) Anal Chem. 2020;92(3):2443–2451. doi: 10.1021/acs.analchem.9b03626. [DOI] [PubMed] [Google Scholar]
- 45.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 46.Cheemerla S, Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Dis (Hoboken) 2021;17(5):365–370. doi: 10.1002/cld.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An R, Wang X, Yang L, et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449 doi: 10.1016/j.tox.2020.152665. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Zhu S, Liu Q, et al. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/beta-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. 2020;265(Pt A) doi: 10.1016/j.envpol.2020.115025. [DOI] [PubMed] [Google Scholar]
- 49.El-Ashmawy NE, Al-Ashmawy GM, Fakher HE, Khedr NF. The role of WNT/beta-catenin signaling pathway and glutamine metabolism in the pathogenesis of CCl4-induced liver fibrosis: repositioning of niclosamide and concerns about lithium. Cytokine. 2020;136 doi: 10.1016/j.cyto.2020.155250. [DOI] [PubMed] [Google Scholar]
- 50.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutz P, Nischalke HD, Strassburg CP, Spengler U. Spontaneous bacterial peritonitis: the clinical challenge of a leaky gut and a cirrhotic liver. World J Hepatol. 2015;7(3):304–314. doi: 10.4254/wjh.v7.i3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu H, Chen A, Song X, et al. How Escherichia coli lands and forms cell clusters on a surface: a new role of surface topography. Sci Rep. 2016;6:29516. doi: 10.1038/srep29516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol. 2018;9:2733. doi: 10.3389/fimmu.2018.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krombach F, Munzing S, Allmeling AM, et al. Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect. 1997;105(suppl 5):1261–1263. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loehry CA, Axon AT, Hilton PJ, Hider RC. Creamer B. Permeability of the small intestine to substances of different molecular weight. Gut. 1970;11(6):466–470. doi: 10.1136/gut.11.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.