Abstract
Polycystic ovary syndrome is the most common endocrine disorder in women of reproductive age, which is still incurable. However, the symptoms can be successfully managed with proper medication and lifestyle interventions. Despite its prevalence, little is known about its etiology. In this review article, the up-to-date diagnostic features and parameters recommended on the grounds of evidence-based data and different guidelines are explored. The ambiguity and insufficiency of data when diagnosing adolescent women have been put under special focus. We look at some of the most recent research done to establish relationships between different gene polymorphisms with polycystic ovary syndrome in various populations along with the underestimated impact of environmental factors like endocrine-disrupting chemicals on the reproductive health of these women. Furthermore, the article concludes with existing treatments options and the scopes for advancement in the near future. Various therapies have been considered as potential treatment through multiple randomized controlled studies, and clinical trials conducted over the years are described in this article. Standard therapies ranging from metformin to newly found alternatives based on vitamin D and gut microbiota could shine some light and guidance toward a permanent cure for this female reproductive health issue in the future.
Keywords: etiology, polycystic ovary syndrome, polycystic ovary syndrome diagnosis, polycystic ovary syndrome, reproductive health, treatment options
Introduction
Polycystic ovary syndrome (PCOS) is a widely prevalent metabolic and endocrine disorder diagnosed in reproductive-aged females. The disease is distinguished by the presence and degree of three major features: irregular menstruation, hyperandrogenism, and polycystic ovarian morphology (PCOM). 1 The prevalence of PCOS is known to be around 5%–20%, depending on the varying definitions used. 2 Despite many advances and adaptations in developing the diagnostic criteria and interpreting the condition’s pathophysiology, PCOS remains a less-understood disorder in terms of criteria for uniform diagnosis and treatment. 3 The multifaceted effects of the disease are spread across a woman’s lifetime beginning from conception and extending across the years following menopause. 4 A majority of the studies related to PCOS were performed to develop a timely and efficient diagnosis, particularly for adolescents, effective treatment, and management of comorbidities associated with PCOS that gravely impact the quality of life, and a homogeneous protocol that can be implemented by healthcare officials. 5 In this review, the diagnostic procedures and other screening protocols mentioned were centered around the most recent international evidence-based guidelines for PCOS. Furthermore, the article went into detail about the diagnosis of PCOS in adults together with the challenges faced in diagnosing adolescent girls. We have found a lack of age-specific guidelines that is a consequence of insufficient scientific investigations. In addition, the causal links, both genetic and environmental, have been summarized with a brief insight into the pathogenesis of PCOS. Finally, the current state of the treatment is looked at, and the new options with considerable potential have been discussed.
The incentive to work with PCOS came from the understanding that a certain percentage of women are still being misdiagnosed or left undiagnosed due to unawareness and misunderstanding. While working on this article, it was made clear that many countries, especially Bangladesh, are yet to take PCOS seriously. This was demonstrated through the lack of research in these geographical regions. Thus, we believe that putting forth the actual situation about the condition would help narrow the chasm in recognition and pave the way for better answers.
Diagnosis of PCOS
Introduction to different diagnostic criteria
PCOS is a recurrent endocrinopathy prevalent in approximately 8%–13% (varying across different populations) of women in the reproductive age bracket.6–9 Despite its frequency, guidance regarding implementing the diagnostic procedures for detecting PCOS is relatively obscure and inconsistent among health professionals. As a result, up to 70% of these women are known to remain undiagnosed. 8 It further adds to the problem of unestablished etiology or origin of PCOS. However, over the past decades, the characteristic traits observed in women with PCOS have been analyzed, and eventually resulted in the development of three diagnostic criteria based on the hallmarks of PCOS.
The three distinctive features of this endocrinologic condition include ovulatory dysfunction, hyperandrogenism, and PCOM in accordance with the consensus-based international guidelines.10–12 In addition, PCOS usually manifests in the form of hirsutism, oligo-anovulation, amenorrhea or irregular menstrual cycle, and infertility. 13 Because of the heterogenic characteristics, three classifications of diagnostic criteria were established over time. The criteria are listed as follows:
1. National Institute of Health (NIH) criteria. It was established in 1990 based on the agreement of a veteran panel and the first kind of diagnostic criteria to be ever generated. The NIH criteria authorized two definite features to be representative of PCOS: (a) signs of hyperandrogenism (clinical or biochemical) and (b) oligo-anovulation or oligomenorrhea. 10 This criterion was later revised in 2012, where the third determinant, PCOM, was integrated to conform to the Rotterdam criteria. 14
- 2. Rotterdam criteria. It was proposed in 2003 by the European Society of Human Reproduction and Radiology (ESHRE) and the American Society for Reproductive Medicine (ASRM), in which the third determinant of PCOS was added, that is the PCOM upon ultra-sonogram imaging. The Rotterdam criteria signified the presence of any two among the three factors to be present in a PCOS diagnosis. Diagnosis using this criterion allows further classification into four different phenotypes from A to D: 11
- • Phenotype A—Hyperandrogenism + Ovulatory Dysfunction + PCOM
- • Phenotype B—Hyperandrogenism + Ovulatory Dysfunction
- • Phenotype C—Hyperandrogenism + PCOM
- • Phenotype D—Ovulatory Dysfunction + PCOM
The Rotterdam criteria are widely used by gynecologists, obstetricians, and other healthcare personnel; it was also adopted by the 2018 International PCOS guideline and other guidelines.15,16 Furthermore, the criteria were also suggested by the NIH evidence-based methodology PCOS workshop held in 2012, alongside phenotype identification in all researches. 14
3. AE-PCOS Criteria. It was put forward in 2006 by the Androgen Excess and PCOS Society. The third criteria suggested the presence of hyperandrogenism along with any one or two of the remaining determinants (ovulatory dysfunction and/or PCOM) for PCOS diagnosis. 6
In short, the three diagnostic criteria present the identification and quantification of the classical features of PCOS (hyperandrogenism, ovulatory dysfunction, and PCOM) for a definitive diagnosis of the condition. However, it is worth noting that diagnosis through any one of the above-mentioned three criteria will only be conclusive of the condition provided that other endocrine disorders such as hyperprolactinemia, thyroid disease, Non-classical Congenital Adrenal Hyperplasia (NCAH), Cushing’s syndrome/disease, hypogonadotropic hypogonadism or androgen producing tumors which exhibit similar manifestations (clinical/biochemical/morphological) as that of PCOS are ruled out.14,15 For instance, NCAH that manifests in hirsutism or irregular menstruation can be tested by measuring 17-hydroxyprogesterone (17 OHP) levels with an additional ACTH stimulation test in borderline cases. 17 Similarly, hyperprolactinemia can be detected if a prolactin level exceeding the threshold of 500 μg/L is found, exhibiting galactorrhea symptoms and irregular periods. 18 In addition, thyroid diseases can be ruled out by calculating the levels of the thyroid-stimulating hormone (TSH). 14 On the other hand, Cushing’s syndrome is a relatively severe condition that displays obesity, high blood pressure, and amenorrhea features. In addition, it is associated with over secretion of cortisol. Thus, an overnight dexamethasone suppression test or midnight salivary cortisol test will assist in distinguishing this condition from PCOS. 19
Diagnostic features in adults
Data from a recent meta-analysis and systematic review revealed a clear picture of the overall prevalence of PCOS based on the three available diagnostic features where ovulatory dysfunction, hirsutism, biochemical hyperandrogenism, and PCOM were found to be in 12%, 13%, 11%, and 28% of women, respectively. 9 The major diagnostic features observed in women with PCOS in a spectrum of degrees are discussed in the succeeding parts of the article.
Ovulatory dysfunction
A staggering proportion of approximately 75% of PCOS individuals is known to have ovulatory dysfunction. 20 It is described as a state of irregular menstrual cycle. 14 In a standard ovulation cycle, menstruation begins by the 24th/25th day. 21 In an adult, irregular menstruation may be indicated by a cycle consisting of <21 or >35 days, or less than eight menstrual cycles per year in a few cases where the gynecologic age is relatively higher.16,22 Continued irregular menstruation indicates anovulation or oligo-anovulation, which can later aggravate a PCOS condition. 14
Contrastingly, regular menstruation reflecting normal ovulatory cycles has also been noticed in women with PCOS. 23 The phenomenon is known as subclinical ovulatory dysfunction and may be explained by hyperandrogenism. This poses a challenge in the diagnosis of PCOS. 24 On the other hand, serum progesterone level above 5 ng/mL (during days 21–24 of the cycle or the luteal phase of the menstrual cycle) may function as a confirmatory test for ovulation in women with PCOM or hirsutism. 25
Hyperandrogenism
Excessive serum androgen level is another salient feature of PCOS, as stated by the Rotterdam criteria. A significant proportion (about 60%–100%) of PCOS-afflicted women are likely to be suffering from hyperandrogenism (clinical and/or biochemical). 15 Hyperandrogenism in PCOS women may be assessed by their clinical signs or biochemical tests.
Clinical hyperandrogenism
Clinical hyperandrogenism observed in the form of hirsutism, acne, or alopecia usually represents low-to-average levels of androgen excess: 26
Hirsutism appears more frequently than the remaining two in about 80% of people with hyperandrogenism. It refers to the visually detectable growth of terminal hair (hair that can grow beyond 5 mm, if untroubled). 25 The extent of hirsutism is evaluated via the modified Ferriman–Gallwey (mFG) score. 27 This involves visual evaluation of the body’s nine areas (chin, chest, upper lips, upper arms, thighs, upper and lower abdomen, and back) with a score ranging from 0 to 4.28,29 The scores are then presented in the form of a graphical image. The state of hirsutism is conclusive if the total mFG score is ranged within ⩾4–6; this range was extended to a score of eight to adjust for variation in ethnicities.30,31
Comedonal acne is a widespread issue among women, especially adolescent girls. It was estimated that around 40% of the acne incidence could be traced back to a susceptible PCOS condition. 32 Although acne can be linked with biochemical hyperandrogenism, there is no specialized measuring tool to assess this condition as of now. 26
Alopecia is the least common feature among the three. Only 22% of women displaying male-like hair loss were discovered during PCOS diagnosis. 32 Many factors other than hyperandrogenemia may be associated with alopecia. The Ludwig scale is a measurement tool for hair loss around the scalp that rates the degree of hair loss from grades I to III upon visual assessment. 1
The subjective variability, racial/ethnic differences, and the existence of a condition named idiopathic hirsutism (hirsutism devoid of hyperandrogenism) play a significant role in determining the clinical signs of PCOS diagnosis and thus require more well-defined data. 33
Biochemical hyperandrogenism
When the clinical signs of hyperandrogenism are obscure, women are assessed for signs of biochemical hyperandrogenism. 12 This diagnostic criterion relies on one of the characteristic traits of PCOS involving elevated serum androgen levels. About 60%–80% of women with PCOS are known to exhibit features of biochemical hyperandrogenism. 6 According to the evidence-based recommendation, the condition can be detected through measurement of total testosterone (TT), free androgen index (FAI), calculated free testosterone (fT), and/or calculated bioavailable testosterone. 12 Previous data suggest that serum-free testosterone is the most sensitive parameter for detecting biochemical hyperandrogenism compared to the other parameters such as total testosterone, dehydroepiandrosterone sulfate (DHEAS), or androstenedione (A4). The latter two have been observed to be elevated in women with PCOS and are particularly useful when a high testosterone level is not detected. Furthermore, DHEAS and A4 facilitate the exclusion of other hyperandrogenic conditions.15,26 Then again, FAI is another indirect means of evaluating the free testosterone level; it is the ratio of the total testosterone and Sex Hormone Binding Globulin (SHBG) multiplied by 100. 34
Total circulating or free testosterone levels can be measured using high-quality assays like extraction/chromatography immunoassays, liquid chromatography-mass spectrometry (LCMS) or mass spectrometry. Other automated direct assays include enzyme-linked immune sorbent assay (ELISA), chemiluminescence assay (CLIA), and radioimmunoassay (RIA). However, these assays exhibit reduced sensitivity and therefore provide imprecise results.35–37 Furthermore, cut-off values vary widely within laboratories and with the method used. Normal thresholds may be derived from a healthy population of women. 12 To conclude, the lack of cut-off values based on evidence, the choice of androgen to test, and the consensus regarding the use of assessment techniques form the major areas of uncertainty in the evaluation of biochemical hyperandrogenism.
