Abstract
Background
As the COVID-19 pandemic spread across the United States, America’s pharmacists and their teammates expanded their clinical services to help their communities from every practice setting: community and ambulatory care, inpatient, long-term care, academia, public health, and many others.
Objectives
The objective of the study is to begin to quantify contributions of U.S. pharmacists in providing clinical interventions that mitigate and control the pandemic. These interventions span the gamut of diagnosis, prevention, treatment, and support, intervening patient by patient with vaccines, diagnostic tests, convalescent plasma, monoclonal antibodies, antiviral medications, and supportive therapies.
Methods
Review of published literature, relevant web pages, and queries to national and state professional pharmacy associations and government agencies.
Results
From February 2020 through September 2022, pharmacists and their teammates conducted >42 million COVID-19 tests, provided >270 million vaccinations (including 8.1 million COVID-19 vaccinations for long-term care residents) within community pharmacy programs alone, and provided >50 million influenza and other vaccinations per year. Pharmacists plausibly accounted for >50% of COVID-19 vaccinations in the United States. Pharmacists prescribed, dispensed, and administered an uncounted number of antibody products and antiviral medications, including care for 5.4 million inpatients and innumerable outpatients. Using conservative estimates, pandemic interventions by pharmacists and teammates averted >1 million deaths, >8 million hospitalizations, and $450 billion in health care costs.
Conclusions
Pharmacists and their teammates contributed to America’s health and recovery during the COVID-19 pandemic by providing >350 million clinical interventions to >150 million people in the form of testing, parenteral antibodies, vaccinations, antiviral therapies, and inpatient care. The number of lives touched and people cared for by pharmacists continues to rise.
Key Points.
Background
-
•
America’s pharmacists and their teammates provided COVID-19 interventions from every practice setting, including community and ambulatory care, inpatient, long-term care, academia, and public health.
-
•
These interventions span the gamut of diagnosis, prevention, treatment, and support.
Findings
-
•
A total of >270 million COVID-19 vaccinations (45% of U.S. total) given by community pharmacists and teams, overall >50% via pharmacist-led programs, Dec 2020—Sep 2022.
-
•
A total of >42 million patient specimens tested by pharmacists for COVID-19, Apr 2020—Jul 2022.
-
•
A total of >100,000 COVID-19 monoclonal antibody treatments provided by pharmacists, Nov 2020—Jun 2022.
Essential workers, selfless providers, neighborhood lifelines, frontline heroes
In February and March 2020, the United States realized how relentlessly a novel coronavirus was spreading across the country and around the world.1 The need to avoid gatherings and slow down viral transmission became preeminent. Restaurants, theaters, businesses, schools, and even houses of worship closed or drastically curtailed their activities. Yet society realized that some community services could not stop, such as those in grocery stores and pharmacies. “Essential services,” people called them. And the people who put their own health at risk to maintain continuity of care and allow prescriptions and medication-consulting services to keep flowing amid the contagious outbreak rightly were called heroes.
No event since the influenza pandemic of 1918–20,2, 3, 4 not even World War II, disrupted day-to-day events within the United States as much as the COVID-19 pandemic, caused by a novel virus that would be designated SARS-CoV-2. America’s pharmacists met the challenge to help their communities in every setting where pharmacists practice: community and ambulatory care, inpatient, long-term care, academia, public health, and many other forms of pharmacy practice. The tenets embodied in the “immunization neighborhood”—collaboration, coordination, and communication—proved to be essential in protecting health and preventing disease.
This document begins to quantify the contributions of pharmacists in providing clinical interventions that would mitigate and control the COVID-19 pandemic. The passages below describe several types of statistics, interspersed with vignettes to help bring dry numbers to life. The goal is to describe the reach and clinical competence of pharmacists, all in service of the public. Regrettably, the pandemic continues to extract its dreadful toll,5 so this is an interim report understating the eventual total contribution.
The word pharmacy is used here primarily to describe the profession itself, a more expansive construct than any building or department within an organization. Indeed, the remarkable contributions described here were accomplished by pharmacists’ teams, comprised of pharmacists, pharmacy residents, student pharmacists, pharmacy managers, pharmacy technicians, clerks, support personnel, stockers, and the vast chains of logisticians who supplied them.
These teams of dedicated care providers operated in buildings that variously bore the names of community pharmacies, both independent pharmacies and national and regional chains of traditional drug stores and mass merchants and grocery stores with pharmacies, ambulatory-care clinics, bedrock community hospitals, major research hospitals, federal facilities (e.g., Veterans Affairs [VA], Department of Defense [DoD], and Indian Health Service [IHS]), nursing homes, assisted-living communities, community-based clinics, colleges, health departments, and military units. When that was not enough, countless pharmacists’ teams packed up mobile units with kits and coolers and traveled to where they could reach even more people: arenas, sports stadiums, community centers, schools, rural outposts, houses of worship, and wherever people congregate.
The contributions of America’s pharmacists and teammates have been myriad, spanning the gamut of diagnosis, prevention, treatment, and support. Pharmacists’ teams used a remarkable variety of tools to keep people healthy or help them recover from COVID-19: primary vaccinations, booster vaccinations, diagnostic tests, convalescent plasma, monoclonal antibodies, antiviral medications, and supportive therapies. They injected, they swabbed, they tested, they provided, they prescribed, they dispensed, they coached, they taught, they explained, they listened, they explained some more.6, 7, 8, 9 Often, pharmacists reached where few others could, crossing vast distances and crossing cultural or linguistic divides to reach disadvantaged communities.
Methods
Statistics on clinical interventions were gathered from April 2022 through September 2022 from publications and web pages of federal agencies describing authorization and distribution of COVID-19 medical countermeasures, including those of U.S. Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), Government Accountability Office (GAO), and the Department of Health and Human Services (DHHS). Literature published in professional journals was also searched using terms such as {COVID-19 and (pharmacist) and (plasma or monoclonal or vaccine or antiviral)}. Reference lists were scanned for other relevant documents. Additional input was sought through queries to senior leaders of the constituent organizations (Academy of Managed Care Pharmacy, American Association of Colleges of Pharmacy, American College of Apothecaries, American College of Clinical Pharmacy, Accreditation Council for Pharmacy Education, American Pharmacists Association, American Society of Consultant Pharmacists, American Society of Health-System Pharmacists, College of Psychiatric/Neurologic Pharmacists, Hematology/Oncology Pharmacy Association, National Association of Boards of Pharmacy, National Community Pharmacists Association, National Alliance of State Pharmacy Associations) of the Joint Commission of Pharmacy Practitioners (jcpp.net), other relevant stakeholders, and searches of their organizations’ websites. Findings related to educational efforts and program advocacy were considered out of scope.
Spring 2020: COVID-19 pandemic spreads across America
In December 2019 and early 2020, stories about severe pneumonia cases following coronavirus infection filled the news, an outbreak spreading from Wuhan, Hubei province, China. Attempts to contain the virus failed, and it spread worldwide. The World Health Organization declared a Public Health Emergency of International Concern on January 30, 2020, and a global pandemic on March 11, 2020.
The first U.S. cases were diagnosed in mid-January 2020.1 On January 31, 2020, the DHHS Secretary declared a public health emergency for the entire United States to aid in the response of the health care community to the COVID-19 outbreak. On March 1, 2020, the President declared that the COVID-19 outbreak in the United States constituted a national emergency. On March 10, 2020, the Secretary issued a declaration under the Public Readiness and Emergency Preparedness (PREP) Act to enable distribution of specific medical countermeasures against COVID-19, with several amendments and clarifications issued since then.
As March 2020 arrived, nature proceeded in its usual springtime patterns, but human societal patterns across America were already seriously disrupted. The virus spread widely, hospitals filled and overflowed, and supply shortages began to manifest.1 Outpatient medical visit patterns were severely disrupted. The first imperative for pharmacists was to continue providing prescription medications, therapy management, and consulting services for hundreds of millions of Americans each week. Pharmacists hastened to require extra distance between an increased volume of clients, arrange plexiglass barriers, and protect inventory. They moved scarce hand sanitizer, alcohol swabs, masks, thermometers, sterile water (for sleep-apnea devices), and other products behind the pharmacy counter to preclude hoarding.
On a typical pre-pandemic day, America’s pharmacists dispensed 17 million outpatient prescriptions,10 cared for 1 million inpatients,11 and provided medications and advice for 2.6 million residents of long-term care (LTC) facilities.12 The need to provide care to millions of people sickened with COVID-19 and hundreds of millions seeking protection from COVID-19 added to this baseline patient load.
As the pandemic progressed, pharmacists modified hours of operation, staffing, and workflow, while adapting to restricted patient contact, infection-control measures, and other practices to meet their neighbors’ changing needs and circumstances.13, 14, 15, 16, 17, 18, 19, 20, 21 Pharmacists dealt with unexpected medication and supply shortages, patient access challenges, and staffing disruptions as workers contracted COVID-19 or were quarantined after contact with a confirmed infected patient. Multiple anticoagulation, hypertension, osteoporosis, and other clinical care interventions shifted to drive-through and other innovative venues beyond a pharmacy’s 4 walls.17 Pharmacy teams used technology to enable teleconsultations (i.e., telehealth). Interventions for antimicrobial stewardship were a common need.22 In the case of hydroxychloroquine and ivermectin, pharmacists helped protect against limitations on a pharmacist’s professional judgment regarding inappropriate use of prescription medications.23, 24, 25, 26, 27
A survey of U.S. hospital pharmacists found that 46% of respondents increased intensive care unit bed capacity in 2020.28 Almost all had to change their usual pharmacy supply-chain acquisition processes. Hospitals experienced shortages of many medications, including asthma inhalers (60%), sedatives and anesthetic agents (58%), neuromuscular blockers (43%), corticosteroids (34%), cardiovascular agents (24%), investigational agents (24%), and dialysis solutions (6%). Medication-use changes were implemented by 86% of hospitals, most commonly involving guidelines for COVID-19 treatment (79%) and opening compassionate use or investigational drug studies (55%). Shortages of personal protective equipment and other basic supplies compounded the struggle to continue care delivery.
When health systems created ad hoc facilities to provide care during patient surges, their pharmacists’ teams created the requisite alternate care site pharmacies.29 Similar experiences occurred in cities across the country.17 , 30 In long-term care settings, procedures to reduce resident-staff contacts by reducing medication burden (i.e., number of medications and associated bedside encounters) were adopted.31 , 32
Managing shortages amid workflow disruption was a recurring theme. Consistently since March 2020, as each new clinical intervention became available (e.g., diagnostic test, antibody product, vaccine, antiviral), there was insufficient supply for the entire country all at once. Time after time, pharmacists helped triage which patients were most likely to benefit from any given product until manufacturing pipelines could increase their output.33 , 34
COVID-19 testing
On February 4, 2020, FDA issued the first emergency use authorization (EUA) for an in vitro diagnostic test to detect or diagnose COVID-19.35 , 36 By late March 2020, the manufacturing pipeline was producing steadily increasing numbers of tests, allowing distribution across the country. Recognizing the value of rapid diagnosis and the proximity of pharmacists to the American population—90% live within 5 miles of a community pharmacy37, 38, 39, 40—the Secretary of DHHS authorized pharmacists to order and administer FDA-authorized COVID-19 tests on April 8, 2020.
During the 2010s, community pharmacists were growing steadily as providers of tests waived from requirements of the Clinical Laboratory Improvement Amendments (CLIA) of 1988.41 With the pandemic, pharmacists became increasingly common sources for people to access CLIA-waived tests. According to statistics compiled by the National Alliance of State Pharmacy Associations,42 an additional number of 14,522 CLIA waivers were granted to pharmacies between May 2020 and May 2022 (27,091 in total).
In April 2020, the DHHS formed a partnership with community pharmacies for pharmacist-based COVID-19 testing, integrating pharmacists into the Community-Based Testing Sites (CBTS) program, beginning with 362 sites across 45 states and the District of Columbia (DC).37 , 43, 44, 45 The pharmacist-based components of the program grew rapidly37:
-
•
April 2020: 362 sites in 45 states and DC
-
•
June 2020: 623 sites in 48 states, DC, and Puerto Rico (PR); >700,000 samples tested (cumulative)
-
•
January 2021: 3300 sites in 50 states, DC, and PR; >5.6 million samples tested
-
•
March 2021: 6211 sites in 50 states, DC, and PR; >9.8 million samples tested
In January 2021, the DHHS highlighted that more than 70% of pharmacy sites within the CBTS program were located in communities with moderate-to-high social vulnerability.37 , 46
In April 2021, the CBTS program transitioned into the Increasing Community Access to Testing (ICATT) for COVID-19 program.47 The CDC reports that more than 10,000 pharmacy sites participate in ICATT. These locations performed more than 42 million COVID-19 tests from April 2020 through July 2022,37 , 47 receiving high marks for satisfaction from their patients.48
Convalescent plasma
Passive immunization, using preformed antibodies to prevent or ameliorate infection, has been used for more than a century against various microbial pathogens.49 The SARS-CoV-2 virus was untreatable in spring 2020 except to manage symptoms. Therefore, clinicians logically turned to plasma from patients convalescing after COVID-19 disease, expecting it to be a rich source of antibodies to neutralize the SARS-CoV-2 virus.20 , 50, 51, 52
Pharmacists in many hospitals contributed to multidisciplinary efforts to recruit plasma donors, harvest and process the plasma, and administer it to eligible patients.20 , 53 Eventually, Expanded Access Protocols (EAPs) for COVID-19 convalescent plasma encompassed more than 2700 hospitals through August 2020.52 , 54 The EAPs were succeeded by an EUA issued by FDA on August 23, 2020.
