Abstract
Chalcone derivatives are considered valuable species because they possess a ketoethylenic moiety, CO–CH=CH–. Due to the presence of a reactive α,β-unsaturated carbonyl group, chalcones and their derivatives possess a wide spectrum of antiproliferative, antifungal, antibacterial, antiviral, antileishmanial, and antimalarial pharmacological properties. Recent developments in heterocyclic chemistry have led to the synthesis of chalcone derivatives, which had been biologically investigated toward certain disease targets. The major aspect of this review is to present the most recent synthesis of chalcones bearing N, O, and/or S heterocycles, revealing their biological potential during the past decade (2010–2021). Based on a review of the literature, many chalcone–heterocycle hybrids appear to exhibit promise as future drug candidates owing to their similar or superior activities compared to those of the standards. Thus, this review may prove to be beneficial for the development and design of new potent therapeutic drugs based on previously developed strategies.
1. Introduction
The flavonoid family includes chalcones, which are secondary metabolites of an edible or medicinal plant. Chalcones, 1,3-diphenyl-2-propen-1-ones, are based on two aryl moieties bridged via an α,β-unsaturated carbonyl group.1 These molecules have a −C=O–CH=CH– ketoethylenic moiety in their structure. In their aromatic rings, they have a delocalized π-electron-containing order.2 Chalcones mainly consist of polyphenolic compounds whose colors vary from yellow to orange and contribute significantly to the pigmentation of the corolla of some plants. Chalcones, which are naturally found in fruits, spices, teas, and soy-based foods, have gotten a lot of attention owing to their intriguing and potentially beneficial properties. Moreover, these molecules are found in natural products as pheromones, plant allelochemicals, and insect hormones.3,4 Chalcones undergo many chemical reactions as well as being used to synthesize heterocyclic compounds. It is possible to synthesize a wide range of chalcone derivatives by treating aromatic aldehydes with aryl ketones in the presence of an appropriate amount of condensing agents.5 By condensing aryl ketones with aromatic aldehydes and adding appropriate condensing agents, it is possible to synthesize a wide range of chalcone derivatives. Chalcones are used in several biosynthetic pathways as initial intermediate structures in the production of flavonoids, isoflavonoids, and aurones.6 A significant part of medicinal chemistry research in the 21st century has been focused on both natural and synthetic chalcones because of their various pharmacological potential including activities and characteristics such as antibacterial,7−9 anti-inflammatory,10−12 analgesic,13,14 anticholinergic,15 antiplatelet,16 antiulcer,17 antioxidant,18,19 antimalarial,20 anticancer,21,22 antiviral,23−26 antileishmanial,27 antidiabetic,28,29 immunomodulatory,30,31 aldose reductase inhibition,32 estrogenic,33 acetylcholinesterase inhibition,34 and as non-purine xanthine oxidase inhibitors.35 Chalcones are highly attractive molecules because of their simple structure, ease of construction, and promising biological applications. Throughout this review, we present the various synthetic pathways used to synthesize chalcone derivatives over the past few years, including their chemistry and biological activities.
2. Chalcone Synthesis
2.1. Synthesis of Chalcone Derivatives of 2-Acetyl Naphthalene
Chalcones 1a–g were constructed by treating 2-acetyl naphthalene with benzaldehyde and/or substituted benzaldehyde in methanol and potassium hydroxide, and the constructed derivatives exhibited antibacterial and antifungal activities (Scheme 1).36
Scheme 1. Synthesis of Chalcones 1a–g.

2.2. Synthesis of Chalcone Derivatives Bearing Triazine
The trisubstituted triazines 6a–f were constructed by treating aniline with cyanuric acid at 0–5 °C to yield monosubstituted triazine, which was reacted with substituted amine at RT to produce disubstituted triazine 4. Treating the latter 4 with 4-aminoacetophenone afforded the corresponding trisubstituted triazine 5, which was reacted with different aldehydes to provide chalcone derivatives 6a–f (Scheme 2).37
Scheme 2. Synthesis of Triazines 6a–f Containing Chalcone.
2.3. Synthesis of Acetamido Chalcone Derivatives
Treatment of 4-acetamidoacetophenone (9) with substituted aldehydes in potassium hydroxide as the base in ethanol and sonication for 10–15 min using a water bath of ultrasonic cleaner afforded chalcone derivatives 10a–f (Scheme 3).37
Scheme 3. Synthesis of Chalcones 10a–f.

2.4. Synthesis of Methoxyamino Chalcones
The chalcone derivatives 13–29 were synthesized via treatment of acetophenone derivative 11 and benzaldehyde derivative 12 in equal amounts using ethyl alcohol in 40% NaOH solution at 10 °C and stirred for 1 h and then at RT for 4 h; the reaction proceeded via Claisen–Schmidt reactions (Scheme 4).38
Scheme 4. Synthesis of Chalcones Derivatives 13–29.
2.5. Synthesis of Sappanchalcone
Chalcone derivatives 32–44 were constructed via a Claisen–Schmidt reaction by treatment of benzaldehyde derivatives and acetophenone derivatives in methanol and potassium hydroxide and subject to ultrasonic irradiation for 8 h, utilizing a water bath at 80 °C, and exhibited XO inhibitory activity (Schemes 5–7).35
Scheme 5. Construction of Chalcone Derivatives.
Reagents and conditions: (a) KOHaq, MeOH, ultrasound-assisted; (b) KOHaq, ultrasound-assisted.
Scheme 7. Synthesis of Chalcone Derivatives 35–44).
Scheme 6. Synthesis of Sappanchalcone 32.
Reagents and conditions: (a) CH3COOH, polyphosphoric acid, 60 °C, 30 min; (b) 2′,4′-dihydroxyacetophenone, 12 M KOH, ultrasound-assisted, 80 °C, 8 h.
2.6. Synthesis of Chalcone Derivatives from 1,3-Diacetylbenzene and 1,4-Diacetylbenzene
Construction and biological potential of chalcone derivatives 45–47 were reported. These compounds were synthesized through acid-catalyzed one-step condensation of 1,3- and/or 1,4-diacetylbenzene and 1,3,5-triacetylbenzene with 4-hydroxy-3-methoxybenzaldehyde in the presence of acids as acetic acid, concentrated hydrochloric acid, phosphoric acid, and concentrated sulfuric acid. The best result was achieved in case using concentrated sulfuric acid in ethanol (Scheme 8).39
Scheme 8.
Reagents and conditions: (a) H2SO4, 1,3-diacetylbenzene, ethyl alcohol, reflux, 3 h; (b) H2SO4, 1,4-diacetylbenzene, ethyl alcohol, reflux, 3 h; (c) H2SO4, 1,3,5-triacetylbenzene, reflux, 3 h.
2.7. Synthesis of Chalcone Derivatives from 1-(2′,4′-Difluorobiphenyl-4-yl)ethanone
The chalcone derivatives 50a–d were prepared in good yield via solvent-free Claisen–Schmidt condensation reaction of equal amounts of 1-(2′,4′-difluorobiphenyl-4-yl)ethanone with many aldehydes in 40% NaOH, leading to the formation of a sodium adduct which was neutralized by diluted HCl in cold water to afford the corresponding chalcone derivatives 50a–d (Scheme 9).40
Scheme 9. Synthesis of (E)-1-(2′,4′-Difluorobiphenyl-4-yl)-3-arylprop-2-en-1-ones.
2.8. Synthesis of Chalcone from Acetophenone Derivatives
Treatment of hydroxyacetophenone 51 with benzaldehyde derivative 52 in 50% KOH provided the corresponding chalcones 53–56, and the highest yield of chalcones ranged from 93 to 97%.41 On the other hand, chalcone 53 was prepared in a low 32% yield in the presence of KOH as the catalyst,42 whereas chalcone B was synthesized with BF3-Et2O catalyst in a high yield 90%.43 Reaction of veratraldehyde with 4-hydroxyacetophenone yielded chalcone 54 in a high 97% yield.41 However, treatment of 2,4-dihydroxyacetophenone provided the corresponding chalcone 55 in 96% yield and 56 in 93% yield (Scheme 10).41
Scheme 10. Synthesis of Chalcones 53–56.
Chalcone derivatives 59–99 were constructed by treating aldehyde 57 with acetophenone 58 in ethanol containing NaOH 40% or few drops of hydrochloric acid (Scheme 11 and Table 1).44
Scheme 11. Construction of Chalcones 59–99.
Reagents and conditions: ethyl alcohol, 40% NaOH or HCl, room temperature, 12–24 h.