PCOM
PCOM is recognized as the most widely used feature in the diagnosis of PCOS. It was first introduced in the Rotterdam criteria as the third feature of PCOS in 2003. 11 However, this diagnostic criterion is debatable due to the lack of homogeneity in the results regarding its implementation, and its non-applicability for females above the gynecological age of 8 years. Therefore, it has been deemed a non-essential feature in the presence of the remaining two criteria. 15
The ovulation process is ceased due to follicular arrest in adult women with PCOS. The minute follicles resemble cyst-like structures on transvaginal ultrasonography.38–42 Initially, as authorized in the Rotterdam criteria, the cut-off value for identifying a polycystic morphology was a value of 12 or more Follicle Number Per Ovary (FNPO), measuring between the size of 2–9 mm or, an ovary with a volume of 10 cm3 on a transvaginal scan. 26 The FNPO value, a key parameter, was later updated in conformity with the technological advancements that enabled more magnified imaging. When using a high-resolution transducer frequency of 8 MHz or more, FNPO value of 20 or more of the same-sized follicles (2–9 mm) and an ovary volume of 10 cm3 for adult women was recommended by an international evidence-based PCOS guideline consensus held in 2018. 15 The ovarian volume plays a significant role when it is challenging to determine FNPO/Antral Follicle Count (AFC) due to technical complications in imaging, as it is in the case of transabdominal ultrasound. 26
Transvaginal ultrasound is the recommended approach for examining FNPO suggestive of a polycystic ovary. However, it is to be used only in sexually active women. 12 Automatic antral follicle count under 3D scan has shown more accurate results than the 2D grid system method; the information regarding this is still sparse and so is not yet recommended for routine purposes.43–49 Other ultrasound metrics include ovarian stroma and ovarian blood flow; studies concerning these parameters are minimal and hence no cut-off values are available for clinical use. 12
Anti-Mullerian hormone as an alternative diagnostic feature for PCOS
Considering the ambiguity about the efficacy of ultrasound as a diagnostic tool Anti-Mullerian Hormone (AMH) has been suggested as an alternative for detecting PCOM. 15 An upward trend of AMH levels in correlation with the number of antral follicles in PCOS was observed in women with PCOS. It is because AMH is produced by the granulosa cells in ovarian follicles (namely pre-antral and antral), which are elevated in PCOS.50–52 Despite its potential as a valuable diagnostic tool, AMH is still not authorized as a part of routine PCOS diagnosis owing to the inadequate standardization of cut-off values and heterogeneity among the studies 12,53.
Diagnostic challenges in adolescents
Much of the already existing gray areas in PCOS diagnosis for females of all ages can be attributed to its unspecified etiology heterogeneity, the lack of evidence-based cut-off values for the diagnostic features, and the unavailability of clearly defined, universal technology for the most accurate results. 54 Furthermore, a distinct set of diagnostic criteria for adolescents is essential since the existing guidelines mostly comply with features relevant to adults (cystic acne, irregular menstruation, and PCOM). Applying these guidelines for adolescents may lead to over- or under-diagnosis since the manifestation of these features in young girls is also a result of normal pubertal development stemming from an underdeveloped hypothalamic–pituitary–ovarian (HPO) axis. 55 At the outset, the adolescence period has been defined to be the age frame within 10 and 19 years by the World Health Organization. Alternatively, young women within the gynecological age of 8 years were also considered for PCOS studies aimed toward adolescents. 56
A conclusive PCOS condition cannot be diagnosed without the concurrent existence of both menstrual irregularity and hyperandrogenism in PCOS. It is also essential to acknowledge a state of “at-risk” category to be followed up by further age-specific diagnosis for PCOS as a means to avoid over or under-diagnosis of young women.56,57
Problems with defining ovulatory dysfunction in adolescence
Diagnosis of PCOS has been recommended to women with persistent irregular menstruation; however, defining irregular menstruation as reflective of ovulatory dysfunction in adolescents is much of a challenge in itself. 58 Definition of irregular menstruation following the gynecological age is tabulated in Figure 1. 56
Figure 1.
Definitions of irregular menstruation according to gynecologic age.
Irregular menstruation is common in young adults after menarche, which is common in the first 2 years, but it can extend as long as 5 years post-menarche. Therefore, the presence of this physiological event cannot be considered a prerequisite for PCOS diagnosis until 2 years succeeding menarche.58–61 However, continued oligomenorrhea even after the 2-year threshold following menarche indicates an “at-risk” status of PCOS in young adults. 55
Determining concurrent anovulation in adolescents is yet another challenge since about 85% of the cycles are known to be anovulatory in the first year following menarche, which shows a downward trend with the number of years post-menarche being 59% and 25% in the second and third year, respectively. 60 In addition, anovulation can be confirmed by serum progesterone level measurement as like in adults. 14 Finally, the variation in age of menarche among females further complicates the assessment and identification of ovulatory dysfunction. 62
Adolescent physiological aspects mimicking hyperandrogenic conditions
As mentioned previously, besides persistent irregular menstruation, androgen excess is a valuable indication of PCOS in adolescents, which may present itself as visible clinical signs (hirsutism, severe acne, and/or rarely alopecia) or elevated serum androgen level.
Clinical hyperandrogenism
Acne is a common condition in adolescents and therefore not a definitive diagnostic criterion for PCOS unless accompanied by other features. However, this may indicate hyperandrogenism if the degree of acne ranges from moderate to severe and is not responsive toward topical dermatologic therapy.58,63
Alopecia in adolescents is still not well understood
Although hirsutism has been linked with hyperandrogenism when in association with menstrual irregularity, 64 the presence of various confounding factors such as genetic and ethnic variations deem it to be a less prominent feature in diagnosing a hyperandrogenic status.29,65,66 Moreover, the modified Ferriman–Gallwey scoring system may not accurately assess the degree of hirsutism in adolescents since it was structured using data from a population of adult, particularly Caucasian women, which may not apply to women from other ethnicities.67–69
Biochemical hyperandrogenism
Assessment of biochemical hyperandrogenism has its own set of complications due to lack of standardization, technical difficulties, interference with other steroid hormones, and effect of SHBG on testosterone level, all of which are irrespective of a woman’s age. Despite the physiological impact of puberty leading to a rise in testosterone levels, the same determinants (free and/or total testosterone) are used to gauge androgen excess in adolescents. These methods are limited by the lack of specifically designed studies for adolescents and well-adjusted thresholds.
Controversies regarding PCOM in adolescents
According to the International guidelines on PCOM, transabdominal ultrasound is not recommended for use as a diagnostic criterion until at least 8 years post-menarche, mainly due to the high prevalence of characteristic follicular increase in young adults and an enlarged ovarian volume during this period.12,70–73 In addition, the implementation of adult thresholds adjusted for the transvaginal route may lead to over-diagnosis of PCOM in adolescents; hence, PCOM in adolescents is not a reliable diagnosis of PCOS. 74
Risks associated with PCOS
PCOS has been associated with the potential risk of cardiovascular and cerebrovascular events, type 2 diabetes mellitus (T2D), impaired glucose tolerance (IGT), pregnancy-linked complications, gestational diabetes, venous thromboembolism, and endometrial cancer. 1 Many of these metabolic and reproductive conditions stem out from an intrinsic feature of PCOS-insulin resistance (IR).75–77
Cardiometabolic events
Cardiometabolic impacts of PCOS described below are primarily linked with dysglycemia resulting from the insulin-resistant characteristic of PCOS. Insulin resistance or consequential hyperinsulinemia in turn is a state heavily influenced by the hyperandrogenic mechanism of the PCOS pathophysiology. 77 Despite the paucity of comparative studies concerning cardiovascular disease (CVD) risk factors with and without PCOS, it is noteworthy that the cardiometabolic conditions are prominent in PCOS, which pose a risk of developing CVDs. According to clinical consensus recommendation by the PCOS guideline group (2018), the manifestation of the cardiometabolic risk factors such as impaired glucose tolerance (IGT), dyslipidemia, hypertension, smoking, obesity, other metabolic syndromes, and a sedentary lifestyle in PCOS women allocates them in the vulnerable category. 15
IGT/T2D
According to past meta-analyses, the prevalence of IGT and T2D in PCOS-affected women has been observed to be independent of, but made worse with body mass index (BMI). It can be traced back to the correlation of PCOS with dysglycemia, which refers to the aberrant blood glucose level. Thus, estimation of the glycemic status of women with PCOS has been recommended by the International Evidence-based Guideline (2018) at a frequency of 1–3 years depending on the presence of other diabetic confounding factors. In addition to that, screening for T2D has been suggested by all consensus recommendations (Endocrine Society, International Evidence-based Guideline in Australia, as well as Androgen Excess and PCOS society).61,78 However, the screening method is still undecided among Oral Glucose Tolerance Test (OGTT), fasting glucose, and HbA1c test. 15
Dyslipidemia
Dyslipidemia is a recurring CVD risk factor identified in women with PCOS. A significant proportion of women (70%) with PCOS were known to exhibit dyslipidemia in a past report. 79 Furthermore, a meta-analysis of 30 studies demonstrated higher levels of lipids in women with PCOS (age < 45 years), particularly high-density lipoprotein (HDL), low-density lipoprotein (LDL), non-HDL-C, LDL-C, and triglyceride (TG). Moreover, TG and HDL-C were substantially higher in the obese stratum indicating a possible link of PCOS with obesity.80,81 Therefore, the latest guidelines suggest women of all ages are diagnosed to undertake a lipid profile. 12
Hypertension
The association between hypertension and PCOS is somewhat complex and influenced by many other factors. The inconsistency between the studies necessitates the requirement for more investigation.67,82–84 However, the recent international evidence-based guideline recommends annual blood pressure measurement considering the significance of hypertension in cardiovascular events. 12
Obesity
Despite its frequency in women with PCOS, there is surprisingly no solid evidence of their causal relationship. However, obesity has been linked to some of the severe PCOS manifestations, including CVD.85–87 Therefore, regular monitoring of body weight has been suggested by the most recent guideline. 12
Other risks
Sympathetic nervous system dysfunction, chronic inflammation, oxidative stress, and vitamin D deficiency are emerging risk factors of PCOS, paving the path for further research. 77
CVDs
The combined effect of a low number of studies and the relatively young population of women restricts the development of a concrete relationship between PCOS and CVD and therefore calling for more research. Nonetheless, the importance of screening for CVDs in PCOS women has been acknowledged. Quantitative research on the clinical manifestations of CVD is insufficient despite researches based on the sub-clinical CVD.15,77
Fertility-related complications in women with PCOS
PCOS comes with lifelong repercussions for women, as stated earlier. Gestational complications associated with PCOS are gradually being recognized, some of which include preeclampsia, gestational diabetes, pregnancy-induced hypertension, and even miscarriage. 88 It is estimated that much of the pregnancy-related inconveniences are partly ramifications of the already existing metabolic and endocrine effects of PCOS in women well ahead of pregnancy, such as hyperandrogenism and increased BMI. 89 Manifestation of gestational diabetes mellitus (GDM) in expecting women with relatively high weight was observed to be more alarming than their counterparts of lower weight. However, it is worth noting some of these complications are also influenced by other independent factors such as age, obesity, ongoing fertility treatment, and ethnicity. 88 Apart from this, several studies have also reported atypical newborn anthropometrics in children of women with PCOS.90–92
Although screening for GDM and hypertension in women with PCOS (pre-conception and antenatal) has been mentioned in the recommended guidelines, other avenues of pre-conception and antenatal screening for PCOS are still underdeveloped as they are not supported by enough evidence.15,88
Endometrial cancer
Malignancies associated with PCOS are rather indirect and stem from PCOS-induced infertility in women. Of these, endometrial cancer has been acknowledged to have an association with PCOS. 93 Although its occurrence is multifactorial and influenced by other morbidities (T2D, obesity, infertility, and the administered PCOS treatment methods), it has been shown to rise by 2–6 times in women with PCOS. This may be attributed to the anovulatory cycles where the endometrium is exposed to a continuous flux of estrogen.94–96 Despite the correlation between the two, routine screening for endometrial cancer has not yet been recommended. However, awareness on this issue is encouraged. 15
OSA
OSA is a chronic sleeping disorder accompanied by disruptive upper airway function and consequential hypoxia and erratic sleeping pattern. 97 It has also been linked with modified heart rate, sympathetic activity, and altered blood pressure, ultimately extending toward more severe outcomes such as CVD and hypertension.98–100 A positive correlation between women with PCOS and OSA was demonstrated through several systematic reviews and meta-analyses. 101 The evaluation of this condition is based on qualitative analysis and a screening tool involving the Berlin Questionnaire. As of present, treatment for OSA only involves the management of distinctive patient symptoms without therapeutic remedy of the linked metabolic diseases. 12
Etiology of PCOS
The current literature emphasizes the role of genetics in PCOS. Many genes have been said to directly or indirectly contribute to the progression of the disease. But to date, no penetrant gene has been identified. 102 Studies conducted in multiple families show low penetrance linked with hormonal/environmental factors or other co-variants. Many studies have suggested that PCOS is a polygenic, multifactorial disorder. Single genes, gene–gene interactions, and gene–environment interactions have been reported to pave the way for the development of the disease. 102 This part of the article will review the current genetic understanding of the disease and some of the environmental determinants explored later in the article.
