Abstract
Purpose
To characterize global and segmental circumferential systolic strain (CS) measured by cardiac MRI in athletes after SARS-CoV-2 infection.
Materials and Methods
This retrospective observational cohort study included 188 soldiers and collegiate athletes referred for cardiac MRI after SARS-CoV-2 infection (C19+) between July 2020 and February 2021 and a control group of 72 soldiers, collegiate, and high school athletes who underwent cardiac MRI from May 2019 to February 2020, prior to the first SARS-CoV-2 case detected in our region (C19-). Global and segmental CS were measured by feature tracking, then compared between each group using unadjusted and multivariable- adjusted models. Acute myocarditis was diagnosed according to the modified Lake Louise criteria and the location of pathologic late gadolinium enhancement (LGE) was ascertained.
Results
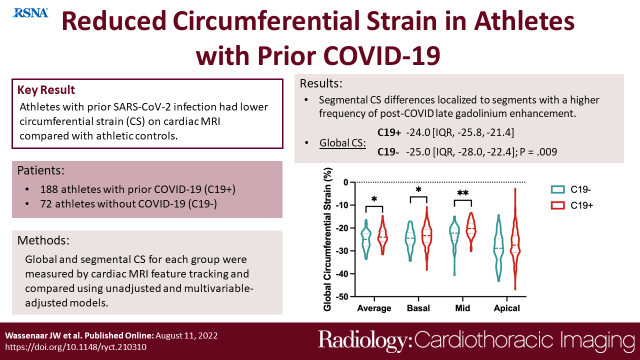
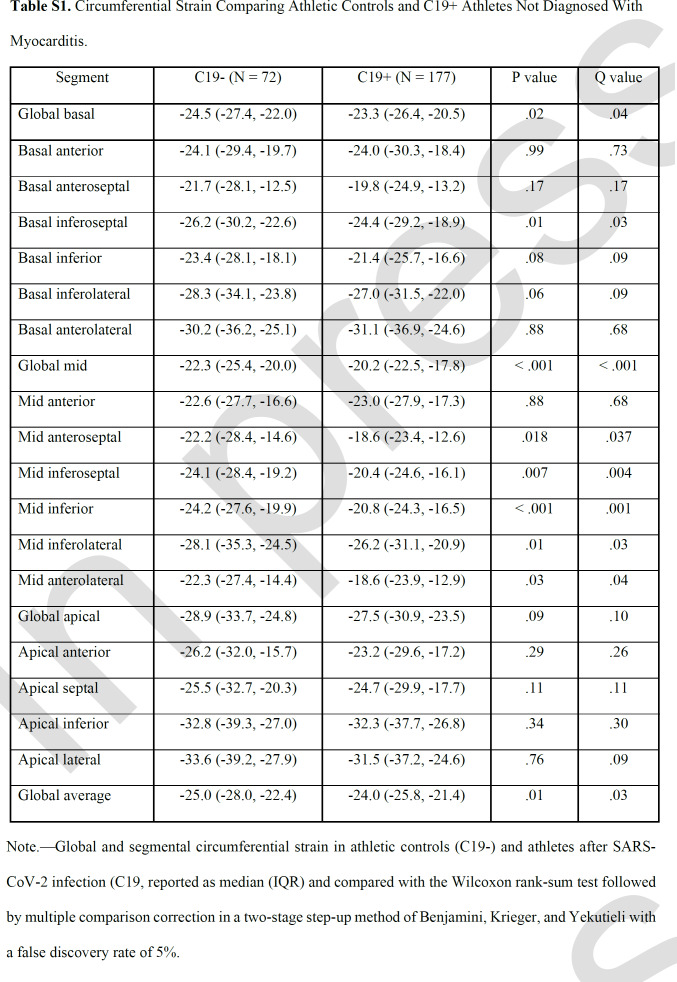
Among the 188 C19+ athletes (median age, 25 years [IQR, 23-30]; 131 men), the majority had mild illness. Global CS significantly differed between C19+ and C19- groups, with a median of -24.0 (IQR -25.8, -21.4) versus. -25.0 (-28.0, -22.4), respectively (p = .009). This difference in CS persisted following adjustment for age, sex, body mass index, heart rate, and systolic blood pressure β coefficient 1.29 [95% CI: 0.20, 2.38], p = .02). In segmental analysis, the basal- and mid- inferoseptal, septal and inferolateral segments were significantly different (p < .05), which had a higher frequency of post-COVID late gadolinium enhancement. The global and segmental differences were similar after exclusion of athletes with myocarditis.
Conclusion
Among athletes, SARS-CoV-2 infection was associated with a small but statistically significant reduced CS.
Summary Statement:
Athletes with prior SARS-CoV-2 infection had reduced circumferential strain (CS) on cardiac MRI compared with athletic controls.
Key Points:
■ Mild SARS-CoV-2 infection in athletes is associated with reduced (less negative) global circumferential strain measured by cardiac MRI (β coefficient 1.29 [95% CI: 0.20, 2.38], p = .02).
■ Strain differences were significantly different in the basal- and mid- inferoseptal, septal, and inferolateral segments (q < .05 for each), which had a higher frequency of post-COVID late gadolinium enhancement.
