Abstract
Background:
Objective biomarkers of cognitive vulnerabilities related to suicidal ideation (SI) may assist in early prevention in adolescents. Previously, we found that smaller gray matter volumes (GMVs) of the dorsal striatum prospectively predicted implicit SI, measured using a computerized implicit association test (IAT) assessing associations between “self” and “death,” in a community sample of adolescents. Here, we sought to replicate these findings in an independent sample of depressed adolescents.
Methods:
53 depressed adolescents who varied in severity of suicidal thoughts and behaviors completed high-resolution structural MRI. Caudate, putamen, and nucleus accumbens GMVs were estimated using FreeSurfer 6.0. Robust linear regressions were used to examine associations between striatal GMVs and implicit and explicit SI, covarying for sex, age, total intracranial volume, medication use, and depression severity. Significance was determined using Bonferroni correction. Finally, LASSO regression was used to identify which striatal GMV contributed most to prediction of SI.
Results:
Smaller bilateral caudate and right nucleus accumbens GMVs were associated with stronger IAT scores (all ps<0.001). Smaller putamen and nucleus accumbens GMVs were not associated with implicit or explicit SI. Our LASSO analysis indicated that right caudate GMV contributed most to the prediction of IAT scores.
Conclusions:
This study is the first to demonstrate that caudate GMVs are significantly associated with implicit self-associations with death in a sample of depressed adolescents. When considered with our previous work, smaller caudate GMVs may be a robust biomarker of SI in adolescents, with clinical implications for early identification of youth at risk for engaging in suicidal behaviors.
Keywords: implicit association test, caudate, adolescents, suicidal ideation
Introduction
Suicide exceeds all physical diseases as a cause of death in adolescents (ages 10–24 years; Mokdad, 2016; WHO, 2014). Although suicide has a relatively low prevalence, for every suicide there are at least 50 non-fatal suicide attempts, and many more adolescents who experience suicidal thoughts or engage in self-harming behaviors (Shain, 2016). Moreover, current global estimates are that almost 75% of college-aged individuals with suicidal thoughts and behaviors experienced their first onset prior to 16 years (Mortier et al., 2018). Collectively, these data underscore the urgent need to identify reliable risk factors in adolescents prior to attempts.
In this context, suicidal ideation (SI) is a significant risk factor for suicide attempts and other self-harming behaviors; however, it is important to recognize that SI is distinct from suicide attempts, with both shared and unique risk factors (Klonsky et al., 2016), and, furthermore, is a distressing symptom that warrants treatment. Indeed, rates of SI are rising among adolescents, with recent lifetime estimates of 8.4% in youth ages 9–10 years (Janiri et al., 2020), which underscores the urgent need to identify predictors and markers of vulnerability to SI. There are several challenges, however, in measuring SI in adolescents, including the transient nature of such thoughts, societal stigma, and the desire to conceal endorsement of SI (Nock et al., 2010). Consequently, researchers have increasingly leveraged measures of SI that do not rely solely on self-report in order to assess cognitive propensities for engaging in SI and self-harming behaviors (Cha et al., 2010; Barnes et al., 2016; Harrison et al., 2018). One such measure is the death-version of the Implicit Association Test (IAT), which measures response latencies to speeded judgments of pairs of words related to death (e.g., “funeral”) and to the self (e.g., “me”; Glenn et al., 2017; Nock et al., 2010). Higher IAT scores reflect greater standardized differences in response latencies to “self/death” pairs than to “self/life” pairs, and, thus, are posited to index a cognitive vulnerability to engaging in suicidal thoughts and/or behaviors. While there is conflicting evidence regarding the clinical utility of IAT scores and whether they provide additional predictive power over other risk factors, including prior attempts and patient self-report (Harrison et al., 2018), some studies have reported that higher IAT scores are associated with future suicide attempts and other risk behaviors (Harrison et al., 2014; Nock et al., 2010; see also Tello et al., 2020, which is a direct replication of the study by Nock et al., 2010), and with subsequent changes in suicidal ideation (Glenn et al., 2017). Thus, elucidating the neurobiological correlates of performance on the IAT may provide insight into biomarkers of cognitive vulnerability for suicidal thoughts and behaviors that, importantly, do not rely solely on self-report.
Virtually no neuroimaging studies have examined suicide risk in the context of IAT (for reviews, see Cox Lippard et al., 2014, and Schmaal et al., 2020). In the only study to date to do so, researchers focused on young adolescents (ages 9–13 years) recruited from the community with minimal psychiatric histories and symptoms. In that study, smaller gray matter volumes (GMVs) of the putamen and caudate significantly predicted IAT scores 2 years later; in contrast, GMVs of the putamen and caudate did not predict explicit self-report measures of SI (Ho et al., 2018). Surprisingly, GMVs of the nucleus accumbens (NAcc) did not predict either IAT scores or explicit SI as we had expected given previous work linking this region with adolescent depression (Forbes & Dahl, 2012; Pan et al., 2017; Stringaris et al., 2015; Whittle et al., 2014) and impulsivity (Ballard & Knutson, 2009; Galtress & Kirkpatrick, 2010), which are two major risk factors for suicidality (Franklin et al., 2017). Because of the high density of dopamine receptors found in these striatal structures, we interpreted these findings as consistent with post-mortem evidence showing dopaminergic alterations in the striatum in suicide victims (Oquendo et al., 2014).
It is important to note that our prior study examined a non-clinically recruited sample with minimal symptoms and diagnoses. Thus, it is unclear if the associations we found between smaller dorsal striatum GMVs and higher implicit SI will also evidence in a clinical sample. Thus, to extend our previous investigation, we recruited a sample of depressed adolescents with varying history of endorsement of suicidal thoughts and behaviors. Although not all adolescents suffering from SI experience depression symptoms, depression is a major risk factor for SI (Franklin et al., 2017; Klonsky et al., 2016). Thus, identifying neuroanatomical markers that do and do not converge with those found in our previous investigation will validate whether those findings generalize to depressed adolescents. Based on our previous findings, we hypothesized that smaller putamen and caudate GMV, but not NAcc GMV, will be associated with vulnerability to SI, as indexed by higher IAT scores, but will not be associated with explicit SI.