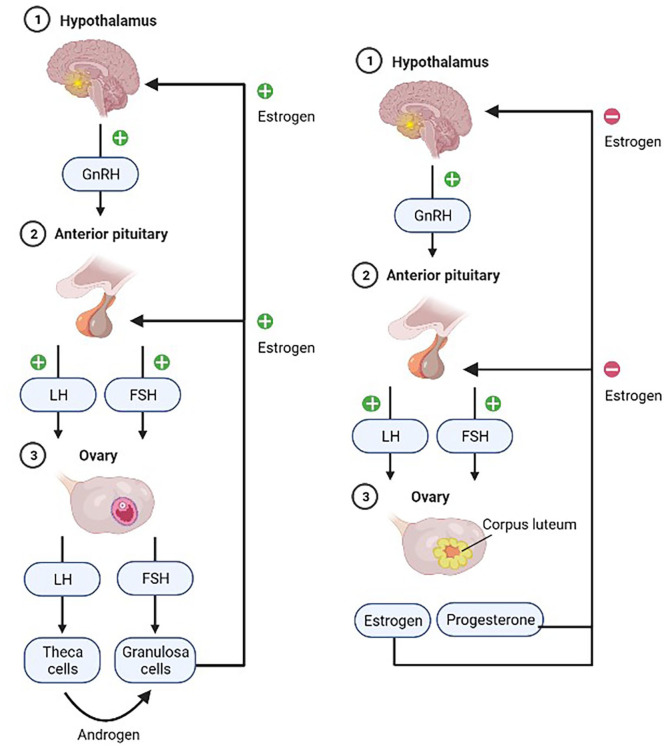
Understanding the roles of these genes better helps to look at some aspects of PCOS pathogenesis, the ovaries, and hormonal metabolism. The ovaries are the primary reproductive organ that releases eggs meant to be fertilized by sperms. They also produce estrogen and progesterone, which help regulate the monthly menstrual cycle, and tiny amounts of the male hormone testosterone, one of the five kinds of androgen. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are gonadotropin hormones released by the pituitary gland in response to gonadotropin-releasing hormone (GnRH) secretion by the hypothalamus. These two hormones control ovulation; FSH primarily stimulates the growth of follicles into proper eggs while LH triggers the release of these eggs. Their hormonal interplay in the body is illustrated in Figure 2. PCOS is a syndrome (or a group of symptoms) that interferes with ovaries and ovulation, in brief. PCOS predominantly has three features: irregular/missed periods, high androgen levels, and cysts, which are fluid-filled sacs in the ovaries. These sacs are essentially immature follicles that never see ovulation. Thus, the lack of ovulation disturbs the hormonal harmony in the body. On top of this, raised androgen levels disrupt the monthly cycles. The underlying justification behind upsetting hormonal balance has been pointed toward genetic alterations, environmental determinants, and epistatic changes.
Figure 2.
The hormonal cycle in the female body illustrated with the positive and negative feedback mechanisms. The diagram on the left shows a state prior to ovulation and the right after ovulation.
Biosynthesis of hormones in the ovary in brief
In a secondary follicle, thecal and granulosa cells work in conjunction to produce estrogen, progesterone, and testosterone. The process has been outlined in Figure 3. There are five types of androgens: dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), androstenedione, and testosterone. LH and FSH secreted by the pituitary gland activate these cells. The thecal cells express LH receptors for LH to bind. Granulosa cells, on the other hand, bind with FSH. When activated by LH, thecal cells increase their absorption of LDLs from the bloodstream. The cholesterol is then used in the synthesis of steroids, like progesterone. Progesterone is then enzymatically converted in a series of steps into androgens. Due to the lack of aromatase enzymes, thecal cells are unable to produce estrogen independently. Thus, the androgens diffuse into the blood and granulosa cells, where it successfully gets converted into estrogen via aromatase. This estrogen later enters the blood. The hypothalamus is stimulated in a positive feedback mechanism; consequently, the characteristic LH surge in the menstrual cycle is seen.
Figure 3.
The biosynthesis of androgen and estrogen inside the ovary.
The granulosa cells also have LH receptors but cannot pick up LDLs from the blood; LDLs cannot easily move past the basal membrane. When the follicle ruptures during ovulation, the membrane is destroyed enabling LDL absorption and progesterone production. However, the cells do lack the enzymes needed to convert progesterone into androgens. Thus, the majority of the progesterone diffuses into the blood, which explains the rapid rise in its level post-ovulation. After ovulation, both cells produce progesterone and, to a lesser extent, androgens.
Cholesterol is the precursor of all steroid hormones classified into three categories: glucocorticoids, mineralocorticoids, and gonadocorticoids (or sex hormones). Sex steroids are mainly androgens, estrogens, and progesterone. All steroid hormones are hydrophobic and require a protein carrier when transported in the blood. These are albumin, corticosteroid-binding globulin, and sex hormone–binding globulin. Cholesterol is a 27-carbon compound that undergoes a multi-step process and gets shortened and hydroxylated eventually. The series of conversions is shown briefly in Figure 4. These enzymes (e.g. cytochrome p450 members) are involved here that are targeted by studies; polymorphisms in their coding region can ultimately affect hormonal metabolism and lead to hyperandrogenism. The idea is that some defect in the hormonal pathway causes the classic characteristics of PCOS; these avenues are probable areas for research. Numerous studies on the relationship between gene polymorphisms and PCOS have been conducted. Some of the significant ones have been discussed later in the review.
Figure 4.
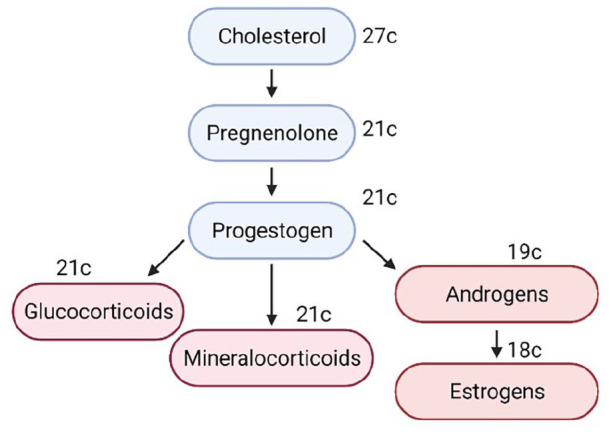
Summary of steroidogenesis with the end-products shown.
While ovaries are generally considered the primary source of androgens, the adrenal glands also contribute. Increased adrenal androgens (DHEA and DHEAS) are consistent with 20%–30% of the PCOS population 103 —a phenomenon called adrenal hyperandrogenism.
Hormonal association in PCOS
Hormones play a crucial role in the normal functioning of the ovary and the regulation of menstruation. If hormonal disturbances persist, the ovary’s function is interrupted, leading to the formation of a cyst inside of its sac. 104 PCOS patients exhibit an imbalance in levels of GnRH, FSH, LH, androgen, and prolactin. 105 The progression of PCOS and its severity rises with an increasing level of insulin and testosterone. Hyperinsulinemia is known to affect ovarian theca cells inflating androgen concentration. 106 Then again, elevated androgen levels trigger visceral adipose tissue (VAT), that is, responsible for the production of free fatty acids (FFA), which in turn elicits insulin resistance. 107 Due to insulin resistance and its consequent outcome of elevated insulin levels, androgen levels rise, which leads to anovulation. 7
To support the hormonal association with PCOS, a cross-sectional study in Pakistan examined healthy and affected women. Blood samples were drawn from individuals, and hormonal analysis was performed using immunoradiometric assay and radioimmunoassay. Their findings stated that FSH, LH, prolactin levels, and BMI were higher in PCOS cases. The current diagnosis of PCOS involves looking at FSH, LH, and androgen levels. 108 We know that raised LH levels result in higher androgen levels, which gives rise to PCOS, among other reasons.
The genetic connection
There are several genes involved in the etiology of PCOS. At present, there are three databases manually curated and published: PCOSKBR2 (2020), PCOSBase (2017), and PCOSDB (2016).109–111 PCOSDB had been inaccessible at the time of writing. The three databases are compared in Table 1.
Table 1.
A brief comparison of the three PCOS databases published so far.
| Attributes | PCOSKBR2 | PCOSBase | PCOSDB |
|---|---|---|---|
| 1. Official name | PolyCystic Ovary Syndrome KnowledgeBase | PolyCystic Ovary Syndrome Base | PolyCystic Ovary Syndrome Database |
| 2. Database URL | http://www.pcoskb.bicnirrh.res.in/ | http://pcosbase.org/ | http://www.pcosdb.net |
| 3. Overall content | 533 genes, 145 SNPs, 29 miRNAs, 1150 pathways, and 1237 PCOS-associated diseases Also included are more 4023 genes identified from microarray expression studies on PCOS |
8185 PCOS-related proteins, 7936 domains, 1004 pathways, 1928 PCOS-associated diseases, 29 disease classes, and 91 tissues | 208 genes, 427 molecular alterations including detailed annotations, 46 associated phenotypes |
PCOS: polycystic ovary syndrome; SNP: single nucleotide polymorphism.
As per PCOSKB R2 , 241 genes and 114 SNPs are closely involved in PCOS. 109 Changes like polymorphisms negatively affect the transcriptional activity of the gene, ultimately leading to PCOS. Now, the genes suitable for PCOS study are those that code for receptors of hormones like androgen, LH, FSH, and even, leptin. 112 Genes, namely, AR, CAPN10, FTO, follicle-stimulating hormone receptor (FSHR), cytochrome family P450, and insulin genes, have been widely discussed.
This review has discussed some genes commonly involved in ovarian and adrenal steroidogenesis, gonadotropin action and regulation, insulin action and secretion, and a few notables. Table 2 details the research on these groups of genes.
Table 2.
A rundown on the associations of different polymorphisms with PCOS found by most recent studies.
| SL. | Category | Gene | Year | Sample Size and ethnicity/location | Findings/Conclusion | Key methods | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Involved in ovarian and adrenal steroidogenesis | CYP11A | 2014 | 267 cases versus 275 controls “South Indian” |
Fifteen different alleles were identified with repeats ranging from 2 to 16. Repeats greater than 8 were thrice more likely to be susceptible to PCOS and have been found comparatively more in the patients. CYP11A1 (ttta)n repeat polymorphism is likely to be a potential molecular marker for PCOS diagnosis. |
DNA extracted from blood and genotyped by PCR-PAGE | Reddy et al. 113 | |
| 2 | 2014 | 1236 cases versus 1306 controls “Asian and Caucasian” Argentina, China, Greece, India, Spain, United Kingdom |
Positive association between CYP11A1 (tttta)n repeat polymorphism and PCOS. | Meta-analysis of nine studies published between 2000 and 2010 | Yu et al. 114 | |||
| 3 | 2014 | 1571 cases versus 1918 controls “Asian and Caucasian” China, India, Greece, Spain, Turkey, United Kingdom, United States |
CYP11A1 microsatellite (ttta)n repeat polymorphism (along with CYP1A1 MspI) showed significant association with PCOS risk in the Caucasian population. | Meta-analysis of 13 studies published between 2000 and 2010 | Shen et al. 115 | |||
| 4 | 2012 | 314 cases versus 314 controls “Chinese” |
SNP rs4077582 in CYP11A1 was found to be strongly associated with PCOS susceptibility. No association was observed in rs11632698. | PCR-RFLP | Assessed the association of SNPs rs4077582 and rs11632698 in CYP11A1 with PCOS | Zhang et al. 116 | ||
| 5 | CYP21 | 2013 | 197 patients | The study looked into 14 molecular defects of the CYP21A2 gene and concluded that its contribution to PCOS is unsubstantiated. | Allele-specific PCR | The study investigated the contribution of CYP21A2 heterozygous mutations to PCOS pathogenesis | Settas et al. 117 | |
| 6 | 2010 | 50 cases versus 60 controls “Italian” |
The data suggested a lack of association between CYP21 V281L polymorphism and PCOS. | PCR-RFLP | Pucci et al. 118 | |||
| 7 | 2005 | 114 cases versus 95 controls “Non-Hispanic White” |
CYP21 mutations were found to play a limited role in the development of PCOS. | Prospective case-control study | Witchel et al. 119 | |||
| 8 | 2000 | n < 50 | CYP21 V281L mutations seemingly manifested PCOS-like phenotype. | Witchel and Aston 120 | ||||
| 9 | CYP17 | 2021 | 394 cases versus 306 controls “Kashmiri” |
Mutant genotype is associated with hyperandrogenism. | PCR-RFLP | T/C polymorphism in 5′UTR of CYP17 gene was analyzed to find its connection to hyperandrogenism and PCOS | Ashraf et al. 121 | |
| 10 | 2021 | 204 cases versus 100 controls “Pakistani” |
rs743572 polymorphism is significantly associated with PCOS. | PCR-RFLP | 5′UTR region (MspA1) of CYP17 gene was analyzed | Munawar Lone et al. 122 | ||
| 11 | 2019 | 50 cases versus 109 controls “Kurdish” West Iran |
Data suggested a positive link of CYP17 T-34C polymorphism with PCOS risk. | PCR-RFLP; chemiluminescent method for hormone measurements | Rahimi and Mohammadi 123 | |||
| 12 | 2018 | 250 cases versus 250 controls North India |
Data suggested that − 34T > C polymorphism in CYP17A1 is associated with PCOS in North Indian females. | PCR-RFLP; lipid profile via biochemical analyzer | Kaur et al. 124 | |||
| 13 | 2012 | 287 cases versus 187 controls “Caucasian” |
The gene has been suggested as a non-major risk factor. | SNP genotyping and haplotype determination | Four SNPs of CYP17 analyzed | Chua et al. 125 | ||
| 14 | CYP19 | 2020 | 204 cases versus 100 controls “Pakistani” |
The polymorphism, rs2414096 (genotype GA) of the CYP 19 gene was considerably associated with PCOS vulnerability. | PCR-RFLP | The study looked at both CYP17 and CYP19 genes | Munawar Lone et al. 122 | |
| 15 | 2019 | 50 cases versus 109 controls “Kurdish” West Iran |
No statistical significance was found in the association of CYP19A1 with PCOS risk. | PCR-RFLP; chemiluminescent method for hormone measurements | Rahimi and Mohammadi 123 | |||
| 16 | 2018 | 250 cases versus 250 controls North India |
Variations of CYP19A1 were not statistically relevant with PCOS. | Kaur et al. 124 | ||||
| 17 | 2017 | 70 cases versus 70 controls Iran |
The study concluded that SNP rs2414096 in CYP19 is likely to play a role in developing PCOS in Iranian women. | PCR-RFLP; enzyme digestion with HSP92II | Mehdizadeh et al. 126 | |||