INTRODUCTION
Infection with the SARS-CoV-2 virus is known to cause a wide range of symptoms spanning from asymptomatic infection to severe respiratory illnesses, acute respiratory distress syndrome, and death. Similarly, cardiovascular involvement associated with SARS-CoV-2 has been shown to be highly variable–ranging from radiographic changes in patients with mild respiratory disease without specific cardiac complaints (1) to acute fulminant myocarditis (2, 3). Cardiac MRI has been used to indicate evidence of direct myocardial injury in patients with COVID-19 with abnormal multiparametric (T1, T2, and extracellular volume [ECV]) mapping and late gadolinium enhancement (LGE) suggesting myocardial edema, inflammation and fibrosis (1), consistent with acute myocarditis. Due to the high prevalence of myocardial inflammation by cardiac MRI shown in an initial series of patients diagnosed with SARS-CoV- 2 (1) and evidence of myocarditis as a known cause of sudden cardiac death in competitive athletes (4, 5), there has been great interest in the development of appropriate screening algorithms for collegiate and professional athletes who have recovered from this infection (6, 7). Suggested algorithms include electrocardiography (ECG), high sensitivity cardiac troponin (TnI) assay, echocardiography, and cardiac MRI. While initial reports suggested myocarditis rates as high as 15%, subsequent studies in collegiate athletes recovering from SARS-CoV-2 (8, 9), including from our institution (10), suggest a lower incidence in the range of 1.5-3%.
Strain imaging detects subclinical myocardial dysfunction and has been shown to improve the prognostic value of cardiac MRI in acute myocarditis (11, 12). The objective of this follow-up study was to characterize global and segmental circumferential systolic strain (CS) in highly trained athletes, including competitive collegiate athletes and active-duty military soldiers.
METHODS
Study Design and Patients
A retrospective observational cohort study was performed that included 188 athletes (active duty military soldiers and collegiate athletes) with prior SARS-CoV-2 infection (C19+) who were referred for cardiac MRI examination from a single institution between July 2020 and February 2021 and a control group (C19-) consisting of 72 healthy soldiers and high school or collegiate athletes. The collegiate athletes with SARS-CoV-2 underwent mandatory universal cardiac screening comprised of TnI measurement, ECG, echocardiography, and cardiac MRI. The control group underwent cardiac MRI if deemed clinically appropriate by a provider not involved in this study. The C19- group was retrospectively identified from patients who underwent cardiac testing for low acuity symptoms in the presence of strenuous training at our institution from May 2019 to February 2020, prior to the first case of SARS-CoV-2 reported locally. Control patients were included if no evidence of myocardial pathology were found. All athletes participated in > 6 hours of endurance activity per week by self-report or retrospective confirmation. Punctate LGE in the inferoseptal right ventricular (RV) insertion point was not considered to be pathological, as it has been previously observed in athletes (10, 13). Demographics and cardiac MRI volumetrics of a subset of both C19- (n = 60) and C19+ (n = 59) athletes has previously been reported, but circumferential strain in these patients is reported only in this study (10, 14). The study was approved by the institutional review board with a waiver of consent for retrospective enrollment.
Cardiac MRI Protocol
A comprehensive cardiac MRI with contrast was performed on a 1.5 Tesla Siemens Avanto Fit scanner (Siemens Healthcare Sector, Erlangen, Germany). The cardiac MRI protocol consisted of cine cardiac MRI balanced steady-state free precession imaging to calculate left and right ventricular volumes, left ventricular ejection fraction (LVEF), and myocardial mass. Intravenous gadolinium contrast (gadobutrol, Gadavist, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA at a dose of 0.15 mmol/kg) was administered through a peripheral intravenous line. LGE was performed using segmented inversion recovery (optimized inversion time to null myocardium) and single shot phase sensitive inversion recovery (inversion time of 300ms) imaging in standard long-axis planes and a short-axis stack. Native T1 mapping, T2 mapping, and post- contrast (15 minutes after contrast administration) T1 mapping was performed. T1 mapping was performed using a modified Look-Locker inversion recovery sequence acquired using a 5(3s)3 protocol before contrast and 4(1)3(1)2 protocol after contrast.
Cardiac MRI Postprocessing
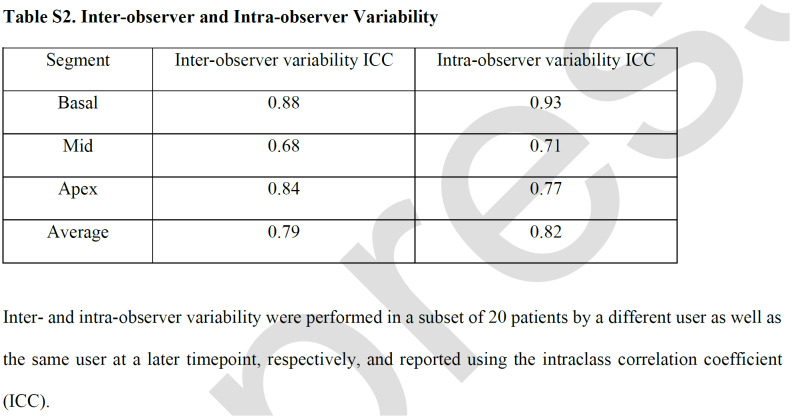
Cardiac MRI post-processing was performed using Medis Suite MR 2.1 (Medis, Leiden, The Netherlands). CS was calculated by feature tracking (FT) using Medis QStrain from each of the 16 short-axis myocardial segments, following the standard model as proposed by the American Heart Association (15), excluding the apical segment. Strain analysis was performed by a single reader (K G.-D.), who has 4 years of experience analyzing strain and was blinded to SARS-CoV-2 status. Strain measurements were repeated in a subset of 20 patients more than 6 months from initial analysis by the same user to assess for intra-observer variability; a second user (J.S.) repeated analysis of those 20 patients to assess for inter-observer variability. Assessment of presence or absence of LGE, as well as its location within the 17-segment model, was done by two cardiologists (S.H. and J.D.) each with over 10 years of experience reading cardiac MRI. If there were disagreements, a third cardiologist (D.C.) repeated assessment of LGE. Acute myocarditis was diagnosed according to the modified Lake Louise Criteria (LLC) (16).
