Abstract
Postoperative cognitive dysfunction (POCD) is common, occurring in around 10-54% of individuals within first few weeks after surgery. Although the majority of POCD is less commonly persistent later than 3 months following surgery, the condition increases length of stay (LOS), mortality and long-term cognitive decline, raising the need for a broad screening to identify individuals at risk for POCD during the perioperative period. In this narrative review, we summarize preoperative, intraoperative and postoperative risk factors for POCD reported in last 5 years and discuss neuropsychological tools and potential biomarkers and time points for assessment that might be suitable for clinical use. We aim to provide crucial information for developing a strategy of routine screening for POCD, which may assist with better identification of at-risk individuals for early interventions. Very importantly, the utilization of a standardized strategy may also allow higher consistency and comparability across different studies.
Keywords: assessment, postoperative cognitive dysfunction, risk factor, screening, tool
Introduction
Perioperative neurocognitive disorder (PND) is an umbrella term in which cognitive impairment occurs or changes preoperatively or postoperatively. 1 It includes cognitive impairment diagnosed before operation (neurocognitive disorder, NCD), acute event (postoperative delirium, POD), cognitive decline diagnosed up to 30 days (delayed neurocognitive recovery) or up to 12 months (postoperative NCD) after surgical procedure. 1 Postoperative cognitive dysfunction (POCD), which occurs at a variety of time points after surgery, 2 is a term used in research studies to describe objectively measurable cognitive decline following surgery using a battery of neuropsychological tests. 1
POCD has been previously reported to occur with a prevalence of 10–54% during first few weeks following surgery, 3 and 12–17% at 3 months,4–6 with the number decreasing to 3% by 12 months. 6 Interestingly, the prevalence may rise again after 12 months, from 9% at 12 months to 30–42% at 5–7.5 years following surgery, as observed in the patients who underwent cardiac surgery.7,8 This needs to be further validated to exclude co-occurrence of dementias, however. Longitudinal studies on individuals who underwent cardiac or non-cardiac surgeries revealed that the condition is associated with prolonged length of stay (LOS), 9 and when persistent at time of discharge or in a longer term, POCD may increase mortality,10–12 as well as risk of long-term cognitive decline. 8 Importantly, interventions for patients with high risk of PND may reduce the risk of occurrence by up to 40%, 13 highlighting the necessity of identifying the at-risk cohort to optimize perioperative management and longitudinal follow-up.
Exploration of risk factors for POCD was initiated decades ago, with some become well-recognized, while others are still under debate. The improvement in utilizing neuropsychological assessment tools as well as newly emerged biomarkers has added new insights into the exploration. In this narrative review, we summarize risk factors for POCD reported in last 5 years to provide the current research status and potential gaps. In addition, we also discuss assessment tools for POCD, including neuropsychological tools and potential biomarkers, and to suggest a strategy in identifying at-risk individuals during perioperative periods.
Preoperative risk factors for POCD
The preoperative risk factors are often the first to be evaluated for POCD, with some demographic features being the most well-recognized factors (Table 1).
Table 1.
The preoperative, intraoperative and postoperative risk factors for POCD.
| Risk factor | Age | Cohort size | Type of surgery | Type of anaesthesia | Finding | Follow-up period | References |
|---|---|---|---|---|---|---|---|
| Preoperative RFs | |||||||
| Age | ⩾18 | 1064 pts, 210 cts | Major non-cardiac surgery ⩾2 h | GA | Independent RF for POCD at 3 months | • ⩽14 days before surgery • Discharge (or 1 week) • 3 months after surgery |
Monk et al. 11 |
| ⩾60 | 1218 pts, 176 cts | Major non-cardiac surgery | GA | RF for both early and late POCD | • Discharge (or 1 week) • 3 months after surgery |
Moller et al. 14 | |
| >18 | 64 pts | Total hip replacement | CSEA | RF affecting POCD | • Admission • 24 h before and after surgery |
Ozalp et al. 15 | |
| Educational level | ⩾18 | 1064 pts, 210 cts | Major non-cardiac surgery ⩾2 h | GA | Independent RF for POCD at 3 months | • ⩽14 days before surgery • Discharge (or 1 week) • 3 months after surgery |
Monk et al. 11 |
| ⩾60 | 1218 pts, 176 cts | Major non-cardiac surgery | GA | RF for early POCD | • Discharge (or 1 week) • 3 months after surgery |
Moller et al. 14 | |
| 45–70 | 131 pts, 40 cts | Valve replacement via CPB | GA | Correlated with POCD | • 1–2 days before surgery • 1 week after surgery |
Tang et al. 16 | |
| Comorbid disease | >18 | 64 pts | Total hip replacement | CSEA | RF affecting POCD | • Admission • 24 h before and after surgery |
Ozalp et al. 15 |
| ASA scores | >18 | 64 pts | Total hip replacement | CSEA | RF affecting POCD | • Admission • 24 h before and after surgery |
Ozalp et al. 15 |
| • Intraoperative RFs | |||||||
| CPB time | 45–70 | 131 pts, 40 cts | Valve replacement via CPB | GA | Correlated with POCD | • 1–2 days before surgery • 1 week after surgery |
Tang et al. 16 |
| Intraoperative hypotension | 65.2 ± 9.6 (low-pressure perfusion) 68.7 ± 8.3 (high-pressure perfusion) |
92 pts | Elective or urgent CABG | GA | Postoperative drop in MMSE score was significantly greater in low-pressure perfusion group | • Before surgery • 48 h after surgery |
Siepe et al. 17 |
| Mean 62.4 (normotensive) Mean 67.9 (hypertensive) |
45 pts | Spinal surgery | GA | Intraoperative mean arterial pressure positive-correlated with 1-day cognitive performance | • Before surgery • 1 day, 1 month after surgery |
Yocum et al. 18 | |
| rScO2 | >65 | 87 pts | Spinal surgery | N/A | Duration of rScO2 <60% of baseline | • 1 day before surgery • 7 days after surgery |
Kim et al. 19 |
| 57–69 | 466 pts | Carotid endarterectomy | GA | rScO2 during carotid artery clamping is a significant predictor | • Before surgery • 1 day after surgery |
Kamenskaya et al. 20 | |
| TOx | ⩾60 | 140 pts | Major elective non-cardiac surgery | GA | Cognitive recovery at 3 days correlated with lower optimal TOx | • 2 weeks before surgery • 3 days after surgery |
Chuan et al. 21 |
| Duration of single longest cerebrovascular autoregulation impairment event | 65 ± 8.7 (non-POCD) 69 ± 6.9 (POCD) |
59 pts | Elective CABG with CPB | GA | Associated with occurrence of POCD | • Before surgery • 10 days after surgery |
Kumpaitiene et al. 22 |
| Anaesthesia method | Middle-aged | 45 pts | Elective back surgery | Intravenous versus inhalation anaesthesia | No difference between two groups in cognitive change | • 1 days before surgery • 1, 6 and 42 days after surgery |
Holeckova et al. 23 |
| ⩾65 | 70 pts | Hip fracture surgery | GA versus subarachnoid anaesthesia | Subarachnoid anaesthesia showed a significant decline in IADL scale and Colour–Word Task at 30 days after surgery | • ⩽24 h before surgery • 30 days after surgery |
Tzimas et al. 24 | |