Although a meta-analysis of multiple clinical trials of COVID-19 convalescent plasma suggested some reduction in risk of death among hospitalized COVID-19 patients, issues related to prompt intervention, identification of high-titer plasma units, donor solicitation and screening, and regulatory hurdles, among others, limited the utility of this approach.50 , 52 , 55, 56, 57, 58, 59 Availability of specific monoclonal antibody products from November 2020 onward offered therapeutic products in ready-to-use formulations with more standardized potency and a clearer basis of evidence.
Monoclonal antibody treatments
FDA issued EUAs for Lilly’s bamlanivimab and Regeneron’s casirivimab with imdevimab (also called REGEN-COV) on November 9 and 21, 2020, respectively, for certain adult and pediatric patients at elevated risk for progressing to severe COVID-19.60 Both products comprised monoclonal antibodies administered by intravenous (I.V.) infusion. The U.S. Government purchased 950,000 doses of bamlanivimab and nearly 3 million doses of casirivimab/imdevimab between July 2020 and September 2021, although it is unclear how many of these doses were administered.
FDA issued EUAs for other monoclonal antibody products as the months went by60:
-
•
February 9, 2021: Bamlanivimab/etesevimab (Lilly), I.V. infusion. Purchases: 388,000 doses (September 2021)
-
•
May 26, 2021: Sotrovimab (Xevudy, GlaxoSmithKline with Vir), I.V. infusion, later intramuscular (IM) injection. Purchases: 1 million doses (November 2021-January 2022)
-
•
December 8, 2021: Tixagevimab/cilgavimab (Evusheld, AstraZeneca), IM injection. Purchases: 1.7 million doses (December 2021-February 2022)
-
•
February 11, 2022: Bebtelovimab (Lilly), I.V. push. Purchases: 600,000 doses (February 2022)
Pharmacists at most U.S. hospitals quickly engaged in patient selection, counseling, administration, monitoring, and other clinical services.61, 62, 63, 64 A pharmacist at the University of Pittsburgh Medical Center helped demonstrate the utility of administering casirivimab/imdevimab as a set of 4 subcutaneous injections and led monoclonal treatments for more than 22,000 patients.63 , 64
In September 2021, anticipating the approach of influenza season, the DHHS Secretary issued the ninth amendment to the medical countermeasures declaration to authorize pharmacists to both order and administer certain COVID-19 therapeutics (Table 1 ). Several states, including Arkansas, Mississippi, and Oregon, responded quickly, implementing statewide standing orders or protocols to allow pharmacists to independently order and administer casirivimab/imdevimab COVID-19 monoclonal antibodies. The Society of Infectious Diseases Pharmacists developed a toolkit informed by the experiences of pharmacists who implemented monoclonal antibody programs at their institutions for treatment, post-exposure prophylaxis, and pre-exposure prophylaxis.67
Table 1.
Extraordinary federal authority within scope of COVID-19 emergency declarations under the Public Readiness and Emergency Preparedness (PREP) Act
| Adapted from American Society of Consultant Pharmacists,32 Department of Health and Human Services,65 National Alliance of State Pharmacy Associations,42 and National Association of Chain Drug Stores66 |
| On January 31, 2020, the Secretary of Health and Human Services declared a public health emergency for the entire United States to aid in the response of the nation’s health care community to the COVID-19 outbreak. On March 10, 2020, the Secretary issued a declaration under the PREP Act for medical countermeasures against COVID-19, amended several times subsequently. Other expansions took the form of DHHS General Counsel advisory opinions or DHHS guidance documents. |
| • DHHS Guidance—COVID-19 Testing, April 8, 2020, Office of the Assistant Secretary for Health |
| ○ Authorizes pharmacists to order and administer FDA-authorized COVID-19 tests, including serology tests. Such pharmacists qualify as “covered persons” under the PREP Act, receiving immunity with respect to claims for loss caused by, arising out of, relating to, or resulting from, the administration or use of such COVID-19 tests. |
| • Third Amendment to COVID-19 PREP Act Declaration—Childhood Vaccines, August 24, 2020, Secretary of the DHHS |
| ○ Authorizes pharmacists to order and administer, and pharmacy interns to administer, ACIP-recommended childhood vaccines (ages 3-18 y). |
| • DHHS Guidance—COVID-19 Vaccines, September 3, 2020, Office of the Assistant Secretary for Health |
| ○ Authorizes pharmacists to order and administer, and pharmacy interns to administer, COVID-19 vaccines. |
| • DHHS Guidance—Pharmacy Technicians and Interns Administer, October 20, 2020 |
| ○ Authorizes qualified pharmacy technicians and pharmacy interns (further clarifies pharmacy interns) to administer both childhood vaccines and COVID-19 vaccines. |
| • Seventh Amendment to COVID-19 PREP Act Declaration—Inactive, Expired, or Lapsed Licenses, March 11, 2021, Secretary of DHHS |
| ○ Authorizes pharmacists and pharmacy interns/student pharmacists who have licenses that are inactive, expired, or lapsed in the past 5 y to prescribe, dispense, and/or administer COVID vaccines (based on previous PREP Act authority) provided the licensee was in good standing before inactivity, expiration, or lapse. |
| • Eighth Amendment to COVID-19 PREP Act Declaration—Authorization for Influenza Vaccine, August 4, 2021, Secretary of DHHS |
| ○ Authorizes pharmacists and qualified pharmacy technicians and pharmacy interns to administer seasonal influenza vaccines under the supervision of a pharmacist to persons aged 19 y and older consistent with ACIP recommendations. |
| • Ninth Amendment (corrected) to COVID-19 PREP Act Declaration: Expanding Access to COVID Therapeutics, September 30, 2021, Secretary of DHHS |
| ○ Authorizes pharmacists to both order and administer certain COVID-19 therapeutics and qualified pharmacy technicians and registered or licensed pharmacy interns to administer these products. |
| • Tenth Amendment to COVID-19 PREP Act Declaration—Seasonal Influenza Vaccine, December 30, 2021, Secretary of DHHS |
| ○ Expands the scope of authority for licensed pharmacists to order and administer and qualified pharmacy interns to administer seasonal influenza vaccines. |
Unfortunately, the SARS-COV-2 virus did not stand still, neither geographically nor antigenically. Genetic mutations produced viral variants that were no longer adequately neutralized by hyperspecific monoclonal antibodies. At various times in January and April 2022, FDA notified clinicians that due to the high frequency of certain variants, casirivimab/imdevimab, bamlanivimab/etesevimab, and sotrovimab were no longer authorized in any U.S. region, until further notice by FDA.60 This situation will likely remain fluid.
The American Pharmacists Association (APhA) created tools to help pharmacists refer eligible patients to infusion centers to receive monoclonal antibody products administered by I.V. infusion.68 Health-system pharmacists staffed many of those infusion centers.69 , 70 Later, products amenable to subcutaneous or intramuscular injection were suitable for administration by outpatient providers, including pharmacists. Pharmacists administered monoclonal antibodies in community, inpatient, and nursing home settings. An Arkansas pharmacist who treated more than 300 patients this way reported that her patients had often been placed on waiting lists at hospitals near their homes but were able to receive the medication conveniently in her community practice.71
December 2020: vaccine doses begin flowing
Research efforts to produce COVID-19 vaccines initiated in spring 2020 started to look promising as fall 2020 arrived. The eventual unprecedented success of these efforts was due in large part to pre-pandemic work with other human coronavirus vaccine candidates, exploration of mRNA vaccine approaches dating back 15 years, the federal government taking the financial risk in case clinical trials did not succeed, manufacturers performing many of the required development steps simultaneously (rather than in sequence), and the willingness of many tens of thousands of people to volunteer for clinical trials quickly.
Finally, in December 2020, America’s ability to control the pandemic took a dramatic turn for the better. FDA granted EUA status to messenger ribonucleic acid (mRNA) vaccines manufactured by Pfizer-BioNTech and by Moderna.72 The CDC’s Advisory Committee on Immunization Practices (ACIP) swiftly recommended the products for widespread use in a phased manner, according to degree of disease risk, as vaccine manufacturing delivered steadily increasing quantities of vaccine.73 Ultra-cold storage temperatures posed special logistical requirements and challenges.
Fortunately, the DHHS and CDC had been planning to include pharmacies and pharmacists in pandemic responses, increasingly since the 2009 influenza A/H1N1 response.37 , 74, 75, 76, 77, 78, 79 In September 2020, the DHHS and CDC started reaching out to pharmacy associations and pharmacy businesses to gauge interest and begin planning large-scale, widely dispersed COVID-19 vaccination programs within pharmacies, as well as having pharmacists administer vaccines in other venues.37
Federal pharmacy partnership for long-term care program
At the top of the priority list for COVID-19 vaccination were residents and staff of LTC facilities, such as nursing homes and skilled nursing facilities. For decades, pharmacists and pharmacies have had consulting relationships with these facilities, so that they already were providing personalized medications and supplies for residents, monthly medication therapy reviews (i.e., drug utilization reviews), and other clinical services. In the first few months of COVID-19 vaccine availability, pharmacists were the most frequent vaccinators at these facilities.
The CDC established the Federal Pharmacy Partnership for Long-Term Care Program in October 2020, an agreement with CVS, Walgreens, and Managed Health Care Associates Inc (a group purchasing organization and pharmacy services administrative organization [PSAO] serving independent LTC pharmacies) to ship and administer COVID-19 vaccines to residents and staff of LTC facilities.80, 81, 82, 83 These partners provided 8.1 million on-site vaccinations for residents and staff at more than 62,000 LTC and assisted-living facilities from December 21, 2020, until April 23, 2021, when the program was consolidated with the Federal Retail Pharmacy Program (FRPP).84
In mid-December 2020, the federal government provided most of the limited supply of vaccine doses to states and jurisdictions and began shipping vaccine to selected pharmacies serving residents and staff in LTC facilities. In mid-January 2021, the CDC issued funding and guidance for all jurisdictions and included as its first recommendation to jurisdictions “increase the number of vaccine provider sites, including through the use of pharmacies.”
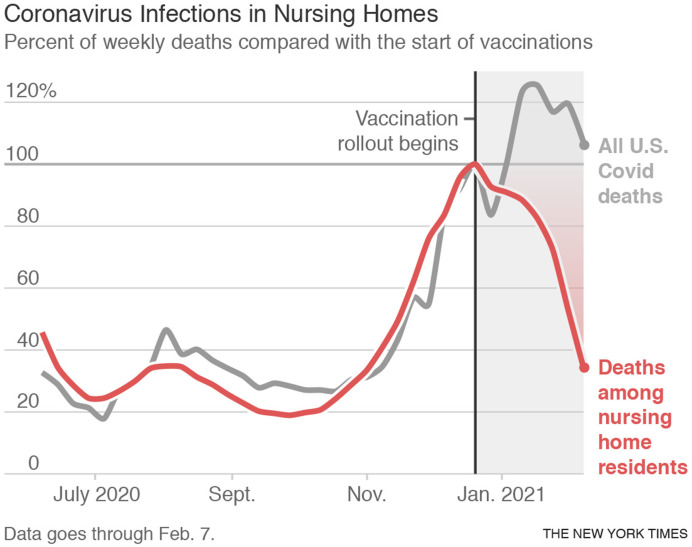
A graph of deaths of nursing-home residents over time published on the front page of the New York Times in late February 2021 looks like a waterfall85 (Figure 1 ), illustrating both the high degree of efficacy of the first mRNA vaccines and the promptness with which providers, especially pharmacists, delivered the vaccines to LTC residents.85
Figure 1.
Proportion of weekly deaths, contrasting nursing home residents with all U.S. COVID-19 deaths, 2020-21. From the New York Times ©2021 The New York Times Company. All rights reserved. Used under license. page A1.85
West Virginia followed a different path than other states for its LTC residents and staff, empowering independent pharmacies with teams who knew the many rural sites across the Mountain State. The West Virginia program was set up promptly, and their pharmacists completed its first round of nursing home vaccination clinics before the end of December 2020, faster than any other state.86, 87, 88, 89, 90, 91 West Virginia’s nearly 250 independent pharmacies administered 28,000 first doses to residents and staff members in 210 LTC facilities, making West Virginia the first state to offer vaccination to all nursing-home residents. These local pharmacists used existing relationships with nursing homes to gather information and leveraged the trust of patients in encouraging them to get vaccinated.