Table 1. Synthesis of Chalcone Derivatives 59–99.
| compound no. | R | R′ | compound no. | R | R′ |
|---|---|---|---|---|---|
| 59 | 3,4-OH | 3-OH | 80 | 3,4-OCH3 | 3,5-F |
| 60 | 3,4-OCH3 | 3-OH | 81 | 4-OCH3 | 3,4-OH |
| 61 | 3,4-OH | 4-NH2 | 82 | 4-OCH3 | ,4-OCH3 |
| 62 | 3,4-OCH3 | 4-NH2 | 83 | 3-OH, 4-OCH3 | 3,4-OH |
| 63 | 3,4-OH | 4-OCH3 | 84 | 3-OH, 4-OCH3 | 3,4-OCH3 |
| 64 | 3,4-OCH3 | 4-OCH3 | 85 | 2,4-OCH3 | 3,4-OH |
| 65 | 3,4-OH | 4-OCH2CH3 | 86 | 2,4-OCH3 | 3,4-OCH3 |
| 66 | 3,4-OCH3 | 4-OCH2CH3 | 87 | 2-OCH3 | 3,4-OH |
| 67 | 3,4-OH | 4-Cl | 88 | 2-OCH3 | 3,4-OCH3 |
| 68 | 3,4-OCH3 | 4-Cl | 89 | 2,3-OCH3 | 3,4-OH |
| 69 | 3,4-OH | 2-F | 90 | 2,3-OCH3 | 3,4-OCH3 |
| 70 | 3,4-OCH3 | 2-F | 91 | 2,5-OCH3 | 3,4-OH |
| 71 | 3,4-OH | 2-Cl | 92 | 2,5-OCH3 | 3,4-OCH3 |
| 72 | 3,4-OCH3 | 2-Cl | 93 | 4-Cl | 3,4-OH |
| 73 | 3,4-OH | 4-F | 94 | 4-Cl | 3,4-OCH3 |
| 74 | 3,4-OCH3 | 4-F | 95 | 3,4-Cl | 3,4-OH |
| 75 | 3,4-OH | 3,4-F | 96 | 3,4-Cl | 3,4-Cl |
| 76 | 3,4-OCH3 | 3,4-F | 97 | 3,4,5-OCH3 | 3,4,5-OCH3 |
| 77 | 3,4-OH | 3,4-OCH3 | 98 | 3,4,5-OCH3 | 3,4,5-OCH3 |
| 78 | 3,4-OCH3 | 3,4-OCH3 | 99 | 3,4-OCH3 | 3,4-OCH3 |
| 79 | 3,4-OH | 3,5-F |
Treatment of benzaldehyde and acetophenone derivatives in acid catalyst or alkaline conditions at 50–100 °C in presence of liquid solvent led to 100 (Scheme 12).45−47
Scheme 12. Claisen–Schmidt Condensation.
2.9. Synthesis of Chalcone from Phenyl Halide
Chalcone 100 was prepared by reaction of phenyl halide and styrene in carbon monoxide and pd catalyst (Scheme 13).48
Scheme 13. Construction of Chalcone Derivative 100.
Treatment of phenylacetylene with benzaldehyde in (BmimOTs) and HBr at 100 °C for 12 h provided the corresponding chalcone 99. The reaction proceeds via a coupling reaction (Scheme 14).49
Scheme 14. Synthesis of Chalcone 100 via a Coupling Reaction.
Chalcone derivative 101 was constructed from the reaction of propargyl alcohol and phenyl halide using microwave irradiation utilizing PdCl2(pph3)2 in THF (Scheme 15).50,51
Scheme 15. Synthesis of Chalcone 101.
2.10. Synthesis of Chalcone Derivatives from Benzoyl Chlorides
Ynones were synthesized by reaction of benzoyl chlorides and phenylacetylenes using Sonogashira conditions described in a literature procedure. Ynones undergo deuterations using the H-Cube system using D2O instead of water (Scheme 16).52,53
Scheme 16. Synthesis of Chalcone 102 via Continuous-Flow Deuteration Reaction.
Chalcone 100 was synthesized by reaction of benzoyl chloride with styrylboronic acid 103 in anhydrous toluene and in presence of Pd(PPh3)4 and CsCO3. Also from the reaction of cinnamoyl chloride 104 and phenylboronic acid in anhydrous toluene and in the presence of Pd(PPh3)4 and CsCO3, the reaction proceeds via the Suzuki–Miyaura coupling reaction (Scheme 17).54
Scheme 17. Synthesis of Chalcone 100 through Suzuki–Miyaura Coupling Reaction.
2.11. Synthesis of Chalcone via One-Pot Synthesis
Treatment of phenylmethanol 105 and acetophenone using CrO3 as the oxidizing agent provided chalcone derivative 100 (Scheme 18).45
Scheme 18. One-Pot Synthesis of Chalcone 100.
Treatment of benzaldehyde and phenylacetylene under microwave irradiation using heterogeneous acid in a catalytic amount in 1,2-dichloroethane as solvent provided the corresponding chalcone 100 (Scheme 19).55
Scheme 19. Synthesis of Chalcone Using Solid Acid Catalyst.
2.12. Some Chalcones from Natural Product
Chalcones occur in natural products derived from plant species, such as Macaranga denticulata, which contains compound 106.56 Also Uvaria siamensis roots contain compound 107;57 compound 108 is derived from Stevia lucida,58 and compound 109 is found in Pongamia pinnata (L.).59 Hydroxychalcone-based sugar functionalities 110–112 were separated from Coreposis lanceolata flowers (Figure 1).60
Figure 1.
Some chalcones from natural products.
3. Biological Activities of Chalcone Derivatives
Some chalcones were investigated and were documented to exhibit many pharmacological activities, such as antioxidant activity,61−64 such as yakuchinones A and curcumin-related enones, which inhibited the prosurvival transcription (Figure 2).
Figure 2.
Some examples of chalcone (1), curcumin (2), JC-3 (3), and yakuchinones B (4).
3.1. Chalcone Derivatives Bearing Five-Membered N-Heterocycles
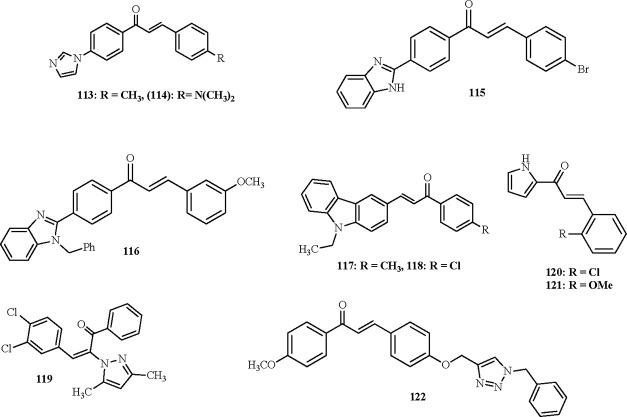
As revealed in Figure 3, chalcone derivatives bearing benzimidazole and imidazole as five-membered N-heterocycle rings were synthesized. Synthesis and evaluation of the imidazolylchalcones for their potential toward recombinant human monoamine oxidase (MAO) A and B inhibition were conducted. Compound 114 provided considerable activity as an inhibitor, whereas derivative 113 was the most active. Compounds 113 (IC50 = 1.6 ± 0.09 μM) showed inhibition activity on MAO-A suppression better than that recorded by Toloxatone (IC50 = 1.10 ± 0.0085 μM) as a standard.65 Moreover, compound 115 with a p-bromo-substituted phenyl ring exhibited insect antifeedant potential (Figure 3).66 Alternatively, N-benzyl-substituted benzimidazole chalcones were prepared following multistep reaction sequences.67 Antibacterial potential of the novel candidates was assessed toward Gram-positive and Gram-negative bacteria. Benzimidazole-based chalcone 116 was effective against Staphylococcus aureus and Bacillus subtilis. Gram-negative bacteria, Escherichia coli and Pseudomonas aeruginosa, are less active against compound 116 (Figure 3).
Figure 3.
Chalcones bearing five-membered N-heterocycles.
The carbazole moiety of chalcone derivatives have been extensively tested as a nonintercalative inhibitor of topoisomerase II and as an inducer of apoptosis.68 The chalcone derivative 117 exhibited moderate topoisomerase II inhibitory activity and inhibited cell proliferation effectively against human cancer cell lines. Conversely, the chalcone derivative 118 exhibited high potential as a topoisomerase II suppressor but recorded less inhibitory potential toward cancerous cell lines. By inducing apoptosis, compounds 117 and 118 can arrest HL-60 cells in the sub-G1 phase, as shown in Figure 3 and Table 2.
Table 2. Anticancer Potential of Carbazole-Bearing Chalcones 117 and 118 and Triazole-Containing Chalcone 122.
| IC50 (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| compound no. | HeLa | A549 | PC-3 | HL-60 | MCF-7 | HepG2 | MIA-Pa-Ca-2 |
| 117 | 0.36 | 2.16 | 0.62 | 0.22 | |||
| 118 | 5.48 | 9.57 | 7.12 | 2.85 | |||
| 122 | 11 | 5 | 9 | 4 | |||
| PI-103 | 8 | 3 | 4 | 6 | |||
Kumari et al.69 had also constructed chalcones containing a pyrazole moiety and tested them for their antioxidant and antimicrobial activity. The pyrazole–chalcone derivative 119 showed significant activity against B. subtilis (ZI = 16 ± 0.82 mm), P. aeruginosa (ZI = 14 ± 1.24 mm), S. aureus (ZI = 13 ± 0.60 mm), and E. coli (ZI = 14 ± 0.83 mm). Furthermore, compound 119 revealed promising radical scavenging potential with an IC50 value equal to 88.04 μg/mL (Figure 3).
The biological activity of chalcone derivatives bearing pyrrole was studied in relation to their inhibition of the CYP1 enzyme.70 Chalcone derivative 120 displayed greater activity against the suppressed CYP1B1 isoform, revealing an IC50 of 0.2 μM. In contrast, chalcone 121 inhibited both CYP1A1 and CYP1B1 isoforms with IC50 values of ∼0.9 μM in similar conditions. Further, compound 121 inhibited the toxicity of benzo[a]pyrene in addition to resistance of reversed cisplatin in human cells (Figure 3).
Chalcone-based 1,2,3-triazoles were synthesized using catalytic copper nanoparticles supported on cellulose in an aqueous medium. From these compounds, compound 122 exhibited promising anticancer activity compared to the reference drug. Moreover, this triazolo chalcone derivative 122 was recorded to promote apoptosis and G2/S arrest, triggering loss of mitochondrial potential in pancreatic cells MIA-Pa-Ca-2,71 as presented in Figure 3 and Table 2.