| 18 | 2014 | 62 cases versus 60 controls | Polymorphism of rs2414096 in CYP19 was found to be associated with the pathogenesis of PCOS. | PCR-RFLP; statistical analysis by SPSS | Hemimi et al. 127 | |||
| 19 | Involved in steroid hormone effects | AR | 2013 | 114 cases versus 1409 controls Diverse ethnicities |
CAG variants in the AR gene were unassociated with PCOS risk while they may be related to the T levels in PCOS. | Meta-analysis of 11 studies published between 2000 and 2012 | Zhang et al. 128 | |
| 20 | SHBG | 2020 | 1660 cases versus 1312 controls | rs6259 and rs727428 polymorphisms in SHBG are not associated with PCOS susceptibility. | Meta-analysis of seven studies published between 2007 and 2019 | Liao and Cao 129 | ||
| 21 | Involved in insulin secretion and action | CAPN10 | 2017 | 169 cases versus 169 controls South India |
No association of rs2975766 and rs7607759 with PCOS. | RT-PCR | The study looked at other gene polymorphisms too | Thangavelu et al. 130 |
| 22 | 2014 | 127 cases versus 150 controls Tunisia |
UCSNP-43 (rs3792267), UCSNP-19 (rs3842570), and UCSNP-63 (rs5030952) were investigated along with their haplotypes. No significant association, except one with obese PCOS subjects, was found. | PCR-RFLP | Ben Salem et al. 131 | |||
| 23 | 2014 | 668 cases versus 200 controls “Caucasian Greek” |
There was no correlation of CAPN 10 polymorphism (UCSNP-43) with the incidence of PCOS. Additionally, the gene polymorphism could not be associated with any biochemical, clinical, hormonal, or ovarian features of PCOS. | Primer extension; MALDI-TOF mass spectrometry | Anastasia et al. 132 | |||
| 24 | 2013 | 2123 cases versus 3612 controls | Nine common SNPs were examined. UCSNP 19/63/44 is likely to be associated with increased PCOS risk among Asians. No statistically significant association with UCSNP-22, UCSNP-43, UCSNP-45, UCSNP-56, UCSNP-58, and UCSNP-110 polymorphisms. |
Meta-analysis of 14 studies published between 2002 and 2013 | The review is comprehensive and helpful | Shen et al. 133 | ||
| 25 | 2009 | 88 cases “Brazilian” |
Data provide evidence that UCSNP-43 may play a role in PCOS while UCSNP-19 and UCSNP-63 remained unassociated with phenotypic traits in PCOS. | Wiltgen et al. 134 | ||||
| 26 | 2008 | 50 cases versus 70 controls “Chilean” |
Data suggests a contribution of UCSNP-43 polymorphism to PCOS in Chilean women. | PCR-RFLP | The study looked at UCSNP-19 and UCSNP-63 as well | Márquezet al. 135 | ||
| 27 | IRS | 2016 | 2975 cases versus 3011 controls “Asian and Caucasian” |
The findings suggested that IRS-1 Gly972Arg polymorphism be associated with PCOS in the Caucasian ethnicity, and IRS-2 Gly1057Asp polymorphism correlated with PCOS in the Asians. | A meta-analysis of 28 studies published between 2001 and 2014 | Shi et al. 136 | ||
| 28 | 2013 | 150 cases versus 175 controls Croatia |
Data did not support an association between Gly792Arg IRS-1 (along with VNTR INS, C/T INSR) polymorphisms and PCOS. Nor did it find any correlation with insulin resistance in Croatian women with PCOS. | Skrgatić et al. 137 | ||||
| 29 | INSR | 2016 | 2975 cases versus 3011 controls “Asian and Caucasian” |
The INSR polymorphism, His 1058 C/T, was not found to be associated with PCOS development. | A meta-analysis of 28 studies published between 2001 and 2014 | Shi et al. 136 | ||
| 30 | 2015 | 17,460 cases versus 23,845 controls | The meta-analytical data suggested no significant correlation between rs1799817/rs2059806 SNPs and PCOS susceptibility. On the other hand, it was concluded that rs2059807 could be a promising SNP involved in PCOS susceptibility. | A meta-analysis of 12 studies published between 1994 and 2013 | Feng et al. 138 | |||
| 31 | Involved in gonadotropin action and regulation | AMH | 2020 | 383 cases versus 433 controls “Chinese” |
Fifteen rare, but known AMH variants were identified, along with seven novel heterozygous variants. Researchers conclude that AMH can play a role in PCOS development. | Sanger sequencing | Qin et al. 139 | |
| 32 | 2019 | 608 case versus 142 controls | The AMH signaling cascade was deduced as a key player in PCOS etiology. Variants have been identified. | Targeted sequencing | Regions of AMH and AMHR2 were looked at | Gorsic et al. 140 | ||
| 33 | 2017 | 643 case versus 153 controls | Rare genetic variants of AMH related to PCOS were identified. | Targeted sequencing | Replication of whole-genome sequencing | Gorsic et al. 141 | ||
| 34 | 2017 | 2042 cases versus 1071 controls | Meta-analytical data showed no association of AMH (or AMHR2) with a heightened risk of PCOS. | A meta-analysis of five studies published between 2002 and 2013 | Wang et al. 142 | |||
| 35 | FSHR | 2021 | 130 cases Iran |
FSHR polymorphisms Ala307Thr and Asn680Ser were concluded to be statistically associated with PCOS women. | PCR followed by sequencing | Seyed Abutorabi et al. 143 | ||
| 36 | 2020 | 1882 cases versus 708 controls “Chinese” |
rs2300441 was found to be a primary contributor. | – | GWAS | Yan et al. 144 | ||
| 37 | 2018 | 93 cases versus 52 controls “Kurdish” Northern Iraq |
Significant differences were found in FSH and LH levels in PCOS patients with different genotypes of Ala307Thr polymorphism. No relationship was established between polymorphism and PCOS. |
PCR-RFLP; Eam1105I enzymatic digestion | Baban et al. 145 | |||
| 38 | 2017 | 377 women versus 388 controls “Korean” |
Findings suggested a significant association between FSHR gene polymorphisms (Thr307Ala or Asn680Ser) and PCOS. | RT-PCR | Kim et al. 146 | |||
| 39 | 2014 | 1760 cases versus 4521 controls “Asian and Caucasian” China, Italy, Korea, Netherlands, Turkey, United Kingdom |
Results showed no association between Thr307Ala and Asn680Ser polymorphisms of FSHR with PCOS susceptibility. | A meta-analysis of 10 studies published between 1999 and 2013 | Chen et al. 147 | |||
| 40 | 2014 | 215 cases versus 205 controls “Han Chinese” North China |
The Ala307Thr and Ser680Asn polymorphisms of FSHR are not related to PCOS in Han ethnic Chinese women. | PCR-RFLP; direct sequencing | Another study in North China with slightly more PCOS cases has found similar results 148 | Wu et al. 149 | ||
| 41 | LHCGR | 2015 | 203 cases versus 211 controls “Bahraini Arab” |
The first study suggested an association of LHCGR polymorphisms (rs7371084 and rs4953616) with PCOS. The study also added a strong association of FSHR (rs11692782). | RT-PCR | Almawi et al. 150 | ||
| 42 | Other genes | FTO | 2019 | 55 cases versus 110 controls “Srilankan” |
FTO gene rs9939609 polymorphism was significantly more common among PCOS subjects. | Allele-specific real-time quantitative PCR (AS-qPCR) | Branavan et al. 151 |
PCR: polymerase chain reaction; PCOS: polycystic ovary syndrome; SNP: single nucleotide polymorphism; GWAS: genome-wide association studies; PAGE: polyacrylamide gel electrophoresis; RFLP: restriction fragment length polymorphism; RT: reverse transcription.
Red text means a negative association or no association, the orange text implies a weak link to PCOS, and the green text indicates a strong correlation with PCOS.
Table 2 focuses on some of the recent and unique studies that have been carried out. The genes discussed are merely a fraction of the entire set of genes that are likely to play a role in PCOS pathogenesis. However, it begs the question of what makes a gene suitable or a target for investigation. For example, cytochromes P450 are a superfamily of enzymes that play a significant role in steroid conversion; it aids in converting androgen into estrogen. Any defect in this pathway will cease the conversion. 152 The human genome includes 18 CYP families, each having a sub-family of its own. 153 The CYP families are CYP1-8, CYP11, CYP17, CYP19-21, CYP24, CYP26-27, CYP39, CYP46, and CP51. The aromatase genes frequently reported in PCOS databases are CYP11A1, CYP11B2, CYP17A1, CYP19A1, CYP1A1, CYP21A2, and CYP3A7. 154 Thus, any abnormality in this gene family can potentially lead to PCOS. Aside from the studies condensed in the table, similar investigations have taken place earlier, most of which have been included in the meta-analytical reviews mentioned. Similarly, elevated androgen levels have been commonly seen in PCOS cases. Thus, the genes that are usually targeted are somehow connected with androgen, its receptor, or its metabolism. Any defect or polymorphism in their coding regions could lead to an explanation for the increased androgen levels. Examples of these genes are CYP1A1, CYP11A, CYP17, CAPN10, INSR, SHBG, and so on. The genes summarized in Table 3, when studied, will reveal some form of a connection that could potentially pave the way for PCOS pathogenesis. In this way, possible avenues for genes to be involved in the defective mechanism of PCOS are identified and assessed. Genetic links can be sought anywhere as long as it is relevant to PCOS; the genes that code for enzymes involved in the metabolism of different hormones, the genes responsible for insulin action, and so on. Insulin is a key player in androgen production by the theca cells. Like LH, a higher level of insulin enhances androgen synthesis.
Table 3.
A list of common endocrine-disrupting chemicals (EDCs) accompanied with their uses.
| Endocrine disruptor | Use |
|---|---|
| Bisphenol A (BPA) | Epoxy resins are found in many plastic products, including food storage containers. |
| Dioxins | A by-product of herbicide manufacture and paper bleaching, released during the burning of waste and wildfires. |
| Parabens | Cosmetics, personal care products. |
| Per- and polyfluoroalkyl chemicals (PFAS) | Non-stick cookware, waterproof clothing, food packaging. |
| Phthalates | Cosmetics, children’s toys, food packaging. |
| Polybrominated diphenyl ethers (PBDE) | Flame retardants. |
| Polychlorinated biphenyls (PCB) | Electrical equipment like transformers, hydraulic fluids, lubricants, etc. |
| Triclosan | Anti-microbial and personal care products. |
In conclusion, the databases mentioned earlier can be a useful starting point in finding susceptible genes. The pattern in studies that aim to establish an association between different gene polymorphisms and PCOS is that every study narrows its subjects to a specific geographical location or ethnicity, collects blood samples, and analyzes the DNA in whichever way is feasible. This process led to many individual studies with inconsistent findings; some suggest a genetic link to PCOS, while others disagree with the same conclusion. Numerous studies have been carried out to date to find novel polymorphisms or support existing data. Systematic reviews and meta-analyses come in very useful too.
In addition, strong emphasis on the outcomes of GWAS has been placed. However, in the end, all papers demand large-scale studies to be undertaken. Often these researches look at multiple genes together. Perhaps, other than looking at the genes and how they may correlate with PCOS or subjects’ biochemical profile, researchers can start looking at the environmental risk factors the subjects could have been exposed to simultaneously. PCOS is said to be a multifactorial, polygenic complex disease. To fully elucidate its etiology, all elements in play should be investigated as much as possible.
Environmental determinants of PCOS
We have already discussed the genetic susceptibility associated with PCOS. With this in mind, it is likely that the environment is an active player in the expression of PCOS-related genes.155,156 Some evidence already supports the fact that environmental toxins play a role in disrupting reproductive health. But the research linking these to the development of PCOS is very limited. Furthermore, these environmental risk factors can eventually trigger or aggravate PCOS pathology. 157 Therefore, this section of the review has briefly discussed the environmental determinants, especially the endocrine disruptors potentially involved in the etiology and modulation of PCOS.
Prenatal exposure
Individuals can be exposed to environmental risks during prenatal or postnatal periods of life. For example, intrauterine exposure to excess androgens/glucocorticoids at critical phases of fetal development may lead to PCOS symptoms and determine phenotypic expression in adulthood, according to experimental studies. 156 One way to explain this would be that intrauterine growth restriction (IUGR) can cause increased prenatal exposure of androgens and glucocorticoids, which could possibly induce PCOS programming in the fetus.156,158 Of course, it goes without saying that studies concerning prenatal exposure have their implementation-related difficulties.