| Anaesthesia type | ⩾65 | 96 pts | Open surgery | GA with sufentanil versus fentanyl | Sufentanil group had a lower POCD incidence at 1 day after surgery | • 1 day before surgery • 1 and 7 days after surgery |
Zhang et al. 25 |
| ⩾65 | 296 pts | Hip arthroplasty | Lumbosacral plexus + T12 paravertebral block with dexmedetomidine versus propofol | Dexmedetomidine group had a lower POCD incidence | • Before surgery • 3 and 7 days after surgery |
Mei et al. 26 | |
| ⩾65, <90 | 379 pts | Major cancer surgery | GA ⩾2 h with propofol versus sevoflurane | Propofol group had a lower incidence of delayed neurocognitive recovery at 1 week | • 1 day before surgery • 1 week after surgery |
Zhang et al. 27 | |
| >60 | 622 pts | Major abdominal surgery | GA with remifentanil versus fentanyl boluses | No difference between two groups | • 1 and 7 days after surgery | De Cosmo et al. 28 | |
| Amounts of EEG suppression during GA | Young- to middle-aged | 27 healthy individuals | No surgery | GA for 3 h | Not correlated with cognitive performance | • At 30 min intervals | Shortal et al. 29 |
| Depth of anaesthesia | ⩾65 | 198 pts | Hip arthroplasty | GA (deep versus light) + plexus block | Deep GA group had a higher POCD incidence | • 1 day before surgery • 3 and 7 days after surgery |
Mei et al. 30 |
| Duration of anaesthesia | >18 | 64 pts | Total hip replacement | CSEA | RF affecting POCD | • Admission • 24 h before and after surgery |
Ozalp et al. 15 |
| ⩾60 | 1218 pts, 176 cts | Major abdominal surgery | GA | RF for early POCD | • Discharge (or 1 week) • 3 months after surgery |
Moller et al. 14 | |
| • Postoperative RFs | |||||||
| POD | ⩾60 | 225 pts | CABG or valve replacement | N/A | POD pts had lower postoperative cognitive function at 30 days after surgery | • Before surgery • Daily after surgery before discharge • 1, 6 and 12 months after surgery |
Saczynski et al. 31 |
| Postoperative pulmonary complications and infection | ⩾60 | 1218 pts, 176 cts | Major abdominal surgery | GA | RF for early POCD | • Discharge (or 1 week) • 3 months after surgery |
Moller et al. 14 |
| IR | 45–70 | 131 pts, 40 cts | Valve replacement via CPB | GA | Correlated with POCD | • 1–2 days before surgery • 1 week after surgery |
Tang et al. 16 |
| Self-rating depression | 45–70 | 131 pts, 40 cts | Valve replacement via CPB | GA | Correlated with POCD | • 1–2 days before surgery • 1 week after surgery |
Tang et al. 16 |
| Decreased haematocrit levels | ⩾18 | 64 pts | Total hip replacement | GA | RF affecting POCD development | • Admission • 24 h before and after surgery |
Ozalp et al. 15 |
| Analgesics | ⩾60 | 99 pts | Total hip arthroplasty | N/A | Oxycodone group had higher MMSE than sufentanil group | • 1, 3, 5 and 7 days after surgery | Gan et al. 32 |
ASA, American Society of Anaesthesiologists; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; CSEA, combined spinal-epidural anaesthesia; cts, controls; EEG, electroencephalogram; GA, general anaesthesia; IADL, instrumental activities of daily living; IR, insulin resistance; MMSE, Mini-Mental State Examination; N/A, not applicable; POCD, postoperative cognitive dysfunction; POD, postoperative delirium; pts, patients; RF, risk factor; rScO2, regional cerebral oxygen saturation; TOx, tissue oxygenation index.
Age
Systematic reviews, meta-analyses and multi-centre studies have all indicated age to be a risk factor for POCD in both short-term and long-term settings.4,14,15,33,34 Approximately, one-third of elective surgery was carried out on individuals aged over 65 years, 35 and among older individuals who underwent elective surgeries, around 30–40% developed early POCD, while 10–15% developed late POCD. 11 Given that age is also a risk factor that predisposes vulnerability to cognitive decline and dementia, 36 it remains to be determined how preclinical stage of dementia could be differentiated from POCD, and whether preclinical stage of dementia may potentially be accelerated by surgeries.
Educational level
Lower educational level has also been considered as a critical risk factor for POCD based on multiple clinical studies. 34 International multi-centre study evaluating 1218 patients aged 60 years and older who underwent non-cardiac surgery revealed that little education was a risk factor for early POCD, 14 which was further supported by studies evaluating the patients who underwent valve replacement 16 and meta-analysis. 37 In addition, within a follow-up period of 6 months, a 1-year increase in education was associated with a 10% reduction in POCD risk. 3 These studies support that low-level education is a well-recognized risk factor for POCD, while longer years of education act as a protective factor.
Other factors
Several other factors have also been previously linked to an increased risk for POCD, include (1) hypertension (although lack of consistency in meta-analyses); 37 (2) vascular factors 2 that are known to correlate with risk of dementia; 38 (3) depression;2,39 (4) high American Society of Anesthesiologists (ASA) scores; 15 (5) presence of comorbid diseases, such as diabetes mellitus (DM). 15 Meta-analysis and reviews revealed that in cohorts with male comprising the majority of the group, hypertension was associated with 27% increase of risk, 37 and that vascular factor and depression were contributing factors to POCD.2,39 In addition, one study on 64 patients undergoing total hip replacement surgery revealed that high ASA scores and comorbid diseases were also factors affecting the incidence of POCD. 15 The preoperative risk factors may vary among POCD that is present at different time points after surgery. 33 Specifically, increasing age, little education, depression, previous stroke, hypertension and DM are all associated with early POCD, while increasing age and depression have been reported as preoperative risk factors for late POCD.14,33 The risk factors for long-term cognitive decline remain unclear, as this may potentially overlap with age-related dementia.
Intraoperative risk factors for POCD
Intraoperative factors have long been hypothesized to contribute to risks for POCD (Table 1). Whether anaesthesia and surgery contribute to risk for long-term cognitive decline remains controversial, however, as indicated by limited number of studies.
Type of surgery
The incidence of POCD varies significantly between non-cardiac and cardiac surgeries. Previous studies have shown that in cognitively normal adults who underwent non-cardiac surgery, an incidence of 3.9% 40 and 12% 4 was observed at 2–3 weeks and at 3 months, respectively. In contrast, the incidence of POCD in adults undergoing cardiac surgery may be as high as 60%, 2 with 33–83% of cases developed POCD after cardiac surgery with cardiopulmonary bypass (CPB). 41 The incidence of POCD in patients undergoing stent/angiography remains unclear, because of limited studies available, with one study revealing no difference in late POCD between stent/angiography and coronary artery bypass graft (CABG). 5 In addition, around 10–50% of POCD have been observed to persist for months following cardiac surgery.41–43 The variability of POCD incidence indicated that surgery type predisposed different risks of developing POCD. This may be partly due to the fact that cardiac surgeries, particularly CABG, often lead to more dynamic change of blood flow and brain perfusion during the procedure, as well as longer duration of anaesthesia.