When vaccine was so scarce in the first few months of vaccinating, pharmacists helped quantify the amount of vaccine intentionally overfilled into each vial.92, 93, 94, 95 Overfill is the quantity of vaccine intentionally placed into each vial to assure that the minimum quantity retrievable with syringe and needle does not fall below the number of doses specified on the product labeling. Specific syringe-needle combinations with tight fittings and reduced “dead space” minimized retained fluid that could not be injected. Variability in the syringes provided with vaccine shipments made this challenging to predict. Pharmacists asked to use their aseptic compounding skills to avoid wasting those doses. FDA eventually agreed. These efforts by pharmacists expanded that initial scarce vaccine supply by 10%–20%.
Federal government partners with the community pharmacy
In November 2020, the DHHS released its first list of participating pharmacy organizations in what would come to be known as the FRPP for COVID-19 vaccination. This list primarily included pharmacy chains with large numbers of outlets. These chains had been planning and modeling with federal planners increasingly since 2009 to forge public-private partnerships that enabled rapid roll-out of COVID-19 vaccinations from December 2020 onward. Additional partners were included, particularly independent pharmacies networked through wholesaler-based PSAOs. In January 2021, the White House released updated vaccination plans featuring community pharmacies as prominent providers of COVID-19 vaccinations,37 , 84 , 96 to expand access to COVID-19 vaccines for the American people. This program developed into a partnership with 21 national pharmacy chains, independent pharmacy networks, and LTC pharmacists96 (Table 2 ), offering COVID-19 vaccinations at more than 41,000 community and LTC pharmacy locations across the country. Vaccine distribution through this channel began on February 9, 2021. In addition to the FRPP, state and local jurisdictions directly engaged additional pharmacies not enrolled in the FRPP, as well as FRPP-enrolled pharmacies to expand access and meet specific community needs.
Table 2.
Federal Retail Pharmacy Program partners (as of September 2022)
| Albertsons Companies, Inc (including Osco, Jewel-Osco, Albertsons, Albertsons Market, Safeway, Tom Thumb, Star Market, Shaw’s, Haggen, Acme, Randalls, Carrs, Market Street, United, Vons, Pavilions, Amigos, Lucky’s, Pak n Save, Sav-On) |
| Costco Wholesale Corporation |
| CPESN USA, LLC (Community Pharmacy Enhanced Services Network) |
| CVS Pharmacy, Inc (including Long’s) |
| GeriMed (long-term care and community pharmacies) |
| Good Neighbor Pharmacy and AmerisourceBergen Drug Corporation’s pharmacy services administrative organization (PSAO), Elevate Provider Network |
| Health Mart Pharmacies |
| H-E-B, LP |
| Hy-Vee, Inc |
| Innovatix (long-term care pharmacies) |
| Kroger Co (including Kroger, Harris Teeter, Fred Meyer, Fry’s, Ralphs, King Soopers, Smiths, City Market, Dillons, Mariano’s, Pick-n-Save, Copps, Metro Market, QFC) |
| LeaderNET and The Medicine Shoppe Pharmacy, Cardinal Health’s PSAO |
| Managed Health Care Associates (PSAO for community and long-term care pharmacies) |
| Meijer, Inc |
| Publix Super Markets, Inc |
| Retail Business Services, LLC (including Food Lion, Giant Food, The Giant Company, Hannaford Bros Co, Stop & Shop) |
| Rite Aid Corporation |
| Southeastern Grocers (Winn-Dixie, Harveys, Fresco Y Mas) |
| Topco Associates, LLC (including Acme Fresh Markets, Associated Food Stores, Bashas, Big-Y Pharmacy and Wellness Center, Brookshire’s Pharmacy, Super One Pharmacy, FRESH by Brookshire’s Pharmacy, Coborn’s Pharmacy, Cash Wise Pharmacy, MarketPlace Pharmacy, Giant Eagle, Hartig Drug Company, King Kullen, Food City Pharmacy, Ingles Pharmacy, Raley’s, Bel Air, Nob Hill Pharmacies, Save Mart Pharmacies, Lucky Pharmacies, SpartanNash, Price Chopper, Market 32, Tops Friendly Markets, ShopRite, Wegmans, Weis Markets, Inc) |
| Walgreens (including Duane Reade) |
| Walmart, Inc (including Sam’s Club) |
Source: Centers for Disease Control and Prevention.96
The Federal Transfer Program with Jurisdiction(s) for COVID-19 Vaccination allowed states to transfer allocated vaccine doses to federal pharmacy partners to minimize vaccine wastage, as an interim solution as the FRPP took shape. At least 7 pharmacy chains participated in this program.37
As spring 2021 proceeded, the supply of vaccine was expanding, and vaccinations no longer needed to be centralized to a few large mass-vaccination sites. Mass-vaccination sites administered 5.6 million doses between February and June 2021, when most were disbanded.84 In April 2021, the CDC issued further guidance and funding to jurisdictions urging them to “improve access to COVID-19 vaccines” by “using multiple types of locations and with flexible hours that are accessible to and frequented by the identified communities of focus” including pharmacies. As expanding supply enabled distribution to more sites, the proportion of vaccine doses distributed through the CDC’s FRPP increased.
Between mid-December 2020 and September 2022, pharmacists’ teams administered more than 270 million doses of the COVID-19 vaccine. These doses include 8.1 million doses administered on site at LTC facilities.84 , 96 , 97 During this interval, the total number of COVID-19 vaccinations reported across all 50 states and U.S. territories numbered 606 million.97 Thus, through the FRPP alone, community pharmacists and their teammates delivered 45% of COVID-19 vaccinations across the United States, although that proportion has been higher or lower at specific points in time. For example, between April and July 2021, the proportion of vaccine doses distributed through states and other jurisdictions decreased from 62% in early April 2021 to 6% in mid-July 2021.84 During that same time, the proportion of vaccine doses distributed through the FRPP increased from 29% to 92%, according to a GAO analysis.83 The oft-quoted statistic of “more than 70% by pharmacists” was based on the Fall 2021 interval.
That 45% proportion for the FRPP does not include COVID-19 vaccinations conducted at the hands of pharmacists or under their direct supervision outside of the FRPP.84 , 98, 99, 100, 101, 102, 103 For example, at least 20 pharmacy chains took part in a state- or jurisdiction-level program for COVID-19 vaccination, typically focused on large and underserved populations.37 Other doses attributed to municipalities, states, or government agencies (e.g., VA, DoD, IHS) were administered by the hands of or at the direction of pharmacists. Pharmacists within health systems often led their institutions’ vaccination programs for workers and surrounding communities.100, 101, 102, 103, 104, 105 Through August 22, 2021, CDC officials reported that pharmacists had administered 3,203,104 vaccine doses at 11,449 mobile clinics across the country.106 The additional doses represented by these and multiple other efforts plausibly expand the proportion of pharmacist-directed COVID-19 vaccinations beyond 50% of the national total.
Equity in vaccine distribution
GAO analysis of FRPP data found that participating pharmacists fully vaccinated 51,554,294 people from February 11, 2021, through September 4, 2021.106 About 74% of those vaccination records contained information about race and ethnicity. Of those with documented race and ethnicity, 43% were from racial and ethnic groups other than non-Hispanic white. The GAO analysis suggests that pharmacists fully vaccinated a disproportionately greater share of non-Hispanic Asian and Hispanic or Latino persons. Non-Hispanic black and non-Hispanic white persons represented a slightly smaller share of persons fully vaccinated through pharmacists relative to their population shares.
The CDC uses several tools to assess the equity of COVID-19 vaccine distribution during the COVID-19 pandemic. The CDC calculates the social vulnerability index (SVI) at the county level based on data from the U.S. Census Bureau.107 Because there can be considerable heterogeneity within counties, the equitable distribution index (EDI) score assesses socioeconomic conditions more finely, at the zip-code level. The CDC’s analytical software anonymously records the EDI of the residence of people vaccinated against COVID-19 and the EDI of the location where they were vaccinated.
Pharmacists’ ability to equitably contribute to COVID-19 vaccine distribution can be assessed, in part, by considering the pharmacies in the Community Pharmacy Enhanced Services Network (CPESN) distributed across 46 States and the DC. These CPESN pharmacists and teammates administered 5.60 million doses as part of the FRPP between February 8, 2021, and June 8, 2022. Each of the 1961 participating CPESN pharmacies administered an average of 2856 vaccinations during this interval [108; CPESN FRPP unpublished data, June 8, 2022].
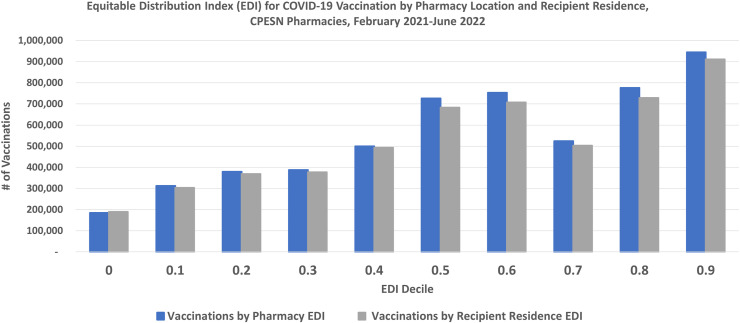
Figure 2 compares the EDI of vaccine recipients’ residences to the EDI of the CPESN pharmacies for these 5.60 million vaccinations. The concordance of the 2 sets of bars shows that CPESN pharmacists vaccinated people who lived in settings with vulnerability indices similar to their pharmacy’s neighborhood. A similar high degree of concordance was also apparent early in the vaccination program, from February 2021 through April 2021, when vaccine supplies were limited and concerns about equitable distribution were most acute (data not shown). The 1961 CPESN pharmacies include 1377 pharmacies (70.2%) sited in zip codes in the upper half of the EDI vulnerability range (i.e., EDI deciles 0.5 through 0.9), of which 942 (48.0%) are in the 3 highest deciles of vulnerability (i.e., 0.7 through 0.9). Of the 5.48 million vaccinations with a known pharmacy EDI, 67.9% were administered in pharmacies in the upper half of the EDI range and 40.9% with the EDI of 0.7 or greater. Of the 5.26 million vaccinations with a known recipient residence EDI, 67.1% were administered to people whose residence was in the upper half of the EDI range and 40.7% with the EDI of 0.7 or greater.
Figure 2.
COVID-19 vaccinations by the equitable distribution index (EDI) of recipient residence and pharmacy location, Community Pharmacy Enhanced Services Network (CPESN), United States, February 2021 through June 2022. Source: CPESN USA LLC (unpublished data). Notes: Data from 1961 pharmacies vaccinating between February 8, 2021, and June 8, 2022. Excludes vaccinations with unknown residence information for recipient and zip codes with fewer than 10 vaccinations each. The EDI assesses socioeconomic vulnerability at the zip-code level, with 0 being the decile of least vulnerability and 0.9 the decile of most vulnerability.
Pharmacists in all practice settings made countless contributions in explaining vaccines to the public, offering reassurance and reliable information from a trusted local source, while helping resolve uncertainty in the direction of accepting vaccination.109, 110, 111, 112 A health equity report authored by National Association of Chain Drug Stores (NACDS) and the Johns Hopkins Center for Health Security noted the many ways pharmacists and pharmacies worked to enhance equity throughout the COVID-19 response and vaccination campaign: outreach to homebound individuals, pop-up clinics, partnerships with schools, community centers, places of worship, employers, community leaders, faith-based organizations, and organizations representing racial and ethnic minority groups.113 Pharmacies collaborated with rideshare companies, deployed mobile vaccination units, and went door to door through underserved communities. Community pharmacies helped address the needs of those with limited mobility, such as the elderly or people with disabilities. In multiple instances, people opted to receive their second COVID-19 vaccine dose from local, trusted, and easy-to-access community pharmacies, rather than returning to a mass-vaccination clinic or another venue.
The APhA posted dozens of video and print success stories of communications and vaccinations at its Vaccine Confident website (vaccineconfident.pharmacist.com), including at a Disneyland parking lot, at houses of worship, in multiple languages, and at centers serving the underserved.114 The American Society of Health-System Pharmacists, the American College of Clinical Pharmacy, and other organizations did likewise.17 , 115 , 116
Between October 29, 2021, when the Pfizer-BioNTech pediatric COVID-19 vaccine received EUA status for children aged 5-11 years in the United States, and January 18, 2022, pharmacists and their teammates administered 46.4% of doses to this pediatric age group.117 This included 48.7% of doses in areas of high social vulnerability and 44.4% in low SVI areas. Pharmacy vaccine providers were permitted to bill COVID-19 vaccine administration fees for uninsured children to Medicaid rather than to the Vaccines for Children (VFC) program, which prevented unnecessary obstacles to care. CDC researchers noted, “Pharmacy providers were critical in addressing high initial demand for COVID-19 vaccine among this age group [i.e., 5-11 years old], including during evenings, weekends, and over holidays, when other providers might be less available.” Pharmacists also played a larger role in provision of COVID-19 vaccine to children aged 5-11 years compared with administration of routine vaccines: 46.4% of all COVID-19 pediatric vaccine doses were administered by pharmacy partners, whereas 12.3% of pediatric seasonal influenza vaccine doses were administered to children aged 5-12 years in pharmacies during 2020-21. Pharmacies are helping vaccinate children aged 6 months through 4 years now that vaccine is authorized for this age group.”72, 117
A subsequent analysis of vaccine provider type between November 2021 and April 2022 found that 87.5% of U.S. counties had at least one active provider of COVID-19 vaccination for children 5 through 11 years of age, with pharmacies being the most common provider type (69.1%).118 The median number of pharmacy vaccine providers per county with a pharmacy provider was 3 (interquartile range: 1–8).