Exposure after birth and in adulthood
Evidence to investigate potential prenatal risk factors for humans is lacking. However, an increasing amount of research is being carried out to study the effects of postnatal exposure. Obesity and low physical activity are harmful lifestyle factors that are targeted in disease management. Obesity has been found to exacerbate the metabolic and ovulatory dysfunction in PCOS.159,160 On the other side, weight loss restores ovulation and improves hyperandrogenism.161,162 Moreover, phenotypical variations between ethnicities suggest that cultural factors play a more substantial role in the metabolic consequences than previously thought.
One such postnatal exposure is environmental toxins. Environmental toxins are chemical pollutants present in the environment that enter living organisms via inhalation, ingestion, or absorption through skin/mucous membranes, ultimately having a detrimental impact on them. 163 An emerging body of evidence points to the lasting effects of environmental toxins on human reproductive health.164–166 Common pollutants include mercury, lead, pesticides, chlorofluorocarbons (CFCs), and so forth. Nevertheless, when it comes to PCOS, a specific group of chemicals known as endocrine-disrupting chemicals (EDCs) have gained particular interest and are the main focus in this review section. They have been proposed in their etiology as they can interfere with the hormone system. Some compounds have been described in Table 3 and are accompanied by examples of their uses in our daily lives. It has been estimated that of all synthetic chemicals, about 1000 of them are likely to exhibit endocrine-acting properties; 167 these compounds can be categorized under groups such as phthalates, xenoestrogens, and so forth. The compounds are a heterogenic group of molecules that interfere with steroid hormone synthesis and interact with hormone receptors. 168 As a consequence of their lipophilic structure, they tend to bio-accumulate in the adipose tissue. Thus, humans being at the end of the food chain become the most exposed to these toxins. Apart from adipose tissue, endocrine disruptors have been detected in amniotic fluid, 169 milk, 170 serum, 171 and urine. 172
BPA or Bisphenol A is an endocrine disruptor. BPA is produced globally in abundance; production exceeds 6 billion pounds every year. 173 It is a xenoestrogen, a chemical that mimics natural estrogen, owing to its phenolic structure, enabling it to bind to estrogen receptors (ER). Higher serum level of BPA has been found among PCOS women compared with non-PCOS women. 174 Animal studies show an association between neonatal BPA exposure and PCOS-like symptoms. 175 To make matters worse, BPA directly stimulates the synthesis of androgens in ovarian theca-interstitial cells. 176 Furthermore, a correlation between testosterone and BPA levels has been seen in the serum of women with PCOS. 174 Data from rat studies have shown that BPA can increase testosterone production in theca-interstitial cells and decrease estradiol formation in granulosa cells. These effects can be explained via some form of upregulation induced by BPA on the key genes involved in ovarian steroidogenesis—CYP17A1, CYP11A1 177 and downregulation of CYP19A1. 178 BPA is also known to interact with sex hormone–binding globulin, whose gene is another PCOS candidate gene. 179
A study in 2019 focusing on adolescents looked at BPA levels in 62 girls with PCOS and 33 control subjects in the age range, 12–18 years. 180 High-performance liquid chromatography was employed to measure urinary BPA concentrations. The adolescent patients demonstrated markedly greater BPA levels (case: 15.89 μg/g versus control: 7.30 μg/g creatinine). A similar study using serum BPA from adolescents has found similar results too. 181 On the contrary, a study focusing on several EDCs failed to establish an association of urinary BPA level with PCOS in relatively older women (18–45 years). 182 There are many other studies available linking specific groups of EDC with PCOS. For instance, two case-control studies delved into finding the correlation between the concentrations of EDCs and PCOS.182,183 Both studies revealed a significantly higher serum level of per-fluorinated compounds in PCOS women as compared to control subjects. It is to be noted no causal relationship was proven. Similar to the genetic links, the ultimate findings of the studies are inconsistent. Although it is true EDCs negatively impact the reproductive health of humans, the mechanism as to how these chemicals upset the hormonal balance or interfere with their receptors is yet to be elucidated. A few common ones are listed in Table 3.167,184,185
Treatment options for PCOS
The remedy for this endocrinopathy has been on the lookout ever since its gravity was recognized. There is no permanent cure for this particular disorder, so far. Treatment is adjusted as per an individual’s need to tackle the symptoms and allow the patient to live a less cumbersome life. Below are listed some possible medications suggested for PCOS patients.
Current therapies
Metformin
For decades, metformin has been used to induce ovulation and fight against insulin resistance, a salient feature detected in women battling PCOS. Metformin belongs to a class of drugs called biguanide and is mostly prescribed to individuals with T2D. Through various experimental evaluations conducted over the years, metformin has proven to be beneficial by increasing the overall glucose uptake in the body, leading to improved insulin sensitivity, reduction in serum androgen level, and proper regulation of the menstrual cycle.186,187. However, its mode of action remains unclear when used exclusively or in combination with other drugs while handling complications like infertility or the clinical live birth rate. According to a cohort study conducted by a hospital in Riyadh, Saudi Arabia, it was notified that metformin did not demonstrate much significant result when it is used as a co-treatment for improving the pregnancy rate in women seeking in vitro fertilization (IVF). 188 However, based on another systematic analysis conducted, 189 metformin was found to be successful in eradicating the risk of ovarian hyperstimulation syndrome for a pregnant woman. Still, nonetheless, it had no association with the clinical live birth rate. On the other hand, Sun et al. 190 reported that linking metformin with drugs like clomiphene citrate was better at reforming infertility and ovulation rate, but at the same time, Kar 191 found no difference in their combined effect. All these evidences imply that the potency of this drug is debatable and requires thoughtful systematic analysis to procure better results.
Spironolactone
The most notable effects of spironolactone involve the reduction of androgen level, improvement of hirsutism, and acne when used as a form of treatment. 192 An aldosterone antagonist predominantly used as a diuretic, 193 the basic concept behind its complex mode of action involves blocking androgenic receptors, partly obstructing adrenal steroidogenesis, and blocking 5α reductase, thereby increasing the level of SHBG (sex hormone–binding globulin) protein. 194 Unlike metformin, the dosage needs to be considered and supplemented precisely to avoid health issues; this is why spironolactone is suggested to be taken with an oral contraceptive while avoiding pregnancy so as not to promote complications like feminization of male fetus.194,195 Other side effects found to be involved with high doses were hypokalemia and menstrual disturbances. 196 According to a study conducted by C Sabbadin et al. 194 regarding the effects of spironolactone on estradiol levels and intermenstrual bleeding on 30 individuals with normal BMI, it was concluded that certain individuals did exhibit intermenstrual bleeding as a form of side effect due to lower estradiol levels and endometrial thickness. But then again, it was also detected that those individuals who displayed this effect had pre-treatment estradiol values lesser than those who did not face this issue. For this reason, this particular proposition requires further assessment in order to provide confirmatory evidence. Based on another study conducted by Zulian et al., 197 this drug significantly improved glucose and metabolic lipid profile when experimented on 25 PCOS patients over 12 months. Therefore, this medication still continues to be prescribed when it comes down to tackling hormonal imbalance and disease management.
Some of the other commonly prescribed drugs are listed in Table 4 concerning the metabolic problems they target, their dosages, and their side effects.
Table 4.
Treatment options for various symptoms listed along with the administration dosage and side effects.
| Metabolic syndrome | Treatment | Dosage | Side effect | Ref. |
|---|---|---|---|---|
| Insulin resistance | Metformin | 500 mg (starting) Over the course of 1–2 weeks |
Nausea, bloating | Lashen 198 |
| Menstrual irregularity | Oral contraceptive pill | 20–35µg | Weight gain | Nader 199 , Domecq et al. 200 |
| Hirsutism | Cyproterone acetate | 50–100 mg (alone) Ethinylestradiol (combined) 20–50 µg |
Headaches, breast tenderness | Nader 199 |
| Infertility | Clomiphene citrate | 50–150 mg for 5 days | Nausea, mood swings | Nader 199 , Practice Committee of the American Society for Reproductive Medicine 201 |
| Hyperandrogenism | Spironolactone, flutamide | Spironolactone 100 mg Flutamide 500 mg + OCP |
Spironolactone: Fatigue, menstrual irregularity (in high dosages) Flutamide: Hepatotoxicity (if not maintained properly) |
Nader 199 , Domecq et al. 200 |
Newly emerging therapies
There are various therapies in consideration with the hopes of being implemented in the near future.
Statins
Statin is an inhibitor and performs its function by impeding 3-hydroxy-3-methylglutaryl coenzyme reductase (HMG-CoA), a rate-determining enzyme involved in cholesterol synthesis, thereby halting its conversion to mevalonate. Atorvastatin specifically has shown significant outcomes when it comes to the reduction of insulin resistance and hyperandrogenism in a 12-week study. The study showed increased levels of vitamin D in PCOS women when compared to control women; headaches were seen as side effects. However, another study conducted in 2013 validated that while atorvastatin improved lipid profile and chronic inflammation, it did not do much in the case of insulin sensitivity. Despite its promising result, more clinical studies need to be conducted in order to determine its efficacy.202,203
Surgical method
Procedures like ovarian drilling and bariatric surgery have been considered for treating PCOS despite the risks of reduced ovarian cells and decreased fertility these methods carry. 193 Even though laparoscopic ovary drilling has improved ovulation and reduced androgens, various clinical trials conducted over the years were somewhat inconsistent. They failed to provide concrete evidence to sustain it as a form of treatment. On the other hand, bariatric surgery does promote excessive weight loss for obese individuals leading to improved ovulation and reduced risk of T2D.204,205
Inositol
The most commonly described forms of inositol are myo-inositol and d-chiro-inositol. These sugar molecules function as a messenger that mostly up-regulates glucose intake and synthesis. Myo-inositol has been used as a supplement in PCOS women and has proven to improve insulin sensitivity and menstrual cycles and cause lesser gastric issues than metformin. However, a Cochrane Systematic Review could not display any promising role of inositol when tested on 1472 sub-fertile PCOS females.202,203,205
Vitamin D
Few studies are backing up the fact that vitamin D deficiency plays a role in insulin resistance, thereby being connected to the pathogenesis of PCOS. When a dosage was given, it did ameliorate insulin resistance; 195 nonetheless, its role remains contradictory based on many RCTs (randomized controlled trials) conducted previously. For example, according to a double-blind, randomized, placebo-controlled trial executed by Trummer et al. 206 at a medical university in Austria, no significant results were achieved in attesting the effects of vitamin D on different metabolic parameters. While on the other hand, a similar trial conducted by Gupta et al. 207 showed vitamin D supplements enhance the menstrual cycle, ovulatory dysfunction, and fasting blood sugar level.
Gut microbiota
There have been widespread speculations regarding the alterations in gut microbiota being related to the metabolic syndromes of PCOS or vice versa. There are some key features that have been reported to provide a better understanding of this hypothesized linkage.
Studies have come forward with contradictory results regarding the imbalance between gut microbial diversities (α, β) caused by gut microbiota partaking in the regulation of sex hormones leading to its dysbiosis or circulating sex hormones alone alters the gut microbiota.
Other than being observed in obese patients, PCOS also gives rise to the aspect of gene encoding pro-inflammatory cytokines like TNF-α (tumor necrosis factor) and IL-6 (interleukin 6) being associated with the triggering of the immune system and causing intestinal permeability has been pinpointed, that leads to the elevation of LPS (lipopolysaccharide) which ends up causing inflammation by pooling the endotoxin in the blood circulation hindering the metabolic system ultimately leading to ovarian inflammation too. This particular theory has been related to the imbalance of microbial composition. 208
Probiotics stood out and were chosen as a form of treatment to deal with gut microbial-related issues based on the benefits it comes along with. It has displayed anti-inflammatory activities and healthy regulation of gut flora. Based on randomized trials conducted, it has also been shown to alleviate insulin resistance overall, keeping the lipid profile balanced. According to a study initiated by Jing Xue et al., 209 the efficacy of inulin (probiotic) and metformin was assessed by targeting gut microbiota in PCOS mice. The result did provide promising evidence when it came down to reducing pro-inflammatory cytokines through a surge of anti-inflammatory cytokines, in turn decreasing ovarian inflammation.208,209
Conclusion
PCOS looks different for everyone to some extent. It is currently incurable and continues way beyond the child-bearing age or post-menopause. Research points toward substantial genetic implications, but the dots are yet to be connected to give us the complete picture. It is likely that once etiological grounds are explicated, the diagnostics, treatment, and disease management will be subject to change dramatically. It had come a long way since 1935, when American gynecologists Irvin F Stein, Sr and Michael L Leventhal first officially described it. With advancements in diagnostic technology, it has been easier to treat patients, yet genetic, ethnic, and so on, variations pose difficulty in instituting universal “laws” of the disease. In spite of progress, accounts of dissatisfaction related to diagnosis are still significant. The gaps in the field of PCOS diagnosis can be attributed to a plethora of causes, including the heterogeneity of the condition itself, discrepant use of the diagnostic criteria and tools, vagueness in the assessment of the salient features, and due to lack of clarity in adolescent diagnosis. Knowledge around these discrepancies and a multidisciplinary intervention must be adapted to reduce delayed and poor PCOS diagnosis in women. It is also worth mentioning that all the guidelines developed to date are predominantly based upon consensus veteran opinion, clearly indicating the need to generate evidence-based data. The development of PCOS has a strong genetic component. Results from twin studies and familial clustering ground a strong genetic basis for PCOS; having a mother or a sister with PCOS increases the risk of developing PCOS by 30%–50%. Following the analysis of the studies conducted on different gene polymorphisms, it can be said that some of these polymorphisms could potentially serve as biomarkers for the diagnosis and prognosis of PCOS. Given that many studies link genes with PCOS pathogenesis, it is essential that this research be extrapolated into projects with much larger sample sizes. PCOS phenotype does vary with ethnicity, geographical region, and probably socioeconomic status. Thus, this would further add to the complex polygenic nature of PCOS. The importance of GWAS is already established in discovering a new locus for susceptibility. The next step would be to link the genetic factors in question with other determinants and the patient history to establish whether PCOS is indeed a polygenic disease and what genes can truly be called key triggers.