Intraoperative hypotension, cerebral perfusion and cerebral autoregulation
Intraoperative hypotension has been associated with increased incidence of postoperative cognitive changes,17,18 although this was not consistent across all studies.13,44,45 Of note, a previous study has suggested that the definition of hypotension should be individualized 13 for evaluating the correlation between hypotension and POCD within an individual.
The brain plays a central and critical role in cognition, and any potential change to the brain may affect its functions. Decreased cerebral perfusion, which is commonly determined by near-infrared spectroscopy (NIRS), 13 has been associated with postoperative cognitive change. 19 Specifically, in adults who underwent carotid endarterectomy, a 20% or more decrease in regional cerebral oxygen saturation (rScO2) during temporary internal carotid artery clamping led to an eightfold increase in risk for cognitive disorders. 20 Although these studies support a potential role cerebral perfusion plays in occurrence of POCD, conflicting result was reported by one study revealing no difference in incidence of POCD among patients with different rScO2. 46
Cerebral autoregulation (CA) is the brain’s ability to maintain constant cerebral perfusion when encountering changes of blood pressure, 47 and an impairment in such functions may be correlated with decreased perfusion of the brain. As the average arterial blood pressure is usually targeted at 50–70 mmHg for CPB, patients who present underlying cerebral vascular diseases are more likely to encounter autoregulation impairment if undergoing such surgeries. 48 In adults who underwent elective CABG with CPB, duration of single longest CA impairment event was associated with occurrence of POCD, 22 indicating that impairment of CA may be related to risk for POCD. In addition, analysis on non-cardiac surgery also revealed that individuals who presented POCD at 3 days after surgery showed lower optimal tissue oxygenation index (TOx) of dynamic autoregulation value compared with those that did not recover, 21 raising the importance of cerebral perfusion and CA. Importantly, meta-analysis on randomized controlled trials revealed that intraoperative management guided by use of cerebral oximetry was associated with reduction of POCD incidence, 49 suggesting this factor to be crucial and may serve as a target in reducing incidence of POCD.
Anaesthetics
Anaesthetic agents have long been considered to be potentially involved in pathogenesis of POCD, with volatile anaesthetic agents considered as potential causative agents for POCD, possibly through the alteration of tubulin and phosphorylation of tau, because multiple binding sites for volatile anaesthetics have been identified on α- and β-tubulin. 50 Although existing meta-analysis did not show evidence that a particular volatile anaesthetic agent increased risk of PND, 13 animal studies showed that a single exposure to isoflurane led to neuronal apoptosis in hippocampus of older animals. 51 In addition, sevoflurane and isoflurane have been shown to increase accumulation of amyloid-β 1–42 (Aβ1–42) and apoptosis in both transgenic and wild-type mice. 52 Other animal models showed that sevoflurane was associated with accumulation of phosphorylated tau in hippocampus. 53 Different anaesthetics may present different effects on POCD development, with one study on 96 elderly patients who underwent open surgery under general anaesthesia showing that patients anesthetized with sufentanil presented lower ratio of POCD incidence than those with fentanyl at day 1 after surgery. 25 Given that POCD may occur at a later stage following surgery, however, the differences of these anaesthetics require longer longitudinal follow-up. Conflicting results are also present in different studies. For instance, in a study on 296 elderly patients who underwent hip arthroplasty, patients supplemented with propofol during lumbosacral plexus + T12 paravertebral block presented a higher incidence of POCD on days 3 and 7 after surgery, compared with those administered with dexmedetomidine. 26 In contrast, another study examining elderly patients undertaking hip or knee replacement under spinal anaesthesia revealed a lower incidence in patients administered with propofol, compared with those administered with dexmedetomidine or midazolam. 54 This was further supported by a larger cohort of older patients who underwent major cancer surgery under over 2 h of general anaesthesia, showing that patients administered with propofol presented a lower incidence of delayed neurocognitive recovery compared with those administered with sevoflurane. 27
Anaesthetic methods
Currently, whether the incidence of POCD differs between regional and general anaesthesia remains controversial. Although several studies revealed no difference between anaesthesia methods on cognitive dysfunctions,23,28 one systematic review and meta-analysis in patients undergoing cardiac surgery with CPB revealed that the Mini-Mental State Examination (MMSE) score of inhalation anaesthesia group was significantly higher than that of total intravenous anaesthesia group. 55 In addition, in older patients who underwent hip fracture surgery, the instrumental activities of daily living (IADL) scale and Colour–Word Task score on day 30 after surgery in subarachnoid anaesthesia group was significantly lower than that in general anaesthesia group, although the overall cognitive test scores did not differ between these two methods, 24 suggesting that the effect of anaesthetic methods on risk of POCD may vary in different surgical types and may affect particular cognitive domains. These findings, however, were not consistent across all studies, with some studies showing no difference in incidence of POCD in general anaesthesia with and without epidural analgesia. 56 Therefore, further investigations were needed to confirm whether anaesthetic methods contribute to risk of POCD.
Duration and depth of anaesthesia
The duration and depth of anaesthesia have been an interest, with previous study revealing that duration of surgery may be a risk factor for POCD, 15 indicating that anaesthesia duration may be related to risk of POCD. The correlation remains controversial, however, as in a small cohort of young- to middle-aged healthy humans who underwent 3 h of general anaesthesia with isoflurane, electroencephalogram (EEG) monitoring during surgical process revealed that the amounts of EEG suppression, which occurs under general anaesthesia, were not correlated with a decrease in cognitive performance. 29 Similarly, the correlation between depth of anaesthesia and risk of POCD also remains unclear. Most studies used Bispectral Index (BIS), which was a processed electroencephalogram that measured level of consciousness, 57 to reflect depth of anaesthesia. One systematic review and meta-analysis revealed no difference in MMSE score between ‘light’ and ‘deep’ anaesthesia. 58 In contrast, one study on elderly patients over 65 years of age who underwent hip arthroplasty with general anaesthesia in conjunction with plexus block revealed that light general anaesthesia with BIS between 60 and 80 presented lower incidence of POCD compared with deep general anaesthesia with BIS between 40 and 60. 30 In addition, total time spent with BIS readings lower than 40 has been suggested to be potentially correlated with risk of POCD. 2 Furthermore, using BIS 40–50 and 55–65 as grouping reference, one study revealed that lighter anaesthesia reduced rate of POCD. 59 Together, although inconclusive, these studies suggest that duration and depth of anaesthesia may present combined effect and that the utilization of BIS in the high-risk group for POCD during preoperative screening may not only be a potential marker to predict development of POCD but also be a suitable marker to monitor anaesthetic adjustment to reduce risk of POCD.
