Abstract
Acute Graft versus host disease (GVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo HSCT), a potent form of cellular therapy that has the potential to cure malignant and benign hematological conditions. Gastrointestinal (GI) GVHD is the principal cause of non-relapse mortality (NRM) after allo HSCT. Allo HSCT alters the intestinal microbiota and recent research uncovered a microbiome-metabolome axis that can affect intestinal homeostasis and mitigate the severity of experimental GI GVHD. This axis can potentially be manipulated via dietary intervention or through probiotics or postbiotics or antibiotics in humans. In this review we summarize major findings of how microbial metabolites and particularly short chain fatty acids (SCFAs) could impact acute GI GVHD.
Acute GVHD results from an allo-immune response driven by donor T cells causing tissue damage in target organs such as in the GI tract[1]. Significant improvements in understanding the role of donor and host immune cells, with better conditioning and supportive care regimens, have resulted in reduction of transplant-related NRM after allo HSCT[2]. However, despite major strides in understanding the pathogenesis of acute GVHD and the development of novel immunosuppressants, GI GVHD related NRM remains significant [3].
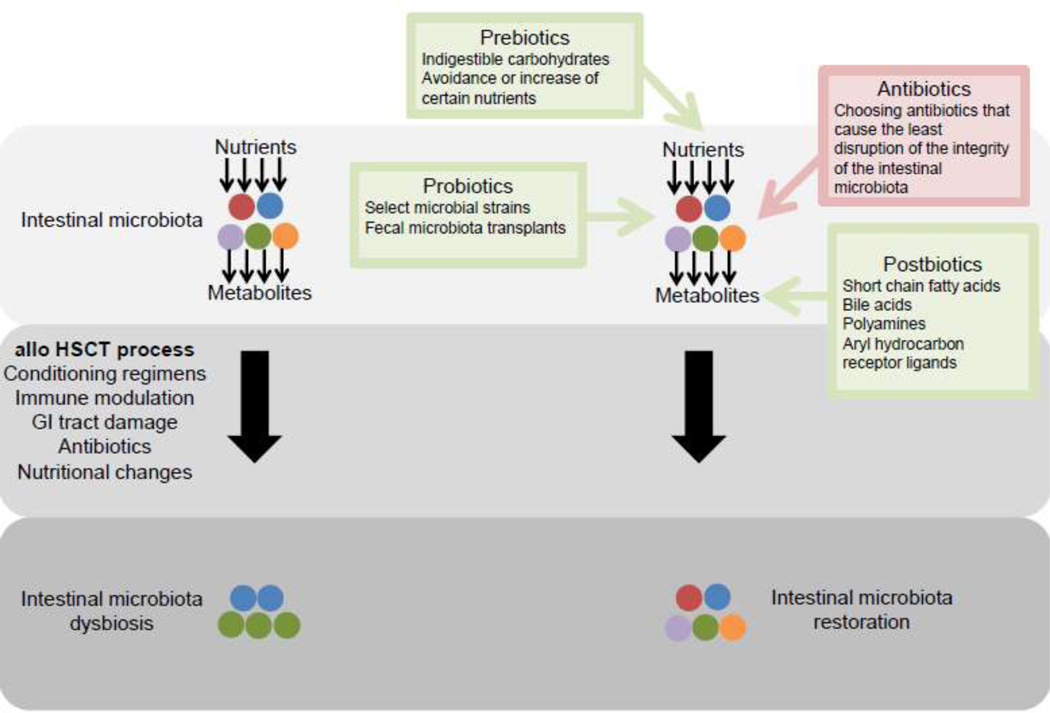
Recent studies have brought into focus the potential role played by the host microbiome on GVHD outcomes[4]. The intestinal microbiota interacts with the host intestinal immune and non-immune cells and contributes to a broad range of functions in the host, including, digestion of nutrients, production of microbial metabolites and maintenance of the intestinal homeostasis[5]. Emerging data have demonstrated a strong correlation between aberrant shifts in intestinal microbiota composition and several disease processes including autoimmune, metabolic diseases, cancer, and acute GI GVHD after allo HSCT [4, 6]. Several clinical strategies (Figure 1), such as diet and prebiotics, antibiotics, probiotics, and fecal microbiota transplantation (FMT), can be employed to manipulate the architecture of the intestinal microbiota [7]. The mechanisms by which the intestinal microbiota modulates acute GI GVHD remain poorly understood[8]. One mechanism could be related to the ability of the intestinal microbiota to produce beneficial or harmful metabolites by breaking down host nutrients[7]. Recent evidence has brought into play the role of microbial metabolites, specifically SCFAs, in the severity of acute GI GVHD[9, 10]. In this review, we summarize data investigating the roles that microbial metabolites, and in particular SCFAs, play in impacting host intestinal immune and non-immune cells, and how these metabolites may regulate acute GI GVHD.