Researchers are continually trying to dig deep to get to the root cause behind this complex metabolic syndrome. With new discoveries flow in tougher challenges, and it all comes down to selecting an effective solution through various trials and errors. The therapies mentioned above do give assuring outcomes, but large-scale, properly structured and funded trials/studies need to be carried out so that this particular disorder can be demystified even more while taking contributive factors like ethnicity, environmental exposures, familial history, and more into account. Keeping the diverse outcomes of PCOS in mind, it can be suggested that a team comprising of a physician, a gynecologist, an endocrinologist, and a reproductive medicine specialist will help these patients manage their lives much better.
Footnotes
ORCID iD: Fahim Kabir Monjurul Haque  https://orcid.org/0000-0001-6347-1836
https://orcid.org/0000-0001-6347-1836
Declarations
Ethics approval and consent to participate: An ethics statement is not applicable because this study is a review and is based exclusively on published literature.
Consent for publication: Not applicable.
Author contribution(s): Hiya Islam: Conceptualization; Writing – original draft; Writing – review & editing.
Jaasia Masud: Formal analysis; Writing – original draft.
Yushe Nazrul Islam: Formal analysis; Writing – original draft.
Fahim Kabir Monjurul Haque: Conceptualization; Supervision; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
Guarantor: None.
References
- 1. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057. [DOI] [PubMed] [Google Scholar]
- 2. Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected Black and White women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998; 83(9): 3078–3082. [DOI] [PubMed] [Google Scholar]
- 3. Dokras A, Saini S, Gibson-Helm M, et al. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril 2017; 107(6): 1380–1386. [DOI] [PubMed] [Google Scholar]
- 4. Witchel SF, Teede HJ, Peña AS. Curtailing PCOS. Pediatr Res 2020; 87(2): 353–361. [DOI] [PubMed] [Google Scholar]
- 5. Azziz R, Marin C, Hoq L, et al. Healthcare-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab 2005; 90(8): 4650–4658. [DOI] [PubMed] [Google Scholar]
- 6. Azziz R, Carmina E, Dewailly D, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab 2006; 91(11): 4237–4245. [DOI] [PubMed] [Google Scholar]
- 7. Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine 2006; 30(1): 19–26. [DOI] [PubMed] [Google Scholar]
- 8. March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25(2): 544–551. [DOI] [PubMed] [Google Scholar]
- 9. Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016; 31(12): 2841–2855. [DOI] [PubMed] [Google Scholar]
- 10. Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome (PCOS): towards a rational approach. In: Dunaif A, Givens JR, Haseltine F. (eds) Current issues in endocrinology and metabolism: polycystic ovary syndrome. Boston, MA: Blackwell, 1992, pp. 377–384. [Google Scholar]
- 11. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81(1): 19–25. [DOI] [PubMed] [Google Scholar]
- 12. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; 33(9): 1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018; 14(5): 270–284. [DOI] [PubMed] [Google Scholar]
- 14. Nicolaides NC, Matheou A, Vlachou F, et al. Polycystic ovarian syndrome in adolescents: from diagnostic criteria to therapeutic management. Acta Bio Medica: Atenei Parmensis 2020; 91(3): e2020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monash University. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018, 2018, https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence-Based-Guidelines_20181009.pdf [Google Scholar]
- 16. Wang R, Mol BWJ. The Rotterdam criteria for polycystic ovary syndrome: evidence-based criteria? Hum Reprod 2017; 32(2): 261–264. [DOI] [PubMed] [Google Scholar]
- 17. Kyritsi EM, Dimitriadis GK, Kyrou I, et al. PCOS remains a diagnosis of exclusion: a concise review of key endocrinopathies to exclude. Clin Endocrinol 2017; 86(1): 1–6. [DOI] [PubMed] [Google Scholar]
- 18. Lane DE. Polycystic ovary syndrome and its differential diagnosis. Obst Gynecol Surv 2006; 61(2): 125–135. [DOI] [PubMed] [Google Scholar]
- 19. Shimon I. Screening for Cushing’s syndrome: is it worthwhile. Pituitary 2015; 18(2): 201–205. [DOI] [PubMed] [Google Scholar]
- 20. Apter D, Vihko R. Serum pregnenolone, progesterone, 17-hydroxyprogesterone, testosterone and 5α-dihydrotestosterone during female puberty. J Clin Endocrinol Metab 1977; 45(5): 1039–1048. [DOI] [PubMed] [Google Scholar]
- 21. Lemarchand-Béraud T, Zufferey M-M, Reymond M. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab 1982; 54(2): 241–246. [DOI] [PubMed] [Google Scholar]
- 22. Hamilton-Fairley D, Taylor A. ABC of subfertility: anovulation. BMJ: Br Med J 2003; 327(7414): 546–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women. Obstet Gynecol 2003; 102(2): 317–318. [DOI] [PubMed] [Google Scholar]
- 24. Balen AH, Conway GS, Kaltsas G, et al. Andrology: polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reproduction 1995; 10(8): 2107–2111. [DOI] [PubMed] [Google Scholar]
- 25. Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009; 91(2): 456–488. [DOI] [PubMed] [Google Scholar]
- 26. Rao P, Bhide P. Controversies in the diagnosis of polycystic ovary syndrome. Ther Adv Reprod Health 2020; 14: 13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neven ACH, Laven J, Teede HJ, et al. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med 2018; 36: 5–12. [DOI] [PubMed] [Google Scholar]
- 28. Ferriman D, Gallwey J. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21(11): 1440–1447. [DOI] [PubMed] [Google Scholar]
- 29. Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010; 16(1): 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2014; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lumezi BG, Berisha VL, Pupovci HL, et al. Grading of hirsutism based on the Ferriman-Gallwey scoring system in Kosovar women. Postepy Dermatol Alergol 2018; 35(6): 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lauritsen M, Bentzen J, Pinborg A, et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Müllerian hormone. Hum Reprod 2014; 29(4): 791–801. [DOI] [PubMed] [Google Scholar]
- 33. Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2011; 18(2): 146–170. [DOI] [PubMed] [Google Scholar]
- 34. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10): 3666–3672. [DOI] [PubMed] [Google Scholar]
- 35. Endocrinologists AAoC. Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Endoc Pract 2015; 21(1): 1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keevil BG. How do we measure hyperandrogenemia in patients with PCOS? J Clin Endocrinol Metab 2014; 99: 777–779. [DOI] [PubMed] [Google Scholar]
- 37. Rosner W, Auchus RJ, Azziz R, et al. Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007; 92(2): 405–413. [DOI] [PubMed] [Google Scholar]
- 38. Dewailly D, Catteau-Jonard S, Reyss AC, et al. The excess in 2–5 mm follicles seen at ovarian ultrasonography is tightly associated to the follicular arrest of the polycystic ovary syndrome. Hum Reprod 2007; 22(6): 1562–1566. [DOI] [PubMed] [Google Scholar]
- 39. Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update 2004; 10(2): 107–117. [DOI] [PubMed] [Google Scholar]
- 40. Li J, Li R, Yu H, et al. The relationship between serum anti-Müllerian hormone levels and the follicular arrest for women with polycystic ovary syndrome. Syst Biol Reprod Med 2015; 61(2): 103–109. [DOI] [PubMed] [Google Scholar]
- 41. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 2003; 88(12): 5957–5962. [DOI] [PubMed] [Google Scholar]
- 42. Welt CK, Taylor AE, Fox J, et al. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab 2005; 90(10): 5582–5587. [DOI] [PubMed] [Google Scholar]
- 43. Allemand MC, Tummon IS, Phy JL, et al. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril 2006; 85(1): 214–219. [DOI] [PubMed] [Google Scholar]
- 44. Ng EH, Chan CC, Ho PC. Are there differences in ultrasound parameters between Chinese women with polycystic ovaries only and with polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol 2006; 125(1): 92–98. [DOI] [PubMed] [Google Scholar]
- 45. Lam PM, Johnson IR, Raine-Fenning NJ. Three-dimensional ultrasound features of the polycystic ovary and the effect of different phenotypic expressions on these parameters. Hum Reprod 2007; 22(12): 3116–3123. [DOI] [PubMed] [Google Scholar]
- 46. Lam P, Raine-Fenning N, Cheung L, et al. Three-dimensional ultrasound features of the polycystic ovary in Chinese women. Ultrasound Obstet Gynecol 2009; 34(2): 196–200. [DOI] [PubMed] [Google Scholar]
- 47. Sun L, Fu Q. Three-dimensional transrectal ultrasonography in adolescent patients with polycystic ovarian syndrome. Int J Gynaecol Obstet 2007; 98(1): 34–38. [DOI] [PubMed] [Google Scholar]
- 48. Pascual MA, Graupera B, Hereter L, et al. Assessment of ovarian vascularization in the polycystic ovary by three-dimensional power Doppler ultrasonography. Gynecol Endocrinol 2008; 24(11): 631–636. [DOI] [PubMed] [Google Scholar]
- 49. Battaglia C, Battaglia B, Morotti E, et al. Two-and three-dimensional sonographic and color Doppler techniques for diagnosis of polycystic ovary syndrome: the stromal/ovarian volume ratio as a new diagnostic criterion. J Ultrasound Med 2012; 31(7): 1015–1024. [DOI] [PubMed] [Google Scholar]
- 50. Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod 2011; 26(11): 3123–3129. [DOI] [PubMed] [Google Scholar]
- 51. Eilertsen TB, Vanky E, Carlsen SM. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced. Hum Reprod 2012; 27(8): 2494–2502. [DOI] [PubMed] [Google Scholar]
- 52. Iliodromiti S, Kelsey TW, Anderson RA, et al. Can anti-Müllerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 2013; 98(8): 3332–3340. [DOI] [PubMed] [Google Scholar]
- 53. Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2014; 20(3): 334–352. [DOI] [PubMed] [Google Scholar]
- 54. Azziz R. Polycystic ovary syndrome. Obstet Gynecol 2018; 132(2): 321–336. [DOI] [PubMed] [Google Scholar]
- 55. Evans A, Hoeger KM. PCOS in adolescence: towards a better diagnosis and treatment. Curr Opin Endoc Metab Res 2020; 12: 105–111. [Google Scholar]
- 56. Peña AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020; 18: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102(2): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Witchel SF, Oberfield S, mar RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015; 83(6): 376–389. [DOI] [PubMed] [Google Scholar]
- 59. Metcalf MG, Skidmore DS, Lowry GF, et al. Incidence of ovulation in the years after the menarche. J Endocrinol 1983; 97(2): 213–219. [DOI] [PubMed] [Google Scholar]
- 60. Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metab 1998; 9(2): 58–61. [DOI] [PubMed] [Google Scholar]
- 61. Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013; 98(12): 4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carroll J, Saxena R, Welt CK. Environmental and genetic factors influence age at menarche in women with polycystic ovary syndrome. J Pediatr Endocrinol Metab 2012; 25(5–6): 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010; 203(3): 201.e1–201.e5. [DOI] [PubMed] [Google Scholar]
- 64. Merino PM, Codner E, Cassorla F. A rational approach to the diagnosis of polycystic ovarian syndrome during adolescence. Arq Bras Endocrinol Metabol 2011; 55(8): 590–598. [DOI] [PubMed] [Google Scholar]
- 65. Engmann L, Jin S, Sun F, et al. Racial and ethnic differences in the polycystic ovary syndrome metabolic phenotype. Am J Obstet Gynecol 2017; 216(5): 493.e1–493.e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li R, Qiao J, Yang D, et al. Epidemiology of hirsutism among women of reproductive age in the community: a simplified scoring system. Eur J Obstet Gynecol Reprod Biol 2012; 163(2): 165–169. [DOI] [PubMed] [Google Scholar]
- 67. Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 1. Endocr Pract 2015; 21(11): 1291–1300. [DOI] [PubMed] [Google Scholar]
- 68. Biro FM, Emans SJ. Whither PCOS? The challenges of establishing hyperandrogenism in adolescent girls. J Adolesc Health 2008; 43(2): 103–105. [DOI] [PubMed] [Google Scholar]
- 69. Akgül S, Düzçeker Y, Kanbur N, et al. Do different diagnostic criteria impact polycystic ovary syndrome diagnosis for adolescents. J Pediatr Adolesc Gynecol 2018; 31(3): 258–262. [DOI] [PubMed] [Google Scholar]
- 70. Codner E, Villarroel C, Eyzaguirre FC, et al. Polycystic ovarian morphology in postmenarchal adolescents. Fertil Steril 2011; 95(2): 702–706. [DOI] [PubMed] [Google Scholar]
- 71. Ersen A, Onal H, Yildirim D, et al. Ovarian and uterine ultrasonography and relation to puberty in healthy girls between 6 and 16 years in the Turkish population: a cross-sectional study. J Pediatr Endocrinol Metab 2012; 25(5-6): 447–451. [DOI] [PubMed] [Google Scholar]