3.2. Chalcone Derivatives Based on Six-Membered N-Heterocycles
Pyridine-bearing chalcone derivatives were prepared and biologically screened for their antiviral potential. Antiviral properties were observed against cucumber mosaic virus (CMV) for the majority of the newly constructed compounds.72 Most of the prepared compounds showed antiviral activity toward cucumber. The chalcone derivative 123 exhibited antiviral activity in terms of curative, inactivation, and protection properties. Furthermore, chalcone 124 demonstrated a high level of activity against CMV (Figure 4).
Figure 4.
Chalcone derivatives bearing six-membered N-heterocycles.
Chalcone derivatives based on acridine were constructed and examined toward Plasmodium falciparum to test their antimalarial effect. The tested compounds displayed 100% antimalarial potential, while chalcones 125–127 showed inhibition of >71% at 2 and 0.4 μg/mL. Chalcone 125 demonstrated an inhibitory activity of 71.4% against the parasite (Figure 4).73
A chalcone derivative containing piperidine was constructed and evaluated as an inhibitor of anti-PI3Kδ activity and normal basophil degranulation. Chalcone derivative 128 was found to inhibit normal basophil degranulation and revealed activity comparable to that of betamethasone as a drug reference (Figure 4).74 A set of chalcone–pyrazine hybrids was constructed and biologically tested. Chalcones with nitro substituents 129 and 130 were effective against Trychophyton mentagrophytes fungi compared with fluconazole as a reference drug. Additionally, the synthesized chalcones were screened for their antimycobacterial potential. Compounds 129 and 130 were recorded to show activity against Mycobacterium tuberculosis H37Rv (ATCC 27294) (Figure 4).75
An investigation was conducted on the antibacterial activity of piperazine chalcone derivatives containing dithiocarbamate, morpholine, and piperazine moieties against multi-drug-resistant Gram-negative bacteria. The chalcone piperazine 131 inhibited Klebsiella pneumonia and Pseudomonas aeruginosa (Ps12). As a result, chalcone 132 exhibited a high level of antibacterial activity and a higher affinity for DNA binding than doxorubicin (Figure 4).76
There are many quinazoline moieties revealing anticancer activity reported in literature, such as Erlotinib and Gefitinib.77−79 From 2-aminobenzoic acid, chalcone derivatives bearing a quinazoline moiety were prepared. Four human cancer cell lines MCF-7, A549, HT-29, and A375 tested with the derivatives 133–136 showed antiproliferative potential higher than that of Combretastatin-A4 as a reference compound,80 as illustrated in Figure 4 and Table 3. Compounds 133–136 were demonstrated to possess higher or comparable anticancer potential with regard to A549, HT-29, MCF-7, and A375 human cancer cell lines when compared with the reference drug Combretastatin-A4. Compounds 134–136 inhibited MCF-7 human breast adenocarcinoma cells with IC50 values of 0.17, 0.14, and 0.16 μM, respectively, compared with the control drug with IC50 = 0.18 μM toward MCF-7 cancer cell line.
Table 3. Anticancer Potential (IC50) of Chalcone Based on Quinazoline Heterocycles 133–136.
| compound no. | A549 | HT-29 | MCF-7 | A375 |
|---|---|---|---|---|
| 133 | 2.90 | 0.18 | 1.89 | |
| 134 | 0.10 | 0.13 | 0.17 | 1.34 |
| 135 | 0.10 | 1.56 | 0.14 | 0.19 |
| 136 | 2.10 | 2.89 | 0.16 | 1.37 |
| Combretastatin-A4 | 0.11 | 0.93 | 0.18 | 0.21 |
Another group of researchers81 recently synthesized analogues of chalcones containing a 4-oxoquinazolinyl moiety and screened these candidates for their antiproliferative potential. It was determined that out of 38 compounds synthesized, compound 137 recorded the greatest potency with anticancer potential toward HCT-116 (IC50 = 3.56 μM) and MCF-7 cells (IC50 = 4.08 μM). Compound 137 induced apoptosis in the sub-G1 phase and in the mitochondrial death pathway (Figure 4).
The antitubercular and antimicrobial properties of chalcone having a quinoxaline moiety were assessed. In comparison with the reference drugs ciprofloxacin and pyrazinamide, chalcone derivatives 138 and 139, which contain a hydroxyl group in their rings, displayed significantly lower antitubercular activities. Compound 138 is not active against fungal strains, but it recorded an antibacterial potential against Gram-negative and Gram-positive bacteria. It is noteworthy that compound 139 exhibited both antifungal and antibacterial effects (Figure 4 and Table 4).82
Table 4. Antimicrobial Potential of Chalcone Derivatives 138 and 139.
| compound no. | S. aureus | E. coli | C. albicans |
|---|---|---|---|
| 138 | 5 | 5 | |
| 139 | 10 | 5 | 10 |
| ciprofloxacin | 2 | 2 | |
| fluconazole | 16 |
3.3. Chalcones Hybridized with Other Types of N-Heterocycles
The antimalarial, antileishmanial, and antitrypanosomal activity of chalcone derivatives bearing a caffeine moiety has been evaluated.83 Chalcone derivatives 140 and 141 were reported to exhibit remarkable activity against Leishmania panamensis (Figure 4). In contrast, chalcone 142 containing a trimethoxy group showed activity against Acrypanozoma cruzi at very low concentrations. Despite the fact that compounds 140–142 did not exhibit significant antimalarial activity, several new tricyclic pyrido[3,4-b]indoles hybridized with chalcone derivatives were constructed and biologically evaluated for their anticancer activity and DNA binding affinity.84 A majority of the synthesized derivatives were found to possess promising cytotoxic potential toward the lung adenocarcinoma cell line A549 with IC50 below 10 μM. Compound 143, having a trimethoxy substituent on both the chalcone skeleton and the C-1 position of β-carboline, recorded significant anticancer potential toward lung adenocarcinoma A549 (IC50 = 5.30 μM), B-16 melanoma (IC50 = 6.37 μM), human prostate PC-3 cancer cells (IC50 = 19.59 μM), human colorectal cancer cells (IC50 = 23.08 μM), and HeLa cervical cancer cells (IC50 = 44.26 μM). Despite this, compounds 144–151 showed significant elevation of ΔTm of DNA compared to Adriamycin, suggesting a significant interaction as well as DNA stabilization. A further series of chalcone derivatives derived from β-carboline via the C-1 position were synthesized and evaluated as potential anticancer and antibacterial agents.85
From the constructed β-carboline–chalcone conjugates, compound 152 containing a trimethoxy substituent and the molecule as a bromide salt was evaluated and determined to show the highest activity toward a panel of cancerous and noncancerous cell lines with IC50 values of 20 ± 2.1 μM (BxPC-3), 22.1 ± 3.23 μM (HeLa), 22.02 ± 3.25 μM (PC-3), 17.18 ± 2.98 μM (HEK293T), 15.95 ± 3.41 μM (MDA-MB-231), and 55.23 ± 5.8 μM (NIH3T3). Meanwhile, compound 153 with a p-methoxy group was found to be the most effective analogue for inhibiting both Gram-positive and Gram-negative bacteria. The high antibacterial potential of this candidate was achieved when it inhibited S. aureus with a zone inhibition diameter of 15 mm and a MIC equal to 440 μg/mL (Figure 5).
Figure 5.
Chalcones bearing some other ring-type N-heterocycles.
New chalcones hybridized with purines had been designed and constructed.86 By means of an amide bridge, chalcones and purines were linked. Antiviral evaluations of the synthesized compounds had been performed against tobacco mosaic virus (TMV) and CMV. Among them, compounds 154 and 155 exhibited an inactivating potential toward TMV higher than that recorded by ribavirin, as recorded in Table 5. Furthermore, compound 154 recorded a moderate affinity for the coat protein of TMV, which is in accordance with its inactivating property (Figure 5). The same group of researchers constructed novel chalcone–purine hybrids that were evaluated in vivo for their antiviral properties.87 It was found that among the compounds under study, compounds 156–159 displayed higher curative activity against CMV with EC50 values of 301.1, 315.7, 282.3, and 230.5 μg/mL, sequentially compared to that of ribavirin (726.3 ng/mL) and dufulin (373.7 ng/mL). In addition, a fluorescence spectroscopy study revealed that compound 156 had a strong affinity for the TMC coat protein. Zhou et al.88 prepared a set of new chalcones linked with purine and benzenesulfonamide moieties. According to in vivo antiviral assays, some of the derivatives exhibited excellent anti-TMV and anti-CMV properties. Compound 160 demonstrated EC50 = 51.65 μg/mL and was identified as the most potent hybrid with significant inactivating activity against the TMV. This compound exhibited a strong affinity for the TMV coat protein.
Table 5. EC50 (μg/mL) of Chalcone Based on Purine Heterocycles 153 and 154 toward TMV.
| compound no. | curative | protective | inactivation |
|---|---|---|---|
| 154 | 452.4 ± 3.7 | 416.2 ± 3.9 | 241.2 ± 4.9 |
| 155 | 438 ± 3.5 | 418.6 ± 3.3 | 261.7 ± 7.5 |
| ribavirin | 585.8 ± 4.1 | 436.0 ± 4.3 | 268.7 ± 5.1 |
Novel chalcone–indolizines were constructed and tested toward lymphoma cells (U937, Raji, and JeKo-1) for their anticancer potential.89 Among those compounds, candidate 161 was identified as the most potent agent, which resulted in below 60% cell viability at 1 μM against U937 cells. The provided results revealed the important role of the 3,5-dimethoxyphenyl moiety in regards to their anticancer properties. Compounds 162 and 163 with halogen substituents at the meta-position inhibited cell activity more effectively than those at the para-position. Moreover, compound 161 recorded apoptotic inducing potential higher than that of doxorubicin but not as high as that of cisplatin, as registered in Table 6.
