Abstract
In this work, an efficient synthesis of 2-imino-1,3,4-oxadiazolines from acylhydrazides and isothiocyanates is described. In the presence of 4-dimethylaminopyridine (DMAP) and molecular oxygen, various 2-imino-1,3,4-oxadiazolines were produced in good to high yields. The developed method showed a broad substrate scope and was effective on the gram scale. On the basis of the mechanistic studies and previous literature, it was proposed that the mechanism consists of an aerobic oxidation of acylhydrazides facilitated by DMAP and isothiocyanates, followed by a DMAP-mediated annulation of the in situ generated acyldiazenes with isothiocyanates.
Introduction
The 1,3,4-oxadiazole skeleton is an interesting scaffold that plays a crucial role in multifarious areas. Various compounds bearing the 1,3,4-oxadiazole ring have emerged as potent candidates for the preparation of organic light-emitting diodes1 after the first utilization was reported in 1990.2 In organic synthesis, 1,3,4-oxadiazoles are useful building blocks for the stereoselective synthesis of natural products through an intramolecular [4 + 2]/[3 + 2] cycloaddition cascade.3 Moreover, 1,3,4-oxadiazole derivatives are important structural motifs used in the development of new drugs,4 with representative examples including raltegravir (antiretroviral drug for HIV),5 zibotentan (anticancer agent),6 and furamizole (hypnotic drug).7
A number of protocols to synthesize 1,3,4-oxadiazoles have been developed over the years.8 These protocols include the dehydrative cyclization of 1,2-diacylhydrazines,9 desulfurative cyclization of thiosemicarbazides,10 and oxidative cyclization of N-acylhydrazones or their analogues,11 among others.12 However, the construction of 2-imino-1,3,4-oxadiazolines, which can have potent biological activity,13 has been much less investigated.
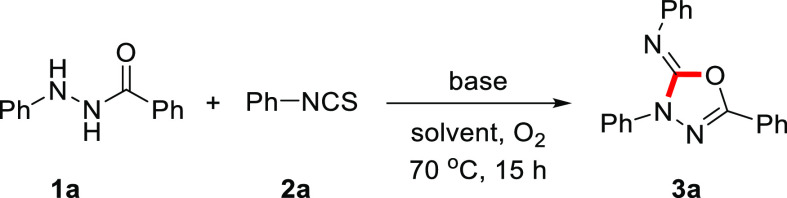
The first syntheses of 2-imino-1,3,4-oxadiazolines were achieved by the cyclization of 1-aroyl-2,4-dimethylthiosemicarbazides using excess amounts of HgO, a highly toxic reagent (Scheme 1a).14 In 2014, palladium-catalyzed aerobic oxidative annulations of hydrazides with isocyanides were achieved by Xu and co-workers, but a precious metal was required, and only tert-butyl-substituted 2-imino-1,3,4-oxadiazolines were able to be synthesized (Scheme 1b).15 The Chang group revealed that 2-imino-1,3,4-oxadiazolines could also be generated by the I2-mediated oxidative annulation of acylhydrazides with isothiocyanates (Scheme 1c).16 However, the production of 2-imino-1,3,4-oxadiazolines having a halogenated phenyl ring at the R1 position or an aliphatic chain at the R3 position was problematic, presumably due to the excessively reactive iodine oxidant. Our group has studied aerobic oxidations of hydrazides and their utility in organic transformations.17 As our previous aerobic oxidations of acylhydrazides showed selective transformations with high functional group tolerance, we envisioned that aerobic oxidative annulation of acylhydrazides with isothiocyanates might provide a practical and efficient route to 2-imino-1,3,4-oxadiazolines with broad substrate scope. In this report, we describe a straightforward synthesis of 2-imino-1,3,4-oxadiazolines through aerobic oxidation of acylhydrazides followed by 4-dimethylaminopyridine (DMAP)-mediated annulation of the in situ generated acyldiazenes with isothiocyanates (Scheme 1d).
Scheme 1. Various Synthetic Methods for 2-Imino-1,3,4-oxadiazolines.
Results and Discussion
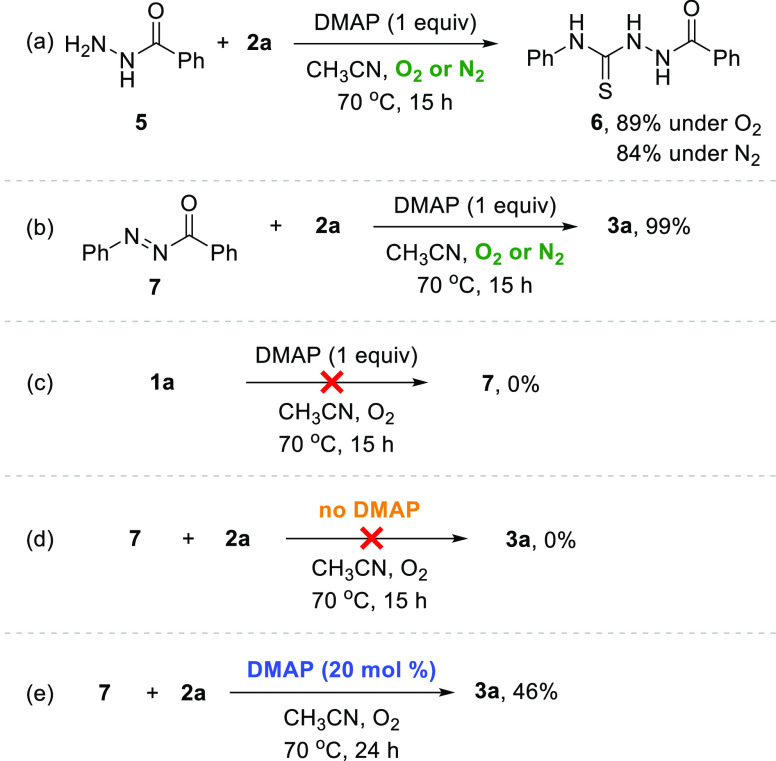
In order to realize our envisioned aerobic method, we initiated the optimization employing N′-phenylbenzohydrazide (1a) and phenyl isothiocyanate (2a) as model substrates (Table 1).18 Gratifyingly, our previously reported CuCl/DMAP system facilitated the aerobic oxidative annulation to produce 3a, albeit in a moderate yield (entry 1, Table 1).17b It is noteworthy that the use of DMAP alone can facilitate the aerobic oxidative annulation without Cu sources (entry 2). Good reactivity was observed using stoichiometric DMAP (entry 3); however, excess DMAP did not result in a higher yield (entry 4). Other bases such as pyridine, DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), Et3N (triethylamine), and K2CO3 were less reactive than DMAP (entries 5–8). A significant increase in yield was afforded when two equivalents of 2a were employed (entry 9). Reducing the temperature from 70 °C to room temperature resulted in decreased yield (entry 10). Among the solvents screened, only toluene showed similar reactivity to CH3CN,19 with other solvents, including DCE (dichloroethane) and DMF (N,N-dimethylformamide), showing inferior results (entries 11–13). Several control experiments were carried out. In the absence of DMAP, the reaction did not produce 3a with no conversion of 1a (entry 14). Under ambient atmosphere, a decreased yield of 3a was observed (entry 15). Unexpectedly, the desired product 3a was synthesized in 28% yield under anaerobic conditions (entry 16). However, the aerobic annulation proceeded ∼4.6-fold faster than the anaerobic annulation. This result indicates that the aerobic oxidative pathway is dominant in spite of the existence of the anaerobic pathway (Figure 1).
Table 1. Optimization of Aerobic Oxidative Annulationa.
| entry | base (equiv) | solvent | yield (%)b |
|---|---|---|---|
| 1c | DMAP (0.2) | CH3CN | 60 |
| 2 | DMAP (0.2) | CH3CN | 37 |
| 3 | DMAP (1.0) | CH3CN | 75 |
| 4 | DMAP (2.0) | CH3CN | 76 |
| 5 | pyridine (1.0) | CH3CN | 5 |
| 6 | DBU (1.0) | CH3CN | 25 |
| 7 | Et3N (1.0) | CH3CN | 39 |
| 8 | K2CO3 (2.0) | CH3CN | 50 |
| 9d | DMAP (1.0) | CH3CN | 92 |
| 10d,e | DMAP (1.0) | CH3CN | 35 |
| 11d | DMAP (1.0) | toluene | 91 |
| 12d | DMAP (1.0) | DCE | 73 |
| 13d | DMAP (1.0) | DMF | 42 |
| 14d | CH3CN | <1 | |
| 15d,f | DMAP (1.0) | CH3CN | 65 |
| 16d,g | DMAP (1.0) | CH3CN | 28 |
Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), and the base in the solvent (2.0 mL) under O2 at 70 °C for 15 h.
Yield of 3a was determined by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane as the internal standard.
In the presence of CuCl (10 mol %).
The use of 1.0 mmol 2a.
At room temperature.
Under air.
Under N2.
Figure 1.