Postoperative risk factors for POCD
Compared with preoperative and intraoperative risk factors, less has been explored on postoperative risk factors for POCD (Table 1). It has been previously shown that patients who underwent cardiac surgery and developed delirium postoperatively presented a significant cognitive decline during the first year after surgery, 31 indicating that POD may be a risk factor for POCD. Another study evaluating the patients who underwent major non-cardiac surgery revealed that POD was associated with risk of POCD at 1 month after surgery, but not at a time point over 1 month after surgery, 60 suggesting that the correlation between POD and POCD may vary among surgery types and time points. Postoperative complications, including postoperative pulmonary complications and infection, have been previously reported as risk factors for POCD. 14 This may partly be due to their potential correlation with POD, which is a potential risk factor for POCD.14,31 In addition, depression has also been shown as a risk factor for POCD as indicated by self-rating depression scale score after operation. 16
Pain management is an important part in postoperative management. 61 The utilization of strong analgesia (e.g. postoperative intravenous administration of opioids versus oral administration) may carry additional risk for developing POCD, 62 which has not been extensively explored. One previous study on elderly patients aged 60 years or older who underwent total hip arthroplasty and administered self-controlled analgesia postoperatively has revealed that individuals administered of intravenous oxycodone (opioid) presented lower incidence of POCD than those administered with sufentanil at up to 3 days following surgery, however. 32 This indicated that the utilization of self-administered analgesia may be a potential risk factor to consider, and that the type of analgesia may play a role in the incidence of POCD. Whether this is linked to a longer effect on development of POCD remains unclear and requires further exploration.
In addition to the more general clinically assessed risk factors, studies on biological changes in blood have also started to emerge, and have shown that higher levels of interleukin (IL)-6 and tumour necrosis factor (TNF)-α at 6 h after operation, as well as decreased levels of postoperative haematocrit, may all contribute to risk for POCD.15,16 Although still at an early stage and need to be differentiated from other causes of such changes, these findings supported the concept that additional biological tests may assist to establish a better model to predict development of POCD.
Risk assessment for POCD
Identification of risk factors
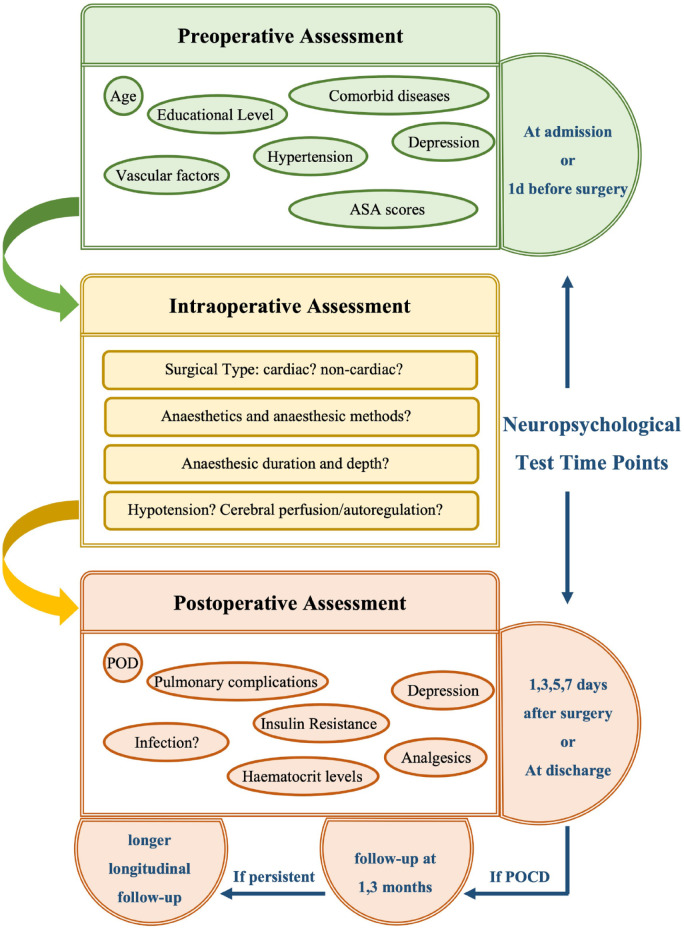
Given that POCD is related to prolonged LOS, increased mortality and elevated risk of long-term cognitive impairment, and that interventions on modifiable risk factors may decrease incidence of POCD, it is crucial to screen patients for the factors that are associated with high risk of POCD to assist with strategy making of surgery, anaesthesia and postoperative management (Figure 1). Although surgical procedures may not be conveniently adjusted, attention could be paid to intraoperative hypotension, decreased cerebral perfusion as well as duration and depth of anaesthesia. Cardiac surgery should be particularly noted, as it poses high incidence of POCD. Other potential factors, such as anaesthesia methods and anaesthetics, should also be noted. Apart from postoperative complications and infection, the presence of POD should be specifically addressed after surgery. Furthermore, POCD at the time of assessment should be recorded to allow longitudinal follow-up after discharge.
Figure 1.
Flow chart of perioperative assessment for the risk of POCD and the time points for neuropsychological tests.
A proposed strategy for identifying risk factors of POCD is presented in the flow chart. The risk factors could be categorized as preoperative, intraoperative and postoperative risk factors and assessed accordingly. Neuropsychological tests should be carried out at admission or 1 day before surgery, 1, 3, 5 and 7 days after surgery or at discharge. When POCD is present at time points after surgery, additional neuropsychological tests will be carried out at follow-up at 1 and 3 months, and may be carried out again during longitudinal follow-up.
Cognitive assessment tools
Cognitive assessment tools play a key role in identification of POCD. At present, a growing number of studies with control groups have used z-score, which evaluates the difference between test scores measured before and after the surgery, to define POCD. 63 In brief, POCD was defined when z-scores of two or more neuropsychological tests or a combined z-score reached 1.96 or more.14,64 In the absence of a control group, early investigations often defined POCD as postoperative cognitive decrement with ⩾1 standard deviation of the whole group at baseline as a cutoff. 1 The lack of consensus on neuropsychological tests specifically used for POCD results in heterogeneity in neuropsychological tests utilized in existing studies, however, which subsequently affects consistency of findings. Various cognitive tests have been developed for identification of cognitive decline and dementia, which present different sensitivity, specificity, covered domains and test duration (for detailed summary, see the review by Zhuang, 2021). 65 Similarly, various neuropsychological tests have been utilized to identify POCD (Table 2), among which, MMSE remains the most frequently used tools.13,17,20,22,24,26,28,30,60,66 The Montreal Cognitive Assessment (MoCA) tool has also been suggested to be one of the most promising assessment tools for preoperative measurement in a clinic, however. 2 In addition, various other cognitive tools have been reported to be utilized in the assessment of POCD,15,16,21,22,24,25,27–29,55,67–75 more frequently as a battery of neuropsychological tests, rather than a single test. The combinations vary among studies, however.
Table 2.