In June 2022, FDA expanded the EUAs for 2 COVID-19 vaccines to encompass young children 6 months and older.72 The ACIP and CDC likewise recommended their vaccination and pharmacists are now part of the effort to vaccinate that youngest cohort.60 , 73
Examples of outreach
A CDC webpage lists examples of how pharmacists helped get America vaccinated against COVID-19 in February and March 2021 as the FRPP gained momentum.96
-
•
Jewel-Osco pharmacists’ teams administered 8600 COVID-19 vaccinations at more than 10 underserved sites in Chicago during a 10-day period.
-
•
Hague Pharmacy, a member of the CPESN, delivered 1300 vaccinations on a single day to African American residents of Norfolk’s Calvert Square neighborhood and another 1700 doses the following day in Chesapeake, VA.
-
•
Hy-Vee pharmacists vaccinated 1000 local Des Moines educators and staff on the Drake University campus.
-
•
Hy-Vee pharmacists also staffed mobile units to vaccinate staff at food-processing plants in Illinois and Iowa.
-
•
Meijer Pharmacy vaccinated 2600 educators at Ford Field in Detroit.
-
•
Giant Eagle Pharmacy vaccinated 4000 Pittsburghers at Heinz Field.
-
•
Walgreens pharmacists vaccinated 7000 people at a 3-day event held at 3 Atlanta Black Churches.
-
•
Walmart Pharmacies conducted 43 vaccination events at 79 locations in 18 states.
A team of 5 from Todd’s Drugs in Adamsville, Tennessee, vaccinated 100 residents of a remote and underserved town in a tent in a parking lot in less than 3 hours. The team returned several more times, administering more than 900 additional doses.119 Several “fearless pharmacy” stories from the Good Neighbor Pharmacy network are comparably compelling.116
Since 1997, America’s student pharmacists have volunteered their extracurricular time to practice the clinical intricacies of vaccination in a program called “Operation Immunization,” a collaboration between the APhA’s Academy of Student Pharmacists (APhA-ASP) and the Student National Pharmaceutical Association. During the 2020-21 Operation Immunization Campaign (August 2020 through June 2021), the group reported that 13,884 student pharmacists, 1716 faculty mentors, and 2158 pharmacist preceptors collaborated to vaccinate 1,286,161 people (1,162,288 against COVID-19 and 82,318 against influenza).120 Several student chapters reported that their Operation Immunization activities were the only in-person activities they were permitted to hold.
One example involves the student pharmacists at the School of Pharmacy at the Notre Dame University of Maryland, based in Baltimore’s Blythewood neighborhood. The student pharmacists vaccinated at large community events on campus, with the Baltimore City Health Department, and at Safeway pharmacies serving the greater Baltimore region, providing more than 50,000 vaccinations. Further, these students provided thousands more vaccinations during more than 100 shifts at 30 independent pharmacies in the metropolitan area. During initial shortages, they compounded alcohol-based sanitizers and led campus-wide mask-making efforts.121
Vaccination against other vaccine-preventable diseases
According to CDC statistics,122 pharmacists administered 35.3 million doses of influenza vaccine to adults during the prepandemic 2019-20 season, increasing to 47.7 million in 2020-21 and softening to 40.5 million in 2021-22. In contrast, the CDC found that physician medical offices administered 36.8 million doses to adults in the prepandemic season, dropping to 32.8 million in 2020-21 and 30.9 million in 2021-22. Of those vaccinated against influenza in the 2020-21 season, about 39% of adults and 12% of children received their influenza vaccination from a community pharmacist.
A CDC report released in May 2020 found a troubling drop in routine childhood vaccination as families missed many in-person clinical visits early in the pandemic.123 This finding spurred the third amendment to the COVID-19 PREP Act Declaration (Table 1),124 preempting any state or local law that effectively prohibited pharmacists who satisfy federal requirements from distributing or administering COVID-19 vaccines, ACIP-recommended routine childhood vaccines, or COVID-19 tests as set forth above.
An analysis of noninfluenza vaccine-claim submissions were 13%-35% lower for adolescents and 17%–40% lower for adults in fall 2020, compared to the same period in 2019, suggesting that many people who should have received recommended vaccines in 2020 did not.125 This analysis found that adolescents and adults in the insurance cohorts studied missed an estimated 26 million doses of recommended vaccines from January to November 2020, compared to the same period in 2019.
The rationale for the preemption was the public health threat posed by the decrease in childhood-vaccination rates and the collateral harm caused by COVID-19. The DHHS actions cited the many states that already enable pharmacists to provide clinical services to children and established a uniform national scope of practice for qualified pharmacists. “Pharmacists are well positioned to increase access to vaccinations, particularly in certain areas or for certain populations that have too few pediatricians and other primary-care providers, or that are otherwise medically underserved. … Pharmacies often offer extended hours and added convenience. … Pharmacists also have strong relationships with local medical providers and hospitals to refer patients as appropriate.”124
In October 2020, the CDC estimated that pharmacies represented 71 sites among 38,000 participating VFC providers.126 , 127 By May 2022, 135 pharmacies had status as VFC providers (CDC unpublished data, May 16, 2022). Enrollment limitations and program requirements have constrained pharmacies’ ability or desire to enroll as VFC providers, to the detriment of vaccine coverage in vulnerable populations.
Antiviral prescribing and dispensing
On May 1, 2020, FDA issued an EUA for the antiviral drug remdesivir (Veklury, Gilead) to treat suspected or laboratory-confirmed COVID-19 in certain adults and children hospitalized with severe disease.60 It is administered by I.V. infusion. As with other therapeutics first available during this pandemic, initial supplies were inadequate and pharmacists helped develop criteria for optimal use.128
The next big advance was the availability of oral antiviral medications.59 On December 22, 2021, FDA issued an EUA for Pfizer’s Paxlovid (nirmatrelvir tablets co-packaged with ritonavir tablets). The next day, December 23, FDA issued an EUA for molnupiravir capsules (Lagevrio, Merck with Ridgeback Bio). These therapeutics involve the traditional paradigm of people being tested and treated by their own health care providers, who can prescribe these oral antivirals to eligible patients. Patients receive those prescriptions and counseling from their pharmacist.
Pharmacists have their usual pivotal role in the safe dispensing of these products, as they address safety considerations, drug interactions, patient education, handling, and reporting. Initially, the terms of the December 2021 EUAs issued by FDA for antivirals are more restrictive than the ninth (September 2021) amendment to the DHHS Secretary’s PREP Act Declaration (Table 1), making pharmacists ineligible to order or prescribe Paxlovid or molnupiravir except under state authorities for standing orders or collaborative practice agreements. The restrictions according to the December 2021 EUA wording restricted oral antiviral uptake, especially in communities of high social vulnerability.129 , 130 Even so, pharmacists generated awareness, answered patient questions, and assessed patients for eligibility after a confirmed COVID-19 infection, referring patients to their provider for a prescription.
Test-to-treat program
The federal test-to-treat program assembled a network of convenient-care clinics and adjacent pharmacists in March 2022 to help speed up access to antiviral medications.131 , 132 Under the structure of this federal program, when people tested positive at test-to-treat sites, they could get a prescription from an authorized health care provider and have the prescription filled at the adjacent pharmacy. People can also bring test results obtained from a home testing kit to test-to-treat sites and be evaluated by a health care provider for treatment. These one-stop test-to-treat sites are available nationwide at more than 2000 pharmacy-based clinics and Health Resources and Services Administration–supported federally qualified health centers.
According to a detailed analysis by the APhA,133 more than 28,000 community pharmacies were located in federally recognized underserved communities in May 2022, yet only 700 had test-to-treat sites. Capitalizing on the pharmacists in the underserved communities could increase access to COVID-19 treatment 39-fold. The least vulnerable areas nationwide host 78% of test-to-treat sites, whereas the most vulnerable communities have only 22% of these locations. These findings were confirmed by the CDC which found that “dispensing rates were lowest in high vulnerability zip codes, despite these zip codes having the largest number of dispensing sites.”134
Recognizing the benefits of improving COVID-19 antiviral access and equity, FDA amended the Paxlovid EUA on July 6, 2022, to allow skilled pharmacists with access to patient-specific information to order and prescribe this medication.135
Direct pharmacy distribution channel
From September 2021 through April 2022, the DHHS Office of the Assistant Secretary for Preparedness and Response used a state- and territory-coordinated distribution system for COVID-19 therapeutics.136 By April 2022, more than 500,000 courses of antivirals had been dispensed in the United States.137 Pfizer noted that prescriptions for Paxlovid in the United States increased from about 8000 patients per week in late February 2022 to nearly 80,000 patients for the week ending in April 22, 2022.138
On April 25, 2022, the DHHS transitioned to a new system that distributed antivirals directly through a pharmacy-specific distribution channel, rather than pharmacies receiving supplies from their state or jurisdiction.139 Expanded antiviral supply permitted expansion of the number of sites dispensing oral antiviral medications, increasing from 20,000 locations (e.g., pharmacies, community health centers, hospitals, urgent care centers, VA, and DoD facilities) in April 2022 toward a goal of 40,000 locations. Pharmacies continued to receive antivirals from state and territorial health departments and through the test-to-treat initiative.
Scope of practice and enabling authority
For pharmacists as skilled providers and pharmacies as centers of clinical care, the COVID-19 pandemic brought substantive expansions of authority. Expanded practice authority enabled that massive number of clinical interventions described above as well as their consistency from state to state. Clinical scopes of practice were standardized between states, bringing more expansive authority to pharmacists in some states. During the pandemic, pharmacies and pharmacists grew their collaborations with public health departments in depth and breadth across the country.37 , 140, 141, 142, 143, 144
Acting under authority of the PREP Act, the Secretary of the DHHS issued several declarations that preempted more restrictive professional authority at the state level for the duration of the COVID-19 pandemic (Table 1).33, 42, 65, 66, 143 Other expansions took the form of DHHS General Counsel advisory opinions and DHHS guidance documents. Many other countries did likewise.145 Pharmacists, student pharmacists (i.e., pharmacy interns), and pharmacy technicians nationwide received remarkable new authority, such as the following:
-
•
Authorizing pharmacists, pharmacy interns, and pharmacy technicians to order or administer FDA-authorized COVID-19 tests
-
•
Authorizing COVID-19 vaccination by pharmacists, pharmacy interns, and pharmacy technicians
-
•
Authorizing any vaccination recommended for children 3 through 18 years old to be ordered and administered by pharmacists and to be administered by pharmacy interns
-
•
Authorizing seasonal influenza vaccination by pharmacy interns and pharmacy technicians under the supervision of a pharmacist to persons aged 19 years and older consistent with ACIP recommendations
-
•
Authorizing pharmacists to order and administer certain COVID-19 therapeutics (e.g., monoclonal antibodies) by injection or I.V. infusion and pharmacy interns and pharmacy technicians to administer these products.
In some cases, state regulatory changes will be needed to retain these practice authorities and their inherent public health value after the COVID-19 emergency ends or the authorities otherwise expire.142 , 144, 145, 146
Contributions of pharmacists internationally
Pharmacists around the world were entrusted with new clinical roles and responsibilities during the COVID-19 pandemic. Published reports describe enhanced authority to handle therapeutic interchange during medication shortages, offer public health services, treat minor ailments, authorize prescription renewals, provide COVID-19 and other testing services, facilitate clinical trials, minimize inappropriate antimicrobial prescribing, and more.55 , 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158 Recognizing the public’s ease of access to a valuable care provider—their pharmacist—is a global phenomenon. The COVID-19 pandemic accelerated the trend since the 2000s for progressively more countries to train and authorize their pharmacists as vaccinators.147 , 153 , 159, 160, 161, 162, 163 In April 2022, Spain’s Ministry of Health awarded the Grand Cross of the Civil Order of Health, Spain’s highest distinction in the field of health, to the General Pharmaceutical Council of Spain for the pandemic contributions of Spain’s pharmacists.