Figure 1.
Points for clinical intervention to alter the intestinal microbiota
The process of allogeneic hematopoietic stem cell transplantation (allo HSCT) results in dysbiosis of the intestinal microbiota. This figure depicts different strategies to restore the architecture of the intestinal microbiota in patients undergoing allo HSCT.
Immune biology of GVHD and the role of the microbiota in intestinal homeostasis
GVHD is a complex interaction between the innate and adaptive immune systems of the host and donor in allo HSCT[1]. In allo HSCT, conditioning regimens are used for facilitating engraftment of donor hematopoietic cells and eliminating residual malignant cells[11]. These regimens cause host tissue injuries which release “danger signals” including pathogen-associated molecular patters (PAMPs) and damage-associated molecular patters (DAMPs). “Danger signals” activate host or donor antigen presenting cells (APCs) which is amplified by microbial stimulation[12]. APCs present allo-antigens to donor T cells via major histocompatibility complex (MHC) class I or class II and further escalate the inflammatory response by producing T-cell stimulating cytokines[13]. Stimulated donor T cells expand and differentiate into effector T cells and these migrate into and damage GVHD target organs such as the GI tract[3]. GI tract cells such as intestinal epithelial cells (IECs), intestinal stem cells (ISCs), and paneth cells, are targeted in GI GVHD[14]. Thus, GVHD is an immunologically mediated process and GI tract damage with subsequent disruptions in intestinal homeostasis, which are further compounded by the use of broad-spectrum antibiotics and alterations in nutrition that occur during the process of allo HSCT, play important roles in augmenting GVHD.
Maintenance of intestinal homeostasis is in part regulated by the intestinal microbiota and its interactions with the host immune system[15–17]. The host immune system has been shown to modulate the intestinal microbiota and its disturbances result in intestinal microbiota dysbiosis. For example, retinoic acid receptor-related orphan receptor- γt (RORγt)+ group 3 innate lymphoid cells (ILC3s) regulate intestinal bacteria by limiting local and systemic inflammation[16] and the absence of intestinal immune cells, particularly isolated lymphoid follicles (ILFs), has been shown to result in alteration of the intestinal microbiota of mice by over representation of gram negative bacteria[18]. The intestinal microbiota also modulates host immune responses. Germ free mice developed an increase in interferon-γ (IFN-γ) after colonization with Escherichia coli (E. coli)[19]. In another study, germ free mice colonized with conventional intestinal microbiota developed structural changes with increase in the depth of the intestinal epithelial crypts as well as expansion in the lamina propria with an increase in immune cells within four days after exposure to intestinal bacteria[20]. Multiple studies linked the intestinal microbiota to regulation of T cells. In one study, it was shown that colonization of mice with segmented filamentous bacteria (SFB) induces CD4(+) T helper cells in the lamina propria[21]. In other studies, several intestinal microbes have been shown to induce forkhead box protein (Foxp3)- expressing regulatory T cells (Tregs)[22–25]. Furthermore, interactions between the intestinal microbiota and the host promote the functions of non-immune cells such as IECs and goblet cells which play important roles in regulating the barrier function of the GI tract[26, 27]. Thus, the intestinal microbiota plays an important role in intestinal homeostasis.