Cognitive and neuropsychological assessment for POCD..
| Assessment tool | Cognitive domains covered | Before surgery | ⩽7 days | ⩽3 months | ⩽12 months |
|---|---|---|---|---|---|
| MMSE | • Orientation • Registration • Attention • Calculation • Immediate recall • Language • Short-term memory • Construct ability |
Kumpaitiene et al. 22 | Kumpaitiene et al. 22 | ||
| De Cosmo et al. 28 | De Cosmo et al. 28 | ||||
| Gan et al. 32 | Gan et al. 32 | ||||
| Mei et al. 26 | Mei et al. 26 | ||||
| Siepe et al. 17 | Siepe et al. 17 | ||||
| Kamenskaya et al. 20 | Kamenskaya et al. 20 | ||||
| Tzimas et al. 24 | Tzimas et al. 24 | ||||
| Saczynski et al. 31 | Saczynski et al. 31 | Saczynski et al. 31 | Saczynski et al. 31 | ||
| Kim et al. 19 | Kim et al. 19 | ||||
| MoCA | • Short-term memory • Visuospatial skills • Executive function • Attention • Concentration • Working memory • Language • Orientation |
Zhang et al. 25 | Zhang et al. 25 | ||
| Gan, et al. 32 | Gan et al. 32 | ||||
| Visuomotor Test of DLOTCA-G | • Visuomotor | Kim et al. 19 | Kim et al. 19 | ||
| RAVLT | • Verbal learning and memory | Kumpaitiene et al. 22 | Kumpaitiene et al. 22 | ||
| De Cosmo et al. 28 | De Cosmo et al. 28 | ||||
| Tang et al. 16 | Tang et al. 16 | ||||
| WAIS | • Verbal comprehension • Perceptual reasoning • Working memory • Processing speed |
Kumpaitiene et al. 22 | Kumpaitiene et al. 22 | ||
| Zhang et al. 27 | Zhang et al. 27 | ||||
| WMS | • Verbal memory • Auditory memory • Visual memory • Short-term memory • Working memory |
Zhang et al. 27 | Zhang et al. 27 | ||
| Schulte Table | • Attention • Visual function |
Kumpaitiene et al. 22 | Kumpaitiene et al. 22 | ||
| MMT | • Orientation • Registration memory • Attention and calculation • Recall • Language |
Ozalp et al. 15 | Ozalp et al. 15 | ||
| DSST | • Visual motor speed • Learning capacity • Sustained attention • Working memory |
Tang et al. 16 | Tang et al. 16 | ||
| Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | ||
| Letter-Digit Coding (based on DSST) | • Working memory | Monk et al. 11 | Monk et al. 11 | Monk et al. 11 | |
| Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |||
| Digit Span Test | • Short-term verbal memory | Saczynski et al. 31 | Saczynski et al. 31 | Saczynski et al. 31 | Saczynski et al. 31 |
| TMT | • Visual attention task switching • Visual search • Scanning • Speed of processing • Mental flexibility • Executive functioning |
Zhang et al. 27 | Zhang et al. 27 | ||
| Tang et al. 16 | Tang et al. 16 | ||||
| Tzimas et al. 24 | Tzimas et al. 24 | ||||
| Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | ||
| SCWT | • Selective attention • Response inhibition • Mental speed • Interference susceptibility |
Tang et al. 16 | Tang et al. 16 | ||
| De Cosmo et al. 28 | De Cosmo et al. 28 | ||||
| Monk et al. 11 | Monk et al. 11 | Monk et al. 11 | |||
| Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |||
| SNST | • Selective attention, • Cognitive flexibility • Processing speed • Executive functioning |
Tzimas et al. 24 | Tzimas et al. 24 | ||
| Grooved Pegboard Test | • Sensory motor integration • Motor processing |
Zhang et al. 27 | Zhang et al. 27 | ||
| Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | ||
| COWAT | • Verbal fluency • Function of the dominant hemisphere (‘left’) |
Tzimas et al. 24 | Tzimas et al. 24 | ||
| Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | ||
| Yocum et al. 18 | Yocum et al. 18 | Yocum et al. 18 | |||
| VVLT | • Word learning | Monk et al. 11 | Monk et al. 11 | Monk et al. 11 | |
| Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |||
| CST | • Cognitive flexibility | Monk et al. 11 | Monk et al. 11 | Monk et al. 11 | |
| Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |||
| CERAD Auditory Verbal Learning Test and Semantic Fluency Test | • Memory and learning • Language • Praxis and executive function |
Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 | Silbert et al. 6 |
| PostopQRS | • Orientation • Verbal memory • Executive functioning • Attention and concentration |
Chuan et al. 21 | Chuan et al. 21 | ||
| IADL scale | • Functional status • Ability of self-care |
Tzimas et al. 24 | Tzimas et al. 24 | ||
| Beck’s Depression Inventory | • Severity of depression | Tzimas et al. 24 | Tzimas et al. 24 | ||
| Three Words–Three Shapes Test | • Learning • Memory • Recall and recognition |
Tzimas et al. 24 | Tzimas et al. 24 | ||
| CDT | • Visuospatial and praxis ability • Attention • Executive functioning |
Tzimas et al. 24 | Tzimas et al. 24 | ||
| Paper and Pencil Memory Scanning Test | • Working memory | Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |
| Four boxes test | • Reaction time | Moller et al. 14 | Moller et al. 14 | Moller et al. 14 | |
| BNT | • Visual confrontation naming | Yocum et al. 18 | Yocum et al. 18 | Yocum et al. 18 | |
| HRNB Trials A and B | • Visual conceptual ability • Visuomotor tracking ability |
Yocum et al. 18 | Yocum et al. 18 | Yocum et al. 18 | |
| Copy portion of the Rey Complex Figure Test | • Perceptual and visuospatial organization • Function of the non-dominant hemisphere (‘right’) |
Yocum et al. 18 | Yocum et al. 18 | Yocum et al. 18 |
BNT, Boston Naming Test; CDT, Clock Drawing Test; CERAD, Consortium to Establish a Registry in Alzheimer Disease; COWAT, Controlled Oral Word Association Test; CST, Concept Shifting Test; DLOTCA-G, Dynamic Lowenstein Occupational Therapy Cognitive Assessment–Geriatric Version; DSST, Digit Symbol Substitution Test; HRNB, Halstead–Reitan Neuropsychological Test Battery; IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; MMT, Mini-Mental Test; MoCA, Montreal Cognitive Assessment; PostopQRS, Postoperative Quality of Recovery Scale; RAVLT, Rey Auditory Verbal Learning Test; SCWT, Stroop Colour–Word Test; SNST, Stroop Neuropsychological Screening Test; TMT, Trail Making Test; VVLT, Visual Verbal Learning Test; WAIS, Wechsler Adult Intelligence Scale; WMS, Wechsler Memory Scale.The significance for shading in Table 2 is to better display the contents of this table and make it seem to be beautiful.
Choosing appropriate time points for cognitive assessment may play a crucial role in identification of POCD. Preoperative assessment should be close to operation. Current studies usually assessed cognition preoperatively at time of admission or within 24 h before surgery.22,23,47 Given that hospitalization before surgery and potential medical treatment as well as psychological stress may affect cognitive performance, the assessment at time of hospital admission may serve as a better point of preoperative assessment.