Methods
Participants
Fifty-three adolescents (35 female assigned at birth) between the ages of 13 and 18 (mean age: 16.25±1.32 years) were recruited from the San Francisco Bay Area community as part of a longitudinal study examining neurobiological mechanisms underlying adolescent stress and depression (K01MH117442; Walker & Teresi, et al., 2020). Potential participants were recruited from the San Francisco Bay Area community using flyers posted at Stanford psychiatry and community clinics, local coffee shops, advertisements on Craigslist, Nextdoor, and Facebook, and an internal referral program. Before being invited for a laboratory visit for more in-depth diagnostic interviewing with the adolescent and their parent(s), a parent completed a screening interview over the telephone where they responded to questions about their child’s current symptoms of depression, and lifetime history of mania, psychosis, and substance dependence, as well as questions related to exclusion criteria of the study (see below). Inclusion criteria at the baseline visit of the study included fluency in English, presence of current threshold or sub-threshold Major Depressive Disorder (MDD) or Dysthymia via child or parent report according to DSM-IV criteria as well as scores above the clinical threshold on the Children’s Depressive Rating Scale—Revised (CDRS-R; see Clinical Assessments, below). Exclusion criteria were premenarchal status (for females), history of concussion within the past 6 weeks or history of any lifetime concussion with loss of consciousness, contraindications to MRI scanning (e.g. braces, metal implants, or claustrophobia), serious neurological or intellectual disorders that could interfere with the participant’s ability to complete study components, or meeting lifetime or current DSM-IV criteria for any Bipolar Disorder, Psychosis, or Alcohol Dependence, or DSM-5 criteria for Moderate Substance Use Disorder with substance-specific threshold for withdrawal. For the present study, participants were included in final analyses if they provided usable structural MRI data (see MRI scans, below) and completed a sufficient number of trials on the IAT (see IAT, below) or the Suicidal Ideation Questionnaire—Junior (see SIQ-JR, below). Of the 53 adolescents who provided usable MRI data (see Table 1 for descriptive statistics), 1 participant failed to successfully complete the IAT but did complete the SIQ-JR. All participants completed the SIQ-JR. The study was approved by the Institutional Review Board (IRB) at Stanford University. All participants and their parents gave written assent and informed consent, respectively, in accordance with the Declaration of Helsinki, and were financially compensated for their participation.
Table 1:
Sample demographic and clinical characteristics for 53 participants with usable MRI data. All values are report as mean ± SD (min – max). Numbers in brackets [] indicate the number of missing or unusable responses.
| Variable | Descriptive statistics |
|---|---|
|
| |
| Age | 16.25 ± 1.32 (13.67 – 18.39) |
| Sex (M/F) | 18/35 |
| SIQ score | 24.91 ± 18.06 (3 – 76) |
| IAT score | −0.13 ± 0.25 (−1 – 0.32) [1] |
| CDRS-R | 47.89 ± 12 (26 – 81) |
| Tanner | 4.50 ± 0.51(3 – 5) [8] |
| L caudate GMV (mm3) | 3687.28 ± 391.98 (3027.6 – 4899.1) |
| R caudate GMV (mm3) | 3807.74 ± 402.46 (3124.2 – 4718.7) |
| L putamen GMV (mm3) | 5026.90 ± 491.32 (3927 – 6155.5) |
| R putamen GMV (mm3) | 5125.27 ± 485.77 (3874.5 – 6172.2) |
| L NAcc GMV (mm3) | 520.08 ± 95.29 (310.5 – 694.3) |
| R NAcc GMV (mm3) | 555.42 ± 79.11 (390.6 – 714) |
| ICV (mm3) | 1.53 × 106 ± 1.71 × 105 (1.18 × 106 – 1.86 × 106) |
| Medication (psychotropic) (%) | 47.17% |
| Antidepressant | 33.96% |
| Stimulant | 11.32% |
| Benzodiazepine | 3.77% |
| Antipsychotic | 5.66% |
| Other1 | 15.09% |
| Concurrent Therapy | 26.42% |
| No response | 9.43% |
| Therapy2 (%) | 49.06% |
| Age of depressive episode onset | 13.73 ± 2.31 (4 – 17) |
| Age of first SI | 13.17 ± 1.93 (8 – 17) [1] |
| Suicide Attempt (%) | 22.64% |
| Age of first attempt | 13.33 ± 2.39 (10 – 17) |
| Number of attempts3 | 2 ± 1.21 (1 – 5) |
| Handedness (% R dominant) | 96.23% |
| Ethnicity | |
| Hispanic or Latino | 16.98% |
| Not Hispanic or Latino | 83.02% |
| Race | |
| White/Caucasian | 47.17% |
| African American or Black | 3.77% |
| Asian | 18.87% |
| American Indian or Alaska Native | 3.77% |
| Native Hawaiian or Other Pacific Islander | 0% |
| Multiracial | 20.75% |
| Other | 5.66% |
| Parental Level of Education | |
| No GED/no high school diploma | 0% |
| GED/high school diploma | 1.89% |
| Some college, no degree | 9.43% |
| Associate’s degree | 3.77% |
| Bachelor’s degree | 26.42% |
| Master’s degree | 33.96% |
| Doctoral or Professional degree | 15.09% |
| No response | 9.43% |
Other medications taken by participants, with () indicating the number of participants on that medication, that did not fit into the above classifications include Gabapentin (1), Trazodone (4), Buspar (1), Dextromethorphan (1), and Cannabidiol (1).
Therapy indicates the percentage of participants who reported attending therapy sessions for their depression in the 2 months prior to their first visit.
Computed only in participants who reported a history of attempt based on the C-SSRS
Clinical Assessments
Trained research assistants interviewed participants at an initial behavioral session to assess study eligibility using the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime (K-SADS-PL; Kaufman 1997, 2000) and Children’s Depression Rating Scale—Revised (CDRS-R; Poznanski & Mokros, 1996). The K-SADS-PL is a semi-structured diagnostic interview used by clinicians to generate reliable and valid DSM-IV Axis I disorder diagnoses in children. For the present study, research assistants administered Screening Interviews and Supplements for the following modules: Major Depressive Disorder, Mania, Psychosis, Cigarette/Tobacco Use, Alcohol Abuse, and Substance Use Disorder (see Kaufman et al., 2016 for DSM-5 Substance Use Disorder module). Every diagnosis was reviewed for accuracy and reliability by a clinically-trained research team.