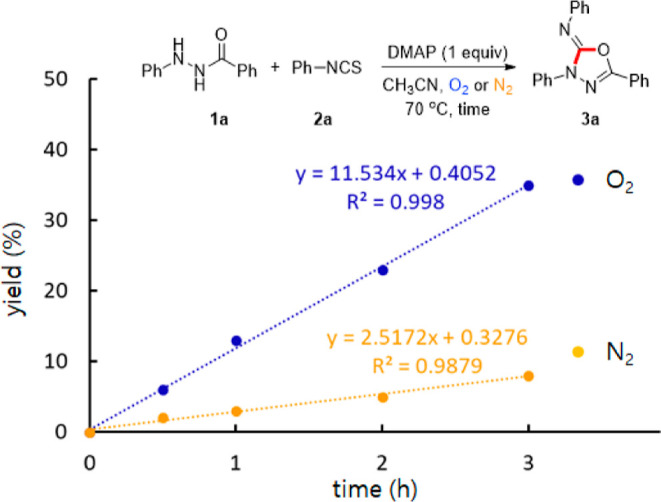
Reaction rates of the oxidative annulation of 1a with 2a under O2 and N2.
With the optimized conditions in hand (Table 1, entry 9), the reactivity of various hydrazides was investigated to gain insights into the substrate scope (Scheme 2). It was revealed that the reactivity for oxidative annulation was largely affected by the electronic nature of the phenyl ring at the R1 position. Relatively electron-rich acylhydrazides showed good yields under the optimized conditions (3a–3e and 3i–3n), while the annulation of electron-poor acylhydrazides was sluggish. However, longer reaction times led to acceptable yields for electron-poor acylhydrazides (3f–3h). No significant increase in conversion and yield was observed after 24 h. It is worth noting that halogenated substrates, which were problematic in previous I2-mediated oxidative annulation,16 were found to be compatible without significant drop in yield (3d, 3e, 3k, and 3n). The acylhydrazide 1o, which was synthesized by the reaction of tert-butyl hydrazine with benzoyl chloride, underwent the annulation in a moderate yield (3o); however, the reaction of N′-acetyl-N-benzoylhydrazide 1p generated neither the annulation product 3p nor the hydrolyzed product 3q.15 Electronic variations of the phenyl ring at the R2 position showed no critical influence on the reactivity of oxidative annulation regardless of ortho, meta, or para substitution (3r–3ac). The reactivity of other aromatic rings such as 2-naphthyl and 2-furyl was also investigated, and the corresponding 2-imino-1,3,4-oxadiazolines were produced in good yields (3ad–3ae). Acylhydrazides bearing cyclohexanecarbonyl or tert-butanecarbonyl were also well tolerated (3af and 3ag).
Scheme 2. Substrate Scope of Hydrazides,
Reaction conditions: 1 (0.5 mmol), 2a (1.0 mmol), and DMAP (0.5 mmol) in CH3CN (2.0 mL) under an O2 balloon at 70 °C for 15 h.
Isolated yields.
For 24 h.
Various isothiocyanates were screened, and the results are delineated in Scheme 3. Phenyl isothiocyanates having electron-donating or electron-withdrawing substituents efficiently underwent the annulation to produce the corresponding 2-imino-1,3,4-oxadiazolines in good to high yields (4a–4o). The annulation was compatible with benzoyl isothiocyanate 2p to produce oxadiazoline 4p in a good yield. Lastly, the aliphatic isothiocyanate 2q could also be employed (4q).
Scheme 3. Substrate Scope of Isothiocyanates,
Reaction conditions: 1a (0.5 mmol), 2 (1.0 mmol), and DMAP (0.5 mmol) in CH3CN (2.0 mL) under an O2 balloon at 70 °C for 15 h.
Isolated yields.
The developed annulation was also effective on a larger scale. We carried out the reaction of 1a with 2a on a 1.0 g scale, and the annulation product 3a was produced with no significant reduction in conversion or yield (Scheme 4).
Scheme 4. Gram-Scale Aerobic Oxidative Annulation.
In order to investigate the roles of reaction parameters and to study the reaction mechanism, several mechanistic experiments were carried out. When benzhydrazide 5 was used as a starting material instead of 1a under the optimized conditions, only benzoyl thiosemicarbazide 6 was produced in high yield with no production of 3q (Scheme 5a). The acyldiazene 7 was separately prepared by a known method17b,17d and then tested under the optimized conditions. Interestingly, the desired product 3a was produced in a quantitative yield under not only oxygen but also nitrogen (Scheme 5b). These results indicate that the plausible intermediate in the present protocol would not be the benzoyl thiosemicarbazide14 but the acyldiazene. Although the oxidative annulation of 1a with 2a showed a faster reaction rate under O2 than under N2 (Figure 1), no significant difference between the reaction rate under O2 and N2 was observed in the annulation of 7 with 2a (Figure 2).
Scheme 5. Control Experiments for Mechanistic Investigation.
Figure 2.
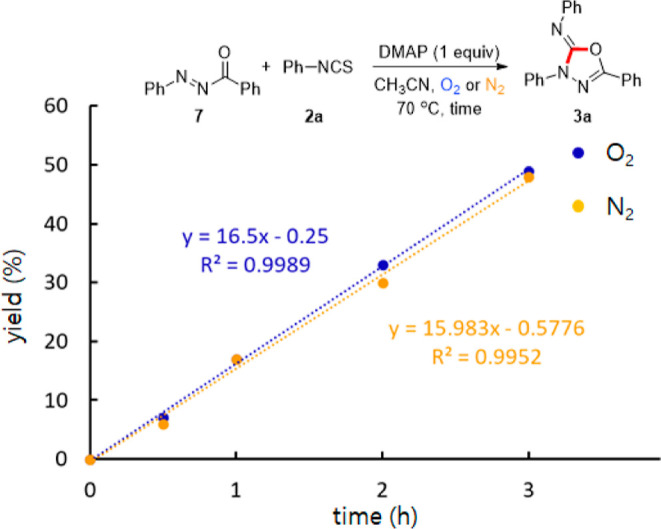
Reaction rates of the annulation of 7 with 2a under O2 and N2.
This suggests that the molecular oxygen plays a role in the oxidation of 1a to 7 but not in the annulation. The oxidation of 1a to 7 was not observed, when the reaction was carried out without 2a (Scheme 5c). Therefore, we believe that 2a might be essential for the aerobic oxidation of 1a to 7. No annulation between 7 and 2a took place without DMAP (Scheme 5d); however, the use of catalytic amounts of DMAP produced 3a in 46% yield (Scheme 5e). These observations in combination with the optimization studies (Table 1, entry 14) suggest that DMAP facilitates not only the oxidation of hydrazine but also the annulation.
Based on our preliminary mechanistic studies and the previous literature, the proposed mechanism of the present protocol is shown in Figure 3. The aerobic oxidation of hydrazide facilitated by DMAP and isothiocyanate produces acyldiazene intermediate A, although at this stage, the detailed oxidation mechanism is not clear. The produced acyldiazene intermediate A reacts with zwitterionic intermediate B which is generated by the activation of isothiocyanate with DMAP,20 and the following cyclization and desulfurization16,21 produce the desired 2-imino-1,3,4-oxadiazoline product.
Figure 3.
Proposed mechanism for aerobic oxidative annulation.
Conclusions
In conclusion, we have developed a novel synthetic method for 2-imino-1,3,4-oxadiazolines from acylhydrazides and isothiocyanates via an aerobic oxidation and DMAP-facilitated annulation sequence. In the presence of DMAP and molecular oxygen, a broad range of 2-imino-1,3,4-oxadiazolines were synthesized through the developed method. The present protocol was effective even on a large scale. Preliminary mechanistic studies revealed that the plausible mechanism consists of an aerobic oxidation of hydrazides into N-acyldiazenes, followed by a DMAP-mediated annulation between the generated N-acyldiazenes and isothiocyanates.
Experimental Section
General Considerations
All commercially available compounds and solvents were purchased and used as received, unless otherwise noted. Analytical thin layer chromatography (TLC) was performed on precoated silica gel 60 F254 plates. Visualization on TLC was achieved by the use of UV light (254 nm) and treatment with phosphomolybdic acid stain followed by heating. Flash chromatography was performed using silica gel (particle size 40–63 μm, 230–400 mesh). 1H and 13C NMR spectra were recorded on 400 MHz NMR (400 MHz for 1H, 101 MHz for 13C). Chemical shift values are given in parts per million relative to internal tetramethylsilane (0.00 ppm for 1H) or CDCl3 (77.06 ppm for 13C). The following abbreviations were used to describe peak splitting patterns when appropriate: br = broad, s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, m = multiplet, dd = double of doublet, dt = double of triplet, and td = triple of doublet. Coupling constants, J, were reported in the hertz unit (Hz). High-resolution mass spectra were obtained from the Korea Basic Science Institute (Daegu) by using the electron ionization method and magnetic sector mass analyzer.
General Procedure for Aerobic Oxidative Annulation of Acylhydrazides with Isothiocyanates
A 10 mL flame-dried test tube (O.D.