Statistical Analysis
Summary statistics were calculated as counts (percentages) or median (interquartile range [IQR]). Between group unadjusted comparisons were made with the chi-squared and Wilcoxon rank-sum tests. All tests were 2-sided, and p<.05 was considered statistically significant. The association between LV mass index (LVMi), RV ejection fraction (RVEF), and RV volume index with average global CS were examined in linear regression models that included prior COVID-19 infection status and adjustment for age, sex, body mass index (BMI), heart rate (HR), and systolic blood pressure (SBP). Among C19+ athletes, the associations between T1, T2, and ECV with CS were also examined in a multivariable-adjusted linear regression. Prior to entry to regression models, the distributions of dependent variables were examined, then log transformed, as appropriate, with normality of residuals tested post regression. When appropriate, multiple comparison correction was performed in a two-stage step-up method of Benjamini, Krieger, and Yekutieli with a false discovery rate of 5%. Inter- and intra-observer variability were calculated using an intraclass coefficient. Statistical analysis was performed using Prism 9 (GraphPad Software, LLC, San Diego, CA, USA).
RESULTS
Study Patient Characteristics
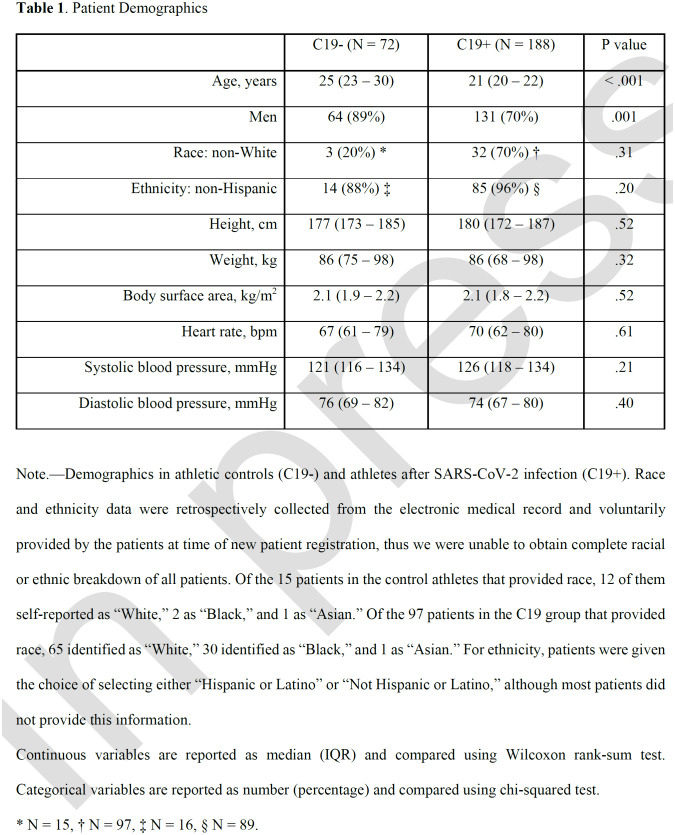
Our cohort included 72 patients in the C19- group and 188 in the C19+ group (Table 1). The median age was 25 years (IQR, 23-30) and 21 years (IQR, 20-22) in the C19- and C19+ groups, respectively (p<0.001). Both groups were majority men, though there were more males in the C19- group (C19+: 131 (70%), C19-: 64 (89%), p<.001). Self-reported race and ethnicity proportions were similar between groups. Height, weight, body surface area, HR, SBP, and diastolic blood pressure were also similar between the two groups.
Table 1.
Patient Demographics
C19+ Athlete Characteristics
The median time from COVID-19 diagnosis to cardiac MRI examination was 30 days (IQR, 15–56). Of the 188 C19+ athletes, the vast majority had mild illnesses and recovered at home; only 5 (3%) required hospitalization, of which 2 (1%) needed intensive care unit-level care, though none were mechanically ventilated. Five C19+ patients (2%) had abnormal TnI (defined as >0.03 ng/mL), 28 (15%) had borderline abnormal ECG as interpreted by a sports cardiologist, and 15 (8%) had an abnormal echocardiogram. Ten (5%) C19+ athletes were diagnosed with acute myocarditis according to the modified LLC (16) using our established normative values for T1 and T2 parametric mapping, as none had an abnormal TnI, ECG, or echocardiogram; none of these athletes were hospitalized.
Cardiac MRI Volumetric, Functional, and Parametric Mapping Comparisons
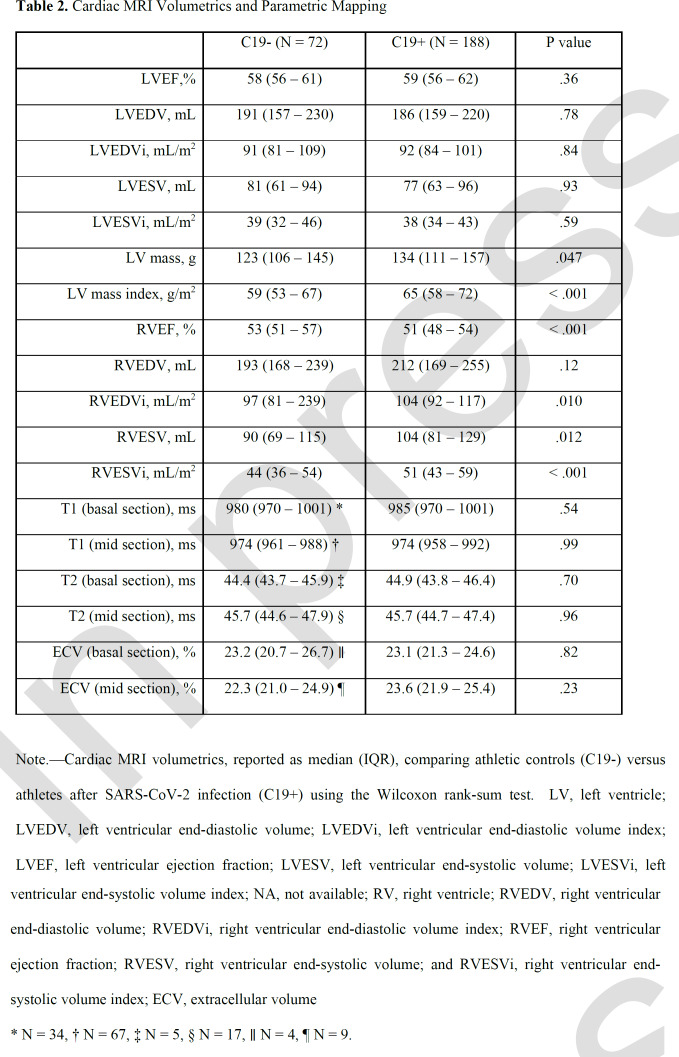
Cardiac volumes, function, and parametric mapping were compared among groups (Table 2). C19+ athletes had similar LV volumes and function, but higher LV mass and RV volumes, and reduced RV ejection fraction compared with the C19- group (RVEF, 51% vs 53%, p < .001). After adjusting for age, sex, BMI, HR and SBP, prior COVID-19 infection independently associated with significantly higher log transformed LV mass index (β coefficient, 0.052 [95% CI: 0.030, 0.074]; p < .001), lower log transformed RVEF (β coefficient, -0.020 [-0.032, -0.008]; p = .001, and higher log transformed RV end systolic index (β coefficient, 0.035 [0.004, 0.065]; p = .02). On parametric mapping, there was no evidence of differences between groups for native T1 relaxation time, T2, or ECV.
