Abstract
This article reports a scalable process development for the production of alkyl esters through the esterification route by utilizing fly ash as a catalyst. The catalyst consisting of mixed oxides such as alumina, iron oxide, calcium oxide, magnesium oxide, and silica was employed for the esterification reaction without modification. The catalyst was evaluated for the conversion of feedstock containing variable amounts of free fatty acids, mono/dibasic acid, and alcohol/polyols into the corresponding alkyl esters. Three types of fly ash catalysts, viz., FS-1, FP-1, and FC-1, were chosen from three different industrial sources. Synthesis of dimethyl adipate was studied as a model reaction. FS-1 fly ash gave the highest yield of dimethyl adipate, whereas FC-1 gave a low yield of dimethyl adipate. The recyclability of FS-1 was evaluated for three cycles, and no loss of yield was observed. Furthermore, the catalyst FS-I was found to be capable of producing good yields for various esterification reactions with different substrates.
1. Introduction
Generation of minimum waste is one of the important characteristics for designing and developing a green process. Another hallmark is developing and utilizing suitable catalysts that accelerate an otherwise slower or improbable reaction. Alkyl esters find application in various industries including oleochem, cosmetics, paints, fuels, emulsifiers, fragrances, and pharmaceuticals.1−5
Fly ash is one of the biggest artificial wastes generated due to industrialization. The source of this waste is the coal used as fuel in the industry. An inorganic that is compositionally different due to the source utilized as fuel is a challenge to the industry with regard to its disposal.
Fly ash consists of mixed metal oxides (mainly SiO2, Al2O3, Fe2O3, CaO, and MgO) and metal silicates.6−8 Metal oxides and mixed metal oxides are known to possess catalytic properties.9−15
The modified catalyst in the form of fly ash-supported metal oxides has been employed as a recyclable solid catalyst for organic reactions, viz., Knoevenagel condensation, esterification, and transesterification reactions.16−22 Scientists have recently developed a patented process for the industrially applicable synthesis of biodiesel and biolubricant base oils using fly ash as a heterogeneous catalyst.23,24 Apart from this, there are limited applications of fly ash as a catalyst, reported from industry.25
As mentioned earlier, reported methods use fly ash as merely a support, and active metal oxides are doped on fly ash with chemical treatment. These additional modification steps may add extra costs, leading to a less economical process.
This article reports a scalable process for the production of alkyl esters through the esterification route by utilizing fly ash as a catalyst. This single-step heterogeneous catalytic method for the production of alkyl esters uses fly ash as a catalyst without any modification of the fly ash. This allows complete conversion of feedstock containing any free mono- or dibasic acid and long-chain fatty acids, alcohols, or diol into the corresponding alkyl esters with minimal downstream processing and generates minimum effluent waste at the end of the process.
Conventionally, alkyl esters are produced through esterification (Scheme 1, equation 1) and transesterification reactions (Scheme 1, equation 2) using acid and alkali catalytic systems.1,26−47
Scheme 1. General Pathway for Esterification and Transesterification Reactions.
In this study, synthesis of dimethyl adipate was taken as a model reaction using adipic acid and methanol as reactants (Scheme 2). Dimethyl adipate is a dibasic ester. Dibasic esters are an important class of alkyl esters and used in different industrial applications, viz., cosmetics, paints, and plasticizers.1−3
Scheme 2. Fly Ash-Catalyzed Synthesis of Dimethyl Adipate.
The fly ash catalyst samples were collected from different industrial sources; those collected from the steel manufacturing industry were named as FS-1, with FP-1 for those from the thermal power industry and FC-1 for those from the alkali chemical industry. After preliminary investigation, the fly ash FS-1 catalyst which gave the highest yield for dimethyl adipate was selected for further process optimization and scale-up studies. Catalyst recovery and recycling were studied in detail along with catalyst characterization before and after the reaction. Other industrially important alkyl esters such as methyl stearate, dioctyl phthalate, and ethylene glycol stearate were also synthesized by this process.
2. Experimental Section
2.1. Chemicals
All chemicals, viz., alcohols (methanol, isopropyl alcohol, ethylene glycol, octanol) and carboxylic acid feedstock (adipic acid, stearic acid, and ptathalic anhydride) were purchased from commercial vendors. GC analytical standards were purchased from Sigma. Individual oxides, viz., silica (SiO2 100–200 mesh size), alumina (C504-type), and iron oxide were purchased from Sigma-Merck. Reactants were used without purification. Various fly ash samples were collected from different industries; those collected from the steel manufacturing industry were named as FS-1, with FP-1 for those from the thermal power industry and FC-1 for those from the alkali chemical industry. All fly ash catalysts were used without modification. During the reuse experiments, the fly ash was calcined at 500 °C for 1 h. For the control esterification reaction, the mixture of oxides was prepared through physical mixing, and the percentage of individual oxides in the mixture was kept as per the composition of the FS-1 fly ash catalyst.