- 72. Hagen CP, Mouritsen A, Mieritz MG, et al. Circulating AMH reflects ovarian morphology by magnetic resonance imaging and 3D ultrasound in 121 healthy girls. J Clin Endocrinol Metab 2015; 100(3): 880–890. [DOI] [PubMed] [Google Scholar]
- 73. Kelsey TW, Dodwell SK, Wilkinson AG, et al. Ovarian volume throughout life: a validated normative model. PLoS ONE 2013; 8(9): e71465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ibáñez L, Oberfield SE, Witchel S, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr 2017; 88(6): 371–395. [DOI] [PubMed] [Google Scholar]
- 75. Cassar S, Misso ML, Hopkins WG, et al. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic–hyperinsulinaemic clamp studies. Hum Reprod 2016; 31(11): 2619–2631. [DOI] [PubMed] [Google Scholar]
- 76. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum Reprod 2013; 28(3): 777–784. [DOI] [PubMed] [Google Scholar]
- 77. Kakoly NS, Moran LJ, Teede HJ, et al. Cardiometabolic risks in PCOS: a review of the current state of knowledge. Expert Rev Endocrinol Metab 2019; 14(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 78. Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010; 95(5): 2038–2049. [DOI] [PubMed] [Google Scholar]
- 79. Detection NCEPEPo and Adults ToHBCi. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): The Program, 2002, https://www.ahajournals.org/doi/10.1161/circ.106.25.3143 [PubMed] [Google Scholar]
- 80. Wild RA, Rizzo M, Clifton S, et al. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 2011; 95(3): 1073.e11–1079.e. [DOI] [PubMed] [Google Scholar]
- 81. Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril 2018; 110(5): 794–809. [DOI] [PubMed] [Google Scholar]
- 82. Meyer C, McGrath BP, Cameron J, et al. Vascular dysfunction and metabolic parameters in polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90(8): 4630–4635. [DOI] [PubMed] [Google Scholar]
- 83. Zimmermann S, Phillips RA, Dunaif A, et al. Polycystic ovary syndrome: lack of hypertension despite profound insulin resistance. J Clin Endocrinol Metab 1992; 75(2): 508–513. [DOI] [PubMed] [Google Scholar]
- 84. Elting MW, Korsen TJ, Bezemer PD, et al. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod 2001; 16(3): 556–560. [DOI] [PubMed] [Google Scholar]
- 85. Legro RS. Obesity PCOS: implications for diagnosis treatment. Semin Reprod Med 2012; 30: 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moran LJ, Misso ML, Wild RA, et al. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010; 16(4): 347–363. [DOI] [PubMed] [Google Scholar]
- 87. Karabulut A, Yaylali GF, Demirlenk S, et al. Evaluation of body fat distribution in PCOS and its association with carotid atherosclerosis and insulin resistance. Gynecol Endocrinol 2012; 28(2): 111–114. [DOI] [PubMed] [Google Scholar]
- 88. Vanky E, Løvvik TS. Polycystic ovary syndrome and pregnancy: from a clinical perspective. Curr Opin Endoc Metab Res 2020; 12: 8–13. [Google Scholar]
- 89. Glintborg D, Hass Rubin K, Nybo M, et al. Morbidity and medicine prescriptions in a nationwide Danish population of patients diagnosed with polycystic ovary syndrome. Eur J Endocrinol 2015; 172(5): 627–638. [DOI] [PubMed] [Google Scholar]
- 90. Qin JZ, Pang LH, Li MJ, et al. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013; 11(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yu HF, Chen HS, Rao DP, et al. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine 2016; 95(51): e4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hjorth-Hansen A, Salvesen Engen Hanem ØLG, Eggebø T, et al. Fetal growth and birth anthropometrics in metformin-exposed offspring born to mothers with PCOS. J Clin Endocrinol Metab 2018; 103(2): 740–747. [DOI] [PubMed] [Google Scholar]
- 93. Hanson B, Johnstone E, Dorais J, et al. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet 2017; 34(2): 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Charalampakis V, Tahrani AA, Helmy A, et al. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol 2016; 207: 220–226. [DOI] [PubMed] [Google Scholar]
- 95. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids 2013; 78(8): 782–785. [DOI] [PubMed] [Google Scholar]
- 96. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract 2016; 2(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc American Thoracic Society 2008; 5(2): 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kumarendran B, Sumilo D, O’Reilly MW, et al. Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population-based cohort study. Eur J Endocrinol 2019; 180(4): 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gunnarsson SI, Peppard PE, Korcarz CE, et al. Obstructive sleep apnea is associated with future subclinical carotid artery disease: thirteen-year follow-up from the Wisconsin sleep cohort. Arterioscler Thromb Vasc Biol 2014; 34(10): 2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. New England Journal of Medicine 2000; 342(19): 1378–1384. [DOI] [PubMed] [Google Scholar]
- 101. Kahal H, Kyrou I, Uthman OA, et al. The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath 2020; 24(1): 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet 2019; 12: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol 2015; 145: 213–225. [DOI] [PubMed] [Google Scholar]
- 104. Reddy R, Deepika MLN, Latha K, et al. Polycystic ovary syndrome: role of aromatase gene variants in South Indian women. Int J Pharm Bio Sci 2015; 6: B1283–B1296. [Google Scholar]
- 105. Marx TL, Mehta AE. Polycystic ovary syndrome: pathogenesis and treatment over the short and long term. Cleve Clin J Med 2003; 70(1): 31–336. [DOI] [PubMed] [Google Scholar]
- 106. Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol 2019; 3: 100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shaikh N, Dadachanji R, Mukherjee S. Genetic markers of polycystic ovary syndrome: emphasis on insulin resistance. Int J Med Gene 2014; 2014: 478972. [Google Scholar]
- 108. Akram M, Roohi N. Endocrine correlates of polycystic ovary syndrome in Pakistani women. J Coll Physicians Surg Pak 2015; 25(1): 22–26. [PubMed] [Google Scholar]
- 109. Sharma M, Barai RS, Kundu I, et al. PCOSKB R2: a database of genes, diseases, pathways, and networks associated with polycystic ovary syndrome. Sci Rep 2020; 10(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Afiqah-Aleng N, Harun S, Nor Muhammad NA, et al. PCOSBase: a manually curated database of polycystic ovarian syndrome. Database 2017; 2017: bax098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jesintha Mary M, Vetrivel U, Munuswamy D, et al. PCOSDB: PolyCystic Ovary Syndrome Database for manually curated disease associated genes. Bioinformation 2016; 12(1): 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xita N, Georgiou I, Tsatsoulis A. The genetic basis of polycystic ovary syndrome. Eur J Endocrinol 2002; 147(6): 717–726. [DOI] [PubMed] [Google Scholar]
- 113. Reddy KR, Deepika ML, Supriya K, et al. CYP11A1 microsatellite (tttta)n polymorphism in PCOS women from South India. J Assist Reprod Genet 2014; 31(7): 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yu M, Feng R, Sun X, et al. Polymorphisms of pentanucleotide repeats (tttta)n in the promoter of CYP11A1 and their relationships to polycystic ovary syndrome (PCOS) risk: a meta-analysis. Mol Biol Rep 2014; 41(7): 4435–4445. [DOI] [PubMed] [Google Scholar]
- 115. Shen W, Li T, Hu Y, et al. Common polymorphisms in the CYP1A1 and CYP11A1 genes and polycystic ovary syndrome risk: a meta-analysis and meta-regression. Arch Gynecol Obstet 2014; 289(1): 107–118. [DOI] [PubMed] [Google Scholar]
- 116. Zhang CW, Zhang XL, Xia YJ, et al. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women. Mol Biol Rep 2012; 39(8): 8379–8385. [DOI] [PubMed] [Google Scholar]
- 117. Settas N, Dracopoulou-Vabouli M, Dastamani A, et al. CYP21A2 mutations in women with polycystic ovary syndrome (PCOS). Horm Metab Res 2013; 45(5): 383–386. [DOI] [PubMed] [Google Scholar]
- 118. Pucci L, Lucchesi D, Longo V, et al. Lack of association between CYP21 V281L variant and polycystic ovary syndrome in Italian women. Gynecol Endocrinol 2010; 26(8): 596–599. [DOI] [PubMed] [Google Scholar]
- 119. Witchel SF, Kahsar-Miller M, Aston CE, et al. Prevalence of CYP21 mutations and IRS1 variant among women with polycystic ovary syndrome and adrenal androgen excess. Fertil Steril 2005; 83(2): 371–375. [DOI] [PubMed] [Google Scholar]
- 120. Witchel SF, Aston CE. The role of heterozygosity for CYP21 in the polycystic ovary syndrome. J Pediatr Endocrinol Metab 2000; 13(Suppl. 5): 1315–1317. [PubMed] [Google Scholar]
- 121. Ashraf S, Rasool SUA, Nabi M, et al. CYP17 gene polymorphic sequence variation is associated with hyperandrogenism in Kashmiri women with polycystic ovarian syndrome. Gynecol Endocrinol 2021; 37(3): 230–234. [DOI] [PubMed] [Google Scholar]
- 122. Munawar Lone N, Babar S, Sultan S, et al. Association of the CYP17 and CYP19 gene polymorphisms in women with polycystic ovary syndrome from Punjab, Pakistan. Gynecological Endocrinology 2021; 37(5): 456–461. [DOI] [PubMed] [Google Scholar]
- 123. Rahimi Z, Mohammadi M, Sc E. The CYP17 MSP AI (T-34C) and CYP19A1 (Trp39Arg) variants in polycystic ovary syndrome: a case-control study. Int J Reprod Biomed 2019; 17(3): 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaur R, Kaur T, Kaur A. Genetic association study from North India to analyze association of CYP19A1 and CYP17A1 with polycystic ovary syndrome. J Assist Reprod Genet 2018; 35(6): 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chua AK, Azziz R, Goodarzi MO. Association study of CYP17 and HSD11B1 in polycystic ovary syndrome utilizing comprehensive gene coverage. Mol Hum Reprod 2012; 18(6): 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mehdizadeh A, Kalantar SM, Sheikhha MH, et al. Association of SNP rs.2414096 CYP19 gene with polycystic ovarian syndrome in Iranian women. Int J Reprod Biomed 2017; 15(8): 491–496. [PMC free article] [PubMed] [Google Scholar]
- 127. Hemimi N, Shaafie I, Alshawa H. The study of the impact of genetic polymorphism of aromatase (CYP19) enzyme and the susceptibility to polycystic ovary syndrome (575.5). FASEB J 2014; 28(S1): 5755. [Google Scholar]
- 128. Zhang T, Liang W, Fang M, et al. Association of the CAG repeat polymorphisms in androgen receptor gene with polycystic ovary syndrome: a systemic review and meta-analysis. Gene 2013; 524(2): 161–167. [DOI] [PubMed] [Google Scholar]
- 129. Liao X, Cao S. Association of the genetic polymorphisms rs6259 and rs727428 of the SHBG gene with polycystic ovary syndrome risk: a meta-analysis. Genet Test Mol Biom 2020; 24(8): 492–501. [DOI] [PubMed] [Google Scholar]
- 130. Thangavelu M, Godla UR, Paul Solomon FD, et al. Single-nucleotide polymorphism of INS, INSR, IRS1, IRS2, PPAR-G and CAPN10 genes in the pathogenesis of polycystic ovary syndrome. J Genet 2017; 96(1): 87–96. [DOI] [PubMed] [Google Scholar]
- 131. Ben Salem A, Attaoua R, Mtiraoui N, et al. Common polymorphisms of calpain-10 and the risk of polycystic ovary syndrome in Tunisian population: a case–control study. Mol Biol Rep 2014; 41(10): 6569–6574. [DOI] [PubMed] [Google Scholar]
- 132. Anastasia K, Koika V, Roupas ND, et al. Association of Calpain (CAPN) 10 (UCSNP-43, rs3792267) gene polymorphism with elevated serum androgens in young women with the most severe phenotype of polycystic ovary syndrome (PCOS). Gynecol Endocrinol 2015; 31(8): 630–634. [DOI] [PubMed] [Google Scholar]
- 133. Shen W, Li T, Hu Y, et al. Calpain-10 genetic polymorphisms and polycystic ovary syndrome risk: a meta-analysis and meta-regression. Gene 2013; 531(2): 426–434. [DOI] [PubMed] [Google Scholar]
- 134. Wiltgen D, Furtado L, Kohek MB, et al. CAPN10 UCSNP-43, UCSNP-19 and UCSNP-63 polymorphisms and metabolic syndrome in polycystic ovary syndrome. Gynecol Endocrinol 2007; 23(3): 173–178. [DOI] [PubMed] [Google Scholar]
- 135. Márquez JL, Pacheco A, Valdés P, et al. Association between CAPN10 UCSNP-43 gene polymorphism and polycystic ovary syndrome in Chilean women. Clin Chim Acta 2008; 398(1–2): 5–9. [DOI] [PubMed] [Google Scholar]
- 136. Shi X, Xie X, Jia Y, et al. Associations of insulin receptor and insulin receptor substrates genetic polymorphisms with polycystic ovary syndrome: a systematic review and meta-analysis. J Obstet Gynaecol Res 2016; 42(7): 844–854. [DOI] [PubMed] [Google Scholar]
- 137. Skrgatić L, Baldani DP, Gersak K, et al. Genetic polymorphisms of INS, INSR and IRS-1 genes are not associated with polycystic ovary syndrome in Croatian women. Colleg Antropol 2013; 37(1): 141–146. [PubMed] [Google Scholar]
- 138. Feng C, Lv P-P, Yu T-T, et al. The association between polymorphism of INSR and polycystic ovary syndrome: a meta-analysis 2015; 16(2): 2403–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Qin L, Zhao S, Yang P, et al. Variation analysis of anti-Müllerian hormone gene in Chinese women with polycystic ovary syndrome. Endocrine 2021; 72(1): 287–293. [DOI] [PubMed] [Google Scholar]