Table 6. Apoptotic Cell Percentage Induced by Compound 160, Doxorubicin, and Cisplatin.
| apoptotic
cells percentage at 10 μM |
|||
|---|---|---|---|
| compound no. | U937 | Raji | Jeko-1 |
| 161 | 51.26 | 33.78 | 46.27 |
| doxorubicin | 74.66 | 95.35 | 87.92 |
| cisplatin | 8.58 | 23.05 | 44.26 |
Many imidazo[1,2-a]pyridine chalcone hybrids were constructed and tested as anticancer agents against the A549 cell line.90 As determined by the MTT assay, compounds 164–169 showed significant inhibitory potential with IC50 values ranging from 7.0 to 42.2 μg/mL. Chalcone derivative 166 was found to be the most active compound among the synthesized molecules, with IC50 = 7.0 ± 2.1 μg/mL.
3.4. Chalcone Derivatives Containing Five-Membered O-Heterocycles
An investigation of the activity of the 36 novel constructed chalcones bearing quinoline, thiofuran, and furan systems against Gram-positive and Gram-negative bacteria was conducted.91 Gram-positive bacteria, including multi-drug-resistant isolates, were selectively inhibited by some derivatives. Streptococcus mutans was significantly inhibited by the target compounds; in particular, 4-chlorophenylfuran derivative 170 (Figure 6) was the most active compound, with MIC = 2 μg/mL. Candidate 170 recorded antibacterial potential similar to that of norfloxacin (MIC = 2 μg/mL) and less activity than oxacillin (MIC = 1 μg/mL).
Figure 6.
Some biologically active chalcones bearing five-membered O-heterocycles.
Chalcone–furan hybrids were designed and prepared to inhibit protein tyrosine phosphatases.92 From these reported chalcones, compounds 171 (IC50 = 2.90 ± 0.12 μM) and 172 (IC50 = 2.49 ± 0.23 μM) inhibited protein tyrosine phosphatase 1B (PTP1B). Generally speaking, the findings indicated that compounds bearing dihydroxy or electron-withdrawing groups had excellent activity against PTP1B (Figure 6).
Many benzofuran–chalcone hybrids have been constructed and studied in vivo with transgenic Caenorhabditis elegans.93 Compounds 173–175 (Figure 6) decreased Aβ aggregates, acetylcholinesterase (AChE) levels, and oxidative stress in the worms. It was demonstrated that these hybrids increased acetylcholine (ACh) levels and protected against chemically induced cholinergic neurodegeneration. Coskun et al.94 constructed a set of 1-(7-ethoxy-1-benzofuran-2-yl)-substituted chalcone derivatives to develop an anticancer candidate by incorporating the chalcone–benzofuran system. A number of compounds containing dimethylamino or trimethoxy substituents have been shown to demonstrate promising effects against cancer cell lines. Compound 176 was identified as the most potent derivative against A549 (IC50 = 9 μM), MCF-7 (IC50 = 2 μM), and PC-3 (IC50 = 10 μM). Approximately 90% of the MCF-7 and A549 cells were in the late apoptotic stage at a concentration of 20 μM, while in PC-3 cells, only 6.45 and 59.70% were in the early and late apoptotic stages, respectively. The sample concentration remains the same (Figure 6).
Compound 177 was the most effective at inhibiting the growth of PC-3 cells for 72 h. A series of benzofuranchalcones were synthesized applying the Claisen–Schmidt reaction to yield chalcone, which was then cross-coupled to many aryl alkynes to afford the benzofuran moiety.95 Moreover, these novel heterocycles had been assessed for their antiproliferative effects in vitro against the MCF-7 cancer cell line and for their inhibitory effect on EGFR phosphorylation and/or tubulin polymerization. In comparison with actinomycin D, almost all of the assessed chalcones recorded excellent an antiproliferative effect on MCF-7; in particular, compounds 178 and 179 demonstrated significant inhibition toward the MCF-7 cell line (Figure 6 and Table 7).
Table 7. IC50 of Compounds 178, 179, Actinomycin D, Colchicine, and Gefitinib toward MCF-7, Tubulin, and EGFR.
| compound no. | MCF (IC50, μM) | tubulin (IC50, μM) | EGFR (IC50, μM) |
|---|---|---|---|
| 178 | 0.55 ± 0.24 | 26.5 | 0.17 ± 0.03 |
| 179 | 3.55 × 10–4 ± 0.07 | 5.51 × 10–5 | 0.09 ± 0.03 |
| actinomycin D | 37.82 ± 1.30 | 0.04 ± 0.03 | |
| colchicine | 9.88 × 10–2 | ||
| gefitinib | 0.03 ± 0.02 |
An investigation of the antiproliferative activity of chalcone derivatives bearing homoserine lactone scaffolds was conducted recently against MCF-7, MGC-803, DU145, and PC-3 cell lines.96 As a result of their in vitro assessment, many derivatives demonstrated selectivity and potency against PC-3 and DU145 prostate cell lines. It was found that compounds 180–182 showed the highest potential recording with IC50 less than 5 μM. It was found that compound 181 inhibited migration and colony formation in DU145 cells to a dose-dependent extent.
3.5. Chalcone Derivatives Containing Six-Membered O-Heterocycles
Coumarin derivatives have been reported to possess a variety of biological properties.97 Therefore, the hybridization of chalcone with coumarin may generate new compounds with potential pharmacological activity. The synthesis of chalcone–coumarin hybrids was carried out, and their anticancer potential was tested in vitro.98 It was reported that some of these derivatives exhibited excellent activities against KB (oral squamous carcinoma), C33A (cervical carcinoma), MCF-7 (breast adenocarcinoma), and A549 (lung carcinoma), as well as mouse embryo fibroblast (NIH3T3). Compounds 183–185 displayed significant performance, with IC50 values ranging from 3.59 to 8.12 μM. With an IC50 of 3.59 μM, compound 185 was the most potent candidate having selectivity against cervical carcinoma C33A 30-fold greater than that of the normal fibroblast NIH3T3 cell line.
The aim of the research is to develop a new class of DNA oxidation inhibitors and radical scavengers.99 In order to achieve that, chalcones containing coumarin frameworks were synthesized. Inhibitory activities were observed for compounds 186–189. It was observed that coumarin clubbed chalcone derivatives were more potent antioxidants in case of presence of incorporating phenolic hydroxy group in the structure. In addition, double OH groups at adjacent positions scavenged ABTS and DPPH radicals and inhibited Cu(II)/glutathione-induced DNA oxidation.100
It has been reported that chalcone–coumarin compounds had been constructed in a trial to act as selective antibacterial agents toward the pathogens causing tenacibaculosis in fish.101 The hybrids 190–192 displayed significant activity against 14 strains of Tenacibaculosis maritimus. The coumarin derivative 192 was found to be the most active candidate with 20-fold MIC increase compared to that of enrofloxacin toward Tenacibaculosis maritimus strains LL01 8.3.1 and LL018.3.8. It should be noted that compounds containing an amino group at the para- or ortho-position to the benzoyl moiety revealed excellent antibacterial potential. An innovative set of chalcone–coumarin fibrates had been prepared and tested as PPARα/γ agonists showing good antioxidant potential.102 Compounds 193–195 were potent dual PPARα and γ agonists. As PPARα agonists, they proved to be more effective than fenofibrate. Antioxidant studies recorded that 194 and 196–199 have antioxidant activity significantly greater than that of trolox, with the IC50 ranging from 9.40 to 18.63 μM.
A group of investigators101 presented two sets of chalcones containing a chromene ring, which included 1-(6-methoxy-2H-chromen-3-yl)-3-phenylpropen-1-ones and 3-(6-methoxy-2H-chromen-3-yl)-1-phenylpropen-1-ones. In biological studies, chloro-substituted 1-(6-methoxy-2H-chromen-3-yl)-3- phenylpropen-1-ones displayed excellent antileishmanial effect toward Leishmania major in a nontoxic dose. Antileishmanial potential of compounds 200–202 with IC50 values below 1 μM was found to be the strongest (Figure 7).
Figure 7.
Chalcones bearing six-membered O-heterocyclic moieties.
3.6. Chalcones Bearing Five-Membered S-Heterocycles
Chalcones containing thiophene were constructed from substituted aromatic aldehydes and 2-acetylthiophene.103 A docking score of −8.46 kcal/mol was achieved for compound 203 following ADME studies, in silico toxicity predictions, and explorations of molecular recognition. The calculated inhibitory constant toward the active site of MAO-B was approximately 0.64 μM.104 Aryl/heteroaryl chalcones derived from 3-arylthiophene-2-carbaldehydes were developed and studied for their anticancer properties. Among the compounds tested, compound 204 was identified as the most effective anticancer compound with an IC50 of 21 μg/mL against the HCT-15 human colon cell line, slightly better than the doxorubicin control. In addition, compound 205 revealed significant potential showing IC50 = 22.8 μg/mL.105 The anticancer effects of chalcone derivatives of 2-acetylthiophene were examined. A 48 h in vitro treatment of the human breast cancer cells MCF-7 and MDA-MB-231 with all chalcone derivatives resulting in significant reductions in cell viability in a dose-dependent manner. From the tested compounds, chalcone 205 was the most potent with IC50 values of 11.76 ± 4.87 and 5.52 ± 4.26 μM against MCF-7 and MDA-MB-231 cells, respectively.