15 mm), which was equipped with a magnetic stir bar and charged with
hydrazide 1 (0.5 mmol) and DMAP (1.0 equiv, 0.5 mmol),
was evacuated and backfilled with oxygen (this process was repeated
three times). After CH3CN (1.0 mL) was added, isothiocyanate 2 (2.0 equiv, 1.0 mmol) and CH3CN (1.0 mL) were
added. Then, the reaction mixture was stirred at 70 °C for 15
h. The mixture was quenched with a saturated aqueous solution of NH4Cl at room temperature and diluted by adding dichloromethane
(DCM). Two layers were separated, and the aqueous layer was extracted
with DCM. The combined organic layer was dried over MgSO4, filtered, and concentrated on a rotary evaporator. The residue
was purified by column chromatography to give 2-imino-1,3,4-oxadiazoline
products.
N,3,5-Triphenyl-1,3,4-oxadiazol-2(3H)-imine (3a)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 8.0 Hz, 2H), 7.85
(d, J = 6.5 Hz, 2H), 7.43 (t, J =
7.2 Hz, 5H), 7.38–7.33 (m, 2H), 7.30 (d, J = 7.5 Hz, 2H), 7.18 (t, J = 7.2 Hz, 1H), 7.08 (t, J = 6.7 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.8, 145.6, 144.4, 137.6, 131.5, 129.1, 129.0, 128.9,
125.9, 124.9, 123.6, 123.3, 123.2, 118.5.
3-(4-Methoxyphenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3b)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.06
(d, J = 9.1 Hz, 2H), 7.84 (d, J =
7.6 Hz, 2H), 7.42 (q, J = 5.8 Hz, 3H), 7.34 (t, J = 7.7 Hz, 2H), 7.29 (d, J = 7.4 Hz, 2H),
7.07 (t, J = 7.1 Hz, 1H), 6.95 (d, J = 9.1 Hz, 2H), 3.79 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 157.0, 152.5, 145.7, 144.7, 131.4, 130.9, 129.0,
128.9, 125.8, 123.6, 123.2, 123.0, 120.4, 114.1, 55.5; HRMS (EI) m/z: calcd for C21H17N3O2 [M]+, 343.1321; found, 343.1322.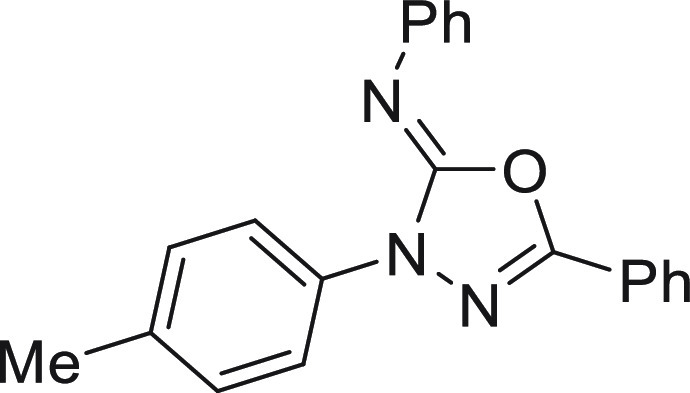
N,5-Diphenyl-3-(p-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3c)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.05 (d, J = 8.5 Hz, 2H), 7.84
(d, J = 7.8 Hz, 2H), 7.46–7.38 (m, 3H), 7.34
(t, J = 7.7 Hz, 2H), 7.29 (d, J =
7.1 Hz, 2H), 7.22 (d, J = 8.2 Hz, 2H), 7.07 (t, J = 7.1 Hz, 1H), 2.34 (s, 3H); 13C NMR (101 MHz,
CDCl3): δ 152.5, 145.7, 144.4, 135.2, 134.5, 131.4,
129.4, 128.9, 125.8, 123.6, 123.3, 123.1, 118.5, 21.0.
3-(4-Chlorophenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3d)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 9.0 Hz, 2H), 7.91
(d, J = 7.0 Hz, 2H), 7.57–7.46 (m, 3H), 7.42
(d, J = 9.1 Hz, 2H), 7.38 (t, J =
7.8 Hz, 2H), 7.30 (d, J = 7.5 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.9, 145.2, 143.9, 136.1, 131.7, 129.9, 129.0, 128.9,
125.9, 123.4, 123.3, 123.2, 119.5.
3-(4-Bromophenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3e)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.15 (d, J = 9.0 Hz, 2H), 7.90
(d, J = 8.0 Hz, 2H), 7.56 (d, J =
9.0 Hz, 2H), 7.52–7.46 (m, 3H), 7.37 (t, J = 7.7 Hz, 2H), 7.30 (d, J = 7.4 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.9, 145.1, 143.9, 136.6, 131.8, 131.7, 129.0, 126.0,
123.4, 123.3, 123.1, 119.8, 117.7.
N,5-Diphenyl-3-[4-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2(3H)-imine (3f)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.39 (d, J = 8.6 Hz, 2H), 7.91
(d, J = 7.0 Hz, 2H), 7.70 (d, J =
8.6 Hz, 2H), 7.55–7.47 (m, 3H), 7.38 (t, J = 7.7 Hz, 2H), 7.31 (d, J = 7.4 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.3, 144.9, 143.7, 140.2, 131.9, 129.0, 126.3 (q, J = 32.5 Hz), 126.08 (q, J = 3.8 Hz), 126.07,
126.06, 124.1 (q, J = 268.7 Hz), 123.6, 123.1, 117.8; 19F NMR (376 MHz, CDCl3): δ −62.09.
4-[5-Phenyl-2-(phenylimino)-1,3,4-oxadiazol-3(2H)-yl]benzonitrile (3g)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.41 (d, J = 8.7 Hz, 2H), 7.90
(d, J = 8.0 Hz, 2H), 7.72 (d, J =
8.8 Hz, 2H), 7.58–7.53 (m, 1H), 7.49 (t, J = 7.1 Hz, 2H), 7.39 (t, J = 7.4 Hz, 2H), 7.30 (d, J = 8.3 Hz, 2H), 7.15 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.6, 144.6,
143.3, 140.8, 133.1, 132.2, 129.1, 129.0, 126.2, 123.9, 123.1, 122.9,
118.9, 118.0, 107.4.
3-(4-Nitrophenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3h)16
Yellow solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 9.3 Hz, 2H), 8.33
(d, J = 9.3 Hz, 2H), 7.92 (d, J =
8.3 Hz, 2H), 7.60–7.54 (m, 1H), 7.51 (t, J = 7.3 Hz, 2H), 7.40 (t, J = 7.8 Hz, 2H), 7.32 (d, J = 7.3 Hz, 2H), 7.16 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.8, 144.4,
143.7, 143.1, 142.4, 132.3, 129.1, 126.2, 124.9, 124.0, 123.1, 122.8,
117.6.
3-(3-Methoxyphenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3i)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 7.91
(s, 1H), 7.85 (d, J = 7.7 Hz, 2H), 7.79 (d, J = 9.1 Hz, 1H), 7.47–7.39 (m, 3H), 7.37–7.30
(m, 5H), 7.09 (t, J = 7.1 Hz, 1H), 6.73 (d, J = 9.5 Hz, 1H), 3.84 (s, 3H); 13C NMR (101 MHz,
CDCl3): δ 160.1, 152.6, 145.4, 144.2, 138.7, 131.5,
129.7, 128.9, 125.9, 123.5, 123.3, 123.2, 110.6, 104.2, 55.4; HRMS
(EI) m/z: calcd for C21H17N3O2 [M]+, 343.1321;
found, 343.1321.
N,5-Diphenyl-3-(m-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3j)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.03
(d, J = 8.2 Hz, 1H), 7.98 (s, 1H), 7.85 (d, J = 6.9 Hz, 2H), 7.47–7.39 (m, 3H), 7.37–7.28
(m, 5H), 7.08 (t, J = 7.1 Hz, 1H), 7.00 (d, J = 7.5 Hz, 1H), 2.40 (s, 3H); 13C NMR (101 MHz,
CDCl3): δ 152.6, 145.6, 144.4, 138.8, 137.5, 131.5,
129.0, 128.9, 128.8, 125.9, 125.7, 123.6, 123.3, 123.1, 119.1, 115.7,
21.8; HRMS (EI) m/z: calcd for C21H17N3O [M]+, 327.1372; found,
327.1368.
3-(3-Chlorophenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3k)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.25 (s, 1H), 8.15 (d, J = 7.9
Hz, 1H), 7.84 (d, J = 7.6 Hz, 2H), 7.47–7.40
(m, 3H), 7.38–7.28 (m, 5H), 7.14–7.08 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 153.0, 145.0,
143.7, 138.5, 134.6, 131.8, 129.9, 129.0, 126.0, 124.6, 123.5, 123.2,
118.2, 116.1.
