Abstract
The immense interindividual clinical variability during any infection is a long-standing enigma. Inborn errors of IFN-γ and IFN-α/β immunity underlying rare infections with weakly virulent mycobacteria and seasonal influenza virus have inspired studies of two common infections: tuberculosis and COVID-19. A TYK2 genotype impairing IFN-γ production accounts for about 1% of tuberculosis cases, and autoantibodies neutralizing IFN-α/β account for about 15% of critical COVID-19 cases. The discovery of inborn errors and mechanisms underlying rare infections drove the identification of common monogenic or autoimmune determinants of related common infections. This “rare-to-common” genetic and mechanistic approach to infectious diseases may be of heuristic value.
Inborn errors associated with rare susceptibility to weakly virulent agents illuminate fundamental immunopathological mechanisms of disease.
Introduction
A key but neglected enigma in the field of microbiology and infectious disease is the immense interindividual clinical variability during any primary infection (Casanova, 2015a; Casanova and Abel, 2021a). We define infection as microbial replication within the host and primary infection as the first infection with a given microorganism. The continuous nature of the microbial world renders this notion more operational than rigorous (e.g., primary infection with SARS-CoV-2 may be different in someone previously infected with SARS-CoV, and the same is true for Mycobacterium tuberculosis infection in someone previously infected with M. bovis). Throughout evolution, primary infections have been the main threat to humankind, with half of all children dying from fever before the age of 15 years and a life expectancy at birth of about 20–25 years until the end of the 19th century (Casanova and Abel, 2005). However, this tragic toll was mostly due to the dazzling diversity of pathogens, rather than their individual virulence. Indeed, life-threatening disease is a rare (<1% of infected individuals) outcome of infection for most (>99%) pathogens, at any given period, and in any given region (Casanova and Abel, 2021b). Plague in the Middle Ages, tuberculosis (TB) between the 17th and 19th centuries, the 1918 flu pandemic, and the current AIDS and COVID-19 pandemics, along with a few other devastating infections, stand out as global exceptions. High mortality may also be restricted to naive populations, on their first exposure to an infectious disease (e.g., measles in native Americans and in Arctic and Polynesian populations) (Christensen et al., 1953; Shanks et al., 2011; Walker et al., 2015). Nevertheless, most infected subjects remained well or suffered benign disease, even for these examples, and mortality only rarely exceeded 30% (Casanova and Abel, 2021a). Ironically, the most important unknown in the field of infectious diseases is, thus, the root cause of life-threatening disease, which occurs in only a fraction of infected people. There is no doubt that an infectious agent is necessary to trigger clinical disease, but there is also no doubt that the infectious agent is far from being sufficient to cause severe disease or death.
Historically, microbiologists and infectious disease specialists have tended not to consider this problem because their attention has been focused elsewhere, on the discovery and characterization of pathogens and the prevention and treatment of infectious diseases (Casanova and Abel, 2020). It is probably fair to say that microbiologists have attempted to analyze the impact of microbes in cells and animal models because these approaches could facilitate the development of vaccines or antimicrobial agents, but they have only rarely considered interindividual variability in the course of infection. In parallel, immunologists have been preoccupied with the pursuit of the “antibody enigma” (Kindt, 1984), focusing on its innate and adaptive components, and largely neglecting the field of infectious diseases after Landsteiner’s seminal breakthrough discovery that Ab responses can be mounted against synthetic structures that do not exist naturally (Landsteiner, 1936). Generations of immunologists have studied immunological responses to various non-infectious antigens. The existence of human determinants of severe infectious disease, highlighted at an unprecedented scale by the impact of immunosuppression from the 1960s onward and by human immunodeficiency virus (HIV) infection from the 1980s onward, has not significantly modified their approach. Moreover, the vast majority of life-threatening primary infections cannot be explained by such acquired immunodeficiencies, which more typically underlie “opportunistic” rather than “idiopathic” infections (Casanova and Abel, 2020, 2021b). These detectable deficits nevertheless suggested that the occurrence of infectious disease in particular patients might have other, hitherto unknown, causes. The search for root causes of severe infectious diseases started some time ago, at the turn of the 20th century. It was historically led by human geneticists, in the wake of or in parallel with the work of plant and animal geneticists (Beutler, 2008; Casanova, 2015a, 2015b; Dangl and Jones, 2001; Haller et al., 2018; Vidal et al., 2008).
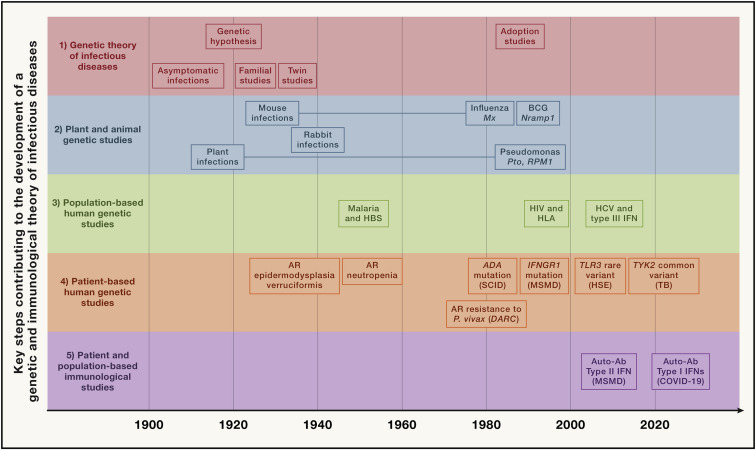
We covered the history of the field of human genetics of infectious diseases in the 19th and 20th centuries in a previous review (Casanova and Abel, 2013) (overview in Figure 1 ). We stressed the importance of classical (from about 1910 onward) and molecular (from about 1950 onward) genetic studies in humans (Casanova, 2015a; Casanova and Abel, 2013, 2021a), together with the considerable influence of pioneering studies in plants and animals (from about 1905 onward) (Dangl and Jones, 2001; Vidal et al., 2008). We reviewed both monogenic and non-monogenic examples of genetic resistance to infection (per se) or disease (in infected individuals) (Casanova and Abel, 2013, 2021a; Quintana-Murci et al., 2007). We also reviewed the three steps, in 1985, 1996, and 2007, by which rare genetic variants underlying monogenic inborn errors of immunity (IEI) in humans were discovered and successively shown to underlie (1) syndromic infectious diseases (i.e., associated with other phenotypes, infectious or otherwise; conditions previously known as “primary immunodeficiencies [PIDs]”), (2) isolated and familial infectious diseases, and (3) isolated and sporadic infectious diseases (Casanova and Abel, 2021a). For these last two categories, IEI underlying the phenotype were found in at least some patients and, sometimes, in single patients, initially with rare infections (from 1996, with disease caused by weakly virulent mycobacteria), or, more recently, with common infections (from 2001, with TB). It should be stressed that these last two steps were inspired by the discovery of infection susceptibility genes by forward genetics in mice between 1986 (mutations of Mx conferring susceptibility to influenza) and 1993 (mutations of Nramp1 conferring susceptibility to mycobacteria) (Staeheli et al., 1986; Vidal et al., 1993) and in plants from 1993 onward (Pto and RPM1 genes involved in resistance to Pseudomonas in tomato and Arabidopsis thaliana, respectively) (Dangl and Jones, 2001; Grant et al., 1995; Martin et al., 1993). We contrasted these patient- and family-based genetic studies generating experimentally validated information about the causal mechanisms of disease with larger, population-based association studies based principally on statistics and yielding modest risk factors at the individual level (Casanova et al., 2013; Thorball et al., 2021; Zhang et al., 2022a).
Figure 1.
Timeline showing some of the key steps that have progressively contributed to the development of a genetic and immunological theory of infectious diseases, grouped into five main parallel fields, from top to bottom
(1) Germ and genetic theories of infectious diseases (red). Following the establishment of the germ theory of disease between 1865 and 1870 (Louis Pasteur) (Pasteur, 1922–1939) and its radical version with Koch’s postulates in 1882 (Robert Koch) (Koch, 1882), it became widely accepted that life-threatening fevers in animals and humans were infectious. Chief among Koch’s postulates was the notion that a pathogen must be found in all patients with a given disease and not in healthy individuals. Once established, this postulate made it difficult to appreciate and understand the importance of the evidence of both latent (non-replicating, dormant germs in tissues of asymptomatic individuals; Clemens von Pirquet and followers) (von Pirquet, 1909) and unapparent (replicating germs in tissues or bloodstream of asymptomatic individuals; Charles Nicolle) (Nicolle, 1933) silent infections. Human geneticists proposed a genetic solution to the problem of asymptomatic infection. Karl Pearson (Pearson, 1912) and other population geneticists and biometricians, in parallel with Archibald Garrod (Garrod, 1931) and other clinical and biochemical geneticists, proposed that the germline genetic background of the host influences or determines susceptibility or resistance to any given microbe. J.B.S. Haldane proposed that infectious diseases had played a major role in natural selection (Haldane, 1949). Various epidemiological and familial approaches were conducted in the first half of the 20th century, supporting this hypothesis. The most remarkable and convincing investigations were probably twin studies comparing monozygotic and dizygotic twins for concordance for a particular infectious phenotype, in particular for tuberculosis (TB) (Puffer, 1944). Adoption studies, which compare the cause of death of patients with that of their biological and foster parents and are equally powerful, were conducted later (Sørensen et al., 1988).
(2) Plant and animal genetic studies (blue). Plant biologists and geneticists also reported early in the 20th century that resistance or susceptibility to infection can be genetically determined (Biffen, 1905; Flor, 1942). From the 1920s onward, other researchers approached the question of the host genetics of infectious diseases from the angle of animal models, including mice and rabbits in particular. Their results led to a similar conclusion, as some strains were vulnerable to the infections tested, whereas others were not (Lurie, 1941; Webster, 1939). These were experimental, as opposed to natural, infections. The experiments were powerful. By the late 1930s, the stage was set for major progress and a transition to molecular genetics, building on multiple independent lines of thought and investigation. Molecular discoveries began in mice in 1986, with the identification, by molecular complementation, of the first monogenic infection susceptibility gene, Mx, mutations of which underlie influenza virus infections (Staeheli et al., 1986), followed by the discoveries, by positional cloning, of the Bcg (Nramp1) (Skamene et al., 1982; Vidal et al., 1993), Cmv (Ly49h) (Brown et al., 2001), and Lps (TLR4) (Poltorak et al., 1998) loci. In parallel, superb studies unraveled the monogenic basis of various infections in various plant species (Dangl and Jones, 2001; Jones et al., 2016), including, in particular, the role of the Pto and RPM1 genes in resistance to Pseudomonas in tomato and Arabidopsis thaliana, respectively (Grant et al., 1995; Martin et al., 1993).
(3) Population-based human genetic studies (green). At the population level, the field of human genetics of infectious diseases began with the discovery, by Allison in 1954, that the sickle cell trait (HbS) provides significant protection against severe forms of P. falciparum malaria (Allison, 1954). Classical genetic epidemiology studies, particularly segregation analyses followed by genome-wide linkage studies, identified major loci for common infectious conditions, such as schistosomiasis and leprosy (Abel and Demenais, 1988; Alcaïs et al., 2007; Marquet et al., 1996; Mira et al., 2004). Very few candidate gene association studies were successful, and only a handful reported odds ratios (ORs) for developing the disease >2 that were replicated in independent populations; among the most remarkable is the association of some HLA class I alleles with AIDS progression, with hazard ratios for protection of about 0.3 (Kaslow et al., 1996). Finally, genome-wide association studies (GWASs) met with variable success, depending on the infectious disease, with many loci identified in leprosy (Zhang et al., 2009, 2011) with ORs below 2, and, more recently, malaria (Malaria Genomic Epidemiology, 2019), with ORs for protection above 0.5, except for the HBB locus. The most remarkable achievement of GWAS in infectious diseases was probably the identification, toward 2010, of IL28B variants (IL28B encodes the type III IFN-λ3) strongly associated with the clearance of hepatitis C virus (Ge et al., 2009) (OR for clearance ∼6). More recently, GWAS in COVID-19 have identified several common variants associated with severe pneumonia, the most significant being located on chromosome 3 with an OR ∼2 (COVID-19 Host Genetics Initiative, 2021; Kousathanas et al., 2022; Pairo-Castineira et al., 2021).
(4) Patient-based human genetic studies (orange). In the early 1950s, pediatricians and clinical geneticists described the first inborn errors of immunity (IEIs)—then referred to as primary immunodeficiencies (PIDs)—as rare, Mendelian, early-onset conditions underlying both multiple, recurrent, and opportunistic infections, and overt, or at least detectable, immunological abnormalities. The blueprint for conventional IEI is widely agreed to be the description of Bruton’s X-linked recessive (XR) agammaglobulinemia in 1952 (Bruton, 1952). Severe congenital neutropenia had, however, been described earlier, in 1950, in children with severe staphylococcal and other bacterial infections and congenital neutropenia, two phenotypes that co-segregated as an autosomal recessive (AR) trait (Kostmann, 1950, 1956). The first IEI conferring predisposition to a single infectious agent was, however, reported even earlier, in 1946, when Wilhelm Lutz described epidermodysplasia verruciformis (EV) as an AR predisposition to skin-tropic viruses (Lutz, 1946) identified in 1978 by Gérard Orth to be weakly virulent human papillomaviruses (HPVs) (Orth et al., 1978). Immunologists did not consider EV to be an IEI, because of the lack of a detectable leukocyte abnormality, until the discovery of EV-causing genes from 2002 onward led to the gradual recognition that keratinocytes contribute to host defense (de Jong et al., 2018; Ramoz et al., 2002). Defects of the membrane attack complex of complement (underlying infections with Neisseria), and X-linked lymphoproliferation (XLP, Epstein-Barr virus) were described later, from different angles, as XLP, like EV, was described as a Mendelian and unexplained (idiopathic) infection (Purtilo et al., 1974, 1977), whereas complement defects were found serendipitously in sporadic cases of Neisseria disease (Lim et al., 1976; Petersen et al., 1976). Mendelian resistance to infectious agents was first detected in the 1970s, with the Duffy antigen receptor for chemokines (DARCs) and P. vivax malaria (Miller et al., 1976), the genetic basis of which was elucidated in 1995 (Tournamille et al., 1995). It was followed, in the mid-1990s, by the discovery of the link between C-C chemokine receptor 5 (CCR5) variants and resistance to human immunodeficiency virus-1 (HIV-1) (Dean et al., 1996; Liu et al., 1996; Samson et al., 1996). The first mutated gene underlying a conventional IEI, ADA, was reported in patients with severe combined immunodeficiency in 1985 (Bonthron et al., 1985). The first mutated gene underlying an isolated infection, IFNGR1, was identified in patients with MSMD in 1996 (Jouanguy et al., 1996; Newport et al., 1996). Since 2007, some rare and sporadic infectious diseases (e.g., HSE) have been shown to be caused by rare monogenic defects, with incomplete penetrance (Casanova and Abel, 2021a; Zhang et al., 2007). Since 2001, rare monogenic defects have also been shown to underlie some common infectious diseases in rare patients (e.g., TB in 2001 [Altare et al., 2001] and COVID-19 in 2020 [Zhang et al., 2020]). Since 2018, monogenic causes of infection have been found in a greater proportion of patients with TB (due to homozygosity for a common TYK2 allele) (Boisson-Dupuis et al., 2018; Kerner et al., 2019, 2021) or COVID-19 (due to hemizygosity for various rare TLR7 alleles) (Asano et al., 2021; Casanova and Abel, 2021a).
