Summary
Background
The circulation of respiratory viruses poses a significant health risk among those residing in congregate settings. Data are limited on seasonal human coronavirus (HCoV) infections in homeless shelter settings.
Methods
We analysed data from a clinical trial and SARS-CoV-2 surveillance study at 23 homeless shelter sites in King County, Washington between October 2019-May 2021. Eligible participants were shelter residents aged ≥3 months with acute respiratory illness. We collected enrolment data and nasal samples for respiratory virus testing using multiplex RT-PCR platform including HCoV. Beginning April 1, 2020, eligibility expanded to shelter residents and staff regardless of symptoms. HCoV species was determined by RT-PCR with species-specific primers, OpenArray assay or genomic sequencing for samples with an OpenArray relative cycle threshold <22.
Findings
Of the 14,464 samples from 3281 participants between October 2019-May 2021, 107 were positive for HCoV from 90 participants (median age 40 years, range: 0·9-81 years, 38% female). HCoV-HKU1 was the most common species identified before and after community-wide mitigation. No HCoV-positive samples were identified between May 2020-December 2020. Adults aged ≥50 years had the highest detection of HCoV (11%) among virus-positive samples among all age-groups. Species and sequence data showed diversity between and within HCoV species over the study period.
Interpretation
HCoV infections occurred in all congregate homeless shelter site age-groups with the greatest proportion among those aged ≥50 years. Species and sequencing data highlight the complexity of HCoV epidemiology within and between shelters sites.
Funding
Gates Ventures, Centers for Disease Control and Prevention, National Institute of Health.
Keywords: Human coronavirus, Homeless shelters, Respiratory viral infections, Homelessness
Research in context.
Evidence before this study
Individuals residing in congregate settings such as homeless shelters are at risk for respiratory viral outbreaks including seasonal human coronaviruses (HCoV). While HCoVs generally cause mild upper respiratory tract symptoms, they can be associated with severe clinical outcomes among those with underlying comorbidities. Homeless shelter residents have a high prevalence of underlying comorbidities which put them at increased risk for severe disease from HCoV infection. We searched PubMed on June 15, 2022, using the search terms (“homeless” OR “shelter” OR “homelessness”) AND ("human coronavirus" OR "seasonal coronavirus" OR "endemic coronavirus" OR "respiratory infection" OR “respiratory pathogen” OR “respiratory disease”) which yielded 102 results; of which, nine discussed respiratory communicable disease in people experiencing homelessness. Three were original research surveillance studies of respiratory pathogens in homeless shelters in Marseille, France which included HCoV infection. These three studies showed that HCoV contributed to the infection burden among residents of homeless shelters but these studies were limited by small sample size.
Added value of this study
Our clinical and genomic characterisation of HCoV epidemiology illustrates the complexity of HCoV infections among residents and staff of homeless shelters. We found a greater proportion of HCoV infections in adults than in paediatric participants before and during the COVID-19 pandemic with the highest percentage detected in shelters housing individuals aged ≥50 years, with decreases in HCoV detection during the early months of the pandemic. HCoV detections began later in the second year of the study compared to the start of the COVID-19 pandemic, possibly related to ongoing community-wide mitigation efforts at the time.
Implications of all the available evidence
Our findings add to the limited data on HCoV epidemiology in congregate settings specifically homeless shelters. We show HCoV infections affect all individuals residing in homeless shelters regardless of age before and during the COVID-19 pandemic with an underappreciated burden of HCoV infection in older adults. In contrast to hospital-based and general community studies, surveillance in congregate settings can provide data on groups of people most at risk for respiratory virus complications in the community. Optimizing prevention and control interventions for all coronavirus infections among homeless shelter residents will rely upon understanding coronavirus epidemiology in these settings. As we enter the next stages of the COVID-19 pandemic, these data establish a baseline for understanding the complexity and diversity of coronavirus circulation in homeless shelters. Novel surveillance studies, such as ours, can further our understanding of the infection burden of respiratory viruses in high-risk populations.