3-(2-Methoxyphenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3l)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 7.85
(d, J = 7.5 Hz, 2H), 7.52 (d, J =
6.2 Hz, 1H), 7.47–7.40 (m, 3H), 7.38–7.33 (m, 1H), 7.30–7.14
(m, 4H), 7.02 (d, J = 8.0 Hz, 3H), 3.83 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 155.4, 153.3,
147.2, 131.3, 130.6, 129.1, 128.9, 128.7, 125.8, 125.2, 123.9, 123.2,
122.7, 120.9, 112.8, 56.0; HRMS (EI) m/z: calcd for C21H17N3O2 [M]+, 343.1321; found, 343.1319.
N,5-Diphenyl-3-(o-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3m)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 7.87
(d, J = 7.6 Hz, 2H), 7.53 (s, 1H), 7.49–7.44
(m, 3H), 7.35–7.28 (m, 5H), 7.24 (d, J = 6.8
Hz, 2H), 7.04 (t, J = 7.0 Hz, 1H), 2.45 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 153.3, 146.2,
145.7, 135.5, 135.3, 131.4, 131.3, 129.0, 128.9, 128.8, 127.0, 126.8,
125.7, 123.8, 123.2, 122.8, 18.5; HRMS (EI) m/z: calcd for C21H17N3O
[M]+, 327.1372; found, 327.1373.
3-(2-Chlorophenyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3n)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 8.0 Hz, 2H), 7.64
(s, 1H), 7.56–7.50 (m, 1H), 7.50–7.41 (m, 3H), 7.39–7.26
(m, 4H), 7.26–7.18 (m, 2H), 7.04 (t, J = 6.4
Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.6,
146.0, 145.5, 134.1, 132.3, 131.5, 130.8, 130.2, 129.5, 128.9, 128.8,
127.7, 125.8, 123.7, 123.1, 123.0.
3-(tert-Butyl)-N,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3o)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 3.8 Hz, 2H), 7.42
(s, 3H), 7.31 (t, J = 7.6 Hz, 2H), 7.24 (d, J = 7.7 Hz, 2H), 7.01 (t, J = 7.2 Hz, 1H),
1.68 (s, 9H); 13C NMR (101 MHz, CDCl3): δ
150.6, 146.5, 146.4, 130.5, 128.7, 125.3, 124.4, 123.1, 122.1, 58.7,
27.6.
5-(4-Methoxyphenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3r)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 8.0 Hz, 2H), 7.82
(d, J = 8.7 Hz, 2H), 7.44 (t, J =
7.8 Hz, 2H), 7.36 (t, J = 7.6 Hz, 2H), 7.30 (d, J = 7.7 Hz, 2H), 7.19 (t, J = 7.3 Hz, 1H),
7.08 (t, J = 7.0 Hz, 1H), 6.95 (d, J = 8.7 Hz, 2H), 3.82 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 162.2, 152.8, 145.7, 144.5, 137.6, 128.9, 128.8,
127.7, 124.6, 123.2, 123.0, 118.3, 115.9, 114.4, 55.4.
N,3-Diphenyl-5-(p-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3s)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 7.9 Hz, 2H), 7.73
(d, J = 8.0 Hz, 2H), 7.42 (t, J =
7.9 Hz, 2H), 7.35 (dd, J = 10.8, 4.4 Hz, 2H), 7.30
(d, J = 7.4 Hz, 2H), 7.18 (dd, J = 14.6, 7.6 Hz, 3H), 7.08 (t, J = 7.0 Hz, 1H),
2.33 (s, 3H); 13C NMR (101 MHz, CDCl3): δ
152.9, 145.6, 144.4, 142.1, 137.7, 129.7, 129.0, 128.9, 125.9, 124.7,
123.3, 120.7, 118.4, 21.7.
5-(4-Chlorophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3t)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.17 (d, J = 7.9 Hz, 2H), 7.77
(d, J = 8.5 Hz, 2H), 7.41 (m, 4H), 7.34 (d, J = 7.3 Hz, 2H), 7.27 (d, J = 7.4 Hz, 2H),
7.18 (t, J = 7.4 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ
151.9, 145.3, 144.0, 137.7, 137.5, 129.4, 129.0, 128.9, 127.2, 125.0,
123.3, 123.2, 122.0, 118.4.
5-(4-Bromophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3u)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.20
(d, J = 7.9 Hz, 2H), 7.77 (d, J =
8.6 Hz, 2H), 7.62 (d, J = 8.5 Hz, 2H), 7.47 (t, J = 7.9 Hz, 2H), 7.37 (t, J = 7.7 Hz, 2H),
7.29 (d, J = 7.6 Hz, 2H), 7.23 (t, J = 7.4 Hz, 1H), 7.10 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.0, 145.3, 144.0, 137.4,
132.3, 129.0, 128.9, 127.2, 126.1, 125.0, 123.3, 123.1, 122.4, 118.4;
HRMS (EI) m/z: calcd for C20H14BrN3O [M]+, 393.0302; found,
393.0300.
N,3-Diphenyl-5-[4-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2(3H)-imine (3v)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.19
(d, J = 7.8 Hz, 2H), 7.99 (d, J =
8.1 Hz, 2H), 7.71 (d, J = 8.2 Hz, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.37 (t, J = 7.8 Hz, 2H),
7.29 (d, J = 7.4 Hz, 2H), 7.22 (t, J = 7.3 Hz, 1H), 7.11 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 151.4, 145.1, 143.8, 137.3,
132.9 (q, J = 33.3 Hz), 129.0, 128.9, 126.8, 126.1,
126.0 (q, J = 3.8 Hz), 125.2, 123.6 (q, J = 273.7 Hz), 123.4, 123.2, 118.5; 19F NMR (376 MHz, CDCl3): δ −63.01; HRMS (EI) m/z: calcd for C21H14F3N3O [M]+, 381.1089; found, 381.1090.
4-[4-Phenyl-5-(phenylimino)-4,5-dihydro-1,3,4-oxadiazol-2-yl]benzonitrile (3w)
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.17 (d, J = 7.8 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H), 7.73 (d, J = 8.5 Hz, 2H), 7.46 (t, J = 8.0 Hz, 2H),
7.37 (t, J = 7.8 Hz, 2H), 7.27 (d, J = 7.5 Hz, 2H), 7.23 (d, J = 7.5 Hz, 1H), 7.11 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 151.0, 144.9, 143.6, 137.2, 132.7, 129.0, 128.9, 127.4,
126.2, 125.4, 123.6, 123.1, 118.5, 117.9, 114.7; HRMS (EI) m/z: calcd for C21H14N4O [M]+, 338.1168; found, 338.1165.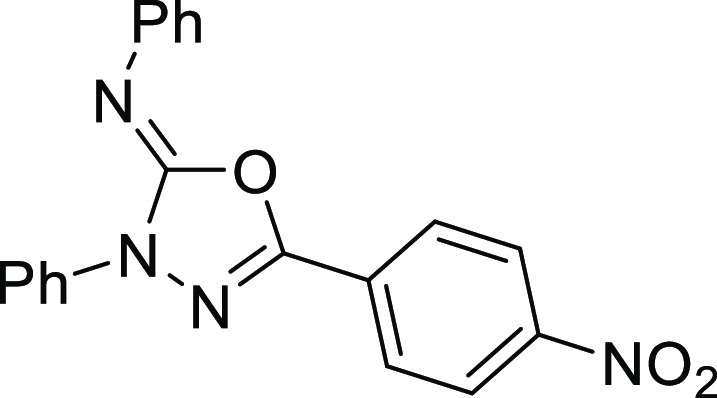
5-(4-Nitrophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3x)
Yellow solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.34
(d, J = 8.8 Hz, 2H), 8.20 (d, J =
7.9 Hz, 2H), 8.07 (d, J = 8.8 Hz, 2H), 7.49 (t, J = 8.0 Hz, 2H), 7.39 (t, J = 7.8 Hz, 2H),
7.29 (d, J = 7.5 Hz, 2H), 7.26 (d, J = 3.8 Hz, 1H), 7.14 (t, J = 7.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 150.8, 149.1, 144.9, 143.6,
137.1, 129.0, 128.9, 126.6, 125.5, 124.3, 123.6, 123.0, 118.6; HRMS
(EI) m/z: calcd for C20H14N4O3 [M]+, 358.1066;
found, 358.1067.
N,3-Diphenyl-5-(m-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3y)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.20
(d, J = 7.9 Hz, 2H), 7.65 (d, J =
12.8 Hz, 2H), 7.43 (t, J = 7.9 Hz, 2H), 7.35 (t, J = 6.8 Hz, 2H), 7.32–7.27 (m, 3H), 7.24 (d, J = 7.5 Hz, 1H), 7.18 (t, J = 7.3 Hz, 1H),
7.08 (t, J = 7.1 Hz, 1H), 2.35 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 152.9, 145.6, 144.3, 138.8,
137.6, 132.4, 129.0, 128.9, 128.8, 126.3, 124.8, 123.4, 123.3, 123.2,
118.4, 21.4; HRMS (EI) m/z: calcd
for C21H17N3O [M]+, 327.1372;
found, 327.1374.