Microbial changes in acute GVHD
Allo HSCT alters the diversity and composition of the intestinal microbiota[28]. There is loss of diversity after allo HSCT with a shift towards Enterococci and a decrease in the obligate anaerobic bacteria, such as those from the phylum Frimicutes[29]. This was more pronounced with the use of antibiotics and the development of GVHD[29]. Acute GVHD has been associated with major fluctuations in the intestinal microbiota including a general loss of diversity and expansion of Enterobacteriales (including Escherichia, Klebsiella, and Enterobacter), Lactobacillales (including Lactobacillus, Enterococcus, and Streptococcus), Proteobacteria and Akkermansia[4]. This expansion is accompanied with loss of obligate anaerobic bacteria from the phylum Firmicutes (including Clostridia and Blautia)[30, 31]. Paneth cell loss in experimental GVHD results in decreased secretion of α-defensins leading to intestinal dysbiosis and that loss of paneth cells and dysbiosis were associated with nonrelapse mortality (NRM) in clinical GVHD[14, 32, 33]. A similar shift post allo HSCT was noted in three more studies[14, 34, 35]. One identified vancomycin-resistent eterococcus (VRE) dominance following antibiotic treatment as a predictor of bacteremia[34]. The second showed that GVHD was associated with a shift towards E. coli[14]. The third study showed expansion of Enterococcus, Streptococcus and various Proteobacteria after allo-HSCT[35]. This study noted that expansion of these bacteria often preceded bloodstream infections by the same organisms, and identified treatment with the antibiotic metronidazole as a risk factor for enterococcal expansion[35]. The intestinal microbiota can also modulate acute GVHD severity. In 1970s, investigators found less GVHD in mice transplanted in germ-free conditions or receiving gut decontamination antibiotics[36, 37]. These studies have not been confirmed in modern day germ-free mice facilities. However, early clinical studies also showed similar beneficial effects of bacterial decontamination in allo HSCT patients[38, 39]. But subsequent studies did not replicate these benefits[40–42]. More recent research made major strides in investigating the specific alterations in the composition of the intestinal mcirobiota and their association with acute GVHD. In one clinical cohort study, high intestinal abundance of the anaerobic bactreria Blautia was correlated with lower rates of GVHD and improved survival[43]. Loss of diversity of the inetstinal microbiota was associated with increased mortality in allo HSCT patients which was attributed to increased death due to GVHD or infection rather than relapse[44]. Another study showed associations between the expansion of certain bacteria Lactobacillales as well as general loss of diversity and acute GVHD severity[30]. This study also showed that expansion of Lactobacillales was not the cause for the development of GVHD since eliminating Lactobacillales from the intestinal flora in mice before allo HSCT caused more severe GVHD and reintroducing Lactobacillales alleviated this effect[30]. But another study found that that modifying the intestinal mcirobiota using the probiotic microorganism Lactobacillus rhamnosus GG reduced experimental GVHD[45]. Similarly, administration of a cocktail of 17 species of Clostridia reduced experimental GVHD and improved survival[9]. These data suggest that probiotic therapy can be used in ameliorating GVHD. As such, limited published early clinical experiences explored the use of probiotics, including via FMT in allo HSCT patients[46, 47].However, such approaches face concerns of safety in an immunocompromised population, as well as challenges related to scalability for widespread application, and large prospective trials evaluating feasibility, safety, and efficacy in allo HSCT patients are needed. A recent study described two patients in whom extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli (E. coli) bacteremia occurred after they had undergone FMT in two independent clinical trials; one of these patients was an allo HSCT recipient who died from severe sepsis[48].
Use of antibiotics
A recent retrospective study showed that broad spectrum antibiotic treatment with imipenem-cilastatin and piperacillin-tazobactam was associated with increased GVHD related mortality in patients and that treatment with imipenem-cilastatin was associated with loss of the protective mucus lining of the colon and a shift towards Akkermansia in mice[49]. Antibiotic agents with more limited spectra of activity such as rifaximin, cefepime and aztreonam were associated with reduced GVHD severity[49, 50]. The use of rifaximin correlated with a lower enterococcal load after allo HSCT and the use of cefepime and aztreonam correlated with less alterations to the composition of the intestinal microbiota post allo-HSCT when compared with broader spectrum antibiotics with increased anaerobic activity[49, 50]. An ongoing prospective clinical study is investigating the best antibiotic choices in protecting the integrity of the intestinal microbiota in patients undergoing allo HSCT (NCT03078010).
Introduction to microbial metabolites in acute GVHD
The intestinal mcirobiota metabolize nutrients ingested by the host and produce microbial metabolites which play critical roles in the microbiota’s interactions with the host and in maintaining intestinal homeostasis[51]. Microbial metabolites include essential fatty acids and amino acids for the host[52]. SCFAs are the most studied microbial metabolites[53]. Examples of other mcirobial metabolites are bile acids, polyamines, and aryl hydrocarbon receptor (AhR) ligands[52]. These metabolites impact both non-immune and immune intestinal cells as well as the microbiome and have varying functions in maintaining the barrier surface of the GI tract, as well as modulating the innate and adaptive host immune responses[51]. There is scarcity of data on microbial metabolites in acute GVHD (Table 1).Herein, we will focus on SCFAs and their roles in acute GVHD.