In addition to the K-SADS-PL, interviewers also administered the CDRS-R, a 17-item clinician-rated semi-structured interview, to assess depression symptom severity, which has shown strong validity and reliability in adolescents (Mayes et al., 2010). For the present study, we administered the 17-item CDRS-R to all participants and an amended 14-item version, which excluded the 3 observational items, to participant’s parents. Clinically trained staff integrated both reports after team discussions to generate a single summary score. Raw summary scores ≥ 30, equivalent to a t-score ≥ 55, indicate that the presence of a depressive disorder is likely to be confirmed in a comprehensive diagnostic evaluation and, therefore, was used as a cut-off for study eligibility for participants who did not meet criteria for a depressive disorder according to the K-SADS-PL, provided that they also endorsed at least 2 symptoms of MDD or Dysthymia in the K-SADS-PL screening. Thus, entry into the study was determined either by meeting DSM-IV criteria for a depressive disorder through the K-SADS-PL or, if meeting for subthreshold depression according to the K-SADS-PL, by a CDRS-R raw cut-off score of 30. Following the initial behavioral session, participants were invited to return to the laboratory at a separate date to complete the MRI scan and IAT (mean interval: 10.98±6.06 days).
Tanner Stage
As in our previous study (Ho et al., 2018), participant’s pubertal development was measured using self-report Tanner staging (Marshall & Tanner, 1969; Marshall & Tanner, 1970; Morris & Udry, 1980). As in prior studies (Ho et al., 2018, 2020), overall pubertal development was calculated by averaging the adrenal (pubic hair) and gonadal (breast/testes) ratings.
SIQ-JR
The Suicidal Ideation Questionnaire—Junior (SIQ-JR; Reynolds, 1987) was administered to participants at their initial behavioral session to evaluate severity of SI based on frequency in the past month. The SIQ-JR is a self-report questionnaire comprised of 15 items (e.g., “I thought about killing myself,” “I thought about how I would kill myself,” etc), each rated on a seven-point scale from 0 (I never had this thought) to 6 (Almost every day), with higher scores indicating more frequent thoughts and, thus, higher risk. A score of 31 is the recommended clinical cut-off. The SIQ-JR is reliable and has strong criterion validity for measuring SI in children and young adolescents (Reynolds & Mazza, 1999).
Implicit Association Test
We administered the death-version of the IAT to examine implicit SI by measuring participants’ response latencies to associations between self- and not self-related stimuli and life- and death-related stimuli (Nock et al., 2010). IAT scores have been demonstrated to have high construct validity (Greenwald et al., 1998; Nosek et al., 2005) and the death-version of the IAT specifically has been demonstrated to have predictive validity of suicidal thoughts and behaviors in adolescents and young adults (Glenn et al., 2017; Harrison et al., 2014; Nock et al., 2010; Tello et al., 2020). Participants completed an in-house MATLAB (2014b; MathWorks, Natick, MA) version of the death IAT, programmed using Psychtoolbox 2 (Brainard, 1997; Pelli, 1997) at a computer station. Participants were instructed to sort words which presented in the center of the screen into four categories (“death”, “life”, “me”, “not me”) by pressing keys with their left and right index fingers. Participants completed practice trials in which they were asked to sort words into only one categorical construct (“Death/Life” or “Me/Not Me”) before moving on to test trials in which they had to sort words into both construct categories simultaneously. Test trial pairings were counterbalanced across participants. Response times (RTs) of the test trial pairings were recorded in milliseconds and analyzed in MATLAB using an in-house scoring script based on the conventional IAT algorithm (Greenwald et al., 2003). Standardized differences (i.e., d-scores) in RTs to “Death/Me” versus “Life/Me” pairings on test trials were computed as an index of implicit SI. With this scoring method, positive scores indicate faster RTs to “Death/Me” pairings versus “Life/Me,” suggesting an implicit association between the self and death; negative scores indicate slower RTs to “Death/Me” pairings relative to “Life/Me” pairings. Participants with more than 18 test trials that were deemed guesses (RT<30 ms) or lapses in attention (RT>10s) were excluded. One participant was excluded based on these criteria (total n=52 for all analyses including IAT). Stimulus sets and code used to score the IAT are available on Github at http://www.github.com/tiffanycheingho/d-IAT/.
MRI Scanning Acquisition and Segmentation
All MRI scans were acquired at the Stanford Center for Cognitive and Neurobiological Imaging (CNI) with a 3T MRI scanner (GE Discovery MR750) and Nova 32-channel head coil. Participants completed a T1-weighted anatomical scan obtained using a spoiled gradient (SPGR) sequence (TR/TE/TI=8.2/3.2/600 ms; flip angle=12°; 156 axial slices; FOV=25.6 cm; matrix=256 mm x 256 mm, isotropic voxel=1 mm, total scan time: 3:40). GMV estimates of the caudate nucleus (Figure 1), putamen, NAcc, and estimates of total intracranial volume (ICV) were calculated using the recon-all function (https://surfer.nmr.mgh.harvard.edu/fswiki/recon-all) from FreeSurfer v. 6.0 (Fischl et al., 2002).
Figure 1.
Representative segmentation of caudate from a single participant.
MRI Quality Control
All MRIs were visually inspected for motion artifact before being subjected to segmentation from FreeSurfer, as described in previous work (Ho et al., 2018; King et al., 2019). Specifically, we: 1) visually inspected each T1-weighted image to determine usability on the basis of motion (i.e., “ringing” artifacts) prior to segmentation; 2) visually inspected each automated segmentation output from FreeSurfer against the T1-weighted volume for accuracy; 3) extracted volumes for each hemisphere and converted them to z-scores and visually examined any segmentations for volumes where z-scores ≥ |2.5|. Any segmentations (left or right, separately) that failed any of these steps were removed from final analyses.