Although a variety of risk factors have been revealed, it remains unknown which factor(s) is(are) associated with the development of POCD in the longer term. As most patients were discharged and followed in a short period of time after surgery, most of these studies only evaluated short-term changes, which were usually up to 7 days after surgery.6,19,25 Some studies explored incidence of POCD at 1–3 months,6,11,18 and occasionally up to 1 year after surgery.6,31 Together, these time points reflect early (occurring at hospital discharge or at 1 week after surgery, whichever occurs earlier) and late (occurring at 1–3 months after surgery) POCD.11,14,76,77 The number of studies on late POCD remains low, however. Longer-term observations have revealed that POCD presented at 3 months after surgery was strongly associated with obvious cognitive decline at 15 months after surgery, 78 suggesting that this time point may serve as a suitable time to predict long-term cognitive dysfunction. Cognitive decline recognized at 1 year after surgery may be interfered by the development of mild cognitive impairment (MCI) or dementia that is irrelevant to the surgery itself, however, and therefore should be interpreted with cautious. Therefore, it has been suggested that the optimal postoperative cognitive testing time is 1 week and 3 months after surgical procedure, 14 but this conclusion remains to be further validated.
Potential biomarkers for POCD
Recent studies have seen progress in utilizing both non-biofluid–based and biofluid-based biomarkers to assist with POCD evaluation, in addition to neuropsychological assessments. One previous study has revealed that event-related potentials, which marks electrophysiological components, is capable of differentiating cognitive functions between older and younger adults, 79 and has been utilized as a marker for cognition when comparing POCD incidence between intravenous and inhalation anaesthesia. 23 In another study, 6-min walk distance measured before surgery has been shown to be lower in those who developed POCD. 80 These findings are still at early stages, and require further validation.
In addition, inflammatory markers are elevated under various insults. Little is known about their correlations with POCD, however. One study using serially collected cerebrospinal fluid (CSF) and blood in patients who underwent elective total hip or knee replacement revealed no association between a single inflammatory marker and neurocognitive outcomes. 81 Principal component (PC) analysis, however, revealed that the two first PC construct outcome variables on CSF biomarkers were associated with long-term cognitive decline at 3 months. 81 In addition, in older adults who underwent selective hip replacement under general anaesthesia, the serum level of matrix metalloproteinase-9 (MMP-9) was higher, and adenosine diphosphate (ADP) level was lower in those that presented POCD on days 1, 2, 3 and 7 after surgery. 82
Oxidative stress markers have also been reported to be potentially related to risk of POCD. In older patients who underwent hip fracture surgery, expression of the oxidative stress marker malondialdehyde at day 1 after surgery has been shown to be an independent risk factor for POCD. 83 In addition, serum level of the anti-oxidant thioredoxin has also been shown to be an independent predictor for POCD in patients who underwent hip fracture surgery. 84
Other factors have also been reported to be correlated with POCD. In patients who underwent CABG with or without CPB that presented POCD at 15 months after surgery, the plasma level of brain fatty acid–binding protein was higher preoperatively, and remained constantly higher during the whole period of observation. 78 In addition, plasma-free haemoglobin at sternal closure was negatively associated with cognitive performance. 78
Conclusion
The risk factors for POCD were widely distributed along the preoperative, intraoperative as well as postoperative period of a surgery, and therefore, screening of risk factors for POCD should be initiated from time of hospital admission prior to surgical procedures. In addition to common preoperative systematic assessment, demographic factors – including age and educational level – should be specifically noted. Vascular factors, hypertension, depression and ASA scores should be evaluated and recorded. Preoperative assessment may not only assist with identification of risk factors of POCD but also assist with planning of surgery and anaesthesia. During operation, hypotension, cerebral perfusion and CA should be closely monitored by NIRS, 13 Doppler ultrasound 48 and other relevant equipment. In addition, anaesthetic duration and depth should be carefully recorded for risk assessment of POCD. Following surgery, POD, postoperative complications and infection, insulin resistance (IR), depression as well as the change of haematocrit levels and the use of analgesics should all be recorded as part of the risk assessment. Neuropsychological assessments should be performed multiple times after surgery. If persistent at time of discharge, longitudinal follow-up for at least 3 months is encouraged, which may be helpful for predicting long-term cognitive decline after surgery. The frequency of testing should be carefully considered when repeating the same neuropsychological tests or batteries during the follow-up periods because of practice effects, however.
The importance of POCD has been increasingly recognized, because of its potential harm in both short- and long-term. Identifying its associating risk factors may allow potential preventions and early interventions. Stratifying these risk factors as preoperative, intraoperative and postoperative factors will facilitate sequential management and collaboration among multidiscipline teams.
Acknowledgments
None.
Footnotes
ORCID iD: Hong Zhang  https://orcid.org/0000-0003-4537-7322
https://orcid.org/0000-0003-4537-7322
Contributor Information
Xiao Yang, Department of Rehabilitation Medicine, Shanghai Fourth People’s Hospital Affiliated to Tongji University School of Medicine, Shanghai, China.
Xinwei Huang, Clinical Research Center for Anesthesiology and Perioperative Medicine, Tongji University, Shanghai, China; Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, China.
Min Li, Department of Rehabilitation Medicine, Shanghai Fourth People’s Hospital Affiliated to Tongji University School of Medicine, Shanghai, China.
Yuan Jiang, Clinical Medical College and The First Affiliated Hospital of Chengdu Medical College, No.278, Baoguang Avenue Middle Section, Xindu District, Chengdu 610599, China.
Hong Zhang, Department of Rehabilitation Medicine, Shanghai Fourth People’s Hospital Affiliated to Tongji University School of Medicine; Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, No. 1279, Sanmen Road, Hongkou District, Shanghai 200434, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All the authors have given their consent for the publication of this manuscript.
Author contribution(s): Xiao Yang: Conceptualization; Writing – original draft.
Xinwei Huang: Conceptualization; Writing – original draft.
Min Li: Writing – review & editing.
Yuan Jiang: Conceptualization; Supervision; Writing – review & editing.