As reported in a previous work,106 a set of 3-acetylthiophene chalcone hybrids was constructed, in vitro tested, and found to possess antitubercular, antifungal, and cytotoxic activity against the DU145 prostate cancer cell line. In terms of antifungal activity, compound 207 was comparable to fluconazole with a MIC value of 4 μg/mL against A. niger and C. tropicalis. In addition, this candidate proved to be the most active toward DU145 (IC50 = 5 ± 1 μg/mL), compared with methotrexate. Compared to pyrazinamide as a general tuberculosis drug, compound 208 recorded the best performance toward M. tuberculosis H37Rv with a MIC of 3.12 μg/mL. Moreover, in the same year, chalcones incorporating a thiophene moiety were constructed and assessed as antifungal agents.107 Among the tested compounds, compound 209 showed notable antifungal potential against C. albicans (MIC = 128 μg/mL) and A. niger (MIC = 64 μg/mL) (Figure 8).
Figure 8.
Chalcone derivatives containing five-membered S-heterocycles.
An evaluation of the construction and biological potential of thieno[3,2-b]thiophene–chalcone, a chalcone derivative attached to a thiophene scaffold, was reported. Compounds 210–213 recorded excellent antibacterial effect toward S. aureus (ZI = 24, 23, 22, and 22 mm, respectively), which was more active than ampicillin (ZI = 20 mm) as a reference antibacterial agent. Compounds 214–216 showed significant potential against A549 and SKNSH cancer cell lines, with IC50 values of 52.40, 51.00, and 47.17 μM, respectively.108 The synthesis and antimicrobial evaluation of thiophene–chalcone hybrids was reported. Among the prepared derivatives, compound 217 demonstrated activity against a wide range of bacteria with IC50 values of 219.1 μg/mL (S. aureus), 441.9 μg/mL (P. aeruginosa), 338.5 μg/mL (Enterococcus faecalis). Nevertheless, none of these values is lower than that of reference drugs (Figure 8).
The antimicrobial activity of benzofused thiophene, also known as benzothiophene, was investigated in vitro for the chalcone structures linked by an amide bond.109 Compounds 218 and 219 demonstrated moderate activity against bacterial strains and significant activity against fungal strains in this series (Figure 8).
4. Conclusion
Chemically, chalcones are easily modified and synthesized to create a variety of compounds with different structures. It is because of these properties that these compounds are very attractive as basic building blocks for the development of molecule-targeting agents. In this review, recent developments in the synthesis of chalcones have been summarized. In addition, it emphasizes how versatile these scaffolds can be in the development of various classes of compounds based on different moieties and synthetic methods. The present review describes the most important in vitro and in vivo biological activities of chalcone derivatives bearing various types of rings. A large number of preclinical studies have not completely established the mechanism of action of chalcone derivatives. Despite being relatively easy to synthesize, novel methods of synthesis must be developed in the future. This will enable the study of new biological properties, the study of molecular mechanisms of action, and, most importantly, the identification of their targets.
Acknowledgments
The authors gratefully acknowledge financial support from Jouf University (Kingdom of Saudi Arabia).
The authors declare no competing financial interest.
References
- Alam M. S.; Rahman S. M.; Lee D.-U. Synthesis, biological evaluation, quantitative-SAR and docking studies of novel chalcone derivatives as antibacterial and antioxidant agents. Chemical Papers. 2015, 69 (8), 1118–1129. 10.1515/chempap-2015-0113. [DOI] [Google Scholar]
- Singh P.; Anand A.; Kumar V. Recent developments in biological activities of chalcones: A mini review. European journal of medicinal chemistry. 2014, 85, 758–777. 10.1016/j.ejmech.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Wadleigh R. W.; Yu S. J. Glutathione transferase activity of fall armyworm larvae toward α, β-unsaturated carbonyl allelochemicals and its induction by allelochemicals. Insect Biochemistry. 1987, 17 (5), 759–764. 10.1016/0020-1790(87)90046-1. [DOI] [Google Scholar]
- Karthikeyan C.; Narayana Moorthy N. S.H.; Ramasamy S.; Vanam U.; Manivannan E.; Karunagaran D.; Trivedi P. Advances in chalcones with anticancer activities. Recent patents on anti-cancer drug discovery. 2014, 10 (1), 97–115. 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- Dhar K.; Saxena A.; Kumar S; Sapra S; Sweety; Nepali K; Suri O.; Sarma G. Synthesis and biological evaluation of chalcones having heterosubstituent (s). Indian journal of pharmaceutical sciences. 2010, 72 (6), 801. 10.4103/0250-474X.84602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya M.; Koketsu M. In Natural Products Ramawat K. G., Ed.; Springer Verlag: Berlin, 2013. [Google Scholar]
- Okolo E. N.; Ugwu D. I.; Ezema B. E.; Ndefo J. C.; Eze F. U.; Ezema C. G.; Ezugwu J. A.; Ujam O. T. New chalcone derivatives as potential antimicrobial and antioxidant agent. Scientific Reports. 2021, 11, 21871. 10.1038/s41598-021-01292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. J.; Bird S. J.; Gowland P.; Collins M.; Cassella J. P. Ferrocenyl chalcone derivatives as possible antimicrobial agents. Journal of antibiotics. 2020, 73 (5), 299–308. 10.1038/s41429-020-0280-y. [DOI] [PubMed] [Google Scholar]
- Benouda H.; Bouchal B.; Challioui A.; Oulmidi A.; Harit T.; Malek F.; Riahi A.; Bellaoui M.; Bouammali B. Synthesis of a series of chalcones and related flavones and evaluation of their antibacterial and antifungal activities. Letters in Drug Design & Discovery. 2018, 16 (1), 93–100. 10.2174/1570180815666180404130430. [DOI] [Google Scholar]
- ur Rashid H.; Xu Y.; Ahmad N.; Muhammad Y.; Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorganic chemistry. 2019, 87, 335–365. 10.1016/j.bioorg.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Nurkenov O.; Ibraev M.; Schepetkin I.; Khlebnikov A.; Seilkhanov T.; Arinova A.; Isabaeva M. Synthesis, structure, and anti-inflammatory activity of functionally substituted chalcones and their derivatives. Russian Journal of General Chemistry. 2019, 89 (7), 1360–1367. 10.1134/S1070363219070028. [DOI] [Google Scholar]
- Ibrahim T. S.; Moustafa A. H.; Almalki A. J.; Allam R. M.; Althagafi A.; Md S.; Mohamed M. F. Novel chalcone/aryl carboximidamide hybrids as potent anti-inflammatory via inhibition of prostaglandin E2 and inducible NO synthase activities: design, synthesis, molecular docking studies and ADMET prediction. Journal of enzyme inhibition and medicinal chemistry. 2021, 36 (1), 1067–1078. 10.1080/14756366.2021.1929201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan B.; Kannappan N.; Subburaju T. Synthesis and biological evaluation of novel chalcones with methanesulfonyl end as potent analgesic and anti-inflammatory agents. Int. J. Pharmaceutical Res. Biosci. 2020, 11 (10), 4974–4981. 10.13040/IJPSR.0975-8232.11(10).4974-81. [DOI] [Google Scholar]
- Higgs J.; Wasowski C.; Marcos A.; Jukič M.; Paván C. H.; Gobec S.; de Tezanos Pinto F.; Colettis N.; Marder M. Chalcone derivatives: synthesis, in vitro and in vivo evaluation of their anti-anxiety, anti-depression and analgesic effects. Heliyon. 2019, 5 (3), e01376. 10.1016/j.heliyon.2019.e01376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaza S.; Mir K. Z.; Tatheer A.; Ullah R. S. Synthesis and evaluation of chalcone and its derivatives as potential anticholinergic agents. Letters in Drug Design & Discovery. 2019, 16 (3), 322–332. 10.2174/1570180815666180523085436. [DOI] [Google Scholar]
- Fakhrudin N.; Pertiwi K. K.; Takubessi M. I.; Susiani E. F.; Nurrochmad A.; Widyarini S.; Sudarmanto A.; Nugroho A. A.; Wahyuono S. A geranylated chalcone with antiplatelet activity from the leaves of breadfruit (Artocarpus altilis). Pharmacia 2020, 67, 173. 10.3897/pharmacia.67.e56788. [DOI] [Google Scholar]
- Choudhary A. N.; Kumar A.; Juy V. Design, synthesis and evaluation of chalcone derivatives as anti-inflammatory, antioxidant and antiulcer agents. Letters in Drug Design & Discovery. 2012, 9 (5), 479–488. 10.2174/157018012800389368. [DOI] [Google Scholar]
- Bale A. T.; Salar U.; Khan K. M.; Chigurupati S.; Fasina T.; Ali F.; Ali M.; Nanda S. S.; Taha M.; Perveen S. Chalcones and Bis-Chalcones Analogs as DPPH and ABTS Radical Scavengers. Letters in Drug Design & Discovery. 2021, 18 (3), 249–257. 10.2174/1570180817999201001155032. [DOI] [Google Scholar]
