Abstract
Pyocin S3 was found to kill exclusively Pseudomonas aeruginosa isolates producing type II pyoverdine (exemplified by strain ATCC 27853). Killing was specifically inhibited by addition of type II ferripyoverdine. All Tn5 mutants resistant to pyocin S3 were defective for pyoverdine-mediated iron uptake and failed to produce an 85-kDa iron-repressed outer membrane protein. We conclude that this protein is probably the type II ferripyoverdine receptor that is used by pyocin S3 to gain entry into the cell.
Pyocins are bacteriocins produced by Pseudomonas aeruginosa that kill strains of the same species. Different types have been described (13, 15); among them are the S-type pyocins (S1, S2, AP41, and S3), which have two components, a protein with DNase activity and an immunity protein which confers protection to the producing strain (9, 20, 24, 25). With Escherichia coli, it is known that colicins gain entry into susceptible cells via specific outer membrane receptors functioning with the Tol or the TonB-ExbB-ExbD translocation system, the latter being necessary for the transport of ferrisiderophores (17). Group E colicins have been shown to use the same receptor as vitamin B12 but not the same translocation system (14). In P. aeruginosa, pyoverdine is translocated thanks to the intervention of TonB (23) while the soluble pyocin AP41 is translocated by the Tol system (8). The observation that killing by pyocins S1 and S2 is greatly increased when the bacterial cells are grown under conditions of iron deficiency has led to the hypothesis that the receptor for those pyocins is also a siderophore receptor (19, 26). When starved of iron, P. aeruginosa produces two siderophores, namely, pyochelin (a thiazolin derivative) (5) and pyoverdine (6), together with their respective outer membrane receptors. Pyoverdine is composed of a quinoline-type chromophore attached to a peptide arm (2, 3). Depending on the nature of the pyoverdine peptide part, P. aeruginosa isolates can be divided into three pyoverdine groups (4, 18). To each type of ferripyoverdine corresponds a type of specific outer membrane receptor (4). In studies of pyocin Sa, an incompletely characterized bacteriocin produced by P. aeruginosa J 1003, some suggested that the pyocin receptor is responsible for ferripyoverdine uptake. A Tn5 insertion mutant, deficient for the pyoverdine receptor of the Sa-susceptible clinical isolate 0:9, was indeed found to be resistant to pyocin Sa (28).
Pyocin S3 is a newly discovered P. aeruginosa bacteriocin homologous to the other S-type pyocins, especially in the translocation and DNase killing domains (9). Preliminary data on pyocin S3 killing specificity suggested some correlation with cellular iron metabolism (9). Screening of a collection of P. aeruginosa cystic fibrosis (CF) isolates revealed that about 40% of the strains were susceptible to pyocin S3 (1a), a percentage close to that found by Meyer et al. for type I and II pyoverdine producers among 88 clinical isolates (18). This result prompted us to investigate whether a correlation exists between the type of pyoverdine or receptor produced and susceptibility to pyocin S3.
Pyocin S3 kills type II pyoverdine producers.
Forty-nine isolates of P. aeruginosa (19 CF, 22 non-CF, and 8 reference strains for pyocin typing) were tested by the spot agar technique on glutamate minimal medium (12) for their susceptibilities to pyocins S1, S2, S3, and AP41. These pyocins were used after partial purification from lysates of recombinant E. coli strains containing the corresponding genes by ammonium sulfate precipitation and ion-exchange chromatography (9). The pyoverdine-containing culture supernatant of each strain grown in iron-poor Casamino Acids (CAA) medium was analyzed by the isoelectric-focusing technique in order to determine the type of pyoverdine produced, as evidenced by the pattern of fluorescent bands representing isoforms of pyoverdine (16, 18). As shown in Table 1, all strains susceptible to pyocin S3 produced and/or could take up type II pyoverdine (isolates which failed to produce pyoverdine were tested for their uptake of the three 59Fe-ferripyoverdines, according to the method described in reference 4). In striking contrast, the pyocin S3-resistant isolates produced either type I or III pyoverdine. No correlation was found between strain susceptibility to other pyocins (S1, S2, AP41) and pyoverdine type production (results not shown). These observations were further confirmed by testing the susceptibilities to pyocin S3 of 40 other strains, which had already been typed for pyoverdine in a previous study (18) (Table 1). As expected, the 15 strains producing type II pyoverdine were all killed by pyocin S3 while the isolates producing type I (n = 10) or type III (n = 10) pyoverdine were all pyocin S3 resistant (Table 1).