2.2. Analytical Techniques
XRD analysis of fly ash was performed using a Panalytical diffractometer equipped with a quartz monochromator and Cu Kα radiation (λ = 0.154059 nm). The X-ray diffraction (XRD) patterns were analyzed using standard ICDD (International Center for Diffraction data) files. Morphology was measured using Philips XL30 scanning electron microscopy (SEM). An energy dispersive X-ray detector (EDX) mounted on the microscope was used for the elemental analysis of the fly ash samples. The elemental composition of various fly ash samples was determined by atomic emission spectrometry with an inductively coupled plasma atomic emission spectrometer (Agilent ICP-MPAES 4010), whereas silica was analyzed gravimetrically. A pH of 5% for the fly ash solution was measured using a pH meter under (200 rpm) constant stirring. Fourier-transform infrared spectroscopy (FT-IR) analysis of fly ash samples was done on a FT-IR spectrometer (Bruker Vertex) using the KBr palate method having an IR scan range of 400–4000 cm–1. The BET measurements of the fly ash catalysts were carried out on a Micromeritics BET analyzer instrument (TriStar II 3020 Version 3.02). The acidity of fly ash catalysts was determined as mmol per gram of the catalyst using n-butyl amine titration.48,49 Catalyst samples were freshly dried at 120 °C and cooled at room temperature in a desiccator before use. Next, 0.2% Hammett indicators and 0.1 M n-butyl amine were prepared in anhydrous benzene. A total of 0.2 g of the dried catalyst was taken in 10 mL of anhydrous benzene. Next, 2 mL of the Hammett indicator was added to the catalyst solution. The solution was titrated against 0.1 M n-butyl amine solution. After stepwise addition of n-butyl amine, the titration mixture was stirred for 4 h at room temperature, and color change was observed. The end point of titration gave the acid strength of the catalyst. Esterification reaction monitoring and product purity analysis were done on a Thermo scientific GC-FID 800+ series machine with a GC column having a 5% phenylpolydimethylsiloxane-bonded stationary phase. The operational temperature was up to 400 °C. The column length was 15 m, and the internal diameter was 0.32 mm. GC standards and reaction product samples were prepared in the THF solvent for GC analysis. GC operation conditions for all ester molecules were as follows (Table 1). The product purity was determined through the % area method.
Table 1. GC-FID Operation Conditions.
| entry | parameters | values |
|---|---|---|
| 1 | injector temperature | 300 °C |
| 2 | injection volume | 1 μL |
| 3 | split ratio | 10 |
| 4 | column temperature program | |
| initial temperature | 50 °C hold 1 min | |
| rate 1 15 °C/min | 180 °C | |
| rate 2 7 °C/min | 230 °C | |
| rate 3 30 °C/min | 300 °C hold 10 min | |
| 5 | flow rate | 3 mL/min |
| 6 | FID temperature | 380 °C |
| 7 | carrier gas | nitrogen |
2.3. Reaction Parameters
For preliminary investigation, the reaction was carried out for 1 mol acid feedstock. To obtain complete conversion of feedstock, experiments were carried out by varying the percentage of the catalyst (2.5–12.5 wt %), temperature (80–220 °C), and reaction time (1–5 h).
2.4. Optimized Process for Dimethyl Adipate for 1 Mol Acid Feedstock
The reaction was performed in a stainless-steel high-pressure reactor (inner volume = 1 L; maximum pressure = 100 bar; maximum temperature = 250 °C). In a typical experiment, 192 g of methanol (6 equivalents) was mixed with 14.5 g of the fly ash catalyst (10% wt/wt of acid feedstock) and transferred into a reactor, followed by addition of 146 g of adipic acid (1 mol). The reactor was closed, and the temperature of the reactor was set at 200 °C with 200 rpm agitation under autogenous pressure. The reaction was carried out for 4 h. After completion of the reaction, the catalyst was recovered by simple filtration. The excess solvent and water generated were evaporated to get the reaction product. The product was analyzed by GC-FID. After filtration, the catalyst was washed with methanol to remove the adsorbed material and heated at 500 °C for 1 h in a muffle furnace and was used for further recycling experiments.
2.5. Process Scale-Up for Dimethyl Adipate
The process was scaled up and validated for 40 mol adipic acid.
As shown in the process flow diagram (Figure 1), 0.590 kg of the FS-1 fly ash catalyst, 5.840 kg of adipic acid (40 mol), and 7.680 kg of methanol (240 mol, 6 equiv) were charged in a 25 L batch high-pressure reactor. The reaction was performed for 4 h at 200 °C under autogenous pressure with agitation. After completion of the reaction, the reaction mass was filtered in a filtration assembly. After filtration, the catalyst was washed with methanol and kept in a furnace at 500 °C for 1 h and reused for the next batch. Catalyst recovery was 98% (0.584 kg), and the remaining was handling loss. The filtrate was again transferred to a reactor, and the excess methanol and water generated during the reaction was removed by vacuum evaporation. A 7.715 kg product with 98.0% yield was obtained after methanol removal against 7.760 kg theoretical yield. The product was stored in a storage tank, while the recovered methanol with water was stored in a recovery storage tank (Figure 1).
Figure 1.
Process flow diagram for dimethyl adipate at the 40 mol scale.
3. Results and Discussion
3.1. Characterization of Fly Ash Samples
The chemical composition of fly ash is shown in Table 2. It mainly consists of mixed metal oxides such as iron oxide, alumina, calcium oxide, magnesium oxide, silica, etc.