- Al Zahrani N. A.; El-Shishtawy R. M.; Elaasser M. M.; Asiri A. M. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules. 2020, 25 (19), 4566. 10.3390/molecules25194566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H.-L.; Zhang Z.-W.; Lekkala R.; Alsulami H.; Rakesh K. Chalcone hybrids as privileged scaffolds in antimalarial drug discovery: a key review. European journal of medicinal chemistry 2020, 193, 112215. 10.1016/j.ejmech.2020.112215. [DOI] [PubMed] [Google Scholar]
- Ouyang Y.; Li J.; Chen X.; Fu X.; Sun S.; Wu Q. Chalcone derivatives: role in anticancer therapy. Biomolecules. 2021, 11 (6), 894. 10.3390/biom11060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čižmáriková M.; Takáč P.; Spengler G.; Kincses A.; Nové M.; Vilková M.; Mojžiš J. New chalcone derivative inhibits ABCB1 in multidrug resistant T-cell lymphoma and colon adenocarcinoma cells. Anticancer Res. 2019, 39 (12), 6499–6505. 10.21873/anticanres.13864. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Liu D.; Zeng H.; Ren X.; Song B.; Hu D.; Gan X. New chalcone derivatives: Synthesis, antiviral activity and mechanism of action. RSC Advances. 2020, 10 (41), 24483–24490. 10.1039/D0RA03684F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsafi M. A.; Hughes D. L.; Said M. A. First COVID-19 molecular docking with a chalcone-based compound: synthesis, single-crystal structure and Hirshfeld surface analysis study. Acta Crystallogr. Sect. C: Structural Chemistry. 2020, 76 (12), 1043–1050. 10.1107/S2053229620014217. [DOI] [PubMed] [Google Scholar]
- Duran N.; Polat M. F.; Aktas D. A.; Alagoz M. A.; Ay E.; Cimen F.; Tek E.; Anil B.; Burmaoglu S.; Algul O. New chalcone derivatives as effective against SARS-CoV-2 agent. Int. J. Clin. Pract. 2021, e14846. 10.1111/ijcp.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalirajan R. Activity of some novel chalcone substituted 9-anilinoacridines against coronavirus (COVID-19): a computational approach. Coronaviruses. 2020, 1, 13–22. 10.2174/2666796701999200625210746. [DOI] [Google Scholar]
- Escrivani D. O.; Charlton R. L.; Caruso M. B.; Burle-Caldas G. A.; Borsodi M. P. G.; Zingali R. B.; Arruda-Costa N.; Palmeira-Mello M. V.; de Jesus J. B.; Souza A. M.; et al. Chalcones identify cTXNPx as a potential antileishmanial drug target. PLoS neglected tropical diseases. 2021, 15 (11), e0009951. 10.1371/journal.pntd.0009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welday Kahssay S.; Hailu G. S.; Taye Desta K. Design, Synthesis, Characterization and in vivo Antidiabetic Activity Evaluation of Some Chalcone Derivatives. Drug Design, Development and Therapy. 2021, 15, 3119. 10.2147/DDDT.S316185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A.; Jain D. Synthesis, characterization and biological evaluation of some new heterocyclic derivatives of chalcone as antihyperglycemic agents. Int. J. Pharmaceutical Sci. Res. 2019, 59, 5700–5706. 10.13040/IJPSR.0975-8232.10(12).5700-06. [DOI] [Google Scholar]
- Bhoj P.; Togre N.; Bahekar S.; Goswami K.; Chandak H.; Patil M. Immunomodulatory Activity of Sulfonamide Chalcone Compounds in Mice Infected with Filarial Parasite, Brugia malayi. Indian Journal of Clinical Biochemistry 2019, 34 (2), 225–229. 10.1007/s12291-017-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S.; Bukhari S. N. A.; Fauzi N. M. Effects of chalcone derivatives on players of the immune system. Drug design, development and therapy 2015, 9, 4761. 10.2147/DDDT.S86242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. R.; Aidhen I. S.; Reddy U. A.; Reddy G. B.; Ingle K.; Mukhopadhyay S. Synthesis of 4-C-β-D-Glucosylated Isoliquiritigenin and Analogues for Aldose Reductase Inhibition Studies. Eur. J. Org. Chem. 2019, 2019 (24), 3937–3948. 10.1002/ejoc.201900413. [DOI] [Google Scholar]
- Shah U.; Patel S.; Patel M.; Gandhi K.; Patel A. Identification of chalcone derivatives as putative non-steroidal aromatase inhibitors potentially useful against breast cancer by molecular docking and ADME prediction. Indian J. Chem. 2020, B59, 283–293. [Google Scholar]
- Aljohani G.; Al-Sheikh Ali A.; Alraqa S. Y.; Itri Amran S.; Basar N. Synthesis, molecular docking and biochemical analysis of aminoalkylated naphthalene-based chalcones as acetylcholinesterase inhibitors. Journal of Taibah University for Science. 2021, 15 (1), 781–797. 10.1080/16583655.2021.2005921. [DOI] [Google Scholar]
- Bui T. H.; Nguyen N. T.; Dang P. H.; Nguyen H. X.; Nguyen M. T. T. Design and synthesis of chalcone derivatives as potential non-purine xanthine oxidase inhibitors. SpringerPlus. 2016, 5 (1), 1789. 10.1186/s40064-016-3485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora V.; Arora P.; Lamba H. Synthesis and biological activities of some 3,5-disubstituted pyrazoline derivatives of 2-acetyl naphthalene. International J. Pharm. and Pharmaceut. Sci. 2012, 4, 303–306. [Google Scholar]
- SG P.; Patil N.; Patil S. M.; Bhusange A.; Ravindranath S. Synthesis, Characterization, Antimicrobial studies and Evaluation of Stability constants of Cu (II), Ni (II) and Co (II) complexes with the ligands derived from chalcone derivatives. Appl. Organomet. Chem. 2022, 36 (2), e6465. [Google Scholar]
- Suwito H.; Jumina; Mustofa; Pudjiastuti P.; Fanani M.; Kimata-Ariga Y.; Katahira R.; Kawakami T.; Fujiwara T.; Hase T.; et al. Design and synthesis of chalcone derivatives as inhibitors of the ferredoxin—Ferredoxin-NADP+ reductase interaction of plasmodium falciparum: Pursuing new antimalarial agents. Molecules. 2014, 19 (12), 21473–21488. 10.3390/molecules191221473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.-C.; Lee Y.; Min D.; Jung M.; Oh S. Practical synthesis of chalcone derivatives and their biological activities. Molecules. 2017, 22 (11), 1872. 10.3390/molecules22111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathimunnisa M.; Manikandan H.; Sivakumar D. An efficient, solvent free synthesis of some chalcone derivatives and their biological evaluation. Asian J. Chem. 2018, 30 (4), 807–810. 10.14233/ajchem.2018.21016. [DOI] [Google Scholar]
- Anwar C.; Prasetyo Y. D.; Matsjeh S.; Haryadi W.; Sholikhah E. N.; Nendrowati N. Synthesis of chalcone derivatives and their in vitro anticancer test against breast (T47D) and colon (WiDr) cancer cell line. Indonesian Journal of Chemistry. 2018, 18 (1), 102–107. 10.22146/ijc.26864. [DOI] [Google Scholar]
- Sultan A.; Raza A. R.; Abbas M.; Khan K. M.; Tahir M. N.; Saari N. Evaluation of silica-H2SO4 as an efficient heterogeneous catalyst for the synthesis of chalcones. Molecules. 2013, 18 (8), 10081–10094. 10.3390/molecules180810081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narender T.; Papi Reddy K. A simple and highly efficient method for the synthesis of chalcones by using borontrifluoride-etherate. Tetrahedron letters. 2007, 48 (18), 3177–3180. 10.1016/j.tetlet.2007.03.054. [DOI] [Google Scholar]
- Wang J.; Huang L.; Cheng C.; Li G.; Xie J.; Shen M.; Chen Q.; Li W.; He W.; Qiu P.; et al. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharmaceutica Sinica B 2019, 9 (2), 335–350. 10.1016/j.apsb.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra D. K.; Bharti S. K.; Asati V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. European journal of medicinal chemistry 2015, 101, 496–524. 10.1016/j.ejmech.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Nasir Abbas Bukhari S.; Jasamai M.; Jantan I. Synthesis and biological evaluation of chalcone derivatives (mini review). Mini reviews in medicinal chemistry. 2012, 12 (13), 1394–1403. 10.2174/138955712804586648. [DOI] [PubMed] [Google Scholar]
- Wang H.Comprehensive Organic Name Reactions; Wiley, 2010. [Google Scholar]
- Wu X.-F.; Neumann H.; Spannenberg A.; Schulz T.; Jiao H.; Beller M. Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc. 2010, 132 (41), 14596–14602. 10.1021/ja1059922. [DOI] [PubMed] [Google Scholar]
- Xu L. W.; Li L.; Xia C. G.; Zhao P. Q. Efficient coupling reactions of arylalkynes and aldehydes leading to the synthesis of enones. Helvetica chimica acta 2004, 87 (12), 3080–3084. 10.1002/hlca.200490276. [DOI] [Google Scholar]
- Takahashi S.; Kuroyama Y.; Sonogashira K.; Hagihara N. A convenient synthesis of ethynylarenes and diethynylarenes. Chemischer Informationsdienst. 1980, 1980 (8), 627–630. [Google Scholar]