TABLE 1.
Correlation between sensitivity to pyocin S3 killing and pyoverdine type
| No. of strains (origin) | Pyoverdine type | No. of CF isolates | No. of other clinical isolates | No. of pyocin typing strains | No. of strains showing pyocin S3 sensitivity |
|---|---|---|---|---|---|
| 10 (Besançon, France)a | I | 2 | 7 | 1 | 0 |
| 9 (Strasbourg, France) | 0 | 9 | 0 | 0 | |
| 24 (Besançon, France)a | II | 9 | 10 | 5 | 24 |
| 16 (Strasbourg, France) | 2 | 14 | 0 | 16 | |
| 15 (Besançon, France)a | III | 8 | 5 | 2 | 0 |
| 10 (Strasbourg, France) | 0 | 10 | 0 | 0 |
All CF and other types of clinical isolates were differentiated by pulsed-field gel electrophoresis and are all genotypically distinct.
Competition between pyoverdine and pyocin S3.
The killing activity of pyocin S3 against strain ATCC 27853 as determined by the spot test assay was clearly inhibited by type II pyoverdine (Fig. 1). This antagonism was more important in the presence of ferripyoverdine than in the presence of pyoverdine. Conversely, the addition of either of the other two pyoverdines (type I and type III) increased the killing activity of S3 on Luria-Bertani medium (compared with that on water [results not shown]), probably by creating a higher iron deficiency (Fig. 1). Two explanations for this result come to mind: either there is a direct competition between the two ligands (type II pyoverdine and pyocin S3) for the same receptor or the cognate ferripyoverdine, being taken up, reduces the iron deficiency, which in turn results in a down regulation of the expression of the receptor gene. The second hypothesis, however, is unlikely, since it has been demonstrated that pyoverdine induces the production of its receptor (10).
FIG. 1.
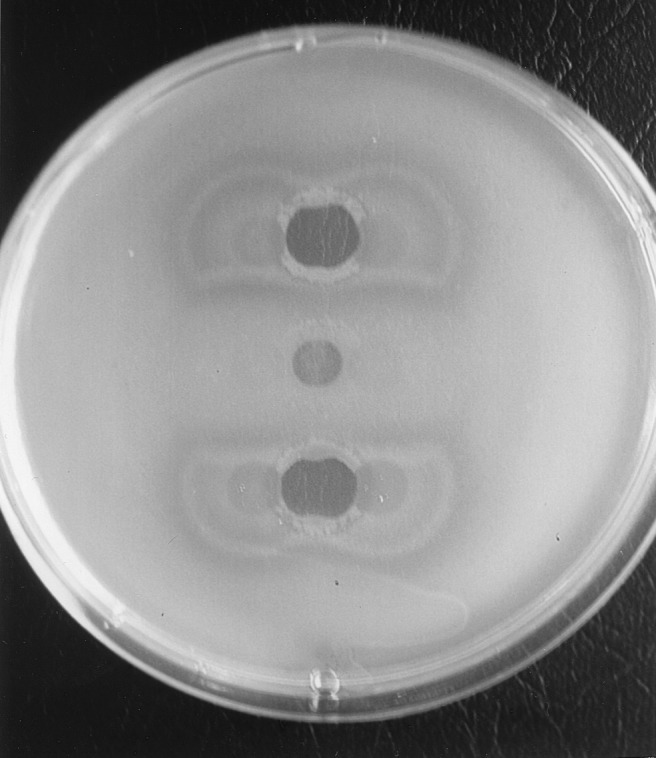
Plate showing the effects of different pyoverdines on the killing of P. aeruginosa ATCC 27853 by pyocin S3. To a lawn of P. aeruginosa ATCC 27853 on Luria-Bertani medium drops of purified pyocin S3 (20 μl, corresponding to 25 U ml−1, defined as the reciprocal of the highest pyocin dilution inhibiting bacterial growth) were deposited at three places in the middle. Next, on each side of the pyocin drop, 20 μl of the following purified pyoverdines (2 μmol) was added: that from PAO1 (top), that from ATCC 27853 (middle), and that from clinical isolate PA6 (bottom). Only the cognate pyoverdine (ATCC 27853), recognized by the pyoverdine receptor, competes with pyocin S3, which uses the same receptor, inhibiting the killing.