(5) Patient- and population-based immunological studies (magenta). Auto-Abs against four cytokines (type I and II IFNs, IL-6, and IL-17A/F) have been shown to underlie infectious phenocopies of the corresponding inborn errors of cytokines or their response pathways (Ku et al., 2020; Puel et al., 2022). The best characterized are probably auto-Abs neutralizing type II IFN, which underlie a phenocopy of MSMD and, more rarely, TB (Puel et al., 2022; Shih et al., 2021). Auto-Abs neutralizing type I IFNs, first described in the early 1980s, were long thought to be clinically silent, except for a 77-year-old woman with disseminated zoster studied by Ion Gresser (Pozzetto et al., 1984). They were found in almost all patients with autoimmune polyendocrine syndrome type 1 (APS-1) (Husebye et al., 2018; Meager et al., 2006). Since 2020, they have been shown to be strong determinants of at least 15% of cases of life-threatening COVID-19 pneumonia (Bastard et al., 2020, 2021a; Zhang et al., 2022a) and a third of adverse reactions to the live-attenuated yellow fever vaccine (Bastard et al., 2021c). More recently, they have also been shown to account for ∼20% of hypoxemic breakthrough COVID-19 cases in fully vaccinated subjects (Bastard et al., 2022b), and ∼5% of cases of severe influenza pneumonia in patients younger than 70 years (Zhang et al., 2022).
Here, we focus on a fourth step forward: the discovery of common determinants of common infectious diseases. Since 2018, studies have shown that homozygosity for the common TYK2 P1104A variant impairs the IL-23-dependent induction of type II IFN and accounts for about 1% of European cases of TB (Boisson-Dupuis et al., 2018; Kerner et al., 2019, 2021). In 2020–2021, at least 1% of cases of critical COVID-19 pneumonia across major ancestries were found to be due to inborn errors of type I IFN immunity (due to rare variants, especially at the X-linked TLR7 locus), and at least 15% were due to the occurrence of auto-Abs neutralizing some type I IFNs and already present before infection (Bastard et al., 2021a; Bastard et al., 2020; Casanova and Abel, 2021b; Zhang et al., 2022a). Monogenic disorders and their autoimmune phenocopies can, thus, account for a sizable proportion (>1%) of at least two common infections. Until these reports, the proportion of patients with a common infection understood at the molecular and cellular levels was negligible. This contrasted with rare infections, some of which were understood for a much larger proportion of cases (e.g., disease caused by weakly virulent mycobacteria). We, thus, place these findings in a more general context, by reviewing the proportion of cases, for given rare infections, that can be explained by rare monogenic lesions displaying complete (Mendelian) or incomplete (non-Mendelian) penetrance. We also discuss how these concepts, methods, and results were applied to common infections, leading to the discovery of rare, causal IEI in small numbers of patients, drawing us along a mechanistic thread to the discovery of common determinants. Finally, we explain how rare IEI underlying common infections can be used like a compass, pointing researchers in the direction of physiological mechanisms that may be disrupted by common determinants, inherited or otherwise.
Rare and monogenic causes of rare and Mendelian bacterial and fungal diseases
Five infectious diseases known to segregate as Mendelian traits were clarified molecularly from 1996 onward (Table 1; Casanova and Abel, 2021a). Mendelian susceptibility to mycobacterial disease (MSMD) is characterized by clinical disease caused by weakly virulent mycobacteria (i.e., live BCG vaccines and environmental mycobacteria) in otherwise healthy patients (Casanova and Abel, 2002). Its prevalence is probably between 10−4 and 10−5 in the general population, depending, in part, on ancestry and the associated level of consanguinity. Its 35 known genetic etiologies, due to germline mutations of 20 genes, disrupt the production of, or the response to IFN-γ, or both (Bustamante, 2020; Martin-Fernandez et al., 2022; Yang et al., 2020) (J.-L.C. and L.A., unpublished data). These inborn errors of IFN-γ immunity account for approximately half the cases of MSMD (Figure 2 A). With hindsight, only five of these etiologies—the three that totally abolish IFN-γ immunity (biallelic null mutations in IFNG, IFNGR1, or IFNGR2) plus autosomal recessive (AR), complete deficiencies of the STAT1 and IRF1 transcription factors, which therefore impair the induction of key IFN-γ-dependent genes (IRF1 itself being STAT1-dependent in this pathway)—are truly Mendelian, as the others appear to have incomplete penetrance, due to residual IFN-γ immunity. Furthermore, patients with complete IRF1 deficiency, unlike those with STAT1 deficiency, display no viral disease, suggesting that IRF1 is not essential for the induction of key IFN-α/β-dependent genes. Incomplete penetrance for MSMD paved the way for the discovery of IEI of IFN-γ immunity underlying TB, which is caused by mycobacterial species that are at least 1,000 times more virulent.
Table 1.
Monogenic causes of rare Mendelian (typically familial) infectious diseases
| Infectious agent | Clinical phenotype | Immunological mechanism | Disease prevalence | Proportion explained |
|---|---|---|---|---|
| BCG vaccines and environmental mycobacteria | Mendelian susceptibility to mycobacterial disease (MSMD) | IFN-γ deficiency | 10−4–10−5 | ∼50%a |
| Epstein-Barr virus | B cell lymphoproliferative disease or lymphoma; hemophagocytosis; hypogammaglobulinemia | cytotoxic CD8+ T cell deficiency | ∼10−5 | ∼50% |
| Human papillomavirus | epidermodysplasia verruciformis tree-man syndrome |
EVER-CIB1 deficiency CD28 deficiency |
<10−5 <10−7 |
∼75% ∼20% |
| Candida | chronic mucocutaneous candidiasis | IL-17A/F deficiency | 10−4–10−5 | ∼50%a |
| Dermatophytes | invasive dermatophytosis | CARD9 deficiency | <10−6 | >90% |
A small proportion of cases are also explained by autoimmune phenocopies of the genetic defects, i.e., autoantibodies neutralizing the corresponding cytokines.
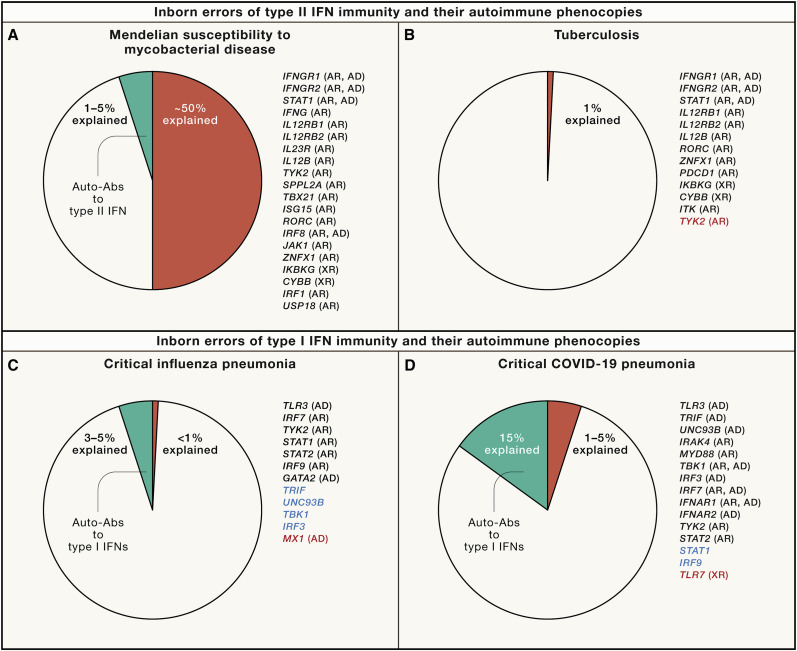
Figure 2.
Proportions of cases of infection explained mechanistically
(A) MSMD. MSMD strikes about 1/50,000 people. Rare germline variants at 20 loci governing type II IFN immunity account for about 50% of cases. Five deficiencies have complete penetrance: autosomal recessive IFN-γ, IFN-γR1, IFN-γR2, STAT1, and IRF1 deficiencies (see text for references). Autoantibodies neutralizing type II IFN (IFN-γ) are found in 1%–5% of cases, with a much greater proportion in individuals of East Asian ancestry, in whom HLA-DRB1 alleles 15:02 and 16:02 are more common.
(B) Tuberculosis. Pulmonary TB strikes about 5%–10% of individuals infected with Mycobacterium tuberculosis. Rare germline variants impairing type II IFN immunity account for less than 0.1% of cases, mostly cases of severe tuberculosis in childhood. These disorders are typically genetic etiologies of MSMD with incomplete penetrance for MSMD. M. tuberculosis is 1,000–10,000 times more virulent than BCG and environmental mycobacteria, and the penetrance of these disorders is, therefore, much higher for TB than for MSMD. Homozygosity for the P1104A variant of TYK2 (red) is found in about 1% of TB patients of European descent. This variant is common in these populations and is homozygous in about 1/600 individuals. Autoantibodies against type II IFN have been reported in very few TB patients to date.
(C) Critical influenza pneumonia. Seasonal influenza has a case-fatality ratio between 0.04% and 0.4%. The human genetic basis of critical influenza pneumonia is largely unknown. Germline mutations of seven genes are causal and all impair the TLR3- and IRF7-dependent induction of type I and III IFNs (black). By inference, genes that are biochemically and immunologically connected to the seven core genes and have also been found to underlie other severe viral infections may be considered influenza susceptibility genes with incomplete penetrance, or, at least, as plausible candidate genes (blue). Autoantibodies against type I IFNs were recently found to account for about 5% of cases of critical influenza pneumonia in patients under 70 years old. Severe avian influenza has been studied genetically and variants of the type I IFN-inducible gene MX1 (red) have been found in ∼9% of the patients studied.
(D) Critical COVID-19 pneumonia. Rare germline variants at loci controlling TLR3- or TLR7-dependent type I IFN immunity underlie critical COVID-19 pneumonia in 1%–5% of patients, this proportion being higher in individuals under the age of 60 years than in older individuals. These defects include AR (IFNAR1, TYK2, TBK1, IRAK4, MYD88, STAT2, and IRF7) and XR disorders (TLR7). The most common defect identified in this context is TLR7 deficiency (red), which has a cumulative frequency in the general population of about 5 × 10−4, but accounts for about 1% of critical cases in males worldwide. By inference, other loci that are biochemically and immunologically related and have been shown to underlie other severe viral infections may also underlie critical COVID-19 (blue). Autoantibodies neutralizing high or low concentrations of type I IFNs have been found in 15%–20% of cases. These antibodies neutralize the 13 forms of IFN-α, or IFN-ω, or both types of IFN. More rarely, they may neutralize IFN-β. These autoantibodies are present before SARS-CoV-2 infection and are also present in uninfected people in the general population. The prevalence of autoantibodies against IFN-α and -ω is between 0.3 (high concentration neutralized) and 1% (low concentration) until the age of 65 years. It then increases sharply, reaching 4% (high concentration) and 7% (low concentration) in people over 80 years old. By contrast, the prevalence of autoantibodies against IFN-β (high concentration) is stable across age groups, at about 0.2%. The risk of critical COVID-19 increases with the number and concentrations of type I IFNs neutralized.
Chronic mucocutaneous candidiasis (CMC) is caused by the fungus Candida albicans. Its prevalence in the general population is between 10−4 and 10−5, excluding acquired forms (e.g., AIDS) and CMC occurring in patients with IEI underlying multiple infections but including both “isolated” (i.e., without any other clinical phenotype) and “syndromic” forms (i.e., associated with at least one other clinical phenotype). Its 14 genetic etiologies, due to mutations of 13 genes impairing IL-17A/F immunity, also account for about half the cases (Li et al., 2019; Puel, 2020; Puel and Casanova, 2021; Puel et al., 2011). The proportion of cases that are understood is probably higher for isolated CMC than for syndromic CMC. Inherited RORC deficiency is at the intersection of MSMD and CMC, as patients with this deficiency suffer from both infections, the encoded RORγC transcription factor being essential for the development of both antifungal Th17 and antimycobacterial Th1∗ cells. Interestingly, IFN-γ and IL-17 are the lymphokines defining the Th1 and Th17 lineages in mice. Studies of the corresponding IEIs have shown that these lymphokines may be largely redundant in human host defense. However, MSMD patients can suffer from other intramacrophagic infections (IFN-γ being more of a macrophage-activating factor than an antiviral IFN) (Nathan et al., 1983), whereas CMC patients occasionally suffer from cutaneous staphylococcal disease.
CMC is a condition of the skin and nails and of the genital and digestive mucosae; C. albicans does not cause invasive disease in patients with IEI of IL-17. This situation contrasts with that for inherited CARD9 deficiency, which underlies various invasive infections, typically caused by actinomycete fungi, including Candida and dermatophytes (Corvilain et al., 2018; Glocker et al., 2009). All patients with invasive dermatophytic disease (IDD)—a very rare Mendelian infectious disease (prevalence of less than 10−6)—tested suffer from inherited CARD9 deficiency (Lanternier et al., 2013). Intriguingly, CARD9 deficiency typically underlies a single type of infection per patient, an observation that remains unexplained. At least a dozen fungi, mostly actinomycetes, can cause severe disease in CARD9-deficient patients (Corvilain et al., 2018; Glocker et al., 2009; Lanternier et al., 2015; Puel, 2020). These invasive fungal diseases are allelic at the CARD9 locus. Most, if not all, cases of IDD (>90%) are explained by this unique genotype. By contrast, other invasive fungal diseases are only rarely explained by CARD9 deficiency. The immunological mechanism of fungal disease in these patients remains unclear but probably involves defective phagocytes, which normally express CARD9 downstream of various cell surface receptors for fungi. Inherited CARD9 deficiency has recently been reviewed (Corvilain et al., 2018; Puel, 2020).