- Braun R. U.; Ansorge M.; Müller T. J. Coupling–isomerization synthesis of chalcones. Chem. Eur. J. 2006, 12 (35), 9081–9094. 10.1002/chem.200600530. [DOI] [PubMed] [Google Scholar]
- Hsieh C.-T.; Otvos S. B.; Wu Y.-C.; Mandity I. M.; Chang F.-R.; Fulop F. Highly Selective Continuous-Flow Synthesis of Potentially Bioactive Deuterated Chalcone Derivatives. ChemPlusChem. 2015, 80 (5), 859–864. 10.1002/cplu.201402426. [DOI] [PubMed] [Google Scholar]
- Ötvös S. B.; Hsieh C.-T.; Wu Y.-C.; Li J.-H.; Chang F.-R.; Fülöp F. Continuous-flow synthesis of deuterium-labeled antidiabetic chalcones: Studies towards the selective deuteration of the alkynone core. Molecules. 2016, 21 (3), 318. 10.3390/molecules21030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selepe M. A.; Van Heerden F. R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 2013, 18 (4), 4739–4765. 10.3390/molecules18044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueping M.; Bootwicha T.; Baars H.; Sugiono E. Continuous-flow hydration–condensation reaction: Synthesis of α, β-unsaturated ketones from alkynes and aldehydes by using a heterogeneous solid acid catalyst. Beilstein journal of organic chemistry. 2011, 7 (1), 1680–1687. 10.3762/bjoc.7.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C.; Zhang L.-B.; Yang J.; Gao L.-X.; Li J.-Y.; Li J.; Hou A.-J. Macdentichalcone, a unique polycyclic dimeric chalcone from Macaranga denticulata. Tetrahedron Lett. 2016, 57 (49), 5475–5478. 10.1016/j.tetlet.2016.10.090. [DOI] [Google Scholar]
- Salae A.-W.; Chairerk O.; Sukkoet P.; Chairat T.; Prawat U.; Tuntiwachwuttikul P.; Chalermglin P.; Ruchirawat S. Antiplasmodial dimeric chalcone derivatives from the roots of Uvaria siamensis. Phytochemistry. 2017, 135, 135–143. 10.1016/j.phytochem.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Chacon Morales P. A.; Santiago Dugarte C.; Amaro Luis J. M. 2′, 3, 4-trihydroxychalcone, phloretin and calomelanone from Stevia lucida. The first chalcones reported in Stevia genus. Biochemical systematics and ecology. 2018, 77, 57–60. 10.1016/j.bse.2018.02.005. [DOI] [Google Scholar]
- Wen R.; Lv H.-N.; Jiang Y.; Tu P.-F. Anti-inflammatory flavone and chalcone derivatives from the roots of Pongamia pinnata (L.) Pierre. Phytochemistry. 2018, 149, 56–63. 10.1016/j.phytochem.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Kim H.-G.; Oh H.-J.; Ko J.-H.; Song H. S.; Lee Y.-G.; Kang S. C.; Lee D. Y.; Baek N.-I. Lanceoleins AG, hydroxychalcones, from the flowers of Coreopsis lanceolata and their chemopreventive effects against human colon cancer cells. Bioorganic Chemistry. 2019, 85, 274–281. 10.1016/j.bioorg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Bhale P. S.; Chavan H. V.; Dongare S. B.; Shringare S. N.; Mule Y. B.; Nagane S. S.; Bandgar B. P. Synthesis of extended conjugated indolyl chalcones as potent anti-breast cancer, anti-inflammatory and antioxidant agents. Bioorganic & medicinal chemistry letters. 2017, 27 (7), 1502–1507. 10.1016/j.bmcl.2017.02.052. [DOI] [PubMed] [Google Scholar]
- Sökmen M.; Akram Khan M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology. 2016, 24 (2), 81–86. 10.1007/s10787-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann E.; Webster J.; Do T.; Kline R.; Snider L.; Hauser Q.; Higginbottom G.; Campbell A.; Ma L.; Paula S. Hydroxylated chalcones with dual properties: Xanthine oxidase inhibitors and radical scavengers. Bioorganic & medicinal chemistry. 2016, 24 (4), 578–587. 10.1016/j.bmc.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. L.; Stevens J. F.; Ivanov V.; McCall M.; Frei B.; Deinzer M. L.; Buhler D. R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. Journal of agricultural and food chemistry. 2000, 48 (9), 3876–3884. 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- Sasidharan R.; Baek S. C.; Sreedharannair Leelabaiamma M.; Kim H.; Mathew B. Imidazole bearing chalcones as a new class of monoamine oxidase inhibitors. Biomedicine & Pharmacotherapy. 2018, 106, 8–13. 10.1016/j.biopha.2018.06.064. [DOI] [PubMed] [Google Scholar]
- Janaki P.; Sekar K.; Thirunarayanan G. Synthesis, spectral correlation and insect antifeedant activities of some 2-benzimidazole chalcones. Journal of Saudi Chemical Society. 2016, 20 (1), 58–68. 10.1016/j.jscs.2012.11.013. [DOI] [Google Scholar]
- Padhy G.; Panda J.; Behera A. Synthesis and characterization of novel benzimidazole chalcones as antibacterial agents. Der Pharma Chemica. 2016, 8 (13), 235–41. [Google Scholar]
- Li P.-H.; Jiang H.; Zhang W.-J.; Li Y.-L.; Zhao M.-C.; Zhou W.; Zhang L.-Y.; Tang Y.-D.; Dong C.-Z.; Huang Z.-S.; et al. Synthesis of carbazole derivatives containing chalcone analogs as non-intercalative topoisomerase II catalytic inhibitors and apoptosis inducers. Eur. J. Med. Chem. 2018, 145, 498–510. 10.1016/j.ejmech.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Kumari S.; Paliwal S. K.; Chauhan R. An improved protocol for the synthesis of chalcones containing pyrazole with potential antimicrobial and antioxidant activity. Current Bioactive Compounds. 2018, 14 (1), 39–47. 10.2174/1573407212666161101152735. [DOI] [Google Scholar]
- Williams I. S.; Joshi P.; Gatchie L.; Sharma M.; Satti N. K.; Vishwakarma R. A.; Chaudhuri B.; Bharate S. B. Synthesis and biological evaluation of pyrrole-based chalcones as CYP1 enzyme inhibitors, for possible prevention of cancer and overcoming cisplatin resistance. Bioorg. Med. Chem. Lett. 2017, 27 (16), 3683–3687. 10.1016/j.bmcl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Yadav P.; Lal K.; Kumar A.; Guru S. K.; Jaglan S.; Bhushan S. Green synthesis and anticancer potential of chalcone linked-1, 2, 3-triazoles. European journal of medicinal chemistry. 2017, 126, 944–953. 10.1016/j.ejmech.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Li P.; Hu D.; Dong L.; Pan J.; Luo L.; Zhang W.; Xue W.; Jin L.; Song B. Synthesis, antiviral activity, and 3D-QSAR study of novel chalcone derivatives containing malonate and pyridine moieties. Arabian Journal of Chemistry. 2019, 12 (8), 2685–2696. 10.1016/j.arabjc.2015.05.003. [DOI] [Google Scholar]
- Tomar V.; Bhattacharjee G.; Kamaluddin; Rajakumar S.; Srivastava K.; Puri S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45 (2), 745–751. 10.1016/j.ejmech.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Dumontet C.; Beck G.; Gardebien F.; Haudecoeur R.; Mathé D.; Matera E.-L.; Tourette A.; Mattei E.; Esmenjaud J.; Boyère C.; et al. Piperidinyl-embeded chalcones possessing anti PI3Kδ inhibitory properties exhibit anti-atopic properties in preclinical models. Eur. J. Med. Chem. 2018, 158, 405–413. 10.1016/j.ejmech.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Kucerova-Chlupacova M.; Kunes J.; Buchta V.; Vejsova M.; Opletalova V. Novel pyrazine analogs of chalcones: synthesis and evaluation of their antifungal and antimycobacterial activity. Molecules. 2015, 20 (1), 1104–1117. 10.3390/molecules20011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayman M.; El-Messery S. M.; Habib E. E.; Al-Rashood S. T.; Almehizia A. A.; Alkahtani H. M.; Hassan G. S. Targeting microbial resistance: Synthesis, antibacterial evaluation, DNA binding and modeling study of new chalcone-based dithiocarbamate derivatives. Bioorganic Chemistry. 2019, 85, 282–292. 10.1016/j.bioorg.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Abdelgawad M. A.; Bakr R. B.; Alkhoja O. A.; Mohamed W. R. Design, synthesis and antitumor activity of novel pyrazolo [3, 4-d] pyrimidine derivatives as EGFR-TK inhibitors. Bioorganic Chemistry. 2016, 66, 88–96. 10.1016/j.bioorg.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Abdellatif K. R.; Abdelall E. K.; Abdelgawad M. A.; Ahmed R. R.; Bakr R. B. Synthesis and anticancer activity of some new pyrazolo [3, 4-d] pyrimidin-4-one derivatives. Molecules. 2014, 19 (3), 3297–3309. 10.3390/molecules19033297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelall E.; BBakr R.; Abdel-Hamid M.; Kandeel M. Enhancement to synthesize, design and dock of novel EGFR inhibitors containing pyrazolo [3, 4-d] pyrimidine cores of expected anticancer activity. OCAIJ. 2014, 10, 470–483. [Google Scholar]