Table 2.
Cardiac MRI Volumetrics and Parametric Mapping
Global Circumferential Strain
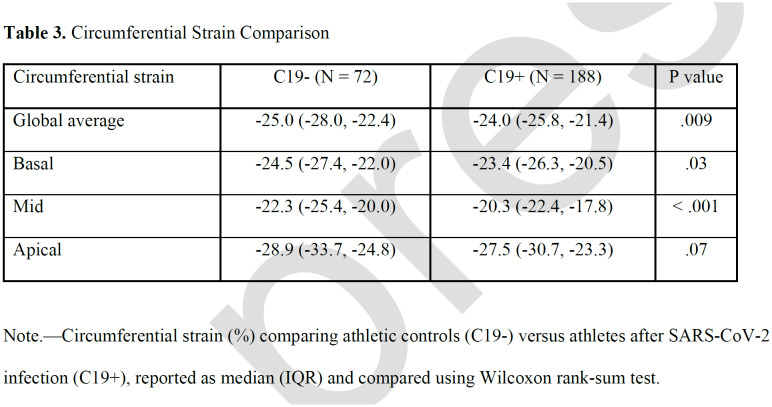
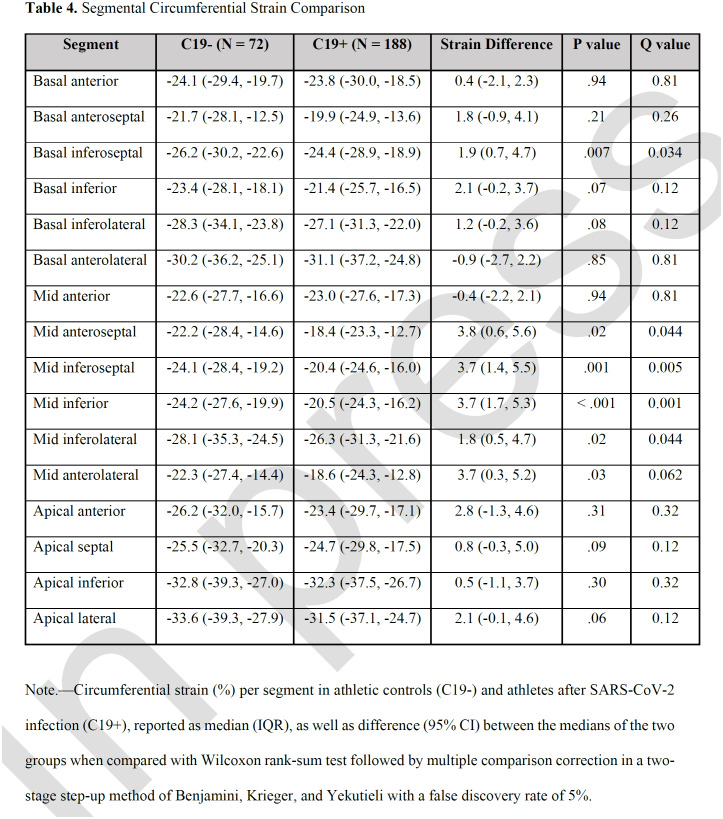
Compared with the C19- group, C19+ athletes had higher (less negative) global CS (median, -24.0 vs -25.0; p=.009), as well as CS of the basal (-23.4 vs – 24.5, p=.03 and mid-left ventricular sections (-20.3 vs -22.3, p<.001; Table 3, Figure 1). This finding persisted even after exclusion of individuals with myocarditis (Table S1). Prior infection remained independently associated with reduced global CS (β coefficient, 1.29 (95% CI: 0.20, 2.38], p = .02) in multivariable regression adjusted for age, sex, BMI, HR, and SBP. Moreover, this effect persisted with further adjustment for LVMi, which was higher in the C19+ group, to the multivariable model (1.32 [0.19, 2.5]; p = .02). In a separate multivariable regression analysis with the addition of parametric mapping data to the above clinical variables, within just the C19+ cohort, CS in the basal or mid section was not significantly associated with T1 relaxation time, T2, or ECV in the respective LV section. Inter- and intra-observer variability analysis performed on a subset of the patients showed strong correlation for global CS as well as for CS at each of the sections (Table S2).
Table 3.
Circumferential Strain Comparison
Figure 1.