Statistical Analyses
All analyses were conducted in R (v 3.5.3; R Core Team). Given the skewed distributions of the SIQ scores and the non-normality of the residuals from linear models where SIQ scores were an outcome measure (assessed with Q-Q plots), we conducted Spearman rank correlations to determine associations between SIQ scores and other variables of interest. All other associations were conducted using Pearson’s correlations. Using the ‘rlm’ function from the MASS package in R, we conducted robust linear regressions fitted with iterated re-weighted least squares to estimate associations between striatal (caudate, putamen, NAcc) GMVs with implicit SI (IAT d-scores) and explicit SI (SIQ-JR scores). We elected to use robust linear regressions to reduce the potential influence of outliers. To evaluate significance of the robust linear regression models, we used ‘f.robftest’ from the sfsmisc package in R. We included age, sex, ICV, psychotropic medication use, and CDRS-R t-scores as covariates in all of our statistical models. To correct for multiple comparisons across 12 tests (bilateral caudate, putamen, and NAcc in relation to SIQ and IAT), we conservatively applied Bonferroni correction and set our significant threshold to p<0.004 (p<0.05/12). We included Tanner scores as a covariate in sensitivity analyses because not all participants completed this measure (see Table 1). To estimate standardized coefficient weights (β), all predictor and response variables were z-scored.
Finally, to test the specificity of our findings to the striatum, we conducted supplemental analyses to determine if the effects were non-significant in non-striatal subcortical structures (i.e., amygdala and hippocampus), as we also observed in our previous investigation. Further, we conducted Least Absolute Shrinkage and Selection Operator (LASSO) regression using the ‘cv.glmnet’ function from the glmnet package in R (Zou & Hastie, 2005) by setting α=1. LASSO (and other related approaches, including ridge regression and elastic net) are preferred over standard linear regression when multicollinearity is present (Hastie et al., 2009). LASSO is ideal for conducting variable selection in the context of generating a sparse solution due to the application of the L1 penalty, which tends to set coefficients of non-relevant predictors to 0; in contrast, ridge regression uses the L2 penalty which shrinks or minimizes coefficients of non-relevant predictors to prevent overfitting (elastic net a data-driven approach that optimizes both L1 and L2 penalties; Hastie et al., 2009; Zou & Hastie, 2005). Given that the goal of this analysis was to identify the striatal structure—left caudate, right caudate, and right NAcc—most strongly associated with IAT scores when including all of these predictor variables in the same model, we elected to use LASSO over ridge regression and elastic net. To estimate the coefficient weights for each predictor in our model, we performed 10-fold cross-validation to optimize the regularization parameter (λ), which controls the magnitude by which the penalty (here, L1) is applied on the basis of minimizing mean squared error (MSE). We conducted this analysis using the complete cases of IAT and caudate and NAcc GMVs in our data set (n=52).
Results
Descriptive statistics
Demographics and clinical characteristics of participants are presented in Table 1. A correlation matrix of our primary outcomes of interest and covariates is presented in Table 2. Neither SIQ scores nor IAT scores were significantly associated with age (all ps>0.665), Tanner scores (all ps>0.075), or CDRS-R scores (all ps>0.516). As expected, all striatal GMVs of interest were highly intercorrelated (all rs>0.40, ps<0.003). Males and females did not differ in SIQ, IAT, or CDRS-R scores (all ps>0.106). Finally, males and females did not differ in bilateral putamen and NAcc GMVs (ps>0.053); however, males had larger bilateral caudate GMV (right: t51=2.190, p=0.033; left: t51=2.681, p<0.010) and total ICV (t51=5.344, p<0.0001).
Table 2:
Summary of zero-order correlations between variables of interest and SIQ scores and IAT scores. All correlations with SIQ scores were assessed using Spearman rank correlation tests and all other correlation were assessed using Pearson’s correlation. No associations were significant at α=0.05 (2-tailed).
| SIQ score | IAT score | |
|---|---|---|
|
| ||
| SIQ score | -- | -- |
| IAT score | −0.036 | -- |
| Age | −0.061 | −0.007 |
| Tanner | 0.029 | −0.271 |
| CDRS-R | 0.045 | 0.092 |
Smaller caudate gray matter volumes are significantly associated with higher IAT scores
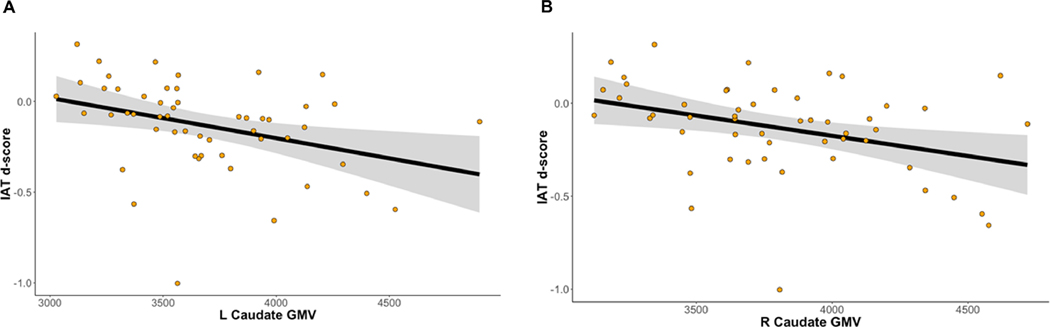
Smaller bilateral caudate GMV was significantly associated with higher IAT scores (left: β=−0.505±0.140, t45=−3.604, p<0.001; right: β=−0.490±0.131, t45=−3.726, p<0.001). See Figure 2 for more details. Bilateral putamen GMV was not associated with IAT scores (all ps>0.583). Neither caudate nor putamen GMV was significantly associated with SIQ scores (all ps>0.118). Smaller right NAcc GMV was associated with higher IAT scores, although the significance of this effect did not survive Bonferroni correction (β=−0.411±0.150, t45=−2.747, p=0.009). Left NAcc GMV was not significantly associated with IAT scores (p=0.861). Bilateral NAcc GMV was not associated with SIQ scores (ps>0.275).
Figure 2.
Smaller GMV of the left (A) and right (B) caudate nucleus are associated with higher IAT d-scores. Raw data and trends are depicted without adjustment for covariates or standardization for the purposes of visualization. Please see Results for more details.
Specificity of the effect of caudate gray matter volumes on IAT scores
Using robust linear regression, we found no associations between left amygdala and bilateral hippocampal GMVs with either IAT (all ps>0.086) or SIQ scores (all ps>0.696). While right amygdala GMV was not associated with IAT scores (p=0.052), larger right amygdala GMV predicted higher SIQ scores (β=0.334±0.153, t46=2.189, p=0.031), although the significance of this effect did not survive Bonferroni correction.