Generation of Tn5 mutants resistant to pyocin S3.
Pb60, a clinical isolate producing type II pyoverdine and susceptible to pyocin S3 (Table 1), was chosen because of its low resistance to kanamycin. Insertion mutants were obtained after conjugational transfer (21) of the suicide plasmid pSUP2021 carrying transposon Tn5 (27) and isolation on Pseudomonas agar medium (Institut Pasteur Productions, Paris, France) containing kanamycin at 400 μg/ml. Two mutants of 500 transconjugants were selected for their inability to grow in the presence of 1 mg of the iron(III) chelator ethylenediamine-di-(o-hydroxyphenylacetic acid) (EDDHA) per ml. One mutant turned out to be susceptible to pyocin S3 (Pb60S), but the other one (Pb60R) was resistant.
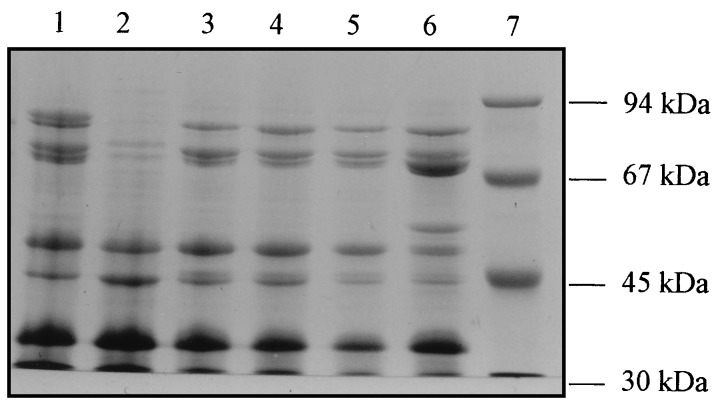
Interestingly, mutant Pb60S, although not producing pyoverdine, was still able to grow in the presence of 1 mg of EDDHA per ml when purified type II pyoverdine was added to the medium while Pb60R (which produced ten times less pyoverdine than the wild type) was not, suggesting that the latter had a defect in the uptake of pyoverdine. In agreement with these results, Pb60R grown in glutamate minimal medium was found to lack an 85-kDa iron-repressed outer membrane protein (IROMP) (results not shown) and to incorporate about 20-fold less 59Fe-pyoverdine than the wild type or Pb60S (Table 2). The levels of pyochelin produced by Pb60 and its two mutants Pb60S and Pb60R were determined after extraction of the acidified growth medium supernatant by ethyl acetate (11) and were found to be nearly identical (results not shown). This result was corroborated by pyochelin-uptake experiments showing normal pyochelin-mediated iron uptake by the two mutants (results not shown). A second insertion mutagenesis was carried out with the type II pyoverdine strain ATCC 27853, which is susceptible to pyocin S3, and a mini-Tn5 with a tetracycline resistance gene (7) as previously described (1). This time, 7 of 1,500 mutants were directly selected for their resistance to pyocin S3. All were partially or totally deficient for pyoverdine production, did not grow in the presence of EDDHA supplemented with type II pyoverdine, and failed to take up type II 59Fe-pyoverdine (Table 2). Furthermore, these mutants all lacked the 85-kDa IROMP in their outer membranes (results for 4 mutants are shown in Fig. 2). The fact that mutants that do not produce the ferripyoverdine receptor are also defective in pyoverdine production has already been demonstrated (22, 28), indicating that the biosynthesis of pyoverdine and the production of the receptor are coregulated.
TABLE 2.