- Madhavi S.; Sreenivasulu R.; Yazala J. P.; Raju R. R. Synthesis of chalcone incorporated quinazoline derivatives as anticancer agents. Saudi Pharmaceutical Journal 2017, 25 (2), 275–279. 10.1016/j.jsps.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Peng B.; Xiao B.-B.; Cao S.-L.; Yang C.-R.; Wang W.-Z.; Wang F.-C.; Li H.-Y.; Yuan X.-L.; Shi R.; et al. Synthesis and evaluation of chalcone analogues containing a 4-oxoquinazolin-2-yl group as potential anti-tumor agents. Eur. J. Med. Chem. 2019, 162, 586–601. 10.1016/j.ejmech.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Desai V.; Desai S.; Gaonkar S. N.; Palyekar U.; Joshi S. D.; Dixit S. K. Novel quinoxalinyl chalcone hybrid scaffolds as enoyl ACP reductase inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg. Med. Chem. Lett. 2017, 27 (10), 2174–2180. 10.1016/j.bmcl.2017.03.059. [DOI] [PubMed] [Google Scholar]
- Insuasty B.; Ramírez J.; Becerra D.; Echeverry C.; Quiroga J.; Abonia R.; Robledo S. M.; Vélez I. D.; Upegui Y.; Muñoz J. A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo [3, 4-b][1, 4] diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. 10.1016/j.ejmech.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Shankaraiah N.; Siraj K.; Nekkanti S.; Srinivasulu V.; Sharma P.; Senwar K. R.; Sathish M.; Vishnuvardhan M.; Ramakrishna S.; Jadala C.; et al. DNA-binding affinity and anticancer activity of β-carboline–chalcone conjugates as potential DNA intercalators: Molecular modelling and synthesis. Bioorganic chemistry. 2015, 59, 130–139. 10.1016/j.bioorg.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Venkataramana Reddy P.O.; Hridhay M.; Nikhil K.; Khan S.; Jha P.N.; Shah K.; Kumar D. Synthesis and investigations into the anticancer and antibacterial activity studies of β-carboline chalcones and their bromide salts. Bioorganic & medicinal chemistry letters 2018, 28 (8), 1278–1282. 10.1016/j.bmcl.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X.; Wang Y.; Hu D.; Song B. Design, synthesis, and antiviral activity of novel chalcone derivatives containing a purine moiety. Chin. J. Chem. 2017, 35 (5), 665–672. 10.1002/cjoc.201600568. [DOI] [Google Scholar]
- Wang Y.-J.; Zhou D.-G.; He F.-C.; Chen J.-X.; Chen Y.-Z.; Gan X.-H.; Hu D.-Y.; Song B.-A. Synthesis and antiviral bioactivity of novel chalcone derivatives containing purine moiety. Chin. Chem. Lett. 2018, 29 (1), 127–130. 10.1016/j.cclet.2017.07.006. [DOI] [Google Scholar]
- Zhou D.; Xie D.; He F.; Song B.; Hu D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorganic & medicinal chemistry letters. 2018, 28 (11), 2091–2097. 10.1016/j.bmcl.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Park S.; Kim E. H.; Kim J.; Kim S. H.; Kim I. Biological evaluation of indolizine-chalcone hybrids as new anticancer agents. Eur. J. Med. Chem. 2018, 144, 435–443. 10.1016/j.ejmech.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Kuthyala S.; Nagaraja G. K.; Sheik S.; Hanumanthappa M.; Kumar S M. Synthesis of imidazo [1, 2-a] pyridine-chalcones as potent inhibitors against A549 cell line and their crystal studies. J. Mol. Struct. 2019, 1177, 381–390. 10.1016/j.molstruc.2018.09.087. [DOI] [Google Scholar]
- Zheng C. J.; Jiang S. M.; Chen Z. H.; Ye B. J.; Piao H. R. Synthesis and Anti-Bacterial Activity of Some Heterocyclic Chalcone Derivatives Bearing Thiofuran, Furan, and Quinoline Moieties. Archiv der Pharmazie. 2011, 344 (10), 689–695. 10.1002/ardp.201100005. [DOI] [PubMed] [Google Scholar]
- Sun L.-P.; Jiang Z.; Gao L.-X.; Sheng L.; Quan Y.-C.; Li J.; Piao H.-R. Synthesis and biological evaluation of furan-chalcone derivatives as protein tyrosine phosphatase inhibitors. Bulletin of the Korean Chemical Society. 2013, 34 (4), 1023–1024. 10.5012/bkcs.2013.34.4.1023. [DOI] [Google Scholar]
- Sashidhara K. V.; Modukuri R. K.; Jadiya P.; Dodda R. P.; Kumar M.; Sridhar B.; Kumar V.; Haque R.; Siddiqi M. I.; Nazir A. Benzofuran–chalcone hybrids as potential multifunctional agents against Alzheimer’s disease: Synthesis and in vivo studies with transgenic Caenorhabditis elegans. ChemMedChem. 2014, 9 (12), 2671–2684. 10.1002/cmdc.201402291. [DOI] [PubMed] [Google Scholar]
- Coskun D.; Erkisa M.; Ulukaya E.; Coskun M. F.; Ari F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: synthesis, characterization and anticancer activity. European journal of medicinal chemistry. 2017, 136, 212–222. 10.1016/j.ejmech.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Mphahlele M. J.; Maluleka M. M.; Parbhoo N.; Malindisa S. T. Synthesis, evaluation for cytotoxicity and molecular docking studies of benzo [c] furan-chalcones for potential to inhibit tubulin polymerization and/or EGFR-tyrosine kinase phosphorylation. International journal of molecular sciences. 2018, 19 (9), 2552. 10.3390/ijms19092552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Liu H.; Kong X.; Chen X.; Wu C. Synthesis of new chalcone-based homoserine lactones and their antiproliferative activity evaluation. Eur. J. Med. Chem. 2019, 163, 500–511. 10.1016/j.ejmech.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Xuan T. D.; Teschke R. Dihydro-5,6-dehydrokavain (DDK) from Alpinia zerumbet: Its Isolation, Synthesis, and Characterization. Molecules. 2015, 20 (9), 16306–16319. 10.3390/molecules200916306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashidhara K. V.; Kumar A.; Kumar M.; Sarkar J.; Sinha S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorganic & medicinal chemistry letters. 2010, 20 (24), 7205–7211. 10.1016/j.bmcl.2010.10.116. [DOI] [PubMed] [Google Scholar]
- Xi G.-L.; Liu Z.-Q. Coumarin moiety can enhance abilities of chalcones to inhibit DNA oxidation and to scavenge radicals. Tetrahedron. 2014, 70 (44), 8397–8404. 10.1016/j.tet.2014.08.063. [DOI] [Google Scholar]
- Vazquez-Rodriguez S.; Lama Lopez R.; Matos M. J.; Armesto-Quintas G.; Serra S.; Uriarte E.; Santana L.; Borges F.; Munoz Crego A.; Santos Y. Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorganic & medicinal chemistry 2015, 23 (21), 7045–7052. 10.1016/j.bmc.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Niu H.; Wang W.; Li J.; Lei Y.; Zhao Y.; Yang W.; Zhao C.; Lin B.; Song S.; Wang S. A novel structural class of coumarin-chalcone fibrates as PPARα/γ agonists with potent antioxidant activities: Design, synthesis, biological evaluation and molecular docking studies. Eur. J. Med. Chem. 2017, 138, 212–220. 10.1016/j.ejmech.2017.06.033. [DOI] [PubMed] [Google Scholar]
- Foroumadi A.; Emami S.; Sorkhi M.; Nakhjiri M.; Nazarian Z.; Heydari S.; Ardestani S. K.; Poorrajab F.; Shafiee A. Chromene-Based Synthetic Chalcones as Potent Antileishmanial Agents: Synthesis and Biological Activity. Chemical biology & drug design. 2010, 75 (6), 590–596. 10.1111/j.1747-0285.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Suresh J.; Mathew G. E.; Haridas A.; Suresh G.; Sabreena P. Synthesis, ADME studies, toxicity estimation, and exploration of molecular recognition of thiophene based chalcones towards monoamine oxidase-A and B. Beni-Suef University Journal of Basic and Applied Sciences. 2016, 5 (4), 396–401. 10.1016/j.bjbas.2015.06.003. [DOI] [Google Scholar]
- Venkataramireddy V.; Shankaraiah V.; Rao A. T.; Kalyani C.; Narasu M. L.; Varala R.; Jayashree A. Synthesis and anti-cancer activity of novel 3-aryl thiophene-2-carbaldehydes and their aryl/heteroaryl chalcone derivatives. Rasayan Journal of Chemistry 2016, 9 (1), 31–39. [Google Scholar]
- Fogaça T. B.; Martins R. M.; Begnini K. R.; Carapina C.; Ritter M.; de Pereira C. M.; Seixas F. K.; Collares T. Apoptotic effect of chalcone derivatives of 2-acetylthiophene in human breast cancer cells. Pharmacological Reports. 2017, 69 (1), 156–161. 10.1016/j.pharep.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Kausar R.; Akhtar N. Synthesis and biological activity of novel 2, 5-dichloro-3-acetylthiophene chalcone derivatives. Ind. J. Pharm. Educ. Res. 2017, 51, 679–683. 10.5530/ijper.51.4.100. [DOI] [Google Scholar]
- Ming L. S.; Jamalis J.; Al-Maqtari H. M.; Rosli M. M.; Sankaranarayanan M.; Chander S.; Fun H.-K. Synthesis, characterization, antifungal activities and crystal structure of thiophene-based heterocyclic chalcones. Chemical Data Collections. 2017, 9, 104–113. 10.1016/j.cdc.2017.04.004. [DOI] [Google Scholar]
- Ritter M.; Martins R. M.; Rosa S. A.; Malavolta J. L.; Lund R. G.; Flores A. F.; Pereira C. M. Green synthesis of chalcones and microbiological evaluation. Journal of the Brazilian Chemical Society 2015, 26, 1201–1210. [Google Scholar]
- Aganagowda G.; Thamyongkit P.; Petsom A. Synthesis and antimicrobial activities of benzothiophene derivatives. Journal of the Chilean Chemical Society. 2012, 57 (1), 1043–1047. 10.4067/S0717-97072012000100019. [DOI] [Google Scholar]