Using 10-fold cross-validation, we identified the optimal regularization parameter from our LASSO analysis that minimized MSE (λmin=0.04). The coefficient weight of right caudate GMV had the largest magnitude in predicting IAT scores, followed by left caudate GMV, and then right NAcc (see Table 3).
Table 3.
Standardized coefficient weights (β) from LASSO results with hyperparameter λ tuned to minimize MSE using 10-fold cross validation (λmin=0.04). In this model, coefficient weights from all covariates were shrunk to 0.
| Variable | β |
|---|---|
|
| |
| R caudate | −6.32 × 10−5 |
| L caudate | −4.10 × 10−5 |
| R NAcc | −1.28 × 10−5 |
Sensitivity analyses
To replicate our previous findings on striatal GMV and implicit SI (Ho et al., 2018), we conducted supplemental robust linear regressions including Tanner score as a covariate in addition to age, sex, ICV, and CDRS-R scores. However, of the 53 participants in the study, 8 participants were missing Tanner scores and 1 was missing IAT. To compare differences in demographics, clinical, and neural characteristics of the 44 participants who were not missing any data on any measure to the 9 who were missing data on at least one measure (n=1 for IAT and n=8 for Tanner scores), we computed effect sizes (Hedge’s g for continuous variables and φ for categorical variables). These effect sizes were negligible or small, with the exception of SIQ scores and age of depression onset, which had medium effect sizes (both gs>0.74), and parental education (Cramer’s φ=0.378), which had a medium effect size. Not surprisingly, all 95% CI for all estimated effect sizes contained 0, suggesting that there is minimal difference between the groups who provided data for all measures and those who were missing Tanner scores. When including Tanner score as a covariate, the effects of bilateral caudate GMV on IAT scores were slightly larger than in our original analyses (left: β=−0.739±0.174, t36=−4.245, p<0.0001; right: β=−0.692±0.153, t36=−4.531, p<0.0001). After including Tanner scores as covariates, the association between right NAcc GMV and IAT scores remained significant without Bonferroni correction (p=0.008); however, the associations between left NAcc GMV and IAT scores, between putamen GMVs and IAT scores, and between all striatal GMVs and SIQ scores remained insignificant (all ps>0.172).
In addition, because suicidality is assessed on the CDRS-R, we also reran all statistical models with CDRS-R as a covariate without including the two items pertaining to suicidal and morbid ideation (items 12 and 13) from the total raw score to yield a measure of depressive symptom severity distinct from suicidal symptoms (Lewis et al., 2020). The significance of all of the findings we report remained unchanged.
Discussion
This study is the first to examine the association between striatal gray matter volumes (GMVs) and implicit and explicit suicidal ideation (SI) in a sample of clinically-assessed adolescents recruited from the community. In a previous investigation in an independent sample of adolescents recruited from the community with minimal clinical symptoms, we found that smaller caudate and putamen GMVs prospectively predicted stronger implicit SI, as assessed by IAT scores (Ho et al., 2018). Based on those findings, we hypothesized that smaller caudate and putamen GMVs would also be associated with implicit, but not explicit, SI in a sample of depressed adolescents. We largely replicated our previous investigation: smaller caudate GMVs were associated with stronger implicit, but not explicit, SI, and smaller putamen GMVs were not associated with explicit suicidal ideation. In contrast to our previous findings, however, smaller putamen GMVs were not associated with implicit SI, but smaller right NAcc GMV was associated with stronger implicit SI, and larger right amygdala GMV was associated with stronger explicit SI (although these results did not survive stringent correction for multiple comparisons). Finally, our LASSO analyses indicate that caudate GMV was a stronger predictor of IAT scores than was NAcc GMV. When considered with our previous findings, the current results suggest that smaller caudate GMVs are a robust marker of cognitive susceptibility to SI that may have important clinical implications for the early detection of suicide risk among adolescents.
It is noteworthy that the majority of neuroimaging studies in the field (the majority of which focused on adult patients) comparing suicide attempters versus non-attempters or healthy controls have reported equivocal results (for reviews, see Auerbach et al., 2020; Schmaal et al., 2020), including a large harmonized meta-analysis across multiple sites recruiting depressed and non-depressed patients that did not report associations between subcortical GMVs and suicidal thoughts and behaviors (Renteria et al., 2017). Interestingly, an older meta-analysis across six structural imaging studies found that adolescents and adults with psychiatric conditions and a history of suicidal behaviors had smaller caudate GMVs than do psychiatric controls who do not have a history of suicidal behaviors (van Heeringen et al., 2014). In our study, however, smaller caudate GMVs were only associated with implicit SI and not with explicit SI nor with history of suicide attempt. Although the two phenomena are correlated, SI is nonetheless distinct from suicidal behaviors (Klonsky et al., 2016). One interpretation of our findings is that smaller caudate GMVs represent a neuroanatomical vulnerability toward broader suicide risk through pathways that are implicated in both SI and attempt (e.g., anhedonia, impulsivity). Unlike the meta-analysis by van Heeringen and colleagues (2014), however, our study assessed only depressed adolescents, 78% of whom did not report a previous attempt. Our study was therefore limited in terms of statistical power in identifying whether caudate volumes differed between attempters and non-attempters in a wider psychiatric population. Thus, an important direction will be to recruit a larger sample size of clinically diverse patients in order to determine whether gray matter volumes of the caudate potentially underlie differences between ideators and attempters (as well as between single and multiple attempters) or whether smaller caudate GMV may represent a broader risk factor for suicidal thoughts and behaviors.
In contrast to our previous investigation in a community sample of adolescents with minimal clinical symptoms, we did not find evidence that putamen GMVs are associated with IAT scores. Given the clinical differences between our two study samples, and evidence from other studies that structural and functional alteration in the putamen is a risk marker for depression (Colich et al., 2017; Pagliaccio et al., 2019) and anhedonia in adolescents (Auerbach et al., 2017; Gabbay et al., 2013), we posit that putamen GMV reflects broader anhedonia-related risk and, as such, may be an important risk factor for both depression and cognitive vulnerabilities to suicidal ideation. In our current study, we also found evidence that smaller right NAcc GMV is associated with stronger implicit SI and that larger right amygdala GMV is associated with more severe explicit SI. Because these findings did not survive stringent Bonferroni correction for multiple tests, it is important that future studies replicate these results and to determine how implicit SI (which appears to be mediated by dorsal striatal systems) is related to the emergence of explicit SI (which may be possibly mediated by amygdala-based systems).