Table 2. Elemental Composition, pH, and BET Surface Area of Various Fly Ash Samples.
| entry | parameters | FS-1 | FP-1 | FC-1 |
|---|---|---|---|---|
| 1 | SiO2% | 58.9 | 60.3 | 18.8 |
| 2 | Al2O3% | 34.5 | 30.7 | 11.0 |
| 3 | Fe2O3% | 4.5 | 4.9 | 2.0 |
| 4 | CaO % | 0.2 | 0.5 | 31.0 |
| 5 | MgO % | 0.5 | 1.5 | 1.4 |
| 6 | Sulfate % | 0.10 | 0.12 | 24.1 |
| 7 | pH of 5% solution | 6.8 | 6.9 | 10.1 |
| 8 | BET surface area (m2/g) | 12.91 | 4.29 | 1.67 |
FS-1 and FP-1 have more amounts of iron oxide, alumina, and silica, which impart an acidic nature to the catalyst. FC-1 fly ash has a higher percentage of calcium oxide, which indicates that FC-1 is basic in nature. This was also confirmed by EDX analysis (Figure 3), which showed that there was a high amount of alumina and silica in FS-1 and FP-1 fly ash samples, while FC-1 has a high amount of calcium and sulfur as compared to others. The pH of the water solution of fly ash samples also confirmed the nature of the catalyst (Table 2). FS-1 and FP-1 show very a mild acidic pH, while FC-1 has a basic pH.
Figure 3.
EDX analysis of fly ash catalysts (a) FS-1, (b) FP-1, and (c) FC-1.
The BET measurement of fly ash catalysts (Table 2) shows that the surface area of the FS-1 catalyst was higher than that of the FP-1 and FC-1 fly ash catalysts. The FT-IR analysis of the fly ash catalyst is shown in Figure 2. In FT-IR analysis, FS-1 and FP-1 catalysts show Si-O-Al asymmetric stretching vibrations at 1072 and 1022 cm–1, respectively, and Si-O-Si bending vibrations at 473 and 465 cm–1, respectively, which confirms the aluminosilicate framework present in both fly ash catalysts. However, in the case of the FS-1 catalyst, the peak at 1072 cm–1 is sharper than that of the FP-1 catalyst with an additional peak at 790 cm–1, which is due to the symmetric stretching vibrations of Si-O-Al. This finding shows that the FS-1 catalyst could have a prominent aluminosilicate framework compared to the FP-1 catalyst, while in the case of the FC-1 catalyst, intense peaks at 1456 cm–1 represent carbonate groups, which may have resulted due to the formation of CaCO3 from Ca(OH)2, which is associated with the CaO present in the catalyst. The intense stretching vibration at 1148 cm–1 is due to the sulfate group present in the FC-1 catalyst. The stretching vibration at 1004 cm–1 with lower intensity indicates that the FC-1 catalyst has a lower percentage of aluminosilicate framework compared to the FS-1 and FP-1 catalysts. The peak at 3629 cm–1 in the FC-1 catalyst is due to OH stretching vibrations due to presence of Ca(OH)2. All three fly ash catalysts show bending vibrations in between 1500 and 1600 cm–1, indicating the presences of a small quantity of water in the fly ash catalyst. All the above-mentioned IR values and their interpretation match with literature data.36,37
Figure 2.

FT-IR analysis of the fly ash catalyst (FS-1, FP-1 and FC-1).
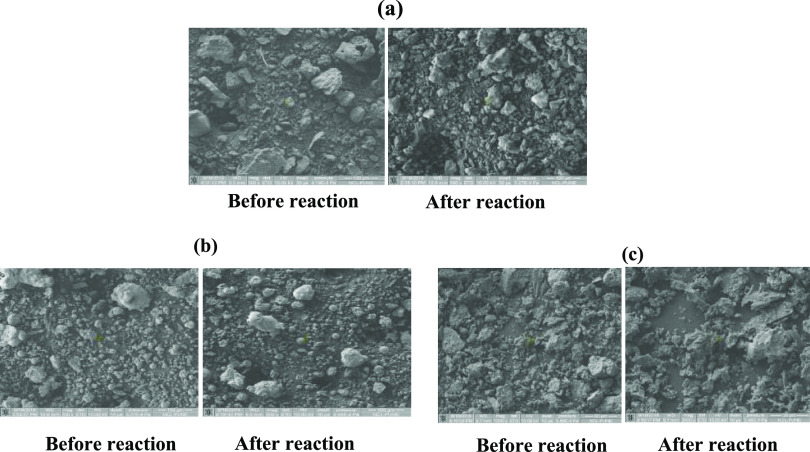
Figure 4 shows the SEM images of FS-1, FP-1, and FC-1, before and after the reaction. The FS-1 catalyst showed irregular particle shape and size in the range of 5–100 μm (Figure 4). However, FP-1 shows spherical particles shape having a range of 2–50 μm. The FC-1 catalyst particles are irregular in shape and size in the range of 10–100 μm.
Figure 4.
SEM images of the fly ash catalyst. (a) FS-1, (b) FP-1, and (c) FC-1.