Alt-text: Unlabelled box
Introduction
Seasonal human coronaviruses (HCoVs) cause acute respiratory illness (ARI) associated with annual community epidemics.1 HCoVs include four globally distributed endemic viral species, which co-circulate with seasonality varying by climate: alphacoronaviruses (HCoV-229E, HCoV-NL63) and betacoronaviruses (HCoV-HKU1, and HCoV-OC43).2,3 Although associated with the “common cold,” severe HCoV disease can occur4 particularly in people with certain underlying medical conditions.5,6
Individuals residing in congregate settings like homeless shelters are at greatest risk for infection and severe health outcomes associated with respiratory viruses.7 In the US, >500,000 people experience homelessness (PEH) nightly with >60% residing in shelters.8 Respiratory virus transmission in shelters can occur because of difficulties with contact tracing, maintaining physical distance and providing effective ventilation.7,9, 10, 11, 12 PEH are disproportionately affected by chronic disease, mental health issues, substance use, limited financial resources and preventive healthcare access.13 SARS-CoV-2 outbreaks in shelters highlight the risk of respiratory viral transmission in these settings.12,14 However, little is known about HCoV epidemiology in homeless shelters.
We describe the HCoV epidemiology among homeless shelters residents and staff in King County, Washington from October 2019-May 2021. We use genome sequencing to evaluate the relationship of HCoV species before and during the COVID-19 pandemic.
Methods
Study design and population
We analysed cross-sectional data from a previously described clinical trial and a SARS-CoV-2 surveillance study in 23 homeless shelters (Supplemental Table 1) in King County, Washington from October 2019-May 2021 (Supplemental Methods).9,15 Before April 1, 2020, eligible participants included shelter residents aged ≥3 months with new or worsening ARI symptoms defined as cough or at least two of the following symptoms: subjective fever, headache, sore throat, runny or stuffy nose, shortness of breath, and muscle or body aches within the last seven days. For participants aged <18 years, diarrhoea, rash and ear pain or discharge were also included as eligible symptoms. Once a month, eligibility expanded to participants regardless of symptoms. During routine surveillance, participants self-enrolled at staffed kiosks within shelter sites. After April 1, 2020, enrolment was opened to shelter residents and staff regardless of symptoms. One-day surge testing events beginning March 30, 2020, were implemented with Public Health – Seattle & King County for SARS-CoV-2 contact tracing efforts within shelter study sites. Consent was obtained from individuals aged ≥18 years or guardians for those aged <18 years; participants aged 13-17 years provided assent.
Questionnaires and a nasal swab were collected at enrolment. Enrolments were limited to once weekly unless new or worsening symptoms developed. Multiple enrolments were permitted with each enrolment constituting a single encounter linked by participant's name and birthdate. Data were de-identified prior to analysis. Our study was approved by the University of Washington Institutional Review Board (Study 00007800) and was prepared using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Questionnaire and shelter site data
Participants or guardians completed a questionnaire administered on an electronic tablet at the time of nasal swab collection, with data stored on Research Electronic Data Capture (REDCap). Self-reported sociodemographic data collected included shelter site location, birthdate, sex, race and ethnicity, pregnancy status, and current tobacco and e-cigarette use status. Questions included self-reported underlying medical conditions (Supplemental Methods). The questionnaire included self-reported ARI symptoms and fatigue, sweats, nausea or vomiting, chills, diarrhoea, rash, and ear pain or discharge in the past week. Loss of taste and smell was added after April 1, 2020.
Specimen collection and respiratory viral testing
Mid-turbinate samples were obtained through July 22, 2020, and then from November 1, 2020, through study end. Anterior nares samples were collected from July 22, 2020-November 1, 2020. Study staff initially collected nasal samples, however due to SARS-CoV-2 community spread, participants self-collected nasal samples beginning March 6, 2020, with study staff supervision. Comparability of a self-collected mid-turbinate swab to clinician-obtained nasopharyngeal sample has previously been described for SARS-CoV-2.16 Samples were extracted and tested using a custom-arrayed RT-PCR platform including influenza viruses (A, B and C), respiratory syncytial virus (RSV A and B), human parainfluenza virus (1-4), HCoV, metapneumovirus, rhinovirus, enterovirus, human bocavirus, human parechovirus and adenovirus (Supplemental Table 2). Beginning November 23, 2020, the OpenArray platform identified HCoV by species. Detailed SARS-CoV-2 testing for this study was previously described.9 From February 25, 2020, onward, samples were tested for SARS-CoV-2 in real-time by a RT-PCR assay. Specimens collected from January 1, 2020-February 25, 2020, were tested retrospectively for SARS-CoV-2. An OpenArray relative cycle threshold (Crt) value was calculated for virus-positive samples. For additional testing details, refer to Supplemental Methods.