5-(3-Chlorophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3z)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.17
(d, J = 8.3 Hz, 2H), 7.83 (s, 1H), 7.71 (d, J = 7.4 Hz, 1H), 7.43 (t, J = 8.1 Hz, 3H),
7.35 (q, J = 7.3 Hz, 3H), 7.28 (d, J = 7.9 Hz, 2H), 7.19 (t, J = 7.1 Hz, 1H), 7.09 (t, J = 7.1 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 151.5, 145.2, 143.8, 137.4, 135.1, 131.5, 130.3, 129.0,
128.9, 125.7, 125.2, 125.0, 123.9, 123.4, 123.2, 118.4; HRMS (EI) m/z: calcd for C20H14ClN3O [M]+, 347.0825; found, 347.0827.
5-(3-Nitrophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3aa)
Yellow solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.67
(s, 1H), 8.29 (d, J = 8.1 Hz, 1H), 8.16 (d, J = 8.2 Hz, 2H), 8.12 (d, J = 7.7 Hz, 1H),
7.62 (t, J = 8.0 Hz, 1H), 7.44 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.6 Hz, 2H), 7.27 (d, J = 7.9 Hz, 2H), 7.21 (t, J = 7.0 Hz, 1H),
7.11 (t, J = 6.9 Hz, 1H); 13C NMR (101
MHz, CDCl3): δ 150.7, 148.5, 144.8, 143.4, 137.2,
131.1, 130.2, 129.1, 129.0, 125.7, 125.3, 125.2, 123.6, 123.1, 120.7,
118.4; HRMS (EI) m/z: calcd for
C20H14N4O3 [M]+, 358.1066; found, 358.1063.
N,3-Diphenyl-5-(o-tolyl)-1,3,4-oxadiazol-2(3H)-imine (3ab)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.22
(d, J = 7.8 Hz, 2H), 7.78 (d, J =
7.7 Hz, 1H), 7.45 (t, J = 7.9 Hz, 2H), 7.35 (t, J = 7.6 Hz, 3H), 7.28 (dd, J = 19.4, 7.0
Hz, 4H), 7.22–7.17 (m, 1H), 7.08 (t, J = 7.0
Hz, 1H), 2.68 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 152.6, 145.4, 143.6, 137.6, 137.4, 131.5, 130.8, 128.6,
128.6, 127.6, 125.9, 124.4, 122.9, 122.8, 121.9, 117.9, 21.9; HRMS
(EI) m/z: calcd for C21H17N3O [M]+, 327.1372; found, 327.1374.
5-(2-Chlorophenyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3ac)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.22
(d, J = 7.9 Hz, 2H), 7.81 (d, J =
7.5 Hz, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.45 (t, J = 8.0 Hz, 2H), 7.40–7.36 (m, 1H), 7.34–7.31
(m, 5H), 7.21 (t, J = 7.4 Hz, 1H), 7.07 (t, J = 6.4 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 150.9, 145.4, 143.9, 137.5, 132.8, 132.0, 131.5, 129.9,
128.9, 127.0, 125.0, 123.3, 122.4, 118.5; HRMS (EI) m/z: calcd for C20H14ClN3O [M]+, 347.0825; found, 347.0828.
5-(Naphthalen-2-yl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3ad)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz,
CDCl3): δ 8.24 (s, 1H), 8.21 (d, J = 5.4 Hz, 2H), 7.92 (d, J = 8.6 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.78 (d, J = 6.7 Hz, 1H),
7.52–7.42 (m, 4H), 7.40 (t, J = 7.8 Hz, 2H),
7.33 (d, J = 7.2 Hz, 2H), 7.20 (t, J = 7.4 Hz, 1H), 7.12 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.8, 145.5, 144.3, 137.6,
134.5, 132.7, 129.0, 128.9, 128.7, 128.0, 127.9, 127.1, 126.2, 124.8,
123.3, 123.2, 122.1, 120.7, 118.5.
5-(Furan-2-yl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3ae)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz,
CDCl3): δ 8.18 (d, J = 7.9 Hz, 2H),
7.59 (s, 1H), 7.44 (t, J = 8.0 Hz, 2H), 7.34 (t, J = 7.7 Hz, 2H), 7.27 (d, J = 7.5 Hz, 2H),
7.20 (t, J = 7.4 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 3.4 Hz, 1H), 6.58–6.51
(m, 1H); 13C NMR (101 MHz, CDCl3): δ 146.2,
145.6, 145.1, 143.3, 138.8, 137.3, 128.9, 128.8, 125.0, 123.3, 123.2,
118.5, 113.8, 112.0.
5-Cyclohexyl-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3af)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.12
(d, J = 8.1 Hz, 2H), 7.41 (t, J =
7.9 Hz, 2H), 7.31 (t, J = 7.7 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.15 (t, J = 7.4 Hz, 1H),
7.04 (t, J = 7.3 Hz, 1H), 2.63 (tt, J = 11.0, 3.4 Hz, 1H), 2.04 (d, J = 11.6 Hz, 2H),
1.82 (dd, J = 9.3, 3.2 Hz, 2H), 1.70 (d, J = 9.5 Hz, 1H), 1.61–1.49 (m, 2H), 1.41–1.25
(m, 3H); 13C NMR (101 MHz, CDCl3): δ 158.8,
145.8, 145.0, 137.6, 128.9, 128.8, 124.4, 123.2, 122.9, 118.1, 35.4,
29.3, 25.6, 25.2; HRMS (EI) m/z:
calcd for C20H21N3O [M]+, 319.1685; found, 319.1688.
5-(tert-Butyl)-N,3-diphenyl-1,3,4-oxadiazol-2(3H)-imine (3ag)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz,
CDCl3): δ 8.13 (d, J = 7.8 Hz, 2H),
7.41 (t, J = 8.0 Hz, 2H), 7.31 (t, J = 7.8 Hz, 2H), 7.26–7.21 (m, 2H), 7.16 (t, J = 7.4 Hz, 1H), 7.04 (t, J = 7.8 Hz, 1H), 1.37 (s,
9H); 13C NMR (101 MHz, CDCl3): δ 161.7,
145.7, 145.1, 137.6, 128.8, 124.4, 123.2, 122.8, 118.2, 32.5, 27.3.
N-(4-Methoxyphenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4a)16
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.22 (d, J = 8.1 Hz, 2H), 7.91
(d, J = 6.2 Hz, 2H), 7.52–7.43 (m, 5H), 7.30–7.25
(m, 2H), 7.20 (t, J = 7.3 Hz, 1H), 6.92 (d, J = 8.8 Hz, 2H), 3.83 (s, 3H); 13C NMR (101 MHz,
CDCl3): δ 155.6, 152.6, 143.9, 138.6, 137.6, 131.4,
128.9, 128.8, 125.9, 124.7, 124.0, 123.6, 118.3, 114.1, 55.5.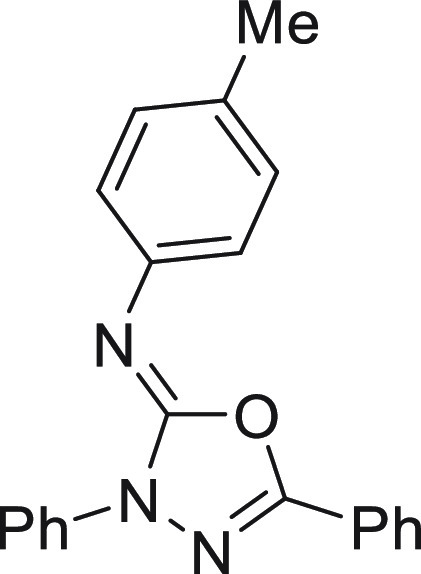
3,5-Diphenyl-N-(p-tolyl)-1,3,4-oxadiazol-2(3H)-imine (4b)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.22 (d, J = 7.9 Hz, 2H), 7.90
(d, J = 7.7 Hz, 2H), 7.53–7.40 (m, 5H), 7.22
(d, J = 8.5 Hz, 3H), 7.16 (d, J =
8.2 Hz, 2H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 152.7, 144.1, 142.8, 137.6, 132.5, 131.4, 129.5, 128.9,
125.9, 124.7, 123.6, 123.0, 118.4, 20.9.
N-(4-Fluorophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4c)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.21
(d, J = 7.8 Hz, 2H), 7.92 (d, J =
7.7 Hz, 2H), 7.56–7.45 (m, 5H), 7.31–7.27 (m, 2H), 7.23
(t, J = 7.4 Hz, 1H), 7.06 (t, J =
8.7 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ
159.0 (d, J = 230.3 Hz), 152.7, 144.5, 141.5, 137.5,
131.6, 129.0, 128.9, 125.9, 124.9, 124.2 (d, J =
8.0 Hz), 123.4, 118.5, 115.5 (d, J = 22.2 Hz); 19F NMR (376 MHz, CDCl3): δ −120.59;
HRMS (EI) m/z: calcd for C20H14FN3O [M]+, 331.1121; found, 331.1121.
N-(4-Chlorophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4d)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.19 (d, J = 7.9 Hz, 2H), 7.90
(d, J = 7.8 Hz, 2H), 7.54–7.42 (m, 5H), 7.31
(d, J = 8.7 Hz, 2H), 7.26–7.20 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 152.8, 144.6,
144.1, 137.3, 131.6, 129.0, 128.9, 128.1, 125.9, 125.0, 124.5, 123.3,
118.5.