Rare and monogenic causes of rare and Mendelian viral diseases
Epidermodysplasia verruciformis was the first IEI to be described, although it was not recognized as such for over 50 years because of its lack of a hematological or immunological phenotype (Lutz, 1946; Orth, 2008). It is characterized by extremely high levels of susceptibility to defective beta-human papillomaviruses (β-HPVs), which cause flat warts and pityriasis-like lesions in these patients that may progress to non-melanoma skin cancers if they occur on areas exposed to the sun (Orth, 2008). Its prevalence is <10−5. In its typical form, the patients are normally resistant to other microbes. Biallelic null mutations of one of three genes account for at least 75% of cases (Béziat et al., 2021a). The products of these genes, EVER1, EVER2, and CIB1, form a complex in keratinocytes that probably mediates cell-intrinsic immunity to HPVs by interacting with E5 or E8, the virulence factors found in HPVs other than beta-HPVs (de Jong et al., 2018). Patients with epidermodysplasia verruciformis (EV) are typically not prone to lesions caused by the other four genera of HPVs. A Mendelian form of clinical disease caused by HPVs of the alpha or gamma genus has recently been described, with inherited CD28 deficiency underlying recalcitrant common HPV4 warts and the giant horns defining the HPV2 “tree-man” syndrome (Béziat et al., 2021b). The three affected patients displayed no detectable lesions caused by beta-HPVs. IEI underlying HPV diseases have recently been reviewed (Béziat et al., 2021a).
Another severe viral illness, fulminant Epstein-Barr virus (EBV) disease, may also be familial, with Mendelian inheritance but variable expressivity. Following EBV infection, the underlying inborn errors can manifest in various ways, including hemophagocytosis, B cell proliferative disease, B cell lymphoma, and hypogammaglobulinemia (Purtilo et al., 1974, 1977). Penetrance is incomplete for all these EBV phenotypes in all affected families, regardless of genetic etiology. The global prevalence of fulminant EBV disease is probably <10−5. The X-linked recessive (XR) form of this disease may be caused by mutations of SAP, XIAP, or MAGT1 (Coffey et al., 1998; Nichols et al., 1998; Sayos et al., 1998), whereas its AR forms are due to mutations of CTPS1, TNFRSF9, CD27, CD70, or ITK (Abolhassani et al., 2017; Alosaimi et al., 2019; Caorsi et al., 2017; Huck et al., 2009; Izawa et al., 2017; Martin et al., 2014; Rodriguez et al., 2019; Somekh et al., 2019; van Montfrans et al., 2012). Together, these mutations probably account for more than half the cases. These genotypes prevent the control of EBV-infected B cells by cytotoxic CD8+ T cells, as neatly demonstrated for SAP deficiency by analyses of both female carriers and revertant male patients (Palendira et al., 2011, 2012). Some deficits preferentially underlie hemophagocytosis (e.g., SAP mutations), whereas others preferentially underlie excessive B cell proliferation (e.g., CD27 and CD70 mutations). The human genetic and immunological determinants of severe EBV manifestations have recently been reviewed (Tangye, 2020; Tangye and Latour, 2020).
These two known Mendelian viral infectious diseases (caused by HPVs and EBV), like the two Mendelian fungal infectious diseases (Candida, dermatophytes), and the only known Mendelian bacterial infectious disease (mycobacteria), are rare conditions, each of which can be genetically explained in most patients, especially in multiplex kindreds (i.e., families with two or more relatives displaying the phenotype considered). As discussed above, not all known genetic etiologies underlie the corresponding infectious diseases with complete penetrance, and the proportion of explained sporadic cases is probably lower, especially in patients suffering from a syndromic form of infection. The discovery of new genetic etiologies in the future is therefore anticipated. We also expect to see the discovery of new Mendelian infectious diseases, particularly in rare multiplex kindreds, together with their genetic etiologies, as illustrated by CD28 deficiency for alpha- and gamma-HPVs considered collectively, and, previously, by the discovery of DBR1 deficiency underlying viral infection of the brainstem with apparently complete penetrance in individual patients for at least one of the three known causal viruses (HSV-1, influenza virus, and norovirus) (Zhang et al., 2018). Indeed, rare multiplex kindreds with other rare and unexplained infections have been reported in the medical literature.
Rare and monogenic causes of rare and non-Mendelian infectious diseases
Since 2007, some rare infectious diseases have been shown to be monogenic but never, or at least exceedingly rarely, Mendelian; their underlying IEI display incomplete penetrance (Table 2 ). Admittedly, there is a continuum between Mendelian (fully penetrant) and non-Mendelian monogenic (incompletely penetrant) IEI, if only because monogenic lesions may be Mendelian in some, but not other populations, making this dichotomy more operational and didactic than rigorous and scientific. The first and best studied of these infections is probably childhood herpes simplex virus 1 (HSV-1) herpes simplex encephalitis (HSE). This disease is typically sporadic, with a prevalence of about 10−4 in children (Zhang, 2020b). It is generally lethal if left untreated, but sequelae are observed in most acyclovir-treated survivors. Forebrain HSE can be caused by IEI of TLR3-dependent type I IFN immunity resulting from mutations of eight genes (Andersen et al., 2015; Audry et al., 2011; Bastard et al., 2021b; Casrouge et al., 2006; Herman et al., 2012; Pérez de Diego et al., 2010; Sancho-Shimizu et al., 2011; Zhang et al., 2007). Other patients carry mutations of SNORA31, defining another pathway (Lafaille et al., 2019). Overall, these genetic etiologies account for <10% of cases in children. Similar forebrain infections in adults remain unexplained; they may be due to the reactivation of HSV-1 from latency, but they may also have a different pathogenesis.
Table 2.
Monogenic causes of rare non-Mendelian (typically sporadic) infectious diseases
| Infectious agent | Clinical phenotype | Immunological mechanism | Prevalence | Proportion explained |
|---|---|---|---|---|
| Neisseria | invasive disease | complement deficiency | 10−3–10−4 | 1%–5% |
| Streptococcus pneumoniae | invasive disease | TLR and IL-1R response deficiency | ∼10−4 | <5% |
| Staphylococcus aureus | invasive disease | TLR2 response deficiency IL-6 response deficiency | <10−5 | <1% |
| Tropheryma whipplei | Whipple’s disease | IRF4 deficiency | <10−6 | <1% |
| Herpes simplex virus 1 | encephalitis | TLR3-IFN-α/β deficiency snoRNA31 deficiency DBR1 deficiencya |
∼10−4 | 5%–10% |
| Human herpes virus 8 | endemic Kaposi sarcoma | OX40 deficiency | <10−5 | <1% |
| Cytomegalovirus | disseminated disease | NOS2 deficiency | <10−5 | <1% |
| Enterovirus | rhombencephalitis | TLR3/MDA5-IFN-α/β deficiency | <10−5 | <1% |
| Varicella zoster virus | encephalitis and pneumonia | POL3RA and POL3RC deficiency | <10−5 | <5% |
| Influenza | severe pneumonia | type I and III IFN deficiency | ∼10−5 | <1% |
| Rhinovirus | recurrent/severe infections | MDA5 deficiency | 10−4–10−5 | <5% |
| Human papillomavirus | recurrent respiratory papillomatosis | NLRP1 gain of function | ∼10−5 | <1% |
| Hepatitis A virus | fulminant hepatitis | IL-18BP deficiency | <10−5 | <1% |
| Live measles and yellow fever vaccines | severe infections | type I IFN response deficiency | <10−6 | <25% |
Inherited DBR1 deficiency underlies brainstem infection with HSV-1, influenza virus, or norovirus.
Brainstem HSE is rarer in children and the only genetic explanation identified to date is an AR deficiency of DBR1, the only known RNA lariat-debranching enzyme, with complete penetrance for at least one of the three viruses detected in the three known families (Zhang et al., 2018). The mechanism by which the excessive accumulation of RNA lariats underlies HSE is unknown but probably involves cell-intrinsic immunity to these viruses in the brainstem. Indeed, monogenic forebrain HSE is due to a deficiency of cell-intrinsic immunity to HSV-1 in cells resident in the central, but not in the peripheral nervous system. This was established by modeling HSE with iPSC-derived cortical and trigeminal neurons (Lafaille et al., 2012; Zimmer et al., 2018). Cortical neurons lacking TLR3 are much more susceptible to HSV-1 than control cortical neurons, but trigeminal neurons with and without TLR3 are equally susceptible to this virus (and more so than control cortical neurons, accounting for the virus infecting trigeminal but not cortical neurons in most infected individuals). The genetic study of HSE revealed that non-leukocytic, cell-intrinsic immunity to viruses in a specific organ, and even a specific territory, can be life-saving (Casanova and Abel, 2021b; Nathan, 2021; Paludan et al., 2021, Zhang et al., 2019). It also revealed the first inborn error of type I IFN immunity, inherited STAT1 deficiency, in a child with a syndromic form of HSE, whose MSMD was due to the abolition of responses to type II IFN (Dupuis et al., 2003).
No other rare, sporadic, severe infectious disease is better understood than HSE, in terms of the proportion of cases explained molecularly (about 5%–10%). Many other rare, severe, sporadic infections are understood in only a single patient (e.g., CMV disease in a patient lacking NOS2; Drutman et al., 2020), a single multiplex kindred (e.g., Whipple’s disease and IRF4 haploinsufficiency; Guérin et al., 2018), or in unrelated patients with mutations of the same single gene (e.g., isolated congenital asplenia and RPSA haploinsufficiency underlying invasive pneumococcal disease; Bolze et al., 2013). A few are explained by mutations of a few genes that, individually, never account for more than 1% of cases (e.g., invasive pneumococcal or staphylococcal disease in patients with IRAK4 or MyD88 deficiency; Picard et al., 2010). Other examples include HHV8-driven Kaposi sarcoma (Jackson et al., 2016), severe respiratory viral infections other than influenza and COVID-19 (Asgari et al., 2017; Lamborn et al., 2017), and varicella zoster virus encephalitis (Ogunjimi et al., 2017). The study of two other viral illnesses—adverse reactions to live attenuated measles and yellow fever vaccines, which are rarely familial (Casanova and Abel, 2021b; Casanova et al., 2022; Duncan et al., 2021), and critical influenza pneumonia, which is typically sporadic (Ciancanelli et al., 2015; Hernandez et al., 2018; Lim et al., 2019)—converged, together with studies of HSE, on type I IFNs, and these discoveries played an important role in the study of COVID-19, as described below.
Rare monogenic causes of common infectious diseases
Two common infectious diseases deserve a special mention because they were found to have rare monogenic causes: TB and COVID-19. The study of their genetic basis has benefited from that of the related rare infectious diseases, MSMD for TB and severe influenza pneumonia for COVID-19 pneumonia. Rare inborn errors identified as causal for MSMD have been found in patients without MSMD, with TB as their sole phenotype (Figure 2B; Boisson-Dupuis, 2020). This finding attested to the incomplete penetrance of these disorders for MSMD, with higher and probably complete penetrance for TB, given that M. tuberculosis is about 1,000–10,000 times more virulent than BCG and environmental mycobacteria (as TB strikes 1/10 infected subjects whereas MSMD strikes between 1/10,000 and 1/100,000 infected individuals). Two equally rare disorders stand out as having been diagnosed in more than one patient with TB: AR complete IL-12Rβ1 and TYK2 deficiencies. Interestingly, both these disorders impair both IL-12- and IL-23-dependent IFN-γ immunity. The penetrance of MSMD by the age of 30 years is probably about 50% for IL-12Rβ1 deficiency (de Beaucoudrey et al., 2010). As both these disorders are rare, with a frequency of less than 10−5 in the general population, and about half the cases are detected on the basis of the MSMD phenotype of the patient, they probably account for only a very small proportion of cases of TB (even though their penetrance for TB is probably very high, if not complete, upon exposure to M. tuberculosis). However, they have provided proof-of-principle that IFN-γ deficiency can underlie isolated TB in humans without MSMD (Altare et al., 2001; Kreins et al., 2015; Ogishi et al., 2022).
The other example is critical COVID-19 pneumonia. The study of this disease built on previous studies of influenza pneumonia that had led to the identification of AR IRF7 (Ciancanelli et al., 2015), IRF9 (Hernandez et al., 2018), STAT1 (Le Voyer et al., 2021), or STAT2 deficiency (Freij et al., 2020), or autosomal dominant TLR3 deficiency (Lim et al., 2019), in children with unexplained, severe seasonal influenza pneumonia (Figure 2C). TLR3 is an endosomal sensor of dsRNA that can be activated by viral intermediates or by-products to induce type I and III IFNs. It can also control baseline, tonic type I IFN levels by hitherto unknown mechanisms in at least some non-leukocytic cell types, including fibroblasts and cortical neurons, and perhaps pulmonary epithelial cells (Gao et al., 2021). STAT1, STAT2, and IRF9 form the ISGF-3 complex, which is activated by type I or III IFNs (STAT1 homodimers also governing cellular responses to type II IFN) (Stark and Darnell, 2012; Stark et al., 1998). IRF7 is the key transcription factor driving the amplification of type I and III IFNs, with only IFN-β being somewhat IRF7-independent. The high levels of constitutive IRF7 expression in plasmacytoid dendritic cells (pDCs) make these cells the most potent known producers of antiviral IFNs among the >400 discernable cell types in the human body. These findings suggested that the disruption of TLR3-dependent type I and/or III IFN immunity might account for life-threatening influenza pneumonia. The penetrance of AD TLR3 deficiency for severe influenza is incomplete, as patients with HSE carrying the same genotype are normally resistant to influenza virus. It was not initially possible to estimate the penetrance of IRF7 and IRF9 deficiencies, as both disorders were described in single patients. A recent study showed that the penetrance of IRF7 deficiency is incomplete (Campbell et al., 2022). STAT1 and STAT2 deficiencies have incomplete penetrance too, suggesting that the penetrance of IRF9 deficiency is also probably incomplete. Severe influenza has, in rare instances, been reported in patients with autosomal dominant GATA2 deficiency (Sologuren et al., 2018), possibly due to the lack of pDCs in these patients (Zhang, 2020a). Finally, deleterious heterozygous variants of the type I IFN-inducible gene MX1 have also been found in 9.7% of patients with severe avian influenza (Chen et al., 2021).