Future research is also needed to determine which mechanistic pathways explain smaller caudate GMV in the context of suicidal thoughts and behaviors in adolescents. At least one study has reported reduced dopamine in response to a dopaminergic agonist in depressed patients who later died by suicide (Pitchot et al., 2001). Thus, dopaminergic signaling and metabolism in the striatum—and in the caudate specifically—may be critical to understanding the emergence of SI (and suicide risk more broadly) in adolescents. Another important direction for future research is to focus on subdivisions of the striatum in order to refine our understanding of the role of these structures with complex behaviors linked with suicidality. For instance, whereas the caudate head has been shown to be involved in affective and cognitive processes, the caudate body/tail is involved more strongly with processing sensory inputs to shape perception and action (Robinson et al., 2012). Similarly, whereas the shell of the NAcc is involved in processing motivational salience and reward-based behaviors through projections to the amygdala and ventral tegmental area, the core of the NAcc, along with the globus pallidus and substantia nigra, is involved in the generation of motor programs (Richard et al. 2013). It is important that future research elucidate which striatal subdivisions and which specific neurotransmitter pathways contribute to suicidality in adolescents.
Although we took a hypothesis-driven approach and focused on the structure of the striatum because of our previous work, a limitation of our current investigation is that we did not examine functional brain correlates of implicit or explicit SI. Markers derived from functional MRI patterns may provide additional information on the neural representations of suicide-related content (see Just et al., 2017 as an example) that may inform more immediate treatment. In contrast, we propose that information derived from structural MRI, such as estimates of caudate GMV, may be more useful in the context of risk identification, particularly in light of our previous findings that smaller caudate GMV prospectively predicts IAT scores 2 years later in a community sample of adolescents (Ho et al., 2018). For MRI-based markers such as smaller caudate GMV to have clinical utility, however, it is imperative that the field establish norms concerning subcortical GMVs, and/or that individuals undergo repeated assessments to determine whether change, or rate of change, in caudate GMVs is associated with significant change in SI that is clinically meaningful. Even if MRI assessments are not part of routine preventative care, it may be possible to implement IAT tests at wellness centers through educational settings, as well as during pediatric visits to provide a cognitive assessment of suicide risk. Finally, despite variability in history and severity of suicidal thoughts and behaviors in our sample, another limitation of our study is that our participants were recruited on the basis of depression symptoms, not on the basis of SI. Although focusing our investigation on a sample of adolescents with depressive disorders reduced clinical heterogeneity, it will be important for future research to examine shared and unique predictors and markers of SI and suicidal behaviors in transdiagnostic samples of adolescents.
Given that thoughts of SI (as well as related self-harming behaviors) are present even in adolescents who do not seek clinical services (Hawton et al., 2012) and that there are challenges in the assessment of SI (Nock et al., 2010), our study is important in demonstrating, for the first time, that GMVs of the caudate are significantly associated with implicit self-associations with death in a sample of depressed adolescents. Considered together with our previous investigation in which we found that smaller caudate and putamen GMVs prospectively predicted stronger implicit SI in a community sample of adolescents, it appears that smaller caudate GMVs are a robust biomarker of SI in adolescents. Future work is needed to address the limitations of our cross-sectional investigation by replicating these findings in larger, prospective studies assessing a patient population with wider variability in suicidal thoughts and behaviors.
Highlights.
Prior work identifying neuroanatomical predictors of implicit suicidal ideation (SI) in a non-clinical sample of adolescents have identified dorsal striatal morphological alterations
We replicated the finding that smaller bilateral caudate gray matter volumes are associated with stronger implicit SI in an independent sample of depressed adolescents
Smaller caudate gray matter volume may be a robust biomarker of SI in adolescents, with clinical implications for early identification of youth at risk for engaging in suicidal behaviors
Acknowledgments
This work was supported by the National Institute of Mental Health (K01MH117442 to Dr. Ho and R37MH101495 to Dr. Gotlib), the Klingenstein Third Generation Foundation (Child and Adolescent Depression Award to Dr. Ho), and Stanford’s Maternal Child Health Research Institute (Early Career Award and K Support Award to Dr. Ho). Dr. Singh has received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, National Institute of Mental Health, National Institute of Aging, Johnson and Johnson, Allergan, and the Brain and Behavior Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We thank Michelle Sanabria, Rachel Weisenburger, Jillian Segarra, Alexess Sosa, Holly Pham, and Miranda Edwards for assistance with data collection and organization. Finally, we thank the participants and their families for their enthusiasm and participation in this study.