N-(4-Bromophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4e)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.18
(d, J = 7.9 Hz, 2H), 7.90 (d, J =
6.6 Hz, 2H), 7.53–7.43 (m, 7H), 7.26–7.16 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 152.8, 144.7,
144.6, 137.3, 131.9, 131.7, 129.0, 128.9, 125.9, 125.1, 124.9, 123.3,
118.6, 115.8; HRMS (EI) m/z: calcd
for C20H14BrN3O [M]+,
391.0320; found, 391.0324.
3,5-Diphenyl-N-[4-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2(3H)-imine (4f)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 8.3 Hz, 2H), 7.91
(d, J = 7.1 Hz, 2H), 7.61 (d, J =
8.4 Hz, 2H), 7.55–7.45 (m, 5H), 7.38 (d, J = 8.2 Hz, 2H), 7.29–7.23 (m, 1H); 13C NMR (101
MHz, CDCl3): δ 153.0, 148.9, 145.2, 137.2, 131.8,
129.1, 129.0, 126.1 (q, J = 3.8 Hz), 126.0, 125.3,
123.3, 123.2, 118.7; 19F NMR (376 MHz, CDCl3): δ −61.76.
4-{[3,5-Diphenyl-1,3,4-oxadiazol-2(3H)-ylidene]amino}benzonitrile (4g)16
White solid,
EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ
8.17 (d, J = 7.8 Hz, 2H), 7.91 (d, J = 8.1 Hz, 2H), 7.63 (d, J = 8.6 Hz, 2H), 7.58–7.45
(m, 5H), 7.36 (d, J = 8.6 Hz, 2H), 7.29–7.24
(m, 1H); 13C NMR (101 MHz, CDCl3): δ 153.2,
150.0, 145.5, 136.9, 133.1, 131.9, 129.1, 129.0, 126.0, 125.6, 123.9,
123.0, 119.6, 118.9, 105.8.
N-(4-Nitrophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4h)16
Yellow solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.25 (d, J = 9.0 Hz, 2H), 8.18
(d, J = 8.6 Hz, 2H), 7.94 (d, J =
7.5 Hz, 2H), 7.58–7.48 (m, 5H), 7.40 (d, J = 9.1 Hz, 2H), 7.30 (d, J = 7.0 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.3, 152.2, 145.9, 143.0,
136.8, 132.1, 129.1, 129.0, 126.0, 125.8, 125.0, 123.5, 122.9, 119.1.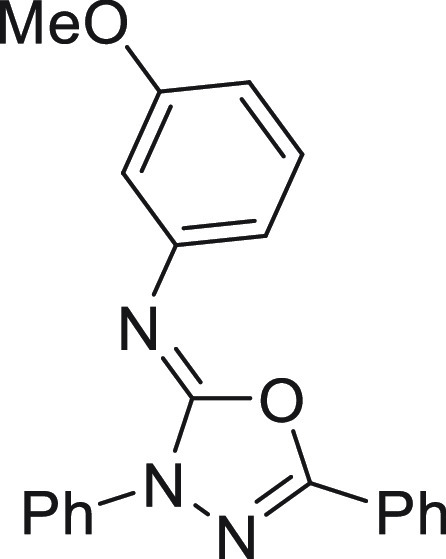
N-(3-Methoxyphenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4i)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.22
(d, J = 8.7 Hz, 2H), 7.92 (d, J =
7.4 Hz, 2H), 7.55–7.43 (m, 5H), 7.28 (d, J = 8.2 Hz, 1H), 7.23 (t, J = 7.0 Hz, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.87 (s, 1H), 6.68 (d, J = 8.2 Hz, 1H), 3.85 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 160.2, 152.8, 146.8, 144.5, 137.5, 131.5, 129.5,
128.9, 128.9, 125.9, 124.9, 123.5, 118.5, 115.6, 109.1, 108.9, 55.3;
HRMS (EI) m/z: calcd for C21H17N3O2 [M]+, 343.1321;
found, 343.1319.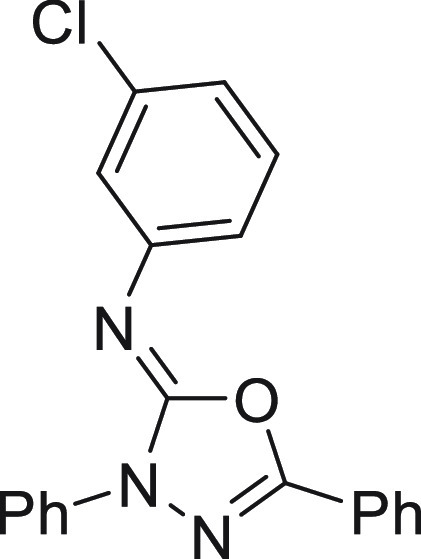
N-(3-Chlorophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4j)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.18
(d, J = 7.8 Hz, 2H), 7.90 (d, J =
7.8 Hz, 2H), 7.52–7.43 (m, 5H), 7.34–7.30 (m, 1H), 7.29–7.24
(m, 1H), 7.24–7.16 (m, 2H), 7.06 (d, J = 7.8
Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 152.9,
146.8, 144.9, 137.3, 134.3, 131.7, 129.8, 129.0, 128.9, 125.9, 125.1,
123.4, 123.3, 123.1, 121.4, 118.6; HRMS (EI) m/z: calcd for C20H14ClN3O [M]+, 347.0825; found, 347.0826.
N-(3-Nitrophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4k)
Yellow solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.13
(d, J = 7.8 Hz, 2H), 7.93 (d, J =
8.3 Hz, 1H), 7.83 (d, J = 7.9 Hz, 2H), 7.57–7.43
(m, 6H), 7.36 (d, J = 8.1 Hz, 1H), 7.28–7.24
(m, 1H), 7.16 (t, J = 7.8 Hz, 1H); 13C
NMR (101 MHz, CDCl3): δ 153.0, 148.9, 146.7, 145.6,
137.0, 131.9, 129.8, 129.4, 129.1, 129.0, 125.9, 125.5, 123.0, 118.8,
118.0, 117.8; HRMS (EI) m/z: calcd
for C20H14N4O3 [M]+, 358.1066; found, 358.1068.
N-(2-Methoxyphenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4l)
White solid, EtOAc/PE
= 1:40, 1H NMR (400 MHz, CDCl3): δ 8.24
(d, J = 7.9 Hz, 2H), 7.85 (d, J =
8.2 Hz, 2H), 7.50–7.41 (m, 5H), 7.24–7.16 (m, 2H), 7.09
(t, J = 7.7 Hz, 1H), 6.97 (t, J =
6.9 Hz, 2H), 3.85 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 152.8, 152.0, 144.9, 137.6, 131.4, 128.9, 128.8, 125.9,
124.7, 123.9, 123.6, 123.5, 120.8, 118.3, 111.7, 55.8; HRMS (EI) m/z: calcd for C21H17N3O2 [M]+, 343.1321; found, 343.1319.
3,5-Diphenyl-N-(o-tolyl)-1,3,4-oxadiazol-2(3H)-imine (4m)16
White solid, EtOAc/PE = 1:40, 1H NMR (400 MHz, CDCl3): δ 8.26 (d, J = 8.6 Hz, 2H), 7.87
(d, J = 8.2 Hz, 2H), 7.50–7.41 (m, 5H), 7.31–7.18
(m, 4H), 7.01 (t, J = 7.4 Hz, 1H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 152.7, 144.3,
143.7, 137.7, 131.6, 131.5, 130.4, 128.9, 128.8, 126.3, 125.9, 124.7,
123.6, 123.2, 121.7, 118.2, 18.7.
N-(2-Chlorophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4n)
White solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.28
(d, J = 8.6 Hz, 2H), 7.88 (d, J =
8.2 Hz, 2H), 7.52–7.43 (m, 6H), 7.36 (d, J = 8.0 Hz, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.23 (d, J = 7.5 Hz, 1H), 7.03 (t, J = 7.7 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 153.0, 144.9,
143.1, 137.3, 131.6, 129.8, 129.0, 128.9, 128.2, 127.1, 126.0, 125.1,
123.9, 123.5, 123.3, 118.5; HRMS (EI) m/z: calcd for C20H14ClN3O [M]+, 347.0825; found, 347.0825.
N-(2-Nitrophenyl)-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4o)
Yellow solid, EtOAc/PE
= 1:20, 1H NMR (400 MHz, CDCl3): δ 8.24
(s, 1H), 8.20 (d, J = 7.9 Hz, 2H), 7.94 (d, J = 6.6 Hz, 3H), 7.62 (d, J = 9.0 Hz, 1H),
7.57–7.46 (m, J = 15.0, 7.7 Hz, 6H), 7.31–7.26
(m, 1H); 13C NMR (101 MHz, CDCl3): δ 153.2,
145.8, 143.6, 140.6, 136.8, 133.3, 131.8, 129.0, 126.0, 125.6, 125.5,
125.0, 123.0, 122.9, 119.0; HRMS (EI) m/z: calcd for C20H14N4O3 [M]+, 358.1066; found, 358.1062.