Table 1.
Known roles of microbial metabolites in acute graft versus host disease (GVHD)
| Microbial metabolites | Short chain fatty acids (SCFAs) | Aryl hydrocarbon receptor (AhR) ligands | Bile Acid metabolite taurine |
|---|---|---|---|
| Known roles in acute GVHD | Direct effect of the SCFAs butyrate and propionate on intestinal epithelial cells (IECs) - > reduced severity of experimental acute GVHD | lower levels of urine 3- indoxyl sulfate -> increased clinical acute GVHD | contributes to the mechanism of NLRP6-mediated GVHD toxicity in mice post allo HSCT |
Short chain fatty acids
A. Effects of SCFAs on intestinal homeostasis
SCFAs (e.g., butyrate, proprionate and acetate) are produced from fermentation of indigestible carbohydrates by intestinal anaerobic commensal bacteria such as Clostridia species and impact both non-immune as well as immune intestinal cells[53]. SCFAs serve as an energy source for the intestinal microbiota as well as the IECs[53]. In the study by Donohoe et al, IECs from germ-free mice were in an energy-deprived state leading to autophagy where the cells degraded their own components for energy and this was reversed by butyrate supplementation[54]. SCFAs also maintain the integrity of the intestinal mucosal barrier. Goblet cells up-regulated their expression of mucin genes in response to SCFAs[55, 56] and inoculating germ-free rats with SCFA-producing Bacteroides thetaiotaomicron or Faecalibacterium prausnitzii induced goblet cell differentiation and mucus production[57]. Furthermore, colonization with Bifidobacterium longum protected mice against death induced by a lethal infection by increasing production of SCFA acetate which maintained the barrier integrity of IECs and inhibited translocation of the E. coli O157:H7 Shiga toxin from the gut lumen into the blood[58]. SCFAs play an important part in innate immunity as they are histone deacetylase (HDAC) inhibitors with anti-inflammatory effects[53]. In the study by Vinolo et al, the exposure of rat neutrophils to SCFAs inhibited HDAC activity which resulted in inactivation of nuclear factor κB (NF-κB) and suppression of nitric oxide (NO) and the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and cytokine-induced neutrophil chemoattractant-2 (CINC-2αβ)[59]. Similarly, exposure of human peripheral blood mononuclear cells to SCFAs and trichostatin A (TSA), a typical HDAC inhibitor, lead to inactivation of NF-κB and down-regulation of TNF-α via prostaglandin E(2) (PGE(2)) secretion[60]. SCFAs also resulted in HDAC inhibition and anti-inflammatory effects in macrophages and dendritic cells (DCs)[61, 62]. In another study, a high fiber diet or treatment with the SCFA acetate in mice increased inflammasome activation and increased levels of IL-18 which promoted epithelial repair[63]. SCFAs play a role in adaptive intestinal immunity as well[53]. They were shown to promote Treg differentiation and anti-inflammatory responses thereby mitigating the severity of colitis[22]. SCFAs butyrate, acetate, and proprionate administration to germ-free mice increased the expression of anti-inflammatory IL-10 producing Foxp3-expressing Tregs through HDAC inhibition[64]. Moreover, Butyrate increased IL-18 expression in epithelial cells, increased IL-10 expression in DCs and macrophages and enabled them to induce the differentiation of Tregs, and protected against colitis[65]. Thus, SCFAs affect both non-immune as well as immune intestinal cells and modulate intestinal homeostasis.
B. Dietary resistant starch and SCFAs
One potential approach for stimulating SCFA production could be via dietary supplementation with carbohydrates that are resistant to degradation by human enzymes but can be metabolized by select microbes in the gut. Multiple studies have reported increased fecal SCFAs, particularly butyrate, by dietary supplementation with different formulations of resistant starch (RS)[66–68]. Another study showed that one such RS prepared from potatoes (RPS) is more potent at increasing average fecal butyrate levels compared to other RS formulations[69]. Butyrate production from RS is a complex multistep process and understanding all the gut microbes involved in this process is an ongoing challenge[69, 70]. Only a limited number of gut bacteria can degrade RS, however, most primary RS degraders are not among the known butyrate producers[71]. Thus, in order for dietary supplementation with RS to stimulate butyrate production, the activities of secondary fermenters are required. These secondary fermenters capture degradation and fermentation products from primary degraders and metabolize them into new molecules, including butyrate[72]. Known primary RS degraders include Ruminococcus bromii, R. lactaris, R. gnavus, and Bifidobacterium spp and known butyrate producers (secondary fermenters) include Roseburia spp, Faecalibacterium prausnitzii, Eubacterium rectale, and Anaerostipes spp[69].