The morphology of all three catalysts remains the same after the reaction, which confirms that the fly ash catalyst remains intact after the reaction. This was further confirmed by XRD analysis of the catalyst (Figure 5). XRD analysis shows the peaks of silica and alumina in all three catalysts, while there are additional peaks in the FC-1 catalyst, which represents calcium similar to the literature value. Even after the reaction, the XRD pattern remains the same for all the catalyst.
Figure 5.
XRD of fly ash catalysts before and after the reaction (a) FS-1, (b) FP-1, and (c) FC-1.
3.2. Catalytic Evaluation and Recycling of the Fly Ash Catalyst for the Model Reaction
Various reaction parameters were studied for the conversion of adipic acid to dimethyl adipate.
3.2.1. Effect of Temperature
Figure 6 shows the effect of the temperature on the conversion of adipic acid for the fly ash catalyst. It is important to note here that no significant conversion of feedstock takes place at a temperature below 100 °C in the presence of the catalyst for all three fly ash samples. As shown in Figure 6, the highest conversion of feedstock was obtained at 200 °C with 100% selectivity. Above 200 °C, no further improvement in conversion was observed, whereas FC-1 showed less conversion at 200 °C compared to FS-1 and FP-1 catalysts. It is noteworthy to mention here that the blank experiment showed approximately 55% of conversion at 200 °C, and their after it remains constant even after an increase in the reaction temperature.
Figure 6.
Effect of the reaction temperature on the % yield of dimethyl adipate. Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, catalyst amount: 10% wt/wt, and reaction time: 4 h.
3.2.2. Effect of the Reaction Time
The effect of the reaction time is shown in Figure 7. Almost 70% reaction was completed in first 2 h. At the 4th h, the conversion was 98% with 100% selectivity for FS-1, 94% for FP-1, and 72% for FC-1. By the 5th h, no further increase was observed in conversion, which means that to obtain the maximum yield, 4 h is needed, whereas for the blank experiment, approximately, 53% of conversion was obtained at 3 h; with a further increase in time, the conversion remains constant.
Figure 7.
Effect of the reaction time on the % yield of dimethyl adipate. Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, reaction temperature: 200 °C, and catalyst amount: 10% wt/wt.
3.2.3. Effect of the Catalyst Amount and Catalyst Nature
The effect of the catalyst amount is shown in Figure 8. Without the catalyst, the conversion was 55%.
Figure 8.
Effect of the catalyst amount on the % yield of dimethyl adipate. Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, reaction temperature: 200 °C, and reaction time: 4 h.
For all three catalysts, 2.5% catalyst amount (wt/wt to the acid feedstock) did not show much increase in conversion. For 5 and 7.5% catalyst amounts, there were increases in conversion. The 10% catalyst gave the highest conversion. Even at a higher catalyst amount, i.e., 12.5%, conversion remained the same.
Under optimized reaction conditions (Table 3), the maximum conversion was given by FS-1 (98%), whereas FP-1 gave 94% and FC-1 fly ash gave the lowest (72%) at 200 °C. This is because FS-1 and FP-1 fly ash samples have a higher percentage of iron oxide, alumina, and silica and negligible percentage of calcium and magnesium oxide. The prominent silica and alumina framework present in the FS-1 fly ash catalyst has a synergistic effect on the reaction with high efficiency. Several Lewis acid systems which have active metal centers such as aluminum, iron, zinc, tin, etc. catalyze the esterification reaction very efficiently.13−15,37−46 The reported heteropolyacid catalytic systems developed for the esterification reaction showed that metal centers (Ti, Al, Fe, Zn, etc.) present in the catalyst possess Lewis acidity, and they have shown the influence of Lewis acidity on catalytic acitivies.44 Similarly, the catalytic system having the aluminosilicate framework which has both Lewis and Bronsted sites showed a synergistic effect in the esterification reaction.38 The FC-1 has a higher percentage of calcium oxide, which is basic in nature. It is known that the esterification reaction slows down in the presence of calcium oxide.47 From the above-mentioned observations, it was found that the FS-1 fly ash catalyst facilitates the reaction completion and gave the highest conversion. As the FS-1 catalyst gave the highest yield of dimethyl adipate, we used the FS-1 fly ash catalyst for further investigation and scale-up studies under optimized reaction conditions. The catalyst after the reaction was further treated and studied for recovery and recyclability.
Table 3. Yield of Dimethyl Adipate under Optimized Conditions for Fly Ash Catalystsa.
| entry | fly ash catalysts | % yield of dimethyl adipate |
|---|---|---|
| 1 | FS-1 | 98 |
| 2 | FP-1 | 94 |
| 3 | FC-1 | 72 |
| 4 | without catalyst | 55 |
Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, catalyst: 10% wt/wt, reaction time: 4 h, and reaction temperature: 200 °C.