Rare genetic disorders underlying critical influenza pneumonia were also found in a small number of patients with the more common critical COVID-19 pneumonia and no history of critical influenza (Figure 2D; Zhang et al., 2020). These two viral infectious diseases of the respiratory system are, thus, allelic at loci governing TLR3- and IRF7-dependent type I IFN immunity. Penetrance is incomplete for critical influenza and, perhaps, also for critical COVID-19. IEI of type I IFN immunity previously identified in patients with severe viral infections other than influenza have also been found in some patients with life-threatening COVID-19 (Zhang et al., 2020). The penetrance of the dominant IEI, which were found in <3% of critical cases, is unknown. Two rare etiologies of critical COVID-19 pneumonia stand out because of their recessive mode of inheritance and associated higher penetrance: IRF7 and IFNAR1 deficiencies. These deficiencies were found in four unrelated previously healthy adults aged 25–50 years hospitalized for COVID-19. Remarkably, up to seven IRF7-deficient patients have recently been diagnosed; all suffered from severe viral respiratory diseases, including influenza and COVID-19, but not from viral illnesses of other tissues (Campbell et al., 2022). Two other unrelated IFNAR1-deficient patients and one TBK1-deficient patient with critical COVID-19 have also been independently reported (Abolhassani et al., 2022; Khanmohammadi et al., 2022; Schmidt et al., 2021). Critical COVID-19 pneumonia strikes about 2%–4% of infected individuals (Zhang et al., 2022a); these recessive genetic disorders are individually rare and account collectively for well below 1% of critical cases (i.e., less than 0.03% of infected subjects), mostly patients under 60 years old. However, they have provided proof-of-principle that type I IFN deficiency can underlie life-threatening COVID-19 pneumonia. Recent genetic studies of multisystem inflammatory syndrome in children (MIS-C) have also successfully identified AR deficiencies of multiple genes in the type I IFN-inducible OAS-RNase L pathway (J.-L.C. and L.A., unpublished data). The study of MSMD led to the discovery of rare AR IEI of type II IFN immunity in rare patients with TB, whereas that of severe influenza led to the discovery of rare AR IEI of type I IFN immunity in rare patients with critical COVID-19. These rare patients may be negligible in terms of public health, but they have provided mechanistic insights that have proved invaluable.
Common monogenic determinants of tuberculosis
TB has killed more humans than any other single infectious disease, accounting for at least one billion deaths in human history (Paulson, 2013). European populations were decimated by TB between the 15th and 20th centuries. This condition was favored worldwide by the aggregation of humans into ever-expanding communities during the Neolithic period. However, less than 10% of individuals infected with M. tuberculosis develop TB, which, if left untreated, probably kills at least half of those affected. M. tuberculosis is a bacterium that can reside and persist in macrophages. The discovery that rare inborn errors of IFN-γ immunity could underlie TB in a few patients without MSMD taught us that IFN-γ was essential for protective immunity to M. tuberculosis. This led to the discovery that homozygosity for the common P1104A variant of TYK2 is found almost exclusively in TB patients of various ancestries (Table 3 ; Boisson-Dupuis et al., 2018). Homozygosity for P1104A also underlies MSMD, with a much lower penetrance than for TB, because of the higher virulence of M. tuberculosis than of BCG and environmental mycobacteria. This variant was further shown to impair (but not abolish) the IL-23 response pathway, as profoundly as a complete deficiency of TYK2 but selectively, thereby impairing IFN-γ production by specific lymphocyte subsets without damaging the IL-12 response pathway (or the IL-10 and type I IFN pathways) (Martínez-Barricarte et al., 2018; Ogishi et al., 2022).
Table 3.
Common monogenic and autoimmune determinants of two common infectious diseases
| Infectious agent | Clinical phenotype | Immunological mechanism | Disease prevalencea | Proportion explained |
|---|---|---|---|---|
| Mycobacterium tuberculosis | tuberculosis | IL-23-dependent IFN-γ deficiency (homozygosity for TYK2 P1104A) | 5%–10% | 1%b |
| SARS-CoV-2 | life-threatening COVID-19 pneumonia |
type I IFN deficiency (inborn errors) type I IFN deficiency (autoimmune) |
2%–4% | 1%–5%c 15%–20% |
Prevalence is provided for individuals infected with the corresponding microbe.
In individuals of European origin.
In individuals under the age of 60 years.
The frequency of this variant is highest in Europeans, about 4% of whom carry it, leading to a prevalence for homozygosity of about 1/600. It is absent in sub-Saharan Africa and very rare in East Asia and has a prevalence of about 1% in other regions of the world. Consistent with these findings, the patients with TB due to P1104A homozygosity in the original study were from Latin America, Europe, and the Greater Middle East. They accounted for about 1% of the total number of cases in this highly diverse cohort of patients. In a subsequent study focusing on a European population, homozygosity for P1104A was found to account for about 1% of cases of TB in British individuals after World War II (Kerner et al., 2019). This unbiased, population-based study was made possible by the data of the UK Biobank (Bycroft et al., 2018). Lifetime penetrance for the development of TB upon infection was estimated at about 80% for P1104A homozygotes.
Finally, the P1104A allele was shown to originate from a founder effect about 30,000 years ago in Western Eurasians (Kerner et al., 2021). The haplotype carrying this variant is small (6 kb) and limited to three single-nucleotide variants upstream of P1104A and another three downstream. The study of ancient DNA has revealed that the frequency of P1104A increased to about 10% around 3,000 years ago, possibly by genetic drift. Its decrease in frequency over the last 2,000 years has been one of the most rapid in this prevalence category, strongly suggesting that negative selection was involved. The cause of this negative selection is unknown and open to speculation, but these results are consistent with a decline in P1104A prevalence due to purging driven by the historically very high burden of TB in Europe (Kerner et al., 2021). About one billion Europeans are estimated to have died from TB in the last 2,000 years (Kerner et al., 2019; Paulson, 2013). Overall, the study of MSMD, including the discovery of IEI through analyses of single patients, has led to the discovery of a common monogenic etiology accounting for about 1% of past and present cases of TB in humans of European descent. The P110A4 variant of TYK2 is common in Europeans (>1%), and although European homozygotes for this variant are rare (<1%, 1/600), they account for about 1% of cases of TB.
Common monogenic determinants of COVID-19
The other infectious disease for which the cause of a sizable proportion of cases has been clarified through earlier studies of rare IEI is COVID-19 critical pneumonia. About 0.5%–1% of people infected with COVID-19 die, with considerable between-country variation (COVID-19 National Preparedness Collaborators, 2022), with life-threatening disease occurring in about 2%–4% of infected individuals, and a doubling of the risk for every 5 years of age, from childhood onward (O'Driscoll et al., 2021). The causal virus, SARS-CoV-2, is an RNA virus that infects the respiratory tract, whence it can spread to other tissues via the bloodstream. Based on previous discoveries of rare inherited TLR3, IRF7, STAT1, STAT2, and IRF9 deficiencies in children with life-threatening influenza pneumonia (Campbell et al., 2022; Le Voyer et al., 2021; Zhang, 2020a), rare autosomal IEI affecting TLR3-dependent type I IFN immunity were discovered in patients with life-threatening COVID-19 (Zhang et al., 2020). Remarkably, AR and complete defects of IFNAR1, IRF7, and TBK1 were found in previously healthy patients (Abolhassani et al., 2022; Khanmohammadi et al., 2022; Schmidt et al., 2021; Zhang et al., 2020, 2022b). All these disorders, including the recessive defects, were rare, with genotype frequencies <10−5 in the general population and probably <10−3 in patients with critical disease (Zhang et al., 2020). Most of these patients were less than 60 years old. TLR3, which is not expressed by blood pDCs, is expressed by respiratory epithelial cells (RECs), in which it is essential for type I IFN immunity to influenza virus (Lim et al., 2019) and, by inference from experiments on fibroblasts, for immunity to SARS-CoV-2 (Zhang et al., 2020).
In a subsequent, unbiased approach, XR TLR7 deficiency was found to underlie critical COVID-19 in as many as 1% of men under the age of 60 years (Asano et al., 2021). The cumulative frequency of TLR7 deficiency in the general population is about 5 × 10−4. TLR7 is expressed by pDCs, but not RECs. Type I IFN immunity, which may be TLR3- and REC-dependent, or TLR7- and pDC-dependent, is therefore essential for host defense against SARS-CoV-2 in the respiratory tract. The candidate gene approach-based discovery of AR IFNAR1 and IRF7 deficiencies in four unrelated adults with critical COVID-19 aged 25–50 years who had previously been healthy provided the fundamental information that type I IFN is essential for host defense against SARS-CoV-2, but potentially otherwise redundant in host defense, even over several decades. This redundancy was confirmed not only by reports of other patients with IFNAR1 or IRF7 deficiency (Campbell et al., 2022) but also by the surprising observation of common loss-of-function (LOF) alleles of IFNAR1 and IFNAR2 in the Pacific and Arctic regions, respectively (Bastard et al., 2022a; Duncan et al., 2022). Recessive inborn errors of type I IFNs, including XR TLR7 and AR IFNAR1, STAT2, and TYK2 deficiencies, were even found in about 10% of international children hospitalized for COVID-19 pneumonia (Zhang et al., 2022b). Who would have predicted such a level of redundancy of type I IFNs and, in the case of IRF7 deficiency, of combined type I and III IFNs? The discovery, by genome-wide analysis, of TLR7 deficiency alone did not necessarily incriminate type I IFNs, but its concomitant association with IFNAR1 and IRF7 deficiency did. Moreover, no enrichment in TLR8 variants was found in patients with critical COVID-19, although both genes are X-linked and share largely overlapping agonists and signaling pathways. The key difference is that TLR7, unlike TLR8, is expressed in type I IFN-producing pDCs.
The identification of various AR inborn errors (IFNAR1, IRF7, TBK1), with a frequency probably <10−5 in the general population and <10−3 in patients with critical disease preceded the discovery, in an unbiased approach, of a single etiology, XR TLR7 deficiency, with frequencies of about 5 × 10−4 and 10−2, respectively, in the two corresponding groups of male subjects. Overall, TLR7 deficiency accounts for the same proportion (1%) of critical COVID-19 cases in men across major ancestries as P1104A TYK2 homozygosity accounts for cases of TB in Europeans. The deleterious TLR7 variants are, however, rare individually (<0.02%) and collectively (cumulative frequency <0.07%), contrasting with the common P1104A TYK2 allele (>1%). Hemizygosity for TLR7 LOF variants (1/1,500) is also collectively rarer than homozygosity for the P1104A variant (1/600). In different ways, these two recessive defects are common etiologies accounting for the same proportion (1%) of different forms of the corresponding common infectious diseases: critical COVID-19 pneumonia for TLR7 in men worldwide and pulmonary TB for TYK2 in Europeans. The negative selection operating on the TLR7 locus globally (Barreiro et al., 2009) and P1104A TYK2 specifically (Kerner et al., 2021) attests to the fitness cost of the corresponding deleterious genotypes.
Common autoimmune determinants of COVID-19
The discovery of AR IRF7 deficiency in a child with critical influenza pneumonia thus led to that of AR IRF7, IFNAR1, and XR TLR7 deficiencies, and AD TLR3 pathway deficiencies, in adults with critical COVID-19 pneumonia. These disorders implicated the disruption of TLR3- and TLR7-dependent type I IFN immunity as a core mechanism of disease. This key causal and mechanistic insight led to the discovery that circulating auto-Abs capable of neutralizing the 13 IFN-α and/or IFN-ω at a concentration of 10 ng/mL in vitro (using plasma diluted 1/10) account for at least 10% of cases of critical COVID-19 pneumonia (Bastard et al., 2020). Half the affected patients were found to be over 65 years of age, and most were men. As a control, such auto-Abs were not found in SARS-CoV-2-infected subjects with silent or mild upper respiratory tract infections. In a subsequent study, the neutralization of lower, more physiological concentrations of IFN-α and/or IFN-ω (100 pg/mL, again with plasma diluted 1/10), and of IFN-β, was found to underlie 15% of critical cases, and 20% of cases in patients over the age of 80 years (Bastard et al., 2021a). These studies have been replicated in at least 26 other cohorts in the Americas, Europe, and Asia (Abers et al., 2021, Acosta-Ampudia et al., 2021, Akbil et al., 2022, Bastard et al., 2021, Busnadiego et al., 2022, Carapito et al., 2021, Chang et al., 2021, Chauvineau-Grenier et al., 2022, Eto et al ., 2022, Frasca et al., 2022, Goncalves et al., 2021, Koning et al., 2021, Lamacchia et al., 2022, Lemarquis et al., 2021, Mathian et al., 2022, Meisel et al., 2021, Raadsen et al., 2022, Savvateeva et al., 2021, Simula et al., 2022, Solanich et al., 2021, Soltani-Zangbar et al., 2022, Troya et al., 2021, van der Wijst et al., 2021, Vazquez et al., 2021, Wang et al., 2021, Ziegler et al., 2021.
These auto-Abs had been known for 40 years, in patients treated with recombinant IFN-α or -β (Vinh et al., 2021, Zhang et al., 2022a), or with autoimmune conditions, such as systemic lupus erythematosus (SLE), myasthenia gravis, thymoma, autoimmune polyendocrinopathy syndrome type 1 (APS-1), immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX), or RAG1/RAG2 hypomorphic mutations (Bastard et al., 2020, 2021d). They were generally thought to be clinically silent, with the exception of one case reported by Ion Gresser (Pozzetto et al., 1984) (an otherwise healthy patient with unexplained auto-Abs) and a small series reported by Luigi Notarangelo (patients whose RAG mutations and combined T and B cell immunodeficiency may also have directly underlain viral illnesses) (Walter et al., 2015). However, auto-Abs against four other cytokines had previously been shown to underlie infectious phenocopies of the corresponding inborn errors of cytokines or their response pathways (Ku et al., 2020; Puel et al., 2022). Perhaps the best characterized and, arguably, the most closely related example is mycobacterial disease, which can be caused by inborn errors of type II IFN or auto-Abs neutralizing type II IFN (Ku et al., 2020; Puel et al., 2022; Figure 2A). These auto-Abs underlie severe disease caused by weakly virulent mycobacteria and, more rarely, TB (Puel et al., 2022; Shih et al., 2021), especially in East Asian populations, in which HLA-DRB1 alleles 15:02 and 16:02 are common (Ku et al., 2016; Shih et al., 2021).