N-[3,5-Diphenyl-1,3,4-oxadiazol-2(3H)-ylidene]benzamide (4p)16
White solid, EtOAc/PE = 1:40, 1H NMR
(400 MHz, CDCl3): δ 8.29 (d, J =
8.3 Hz, 2H), 8.20 (d, J = 8.6 Hz, 2H), 8.08 (d, J = 8.1 Hz, 2H), 7.60–7.50 (m, 6H), 7.47 (t, J = 7.2 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 173.4, 155.7,
151.6, 136.3, 136.2, 132.3, 132.2, 129.9, 129.1, 129.0, 128.2, 126.9,
126.6, 122.5, 120.5.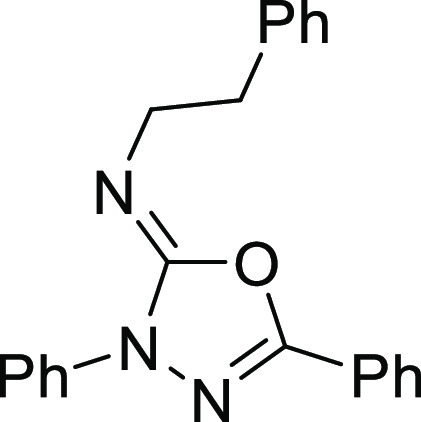
N-Phenethyl-3,5-diphenyl-1,3,4-oxadiazol-2(3H)-imine (4q)
White solid, EtOAc/PE = 1:20, 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 8.0 Hz, 2H), 7.87 (d, J = 7.5 Hz, 2H), 7.50–7.45 (m, 3H), 7.42 (t, J = 7.9 Hz, 2H), 7.36–7.28 (m, 4H), 7.22–7.12 (m, 2H), 3.76 (t, J = 7.5 Hz, 2H), 3.01 (t, J = 7.5 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 152.3, 145.6, 140.7, 138.0, 131.1, 129.0, 128.9, 128.80, 128.3, 126.0, 125.7, 124.0, 117.6, 49.6, 38.1; HRMS (EI) m/z: calcd for C22H19N3O [M]+, 341.1528; found, 341.1525.
Procedure for Aerobic Oxidative Annulation on a Large Scale
A 250 mL round-bottom flask, which was equipped with a magnetic stir bar and charged with hydrazide 1a (4.7 mmol, 1.0 g) and DMAP (1.0 equiv, 4.7 mmol), was evacuated and backfilled with oxygen (this process was repeated three times). After 10 mL of CH3CN was added, 2a (2.0 equiv, 9.4 mmol) and CH3CN (5 mL) were added in sequence. The reaction mixture was stirred under an O2 balloon at 70 °C for 15 h. The mixture was quenched with a saturated aqueous solution of NH4Cl at room temperature and diluted by adding DCM. Two layers were separated, and the aqueous layer was extracted with DCM. The combined organic layer was dried over MgSO4, filtered, and concentrated on a rotary evaporator. The residue was purified by column chromatography to give 2-imino-1,3,4-oxadiazoline products (EtOAc/PE = 1:20).
Acknowledgments
This work is dedicated to Prof. Sukbok Chang on the occasion of his 60th birthday. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2021R1A2C4002062). This work was also supported by the Incheon National University RIBS Grant in 2019.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02323.
Preparation of acylhydrazides, detail optimizations, and copies of 1H, 13C, and 19F NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Dumur F.; Goubard F. Triphenylamines and 1,3,4-oxadiazoles: a versatile combination for controlling the charge balance in organic electronics. New J. Chem. 2014, 38, 2204–2224. 10.1039/c3nj01537h. [DOI] [Google Scholar]; b Paun A.; Hadade N. D.; Paraschivescu C. C.; Matache M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. 10.1039/c6tc03003c. [DOI] [Google Scholar]
- Adachi C.; Tsutsui T.; Saito S. Blue light-emitting organic electroluminescent devices. Appl. Phys. Lett. 1990, 56, 799–801. 10.1063/1.103177. [DOI] [Google Scholar]
- a Wilkie G. D.; Elliott G. I.; Blagg B. S. J.; Wolkenberg S. E.; Soenen D. R.; Miller M. M.; Pollack S.; Boger D. L. Intramolecular Diels-Alder and Tandem Intramolecular Diels-Alder/1,3-Dipolar Cycloaddition Reactions of 1,3,4-Oxadiazoles. J. Am. Chem. Soc. 2002, 124, 11292–11294. 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]; b Elliott G. I.; Fuchs J. R.; Blagg B. S. J.; Ishikawa H.; Tao H.; Yuan Z.-Q.; Boger D. L. Intramolecular Diels-Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles. J. Am. Chem. Soc. 2006, 128, 10589–10595. 10.1021/ja0612549. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sasaki Y.; Kato D.; Boger D. L. Asymmetric Total Synthesis of Vindorosine, Vindoline, and Key Vinblastine Analogues. J. Am. Chem. Soc. 2010, 132, 13533–13544. 10.1021/ja106284s. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lajiness J. P.; Jiang W.; Boger D. L. Divergent Total Syntheses of (−)-Aspidospermine and (+)-Spegazzinine. Org. Lett. 2012, 14, 2078–2081. 10.1021/ol300599p. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xie J.; Wolfe A. L.; Boger D. L. Total Synthesis of Kopsinine. Org. Lett. 2013, 15, 868–870. 10.1021/ol303573f. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Campbell E. L.; Skepper C. K.; Sankar K.; Duncan K. K.; Boger D. L. Transannular Diels–Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles: Total Synthesis of a Unique Set of Vinblastine Analogues. Org. Lett. 2013, 15, 5306–5309. 10.1021/ol402549n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Boström J.; Hogner A.; Llinàs A.; Wellner E.; Plowright A. T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. 10.1021/jm2013248. [DOI] [PubMed] [Google Scholar]; b Vaidya A.; Pathak D.; Shah K. 1,3,4-oxadiazole and its derivatives: A review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591. 10.1111/cbdd.13795. [DOI] [PubMed] [Google Scholar]
- Summa V.; Petrocchi A.; Bonelli F.; Crescenzi B.; Donghi M.; Ferrara M.; Fiore F.; Gardelli C.; Gonzalez Paz O. G.; Hazuda D. J.; Jones P.; Kinzel O.; Laufer R.; Monteagudo E.; Muraglia E.; Nizi E.; Orvieto F.; Pace P.; Pescatore G.; Scarpelli R.; Stillmock K.; Witmer M. V.; Rowley M. Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection. J. Med. Chem. 2008, 51, 5843–5855. 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- James N. D.; Growcott J. W. Zibotentan. Drugs Future 2009, 34, 624–633. 10.1358/dof.2009.034.08.1400202. [DOI] [Google Scholar]
- a Hirao I. Synthesis of 5 [2-(5-Nitro-2-furyl)-1-(2-furyl)vinyl] -2-amino 1, 3, 4-oxadiazole. Nippon Kagaku Zasshi 1967, 88, 574–575. 10.1246/nikkashi1948.88.5_574. [DOI] [Google Scholar]; b Ogata M.; Atobe H.; Kushida H.; Yamamoto K. In vitro sensitivity of mycoplasmas isolated from various animals and sewage to antibiotics and nitrofurans. J. Antibiot. 1971, 24, 443–451. 10.7164/antibiotics.24.443. [DOI] [PubMed] [Google Scholar]
- a Jakopin Z.; Dolenc M. S. Recent Advances in the Synthesis of 1,2,4- and 1,3,4-Oxadiazoles. Curr. Org. Chem. 2008, 12, 850–898. 10.2174/138527208784911860. [DOI] [Google Scholar]; b Patel K. D.; Prajapati S. M.; Panchal S. N.; Patel H. D. Review of Synthesis of 1,3,4-Oxadiazole Derivatives. Synth. Commun. 2014, 44, 1859–1875. 10.1080/00397911.2013.879901. [DOI] [Google Scholar]
- For selected dehydrative cyclizations, see:; a Borg S.; Vollinga R. C.; Labarre M.; Payza K.; Terenius L.; Luthman K. Design, Synthesis, and Evaluation of Phe-Gly Mimetics: Heterocyclic Building Blocks for Pseudopeptides. J. Med. Chem. 1999, 42, 4331–4342. 10.1021/jm990197+. [DOI] [PubMed] [Google Scholar]; b Pouliot M.-F.; Angers L.; Hamel J.-D.; Paquin J.-F. Synthesis of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using [Et2NSF2]BF4 as a practical cyclodehydration agent. Org. Biomol. Chem. 2012, 10, 988–993. 10.1039/c1ob06512b. [DOI] [PubMed] [Google Scholar]; c Chaudhari P. S.; Pathare S. P.; Akamanchi K. G. o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. J. Org. Chem. 2012, 77, 3716–3723. 10.1021/jo2025509. [DOI] [PubMed] [Google Scholar]; d Prabhu G.; Sureshbabu V. V. Hypervalent iodine(V) mediated mild and convenient synthesis of substituted 2-amino-1,3,4-oxadiazoles. Tetrahedron Lett. 2012, 53, 4232–4234. 10.1016/j.tetlet.2012.05.154. [DOI] [Google Scholar]