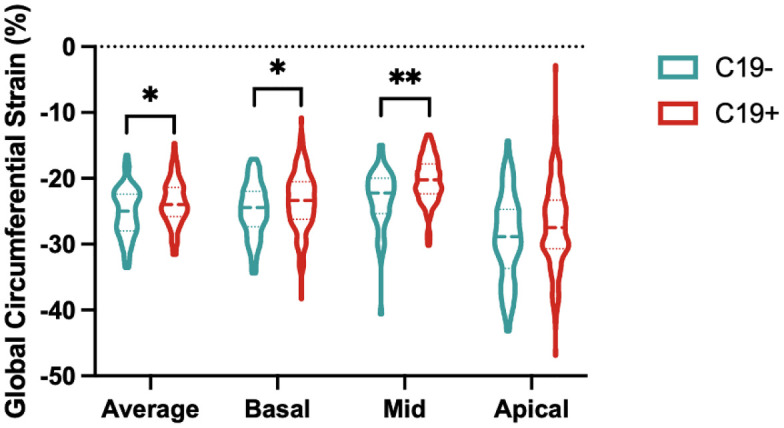
Global circumferential strain comparison. Average global circumferential strain and from each section in athletic controls (C19-) and athletes after SARS-CoV-2 infection (C19+) are shown with dashed lines representing median and IQR. * p < .05 and ** p < .01 when compared by Wilcoxon rank-sum test.
Segmental Circumferential Strain
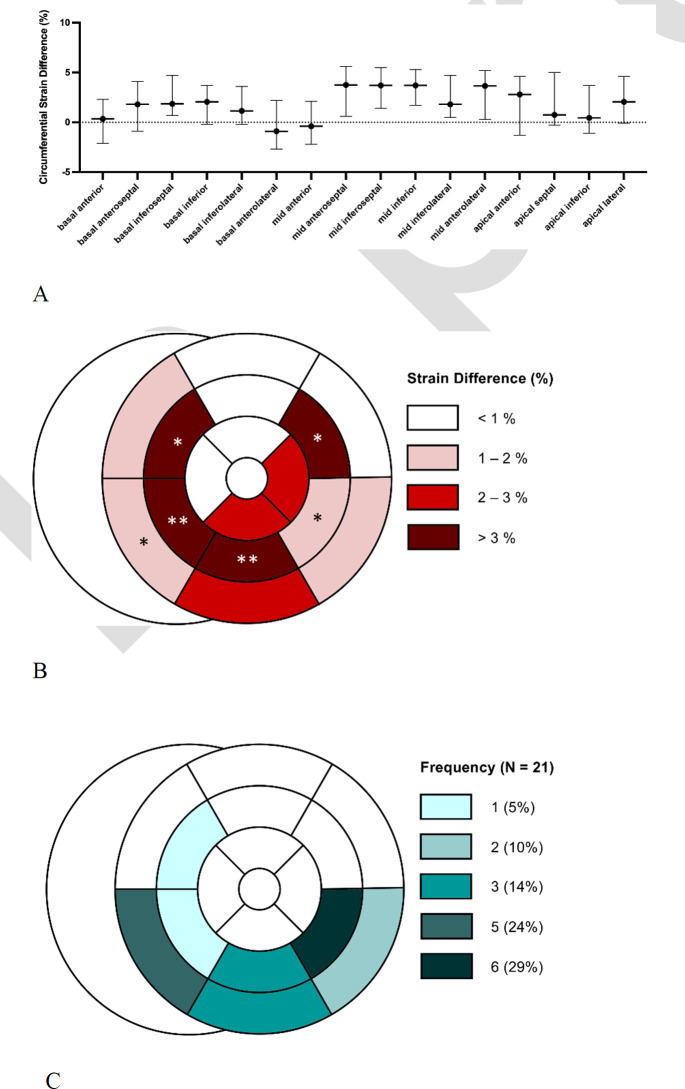
After correcting for multiple comparisons, segmental CS was found to be higher (less negative) in the basal- to-mid-inferoseptal, mid-anteroseptal, mid-inferior, and mid-inferolateral segments in unadjusted analysis comparing C19+ with C19- athletes (q<0.05 for each, Table 4, Figure 2A and 2B). A similar difference was observed when comparing C19- to C19+ athletes without myocarditis (Table S1). Pathologic LGE in C19+ athletes with myocarditis (n = 10) was most frequently located in the basal- and mid-inferoseptum, mid-inferior, and mid-inferolateral segments (Figure 2C), in a similar pattern to the abnormal segmental CS findings.
Table 4.
Segmental Circumferential Strain Comparison
Figure 2.
Location of circumferential strain differences and pathologic LGE within the 17-segment model. (A-B) Segmental circumferential strain difference (95% CI) between athletes after SARS-CoV-2 (C19+) and athletic controls (C19-), * q < .05 and ** q < .01 when compared using the Wilcoxon rank-sum test followed by multiple comparison correction in a two-stage step-up method of Benjamini, Krieger, and Yekutieli with a false discovery rate of 5%. C) Frequency of the location of pathologic late gadolinium enhancement (LGE) in C19+ athletes diagnosed with myocarditis.
DISCUSSION
This study aimed to compare global and segmental CS measured by cardiac MRI in athletes after SARS- CoV-2 infection and athletic controls. SARS-CoV-2 positivity was associated with lower global (β coefficient, 1.29 [95% CI: 0.20, 2.38], p = .02) and segmental CS. Segmental analyses showed that COVID- 19 had a predilection for the basal-mid inferior myocardial segments, with strain abnormalities and LGE localized to these regions, for which COVID-19 has previously been shown to have a predilection for (17).