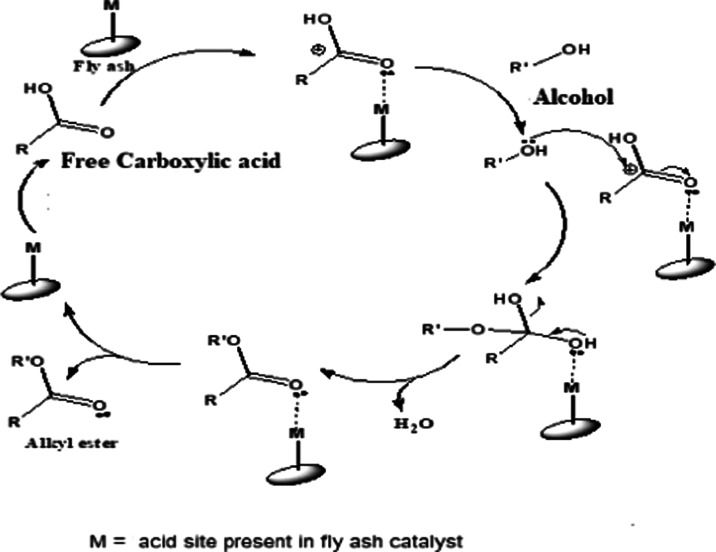
To support our observations for the FS-1 catalyst, we have carried out the dimethyl adipate model reaction with individual oxide and their mixture under optimized reaction conditions (Table 4). It was observed that silica gave 89% yield of dimethyl adipate, which was higher compared to that of individual alumina and iron oxide. However, the mixture of all three oxides gave 82% yield of dimethyl adipate. Silica comprises milder acidity due to the acidic proton, whereas aluminum metal imparts Lewis acidity to alumina due to its empty d orbital, which catalyzes the reaction toward the product side. Furthermore, the acidity of the fly ash catalyst and mixture of individual oxides were determined using n-butyl amine titration with different Hammett indicators to support our observations (Table 5). All the catalysts gave positive results to the methyl red indicator, but the other two indicators were nonresponsive to the tested solutions. The analysis shows that the FS-1 catalyst has a higher acidity (2.93 mmol/g) than that of FP-1, FC-1, and the mixture of individual oxides. The acidity data of the catalysts are in agreement with our observation for the catalytic activity of the FS-1 catalyst, which was the highest among the tested catalysts for the esterification reaction. Similarly, the BET surface area of the FS-1 catalyst was higher than that of the FP-1 and FC-1 fly ash catalysts. These observations indicate that the FS-1 fly ash catalyst is more active than the other tested catalysts. The silica and alumina framework present in the FS-1 fly ash catalyst has a synergistic effect in the esterification reaction, giving a higher yield of dimethyl adipate (98%) than the individual oxides and physical mixture of oxides. The probable reaction mechanism is given in Figure 9.
Table 4. Synthesis of Dimethyl Adipate Using Individual Oxides and Mixture of Oxidesa.
| entry | catalysts | dimethyl adipate (% yield) |
|---|---|---|
| 1 | SiO2 | 89 |
| 2 | Al2O3 | 84 |
| 3 | Fe2O3 | 78 |
| 4 | SiO2 65 % + 35 % Al2O3 mixture | 86 |
| 5 | SiO2 60 % + 35 % Al2O3 + 5 % Fe2O3 mixture | 82 |
Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, catalyst: 10% wt/wt, reaction time: 4 h, and reaction temperature: 200 °C.
Table 5. Acidity Determination of the Catalysts Using n-Butyl Amine Titration.
| acid
strength of the catalysts (mmol/g) |
||||||
|---|---|---|---|---|---|---|
| entry | Hammett indicator | color of the indicator after addition in the catalyst test solution | FS-1 | FP-1 | FC-1 | SiO2 + Al2O3 + Fe2O3 60% + 35% + 5% |
| 1 | methyl red (pKa = +4.8) red in acid forms yellow in the base form | red to all catalysts (+) | 2.93 | 2.20 | 1.12 | 0.78 |
| 2 | crystal violet (pKa = +0.8) yellow in acid forms blue in the base form | blue to all catalysts (−) | no color change | no color change | no color change | no color change |
| 3 | p-nitro chlorobenzene (pKa = −12.70) yellow in acid forms colorless in the base form | colorless to all catalysts (−) | no color change | no color change | no color change | no color change |
Figure 9.
Probable reaction mechanism for the fly ash-catalyzed esterification reaction.
The acid sites present on the silica and alumina framework of the fly ash catalyst interact with carbonyl oxygen of free acids.13,38 This interaction makes carbonyl carbon more electron-deficient. Alcohol is introduced in the reaction, which acts as a nucleophile and attacks the electron-deficient carbonyl carbon of free acid. As the reaction proceeds, the dehydration reaction takes place with loss of water molecules, giving alkyl ester as the end product.
3.3. Catalyst Recycling
Catalyst recycling was investigated for three cycles (Figure 10). For the first two cycles, the catalyst showed almost similar activity (98% conversion) with 100% selectivity as compared to the fresh catalyst. In the third recycling, catalyst activity decreased slightly (97% conversion). From recycling experiments, it was found that the fly ash catalyst was successfully recycled for three cycles.
Figure 10.
Effect of catalyst recycling on the % yield of dimethyl adipate. Reaction conditions: adipic acid: 1 mol, methanol: 6 mol, catalyst: 10% wt/wt, reaction time: 4 h, and reaction temperature: 200 °C.
3.4. Specifications of Dimethyl Adipate Synthesized in Scale-Up Studies
The important properties and specification of dimethyl adipate obtained at the 40 mole scale process are shown in Table 6. Dimethyl adipate obtained by our process has 98% purity and meets the specification required for different industrial applications. Metal leaching from the fly ash catalyst into the product was determined and found to be below the detection level.