- For selected desulfurative cyclizations, see:; a Coppo F. T.; Evans K. A.; Graybill T. L.; Burton G. Efficient one-pot preparation of 5-substituted-2-amino-1,3,4-oxadiazoles using resin-bound reagents. Tetrahedron Lett. 2004, 45, 3257–3260. 10.1016/j.tetlet.2004.02.119. [DOI] [Google Scholar]; b Dolman S. J.; Gosselin F.; O’Shea P. D.; Davies I. W. Superior Reactivity of Thiosemicarbazides in the Synthesis of 2-Amino-1,3,4-oxadiazoles. J. Org. Chem. 2006, 71, 9548–9551. 10.1021/jo0618730. [DOI] [PubMed] [Google Scholar]; c Yang S. J.; Lee S. H.; Kwak H. J.; Gong Y. D. Regioselective Synthesis of 2-Amino-Substituted 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives via Reagent-Based Cyclization of Thiosemicarbazide Intermediate. J. Org. Chem. 2013, 78, 438–444. 10.1021/jo302324r. [DOI] [PubMed] [Google Scholar]; d Long B.; Tian B.; Tang Q.; Hu X.; Han L.; Wang Z.; Wang C.; Wu Y.; Yu Y.; Gan Z. A KHSO4 mediated facile synthesis of 2-amino-1,3,4-oxadiazole derivative. Tetrahedron 2021, 96, 132382. 10.1016/j.tet.2021.132382. [DOI] [Google Scholar]
- For selected oxidative cyclizations, see:; a Guin S.; Ghosh T.; Rout S. K.; Banerjee A.; Patel B. K. Cu(II) Catalyzed Imine C–H Functionalization Leading to Synthesis of 2,5-Substituted 1,3,4-Oxadiazoles. Org. Lett. 2011, 13, 5976–5979. 10.1021/ol202409r. [DOI] [PubMed] [Google Scholar]; b Shang Z.; Ha J.; Tao X.; Xu L.; Liu Q.; Wang P. (PhIO)n Mediated Annulations of Aromatic Aldehyde N-Acylhydrazones for the Synthesis of 1,3,4-Oxadiazoles. Bull. Korean Chem. Soc. 2013, 34, 1879–1882. 10.5012/bkcs.2013.34.6.1879. [DOI] [Google Scholar]; c Fan Y.; He Y.; Liu X.; Hu T.; Ma H.; Yang X.; Luo X.; Huang G. Iodine-Mediated Domino Oxidative Cyclization: One-Pot Synthesis of 1,3,4-Oxadiazoles via Oxidative Cleavage of C(sp2)–H or C(sp)–H Bond. J. Org. Chem. 2016, 81, 6820–6825. 10.1021/acs.joc.6b01135. [DOI] [PubMed] [Google Scholar]
- For other preparations for 1,3,4-oxadiazoles, see:; a Zeevaart J. G.; Wang L.; Thakur V. V.; Leung C. S.; Tirado-Rives J.; Bailey C. M.; Domaoal R. A.; Anderson K. S.; Jorgensen W. L. Optimization of Azoles as Anti-Human Immunodeficiency Virus Agents Guided by Free-Energy Calculations. J. Am. Chem. Soc. 2008, 130, 9492–9499. 10.1021/ja8019214. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Diao P.; Ge Y.; zhang W.; Xu C.; Zhang N.; Guo C. Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles by visible-light-mediated decarboxylation–cyclization of hydrazides and diketone. Tetrahedron Lett. 2018, 59, 767–770. 10.1016/j.tetlet.2018.01.037. [DOI] [Google Scholar]; c Li J.; Lu X.-C.; Xu Y.; Wen J.-X.; Hou G.-Q.; Liu L. Photoredox Catalysis Enables Decarboxylative Cyclization with Hypervalent Iodine(III) Reagents: Access to 2,5-Disubstituted 1,3,4-Oxadiazoles. Org. Lett. 2020, 22, 9621–9626. 10.1021/acs.orglett.0c03663. [DOI] [PubMed] [Google Scholar]; d Li J.; Wen J.-X.; Lu X.-C.; Hou G.-Q.; Gao X.; Li Y.; Liu L. Catalyst-Free Visible-Light-Promoted Cyclization of Aldehydes: Access to 2,5-Disubstituted 1,3,4-Oxadiazole Derivatives. ACS Omega 2021, 6, 26699–26706. 10.1021/acsomega.1c04098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao Y.; Hirata T.; Goto S.; Kakehi A.; Iizuka K.; Shiro M. Intramolecular Nonbonded S···O Interaction Recognized in (Acylimino)thiadiazoline Derivatives as Angiotensin II Receptor Antagonists and Related Compounds. J. Am. Chem. Soc. 1998, 120, 3104–3110. 10.1021/ja973109o. [DOI] [Google Scholar]
- Kane J. M.; Staeger M. A. An Improved Method for the Synthesis of 5-Aryl-3-methyl-2-methylimino-1,3,4-Oxadiazoles. Synth. Commun. 1992, 22, 1–11. 10.1080/00397919208021073. [DOI] [Google Scholar]
- Fang T.; Tan Q.; Ding Z.; Liu B.; Xu B. Pd-Catalyzed Oxidative Annulation of Hydrazides with Isocyanides: Synthesis of 2-Amino-1,3,4-oxadiazoles. Org. Lett. 2014, 16, 2342–2345. 10.1021/ol5006449. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Ren L.; Hou J.; Yu W.; Chang J. Annulation Reactions of In-Situ-Generated N-(Het)aroyldiazenes with Isothiocyanates Leading to 2-Imino-1,3,4-oxadiazolines. Org. Lett. 2019, 21, 210–213. 10.1021/acs.orglett.8b03663. [DOI] [PubMed] [Google Scholar]
- a Jung D.; Kim M. H.; Kim J. Cu-Catalyzed Aerobic Oxidation of Di-tert-butyl Hydrazodicarboxylate to Di-tert-butyl Azodicarboxylate and Its Application on Dehydrogenation of 1,2,3,4-Tetrahydroquinolines under Mild Conditions. Org. Lett. 2016, 18, 6300–6303. 10.1021/acs.orglett.6b03166. [DOI] [PubMed] [Google Scholar]; b Kim M. H.; Kim J. Aerobic Oxidation of Alkyl 2-Phenylhydrazinecarboxylates Catalyzed by CuCl and DMAP. J. Org. Chem. 2018, 83, 1673–1679. 10.1021/acs.joc.7b03119. [DOI] [PubMed] [Google Scholar]; c Jung D.; Jang S. H.; Yim T.; Kim J. Oxidation Potential Tunable Organic Molecules and Their Catalytic Application to Aerobic Dehydrogenation of Tetrahydroquinolines. Org. Lett. 2018, 20, 6436–6439. 10.1021/acs.orglett.8b02749. [DOI] [PubMed] [Google Scholar]; d Jo G.; Kim M. H.; Kim J. A practical route to azo compounds by metal-free aerobic oxidation of arylhydrazides using an NOx system. Org. Chem. Front. 2020, 7, 834–839. 10.1039/d0qo00043d. [DOI] [Google Scholar]; e Kim J.; Lee D. H.; Kim J. Cu-Catalyzed Aerobic Oxidative Azo-Ene Cyclization. Adv. Synth. Catal. 2021, 363, 4728–4733. 10.1002/adsc.202100687. [DOI] [Google Scholar]
- See the Supporting Information for details.
- When the reaction carried out for 6 h, the CH3CN showed a better yield (70%) than toluene (48%).
- a Zhang Q.; Meng L.-G.; Zhang J.; Wang L. DMAP-Catalyzed [2 + 4] Cycloadditions of Allenoates with N-Acyldiazenes: Direct Method to 1,3,4-Oxadiazine Derivatives. Org. Lett. 2015, 17, 3272–3275. 10.1021/acs.orglett.5b01237. [DOI] [PubMed] [Google Scholar]; b Ren Y.; Meng L.-G.; Peng T.; Zhu L.; Wang L. 4-Dimethylaminopyridine-Catalyzed Regioselective [3+2] Cycloaddition of Isatin-Derived Morita–Baylis–Hillman Adducts with Azo Esters: A Simple Protocol to Access 3-Spiropyrazole-2-oxindoles. Adv. Synth. Catal. 2018, 360, 3176–3180. 10.1002/adsc.201800552. [DOI] [Google Scholar]
- Huang Z.; Zhang Q.; Zhao Q.; Yu W.; Chang J. Synthesis of 2-imino-1,3,4-thiadiazoles from hydrazides and isothiocyanates via sequential oxidation and P(NMe2)3-mediated annulation reactions. Org. Lett. 2020, 22, 4378–4382. 10.1021/acs.orglett.0c01393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.