Strain analysis by FT has been shown to improve cardiac MRI diagnostic accuracy of acute myocarditis, especially when the LVEF is preserved (18-21), although abnormal strain is not currently incorporated as part of the LLC (16). The location and extent of LGE is strongly predictive of future major adverse cardiac events (MACE) post-myocarditis in patients with preserved LVEF (22, 23). More recently, studies have shown that strain quantified by FT MRI is an independent prognosticator of long-term MACE and LV functional recovery after myocarditis in both preserved and reduced EF (11, 12). In acute myocarditis, global CS has been shown to correlate with T1 and T2 relaxation times, indicative of myocardial edema and inflammation, as well as extracellular matrix expansion (20). Segments with visually identifiable LGE or edema had reduced CS (20).
In our study, athletes with prior SARS-CoV-2 infection had significantly worse global and segmental CS compared with athletic controls. This difference was observed even among C19+ athletes without cardiac MRI evidence of myocarditis. While the absolute difference in strain was small (-1.0 difference between C19+ and C19- group), this represents a relative reduction of 4% of global CS. By comparison, a relative reduction of 12% in global longitudinal strain has been used as the cutoff for evidence of chemo-induced cardiotoxicity, and initiation of cardioprotective therapy using strain-guided management has been shown to be superior to EF-guided management (24). Furthermore, a -1.0 absolute difference in strain by cardiac MRI is associated with a 21% increased risk of MACE after myocarditis, independent of other traditional features of cardiac MRI such as LVEF or LGE extent (11). Longitudinal outcome studies are necessary to fully understand the clinical significance of these findings, which will provide better understanding of myocardial changes post-COVID.
Reduction in global strain parameters and increased ECV in the absence of formal criteria for myocarditis was reported in a study of 40 patients who recovered from moderate or severe SARS-CoV-2 infection (25). These findings suggest that viral involvement of SARS-CoV-2 in the myocardium may occur at a nominal level, resulting in a subclinical change in myocardial function, and that cardiac MRI with strain is a sensitive method for studying post-SARS-CoV-2 cardiac sequelae. In an exploratory analysis, CS was not associated with T1, T2, or ECV within the C19+ group after adjusting for clinical variables (age, sex, HR, BMI, and BP). While speculative, the worse systolic strain observed in C19+ athletes may suggest a relatively greater effect of SARS-CoV-2 on cardiomyocyte function compared to the interstitial space. Of note, direct infection of SARS-CoV-2 in cardiomyocytes has been demonstrated in vitro through an angiotensin converting enzyme 2 dependent pathway, resulting in increased cell size and decreased contraction (26, 27). However, further investigation into the clinical significance of these strain differences in the absence of visible LGE is needed.
The diagnosis of myocarditis in athletes post SARS-CoV-2 presents a unique challenge as intensive athletic training can lead to adaptive changes that must not be confounded with pathology (28). In our study, none of the athletes diagnosed with myocarditis had an abnormal TnI, ECG, or echocardiogram, although testing was done in the convalescent period after the acute infectious period had resolved. Furthermore, mostpatients diagnosed with SARS-CoV-2 had relatively mild infections with nonspecific symptoms that may overlap with those of myocarditis, thus requiring a higher index of suspicion to pursue further testing.
Our study included athletes undergoing mandatory cardiac MRI post SARS-CoV-2 as part of return-to-play for collegiate athletes and clinically-indicated cardiac MRI for tactical military soldiers, who both had a similar rate (5%) of myocarditis, consistent with prior studies in athletic populations (8, 9). This contrasts with previous studies that reported a much higher incidence of myocarditis in post SARS-CoV-2 patients who were older with more comorbidities (1), had more severe illnesses, and were not competitive athletes (29). By comparison, a study of a return-to-play screening algorithm without universal cardiac MRI in professional athletes reported a much lower incidence of myocarditis (30), and a large, multicenter collegiate athlete cohort in which only 7% underwent cardiac MRI similarly found low rates of myocarditis (31).
Cardiac remodeling is known to occur in competitive athletes (31) with both volumetric and parametric mapping changes; thus, an athletic control group is critical to detect true pathologic differences. Like prior studies, our data demonstrated increased RV volumes and decreased RVEF compared with athletic controls, though the median values remained in the normal range (1, 29). Whether these differences result from direct myocardial effects of SARS-CoV-2 or secondary effects from residual pulmonary abnormalities is unclear. Our data also demonstrate significantly increased indexed LV mass after SARS-CoV-2, but no evidence of differences in LV volumes or function.
There are inherent limitations of our retrospective observational study design with limited follow-up due to study of an emerging post-viral syndrome from the SARS-CoV-2 pandemic. Despite multivariable- adjustment, residual confounding may exist. Race and ethnicity were self-reported, and the majority of patients in the C19- group and approximately half of the C19+ athletes chose not to report these demographics. Since athletic participation was self-reported, there could be difference in athletic conditioning between C19+ and C19- groups, which could contribute to differences in volumetrics, LVMi, and CS. Lastly, cardiac MRI was obtained in the C19- group for various clinical indications; thus, the control athletes did not undergo a standardized protocol that required parametric mapping, and as such, T1, T2, and ECV data were not available for some patients. Due to the missing data, multivariable analysis to account for effects of T1 relaxation time, T2 relaxation time, or ECV on global CS was only performed within the C19+ cohort.
In conclusion, differences in myocardial systolic strain exist in athletes with prior SARS-CoV-2 infection compared with athletes without prior infection, independent of demographics, hemodynamic parameters, and LV mass. Reduced myocardial strain co-localized to segments shown to be most likely affected by SARS-CoV-2 infection, even in the absence of detectable myocardial inflammation or LGE. Further study is necessary to determine the clinical significance of this small but statistically significant worsening of myocardial strain.
Supplemental
Table S1.
Circumferential Strain Comparing Athletic Controls and C19+ Athletes Not Diagnosed With Myocarditis.
Table S2.