Table 6. Important Properties of Dimethyl Adipate Synthesized from Scale-Up Operations.
| entry | product properties | results |
|---|---|---|
| 1 | color and appearance | pale-yellow clear liquid |
| 2 | melting point | 9.0–11.00 °C |
| 3 | purity | 98% by GC |
| 4 | moisture | 0.10% max |
| 5 | acid value | not more than 5.0 |
| 6 | density | 1.062 g/cc |
| 7 | metal impurities (Ca, Mg, Al, Fe, Si) | below the detection limit |
3.5. Process Scope for Different Industrially Important Alkyl Esters
We further synthesized industrially important esters from long-chain free fatty acids/dibasic acids and alcohol/diol with an average yield of 97% (Table 7), which demonstrates the versatility and scalability of our process.
Table 7. Industrially Important Alkyl Esters Synthesized Using the Fly Ash FS-1 Catalysta.
| entry | carboxylic acid | alcohol | products | yield (%) |
|---|---|---|---|---|
| 1 | adipic acid | methanol | dimethyl adipate | 98 |
| 2 | stearic acid | methanol | methyl stearate | 98 |
| 3 | stearic acid | isopropyl alcohol | isopropyl stearate | 95 |
| 4 | stearic acid | ethylene glycol | ethylene glycol stearate | 95 |
| 5 | phthalic anhydride | n-octanol | dioctyl phthalate | 97 |
Reaction Conditions: carboxylic acid: 1 mol, alcohol: 6 mol for dibasic esters, alcohol: 3 mol for monobasic esters, alcohol: 1.5 mol for diol ester, catalyst: FS-1, 10% wt/wt, reaction time: 4 h, and reaction temperature: 200 °C.
4. Conclusions
This study demonstrates the utilization of fly ash as a catalyst, which is able to convert the feedstock having free mono/dibasic acid and alcohols/polyols into alkyl esters with the highest selectivity and yield. The use of this fly ash catalyst for the esterification reaction is more economic and advantageous over processes using modified fly ash catalysts. The catalyst can be separated by simple filtration after completion of the reaction, with no water requirement. The catalyst is successfully recovered and recycled. Among all fly ash catalysts, the highest conversion was seen with FS-1 (98%) followed by FP-1 and FC-1 fly ash catalysts. Successful demonstration of the process at the kilogram scale for dimethyl adipate as a model molecule by utilizing the FS-1 fly ash catalyst is achieved. The product obtained by our process meets the industrial specification.
Acknowledgments
We gratefully acknowledge Tata Chemicals Limited, Innovation Centre for financial support, and the Manipal Academy of Higher Education.
Author Contributions
The article was written through contributions of all authors.
The authors declare no competing financial interest.
References
- Riemenschneider W.; Bolt H. M.. Esters, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, 2005; pp 245–266. [Google Scholar]
- Mitsui T.Raw Materials of Cosmetics. In New Cosmetic Science; Elsevier B.V. publication, 1997; pp 121–147. [Google Scholar]
- Krauskopf L. G.; Godwin A.. Plasticizers. In PVC Handbook; Wilkes C.; Summers J.; Daniels C., Eds.; Hanser Publication: Munich, 2005; pp 173–193. [Google Scholar]
- Hossain M. A.; Iqbal A.; Julkapli N. M.; Kong P. S.; Ching D. J.; Lee H. V. Development of catalyst complexes for upgrading biomass into ester-based bio lubricants for automotive applications a review. RSC Adv. 2018, 8, 5559–5577. 10.1039/C7RA11824D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttig A.; Wenning L.; Broker D.; Steinbuchel A. Fatty acid alkyl esters perspectives for production of alternative biofuels. Appl. Microbiol. Biotechnol. 2010, 85, 1713–1733. 10.1007/s00253-009-2383-z. [DOI] [PubMed] [Google Scholar]
- Yao Z. T.; Ji X. S.; Sarker P. K.; Tang J. H.; Ge L. Q.; Xi M. S.; Xi Y. Q. Comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 41, 105–121. 10.1016/j.earscirev.2014.11.016. [DOI] [Google Scholar]
- Dwivedi A.; Jain M. K.. Fly ash waste management and overview a review Recent Research in Science and Technology, 2014; Vol. 6, pp 30–35.