Several other lines of evidence indicate that auto-Abs against type I IFNs are causal for critical COVID-19 (Figure 2D; Manry et al., 2022). First, they were found in samples taken before infection, in the cases for which such samples were available and tested (Bastard et al., 2020). Second, they appear early in childhood in patients with APS-1, in whom the course of COVID-19 is very severe (Bastard et al., 2021d). Third, they can underlie adverse reactions to the live attenuated yellow fever vaccine in patients not infected with SARS-CoV-2 (Bastard et al., 2021c); they were also recently found in about 20% of hypoxemic breakthrough COVID-19 cases in fully vaccinated subjects (Bastard et al., 2022b), and in 5% of patients with critical influenza pneumonia under 70 years old (Zhang et al., 2022). Fourth, they are found in the nasal mucosae of patients during the first few days of infection with SARS-CoV-2, during which they actually impair type I IFN responses (Lopez et al., 2021). Fifth, these auto-Abs are found in the general population, their frequency increasing with age and reaching 4% after the age of 70 years and 7% between the ages of 80 and 85 years (Bastard et al., 2021a). Overall, they account for 20% of COVID-19 deaths across all age groups. The discovery of exceedingly rare IEI of type I IFN immunity, first in patients with critical influenza and then in patients with critical COVID-19, led to the discovery of a common genetic etiology of critical COVID-19 pneumonia (XR TLR7 deficiency, underlying 1% of cases in male patients), and then to the detection of auto-Abs against type I IFNs, which underlie about 20% of COVID-19 deaths.
Concluding remarks
With a molecular explanation for about 1% of cases of pulmonary TB in European adults and 20% of cases of critical COVID-19 pneumonia across patients of most ages and ancestries (1%–5% due to inborn errors of type I IFN immunity and 15%–20% due to auto-Abs against type I IFNs), we are beginning to unravel the root causes of at least two common infectious diseases at individual level, and in a sizable proportion of individuals at population level. The proportion of cases of TB explained by HIV infection in developing countries may be as high as 15% (Kwan and Ernst, 2011), which, incidentally, also indicates that the development of TB is conditional on a human immunodeficiency, whether inherited or acquired. By analogy with COVID-19, it is tempting to speculate that auto-Abs against type II IFN might underlie a proportion of TB cases. This recent progress was made possible by the study of small numbers, and even, sometimes, single patients with either of these common infections (Casanova et al., 2014), and this approach was dependent on earlier studies of even fewer patients with rarer, related infections. The study of TB built on studies of MSMD, whereas that of COVID-19 built on studies of influenza. Why did this approach succeed? Probably because it led to the discovery of mechanisms of disease in individual patients, at the molecular, cellular, and immunological levels, with disease actually occurring in individual patients—“whole organisms”—rather than being considered in populations of patients, who do not form a “meta-organism.” Incidentally, the success of the patient-based, mechanistic approach, focusing on causes and consequences from atomic to whole-organism levels, may, to some extent, explain the difficulties encountered by the population-based, statistical approach, which is a topic to be discussed elsewhere. Hopefully, a confluence of the two approaches may be in sight, for the greater good of science and medicine.
Does it matter that we now understand these two life-threatening infectious diseases in a significant proportion of patients? The question can be posed, as the fields of microbiology and infectious diseases have historically focused on three other goals: (1) the discovery of microbes, their life cycle (cellular microbiology in vitro and animal models in vivo), and pharmacological approaches to interrupting this life cycle (drugs); (2) the development of vaccines: live attenuated microbes, then heat-killed microbes, recombinant proteins, and, more recently, mRNAs; and (3) the analysis of T and B cell adaptive responses to microbes, as a way of using antibodies or T cells to prevent or treat infection. The human genetic study of infectious diseases, a fourth approach that remains dwarfed by the others in terms of the volume of scientific publications, has always been seen as a third wheel. After all, why do we need to understand the root cause—the host determinant of infectious diseases—if we can prevent or treat these diseases without needing to resort to this knowledge? Investigators in this field have therefore sometimes been perceived as “conquerors of the useless” (Terray, 1961, 2008). COVID-19 provides a good example. We have witnessed over the last 2 years how research on COVID-19 has been extraordinarily focused on ways to prevent or treat this disease—as for all infections. Now that vaccines against SARS-CoV-2 seem to work relatively well, apparently even in type I IFN-deficient individuals, why does it matter that critical disease can be caused by deficiencies of type I IFNs? Would somatic oncogenes or germline tumor suppressor genes (e.g., BRCA1) have been discovered or their discovery found to be of interest if some herb tea could prevent cancer? We can think of two responses to this legitimate interrogation.
The first answer is medical. Fully understanding infectious diseases, from the angle of human determinants, has diagnostic and therapeutic implications. Microbes are becoming increasingly resistant to anti-infectious agents, as illustrated by the rapid spread of multidrug-resistant TB. Microbes can also resist vaccines, as illustrated by cases of “breakthrough” COVID-19 hypoxemic pneumonia (Bastard et al., 2021a; Bergwerk et al., 2021), or may escape vaccines altogether, as illustrated by the spread of pneumococcal serotypes not covered by current vaccines. Efficient vaccines can also be very difficult to develop in the first place, as illustrated for TB, malaria, and HIV. In the long term, it is not certain that we can win the arms race with microbes using only drugs and vaccines. Vaccines, in particular, disrupt both the current biodiversity and the natural evolution of the microbes they target. Viewed from this angle, it is interesting that the two infections for which we have developed a molecular understanding are caused by defects of immunity mediated by type I IFNs—antiviral molecules—and type II IFN, an antimycobacterial molecule, both of which are commercially available. The use of type II IFN in patients with TB or, as a preventive measure, in P1104A homozygotes living in areas of endemic TB would appear to be a reasonable approach worth testing. Likewise, type I IFN might be useful in patients prone to severe COVID-19 and infected with the virus, provided it is administered at an early stage of infection. The specific type I IFN to be used may be determined by the nature of the predisposition. IFN-α2 and IFN-β are good options in patients with a functional response pathway and without neutralizing auto-Abs against the IFN concerned, whereas mAbs neutralizing the virus would be a better option in patients with a defective type I IFN responsive pathway (Lévy et al., 2021).
The other response to the question is biological. The discovery of the molecular and cellular basis of interindividual clinical variability in the course of primary infection allows us to revisit immunology from the angle of host defense against pathogens at individual, whole-organism level, and in the conditions of a natural ecosystem (Casanova and Abel, 2004; Nathan, 2021; Pulendran and Davis, 2020; White and Caskey, 1988). This contrasts with the history of immunology, which, since 1881, has been constructed largely from the angle of vaccination and therefore from the perspective of antigen-specific responses but also on the basis of experiments conducted in vitro or in animal models in vivo, and, implicitly, at the level of a population considered to be uniform. Indeed, immunology has been built from the perspective of typology, with the idea that general principles apply not only to all individuals of a species but also to most, if not all animal species, and if not to all vertebrates, then at least all mammals. This assumption has proved to be largely correct for the general architecture of immunity and was spectacularly useful as a first step to combating infections medically. However, this paradigm obviously does not explain interindividual variability in the course of infection, whether in humans or in other species, and this is, or at least should be the quintessential immunological enigma. If anything, this paradigm prevents us from understanding this enigma because of its typologist nature. The host genetic approach is based on nominalism, which is itself rooted in the idea that each living organism is unique in space and time, as is each natural interaction between a microbe and a host, accounting for their infinite potential outcomes. The human genetic approach—a pathogen-centered, natural, nominalist approach—is already revealing increasing numbers of findings at odds with major immunological notions established in the antigen-centered, experimental, typologist tradition (Béziat et al., 2021b; Israel et al., 2017; Notarangelo et al., 2020; Yang et al., 2020; Zhang et al., 2018), potentially heralding a shift in the immunological paradigm (Kuhn, 1962; Polanyi, 1958; Timmins, 2013).
Using Stokes’ classification of science (Stokes, 1997), one can probably consider the search for human genetic and immunological determinants of infectious diseases as driven more by a “quest for fundamental understanding” than “considerations of use.” Indeed, the principles governing the prevention and cure of infectious diseases were established in 1881 by Louis Pasteur with the discovery of vaccination (Pasteur, 1922–1939) and in 1932 by Gehrard Domagk with the discovery of anti-infectious agents (Domagk, 1935). This contrasts with other fields of medical research, in which progress in medicine lags behind that in science. The field of infectious diseases is specific in that regard, and one might even wonder if its early medical success has not hampered its subsequent scientific development. This singularity is due to another specific feature of infectious diseases: their environmental component, the pathogen, is both known and necessary. There is little doubt that their infectious nature has also facilitated the discovery of underlying IEI, by permitting the study of patients sharing at least the same environmental cause of disease. Despite these two related and notable differences between infectious and non-infectious diseases, this “rare-to-common” genetic and mechanistic approach has been inspired by the study of other diseases. Indeed, the general mechanism of at least three other common conditions—hypertension (Lifton, 2004–2005), hypercholesterolemia (Goldstein and Brown, 2015; Horton et al., 2007), and diabetes (Yang and Chan, 2016)—was elegantly cracked by a similar “rare-to-common” approach. Collectively, these findings are of heuristic value. The documentation of causality between an inborn error and a clinical phenotype, together with clarification of the molecular and cellular mechanism of disease, in a few patients or even a single patient, can pave the way for the discovery of other causes, genetic, or otherwise, including autoimmune phenocopies, triggering the same mechanism of disease in many more patients. In other words, developing a deep understanding of disease in a single patient appears to be a better starting point than developing a superficial understanding of thousands of patients (Casanova et al., 2014). Decoding the molecular and cellular mechanisms of disease in a single patient seems to be more insightful and fruitful than estimating associations between genomic regions and disease in large populations. For physiological studies, the legacy of Mendel and Garrod has surpassed that of Galton and Pearson, whose legacy evidently turned out to be greater for evolutionary studies (Pickrell and Reich, 2014; Quintana-Murci, 2019).
Acknowledgments
We thank past and present members of the laboratory for stimulating discussions and incessant activity, and Jacques Fellay (University of Lausanne and EPFL, Switzerland), Philippe Gros (McGill University, Canada), and Isabelle Meyts (KU Leuven, Belgium) for very helpful suggestions on an earlier draft of this manuscript; Helen Hobbs (UTSW, TX) for helpful discussions; members of the COVID Human Genetic Effort; three anonymous referees for very constructive comments. The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, The Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (P01AI061093, R01AI088364, R01AI095983, R01AI127564, R01AI143810, R01AI163029, R01NS072381, R21AI137371, and U19AI162568), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1TR001866), the Fisher Center for Alzheimer's Research Foundation, the Meyer Foundation, the JPB Foundation, the Robertson Therapeutic Development Fund, the Tri-Institutional Stem Cell Initiative fund (Tri-SCI), the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), the FRM and ANR GENCOVID project (ANR-20-COVI-0003), ANRS Nord-Sud (ANRS-COV05), ANR grants SEAeHostFactors (ANR-18-CE15-0020-02), LTh-MSMD-CMCD (ANR-18-CE93-0008), CNSVIRGEN (ANR-19-CE15-0009-01), GENVIR (ANR-20-CE93-003), GENMSMD (ANR-16-C17-005), AABIFNCOV (ANR-20-CO11-0001), and GenMIS-C (ANR-21-COVR-0039), the ANR-RHU program (ANR-21-RHUS-08), the European Union’s Horizon 2020 research and innovation program under grant agreement no. 824110 (EASI-genomics), the HORIZON-HLTH-2021-DISEASE-04 program under grant agreement 01057100 (UNDINE), the Square Foundation, Grandir – Fonds de solidarité pour l’enfance, the SCOR Corporate Foundation for Science, Fondation du Souffle, Institut National de la Santé et de la Recherche Médicale (INSERM), REACTing-INSERM, and the University of Paris.
Author contributions
J.-L.C. and L.A. co-wrote the manuscript and secured the funding support.
Declaration of interests
J.-L.C. served on the scientific advisory boards of ADMA Biologics, Kymera Therapeutics, and Elixiron Immunotherapeutics. J.-L.C. is an inventor on patent application PCT/US2021/042741, filed July 22, 2021, submitted by The Rockefeller University that covers diagnosis of susceptibility to, and treatment of, viral disease and viral vaccines, including COVID-19 and vaccine-associated diseases.