Hong Zhang: Conceptualization; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Shanghai Hongkou District Health Committee Traditional Chinese Medicine Research Project (no. HKQ-ZYY-2020-20), Scientific research start-up foundation of Shanghai Fourth People’s Hospital, School of Medicine, Tongji University (no. sykyqd01001), the Scientific Research Project of Shanghai Hongkou District Health Committee (no. Hongwei 2003-05) , Shanghai Sailing Program (21YF1435600), and the Scientific Research Promotion Program of Shanghai Fourth People’s Hospital Affiliated to Tongji University (no. SY-XKZT-2020-1006)
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018; 121: 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth 2017; 119: i115–i125. [DOI] [PubMed] [Google Scholar]
- 3. Feinkohl I, Winterer G, Spies CD, et al. Cognitive Reserve and the Risk of Postoperative Cognitive Dysfunction. Dtsch Arztebl Int 2017; 114: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paredes S, Cortinez L, Contreras V, et al. Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand 2016; 60: 1043–1058. [DOI] [PubMed] [Google Scholar]
- 5. Evered L, Scott DA, Silbert B, et al. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg 2011; 112: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 6. Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology 2015; 122: 1224–1234. [DOI] [PubMed] [Google Scholar]
- 7. Evered LA, Silbert BS, Scott DA, et al. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology 2016; 125: 62–71. [DOI] [PubMed] [Google Scholar]
- 8. Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344: 395–402. [DOI] [PubMed] [Google Scholar]
- 9. Boone MD, Sites B, von Recklinghausen FM, et al. Economic burden of postoperative neurocognitive disorders among US Medicare patients. JAMA Netw Open 2020; 3: e208931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009; 110: 548–555. [DOI] [PubMed] [Google Scholar]
- 11. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108: 18–30. [DOI] [PubMed] [Google Scholar]
- 12. Ruggiero C, Bonamassa L, Pelini L, et al. Early post-surgical cognitive dysfunction is a risk factor for mortality among hip fracture hospitalized older persons. Osteoporos Int 2017; 28: 667–675. [DOI] [PubMed] [Google Scholar]
- 13. Berger M, Schenning KJ, Brown CH, 4th, et al. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg 2018; 127: 1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study: ISPOCD Investigators – International Study of Post-operative Cognitive Dysfunction. Lancet 1998; 351: 857–861. [DOI] [PubMed] [Google Scholar]
- 15. Ozalp Horsanali B, Ozkalkanli MY, Tekgul ZT, et al. Effect of preoperative hospitalisation period on postoperative cognitive dysfunction in patients undergoing hip surgery under regional anaesthesia. Int J Clin Pract 2021; 75: e14032. [DOI] [PubMed] [Google Scholar]
- 16. Tang N, Jiang R, Wang X, et al. Insulin resistance plays a potential role in postoperative cognitive dysfunction in patients following cardiac valve surgery. Brain Res 2017; 1657: 377–382. [DOI] [PubMed] [Google Scholar]
- 17. Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg 2011; 40: 200–207. [DOI] [PubMed] [Google Scholar]
- 18. Yocum GT, Gaudet JG, Teverbaugh LA, et al. Neurocognitive performance in hypertensive patients after spine surgery. Anesthesiology 2009; 110: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim J, Shim JK, Song JW, et al. Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth Analg 2016; 123: 436–444. [DOI] [PubMed] [Google Scholar]
- 20. Kamenskaya OV, Loginova IY, Lomivorotov VV. Brain oxygen supply parameters in the risk assessment of cerebral complications during carotid endarterectomy. J Cardiothorac Vasc Anesth 2017; 31: 944–949. [DOI] [PubMed] [Google Scholar]
- 21. Chuan A, Short TG, Peng AZY, et al. Is cerebrovascular autoregulation associated with outcomes after major noncardiac surgery? A prospective observational pilot study. Acta Anaesthesiol Scand 2019; 63: 8–17. [DOI] [PubMed] [Google Scholar]
- 22. Kumpaitiene B, Svagzdiene M, Sirvinskas E, et al. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypass are related to postoperative cognitive deterioration: prospective observational study. Minerva Anestesiol 2019; 85: 594–603. [DOI] [PubMed] [Google Scholar]
- 23. Holeckova I, Kletecka J, Stepanek D, et al. Cognitive impairment measured by event-related potentials during early and late postoperative period following intravenous or inhalation anaesthesia. Clin Neurophysiol 2018; 129: 246–253. [DOI] [PubMed] [Google Scholar]
- 24. Tzimas P, Samara E, Petrou A, et al. The influence of anesthetic techniques on postoperative cognitive function in elderly patients undergoing hip fracture surgery: General vs spinal anesthesia. Injury 2018; 49: 2221–2226. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Chen L, Sun Y, et al. Comparative effects of fentanyl versus sufentanil on cerebral oxygen saturation and postoperative cognitive function in elderly patients undergoing open surgery. Aging Clin Exp Res 2019; 31: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 26. Mei B, Meng G, Xu G, et al. Intraoperative sedation with dexmedetomidine is superior to propofol for elderly patients undergoing hip arthroplasty: a prospective randomized controlled study. Clin J Pain 2018; 34: 811–817. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth 2018; 121: 595–604. [DOI] [PubMed] [Google Scholar]
- 28. De Cosmo G, Sessa F, Fiorini F, et al. Effect of remifentanil and fentanyl on postoperative cognitive function and cytokines level in elderly patients undergoing major abdominal surgery. J Clin Anesth 2016; 35: 40–46. [DOI] [PubMed] [Google Scholar]
- 29. Shortal BP, Hickman LB, Mak-McCully RA, et al. Duration of EEG suppression does not predict recovery time or degree of cognitive impairment after general anaesthesia in human volunteers. Br J Anaesth 2019; 123: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mei B, Zha H, Lu X, et al. Peripheral Nerve Block as a supplement to light or deep general anesthesia in elderly patients receiving total hip arthroplasty: a prospective randomized study. Clin J Pain 2017; 33: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 31. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gan J, Tu Q, Miao S, et al. Effects of oxycodone applied for patient-controlled analgesia on postoperative cognitive function in elderly patients undergoing total hip arthroplasty: a randomized controlled clinical trial. Aging Clin Exp Res 2020; 32: 329–337. [DOI] [PubMed] [Google Scholar]
- 33. Greaves D, Psaltis PJ, Davis DHJ, et al. Risk factors for delirium and cognitive decline following coronary artery bypass grafting surgery: a systematic review and meta-analysis. J Am Heart Assoc 2020; 9: e017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown CtDeiner S. Perioperative cognitive protection. Br J Anaesth 2016; 117: iii52–iii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA 2014; 311: 2110–2120. [DOI] [PubMed] [Google Scholar]
- 36. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Publ Health 2020; 5: e661–e671. [DOI] [PubMed] [Google Scholar]
- 37. Feinkohl I, Winterer G, Pischon T. Hypertension and risk of post-operative cognitive dysfunction (POCD): a systematic review and meta-analysis. Clin Pract Epidemiol Ment Health 2017; 13: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology 2009; 72: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhushan S, Li Y, Huang X, et al. Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: a review article. Int J Surg 2021; 95: 106163. [DOI] [PubMed] [Google Scholar]
- 40. Awada HN, Luna IE, Kehlet H, et al. Postoperative cognitive dysfunction is rare after fast-track hip- and knee arthroplasty: but potentially related to opioid use. J Clin Anesth 2019; 57: 80–86. [DOI] [PubMed] [Google Scholar]
- 41. Gao L, Taha R, Gauvin D, et al. Postoperative cognitive dysfunction after cardiac surgery. Chest 2005; 128: 3664–3670. [DOI] [PubMed] [Google Scholar]