- Bhatt A.; Priyadarshinia S. B.; Mohanakrishnana A. A.; Abria A.; Sattlera M.; Techapaphawit S. Physical, chemical, and geotechnical properties of coal fly ash a global review. Case Stud. Constr. Mater. 2019, 11, e00263 10.1016/j.cscm.2019.e00263. [DOI] [Google Scholar]
- Gawande M. B.; Pandey R. K.; Jayaram R. V. Role of mixed metal oxides in catalysis science versatile applications in organic synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. 10.1039/C2CY00490A. [DOI] [Google Scholar]
- Védrine J. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341–366. 10.3390/catal7110341. [DOI] [Google Scholar]
- Wang S. Application of solid ash based catalysts in heterogeneous catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063. 10.1021/es801312m. [DOI] [PubMed] [Google Scholar]
- Mostafa Hosseini Asl S.; Ghadia A.; Baei M. S.; Javadian H.; Maghsudi M.; Kazemian H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications a review. Fuel 2018, 217, 320–342. 10.1016/j.fuel.2017.12.111. [DOI] [Google Scholar]
- Mello V. M.; Pousa G.; Pereira M.; Dias I.; Suarez P. Metal oxides as heterogeneous catalysts for esterification of fatty acids obtained from soybean oil. Fuel Process. Technol. 2011, 92, 53–57. 10.1016/j.fuproc.2010.08.019. [DOI] [Google Scholar]
- Putra M. D.; Irawan C.; Udiantorob; Ristianingsiha Y.; Nata I. F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Cleaner Prod. 2018, 195, 1249–1258. 10.1016/j.jclepro.2018.06.010. [DOI] [Google Scholar]
- Kuriacose J. C.; Jewur S. S. Esterification of alcohols with acetic acid over ferric oxide. Int. J. Chem. Kinet. 1977, 9, 641–650. 10.1002/kin.550090412. [DOI] [Google Scholar]
- Jain D.; Khatri C.; Rani A. Fly ash supported calcium oxide as recyclable solid base catalyst for Knoevenagel condensation reaction. Fuel Process. Technol. 2010, 91, 1015–1021. 10.1016/j.fuproc.2010.02.021. [DOI] [Google Scholar]
- Khatri C.; Mishra M.; Rani A. Synthesis and characterization of fly ash supported sulfated zirconia catalyst for benzylation reactions. Fuel Process. Technol. 2010, 91, 1288–1295. 10.1016/j.fuproc.2010.04.011. [DOI] [Google Scholar]
- Chakraborty R.; Bepari S.; Banerjee A. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem. Eng. J. 2010, 165, 798–805. 10.1016/j.cej.2010.10.019. [DOI] [Google Scholar]
- Deka B.; Bhattacharyya K. Using coal fly ash as a support for Mn(II), Co(II) and Ni(II) and utilizing the materials as novel oxidation catalysts for 4-chlorophenol mineralization. J. Environ. Manage. 2015, 150, 479–488. 10.1016/j.jenvman.2014.12.037. [DOI] [PubMed] [Google Scholar]
- Jain D.; Hada R.; Rani A. Surface Modification of fly ash for active catalysis. J. Catal. 2013, 2013, 723957 10.1155/2013/723957. [DOI] [Google Scholar]
- Thirunarayanan G.; Sekar K.. Solid fly-ash: H2SO4 is an efficient catalyst for cyclization of α, β-unsaturated ketones: Solvent-free synthesis of some oxazine amine derivatives Der Pharma Chemica 2013, 5, 142–148..
- Srivastava k.; Devra V.; Rani A. Fly ash supported vanadia catalyst: An efficient catalyst for vapor phase partial oxidation of toluene in a micro-reactor. Fuel Process. Technol. 2014, 121, 1–8. 10.1016/j.fuproc.2013.12.002. [DOI] [Google Scholar]
- Kumar R.; Mal N. K.; Nagabhushana K. S.; Shinde T.; Rautaray D.. A process for production of biodiesel. WO2010/082211A2, 2010.
- Nagabhushana K. S.; Mal N. K.; Shinde T.; Dapurkar S. E.; Kumar R.. A process for production of bio lubricant using fly ash as a catalyst. WO2011/007361A, 2011.
- Nagarkar R. A.; Nagabhushana K. S.; Chaudhari P.. A process for production of alkyl esters. MU2015/4274A, 2015.
- Matsumoto K.; Yanagi R.; Oe Y.. Recent Advances in the Synthesis of Carboxylic Acid Esters. In Carboxylic Acid - Key Role in Life Sciences; Intechopen publisher, 2018 10.5772/intechopen.68350. [DOI] [Google Scholar]
- Salimon J.; Salih N.; Yousif E. Industrial development and applications of plant oils and their bio based oleochemicals. Arabian J. Chem. 2012, 5, 135–145. 10.1016/j.arabjc.2010.08.007. [DOI] [Google Scholar]
- Khire S.; Bhagwat P. V.; Fernandes M.; Gangundi P. B.; Vadalia H. Esterification of lower aliphatic alcohols with acetic acid in presence of different acid catalyst. Indian J. Chem. Technol. 2012, 19, 342–350. [Google Scholar]
- Chan K. W.; Tsai Y. T.; Lin H. M.; Lee M. J. Esterification of adipic acid with methanol over Amberlyst 35. J. Taiwan Inst. Chem. Eng. 2010, 41, 414–420. 10.1016/j.jtice.2009.12.001. [DOI] [Google Scholar]