References
- Abel L., Demenais F. Detection of major genes for susceptibility to leprosy and its subtypes in a Caribbean island: Desirade island. Am. J. Hum. Genet. 1988;42:256–266. [PMC free article] [PubMed] [Google Scholar]
- Abers M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 2021;99:917–921. doi: 10.1111/imcb.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolhassani H., Edwards E.S., Ikinciogullari A., Jing H., Borte S., Buggert M., Du L., Matsuda-Lennikov M., Romano R., Caridha R., et al. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J. Exp. Med. 2017;214:91–106. doi: 10.1084/jem.20160849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolhassani H., Landegren N., Bastard P., Materna M., Modaresi M., Du L., Aranda-Guillén M., Sardh F., Zuo F., Zhang P., et al. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J. Clin. Immunol. 2022;42:471–483. doi: 10.1007/s10875-022-01215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 2021;118:102598. doi: 10.1016/j.jaut.2021.102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbil B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. Early and rapid identification of COVID-19 patients with neutralizing Type I interferon auto-antibodies. J. Clin. Immunol. 2022 doi: 10.1007/s10875-022-01252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaïs A., Alter A., Antoni G., Orlova M., Nguyen V.T., Singh M., Vanderborght P.R., Katoch K., Mira M.T., Vu H.T., et al. Stepwise replication identifies a low-producing lymphotoxin-alpha allele as a major risk factor for early-onset leprosy. Nat. Genet. 2007;39:517–522. doi: 10.1038/ng2000. [DOI] [PubMed] [Google Scholar]
- Allison A.C. Protection afforded by sickle cell trait against subtertian malarian infection. BMJ. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosaimi M.F., Hoenig M., Jaber F., Platt C.D., Jones J., Wallace J., Debatin K.M., Schulz A., Jacobsen E., Möller P., et al. Immunodeficiency and EBV-induced lymphoproliferation caused by 4–1BB deficiency. J. Allergy Clin. Immunol. 2019;144:574–583.e5. doi: 10.1016/j.jaci.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F., Ensser A., Breiman A., Reichenbach J., Baghdadi J.E., Fischer A., Emile J.F., Gaillard J.L., Meinl E., Casanova J.L. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J. Infect. Dis. 2001;184:231–236. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- Andersen L.L., Mørk N., Reinert L.S., Kofod-Olsen E., Narita R., Jørgensen S.E., Skipper K.A., Höning K., Gad H.H., Østergaard L., et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J. Exp. Med. 2015;212:1371–1379. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021;6:eabl4348. doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S., Schlapbach L.J., Anchisi S., Hammer C., Bartha I., Junier T., Mottet-Osman G., Posfay-Barbe K.M., Longchamp D., Stocker M., et al. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc. Natl. Acad. Sci. USA. 2017;114:8342–8347. doi: 10.1073/pnas.1704259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audry M., Ciancanelli M., Yang K., Cobat A., Chang H.H., Sancho-Shimizu V., Lorenzo L., Niehues T., Reichenbach J., Li X.X., et al. NEMO is a key component of NF-kappaB- and IRF-3-dependent TLR3-mediated immunity to herpes simplex virus. J. Allergy Clin. Immunol. 2011;128:e611–e614. doi: 10.1016/j.jaci.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro L.B., Ben-Ali M., Quach H., Laval G., Patin E., Pickrell J.K., Bouchier C., Tichit M., Neyrolles O., Gicquel B., et al. Evolutionary dynamics of human toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 2021;6:eabl4340. doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Hsiao K.C., Zhang Q., Choin J., Best E., Chen J., Gervais A., Bizien L., Materna M., Harmant C., et al. A loss-of-function IFNAR1 allele in Polynesia underlies severe viral diseases in homozygotes. J. Exp. Med. 2022;219:e20220028. doi: 10.1084/jem.20220028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Manry J., Chen J., Rosain J., Seeleuthner Y., AbuZaitun O., Lorenzo L., Khan T., Hasek M., Hernandez N., et al. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Invest. 2021;131:e139980. doi: 10.1172/JCI139980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021;218:e20202486. doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021;218 doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021;218:e20210554. doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Vazquez S., Liu J., Laurie M.T., Gervais A., Le Voyer T., Bizien L., Zamecnik C., Philippot Q., Rosain J., et al. Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-Abs neutralizing type I IFNs. Sci. Immunol. 2022;7:abp8966. doi: 10.1126/sciimmunol.abp8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. Springer; 2008. Immunology, Phenotype First : How Mutations Have New Principles and Pathways in Immunology. [Google Scholar]
- Béziat V., Casanova J.L., Jouanguy E. Human genetic and immunological dissection of papillomavirus-driven diseases: new insights into their pathogenesis. Curr. Opin. Virol. 2021;51:9–15. doi: 10.1016/j.coviro.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat V., Rapaport F., Hu J., Titeux M., Bonnet des Claustres M., Bourgey M., Griffin H., Bandet É., Ma C.S., Sherkat R., et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell. 2021;184:3812–3828.e30. doi: 10.1016/j.cell.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffen R.H. Mendel's laws of inheritance and wheat breeding. J. Agric. Sci. 1905;1:4–48. [Google Scholar]
- Boisson-Dupuis S. The monogenic basis of human tuberculosis. Hum. Genet. 2020;139:1001–1009. doi: 10.1007/s00439-020-02126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dupuis S., Ramirez-Alejo N., Li Z., Patin E., Rao G., Kerner G., Lim C.K., Krementsov D.N., Hernandez N., Ma C.S., et al. Tuberculosis and impaired IL-23-dependent IFN-gamma immunity in humans homozygous for a common TYK2 missense variant. Sci. Immunol. 2018;3:eaau8714. doi: 10.1126/sciimmunol.aau8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolze A., Mahlaoui N., Byun M., Turner B., Trede N., Ellis S.R., Abhyankar A., Itan Y., Patin E., Brebner S., et al. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science. 2013;340:976–978. doi: 10.1126/science.1234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D.T., Markham A.F., Ginsburg D., Orkin S.H. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J. Clin. Invest. 1985;76:894–897. doi: 10.1172/JCI112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.G., Dokun A.O., Heusel J.W., Smith H.R., Beckman D.L., Blattenberger E.A., Dubbelde C.E., Stone L.R., Scalzo A.A., Yokoyama W.M. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- Bruton O.C. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [PubMed] [Google Scholar]
- Busnadiego I., Abela I.A., Frey P.M., Hofmaenner D.A., Scheier T.C., Schuepbach R.A., Buehler P., Brugger S.D., Hale B.G. Herpesvirus reactivations in Critically-1 ill COVID-19 patients with autoantibodies neutralizing Type I interferons. medRxiv. 2022 doi: 10.1101/2022.03.19.22272532. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. Mendelian susceptibility to mycobacterial disease: recent discoveries. Hum. Genet. 2020;139:993–1000. doi: 10.1007/s00439-020-02120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K., Motyer A., Vukcevic D., Delaneau O., O'Connell J., et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T.M., Liu Z., Zhang Q., Moncada-Velez M., Covill L.E., Zhang P., Alavi Darazam I., Bastard P., Bizien L., Bucciol G., et al. Respiratory viral infections in otherwise healthy humans with inherited IRF7 deficiency. J. Exp. Med. 2022;219:e20220202. doi: 10.1084/jem.20220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caorsi R., Rusmini M., Volpi S., Chiesa S., Pastorino C., Sementa A.R., Uva P., Grossi A., Lanino E., Faraci M., et al. CD70 deficiency due to a novel mutation in a patient with severe chronic EBV infection presenting as a periodic fever. Front. Immunol. 2017;8:2015. doi: 10.3389/fimmu.2017.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapito R., Li R., Helms J., Carapito C., Gujja S., Rolli V., Guimaraes R., Malagon-Lopez J., Spinnhirny P., Lederle A., et al. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl. Med. 2021;14:eabj7521. doi: 10.1126/scitranslmed.abj7521. [DOI] [PubMed] [Google Scholar]
- Casanova J.L. Human genetic basis of interindividual variability in the course of infection. Proc. Natl. Acad. Sci. USA. 2015;112:E7118–E7127. doi: 10.1073/pnas.1521644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc. Natl. Acad. Sci. USA. 2015;112:E7128–E7137. doi: 10.1073/pnas.1521651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 2004;4:55–66. doi: 10.1038/nri1264. [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Inborn errors of immunity to infection: the rule rather than the exception. J. Exp. Med. 2005;202:197–201. doi: 10.1084/jem.20050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu. Rev. Genomics Hum. Genet. 2013;14:215–243. doi: 10.1146/annurev-genom-091212-153448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity? Hum. Genet. 2020;139:681–694. doi: 10.1007/s00439-020-02184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Lethal infectious diseases as inborn errors of immunity: Toward a synthesis of the germ and genetic theories. Annu. Rev. Pathol. 2021;16:23–50. doi: 10.1146/annurev-pathol-031920-101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Mechanisms of viral inflammation and disease in humans. Science. 2021;374:1080–1086. doi: 10.1126/science.abj7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L., Quintana-Murci L. Immunology taught by human genetics. Cold Spring Harbor Symp. Quant. Biol. 2013;78:157–172. doi: 10.1101/sqb.2013.78.019968. [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Conley M.E., Seligman S.J., Abel L., Notarangelo L.D. Guidelines for genetic studies in single patients: lessons from primary immunodeficiencies. J. Exp. Med. 2014;211:2137–2149. doi: 10.1084/jem.20140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Zhang Q., Bastard P., Jouanguy E. In memoriam: Stephen J Seligman, MD : adverse reactions to the yellow fever vaccine: from epidemiological risk factors to causes and mechanisms. J. Clin. Immunol. 2022;42:437–440. [Google Scholar]
- Casrouge A., Zhang S.Y., Eidenschenk C., Jouanguy E., Puel A., Yang K., Alcais A., Picard C., Mahfoufi N., Nicolas N., et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Chang S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021;12:5417. doi: 10.1038/s41467-021-25509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvineau-Grenier A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., Cobat A., Casanova J.L., Rossi B. Autoantibodies neutralizing Type I interferons in 20% of COVID-19 deaths in a French hospital. J. Clin. Immunol. 2022;42:459–470. doi: 10.1007/s10875-021-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Graf L., Chen T., Liao Q., Bai T., Petric P.P., Zhu W., Yang L., Dong J., Lu J., et al. Rare variant MX1 alleles increase human susceptibility to zoonotic H7N9 influenza virus. Science. 2021;373:918–922. doi: 10.1126/science.abg5953. [DOI] [PubMed] [Google Scholar]
- Christensen P.E., Schmidt H., Jensen O., Bang H.O., Andersen V., Jordal B. An epidemic of measles in Southern Greenland, 1951. I. Measles in virgin soil. Acta Med. Scand. 1953;144:313–322. doi: 10.1111/j.0954-6820.1953.tb15701.x. [DOI] [PubMed] [Google Scholar]
- Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S., Lafaille F.G., Trouillet C., Schmolke M., Albrecht R.A., et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey A.J., Brooksbank R.A., Brandau O., Oohashi T., Howell G.R., Bye J.M., Cahn A.P., Durham J., Heath P., Wray P., et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- Corvilain E., Casanova J.L., Puel A. Inherited CARD9 deficiency: invasive disease caused by ascomycete fungi in previously healthy children and adults. J. Clin. Immunol. 2018;38:656–693. doi: 10.1007/s10875-018-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 National Preparedness Collaborators Pandemic preparedness and COVID-19: an exploratory analysis of infection and fatality rates, and contextual factors associated with preparedness in 177 countries, from Jan 1, 2020, to Sept 30, 2021. Lancet. 2022;399:1489–1512. doi: 10.1016/S0140-6736(22)00172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J.L., Jones J.D. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L., Samarina A., Bustamante J., Cobat A., Boisson-Dupuis S., Feinberg J., Al-Muhsen S., Jannière L., Rose Y., de Suremain M., et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong S.J., Créquer A., Matos I., Hum D., Gunasekharan V., Lorenzo L., Jabot-Hanin F., Imahorn E., Arias A.A., Vahidnezhad H., et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to beta-papillomaviruses. J. Exp. Med. 2018;215:2289–2310. doi: 10.1084/jem.20170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Carrington M., Winkler C., Huttley G.A., Smith M.W., Allikmets R., Goedert J.J., Buchbinder S.P., Vittinghoff E., Gomperts E., et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Domagk G. Ein Beitrag zur Chemotherapie der Bakteriellen Infektionen. Dtsch. Med. Wochenschr. 1935;61:250–253. [Google Scholar]
- Drutman S.B., Mansouri D., Mahdaviani S.A., Neehus A.L., Hum D., Bryk R., Hernandez N., Belkaya S., Rapaport F., Bigio B., et al. Fatal Cytomegalovirus infection in an adult with inherited NOS2 deficiency. N. Engl. J. Med. 2020;382:437–445. doi: 10.1056/NEJMoa1910640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C.J.A., Randall R.E., Hambleton S. Genetic lesions of Type I interferon signalling in human antiviral immunity. Trends Genet. 2021;37:46–58. doi: 10.1016/j.tig.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C.J.A., Skouboe M.K., Howarth S., Hollensen A.K., Chen R., Børresen M.L., Thompson B.J., Stremenova Spegarova J., Hatton C.F., Stæger F.F., et al. Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J. Exp. Med. 2022;219:e20212427. doi: 10.1084/jem.20212427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis S., Jouanguy E., Al-Hajjar S., Fieschi C., Al-Mohsen I.Z., Al-Jumaah S., Yang K., Chapgier A., Eidenschenk C., Eid P., et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- Eto S., Nukui Y., Tsumura M., Nakagama Y., Kashimada K., Mizoguchi Y., Utsumi T., Taniguchi M., Sakura F., Noma K., et al. Neutralizing Type I interferon autoantibodies in Japanese patients With severe COVID-19. Res Sq. 2022 doi: 10.21203/rs.3.rs-1430985/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H.H. Inheritance of pathogenicity in a cross between physiologic races 22 and 24 of Melampsora lini. Phytopathology. 1942;32:5. [Google Scholar]
- Frasca F., Scordio M., Santinelli L., Gabriele L., Gandini O., Criniti A., Pierangeli A., Angeloni A., Mastroianni C.M., d'Ettorre G., et al. Anti-IFN-alpha/-omega neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 2022;52:1120–1128. doi: 10.1002/eji.202249824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freij B.J., Hanrath A.T., Chen R., Hambleton S., Duncan C.J.A. Life-threatening influenza, hemophagocytic lymphohistiocytosis and probable vaccine-strain varicella in a novel case of homozygous STAT2 deficiency. Front. Immunol. 2020;11:624415. doi: 10.3389/fimmu.2020.624415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Ciancanelli M.J., Zhang P., Harschnitz O., Bondet V., Hasek M., Chen J., Mu X., Itan Y., Cobat A., et al. TLR3 controls constitutive IFN-beta antiviral immunity in human fibroblasts and cortical neurons. J. Clin. Invest. 2021;131:e134529. doi: 10.1172/JCI134529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod A.E. Clarendon Press; 1931. The Inborn Factors in Disease. [Google Scholar]