- 140. Gorsic LK, Dapas M, Legro RS, et al. Functional genetic variation in the anti-Müllerian hormone pathway in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2019; 104(7): 2855–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gorsic LK, Kosova G, Werstein B, et al. Pathogenic anti-Müllerian hormone variants in polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102(8): 2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang F, Niu W-B, Kong H-J, et al. The role of AMH and its receptor SNP in the pathogenesis of PCOS. Mol Cell Endocrinol 2017; 439: 363–368. [DOI] [PubMed] [Google Scholar]
- 143. Seyed Abutorabi E, Hossein Rashidi B, Irani S, et al. Investigation of the FSHR, CYP11, and INSR mutations and polymorphisms in Iranian infertile women with polycystic ovary syndrome (PCOS). Rep Biochem Mol Biol 2021; 9(4): 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yan J, Tian Y, Gao X, et al. A genome-wide association study identifies FSHR rs2300441 associated with follicle-stimulating hormone levels. Clin Genet 2020; 97(6): 869–877. [DOI] [PubMed] [Google Scholar]
- 145. Baban ASS, Korsheed SH, Al Hayawi AY. The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn Al-Haitham J Pure Appl Sci 2018; 2018: 257–272. [Google Scholar]
- 146. Kim JJ, Choi YM, Hong MA, et al. FSH receptor gene p. Thr307ala and P. Asn680ser polymorphisms are associated with the risk of polycystic ovary syndrome. Journal of Assisted Reproduction and Genetics 2017; 34(8): 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen DJ, Ding R, Cao JY, et al. Two follicle-stimulating hormone receptor polymorphisms and polycystic ovary syndrome risk: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014; 182: 27–32. [DOI] [PubMed] [Google Scholar]
- 148. Fu L, Zhang Z, Zhang A, et al. Association study between FSHR Ala307Thr and Ser680Asn variants and polycystic ovary syndrome (PCOS) in Northern Chinese Han women. J Assist Reprod Genet 2013; 30(5): 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wu XQ, Xu SM, Liu JF, et al. Association between FSHR polymorphisms and polycystic ovary syndrome among Chinese women in north China. J Assist Reprod Genet 2014; 31(3): 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Almawi WY, Hubail B, Arekat DZ, et al. Leutinizing hormone/choriogonadotropin receptor and follicle stimulating hormone receptor gene variants in polycystic ovary syndrome. J Assist Reprod Genet 2015; 32(4): 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Branavan U, Muneeswaran K, Wijesundera S, et al. Identification of selected genetic polymorphisms in polycystic ovary syndrome in Sri Lankan women using low cost genotyping techniques. PLoS ONE 2019; 13(12): e0209830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Harada N, Ogawa H, Shozu M, et al. Genetic studies to characterize the origin of the mutation in placental aromatase deficiency. Am J Hum Genet 1992; 51(3): 666–672. [PMC free article] [PubMed] [Google Scholar]
- 153. Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 2013; 368(1612): 120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Joseph S, Barai RS, Bhujbalrao R, et al. PCOSKB: A KnowledgeBase on genes, diseases, ontology terms and biochemical pathways associated with PolyCystic Ovary Syndrome. Nucleic Acids Research 2016; 44(D1): D1032–D1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33(6): 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. de Melo AS, Dias SV, Cavalli Rde C, et al. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction 2015; 150(1): R11–R24. [DOI] [PubMed] [Google Scholar]
- 157. Escobar-Morreale HF, Luque-Ramirez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 2005; 26(2): 251–282. [DOI] [PubMed] [Google Scholar]
- 158. Melo AS, Vieira CS, Barbieri MA, et al. High prevalence of polycystic ovary syndrome in women born small for gestational age. Hum Reprod 2010; 25(8): 2124–2131. [DOI] [PubMed] [Google Scholar]
- 159. Moran LJ, Pasquali R, Teede HJ, et al. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 2009; 92(6): 1966–1982. [DOI] [PubMed] [Google Scholar]
- 160. Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013; 14(2): 95–109. [DOI] [PubMed] [Google Scholar]
- 161. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011(7): CD007506. [DOI] [PubMed] [Google Scholar]
- 162. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008; 89(3): 505–522. [DOI] [PubMed] [Google Scholar]
- 163. Merkin SS, Phy JL, Sites CK, et al. Environmental determinants of polycystic ovary syndrome. Fertil Steril 2016; 106(1): 16–24. [DOI] [PubMed] [Google Scholar]
- 164. Di Renzo GC, Conry JA, Blake J, et al. International federation of gynecology and obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynaecol Obstet 2015; 131(3): 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009; 30(4): 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Boekelheide K, Blumberg B, Chapin RE, et al. Predicting later-life outcomes of early-life exposures. Environ Health Perspect 2012; 120(10): 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hormone Health Network. Endocrine-disrupting chemicals (EDCs), https://www.endocrine.org/patient-engagement/endocrine-library/edcs#:~:text=Endocrine%2Ddisrupting%20chemicals%20(EDCs)%20are%20substances%20in%20the%20environment,of%20your%20body's%20endocrine%20system.
- 168. Rutkowska A, Rachoń D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS). Gynecol Endocrinol 2014; 30(4): 260–265. [DOI] [PubMed] [Google Scholar]
- 169. Ikezuki Y, Tsutsumi O, Takai Y, et al. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002; 17(11): 2839–2841. [DOI] [PubMed] [Google Scholar]
- 170. Vandenberg LN, Hauser R, Marcus M, et al. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24(2): 139–177. [DOI] [PubMed] [Google Scholar]
- 171. Cobellis L, Latini G, De Felice C, et al. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod 2003; 18(7): 1512–1515. [DOI] [PubMed] [Google Scholar]
- 172. Yokota H, Iwano H, Endo M, et al. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J 1999; 340(Pt. 2): 405–409. [PMC free article] [PubMed] [Google Scholar]
- 173. Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010; 118(8): 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kandaraki E, Chatzigeorgiou A, Livadas S, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab 2011; 96(3): E480–E484. [DOI] [PubMed] [Google Scholar]
- 175. Fernández M, Bourguignon N, Lux-Lantos V, et al. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 2010; 118(9): 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou W, Liu J, Liao L, et al. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol 2008; 283(1–2): 12–18. [DOI] [PubMed] [Google Scholar]
- 177. Nilsson E, Larsen G, Manikkam M, et al. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS ONE 2012; 7(5): e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ehrlich S, Williams PL, Missmer SA, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect 2012; 120(7): 978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Déchaud H, Ravard C, Claustrat F, et al. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids 1999; 64(5): 328–334. [DOI] [PubMed] [Google Scholar]
- 180. Akgül S, Sur Ü, Düzçeker Y, et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol Endocrinol 2019; 35(12): 1084–1087. [DOI] [PubMed] [Google Scholar]
- 181. Akın L, Kendirci M, Narin F, et al. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr 2015; 104(4): e171–e177. [DOI] [PubMed] [Google Scholar]
- 182. Vagi SJ, Azziz-Baumgartner E, Sjödin A, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC Endocr Disord 2014; 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yang Q, Zhao Y, Qiu X, et al. Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): a case–control study. Hum Reprod 2015; 30(8): 1964–1973. [DOI] [PubMed] [Google Scholar]
- 184. National Institute of Environmental Health Sciences. Endocrine disruptors, https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm [Google Scholar]
- 185. Endocrine Society. PFAS chemicals: EDCs contaminating our water and food supply. 2019, https://www.endocrine.org/topics/edc/what-edcs-are/common-edcs/pfas [Google Scholar]
- 186. Kyrou I, Weickert MO, Randeva HS. Diagnosis and management of polycystic ovary syndrome (PCOS). In: Ajjan R, Orme S. (eds) Endocrinology and diabetes. Springer: London, 2015, pp. 99–113. DOI: 10.1007/978-1-4471-2789-5_13 [Google Scholar]
- 187. Daneshjou D, Soleimani Mehranjani M, Zadeh Modarres S, et al. Sitagliptin/metformin: a new medical treatment in polycystic ovary syndrome. Trends Endocrinol Metab 2020; 31: 890–892. [DOI] [PubMed] [Google Scholar]
- 188. Al-Ruthia YS, Al-Mandeel H, AlSanawi H, et al. The effect of metformin use on pregnancy rates among polycystic ovary syndrome patients undergoing in vitro fertilization: a retrospective-cohort study 2017; 25(6): 906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wu Y, Tu M, Huang Y, et al. Association of metformin with pregnancy outcomes in women with polycystic ovarian syndrome undergoing in vitro fertilization: a systematic review and meta-analysis 2020; 3(8): e2011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sun X, Zhang D, Zhang W. Effect of metformin on ovulation and reproductive outcomes in women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet 2013; 288(2): 423–430. [DOI] [PubMed] [Google Scholar]
- 191. Kar SJF. Clomiphene citrate, metformin or the combination of both, as first line ovulation induction drug in polycystic ovarian syndrome: a randomised controlled trial. Fertil Steril 2013; 100(3): S359–S360. [Google Scholar]
- 192. Armanini D, Andrisani A, Bordin L, et al. Spironolactone in the treatment of polycystic ovary syndrome 2016; 17(13): 1713–1715. [DOI] [PubMed] [Google Scholar]
- 193. Ganie M, Chakraborty S, Rehman HJCI. Treatment of polycystic ovary syndrome: recent trial results. Clin Trial Outc 2015; 5(3): 337–350. [Google Scholar]
- 194. Sabbadin C, Andrisani A, Zermiani M, et al. Spironolactone and intermenstrual bleeding in polycystic ovary syndrome with normal BMI. J Endocrinol Invest 2016; 39(9): 1015–1021. [DOI] [PubMed] [Google Scholar]
- 195. Bargiota A, Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther Adv Endocrinol Metab 2012; 3(1): 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Diri H, Karaburgu S, Acmaz B, et al. Comparison of spironolactone and spironolactone plus metformin in the treatment of polycystic ovary syndrome. Gynecol Endocrinol 2016; 32(1): 42–45. [DOI] [PubMed] [Google Scholar]
- 197. Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest 2005; 28(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 198. Lashen HJ. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2010; 1(3): 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Nader S. Treatment for polycystic ovary syndrome: a critical appraisal of treatment options. Expert Rev Endocrinol Metab 2008; 3(3): 349–359. [DOI] [PubMed] [Google Scholar]
- 200. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98(12): 4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: a committee opinion. Fertil Steril 2013; 100(2): 341–348. [DOI] [PubMed] [Google Scholar]
- 202. Abdalla MA, Deshmukh H, Atkin S, et al. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2020; 11: 38305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Banaszewska B, Pawelczyk L, Spaczynski R. Current and future aspects of several adjunctive treatment strategies in polycystic ovary syndrome. Reprod Biol 2019; 19(4): 309–315. [DOI] [PubMed] [Google Scholar]
- 204. Jin P, Xie YJGE. Treatment strategies for women with polycystic ovary syndrome 2018; 34(4): 272–277. [DOI] [PubMed] [Google Scholar]
- 205. Glintborg D, Andersen MJ. Medical treatment and comorbidity in polycystic ovary syndrome: an updated review. Curr Opin Endoc Metab Res 2020; 12: 33–40. [Google Scholar]
- 206. Trummer C, Schwetz V, Kollmann M, et al. Effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS: a randomized-controlled trial. Eur J Nutr 2019; 58(5): 2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Gupta T, Rawat M, Gupta N, et al. Study of effect of vitamin D supplementation on the clinical, hormonal and metabolic profile of the PCOS women. J Obstet Gynaecol India 2017; 67(5): 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Yurtdaş G, Akdevelioğlu Y. A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr 2020; 39(4): 371–382. [DOI] [PubMed] [Google Scholar]
- 209. Xue J, Li X, Liu P, et al. Inulin and metformin ameliorate polycystic ovary syndrome via anti-inflammation and modulating gut microbiota in mice. Endocr J 2019; 66(10): 859–870. [DOI] [PubMed] [Google Scholar]