C. SCFA and acute GVHD
A recent murine study explored the role of microbial metabolites, specifically SCFAs, in acute GVHD severity and found that the SCFA butyrate was significantly decreased in the IECs of mice experiencing acute GVHD[9]. Restoring butyrate levels, either by direct administration of butyrate or by increasing intestinal butyrate-producing bacteria, reduced acute GVHD. Two SCFAs, butyrate and propionate, were shown to directly protect IECs and reduce acute GVHD severity in mice[9, 10]. A small clinical study found that fecal butyrate and propionate levels are decreased in patients after allo HSCT and are lower with anti-anaerobic antibiotic exposure[73]. Another recent clinical study examined fecal SCFA concentrations after allo HSCT and found that higher levels of butyrate and other SCFAs correlated with abundance of butyrate producing organisms in the intestinal microbiota and this abundance was associated with resistance against lower tract respiratory infections[74].However, the impact of SCFA levels on acute GVHD has yet to be examined in patients post allo HSCT.
D. Dietary manipulation of the microbiome-metabolome axis and acute GVHD
Diet affects the composition of the intestinal mcirobiome and the associated metabolome is correspondingly altered[75, 76]. In one study, high protein and low carbohydrate diet was associated with loss of members of Clostridiales, including Roseburia, Faecalibacterium, Ruminococcus, and Blautia[77]. Similar patterns were seen with diets derived entirely from animal products[75].
Turnbaugh et al showed that changes in diet from a low-fat, plant polysaccharide-rich diet to a high- fat, high-sugar diet in mice that were colonized with human feces altered the intestinal microbiota composition and this was accompanied by changes in the metabolic milieu[78]. In two other studies, researchers showed that total parenteral nutrition resulted in a pro-inflammatory pattern of immune responses in mice and humans[79, 80]. These data suggest that diet can alter the intestinal microbiota as well as the metabolome and thereby regulate intestinal homeostasis.
Specific elements of the diet called prebiotics, which usually refer to indigestible carbohydrates that are metabolized by the intestinal microbiota to produce SCFAs, are particularly influential in modulating the structure and function of the intestinal microbiota[53]. There is lack of published clinical data on the effect of prebiotics in acute GVHD. However, several studies showed that the prebiotics inulin and fructo-oligosaccharides increased intestinal microbiota diversity and decreased disease activity in inflammatory bowel disease patients[81, 82]. These studies along with the evidence that SCFAs protect IECs and reduce experimental acute GVHD severity[9, 10], provide a compelling rationale for studies of dietary manipulation of the microbiome-metabolome axis using prebiotics to mitigate acute GVHD in patients receiving allo HSCT. Of interest, a small study showed that RPS induced the production of the SCFA butyrate from the intestinal microbiota of healthy volunteers[66]. Importantly, an abstract presented at the American Society of Hematology (ASH) conference in 2019 reported that RPS administration is feasible and safe and increased fecal RPS-degrading and butyrate-producing bacteria with a concomitant increase in butyrate levels in a pilot study in allo HSCT recipients[83]. As such, a prospective phase II clinical trial to determine whether nutritional modulation of the microbiome-metabolome axis using RPS can reduce acute GVHD, is currently underway (www.clinicaltrials.gov: NCT02763033). Similarly, a trial of Fructooligosaccharides, to assess this prebiotic’s safety and tolerability in allo HSCT patients is undergoing (NCT02805075). Notably, a recent study showed that a diet free of the disaccharide lactose, a carbohydrate source for the growth and expansion of Enterococcus faecalis and Enterococcus faecium, decreased the Enterococcus bloom observed by this group in GVHD and attenuated GVHD in mice after allo HSCT[84]. This study also describes a high incidence of enterococcal expansion in allo HSCT patients which was associated with GVHD and mortality[84].Furthermore, patients in this study who were carriers of lactose-nonabsorber genotypes showed compromised clearance of post-antibiotic Enterococcus domination[84]. However, this study did not report whether there was an association between these lactose-nonabsorber genotypes and GVHD and mortality in patients and did not investigate the effect of a lactose free diet on GVHD in patients[84].