Footnotes
Uncited References:
(Francis et al., 2015b, Ho et al., 2020, Kaufman et al., 2000, Kerestes et al., 2016, Martin-Soelch, 2009, Miller et al., 2018, Ordaz et al., 2018, Pizzagalli et al., 2009, Core Team, 2019, Schreiner et al., 2019, Walker et al., 2020, Gotlib, 2017)
Declaration of Competing Interest
Dr. Singh is also on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from the American Psychiatric Association Publishing. All other authors report no biomedical conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auerbach RP, Pagliaccio D, Allison GO, Alqueza KL, & Alonso MF. (2020). Neural Correlates Associated With Suicide and Nonsuicidal Self-injury in Youth. 10.1016/j.biopsych.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, & Pizzagalli DA. (2017). Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology, 42(10), 2087–2095. 10.1038/npp.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K, & Knutson B. (2009). Dissociable neural representations of future reward magnitude and delay during temporal discounting. NeuroImage, 45(1), 143–150. 10.1016/j.neuroimage.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SM, Bahraini NH, Forster JE, Stearns-Yoder KA, Hostetter TA, Smith G, Nagamoto HT, & Nock MK. (2016). Moving Beyond Self-Report: Implicit Associations about Death/Life Prospectively Predict Suicidal Behavior among Veterans. Suicide and Life-Threatening Behavior, 47(1), 67–77. 10.1111/sltb.12265 [DOI] [PubMed] [Google Scholar]
- Brainard DH. (1997). The Psychophysics Toolbox. Spatial Vision, 10 (4), 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Cha CB, Najmi S, Park JM, Finn CT, & Nock MK. (2010). Attentional bias toward suicide-related stimuli predicts suicidal behavior. Journal of Abnormal Psychology, 119(3), 616–622. 10.1037/a0019710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Ho TC, Ellwood-Lowe ME, Foland-Ross LC, Sacchet MD, LeMoult JL, Gotlib IH. (2017). Like mother like daughter: putamen activation as a mechanism underlying intergeneraitonal risk for depression. Social Cognitive and Affective Neuroscience, 12(9):1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox Lippard ET, Johnston JAY, & Blumberg HP. (2014). Neurobiological risk factors for suicide: Insights from brain imaging. American Journal of Preventive Medicine, 47(3 SUPPL. 2), S152. 10.1016/j.amepre.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE. (2012). Research Review: Altered reward function in adolescent depression: What, when and how? In Journal of Child Psychology and Psychiatry and Allied Disciplines (Vol. 53, Issue 1, pp. 3–15). 10.1111/j.1469-7610.2011.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O’Donnell P, Kravitz A, & Lobo MK. (2015b). Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biological Psychiatry, 77(3), 212–222. 10.1016/j.biopsych.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, Musacchio KM, Jaroszewski AC, Chang BP, & Nock MK. (2017). Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychological Bulletin, 143(2), 187–232. 10.1037/bul0000084 [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. (2013). Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academic of Child and Adolescent Psychiatry, 52(6):628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, & Kirkpatrick K. (2010). The Role of the Nucleus Accumbens Core in Impulsive Choice, Timing, and Reward Processing. Behavioral Neuroscience, 124(1), 26–43. 10.1037/a0018464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Kleiman EM, Coppersmith DDL, Santee AC, Esposito EC, Cha CB, Nock MK, & Auerbach RP. (2017). Implicit identification with death predicts change in suicide ideation during psychiatric treatment in adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(12), 1319–1329. 10.1111/jcpp.12769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, & Schwartz JLK. (1998). Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology, 74(6), 1464–1480. doi: 10.1037/0022-3514.74.6.1464 [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, & Banaji MR. (2003). Understanding and using the Implicit Association Test: I. An improved scoring algorithm. Journal of Personality and Social Psychology, 85(2), 197–216. 10.1037/0022-3514.85.2.197 [DOI] [PubMed] [Google Scholar]
- Harrison DP, Stritzke WGK, Fay N, & Hudaib AR. (2018). Suicide risk assessment: Trust an implicit probe or listen to the patient? Psychological Assessment, 30(10), 1317–1329. 10.1037/pas0000577 [DOI] [PubMed] [Google Scholar]
- Harrison DP, Stritzke WGK, Fay N, Mark Ellison T, & Hudaib AR. (2014). Probing the implicit suicidal mind: Does the death/suicide implicit association test reveal a desire to die, or a diminished desire to live? Psychological Assessment, 26(3), 831–840. 10.1037/pas0000001 [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, & Friedman J. (2009). The elements of statistical learning: data mining, inference, and prediction (2nd ed.) New York: Springer. [Google Scholar]
- Hawton K, Saunders KEA, & O’connor RC. (2012). Self-harm and suicide in adolescents. Lancet, 379, 2373–2382. 10.1016/S0140-6736(12)60322-5 [DOI] [PubMed] [Google Scholar]
- Ho TC, Cichocki AC, Gifuni AJ, Camacho MC, Ordaz SJ, Singh MK, & Gotlib IH. (2018). Reduced dorsal striatal gray matter volume predicts implicit suicidal ideation in adolescents. Social Cognitive and Affective Neuroscience, 1215–1224. 10.1093/scan/nsy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Colich NL, Sisk LM, Oskirko K, Jo B, & Gotlib IH. (2020). Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Developmental Cognitive Neuroscience, 42, 100773. 10.1016/j.dcn.2020.100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D, Doucet GE, Pompili M, Sani G, Luna B, Brent DA, & Frangou S. (2020). Risk and protective factors for childhood suicidality: a US population-based study. 317. 10.1016/S2215-0366(20)30049-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Pan L, Cherkassky VL, McMakin DL, Cha C, Nock MK, & Brent D. (2017). Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nature Human Behaviour, 1(12), 911–919. 10.1038/s41562-017-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaufman J, Birmaher B, Axelson D, Pereplitchikova F, Brent D, & Ryan N. (2016) The KSADS-PL DSM-5. Kennedy Krieger Institute. Baltimore, MD. https://www.kennedykrieger.org/sites/default/files/library/documents/faculty/ksads-dsm-5-screener.pdf [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. (1997). Schedule for affective disorders and schizophrenia for school-age childrenpresent and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, & Rao U. (2000). K-SADS-PL. Journal of the American Academy of Child & Adolescent Psychiatry, 39(10), 1208. 10.1097/00004583-200010000-00002 [DOI] [PubMed] [Google Scholar]
- Kerestes R, Segreti AM, Pan LA, Phillips ML, Birmaher B, Brent DA, & Ladouceur CD. (2016). Altered neural function to happy faces in adolescents with and at risk for depression. Journal of Affective Disorders, 192, 143–152. 10.1016/j.jad.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Humphreys KL, Camacho MC, &Gotlib IH. (2019). A person-centered approach to the assessment of early life stress: Associations with the volume of stress-sensitive brain regions in early adolescence. Development and Psychopathology, 31(2), 643–655. 10.1017/S0954579418000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED, May AM, & Saffer BY. (2016). Suicide, Suicide Attempts, and Suicidal Ideation. Annu. Rev. Clin. Psychol. 2016, 12, 307–337. 10.1146/annurevclinpsy-021815-093204 [DOI] [PubMed] [Google Scholar]