- 42. Bruggemans EF. Cognitive dysfunction after cardiac surgery: pathophysiological mechanisms and preventive strategies. Neth Heart J 2013; 21: 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int 2014; 111: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng X, Hu J, Hua F, et al. The correlation of intraoperative hypotension and postoperative cognitive impairment: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2020; 20: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langer T, Santini A, Zadek F, et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J Clin Anesth 2019; 52: 111–118. [DOI] [PubMed] [Google Scholar]
- 46. Holmgaard F, Vedel AG, Rasmussen LS, et al. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: a secondary analysis of a randomised trial. Br J Anaesth 2019; 123: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161–192. [PubMed] [Google Scholar]
- 48. Brown CH, 4th, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg 2019; 154: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zorrilla-Vaca A, Healy R, Grant MC, et al. Intraoperative cerebral oximetry-based management for optimizing perioperative outcomes: a meta-analysis of randomized controlled trials. Can J Anaesth 2018; 65: 529–542. [DOI] [PubMed] [Google Scholar]
- 50. Craddock TJ, St George M, Freedman H, et al. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS ONE 2012; 7: e37251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofacer RD, Deng M, Ward CG, et al. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol 2013; 73: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie Z, Xu Z. General anesthetics and beta-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry 2013; 47: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Freche H, Brouillette J, Fernandez-Gomez FJ, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology 2012; 116: 779–787. [DOI] [PubMed] [Google Scholar]
- 54. Li WX, Luo RY, Chen C, et al. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J 2019; 132: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen F, Duan G, Wu Z, et al. Comparison of the cerebroprotective effect of inhalation anaesthesia and total intravenous anaesthesia in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and meta-analysis. BMJ Open 2017; 7: e014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Orhun G, Sungur Z, Koltka K, et al. Comparison of epidural analgesia combined with general anesthesia and general anesthesia for postoperative cognitive dysfunction in elderly patients. Ulus Travma Acil Cerrahi Derg 2020; 26: 30–36. [DOI] [PubMed] [Google Scholar]
- 57. Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med 2008; 358: 1097–1108. [DOI] [PubMed] [Google Scholar]
- 58. Li Y, Zhang B. Effects of anesthesia depth on postoperative cognitive function and inflammation: a systematic review and meta-analysis. Minerva Anestesiol 2020; 86: 965–973. [DOI] [PubMed] [Google Scholar]
- 59. Hou R, Wang H, Chen L, et al. POCD in patients receiving total knee replacement under deep vs light anesthesia: a randomized controlled trial. Brain Behav 2018; 8: e00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology 2019; 131: 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain 2018; 159: S11–S16. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Sands LP, Vaurio L, et al. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry 2007; 15: 50–59. [DOI] [PubMed] [Google Scholar]
- 63. Rudolph JL, Schreiber KA, Culley DJ, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand 2010; 54: 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. Acta Anaesthesiol Scand 2000; 44: 1246–1251. [DOI] [PubMed] [Google Scholar]
- 65. Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol 2021; 268: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 66. Gaulton TG. The older adult with preexisting neurocognitive disorder. Curr Opin Anaesthesiol 2019; 32: 438–442. [DOI] [PubMed] [Google Scholar]
- 67. Koch S, Feinkohl I, Chakravarty S, et al. Cognitive impairment is associated with absolute intraoperative frontal alpha-band power but not with baseline alpha-band power: a pilot study. Dement Geriatr Cogn Disord 2019; 48: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hassan WF, Tawfik MH, Nabil TM, et al. Could intraoperative magnesium sulphate protect against postoperative cognitive dysfunction. Minerva Anestesiol 2020; 86: 808–815. [DOI] [PubMed] [Google Scholar]
- 69. Amado LA, Perrie H, Scribante J, et al. Preoperative cognitive dysfunction in older elective noncardiac surgical patients in South Africa. Br J Anaesth 2020; 125: 275–281. [DOI] [PubMed] [Google Scholar]
- 70. Gregory SH, King CR, Ben Abdallah A, et al. Abnormal preoperative cognitive screening in aged surgical patients: a retrospective cohort analysis. Br J Anaesth 2021; 126: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Decker J, Kaloostian CL, Gurvich T, et al. Beyond cognitive screening: establishing an interprofessional perioperative brain health initiative. J Am Geriatr Soc 2020; 68: 2359–2364. [DOI] [PubMed] [Google Scholar]
- 72. Hasan TF, Kelley RE, Cornett EM, et al. Cognitive impairment assessment and interventions to optimize surgical patient outcomes. Best Pract Res Clin Anaesthesiol 2020; 34: 225–253. [DOI] [PubMed] [Google Scholar]
- 73. Uzoigwe CE, O’Leary L, Nduka J, et al. Factors associated with delirium and cognitive decline following hip fracture surgery. Bone Joint J 2020; 102-B: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 74. Traupe I, Giacalone M, Agrimi J, et al. Postoperative cognitive dysfunction and short-term neuroprotection from blueberries: a pilot study. Minerva Anestesiol 2018; 84: 1352–1360. [DOI] [PubMed] [Google Scholar]
- 75. Culley DJ, Flaherty D, Reddy S, et al. Preoperative cognitive stratification of older elective surgical patients: a cross-sectional study. Anesth Analg 2016; 123: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krenk L, Kehlet H, Bæk Hansen T, et al. Cognitive dysfunction after fast-track hip and knee replacement. Anesth Analg 2014; 118: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 77. Twomey C, Corrigan M, Burlacu C, et al. Nitric oxide index is not a predictor of cognitive dysfunction following laparotomy. J Clin Anesth 2010; 22: 22–28. [DOI] [PubMed] [Google Scholar]
- 78. Kok WF, Koerts J, Tucha O, et al. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia 2017; 72: 359–369. [DOI] [PubMed] [Google Scholar]
- 79. Kieffaber PD, Okhravi HR, Hershaw JN, et al. Evaluation of a clinically practical, ERP-based neurometric battery: application to age-related changes in brain function. Clin Neurophysiol 2016; 127: 2192–2199. [DOI] [PubMed] [Google Scholar]
- 80. Hayashi K, Oshima H, Shimizu M, et al. Preoperative 6-minute walk distance is associated with postoperative cognitive dysfunction. Ann Thorac Surg 2018; 106: 505–512. [DOI] [PubMed] [Google Scholar]
- 81. Danielson M, Wiklund A, Granath F, et al. Neuroinflammatory markers associate with cognitive decline after major surgery: findings of an explorative study. Ann Neurol 2020; 87: 370–382. [DOI] [PubMed] [Google Scholar]
- 82. Xie H, Huang D, Zhang S, et al. Relationships between adiponectin and matrix metalloproteinase-9 (MMP-9) serum levels and postoperative cognitive dysfunction in elderly patients after general anesthesia. Aging Clin Exp Res 2016; 28: 1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu C, Gao B, Gui Y. Malondialdehyde on postoperative day 1 predicts postoperative cognitive dysfunction in elderly patients after hip fracture surgery. Biosci Rep 2019; 39: 190166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu XM, Xu WC, Yu YJ, et al. Postoperative serum thioredoxin concentrations correlate with delirium and cognitive dysfunction after hip fracture surgery in elderly patients. Clin Chim Acta 2017; 466: 93–97. [DOI] [PubMed] [Google Scholar]