- Ge D., Fellay J., Thompson A.J., Simon J.S., Shianna K.V., Urban T.J., Heinzen E.L., Qiu P., Bertelsen A.H., Muir A.J., et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Glocker E.O., Hennigs A., Nabavi M., Schäffer A.A., Woellner C., Salzer U., Pfeifer D., Veelken H., Warnatz K., Tahami F., et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L., Brown M.S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., Pescarmona R., Lombard C., Walzer T., Casanova J.L., et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl. Immunol. 2021;10:e1327. doi: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.R., Godiard L., Straube E., Ashfield T., Lewald J., Sattler A., Innes R.W., Dangl J.L. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science. 1995;269:843–846. doi: 10.1126/science.7638602. [DOI] [PubMed] [Google Scholar]
- Guérin A., Kerner G., Marr N., Markle J.G., Fenollar F., Wong N., Boughorbel S., Avery D.T., Ma C.S., Bougarn S., et al. IRF4 haploinsufficiency in a family with Whipple's disease. eLife. 2018;7:e32340. doi: 10.7554/eLife.32340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane J.B. Disease and evolution. Ric. Sci. 1949;19:68–76. [Google Scholar]
- Haller O., Arnheiter H., Pavlovic J., Staeheli P. The discovery of the antiviral resistance gene Mx: A story of great ideas, great failures, and some success. Annu. Rev. Virol. 2018;5:33–51. doi: 10.1146/annurev-virology-092917-043525. [DOI] [PubMed] [Google Scholar]
- Herman M., Ciancanelli M., Ou Y.H., Lorenzo L., Klaudel-Dreszler M., Pauwels E., Sancho-Shimizu V., Pérez de Diego R., Abhyankar A., Israelsson E., et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 2012;209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Melki I., Jing H., Habib T., Huang S.S.Y., Danielson J., Kula T., Drutman S., Belkaya S., Rattina V., et al. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J. Exp. Med. 2018;215:2567–2585. doi: 10.1084/jem.20180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Cohen J.C., Hobbs H.H. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck K., Feyen O., Niehues T., Rüschendorf F., Hübner N., Laws H.J., Telieps T., Knapp S., Wacker H.H., Meindl A., et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J. Clin. Invest. 2009;119:1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye E.S., Anderson M.S., Kämpe O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 2018;378:2543–2544. doi: 10.1056/NEJMc1805308. [DOI] [PubMed] [Google Scholar]
- COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel L., Wang Y., Bulek K., Della Mina E., Zhang Z., Pedergnana V., Chrabieh M., Lemmens N.A., Sancho-Shimizu V., Descatoire M., et al. Human adaptive immunity rescues an inborn error of innate immunity. Cell. 2017;168:789–800.e10. doi: 10.1016/j.cell.2017.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa K., Martin E., Soudais C., Bruneau J., Boutboul D., Rodriguez R., Lenoir C., Hislop A.D., Besson C., Touzot F., et al. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J. Exp. Med. 2017;214:73–89. doi: 10.1084/jem.20160784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C.C., Dickson M.A., Sadjadi M., Gessain A., Abel L., Jouanguy E., Casanova J.L. Kaposi sarcoma of childhood: inborn or acquired immunodeficiency to oncogenic HHV-8. Pediatr. Blood Cancer. 2016;63:392–397. doi: 10.1002/pbc.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:eaaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- Jouanguy E., Altare F., Lamhamedi S., Revy P., Emile J.F., Newport M., Levin M., Blanche S., Seboun E., Fischer A., et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille calmette-guerin infection. N. Engl. J. Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- Kaslow R.A., Carrington M., Apple R., Park L., Muñoz A., Saah A.J., Goedert J.J., Winkler C., O'Brien S.J., Rinaldo C., et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- Kerner G., Laval G., Patin E., Boisson-Dupuis S., Abel L., Casanova J.L., Quintana-Murci L. Human ancient DNA analyses reveal the high burden of tuberculosis in Europeans over the last 2,000 years. Am. J. Hum. Genet. 2021;108:517–524. doi: 10.1016/j.ajhg.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner G., Ramirez-Alejo N., Seeleuthner Y., Yang R., Ogishi M., Cobat A., Patin E., Quintana-Murci L., Boisson-Dupuis S., Casanova J.L., et al. Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc. Natl. Acad. Sci. USA. 2019;116:10430–10434. doi: 10.1073/pnas.1903561116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanmohammadi S., Rezaei N., Khazaei M., Shirkani A. A case of autosomal recessive interferon alpha/beta receptor alpha chain (IFNAR1) deficiency with severe COVID-19. J. Clin. Immunol. 2022;42:19–24. doi: 10.1007/s10875-021-01166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt T.J., Capra J.D. Plenum Press; 1984. The Antibody Enigma. [Google Scholar]
- Koch R. Die Aetiologie der Tuberkulose. Berl. Klin. Wochenschr. 1882;19:221–230. [Google Scholar]
- Koning R., Bastard P., Casanova J.L., Brouwer M.C., van de Beek D., the Amsterdam U.M.C. COVID-19 Biobank Investigators Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021;47:704–706. doi: 10.1007/s00134-021-06392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostmann R. Hereditär reticulos—en ny systemsjukdom. Sven. Läkartdin. 1950;47:2861. [Google Scholar]
- Kostmann R. Infantile genetic agranulocytosis. Acta Paediatr. Scand. 1956;45:1–78. [PubMed] [Google Scholar]
- Kousathanas A., Pairo-Castineira E., Rawlik K., Stuckey A., Odhams C.A., Walker S., Russell C.D., Malinauskas T., Wu Y., Millar J., et al. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature. 2022;607:97–103. doi: 10.1038/s41586-022-04576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreins A.Y., Ciancanelli M.J., Okada S., Kong X.-F., Ramírez-Alejo N., Kilic S.S., El Baghdadi J., Nonoyama S., Mahdaviani S.A., Ailal F., et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J. Exp. Med. 2015;212:1641–1662. doi: 10.1084/jem.20140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.L., Chi C.Y., von Bernuth H., Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum. Genet. 2020;139:783–794. doi: 10.1007/s00439-020-02180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.L., Lin C.H., Chang S.W., Chu C.C., Chan J.F., Kong X.F., Lee C.H., Rosen E.A., Ding J.Y., Lee W.I., et al. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR∗15. J. Allergy Clin. Immunol. 2016;137:945–948.e8. doi: 10.1016/j.jaci.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Kuhn T.S. First Edition. University of Chicago Press; 1962. The Structure of Scientific Revolutions. [Google Scholar]
- Kwan C.K., Ernst J.D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille F.G., Harschnitz O., Lee Y.S., Zhang P., Hasek M.L., Kerner G., Itan Y., Ewaleifoh O., Rapaport F., Carlile T.M., et al. Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis. Nat. Med. 2019;25:1873–1884. doi: 10.1038/s41591-019-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille F.G., Pessach I.M., Zhang S.Y., Ciancanelli M.J., Herman M., Abhyankar A., Ying S.W., Keros S., Goldstein P.A., Mostoslavsky G., et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia G., Mazzoni A., Spinicci M., et al. Clinical and Immunological Features of SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals Requiring Hospitalization. J. Clin. Immunol. 2022 doi: 10.1007/s10875-022-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamborn I.T., Jing H., Zhang Y., Drutman S.B., Abbott J.K., Munir S., Bade S., Murdock H.M., Santos C.P., Brock L.G., et al. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J. Exp. Med. 2017;214:1949–1972. doi: 10.1084/jem.20161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner K. Dover Publications, Inc; 1936. The Specificity of Serological Reactions. [Google Scholar]
- Lanternier F., Barbati E., Meinzer U., Liu L., Pedergnana V., Migaud M., Héritier S., Chomton M., Frémond M.L., Gonzales E., et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J. Infect. Dis. 2015;211:1241–1250. doi: 10.1093/infdis/jiu412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F., Pathan S., Vincent Q.B., Liu L., Cypowyj S., Prando C., Migaud M., Taibi L., Ammar-Khodja A., Stambouli O.B., et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013;369:1704–1714. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Voyer T., Sakata S., Tsumura M., Khan T., Esteve-Sole A., Al-Saud B.K., Gungor H.E., Taur P., Jeanne-Julien V., Christiansen M., et al. Genetic, immunological, and clinical features of 32 patients with autosomal recessive STAT1 deficiency. J. Immunol. 2021;207:133–152. doi: 10.4049/jimmunol.2001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarquis A., Campbell T., Aranda-Guillen M., Hennings V., Brodin P., Kämpe O., Blennow K., Zetterberg H., Wennerås C., Eriksson K., et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy. Clin. Immunol. 2021;148:96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy R., Zhang P., Bastard P., Dorgham K., Melki I., Hadchouel A., Hartoularos G.C., Neven B., Castelle M., Roy C., et al. Monoclonal antibody-mediated neutralization of SARS-CoV-2 in an IRF9-deficient child. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2114390118. e2114390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ritelli M., Ma C.S., Rao G., Habib T., Corvilain E., Bougarn S., Cypowyj S., Grodecká L., Lévy R., et al. Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-beta. Sci. Immunol. 2019;4:eaax7965. doi: 10.1126/sciimmunol.aax7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifton R.P. Genetic dissection of human blood pressure variation: common pathways from rare phenotypes. Harvey Lect. 2004-2005;100:71–101. [PubMed] [Google Scholar]
- Lim D., Gewurz A., Lint T.F., Ghaze M., Sepheri B., Gewurz H. Absence of the sixth component of complement in a patient with repeated episodes of meningococcal meningitis. J. Pediatr. 1976;89:42–47. doi: 10.1016/s0022-3476(76)80924-9. [DOI] [PubMed] [Google Scholar]
- Lim H.K., Huang S.X.L., Chen J., Kerner G., Gilliaux O., Bastard P., Dobbs K., Hernandez N., Goudin N., Hasek M.L., et al. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 2019;216:2038–2056. doi: 10.1084/jem.20181621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Lopez J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., Villard M., Lina B., Richard J.C., Fassier J.B., et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 2021;218:e20211211. doi: 10.1084/jem.20211211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie M. Heredity, constitution and tuberculosis: an experimental study. Am. Rev. Tuberc. 1941;44:1–125. [Google Scholar]
- Lutz W. A propos de l'épidermodysplasie verruciforme. Dermatologica. 1946;92:30–43. [PubMed] [Google Scholar]
- Malaria Genomic Epidemiology Network Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania. Nat. Commun. 2019;10:5732. doi: 10.1038/s41467-019-13480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manry J., Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2200413119. e2200413119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet S., Abel L., Hillaire D., Dessein H., Kalil J., Feingold J., Weissenbach J., Dessein A.J. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- Martin E., Palmic N., Sanquer S., Lenoir C., Hauck F., Mongellaz C., Fabrega S., Nitschké P., Esposti M.D., Schwartzentruber J. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510:288–292. doi: 10.1038/nature13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.B., Brommonschenkel S.H., Chunwongse J., Frary A., Ganal M.W., Spivey R., Wu T., Earle E.D., Tanksley S.D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Martínez-Barricarte R., Markle J.G., Ma C.S., Deenick E.K., Ramírez-Alejo N., Mele F., Latorre D., Mahdaviani S.A., Aytekin C., Mansouri D., et al. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018;3:eaau6759. doi: 10.1126/sciimmunol.aau6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M., Buta S., Le Voyer T., Li Z., Dynesen L.T., Vuillier F., Franklin L., Ailal F., Muglia Amancio A., Malle L., et al. A partial form of inherited human USP18 deficiency underlies infection and inflammation. J. Exp. Med. 2022;219 doi: 10.1084/jem.20211273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathian A., Breillat P., Dorgham K., et al. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Ann. Rheum. Dis. 2022 doi: 10.1136/ard-2022-222549. [DOI] [PubMed] [Google Scholar]
- Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C., Akbil B., Meyer T., et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 2021;131 doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.H., Mason S.J., Clyde D.F., McGinniss M.H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood- group genotype, FyFy. N. Engl. J. Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Mira M.T., Alcaïs A., Nguyen V.T., Moraes M.O., Di Flumeri C., Vu H.T., Mai C.P., Nguyen T.H., Nguyen N.B., Pham X.K., et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636–640. doi: 10.1038/nature02326. [DOI] [PubMed] [Google Scholar]
- Nathan C. Rethinking immunology. Science. 2021;373:276–277. doi: 10.1126/science.abj5637. [DOI] [PubMed] [Google Scholar]
- Nathan C.F., Murray H.W., Wiebe M.E., Rubin B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport M.J., Huxley C.M., Huston S., Hawrylowicz C.M., Oostra B.A., Williamson R., Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- Nichols K.E., Harkin D.P., Levitz S., Krainer M., Kolquist K.A., Genovese C., Bernard A., Ferguson M., Zuo L., Snyder E., et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle C. Les infections inapparentes. Scientia. 1933:181–271. [Google Scholar]
- Notarangelo L.D., Bacchetta R., Casanova J.L., Su H.C. Human inborn errors of immunity: an expanding universe. Sci. Immunol. 2020;5:eabb1662. doi: 10.1126/sciimmunol.abb1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll M., Ribeiro dos Santos G., Wang L., Cummings D.A.T., Azman A.S., Paireau J., Fontanet A., Cauchemez S., Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- Ogishi M., Arias A.A., Yang R., Han J.E., Zhang P., Halpern J., Mulwa J., Keating N., Chrabieh M., Lainé C. Impaired IL-23-dependent induction of IFN-γ underlies mycobacterial disease in patients with inherited TYK2 deficiency. J. Exp. Med. 2022 doi: 10.1084/jem.20220094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunjimi B., Zhang S.Y., Sørensen K.B., Skipper K.A., Carter-Timofte M., Kerner G., Luecke S., Prabakaran T., Cai Y., Meester J., et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J. Clin. Invest. 2017;127:3543–3556. doi: 10.1172/JCI92280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr. Top. Microbiol. Immunol. 2008;321:59–83. doi: 10.1007/978-3-540-75203-5_3. [DOI] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Favre M., Croissant O., Jarzabek-Chorzelska M., Rzesa G. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc. Natl. Acad. Sci. USA. 1978;75:1537–1541. doi: 10.1073/pnas.75.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., Walker S., Parkinson N., Fourman M.H., Russell C.D., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- Palendira U., Low C., Bell A.I., Ma C.S., Abbott R.J., Phan T.G., Riminton D.S., Choo S., Smart J.M., Lougaris V., et al. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J. Exp. Med. 2012;209:913–924. doi: 10.1084/jem.20112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palendira U., Low C., Chan A., Hislop A.D., Ho E., Phan T.G., Deenick E., Cook M.C., Riminton D.S., Choo S., et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 2011;9:e1001187. doi: 10.1371/journal.pbio.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan S.R., Pradeu T., Masters S.L., Mogensen T.H. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021;21:137–150. doi: 10.1038/s41577-020-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteur L. Masson et Cie; 1922–1939. Oeuvres complètes de Louis Pasteur, réunies par Pasteur Vallery-Radot. [Google Scholar]