- Lewis CP, Port JD, Blacker CJ, Sonmez AI, Seewoo BJ, Leffler JM, Frye MA, Croarkin PE. (2020) Altered anterior cingulate glutamatergic metabolism in depresed adolescents with current suicidal ideation, Translational Psychiatry, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM. (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. doi: 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44(235), 291–303. 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C. (2009). Is depression associated with dysfunction of the central reward system? Biochemical Society Transactions, 37(1), 313–317. 10.1042/BST0370313 [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, & Emslie GJ. (2010). Psychometric properties of the children’s depression rating Scale-Revised in adolescents. Journal of Child and Adolescent Psychopharmacology, 20(6), 513–516. 10.1089/cap.2010.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, & Sheridan MA. (2018). Neural Correlates of Emotion Regulation and Adolescent Suicidal Ideation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(2), 125–132. 10.1016/j.bpsc.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, Kyu HH, Barber RM, Wagner J, Cercy K, Kravitz H, Coggeshall M, Chew A, O’Rourke KF, Steiner C, Tuffaha M, Charara R, Al-Ghamdi EA, Adi Y, Murray CJL. (2016). Global burden of diseases, injuries, and risk factors for young people’s health during 1990−−2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 387(10036), 2383–2401. 10.1016/S0140-6736(16)00648-6 [DOI] [PubMed] [Google Scholar]
- Morris NM, & Udry JR. (1980). Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence, 9(3), 271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- Mortier P, Auerbach RP, Alonso J, Bantjes J, Benjet C, Cuijpers P, Ebert DD, Green JG, Hasking P, Nock MK, O’neill S, Pinder-Amaker S, Sampson NA, Vilagut G, Zaslavsky AM, Bruffaerts R, & Kessler RC. (2018). Suicidal Thoughts and Behaviors Among First-Year College Students: Results From the WMH-ICS Project. In Journal of the American Academy of Child & Adolescent Psychiatry www.jaacap.org (Vol. 263, Issue 4). 10.1016/j.jaac.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL,Dour HJ, & Banaji MR. (2010). Measuring the suicidal mind: Implicit cognition predicts suicidal behavior. Psychological Science, 21(4), 511–517. 10.1177/0956797610364762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek BA, Greenwald AG, & Banaji MR. (2005). Understanding and using the implicit association test: II. Method variables and construct validity. Personality and Social Psychology Bulletin, 31(2), 166–180. 10.1177/0146167204271418 [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Sullivan GM, Sudol K, Baca-Garcia E, Stanley BH, Sublette ME, & Mann JJ. (2014). Toward a biosignature for suicide. In American Journal of Psychiatry (Vol. 171, Issue 12, pp. 1259–1277). American Psychiatric Association. 10.1176/appi.ajp.2014.14020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Goyer MS, Ho TC, Singh MK, & Gotlib IH. (2018). Network basis of suicidal ideation in depressed adolescents. Journal of Affective Disorders, 226, 92–99. 10.1016/j.jad.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Alqueza KL, Marsh R, & Auerbach RP. (2019). Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. Journal of the American Academy of Child and Adolescent Psychiatry, 0(0). 10.1016/j.jaac.2019.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PM, Sato JR, Salum GA, Rohde LA, Gadelha A, Zugman A, Mari J, Jackowski A, Picon F, Miguel EC, Pine DS, Leibenluft E, Bressan RA, & Stringaris A. (2017). Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. American Journal of Psychiatry, 174(11), 1112–1119. 10.1176/appi.ajp.2017.17040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, & Ansseau M. (2001). Role of dopamine in non-depressed patients with a history of suicide attempts. European Psychiatry : The Journal of the Association of European Psychiatrists, 16(7), 424–427. 10.1016/s0924-9338(01)00601-0 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, & Fava M. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–710. 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, & Mokros HB. (1996). Children’s depression rating scale, revised (CDRS-R). Los Angeles: Western Psychological Services. [Google Scholar]
- Rentería ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, De Zubicaray GI, McMahon KL, Medland SE, Gillespie NA, Hatton SN, Lagopoulos J, Veltman DJ, Van Der Wee N, Van Erp TGM, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Hickie IB. (2017). Subcortical brain structure and suicidal behaviour in major depressive disorder: A meta-analysis from the ENIGMA-MDD working group. Translational Psychiatry, 7(5), e1116–e1116. 10.1038/tp.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds WM (1987), Suicidal Ideation Questionnaire (SIQ): Professional Manual. Odessa, FL. Psychological Assessment Resources [Google Scholar]
- Reynolds WM, & Mazza JJ. (1999). Assessment of Suicidal Ideation in Inner-City Children and Young Adolescents: Reliability and Validity of the Suicidal Ideation Questionnaire-JR. In School Psychology Review (Vol. 28, Issue 1). [Google Scholar]
- Richard JM, Castro DC, DiFeliceantonio AG, Robinson MJF, & Berridge KC. (2013). Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. In Neuroscience and Biobehavioral Reviews (Vol. 37, Issue 9, pp. 1919–1931). 10.1016/j.neubiorev.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, & Fox PT. (2012). The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage, 60(1), 117–129. 10.1016/j.neuroimage.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, van Harmelen AL, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, Mazure CM, & Blumberg HP. (2020). Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. In Molecular Psychiatry (Vol. 25, Issue 2, pp. 408–427). Springer Nature. 10.1038/s41380-019-0587-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MW, Klimes-Dougan B, & Cullen KR. (2019). Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide and Life-Threatening Behavior, 49(3), 899–913. 10.1111/sltb.12471 [DOI] [PubMed] [Google Scholar]
- Shain B. (2016). Suicide and suicide attempts in adolescents. In Pediatrics (Vol. 138, Issue 1, p. ePub-ePub). American Academy of Pediatrics. 10.1542/peds.2016-1420 [DOI] [Google Scholar]
- Stringaris A, Belil PVR, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttilä J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banaschewski T, Bokde ALW, Rogers J. (2015). The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry, 172(12), 1215–1223. 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello N, Harika-Germaneau G, Serra W, Jaafari N, & Chatard A. (2020). Forecasting a Fatal Decision: Direct Replication of the Predictive Validity of the Suicide--Implicit Association Test. Psychological Science, 31(1), 65–74. 10.1177/0956797619893062 [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, & Baeken C. (2014). Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in Human Neuroscience, 8(OCT). 10.3389/fnhum.2014.00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Teresi GI, Weisenburger RL, Segarra JR, Ohja A, Kulla A, Sisk LM, Li M, Stein DJ, Rosenberg-Hasson Y, Maecker HT, Gotlib IH, Ho TC. (2020) Study Protocol for Teen Inflammation Glutamate Emotion Research (TIGER) [Manuscript submitted for publication]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Preventing Preventing suicide suicide A global imperative A global imperative. (2014). www.who.int
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, Pantelis C, McGorry P, & Alle NB. (2014). Structural brain development and depression onset during adolescence: A prospective longitudinal study. American Journal of Psychiatry, 171(5), 564–571. 10.1176/appi.ajp.2013.13070920 [DOI] [PubMed] [Google Scholar]
- Zou H, & Hastie T. (2005). Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 67(2), 301–320. 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]