Inter-observer and Intra-observer Variability
Drs. Soslow and Dendy contributed equally as co-senior authors
Funding: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007411 (DEC, DDD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: All authors have no disclosures to report, including any relationship to industry.
Disclosures of conflicts of interest: J.W.W. No relevant relationships. D.E.C. National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL00741. D.D.D. National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL00741. K.G.D. No relevant relationships. A.P. No relevant relationships. D.L.L. No relevant relationships. M.T.B. No relevant relationships. D.K.G. No relevant relationships. S.G.H. No relevant relationships. J.H.S. The project described was supported by CTSA award No. UL1TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. J.M.D. Grant from Centers for Disease Control and Prevention (5% salary support for service on the Clinical Immunization Safety Awareness Team.
Abbreviations:
- ECV
- extracellular volume
- EF
- ejection fraction
- CS
- circumferential strain
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- RV
- right ventricle
References
- 1. Puntmann VO , Carerj ML , Wieters I , Fahim M , Arendt C , Hoffmann J , Shchendrygina A , Escher F , Vasa-Nicotera M , Zeiher AM , Vehreschild M , Nagel E . Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) . JAMA Cardiol 2020. ; 5 ( 11 ): 1265 ‒ 1273 . doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng JH , Liu YX , Yuan J , Wang FX , Wu WB , Li JX , Wang LF , Gao H , Wang Y , Dong CF , Li YJ , Xie XJ , Feng C , Liu L . First case of COVID-19 complicated with fulminant myocarditis: a case report and insights . Infection 2020. ; 48 ( 5 ): 773 ‒ 777 . doi: 10.1007/s15010-020-01424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu H , Ma F , Wei X , Fang Y . Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin . Eur Heart J 2021. ; 42 ( 2 ): 206 . doi: 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrado D , Basso C , Schiavon M , Thiene G . Screening for hypertrophic cardiomyopathy in young athletes . N Engl J Med 1998. ; 339 ( 6 ): 364 ‒ 369 . doi: 10.1056/NEJM199808063390602 [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ , Carney KP , Lever HM , Lewis JF , Barac I , Casey SA , Sherrid MV . Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy . J Am Coll Cardiol 2003. ; 41 ( 6 ): 974 ‒ 980 . doi: 10.1016/s0735-1097(02)02976-5 [DOI] [PubMed] [Google Scholar]
- 6. Phelan D , Kim JH , Elliott MD , Wasfy MM , Cremer P , Johri AM , Emery MS , Sengupta PP , Sharma S , Martinez MW , La Gerche A . Screening of Potential Cardiac Involvement in Competitive Athletes Recovering From COVID-19: An Expert Consensus Statement . JACC Cardiovasc Imaging 2020. ; 13 ( 12 ): 2635 ‒ 2652 . doi: 10.1016/j.jcmg.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poppas A , Chung EH , Kovacs R . COVID-19 and the Athlete: Gaining Ground But Not Yet at the Finish . J Am Coll Cardiol 2021. ; 77 ( 10 ): 1368 ‒ 1371 . doi: 10.1016/j.jacc.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajpal S , Tong MS , Borchers J , Zareba KM , Obarski TP , Simonetti OP , Daniels CJ . Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection . JAMA Cardiol 2021. ; 6 ( 1 ): 116 ‒ 118 . doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Starekova J , Bluemke DA , Bradham WS , Eckhardt LL , Grist TM , Kusmirek JE , Purtell CS , Schiebler ML , Reeder SB . Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging . JAMA Cardiol 2021. . doi: 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark DE , Parikh A , Dendy JM , Diamond AB , George-Durrett K , Fish FA , Slaughter JC , Fitch W , Hughes SG , Soslow JH . COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR) . Circulation 2021. ; 143 ( 6 ): 609 ‒ 612 . doi: 10.1161/CIRCULATIONAHA.120.052573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer K , Obrist SJ , Erne SA , Stark AW , Marggraf M , Kaneko K , Guensch DP , Huber AT , Greulich S , Aghayev A , Steigner M , Blankstein R , Kwong RY , Grani C . Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features . JACC Cardiovasc Imaging 2020. ; 13 ( 9 ): 1891 ‒ 1901 . doi: 10.1016/j.jcmg.2020.04.025 [DOI] [PubMed] [Google Scholar]
- 12. Lee JW , Jeong YJ , Lee G , Lee NK , Lee HW , Kim JY , Choi BS , Choo KS . Predictive Value of Cardiac Magnetic Resonance Imaging-Derived Myocardial Strain for Poor Outcomes in Patients with Acute Myocarditis . Korean J Radiol 2017. ; 18 ( 4 ): 643 ‒ 654 . doi: 10.3348/kjr.2017.18.4.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domenech-Ximenos B , Sanz-de la Garza M , Prat-Gonzalez S , Sepulveda-Martinez A , Crispi F , Duran-Fernandez K , Perea RJ , Bijnens B , Sitges M . Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes . J Cardiovasc Magn Reson 2020. ; 22 ( 1 ): 62 . doi: 10.1186/s12968-020-00660-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark DE , Dendy JM , Li DL , Crum K , Dixon D , George-Durrett K , Parikh AP , Wassenaar JW , Hughes SG , Soslow JH . Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC) . J Cardiovasc Magn Reson 2021. ; 23 ( 1 ): 106 . doi: 10.1186/s12968-021-00798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerqueira MD , Weissman NJ , Dilsizian V , Jacobs AK , Kaul S , Laskey WK , Pennell DJ , Rumberger JA , Ryan T , Verani MS , American Heart Association Writing Group on Myocardial S, Registration for Cardiac Standardized I. myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association . Circulation 2002. ; 105 ( 4 ): 539 ‒ 542 . doi: 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- 16.