Pyoverdine production, growth stimulation by pyoverdine, and uptake of ferripyoverdine of wild-type and pyocin S3-susceptible and -resistant mutants of strains Pb60 and ATCC 27853
| Strain | Pyocin S3 susceptibility | Pyoverdine productiona | Growth on EDDHA with type II pyoverdine | 59Fe-pyoverdine incorporated (pmol)b |
|---|---|---|---|---|
| Pb60 (WT)c | + | 100 | + | 264 |
| Pb60R | − | 10 | − | 9 |
| Pb60S | + | 0 | + | 236 |
| ATCC 27853 (WT) | + | 100 | + | 257 |
| ATCC 27853-5B | − | 0 | − | 7 |
| ATCC 27853-6A | − | 26 | − | 6 |
| ATCC 27853-6B | − | 0 | − | 7 |
| ATCC 27853-9A1 | − | 0 | − | 13 |
| ATCC 27853-14.2 | − | 7 | − | 0 |
| ATCC 27853-16B1 | − | 9 | − | 0 |
| ATCC 27853-16B2 | − | 9 | − | 0 |
Expressed as a percentage of the level produced by the wild type after 48 h of growth in CAA medium and after the absorbency at 400 nm was measured and normalized to growth at an absorbency of 600 nm (15).
Expressed in picomoles of 59Fe incorporated per milligram (dry weight) of cells during a 20-min incubation. The results are means of results of three independent experiments (15).
WT, wild type.
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel (7%) electrophoresis of 10-μg quantities of outer membrane proteins from P. aeruginosa ATCC 27853 grown in CAA medium (lane 1) and in CAA medium plus FeCl3 (50 μM) (lane 2), mutant ATCC 27853-5B (lane 3), mutant ATCC 27853-6A (lane 4), mutant ATCC 27853-9A1 (lane 5), and mutant ATCC 27853-16B1 (lane 6). Lane 7 contains the molecular mass markers (High molecular mass markers; Pharmacia-LKB, Uppsala, Sweden).
Conclusions.
Altogether, our results strongly suggest that pyocin S3 utilizes the receptor for type II ferripyoverdine to enter a bacterial cell, because of (i) the perfect correlation between the ability of an isolate to utilize type II pyoverdine and its susceptibility to pyocin S3, (ii) the inhibition of killing by the addition of type II pyoverdine, and (iii) the fact that mutants resistant to killing by pyocin S3 are all deficient in pyoverdine production and uptake and lack an 85-kDa IROMP.
Work is now in progress to further characterize the Tn5 mutants obtained and to clone the genes involved in the expression of the type II ferripyoverdine receptor.
REFERENCES
- 1.Anjaiah V, Koedam N, Nowak-Thompson B, Loper J E, Höfte M, Tabi Tambong J, Cornelis P. Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn5 derivatives towards Fusarium spp. and Pythiumspp. Mol Plant-Microbe Interact. 1998;11:847–854. [Google Scholar]
- 1a.Baysse, C. Unpublished results.