Other microbial metabolites in acute GVHD
The bile acid metabolite, taurine, has been shown to contribute to the mechanism of NLRP6-mediated GVHD toxicity in mice post allo HSCT[54]. AhR has been shown to be necessary for expansion of IL-22 producing ILCs needed for the clearance of Citrobacter rodentium infection[55]. In allo HSCT, IL-22 producing ILCs were depleted in the intestines of mice with acute GVHD, and treatment with IL-22 enhanced the recovery of intestinal stem cells (ISCs), increased epithelial regeneration, and reduced acute GVHD[56]. However, the role that AhR plays in modulating IL-22 producing ILCs in acute GVHD and whether dietary ligands can modulate that role are yet to be investigated. One clinical study showed that lower levels of urine 3-indoxyl sulfate, an AhR ligand, were associated with higher treatment related mortality and lower overall survival in allo HSCT patients[57].
Future perspectives
Growing evidence suggests the importance of the microbiome-metabolome axis in mitigating acute GVHD[9, 10]. Clinical approaches to rationally alter this axis in allo HSCT patients are needed. As such, dietary manipulation using RPS is currently being tested in this population (www.clinicaltrials.gov: NCT02763033). If the primary endpoint of reducing clinical acute GVHD is met, then follow-up multi-institutional prospective randomized trials will be warranted. Other dietary interventions such as lactose free diet which has been recently shown to ameliorate GVHD in mice[84], need to be investigated in allo HSCT patients. Combining dietary interventions with other approaches such as probiotics, modifications of antibiotic strategies, or FMT can also be explored in this allo HSCT population. However, exploring the safety of FMT in this immunocompromised population needs to be exercised with caution, especially in light of the recent report of an allo HSCT recipient in whom ESBL–producing E. coli bacteremia and death occurred after undergoing FMT[48]. Additionally, more mechanistic studies of microbial metabolite mediated protection in acute GVHD are needed. One tactic is to better understand host metabolism and its impact on the microbiome and subsequent effect on GVHD. Another is to understand microbial metabolism and its impact on host metabolism and GVHD. Notably, a recent study in mice showed the importance of a host-microbial biliary network interaction, which was manipulated by diet, in modulating a specific colonic Treg cell population and ameliorating inflammatory colitis via the resulting gut bile acid pool[85]. This highlights the need for further investigations of the role of other microbial metabolites besides SCFAs, such as bile acids, in acute GVHD. Furthermore, the role of microbial and metabolite geographical variation (small or large bowel) and its impact on GVHD colitis needs to be explored.
Footnotes
Declarations of interest for Mary Riwes and Pavan Reddy: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11(9):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferrara JL, Smith CM, Sheets J, Reddy P, Serody JS. Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J Clin Invest. 2017;127(7):2441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124(10):4162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–32. [DOI] [PubMed] [Google Scholar]
- [7].Riwes M, Reddy P. Microbial metabolites and graft versus host disease. Am J Transplant. 2018;18(1):23–9. [DOI] [PubMed] [Google Scholar]
- [8].Riwes M, Reddy P. Microbes and Their Metabolites Correlate with Hematopoietic Stem Cell Transplantation Outcomes? Biol Blood Marrow Transplant. 2018;24(12):e7–e8. [DOI] [PubMed] [Google Scholar]
- [9].Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fujiwara H, Docampo MD, Riwes M, et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat Commun. 2018;9(1):3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–9. [DOI] [PubMed] [Google Scholar]
- [12].Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11(11):1244–9. [DOI] [PubMed] [Google Scholar]
- [13].Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–13. [PubMed] [Google Scholar]
- [14].Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120(1):223–31. [DOI] [PubMed] [Google Scholar]
- [15].Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bouskra D, Brezillon C, Berard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–10. [DOI] [PubMed] [Google Scholar]
- [19].Wu C, Sartor RB, Huang K, Tonkonogy SL. Transient activation of mucosal effector immune responses by resident intestinal bacteria in normal hosts is regulated by interleukin-10 signalling. Immunology. 2016;148(3):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].El Aidy S, van Baarlen P, Derrien M, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567–79. [DOI] [PubMed] [Google Scholar]
- [21].Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. [DOI] [PubMed] [Google Scholar]
- [23].Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6(220):220ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sefik E, Geva-Zatorsky N, Oh S, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349(6251):993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113–8. [DOI] [PubMed] [Google Scholar]
- [27].Pelaseyed T, Bergstrom JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129(8):927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59(8):1079–87. [DOI] [PubMed] [Google Scholar]