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol 2018;72(24):3158‒3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 17.Li DL, Davogustto G, Soslow JH, Wassenaar JW, Parikh AP, Chew JD, Dendy JM, George-Durrett KM, Parra DA, Clark DE, Hughes SG. Characteristics of COVID-19 Myocarditis With and Without Multisystem Inflammatory Syndrome. Am J Cardiol 2022;168:135‒141. doi: 10.1016/j.amjcard.2021.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baessler B, Schaarschmidt F, Dick A, Michels G, Maintz D, Bunck AC. Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol 2016;85(1):218‒227. doi: 10.1016/j.ejrad.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 19. Weigand J , Nielsen JC , Sengupta PP , Sanz J , Srivastava S , Uppu S. Feature Tracking-Derived Peak Systolic Strain Compared to Late Gadolinium Enhancement in Troponin-Positive Myocarditis: A Case- Control Study . Pediatr Cardiol 2016. ; 37 ( 4 ): 696 ‒ 703 . doi: 10.1007/s00246-015-1333-z [DOI] [PubMed] [Google Scholar]
- 20. Luetkens JA , Schlesinger-Irsch U , Kuetting DL , Dabir D , Homsi R , Doerner J , Schmeel FC , Fimmers R , Sprinkart AM , Naehle CP , Schild HH , Thomas D. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema . Eur Radiol 2017. ; 27 ( 11 ): 4661 ‒ 4671 . doi: 10.1007/s00330-017-4854-4 [DOI] [PubMed] [Google Scholar]
- 21.Andre F, Stock FT, Riffel J, Giannitsis E, Steen H, Scharhag J, Katus HA, Buss SJ. Incremental value of cardiac deformation analysis in acute myocarditis: a cardiovascular magnetic resonance imaging study. Int J Cardiovasc Imaging 2016;32(7):1093‒1101. doi: 10.1007/s10554-016-0878-0 [DOI] [PubMed] [Google Scholar]
- 22.Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol 2017;70(16):1964‒1976. doi: 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G, Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiac C. MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol 2017;70(16):1977‒1987. doi: 10.1016/j.jacc.2017.08.044 [DOI] [PubMed] [Google Scholar]
- 24. Thavendiranathan P , Negishi T , Somerset E , Negishi K , Penicka M , Lemieux J , Aakhus S , Miyazaki S , Shirazi M , Galderisi M , Marwick TH , Investigators S. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy . J Am Coll Cardiol 2021. ; 77 ( 4 ): 392 ‒ 401 . doi: 10.1016/j.jacc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 25. Li X , Wang H , Zhao R , Wang T , Zhu Y , Qian Y , Liu B , Yu Y , Han Y. Elevated Extracellular Volume Fraction and Reduced Global Longitudinal Strains in Participants Recovered from COVID-19 without Clinical Cardiac Findings . Radiology 2021. ; 299 ( 2 ): E230 ‒ E240 . doi: 10.1148/radiol.2021203998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, Nair AP, Heck KA, Rali AS, Simpson L, Saririan M, Hobohm D, Stump WT, Fitzpatrick JA, Xie X, Zhang X, Shi PY, Hinson JT, Gi WT, Schmidt C, Leuschner F, Lin CY, Diamond MS, Greenberg MJ, Lavine KJ. SARS-CoV-2 Infects Human Engineered Heart Tissues and Models COVID- 19 Myocarditis. JACC Basic Transl Sci 2021;6(4):331‒345. doi: 10.1016/j.jacbts.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siddiq MM , Chan AT , Miorin L , Yadaw AS , Beaumont KG , Kehrer T , Cupic A , White KM , Tolentino RE , Hu B , Stern AD , Tavassoly I , Hansen J , Sebra R , Martinez P , Prabha S , Dubois N , Schaniel C , Iyengar-Kapuganti R , Kukar N , Giustino G , Sud K , Nirenberg S , Kovatch P , Albrecht RA , Goldfarb J , Croft L , McLaughlin MA , Argulian E , Lerakis S , Narula J , Garcia-Sastre A , Iyengar R. Functional Effects of Cardiomyocyte Injury in COVID-19 . J Virol 2022. ; 96 ( 2 ): e0106321 . doi: 10.1128/JVI.01063-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schellhorn P , Klingel K , Burgstahler C. Return to sports after COVID-19 infection . Eur Heart J 2020. ; 41 ( 46 ): 4382 ‒ 4384 . doi: 10.1093/eurheartj/ehaa448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kotecha T , Knight DS , Razvi Y , Kumar K , Vimalesvaran K , Thornton G , Patel R , Chacko L , Brown JT , Coyle C , Leith D , Shetye A , Ariff B , Bell R , Captur G , Coleman M , Goldring J , Gopalan D , Heightman M , Hillman T , Howard L , Jacobs M , Jeetley PS , Kanagaratnam P , Kon OM , Lamb LE , Manisty CH , Mathurdas P , Mayet J , Negus R , Patel N , Pierce I , Russell G , Wolff A , Xue H , Kellman P , Moon JC , Treibel TA , Cole GD , Fontana M. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance . Eur Heart J 2021. . doi: 10.1093/eurheartj/ehab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, Rowe D, Bogoch II, Smith PT, Baggish AL, Putukian M, Engel DJ. Prevalence of Inflammatory Heart Disease Among Professional Athletes With Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol 2021. doi: 10.1001/jamacardio.2021.0565 [DOI] [PMC free article] [PubMed]
- 31.Baggish AL, Battle RW, Beckerman JG, Bove AA, Lampert RJ, Levine BD, Link MS, Martinez MW, Molossi SM, Salerno J, Wasfy MM, Weiner RB, Emery MS. Sports Cardiology: Core Curriculum for Providing Cardiovascular Care to Competitive Athletes and Highly Active People. J Am Coll Cardiol 2017;70(15):1902‒1918. doi: doi: 10.1016/j.jacc.2017.08.055 [DOI] [PubMed] [Google Scholar]