- 2.Budzikiewicz H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol Rev. 1993;104:209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 3.Budzikiewicz H. Siderophores of fluorescent pseudomonads. Z Naturforsch. 1997;52:713–720. [PubMed] [Google Scholar]
- 4.Cornelis P, Hohnadel D, Meyer J M. Evidence for different pyoverdine-mediated iron uptake systems among Pseudomonas aeruginosastrains. Infect Immun. 1989;57:3491–3497. doi: 10.1128/iai.57.11.3491-3497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox C D, Rinehart K L, Moore M L, Cook J C. Pyochelin: novel structure of an iron chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1981;78:4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox C D, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative Eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis J J, Lafontaine E R, Sokol P A. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J Bacteriol. 1996;178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duport C, Baysse C, Michel-Briand Y. Molecular characterization of pyocin S3, a novel S-type pyocin from Pseudomonas aeruginosa. J Biol Chem. 1995;270:8920–8927. doi: 10.1074/jbc.270.15.8920. [DOI] [PubMed] [Google Scholar]
- 10.Gensberg K, Hughes K, Smith A W. Siderophore-specific induction of iron uptake in Pseudomonas aeruginosa. J Gen Microbiol. 1992;138:2381–2387. doi: 10.1099/00221287-138-11-2381. [DOI] [PubMed] [Google Scholar]
- 11.Gensberg K, Doyle E J, Perry D J, Smith A W. Uptake of BRL 41897A, a C(7) α-formamido substituted cephalosporin, via the ferri-pyochelin transport system of Pseudomonas aeruginosa. J Antimicrob Chemother. 1994;34:697–705. doi: 10.1093/jac/34.5.697. [DOI] [PubMed] [Google Scholar]
- 12.Govan J R W. Pyocin typing of Pseudomonas aeruginosa. Methods Microbiol. 1978;10:61–91. [Google Scholar]
- 13.Govan J R W. In vivosignificance of bacteriocins and bacteriocin receptors. Scand J Infect Dis. 1986;49:31–37. [PubMed] [Google Scholar]
- 14.Heller K, Kadner R J. Nucleotide sequence of the gene for the vitamin B12receptor protein in the outer membrane. J Bacteriol. 1985;161:896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito S, Kageyama M, Egami F. Isolation and characterization of pyocins from several strains of Pseudomonas aeruginosa. J Gen Appl Microbiol. 1970;16:205–214. [Google Scholar]
- 16.Koedam N, Wittouck E, Gaballa A, Gillis A, Höfte M, Cornelis P. Detection and differentiation of microbial siderophores by isoelectric focusing and chrome azurol S overlay. Biometals. 1994;7:287–291. doi: 10.1007/BF00144123. [DOI] [PubMed] [Google Scholar]
- 17.Lazdunski C, Bouveret E, Rigal A, Journet L, Lloubés R, Bénédetti H. Colicin import into Escherichia coli. J Bacteriol. 1995;180:4993–5002. doi: 10.1128/jb.180.19.4993-5002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer J M, Stintzi A, De Vos D, Cornelis P, Tappe R, Taraz K, Budzikiewicz H. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosapyoverdine systems. Microbiology. 1997;143:35–43. doi: 10.1099/00221287-143-1-35. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa I, Shiga S, Kageyama M. Effect of iron concentration in the growth medium on the sensitivity of Pseudomonas aeruginosato pyocin S2. J Biochem. 1980;87:323–331. doi: 10.1093/oxfordjournals.jbchem.a132740. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa I, Kageyama M, Egami F. Purification and properties of pyocins S2. J Biochem. 1985;73:281–289. [PubMed] [Google Scholar]
- 21.Plesiat P, Grandguillot M, Harayama S, Vragar S, Michel-Briand Y. Cloning, sequencing, and expression of the Pseudomonas testosteroni gene encoding 3-oxosteroid Δ1-dehydrogenase. J Bacteriol. 1991;173:7219–7227. doi: 10.1128/jb.173.22.7219-7227.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole K, Neshat S, Krebes K, Heinrichs D E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol. 1993;175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The Pseudomonas aeruginosa tonBgene encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 24.Sano Y, Kageyama M. Purification and properties of an S-type pyocin, pyocin AP41. J Bacteriol. 1981;146:733–739. doi: 10.1128/jb.146.2.733-739.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano Y, Matsui H, Kobayashi M, Kageyama M. Pyocins S1 and S2, bacteriocins of Pseudomonas aeruginosa. In: Silver S, Chakrabarty A M, Iglewski B, Kaplan S, editors. Pseudomonas: biotransformations, pathogenesis, and evolving biotechnology. Washington, D.C: American Society for Microbiology; 1990. pp. 352–358. [Google Scholar]
- 26.Sano Y, Kobayashi M, Kageyama M. Functional domains of S-type pyocins deduced from chimeric molecules. J Bacteriol. 1993;175:6179–6185. doi: 10.1128/jb.175.19.6179-6185.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R, Prieffer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 28.Smith A W, Hirst P H, Hughes K, Gensberg K, Govan J R W. The pyocin Sa receptor of Pseudomonas aeruginosais associated with ferripyoverdin uptake. J Bacteriol. 1992;174:4847–4849. doi: 10.1128/jb.174.14.4847-4849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]