- Paulson T. Epidemiology: a mortal foe. Nature. 2013;502:S2–S3. doi: 10.1038/502S2a. [DOI] [PubMed] [Google Scholar]
- Pearson K. The Galton Laboratory for national eugenics: Dulau and Co., Ltd.; 1912. Tuberculosis, heredity and environment; p. 46. [Google Scholar]
- Pérez de Diego R., Sancho-Shimizu V., Lorenzo L., Puel A., Plancoulaine S., Picard C., Herman M., Cardon A., Durandy A., Bustamante J., et al. Human TRAF3 adaptor molecule deficiency leads to impaired toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.H., Graham J.A., Brooks G.F. Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J. Clin. Invest. 1976;57:283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., von Bernuth H., Ghandil P., Chrabieh M., Levy O., Arkwright P.D., McDonald D., Geha R.S., Takada H., Krause J.C., et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine. 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell J.K., Reich D. Toward a new history and geography of human genes informed by ancient DNA. Trends Genet. 2014;30:377–389. doi: 10.1016/j.tig.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanyi M. University of Chicago Press; 1958. Personal knowledge. [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C.V., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Pozzetto B., Mogensen K.E., Tovey M.G., Gresser I. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J. Infect. Dis. 1984;150:707–713. doi: 10.1093/infdis/150.5.707. [DOI] [PubMed] [Google Scholar]
- Puel A. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum. Genet. 2020;139:1011–1022. doi: 10.1007/s00439-020-02141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Bastard P., Bustamante J., Casanova J.L. Human autoantibodies underlying infectious diseases. J. Exp. Med. 2022;219:e20211387. doi: 10.1084/jem.20211387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Casanova J.L. Comment on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science. 2021;373:eabi5459. doi: 10.1126/science.abi5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Cypowyj S., Bustamante J., Wright J.F., Liu L., Lim H.K., Migaud M., Israel L., Chrabieh M., Audry M., et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffer R. Vol. 5. Harvard University Press; 1944. Familial Susceptibility to Tuberculosis; Its Importance as a Public Health Problem. [Google Scholar]
- Pulendran B., Davis M.M. The science and medicine of human immunology. Science. 2020;369:eaay4014. doi: 10.1126/science.aay4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D.T., Cassel C., Yang J.P. Letter: fatal infectious mononucleosis in familial lymphohistiocytosis. N. Engl. J. Med. 1974;291:736. doi: 10.1056/nejm197410032911415. [DOI] [PubMed] [Google Scholar]
- Purtilo D.T., DeFlorio D., Jr., Hutt L.M., Bhawan J., Yang J.P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N. Engl. J. Med. 1977;297:1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Quintana-Murci L. Human immunology through the lens of evolutionary genetics. Cell. 2019;177:184–199. doi: 10.1016/j.cell.2019.02.033. [DOI] [PubMed] [Google Scholar]
- Quintana-Murci L., Alcaïs A., Abel L., Casanova J.L. Immunology in natura: clinical, epidemiological and evolutionary genetics of infectious diseases. Nat. Immunol. 2007;8:1165–1171. doi: 10.1038/ni1535. [DOI] [PubMed] [Google Scholar]
- Raadsen M.P., Gharbharan A., Jordans C.C.E., Mykytyn A.Z., Lamers M.M., van den Doel P.B., Endeman H., van den Akker J.P.C., GeurtsvanKessel C.H., Koopmans M.P.G., et al. Interferon-alpha2 auto-antibodies in convalescent plasma therapy for COVID-19. J. Clin. Immunol. 2022;42:232–239. doi: 10.1007/s10875-021-01168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoz N., Rueda L.A., Bouadjar B., Montoya L.S., Orth G., Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002;32:579–581. doi: 10.1038/ng1044. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Fournier B., Cordeiro D.J., Winter S., Izawa K., Martin E., Boutboul D., Lenoir C., Fraitag S., Kracker S., et al. Concomitant PIK3CD and TNFRSF9 deficiencies cause chronic active Epstein-Barr virus infection of T cells. J. Exp. Med. 2019;216:2800–2818. doi: 10.1084/jem.20190678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C., et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Sancho-Shimizu V., Pérez de Diego R., Lorenzo L., Halwani R., Alangari A., Israelsson E., Fabrega S., Cardon A., Maluenda J., Tatematsu M., et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J. Clin. Invest. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvateeva E., Filippova M., Valuev-Elliston V., Nuralieva N., Yukina M., Troshina E., Baklaushev V., Ivanov A., Gryadunov D. Microarray-based detection of antibodies against SARS-CoV-2 proteins, common respiratory viruses and Type I interferons. Viruses. 2021;13:2553. doi: 10.3390/v13122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayos J., Wu C., Morra M., Wang N., Zhang X., Allen D., van Schaik S., Notarangelo L., Geha R., Roncarolo M.G., et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Peters S., Knaus A., Sabir H., Hamsen F., Maj C., Fazaal J., Sivalingam S., Savchenko O., Mantri A., et al. TBK1 and TNFRSF13B mutations and an autoinflammatory disease in a child with lethal COVID-19. NPJ Genom. Med. 2021;6:55. doi: 10.1038/s41525-021-00220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks G.D., Lee S.E., Howard A., Brundage J.F. Extreme mortality after first introduction of measles virus to the Polynesian island of Rotuma, 1911. Am. J. Epidemiol. 2011;173:1211–1222. doi: 10.1093/aje/kwq504. [DOI] [PubMed] [Google Scholar]
- Shih H.P., Ding J.Y., Yeh C.F., Chi C.Y., Ku C.L. Anti-interferon-gamma autoantibody-associated immunodeficiency. Curr. Opin. Immunol. 2021;72:206–214. doi: 10.1016/j.coi.2021.05.007. [DOI] [PubMed] [Google Scholar]
- Simula E.R., Manca M.A., Noli M., Jasemi S., Ruberto S., Uzzau S., Rubino S., Manca P., Sechi L.A. Increased Presence of Antibodies against Type I Interferons and Human Endogenous Retrovirus W in Intensive Care Unit COVID-19 Patients. Microbiol. Spectr. 2022 doi: 10.1128/spectrum.01280-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P.A., St Charles C., Taylor B.A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982;297:506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Solanich X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., Perez-Fernandez X.L., Fuset-Cabanes M.P., Gordillo-Benitez M.Á., Suarez-Cuartin G., et al. Pre-existing autoantibodies neutralizing high concentrations of Type I interferons in almost 10% of COVID-19 patients admitted to intensive care in Barcelona. J. Clin. Immunol. 2021;41:1733–1744. doi: 10.1007/s10875-021-01136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sologuren I., Martínez-Saavedra M.T., Solé-Violán J., de Borges de Oliveira E., Jr., Betancor E., Casas I., Oleaga-Quintas C., Martínez-Gallo M., Zhang S.Y., Pestano J., et al. Lethal influenza in two related adults with inherited GATA2 deficiency. J. Clin. Immunol. 2018;38:513–526. doi: 10.1007/s10875-018-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani-Zangbar M.S., Parhizkar F., Ghaedi E., Tarbiat A., Motavalli R., Alizadegan A., Aghebati-Maleki L., Rostamzadeh D., Yousefzadeh Y., Jadideslam G., et al. A comprehensive evaluation of the immune system response and type-I Interferon signaling pathway in hospitalized COVID-19 patients. Cell. Commun. Signal. 2022;20:106. doi: 10.1186/s12964-022-00903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekh I., Thian M., Medgyesi D., Gülez N., Magg T., Gallón Duque A., Stauber T., Lev A., Genel F., Unal E., et al. CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis. Blood. 2019;134:1510–1516. doi: 10.1182/blood.2019000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T.I., Nielsen G.G., Andersen P.K., Teasdale T.W. Genetic and environmental influences on premature death in adult adoptees. N. Engl. J. Med. 1988;318:727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986;44:147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Stark G.R., Darnell J.E., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G.R., Kerr I.M., Williams B.R., Silverman R.H., Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stokes D.E. Brookings Institution Press; 1997. Pasteur's Quadrant: Basic Science and Technological Innovation. [Google Scholar]
- Tangye S.G. Genetic susceptibility to EBV infection: insights from inborn errors of immunity. Hum. Genet. 2020;139:885–901. doi: 10.1007/s00439-020-02145-3. [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Latour S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood. 2020;135:644–655. doi: 10.1182/blood.2019000928. [DOI] [PubMed] [Google Scholar]
- Terray L. Gallimard; 1961. Les conquérants de l'inutile. Des Alpes à l'Annapurna. [Google Scholar]
- Terray L. Mountaineers Books; 2008. Conquistadors of the useless. From the Alps to Annapurna. [Google Scholar]
- Thorball C.W., Fellay J., Borghesi A. Immunological lessons from genome-wide association studies of infections. Curr. Opin. Immunol. 2021;72:87–93. doi: 10.1016/j.coi.2021.03.017. [DOI] [PubMed] [Google Scholar]
- Timmins A. Why was Kuhn’s structure more successful than Polanyi’s personal knowledge? The J. Int. Soc. Hist. Philos. Sci. 2013;3:306–317. [Google Scholar]
- Tournamille C., Colin Y., Cartron J.P., Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat. Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- Troya J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martínez A., Abel L., Casanova J.L., et al. Neutralizing autoantibodies to Type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J. Clin. Immunol. 2021;41:914–922. doi: 10.1007/s10875-021-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021;13:eabh2624. doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfrans J.M., Hoepelman A.I., Otto S., van Gijn M., van de Corput L., de Weger R.A., Monaco-Shawver L., Banerjee P.P., Sanders E.A., Jol-van der Zijde C.M., et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J. Allergy Clin. Immunol. 2012;129:787–793.e6. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez S.E., Bastard P., Kelly K., Gervais A., Norris P.J., Dumont L.J., Casanova J.L., Anderson M.S., DeRisi J.L. Neutralizing autoantibodies to Type I interferons in COVID-19 convalescent donor plasma. J. Clin. Immunol. 2021;41:1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S.M., Malo D., Marquis J.F., Gros P. Forward genetic dissection of immunity to infection in the mouse. Annu. Rev. Immunol. 2008;26:81–132. doi: 10.1146/annurev.immunol.26.021607.090304. [DOI] [PubMed] [Google Scholar]
- Vidal S.M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Vinh D.C., Abel L., Bastard P., Cheng M.P., Condino-Neto A., Gregersen P.K., Haerynck F., Cicalese M.P., Hagin D., Soler-Palacín P., et al. Harnessing Type I IFN immunity Against SARS-CoV-2 with early administration of IFN-beta. J. Clin. Immunol. 2021;41:1425–1442. doi: 10.1007/s10875-021-01068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Pirquet C. Frequency of tuberculosis in childhood. JAMA. 1909;LII:675–678. [Google Scholar]
- Walker R.S., Sattenspiel L., Hill K.R. Mortality from contact-related epidemics among indigenous populations in Greater Amazonia. Sci. Rep. 2015;5:14032. doi: 10.1038/srep14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J.E., Rosen L.B., Csomos K., Rosenberg J.M., Mathew D., Keszei M., Ujhazi B., Chen K., Lee Y.N., Tirosh I., et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 2015;125:4135–4148. doi: 10.1172/JCI80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Webster L.T. Heredity in infectious disease. J. Hered. 1939;30:365–370. [Google Scholar]
- White R., Caskey C.T. The human as an experimental system in molecular genetics. Science. 1988;240:1483–1488. doi: 10.1126/science.3287625. [DOI] [PubMed] [Google Scholar]
- Yang R., Mele F., Worley L., Langlais D., Rosain J., Benhsaien I., Elarabi H., Croft C.A., Doisne J.M., Zhang P., et al. Human T-bet governs innate and innate-like adaptive IFN-gamma immunity against mycobacteria. Cell. 2020;183:1826–1847.e31. doi: 10.1016/j.cell.2020.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Chan L. Monogenic diabetes: what it teaches us on the common forms of Type 1 and Type 2 diabetes. Endocr. Rev. 2016;37:190–222. doi: 10.1210/er.2015-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Zhang Q., Abel L., Puel A., Casanova J.-L. Human inborn errors of immunity to infection affecting cells other than leukocytes: from the immune system to the whole organism. Curr. Opin. Immunol. 2019;59:88–100. doi: 10.1016/j.coi.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Liu H., Chen S., Low H., Sun L., Cui Y., Chu T., Li Y., Fu X., Yu Y., et al. Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat. Genet. 2011;43:1247–1251. doi: 10.1038/ng.973. [DOI] [PubMed] [Google Scholar]
- Zhang F.R., Huang W., Chen S.M., Sun L.D., Liu H., Li Y., Cui Y., Yan X.X., Yang H.T., Yang R.D., et al. Genomewide association study of leprosy. N. Engl. J. Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Human genetics of life-threatening influenza pneumonitis. Hum. Genet. 2020;139:941–948. doi: 10.1007/s00439-019-02108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., COVID Human Genetic Effort. Cobat A., Casanova J.L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 2022;603:587–598. doi: 10.1038/s41586-022-04447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Matuozzo D., Le Pen J., Lee D., Moens L., Asano T., Bohlen J., Liu Z., Moncada-Velez M., Kendir-Demirkol Y., et al. Recessive inborn errors of type I IFN immunity in children with COVID-19 pneumonia. J. Exp. Med. 2022;219:e20220131. doi: 10.1084/jem.20220131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y. Herpes simplex virus encephalitis of childhood: inborn errors of central nervous system cell-intrinsic immunity. Hum. Genet. 2020;139:911–918. doi: 10.1007/s00439-020-02127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Clark N.E., Freije C.A., Pauwels E., Taggart A.J., Okada S., Mandel H., Garcia P., Ciancanelli M.J., Biran A., et al. Inborn errors of RNA lariat metabolism in humans with brainstem viral infection. Cell. 2018;172:952–965.e18. doi: 10.1016/j.cell.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Pizzorno A., Miorin L., Bastard P., Gervais A., Le Voyer T., Bizien L., Manry J., Rosain J., Philippot Q. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 2022 doi: 10.1084/jem.20220514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Miao V.N., Owings A.H., Navia A.W., Tang Y., Bromley J.D., Lotfy P., Sloan M., Laird H., Williams H.B., et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184:4713–4733.e22. doi: 10.1016/j.cell.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B., Ewaleifoh O., Harschnitz O., Lee Y.S., Peneau C., McAlpine J.L., Liu B., Tchieu J., Steinbeck J.A., Lafaille F., et al. Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc. Natl. Acad. Sci. USA. 2018;115:E8775–E8782. doi: 10.1073/pnas.1809853115. [DOI] [PMC free article] [PubMed] [Google Scholar]