Discussion
In the 1918-20 influenza pandemic, American pharmacists did what they could to help their patients, but there was little to offer beyond symptomatic relief.164 Viruses had not yet been identified; bacterial vaccines were ineffective at preventing influenza.2, 3, 4 , 164, 165, 166, 167, 168, 169 Antipneumococcal sera had some effect in treating or preventing secondary pneumococcal infections.165 , 167 Disinfecting washes with various combinations of camphor, menthol, eucalyptus oil, phenol, and other components were popular, as were many patent medicines.168 , 170, 171, 172 The scarcity of sugar, due to the war effort, constrained the compounding of syrups.166 Treatments included acetylsalicylic acid for pain or fever, opiates for cough, and quinine, laxatives, and medicinal whiskey of dubious value.168 , 170 , 171
At this writing, in September 2022, the COVID-19 pandemic exceeds 2.5 years in duration, and we have had tools to prevent disease by vaccination for the last 22 months. Pharmacists and their teams provided pharmaceutical care in its many forms to more than 5.4 million COVID-19 infected patients hospitalized between March 2020 and September 2022.5 , 172 , 173 This analysis was limited in its ability to quantify pharmacists’ involvement with monoclonal-antibody and antiviral administration—an order-of-magnitude estimate would be a million patients. Numbers of vaccinations and COVID-19 tests are easier to quantify. Overall, pharmacists’ teams contributed more than 350 million interventions to more than 150 million individuals, despite supply constraints, intricate storage requirements, workflow revisions, and workforce shortages. These interventions were equitably distributed in communities with high social vulnerability.37 , 106 , 112 This report does not attempt to describe the many educational efforts conducted by pharmacists’ teams, neither individually nor collectively as organized into professional associations. These hundreds of millions of interventions came at a personal cost to the pharmacists and their teams: personal risk to their health in terms of a COVID-19 infection and an extraordinary increase in workload and stress.16 , 174 , 175
The Commonwealth Fund estimated that the U.S. national COVID-19 vaccination program had averted 2.2 million deaths, 17 million hospitalizations, 66 million infections, and $900 billion in health care costs between December 2020 and March 2022.176 The DHHS estimated that COVID-19 vaccinations among Medicare beneficiaries saved $2.6 billion from reduced hospitalizations in just the short interval from January through May 2021.177
What fraction of this human and economic value can be attributed to pharmacists and their teams? More detailed analysis is needed, but if pharmacists disproportionately reached seniors and the socially vulnerable who have borne the brunt of the pandemic, contributed 45% of all COVID-19 vaccinations via the community-pharmacy channel alone, as well as more vaccinations in other practice settings, pharmacists likely account for at least half of the harm averted and value gained. Pharmacy involvement in testing and monoclonal antibody and antiviral programs will have averted even more morbidity and mortality.
Pharmacists’ ability to provide essential services in pandemic response has been building from foundations laid more than 2 decades ago.75 , 129 , 140 , 141 , 144 The national movement to train pharmacists as vaccinators began in 1996 in Mississippi. Maine became the 50th state to authorize pharmacist-vaccinators in 2009. By the late 2010s, U.S. pharmacists delivered an estimated 50 million vaccinations each year.
During the 2009 influenza A/H1N1 pandemic response, the federal government leveraged the reach of 10 pharmacy chains to distribute 5.5 million doses of federally purchased vaccine among more than 10,700 community locations across the country, accounting for 23% of the vaccine distributed during that time period.73 , 74 In our 2020-22 COVID-19 experience, many more pharmacists over many more months administered almost 50 times that the number of COVID-19 vaccinations plus influenza and other vaccinations. And these numbers continue to rise as the pandemic continues.
In the mid-2010s, the CDC conducted exercises with pharmacists to assess their ability to provide vaccines and other countermeasures during a pandemic178 and recommended that state and local public health authorities actively collaborate with pharmacists to enable rapid vaccination of their populations.75, 76, 77, 78 , 179 Although pharmacists were ultimately underutilized as pandemic vaccinators in 2009,74 that effort established the proof of concept and led to more robust planning efforts. Now, public health officials readily acknowledge the valuable contributions of pharmacists in population health and emergency preparedness.14 , 37 , 75 , 179, 180, 181, 182
Even with the prepandemic planning and multiple 2020-21 planning meetings,37 , 179 actual implementation in December 2020 and early 2021 was hectic, stressful, and yet intensely rewarding to the pharmacists’ teams that participated.16 , 114 Each of the national and state associations of pharmacists devoted innumerable hours to programming, sharing ideas, and supporting their members. Once each of the teams completed its own local learning curve, pharmacists reported that they had more capacity to vaccinate than they had doses to administer.37 , 86
Curiously, pharmacists administered a greater proportion of pediatric COVID-19 vaccinations than of pediatric influenza vaccinations.117 Future research could assess the relative contributions of proximity of pharmacists in settings of elevated social vulnerability, willingness or reluctance of other professions or practice settings to offer vaccination, and other factors contributing to the differential.
The advantages pharmacists offer in providing clinical interventions around several key parameters are as follows:
-
•
Clinical competence: Extensive knowledge- and skills-based experience
-
•
Convenient access: 90% of Americans live within 5 miles of a community pharmacy,37, 38, 39, 40 offering extended hours of availability
-
•
Trust: established relationships with their patients, reaching into underserved communities
-
•
Networked professionals: Pharmacists embedded within collaborative networks of other medical providers that readily share detailed clinical information, both in community pharmacy settings and as members of patient care teams in hospitals and health systems
NACDS issued an interim evaluation of successes and lessons learned in COVID-19 intervention programs in November 2021.37 Additional thoughtful, candid reviews of what worked and what did not (and how to preclude problems during the inevitable next pandemic) will be needed.
The NACDS lessons-learned report identified a key point: the (for some, recently recognized) value of pharmacists networking with public health departments.37
“… in one national survey of pharmacists conducted in 2012, over two-thirds of pharmacists polled had no contact with a state or local public health official in the past year.183 In stark contrast, [NACDS Virtual] Forum participants frequently mentioned that establishing and reinforcing partnerships with state and local public health was a major success factor that will yield benefits beyond the current pandemic. One Forum participant mentioned ‘this was really our first connection with our state Department of Health.’ Several Forum participants noted that it was the first time that public health officials ‘saw what we could do to improve public health. Public health folks are now advocating for what pharmacy used to have to solely advocate for.’ Another commented that ‘this [partnership] gave pharmacy [better ability to collaborate with public health] to pinpoint where there are pockets of needs for vaccine services—this can be grown potentially for other clinical services in future.’
The successes from the COVID-19 interventions described in this report show how pharmacists and their teammates played vital roles as health care providers during a public health crisis. These competencies and capacities can be applied in more normal times to improve health care access and strengthen community health nationwide.129 , 142 , 144 , 184 It will be important for communities to maintain their access to the care of a pharmacist they have come to expect and depend on during the pandemic. Therefore, it is essential to ensure that the scope-of-practice standardizations and sustainability mechanisms that increased the provision of pharmacist services during the pandemic remain in place and are enhanced after the pandemic subsides.
When the final tally of COVID-19 interventions is compiled, pharmacists will be justly recognized for the quality and magnitude of their contributions as care providers who mitigated and eventually controlled this pandemic. The tally in aggregate will show the pharmacist’s value in a programmatic and economic sense, but its most meaningful benefit will manifest in the health of individual neighbors cared for one by one.6
Document your contributions
This report features a small fraction of the ways pharmacists provided care for their communities. Record your contributions and those of your institution, association, or state. The American Institute of the History of Pharmacy (AIHP) invites contributions to its COVID-19 Pandemic Pharmacy Historical Documentation Project (https://aihp.org/collections/aihp-covid19-project/), to preserve pharmacists’ experiences during the pandemic. The AIHP seeks stories, photos, videos, artifacts, and other documentation of the COVID-19 pandemic’s effects on society.
Conclusion
Pharmacists and their teammates contributed essential services to America’s health and recovery during the COVID-19 pandemic as providers of multiple hundreds of millions of clinical interventions to hundreds of millions of people. Pharmacy teams responded quickly and courageously to rapidly changing circumstances, providing personalized care despite risks to their own health from the highly contagious virus. Despite medication shortages, supply shortages, and staffing shortages, pharmacists overcame geographic and cultural distances to provide compassionate care in every pharmacy practice setting.
Dedication
This report is dedicated to the pharmacists and their teammates who persevered in spring 2020, when little was known and much was feared.
Acknowledgments
Recognizing their input, advice, and critique: Nancy A. Alvarez, Scott J. Bergman, Lynette R. Bradley-Baker, Monali Bhosle, Anne Burns, Cody L. Clifton, Kimberly S. Croley, Arthur Daemmrich, Sharon G. Humiston, Christopher John, Lisa M. Koonin, Anne Lin, Michael S. Maddux, Lucinda L. Maine, Eric M. Maroyka, Erin K. McCreary, Sara R. Roszak, Mitchel C. Rothholz, Douglas J. Scheckelhoff, Jann B. Skelton, Gonçalo Sousa Pinto, Alli Jo Shipman, Catherine A. Taglieri, Theresa Taylor, Troy Trygstad, Dennis B. Worthen, and William A. Zellmer.
Biography
John D. Grabenstein, RPh, PhD, FAPhA, FASHP, Vaccine Dynamics, Easton, MD
Footnotes
Commissioned by the American Pharmacists Association Foundation, with guidance from the American Institute of the History of Pharmacy.
Disclosure: The author has received compensation for unrelated consulting services from Bavarian Nordic, Janssen, Seqirus, Takeda, Valneva, and VBI.
Funding: This work was funded in part by a collaborative agreement with the Centers for Disease Control and Prevention (CDC), CoAg # 1 NU50CK000576-01-00. The views presented here are those of the author and do not necessarily represent the views of the CDC or the U.S. Department of Health and Human Services.
Supplementary appendix to the article
Main Points:
>270million COVID-19 vaccinations given by community pharmacists and teams, December 2020-September 202286, 98, 99
45% of U.S. COVID-19 vaccinations given by community pharmacists and teams, December 2020-September 202298, 99
>50% of U.S. COVID-19 vaccinations overall given via pharmacist-led programs, December 2020-September 202286, 98 , 99, 100, 101, 102, 103, 104, 105 , 106, 107, 108
8.1 million COVID-19 vaccinations given by pharmacists and teams at long-term care (LTC) facilities, December 2020-April 202184 , 98, 99
2/3 drop • COVID-19 deaths among LTC residents fell by two-thirds compared to all COVID-19 deaths, December 2020-February 202185
1.3 million vaccinations given by student pharmacists via “Operation Immunization,” December 2020-June 2021120
>42 million patient specimens tested by pharmacists for COVID-19, April 2020-July 202237, 43, 45, 46, 47
Secondary Points:
>100,000 COVID-19 monoclonal antibody treatments provided by pharmacists, November 2020-June 2022
70% of vaccinating pharmacies located in communities with moderate-to-high social vulnerability37
>12 million more influenza vaccinations given by pharmacists to adults in 2020-21 season compared to previous season (47.7 million vs. 35.3 million for 2019-20)122
# 1 • West Virginia was the first state to offer COVID-19 vaccination to all LTC residents: pharmacists from 250 community pharmacies gave 28,000 vaccinations in 210 LTC facilities86, 87, 88, 89, 90 , 91
>5.4million inpatients cared for by pharmacists and teams, February 2020-September 20225 , 172 173
>350 million interventions
>150 million individuals received COVID-19 care provided by a pharmacist
References
- 1.Wikipedia COVID-19 pandemic in the United States. https://en.wikipedia.org/wiki/COVID-19_pandemic_in_the_United_States Available at:
- 2.Crosby A.W. Cambridge University Press; New York, NY: 1989. America’s Forgotten Pandemic: the Influenza of 1918. [Google Scholar]
- 3.Kolata G. Farrar Straus Giroux; New York, NY: 1999. Flu: the Story of the Great Influenza Pandemic of 1918 and the Search for the Virus That Caused It. [Google Scholar]