- [32].Eriguchi Y, Nakamura K, Hashimoto D, et al. Decreased secretion of Paneth cell alpha-defensins in graft-versus-host disease. Transpl Infect Dis. 2015;17(5):702–6. [DOI] [PubMed] [Google Scholar]
- [33].Levine JE, Huber E, Hammer ST, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122(8):1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45(3):577–88. [PubMed] [Google Scholar]
- [37].van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974;52(2):401–4. [DOI] [PubMed] [Google Scholar]
- [38].Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–7. [DOI] [PubMed] [Google Scholar]
- [39].Vossen JM, Heidt PJ, van den Berg H, Gerritsen EJ, Hermans J, Dooren LJ. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis. 1990;9(1):14–23. [DOI] [PubMed] [Google Scholar]
- [40].Petersen FB, Buckner CD, Clift RA, et al. Infectious complications in patients undergoing marrow transplantation: a prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Scand J Infect Dis. 1987;19(5):559–67. [DOI] [PubMed] [Google Scholar]
- [41].Passweg JR, Rowlings PA, Atkinson KA, et al. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 1998;21(12):1231–8. [DOI] [PubMed] [Google Scholar]
- [42].Russell JA, Chaudhry A, Booth K, et al. Early outcomes after allogeneic stem cell transplantation for leukemia and myelodysplasia without protective isolation: a 10-year experience. Biol Blood Marrow Transplant. 2000;6(2):109–14. [DOI] [PubMed] [Google Scholar]
- [43].Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(8):1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103(11):4365–7. [DOI] [PubMed] [Google Scholar]
- [46].Gorshein E, Wei C, Ambrosy S, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2017;31(5). [DOI] [PubMed] [Google Scholar]
- [47].DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2(7):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381(21):2043–50. [DOI] [PubMed] [Google Scholar]
- [49].Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Weber D, Oefner PJ, Dettmer K, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(8):1087–92. [DOI] [PubMed] [Google Scholar]
- [51].Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. [DOI] [PubMed] [Google Scholar]
- [52].Postler TS, Ghosh S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017;26(1):110–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–45. [DOI] [PubMed] [Google Scholar]
- [54].Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52(10):1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gaudier E, Jarry A, Blottiere HM, et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287(6):G1168–74. [DOI] [PubMed] [Google Scholar]
- [57].Wrzosek L, Miquel S, Noordine ML, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–7. [DOI] [PubMed] [Google Scholar]
- [59].Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849–55. [DOI] [PubMed] [Google Scholar]
- [60].Usami M, Kishimoto K, Ohata A, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28(5):321–8. [DOI] [PubMed] [Google Scholar]
- [61].Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111(6):2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Singh N, Thangaraju M, Prasad PD, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285(36):27601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. [DOI] [PubMed] [Google Scholar]
- [64].Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Welters CF, Heineman E, Thunnissen FB, van den Bogaard AE, Soeters PB, Baeten CG. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45(5):621–7. [DOI] [PubMed] [Google Scholar]
- [68].Le Leu RK, Hu Y, Brown IL, Young GP. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr Metab (Lond). 2009;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cockburn DW, Koropatkin NM. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J Mol Biol. 2016;428(16):3230–52. [DOI] [PubMed] [Google Scholar]
- [71].Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. [DOI] [PubMed] [Google Scholar]
- [73].Romick-Rosendale LE, Haslam DB, Lane A, et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2018;24(12):2418–24. [DOI] [PubMed] [Google Scholar]
- [74].Haak BW, Littmann ER, Chaubard JL, et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131(26):2978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1-2):84–96. [DOI] [PubMed] [Google Scholar]
- [77].Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–72. [DOI] [PubMed] [Google Scholar]
- [78].Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229(5):662–7; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47(2):109–16. [PMC free article] [PubMed] [Google Scholar]
- [81].Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55(3):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Casellas F, Borruel N, Torrejon A, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25(9):1061–7. [DOI] [PubMed] [Google Scholar]
- [83].Riwes M SA, Braun T, et al. Rational modification of intestinal microbiome and metabolites after allogeneic hematopoietic stem cell transplantation with resistant starch: a pilot study. American Society of Hematology. 2019;134(ABSTRACT 3276). [Google Scholar]
- [84].Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science. 2019;366(6469):1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. 2020;577(7790):410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]