- Dedè F.; Piccolo O.; Vigo D. Dimethyl fumarate heterogeneous catalysis for the development of an innovative flow synthesis. Org. Process Res. Dev. 2021, 25, 292–299. [Google Scholar]
- jabbari H.; Pesyan N. N. Synthesis of polyol esters by p-toluenesulfonic acid catalyst as synthetic lubricant oils. Asian J. Green Chem. 2017, 1, 41–45. 10.22631/ajgc.2017.89392.1002. [DOI] [Google Scholar]
- José da Silva M.; Cardoso A. L. Heterogeneous tin catalysts applied to the esterification and transesterification reactions. J. Catal. 2013, 2013, 510509 10.1155/2013/510509. [DOI] [Google Scholar]
- Sharma Y. C.; Singh B. Advancements in solid acid catalysts for ecofriendly and economically viable synthesis of biodiesel. Biofuels, Bioprod. Biorefin. 2011, 5, 69–92. 10.1002/bbb.253. [DOI] [Google Scholar]
- Dou Y.; Zhang H.; Zhou A.; Yang F.; Shu L.; She Y.; Li J. Highly efficient catalytic esterification in a −SO3H functionalized Cr(III)-MOF. Ind. Eng. Chem. Res. 2018, 57, 8388–8395. 10.1021/acs.iecr.8b01239. [DOI] [Google Scholar]
- Essamlali Y.; Larzek M.; Essaid B.; Zahouily M. Natural phosphate supported titania as a novel solid acid catalyst for oleic acid esterification. Ind. Eng. Chem. Res. 2017, 56, 5821–5832. 10.1021/acs.iecr.7b00607. [DOI] [Google Scholar]
- Mozgawa W.; Krol M.; Dyczek J.; Deja J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta, Part A 2014, 132, 889–894. 10.1016/j.saa.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Mazumder N. A.; Rano R.; Sarmah G. A green and efficient solid acid catalyst from coal fly ash for Fischer esterification reaction. J. Ind. Eng. Chem. 2015, 32, 211–217. 10.1016/j.jiec.2015.08.020. [DOI] [Google Scholar]
- Su X.; Li L.; Xiao F.; Wei W.; Sun Y. Esterification of salicylic acid with dimethyl carbonate over mesoporous aluminosilicate. Ind. Eng. Chem. Res. 2009, 48, 3685–3691. 10.1021/ie801148v. [DOI] [Google Scholar]
- Melchiorre M.; Cucciolito M. E.; Di Serio M.; Ruffo F.; Tarallo O.; Trifuoggi M.; Esposito R. Homogeneous catalysis and heterogeneous recycling: a simple Zn(ii) catalyst for green fatty acid esterification. ACS Sustainable Chem. Eng. 2021, 9, 6001–6011. 10.1021/acssuschemeng.1c01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R.; Melchiorre M.; Annunziata A.; Cucciolito M. E.; Ruffo F. Emerging catalysis in biomass valorisation: simple Zn(II) catalysts for fatty acids esterification and transesterification. ChemCatChem 2020, 12, 5858–5879. 10.1002/cctc.202001144. [DOI] [Google Scholar]
- Melchiorre M.; Amendola R.; Benessere V.; Cucciolito M. E.; Ruffo F.; Esposito R. Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron(III) dimer complex. Mol.Catal. 2020, 483, 110777 10.1016/j.mcat.2020.110777. [DOI] [Google Scholar]
- Tomita K.; Ida H. Studies on the formation of poly(ethylene terephthalate): 3. Catalytic activity of metal compounds in transesterification of dimethyl terephthalate with ethylene glycol. Polymer 1975, 16, 185–190. 10.1016/0032-3861(75)90051-8. [DOI] [Google Scholar]
- Tian Y.; Wang F.; Xie L.-F.; Xu Y-P.; Duan P-G. Lewis acid-catalyzed in situ transesterification/esterification of tigernut in sub/supercritical ethanol: an optimization study. Fuel 2019, 245, 96–104. 10.1016/j.fuel.2019.02.038. [DOI] [Google Scholar]
- Tao M.; Xue L.; Sun Z.; Wang S.; Wang X.; Shi J. Tailoring the synergistic Bronsted- Lewis acidic effects in heteropolyacid catalysts: applied in esterification and transesterification reactions. Sci. Rep. 2015, 5, 13764 10.1038/srep13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X.; Qi Y.; Qiao X.; Wang G.; Qin Z.; Wang J. Lewis acid-catalyzed transesterification and esterification of high free fatty acid oil in subcritical methanol. Korean J. Chem. Eng. 2007, 24, 311–313. 10.1007/s11814-007-5052-x. [DOI] [Google Scholar]
- Mantri K.; Nakamura R.; Komura K.; Sugi Y. Esterification of long chain aliphatic acids with long chain alcohols catalyzed by multi-valent metal salts. Chem. Lett. 2005, 34, 1502–1503. 10.1246/cl.2005.1502. [DOI] [Google Scholar]
- Kouzu M.; Kasuno T.; Tajika M.; Sugimoto Y.; Yamanaka S.; Hidaka J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. 10.1016/j.fuel.2007.10.019. [DOI] [Google Scholar]
- Yurdakoc M.; Akcay M.; Tonbul Y.; Yurdakoc K. Acidity of silica-alumina catalysts by amine titration using Hammett indicators and FT-IR Study of pyridine adsorption. Turk. J. Chem. 1999, 23, 319–327. [Google Scholar]
- Benesi H. A. Acidity of catalyst surfaces. II. amine titration using hammett indicators. J. Phys. Chem. A 1957, 61, 970–973. 10.1021/j150553a030. [DOI] [Google Scholar]