- 4.Barry J.M. Viking Adult; New York City, NY: 2004. The Great Influenza: the Epic Story of the Deadliest Plague in History. [Google Scholar]
- 5.Centers for Disease Control & Prevention COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home Available at:
- 6.Grabenstein J.D. Remington Honor Medal Address: The Patients (the People) You Touch. J Am Pharm Assoc (2003) 2020;60(5):e1–e4. doi: 10.1016/j.japh.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabenstein J.D. Vaccine humanity. J Am Pharm Assoc (2003) 2022;62(1):286–287. doi: 10.1016/j.japh.2021.11.018. (editorial) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogue M.D. You are heroes. J Am Pharm Assoc (2003) 2021;61(4):e10–e11. doi: 10.1016/j.japh.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Mutakabbir J.C., Casey S., Jews V., et al. A three-tiered approach to address barriers to COVID-19 vaccine delivery in the Black community. Lancet Glob Health. 2021;9(6):e749–e750. doi: 10.1016/S2214-109X(21)00099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IQVIA Institute for Human Data Science The use of medicines in the U.S.: spending and usage trends and outlook to 2025. IQVIA. 2021. www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-use-of-medicines-in-the-us/iqi-the-use-of-medicines-in-the-us-05-21-forweb.pdf Available at:
- 11.American Hospital Association Fast facts on US hospitals. 2021. www.aha.org/system/files/media/file/2021/01/Fast-Facts-2021-table-FY19-data-14jan21.pdf Available at:
- 12.Harris-Kojetin L., Sengupta M., Lendon J.P., Rome V., Valverde R., Caffrey C. Long-term care providers and services users in the United States, 2015–2016. National Center for Health Statistics. 2019. www.cdc.gov/nchs/data/series/sr_03/sr03_43-508.pdf Available at: [PubMed]
- 13.Herzik K.A., Bethishou L. The impact of COVID-19 on pharmacy transitions of care services. Res Social Adm Pharm. 2021;17(1):1908–1912. doi: 10.1016/j.sapharm.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aruru M., Truong H.A., Clark S. Pharmacy Emergency Preparedness and Response (PEPR): a proposed framework for expanding pharmacy professionals’ roles and contributions to emergency preparedness and response during the COVID-19 pandemic and beyond. Res Social Adm Pharm. 2021;17(1):1967–1977. doi: 10.1016/j.sapharm.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strand M.A., Bratberg J., Eukel H., Hardy M., Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi: 10.5888/pcd17.200317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston K., O'Reilly C.L., Cooper G., Mitchell I. The burden of COVID-19 on pharmacists. J Am Pharm Assoc (2003) 2021;61(2):e61–e64. doi: 10.1016/j.japh.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Clinical Pharmacy Clinical pharmacy in action: ACCP members respond to the COVID-19 pandemic. www.accp.com/membership/clinicalPharmacyInAction/covid19.aspx Available at:
- 18.MacArthur R.B., Bentur O.S., MacArthur I.C., et al. CTSA pharmacies: contribution to research and public health during the COVID-19 pandemic. J Clin Transl Sci. 2021;5(1):e108. doi: 10.1017/cts.2021.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do T., Luon S., Boothe K., Stutsky K., Renauer M. Advancing ambulatory pharmacy practice through a crisis: objectives and strategies used in an ambulatory care action team’s response to the COVID-19 pandemic. Am J Health Syst Pharm. 2021;78(8):720–725. doi: 10.1093/ajhp/zxab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enzmann M.O., Erickson M.P., Grindeland C.J., Lopez S.M.C., Hoover S.E., Leedahl D.D. Treatment and preliminary outcomes of 150 acute care patients with COVID-19 in a rural health system in the Dakotas. Epidemiol Infect. 2020;148:e124. doi: 10.1017/S0950268820001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Society of Health-System Pharmacists COVID-19 periodic pharmacy resources survey results. www.ashp.org/covid-19/bi-weekly-ppe-survey-results-covid-19 Available at:
- 22.Langford B.J., So M., Raybardhan S., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills W.R., Creasy S.M., Sender S., et al. Hydroxychloroquine sulfate prescribing trends and pharmacist-led outbreak preparedness in long-term care pharmacy during coronavirus disease 2019. J Am Med Dir Assoc. 2020;21(7):1000–1001. doi: 10.1016/j.jamda.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joint statement of the AMA, APhA, and ASHP on FDA action to remove emergency use authorization of medications to treat COVID-19. 2020. www.ashp.org/news/2020/06/23/ama-apha-ashp-covid-medications Available at:
- 25.Joint statement of the AMA, APhA, and ASHP. AMA, APhA, ASHP call for immediate end to prescribing, dispensing, and use of ivermectin to prevent or treat COVID-19 outside clinical trials. 2021. www.ashp.org/news/2021/09/01/ama-apha-ashp-call-for-end-to-ivermectin-to-prevent-or-treat-covid-19 Available at:
- 26.Burns A., Goodlet K.J., Chapman A., Roberts E.P. A case report of self-medication with over-the-counter fish antibiotic: implications for pharmacists. J Am Pharm Assoc (2003) 2020;60(4):E121–E123. doi: 10.1016/j.japh.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Morrisette T., Lodise T.P., Scheetz M.H., Goswami S., Pogue J.M., Rybak M.J. The pharmacokinetic and pharmacodynamic properties of hydroxychloroquine and dose selection for COVID-19: putting the cart before the horse. Infect Dis Ther. 2020;9(3):561–572. doi: 10.1007/s40121-020-00325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen C.A., Schneider P.J., Ganio M.C., Scheckelhoff D.J. ASHP national survey of pharmacy practice in hospital settings: impact of COVID-19 pandemic on pharmacy operations—2020. Am J Health Syst Pharm. 2021;78(18):1701–1712. doi: 10.1093/ajhp/zxab212. [DOI] [PubMed] [Google Scholar]
- 29.Warr D., Storey E., Denys M., Brown S., Rose C. Providing pharmacy services in a basketball arena: reflections on building a pharmacy in a COVID-19 surge facility. Am J Health Syst Pharm. 2021;78(5):416–425. doi: 10.1093/ajhp/zxaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Razaki H., Mui V., Rao P., Brocavich S. The pivotal role of pharmacists during the 2019 coronavirus pandemic. J Am Pharm Assoc (2003) 2020;60(6):e73–e75. doi: 10.1016/j.japh.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Society of Consultant Pharmacists Field guide to reduce medication burden during COVID-19. www.ascp.com/resource/resmgr/docs/disaster/field_guide_to_reduce_medica.pdf Available at:
- 32.American Society of Consultant Pharmacists Timeline of HHS regulations and legislative activities relating to the COVID-19 pandemic and pharmacy. www.ascp.com/resource/resmgr/docs/disaster/timeline_of_hhs_regulations_.pdf Available at:
- 33.Mun F., Hale C.M., Hennrikus E.F. A survey of US hospitals’ criteria for the allocation of remdesivir to treat COVID-19. Am J Health Syst Pharm. 2021;78(3):235–241. doi: 10.1093/ajhp/zxaa391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traynor K. Pharmacist-managed triage service guides COVID-19 antiviral treatments. Am J Health Syst Pharm. 2022;79(9):e99. doi: 10.1093/ajhp/zxac076. [DOI] [PubMed] [Google Scholar]
- 35.Congressional Research Service COVID-19 testing: frequently asked questions. 2021. https://crsreports.congress.gov/product/pdf/R/R46481 Available at:
- 36.Food & Drug Administration Coronavirus disease 2019 (COVID-19) emergency use authorizations for medical devices. www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices Available at: [PubMed]
- 37.National Association of Chain Drug Stores Community retail pharmacies’ experience during the COVID-19 response: successes and lessons learned to date. 2021. www.nacds.org/pdfs/pharmacy/2021/CommunityRetailPharmaciesExperience-during-the-COVID-19-Response-Successes-and-Lessons-Learned-to-Date.pdf Available at:
- 38.Chevalier J.A., Schwartz J.L., Su Y., Williams K.R. Distributional impacts of retail vaccine availability. J Urban Econ. 2022;127 doi: 10.1016/j.jue.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Association of Chain Drug Stores Face-to-face with community pharmacies. 2022. www.nacds.org/pdfs/about/rximpact-leavebehind.pdf Available at:
- 40.Qato D.M., Zenk S., Wilder J., Harrington R., Gaskin D., Alexander G.C. The availability of pharmacies in the United States: 2007-2015. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klepser N.S., Klepser D.G., Adams J.L., Adams A.J., Klepser M.E. Impact of COVID-19 on prevalence of community pharmacies as CLIA-waived facilities. Res Social Adm Pharm. 2021;17(9):1574–1578. doi: 10.1016/j.sapharm.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Alliance of State Pharmacy Associations COVID-19: federal PREP act actions. https://naspa.us/resource/federal-prep-act-actions/ Available at:
- 43.Goode J.R., Page A., Burns A., Bernard S., Wheawill S., Gatewood S.B.S. The pharmacist’s role in SARS-CoV-2 diagnostic testing. J Am Pharm Assoc (2003) 2020;60(6):e19–e32. doi: 10.1016/j.japh.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor S.K., Healey P., Mark N., Adams J.L., Robinson R., Nguyen E. Developing sustainable workflows for community pharmacy–based SARS-CoV-2 testing. J Am Pharm Assoc (2003) 2022;62(1):253–259. doi: 10.1016/j.japh.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Federal Emergency Management Agency By the numbers – coronavirus pandemic whole-of-America response. 2021. https://content.govdelivery.com/attachments/USDHSFEMA/2021/03/09/file_attachments/1717220/By%20the%20Numbers.COVID.FINAL.Mar.%208.2021.pdf Available at:
- 46.U.S. Department of Health & Human Services HHS continues community based testing sites for COVID-19. 2021. www.hhs.gov/about/news/2021/01/07/hhs-continues-community-based-testing-sites-covid-19.html Available at:
- 47.Centers for Disease Control & Prevention Increasing community access to testing (ICATT) for COVID-19. 2022. www.cdc.gov/icatt/index.html Available at:
- 48.Patel J., Christofferson N., Goodlet K.J. Pharmacist-provided SARS-CoV-2 testing targeting a majority-Hispanic community during the early COVID-19 pandemic: results of a patient perception survey. J Am Pharm Assoc (2003) 2022;62(1):187–193. doi: 10.1016/j.japh.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casadevall A., Dadachova E., Pirofski L.A. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 50.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Focosi D., Franchini M., Pirofski L.A., et al. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses. 2021;13(8):1594. doi: 10.3390/v13081594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers A. 97,000 people got convalescent plasma. Who knows if it works? Wired. 2020. www.wired.com/story/97000-people-got-convalescent-plasma-who-knows-if-it-works/ Available at:
- 53.Blackall D., Wulff S., Roettger T., et al. Rapid establishment of a COVID-19 convalescent plasma program in a regional health care delivery network. Transfusion. 2020;60(10):2203–2209. doi: 10.1111/trf.16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drug Information Group, University of Illinois College of Pharmacy Update: what do pharmacists need to know about convalescent plasma for COVID-19? 2021. https://dig.pharmacy.uic.edu/faqs/2021-2/february-2021-faqs/update-what-do-pharmacists-need-to-know-about-convalescent-plasma-for-covid-19/ Available at:
- 55.Klassen S.A., Senefeld J.W., Johnson P.W., et al. The effect of convalescent plasma therapy on mortality among patients with covid-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paudyal V., Cadogan C., Fialová D., et al. Provision of clinical pharmacy services during the COVID-19 pandemic: experiences of pharmacists from 16 European countries. Res Social Adm Pharm. 2021;17(8):1507–1517. doi: 10.1016/j.sapharm.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin R. Once viewed as a promising covid-19 treatment, convalescent plasma falls out of favor. JAMA. 2022;327(12):1115–1116. doi: 10.1001/jama.2022.3214. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan D.J., Gebo K.A., Shoham S., et al. Early outpatient treatment for COVID-19 with convalescent plasma. N Engl J Med. 2022;386(18):1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estcourt L., Callum J. Convalescent plasma for Covid-19—making sense of the inconsistencies. N Engl J Med. 2022;386:1753–1754. doi: 10.1056/NEJMe2204332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Food & Drug Administration Emergency use authorization. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization Available at:
- 61.Malashock C., Keifer A., Reisbig K., Reha C., Alexander B.T. Pharmacist outreach program for COVID-19 monoclonal antibody distribution. Am J Health Syst Pharm. 2021;78(13):1172–1175. doi: 10.1093/ajhp/zxab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Traynor K. Pharmacists fill caregiver gaps to expand COVID-19 antibody therapy. Am J Health Syst Pharm. 2021;78(23):2097–2099. doi: 10.1093/ajhp/zxab397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCreary E.K., Bariola J.R., Wadas R.J., et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.University of Pittsburgh Medical Center When demand outstrips capacity, injections of Covid-19 treatment instead of IV are justified. 2021. www.upmc.com/media/news/120221-mccreary-kip-subq-mabs Available at:
- 65.U.S. Department of Health & Human Services Public readiness and emergency preparedness act and its amendments: COVID-19 PREP act declarations. https://aspr.hhs.gov/legal/PREPact/Pages/default.aspx Available at:
- 66.National Association of Chain Drug Stores Timeline of key events: pharmacies’ role in advancing access to COVID-19 countermeasures, including vaccine & testing. www.nacds.org/covid-19/timeline/ Available at:
- 67.Society of Infectious Diseases Pharmacists COVID-19 monoclonal antibody toolkit. https://sidp.org/COVID19-Monoclonal-Antibody-Toolkit-Guide Available at:
- 68.American Pharmacists Association COVID-19 monoclonal antibody therapies implementation guide. 2021. www.pharmacist.com/Practice/COVID-19/Know-the-Facts Available at:
- 69.Rivera G., De León M., Bernardelli B., Brannan B., Dicubellis J., Williams C. Rapid implementation of pharmacy infusion services for emergency use authorization COVID-19 treatments at a field hospital. Am J Health Syst Pharm. 2021;78(22):2015–2019. doi: 10.1093/ajhp/zxab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris A.K., Gibson A.M., Spencer B., et al. Rapid implementation of a new ambulatory infusion location for patients with COVID-19 to receive monoclonal antibody therapy. Am J Health Syst Pharm. 2021;78(13):1166–1168. doi: 10.1093/ajhp/zxab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sartain M. Arkansas pharmacy keeps COVID-19 patients out of hospitals with monoclonal antibody therapy. 2021. www.pharmacist.com/Pharmacy-News/arkansas-pharmacy-keeps-covid-19-patients-out-of-hospitals-with-monoclonal-antibody-therapy Available at:
- 72.Food & Drug Administration Coronavirus disease 2019 (COVID-19) www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19 Available at: [PubMed]
- 73.Advisory Committee on Immunization Practices COVID-19 ACIP vaccine recommendations. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html Available at:
- 74.Institute of Medicine . National Academies Press; Washington, DC: 2010. The 2009 H1N1 Influenza Vaccination Campaign: Summary of a Workshop Series. [PubMed] [Google Scholar]
- 75.Koonin L.M., Beauvais D.R., Shimabukuro T., et al. CDC‘s 2009 H1N1 vaccine pharmacy initiative in the United States: implications for future public health and pharmacy collaborations for emergency response. Disaster Med Public Health Prep. 2011;5(4):253–255. doi: 10.1001/dmp.2011.83. [DOI] [PubMed] [Google Scholar]
- 76.Rubin S.E., Schulman R.M., Roszak A.R., Herrmann J., Patel A., Koonin L.M. Leveraging partnerships among community pharmacists, pharmacies, and health departments to improve pandemic influenza response. Biosecur Bioterror. 2014;12(2):76–84. doi: 10.1089/bsp.2013.0082. [DOI] [PubMed] [Google Scholar]
- 77.Centers for Disease Control & Prevention Public health emergency preparedness and response capabilities: national standards for state, local, tribal, and territorial public health. 2018. www.cdc.gov/cpr/readiness/00_docs/CDC_PreparednesResponseCapabilities_October2018_Final_508.pdf Available at:
- 78.Sokolow L.Z., Patel A., Koonin L.M., Graitcer S.B. Scripted surge pharmacy pandemic exercise: testing vaccine administration and antiviral dispensing. Health Secur. 2018;16(4):262–273. doi: 10.1089/hs.2018.0031. [DOI] [PubMed] [Google Scholar]
- 79.Schwerzmann J., Graitcer S.B., Jester B., et al. Evaluating the impact of pharmacies on pandemic influenza vaccine administration. Disaster Med Public Health Prep. 2017;11(5):587–593. doi: 10.1017/dmp.2017.1. [DOI] [PubMed] [Google Scholar]
- 80.Government Accountability Office COVID-19 in nursing homes: HHS has taken steps in response to pandemic, but several GAO recommendations have not been implemented. 2021. www.gao.gov/assets/gao-21-402t.pdf Available at:
- 81.Gharpure R., Guo A., Bishnoi C.K., et al. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the Pharmacy Partnership for Long-Term Care Program—United States, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5):178–182. doi: 10.15585/mmwr.mm7005e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gharpure R., Yi S.H., Li R., et al. COVID-19 vaccine uptake among residents and staff members of assisted living and residential care communities: pharmacy partnership for long-term care program, December 2020-April 2021. J Am Med Dir Assoc. 2021;22(10):2016–2020. doi: 10.1016/j.jamda.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Managed Health Care Associates, Inc MHA selected as COVID-19 vaccinations network administrator for the MHA independent long-term care pharmacy network. 2020. www.mhainc.com/News/MHA_selected_as_COVID-19_vaccinations_network_administrator_for_the_MHA_independent_long-term_care_pharmacy_network/ Available at:
- 84.Government Accountability Office COVID-19: HHS agencies’ planned reviews of vaccine distribution and communication efforts should include stakeholder perspectives. 2021. www.gao.gov/assets/gao-22-104457.pdf Available at:
- 85.Conlen M., Mervosh S., Ivory D. Nursing homes, once hotspots, far outpace U.S. in Covid declines. New York Times, February 25, 2021, page A1. www.nytimes.com/interactive/2021/02/25/us/nursing-home-covid-vaccine.html Available at:
- 86.Government Accountability Office COVID-19: sustained federal action is crucial as pandemic enters its second year. 2021. www.gao.gov/assets/gao-21-387.pdf Available at:
- 87.Gonzales A., Lee E.C., Grigorescu V., Smith S.R., De Lew N., Sommers B.D. Overview of barriers and facilitators in COVID-19 vaccine outreach. 2021. https://aspe.hhs.gov/reports/covid-19-vaccine-outreach Available at:
- 88.Galston W.A. COVID-19 vaccinations: why are some states and localities so much more successful? January 25, 2021. Brookings Institution, Washington, DC. www.brookings.edu/blog/fixgov/2021/01/25/covid-19-vaccinations-why-are-some-states-and-localities-so-much-more-successful/ Available at:
- 89.Rafa M. Pharmacists answer the call for COVID-19 vaccine administration. West Virginia Pharmacists Association. 2020. https://wvpharmacy.org/2020/12/pharmacistsanswercall/ Available at:
- 90.Sweet J. North Dakota and West Virginia have found the secret to a successful vaccine rollout. Healthline. 2021. www.healthline.com/health-news/north-dakota-and-west-virginia-have-found-the-secret-to-a-successful-vaccine-rollout#Collaborating-with-independent-pharmacies Available at:
- 91.Whang O. What the world can learn from West Virginia’s successful vaccine roll-out: in the race to vaccinate, the state’s personal touch is a winner. National Geographic. 2021. www.nationalgeographic.com/culture/article/what-world-can-learn-from-west-virginia-successful-vaccine-roll-out Available at:
- 92.Thomas K. Hospitals discover a surprise in their vaccine deliveries: extra doses. New York Times. 2020. www.nytimes.com/2020/12/16/health/Covid-Pfizer-vaccine-extra-doses.html Available at:
- 93.Dunn L., Beck C. Pharmacists say ‘pooling’ Covid vaccines could save thousands of doses. 2021. www.nbcnews.com/health/health-news/pharmacists-say-pooling-covid-vaccines-could-save-thousands-doses-n1258149 Available at:
- 94.Allen A. To extract more doses per vial, vaccinators put squeeze on FDA to relax vaccine handling advice. Kaiser Health News. 2021. www.healthleadersmedia.com/covid-19/extract-more-doses-vial-vaccinators-put-squeeze-fda-relax-vaccine-handling-advice Available at:
- 95.Traynor K. Children’s hospital celebrates successful COVID-19 vaccine rollout. Am J Health Syst Pharm. 2021;78(10):838–839. doi: 10.1093/ajhp/zxab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Centers for Disease Control & Prevention The federal retail pharmacy program for COVID-19 vaccination: a collaboration between the federal government, states and territories, and 21 national pharmacy partners and independent pharmacy networks nationwide. www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/index.html Available at:
- 97.Centers for Disease Control & Prevention COVID-19 vaccine access in long-term care settings. www.cdc.gov/vaccines/covid-19/long-term-care/pharmacy-partnerships.html Available at:
- 98.Andrade J., Slaby M., DeAngelis J., et al. Implementation of a pharmacist-led COVID-19 vaccination clinic at a community teaching hospital. Am J Health Syst Pharm. 2021;78(12):1038–1042. doi: 10.1093/ajhp/zxab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Federal Emergency Management Agency Community vaccination centers playbook. 2021. www.fema.gov/disaster/coronavirus/governments/community-vaccination-centers-playbook Available at:
- 100.Traynor K. COVID-19 mass vaccination involves unusual venues. Am J Health Syst Pharm. 2021;78(13):1162–1164. doi: 10.1093/ajhp/zxab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gregory N. Pharmacy leadership, expertise imperative for successful COVID-19 vaccine rollout. Am J Health Syst Pharm. 2021;78(14):1264–1266. doi: 10.1093/ajhp/zxab187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gregory N. COVID-19 vaccines in tribal communities save lives, preserve culture. Am J Health Syst Pharm. 2021;78(10):835–839. doi: 10.1093/ajhp/zxab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Traynor K. Health systems quickly vaccinated communities against COVID-19 despite supply constraints. Am J Health Syst Pharm. 2021;78(11):940–942. doi: 10.1093/ajhp/zxab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Centers for Disease Control & Prevention COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total Available at:
- 105.Traynor K. COVID-19 dispatch: lifespan, Rhode Island. 2021. www.ashp.org/news/2021/05/04/rhode-island-pharmacist-earns-leadership-role-in-pandemic Available at:
- 106.Government Accountability Office COVID-19: federal efforts to provide vaccines to racial and ethnic groups. 2022. www.gao.gov/assets/gao-22-105079.pdf Available at:
- 107.Centers for Disease Control and Prevention CDC/ATSDR social vulnerability index. www.atsdr.cdc.gov/placeandhealth/svi/index.html Available at:
- 108.Community Pharmacy Enhanced Services Network Services available from CPESN network pharmacies. https://www.cpesn.com/payors/services-available-from-cpesn-network-pharmacies/ Available at:
- 109.Riley A.C., Campbell H., Butler L., Wisseh C., Nonyel N.P., Shaw T.E. Socialized and traumatized: pharmacists, underserved patients, and the COVID-19 vaccine. J Am Pharm Assoc (2003) 2021;61(6):E2–E5. doi: 10.1016/j.japh.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Traynor K. Trusted sources critical for COVID-19 vaccine outreach. Am J Health Syst Pharm. 2021;78(18):1658–1659. doi: 10.1093/ajhp/zxab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonner L. Looking back: pharmacists share COVID-19 vaccination lessons one year later. Pharm Today. 2022;28:24–29. [Google Scholar]
- 112.Popovian R., Winegarden W., Rivera E., Gavigan K. Accessibility of adult immunizations in pharmacies compared to physician offices in low-income communities. J Am Pharm Assoc (2003) 2022 doi: 10.1016/j.japh.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 113.Johns Hopkins Center for Health Security and National Association of Chain Drug Stores Striving toward health equity in COVID-19: the role of pharmacies in a national response. 2021. www.centerforhealthsecurity.org/news/center-news/2021/2021-10-18-striving-toward-health-equity-in-COVID-19.html Available at:
- 114.American Pharmacists Association Vaccine confident: success stories. https://vaccineconfident.pharmacist.com/Share/Success-Stories Available at:
- 115.American Society of Health-System Pharmacists Pharmacy on the front lines of the COVID-19 pandemic. www.ashp.org/covid-19/stories Available at: [DOI] [PMC free article] [PubMed]
- 116.AmerisourceBergen Corporation Fearless pharmacy: good neighbor pharmacy stores administering COVID-19 vaccinations. www.wearegnp.com/fearless-pharmacy Available at:
- 117.Kim C., Yee R., Bhatkoti R., et al. COVID-19 vaccine provider access and vaccination coverage among children aged 5–11 years—United States, November 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:378–383. doi: 10.15585/mmwr.mm7110a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeCuir J., Meng L., Pan Y., et al. COVID-19 vaccine provider availability and vaccination coverage among children aged 5–11 years — United States, November 1, 2021–April 25, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:847–851. doi: 10.15585/mmwr.mm7126a3. [DOI] [PubMed] [Google Scholar]
- 119.McKesson Corporation Rural pharmacies providing COVID-19 vaccinations: select U.S. health mart pharmacies have started vaccinating patients. www.mckesson.com/Our-Stories/Rural-Pharmacies-Providing-COVID-19-Vaccinations/ Available at:
- 120.American Pharmacists Association—Academy of Student Pharmacists . American Pharmacists Association; 2022. Operation immunization annual report, 2020-2021. Washington, DC. [Google Scholar]
- 121.Notre Dame of Maryland University School of pharmacy dean named health care hero by The Daily Record. 2022. https://www.ndm.edu/news-and-events/news/school-pharmacy-dean-named-health-care-hero-daily-record Available at:
- 122.Centers for Disease Control & Prevention Influenza vaccinations administered in pharmacies and physician medical offices, adults, United States. www.cdc.gov/flu/fluvaxview/dashboard/vaccination-administered.html Available at:
- 123.Santoli J.M., Lindley M.C., DeSilva M.B., et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:591–593. doi: 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
- 124.U.S. Department of Health & Human Services Third amendment to COVID-19 PREP Act declaration—childhood vaccines, August 24, 2020. Washington, DC. www.hhs.gov/sites/default/files/third-amendment-declaration.pdf Available at:
- 125.Liow C., Gillen E.M., Becker L., Trost A., Young J. Updated analysis finds sustained drop in routine vaccines through 2020. 2021. https://avalere.com/insights/updated-analysis-finds-sustained-drop-in-routine-vaccines-through-2020 Available at:
- 126.Association of State & Territorial Health Officials Key considerations for pharmacies and the vaccines for children (VFC) program: summary of interview and survey findings. 2015. www.mysocietysource.org/sites/HPV/ResourcesandEducation/Lists/Clearinghouse/Attachments/516/ASTHO%20VFC%20Pharmacy%20Report_Executive%20Summary.pdf Available at:
- 127.Rodriguez C.H. Feds look to pharmacists to boost childhood immunization rates. Kaiser Health News. 2020. https://khn.org/news/pharmacist-federal-training-program-perform-vaccinations-to-boost-childhood-immunization-rates/ Available at:
- 128.Fox E.R., Shah M., Vinik R., et al. Developing statewide remdesivir use criteria. Am J Health Syst Pharm. 2021;78(8):732–735. doi: 10.1093/ajhp/zxab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abramowitz P.W., Thompson K.K., Cobaugh D.J. Pharmacists: essential providers of COVID-19 care. Am J Health Syst Pharm. 2022;79:927–928. doi: 10.1093/ajhp/zxac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abdul-Mutakabbi J.C., Hirsch E.B., Ko C., et al. A call to action: a need for initiatives that increase equitable access to COVID-19 therapeutics. Lancet Reg Health Am. 2022;11:100263. doi: 10.1016/j.lana.2022.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.U.S. Department of Health & Human Services Test-to-treat. https://aspr.hhs.gov/TestToTreat/Pages/default.aspx Available at:
- 132.Weiland N. With supply more abundant, pharmacies struggle to use up Covid pills. NY Times. 2022. www.nytimes.com/live/2022/04/26/world/mandates-cases-vaccine-covid-19#paxlovid-test-to-treat Available at:
- 133.American Pharmacists Association Inequity to COVID-19 test to treat access—pharmacists can help if permitted. 2022. www.pharmacist.com/Advocacy/Issues/Inequity-to-COVID-19-Test-to-Treat-Access-Pharmacists-can-help-if-permitted Available at:
- 134.Gold J.A.W., Kelleher J., Magid J., et al. Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code–level social vulnerability — United States, December 23, 2021–May 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(25):825–829. doi: 10.15585/mmwr.mm7125e1. [DOI] [PubMed] [Google Scholar]
- 135.Food & Drug Administration Coronavirus (COVID-19) update: FDA authorizes pharmacists to prescribe Paxlovid with certain limitations. 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pharmacists-prescribe-paxlovid-certain-limitations Available at:
- 136.U.S. Department of Health & Human Services State/territory-coordinated distribution of COVID-19 therapeutics. 2022. https://aspr.hhs.gov/COVID-19/Therapeutics/Distribution/Pages/default.aspx Available at:
- 137.Background press call by senior administration officials on new actions to increase access to COVID-19 treatments and boost patient and provider awareness. 2022. www.whitehouse.gov/briefing-room/press-briefings/2022/04/25/background-press-call-by-senior-administration-officials-on-new-actions-to-increase-access-to-covid-19-treatments-and-boost-patient-and-provider-awareness/ Available at:
- 138.CNBC Paxlovid prescriptions to treat Covid increased tenfold in U.S. since late February, Pfizer says. 2022. www.cnbc.com/2022/05/03/pfizer-paxlovid-prescriptions-to-treat-covid-increased-tenfold-in-us-since-late-february.html Available at:
- 139.White House Fact sheet: biden administration increases access to COVID-19 treatments and boosts patient and provider awareness. 2022. www.whitehouse.gov/briefing-room/statements-releases/2022/04/26/fact-sheet-biden-administration-increases-access-to-covid-19-treatments-and-boosts-patient-and-provider-awareness/ Available at:
- 140.Grabenstein J.D. Pharmacists and immunization: increasing involvement over a century. Pharm Hist. 1999;41(4):137–152. [PubMed] [Google Scholar]
- 141.Hogue M.D., Grabenstein J.D., Foster S., Rothholz M. Pharmacist-administered immunizations: a decade of professional advancement. J Am Pharm Assoc (2003) 2006;46:168–182. doi: 10.1331/154434506776180621. Erratum 308. [DOI] [PubMed] [Google Scholar]
- 142.Andrawis M., Carmichael J., Collins C.D., et al. Improving patient care and demonstrating value during a global pandemic: recommendations from leaders of the Pharmacy Accountability Measures Work Group. Am J Health Syst Pharm. 2020;77(23):2003–2005. doi: 10.1093/ajhp/zxaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang W. The history of pharmacy’s expanded role in combatting COVID-19. 2021. https://aihp.org/the-history-of-pharmacys-expanded-role-in-combatting-covid-19/ Available at:
- 144.Pantasri T. Expanded roles of community pharmacists in COVID-19: a scoping literature review. J Am Pharm Assoc (2003) 2022;62(3):649–657. doi: 10.1016/j.japh.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Merks P., Jakubowska M., Drelich E., et al. The legal extension of the role of pharmacists in light of the COVID-19 global pandemic. Res Social Adm Pharm. 2021;17(1):1807–1812. doi: 10.1016/j.sapharm.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lynch M., O'Leary A.C. COVID-19 related regulatory change for pharmacists—the case for its retention post the pandemic. Res Social Adm Pharm. 2021;17(1):1913–1919. doi: 10.1016/j.sapharm.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Czech M., Balcerzak M., Antczak A., et al. Flu vaccinations in pharmacies—a review of pharmacists fighting pandemics and infectious diseases. Int J Environ Res Public Health. 2020;17(21):7945. doi: 10.3390/ijerph17217945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wilkinson E. No going back: how the pandemic is changing hospital pharmacy. Pharmaceutical Journal. 2020. https://pharmaceutical-journal.com/article/feature/no-going-back-how-the-pandemic-is-changing-hospital-pharmacy Available at:
- 149.Malson G. No going back: how the pandemic is changing general practice pharmacy. Pharmaceutical Journal. 2020. https://pharmaceutical-journal.com/article/feature/no-going-back-how-the-pandemic-is-changing-general-practice-pharmacy Available at:
- 150.Moore A. No going back: how the pandemic is changing community pharmacy. Pharmaceutical Journal. 2020. https://pharmaceutical-journal.com/article/feature/no-going-back-how-the-pandemic-is-changing-community-pharmacy Available at:
- 151.Gregory P.A.M., Austin Z. COVID-19: how did community pharmacies get through the first wave? Can Pharm J. 2020;153(5):243–251. doi: 10.1177/1715163520945741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goff D.A., Ashiru-Oredope D., Cairns K.A., et al. Global contributions of pharmacists during the COVID-19 pandemic. J Am Coll Clin Pharm. 2020;3(8):1480–1492. doi: 10.1002/jac5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jordan D., Guiu-Segura J.M., Sousa-Pinto G., Wang L.-N. How COVID-19 has impacted the role of pharmacists around the world. Farm Hosp. 2021;45(2):89–95. doi: 10.7399/fh.11652. [DOI] [PubMed] [Google Scholar]
- 154.Sousa Pinto G., Hung M., Okoya F., Uzman N. FIP's response to the COVID-19 pandemic: global pharmacy rises to the challenge. Res Social Adm Pharm. 2021;17(1):1929–1933. doi: 10.1016/j.sapharm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Song Z., Hu Y., Zheng S., Yang L., Zhao R. Hospital pharmacists' pharmaceutical care for hospitalized patients with COVID-19: recommendations and guidance from clinical experience. Res Social Adm Pharm. 2021;17(1):2027–2031. doi: 10.1016/j.sapharm.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Watson K.E., Schindel T.J., Barsoum M.E., Kung J.Y. COVID the catalyst for evolving professional role identity? A scoping review of global pharmacists' roles and services as a response to the COVID-19 pandemic. Pharmacy (Basel) 2021;9(2):99. doi: 10.3390/pharmacy9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maidment I., Young E., MacPhee M., et al. Rapid realist review of the role of community pharmacy in the public health response to COVID-19. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2021-050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Costa S., Romão M., Mendes M., et al. Pharmacy interventions on COVID-19 in Europe: mapping current practices and a scoping review. Res Social Adm Pharm. 2022;18(8):3338–3349. doi: 10.1016/j.sapharm.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song Z., Hu Y., Ren Z., et al. Optimal management of the public and patients by pharmacists in the era of COVID-19: an evidence-based review and practical recommendations. Front Public Health. 2022;9 doi: 10.3389/fpubh.2021.758325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.International Pharmaceutical Federation (FIP) International Pharmaceutical Federation; Hague, The Netherlands: 2020. An Overview of Pharmacy's Impact on Immunisation Coverage: a Global Report, 2020. [Google Scholar]
- 161.International Pharmaceutical Federation (FIP) International Pharmaceutical Federation; Hague, The Netherlands: 2022. Advocating Expansion of the Pharmacist’s Role in Immunisation: a Focus on Diphtheria-Tetanus-Pertussis Booster, COVID-19 and Meningitis Vaccinations. [Google Scholar]
- 162.International Pharmaceutical Federation (FIP) International Pharmaceutical Federation; Hague, The Netherlands: 2022. FIP Vaccination Reference Guide. Knowledge and Skills to Support Professional Development and Inform Pharmacy Education in Vaccination. [Google Scholar]
- 163.Moore A. Community pharmacy: vaccinating the people that other sites cannot reach. Pharmaceutical Journal. 2021. https://pharmaceutical-journal.com/article/feature/community-pharmacy-vaccinating-the-people-that-other-sites-cannot-reach Available at:
- 164.American Public Health Association Influenza: report of a Special Committee of the American Public Health Association. JAMA. 1918;71(25):2068–2073. [Google Scholar]
- 165.Fischelis R.P. Practical information on biological products for the retail druggists. Am J Pharm. 1918;90:758–764. [Google Scholar]
- 166.Anonymous Philadelphia paragraphs; Indiana information; Boston briefs. Natl Assoc Retail Druggists J. 1918;27:16–17. [Google Scholar]
- 167.Grabenstein J.D., Klugman K.P. A century of pneumococcal vaccination research in humans. Clin Microbiol Infect. 2012;18(Suppl 5):15–24. doi: 10.1111/j.1469-0691.2012.03943.x. [DOI] [PubMed] [Google Scholar]
- 168.MacCara M.E. Combating Spanish influenza: focus on pharmacists, pharmacies and drugs. Can Pharm J. 2020;153:335–342. doi: 10.1177/1715163520960178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tannenbaum J. The Philadelphia College of Pharmacy and the 1918 influenza. www.usciences.edu/news/2021/philadelphia-college-of-pharmacy-and-the-1918-influenza.html Available at:
- 170.Daugherty G. Amid 1918 pandemic, bootleg whiskey became a respectable medicine. A&E Television Networks, LLC. 2020. www.history.com/news/1918-flu-pandemic-whiskey-remedy-prohibition Available at:
- 171.Cecil County [Maryland] Historical Society Pharmacists and drug store clerks were essential when the Spanish flu hit. https://cecilcountyhistory.com/pharmacists-were-essential-when-the-spanish-flu-hit Available at:
- 172.Centers for Disease Control & Prevention Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html Available at:
- 173.Owusu D., Kim L., O'Halloran A., et al. Characteristics of adults aged 18-49 years without underlying conditions hospitalized with laboratory-confirmed coronavirus disease 2019 in the United States: COVID-NET-March-August 2020. Clin Infect Dis. 2021;72(5):e162–e166. doi: 10.1093/cid/ciaa1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Terlap S. Omicron surge is just starting and America’s pharmacists are already burned out. Wall Street Journal. 2021. www.wsj.com/articles/omicron-surge-is-just-starting-andamericas-pharmacists-are-already-burned-out-11640008870 Available at:
- 175.Bakken B.K., Winn A.N. Clinician burnout during the COVID-19 pandemic before vaccine administration. J Am Pharm Assoc (2003) 2021;61:e71–e77. doi: 10.1016/j.japh.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schneider E.C., Shah A., Sah P., et al. Commonwealth Fund; New York, NY: 2022. Impact of U.S. COVID-19 Vaccination Efforts: An Update on Averted Deaths, Hospitalizations, and Health Care Costs Through March 2022, to the Point (Blog) [Google Scholar]
- 177.U.S. Department of Health & Human Services Hospitalization cost savings associated with COVID-19 vaccinations among Medicare beneficiaries in early 2021. 2022. https://aspe.hhs.gov/sites/default/files/documents/b3bd41440bde364040c1293699b31ed8/mcare-hospital-savings-covid-vaccine.pdf Available at:
- 178.Avalere Health LLC Exploring pharmacists’ role in a changing healthcare environment. 2014. www.nacds.org/pdfs/comm/2014/pharmacist-role.pdf Available at:
- 179.Bacci J.L., Odegard P., Arnold J., Stergachis A. Strengthening pandemic preparedness through pharmacy and public health collaborations: findings from a facilitated discussion exercise. J Am Pharm Assoc (2003) 2021;61(3):E99–E106. doi: 10.1016/j.japh.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 180.Bartsch S.M., Taitel M.S., DePasse J.V., et al. Epidemiologic and economic impact of pharmacies as vaccination locations during an influenza epidemic. Vaccine. 2018;36(46):7054–7063. doi: 10.1016/j.vaccine.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Centers for Disease Control & Prevention Pharmacy contributions to improved population health: expanding the public health roundtable. 2020. www.cdc.gov/pcd/issues/2020/20_0350.htm Available at: [DOI] [PMC free article] [PubMed]
- 182.Gessler C.A., Richardson R.M., Hall D.L., Coley K.C. Operationalizing pandemic vaccinations at a regional supermarket chain pharmacy. Disaster Med Public Health Prep. 2021:1–6. doi: 10.1017/dmp.2021.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.SteelFisher G.K., Benson J.M., Caporello H., et al. Pharmacist views on alternative methods for antiviral distribution and dispensing during an influenza pandemic. Health Secur. 2018;16(2):108–118. doi: 10.1089/hs.2017.0068. [DOI] [PubMed] [Google Scholar]
- 184.Ashcraft A.M., Ponte C.D., Farjo S., Dotson S., Murray P.J. The [underutilized] power of independent pharmacies to promote public health in rural communities: a call to action. J Am Pharm Assoc (2003) 2022;62(1):38–41. doi: 10.1016/j.japh.2021.09.002. [DOI] [PubMed] [Google Scholar]