Genomic sequencing and analysis
We attempted genomic sequencing on HCoV-positive samples with Crt values <22. Extracted RNA was purified and underwent shotgun metagenomic sequencing. Shotgun metagenomic sequencing libraries were prepared as previously described.17,18 Samples where genomes were not recovered using shotgun sequencing were sequenced with hybridization capture. Raw sequence reads were assembled into consensus genomes using the Seattle Flu Study (SFS) assembly pipeline (https://github.com/seattleflu/assembly). Processed reads are then mapped to reference genomes representing the four HCoV species. The consensus genome with the least missing data (lowest percent Ns) for each sample was selected for inclusion in further analyses and uploaded to GenBank (Supplemental Table 3).
Among samples with completed genome sequencing, one genome per HCoV species from unique participants was used for further analyses to exclude multiple specimens from the same illness course. Maximum likelihood phylogenetic trees were constructed for each HCoV species (with separate trees for HCoV-HKU1A and HCoV-HKU1B) that included the shelter genomes and all full length (>25,000bp) genomes available in GenBank. Shelter genomes for each HCoV species were compared to assess clustering by shelter site and time of sample collection. Genomic sequences collected before the COVID-19 pandemic (April 2020 and earlier) and during the pandemic (January 2021 and later) were compared. Additional details on genomic sequencing and analysis are provided in the Supplemental Methods.
Seasonal human coronavirus species identification
The reference genome associated with the highest quality consensus genome was presumed to represent the sample's species. These prospective species assignments were confirmed by creating a maximum likelihood phylogenetic tree including all sample genomes and the four reference genomes. All other HCoV samples were sent for primer specific RT-PCR amplification or were determined by multiplex OpenArray platform as described in Supplemental Methods.
Data sources, variables and computational analysis
We defined the co-detection of respiratory viruses as ≥2 respiratory viruses detected in the same specimen. Asymptomatic participants were those without any reported illness course symptoms up until their study encounter. We categorized SARS-CoV-2 inconclusive results as negative. Influenza-like illness (ILI) was defined as the presence of fever with cough or sore throat and COVID-19-like illness (CLI) was defined as the presence of fever with cough or shortness of breath. History of lung disease was defined as asthma, bronchitis or chronic obstructive pulmonary disease. Among participants with multiple HCoV-positive samples, we classified separate infections by the presence of a new HCoV species detection or possible reinfection when the same species detection is separated by ≥1 HCoV-negative sample. We used descriptive statistics to summarize sociodemographic data and underlying medical conditions and the Pearson χ2 test for comparison of categorical variables. HCoV proportions among all samples and among virus-positive samples are presented with 95% confidence intervals (CI). To compare the proportion of HCoV-positive samples in participants aged ≥50 years to younger age groups, we used logistic regression controlling for sex, race, ethnicity and shelter site. We defined p-value <0·05 as statistically significant. We used SAS software version 9·4 (Cary, NC, USA) for general analysis and NextStrain software for genome consensus processing and visualization of phylogenetic trees.
Role of funding source
This study was supported by donations from Gates Ventures and a contract from the Centers for Disease Control and Prevention (CDC; Contract #: 75D30120C09322 AM002 to HYC), National Institute of Health (Grant #: T32 AI007044 to EJC). Funders did not have a role in study design, data collection, data analysis, interpretation, writing of the report or decision to submit. CDC co-authors did contribute to data analysis, interpretation, writing of the report and the decision to submit.
Results
From October 2019-May 2021, there were 14,464 encounters from 3281 unique participants (median age 37 years, range: 0·3-85 years, 38% female; mean of 4·4 encounters per unique participant; Supplemental Figure 1 and 2). Among participants, 17% were shelter staff, 40% White, 15% Hispanic and 69% reported no underlying medical condition (Table 1). The majority of encounters (90%) occurred during routine surveillance testing. Participants from six family shelters and one adolescent shelter, accounted for 35% of all encounters. A total of 1569 encounters (11%) had ≥1 respiratory virus from 992 unique participants (Supplemental Table 4).
Table 1.
Characteristics of unique shelter participants with and without seasonal human coronavirus detection.
| Characteristics | All | Seasonal human coronavirus positive | SARS-CoV-2 positive | Other respiratory virus positivea |
|---|---|---|---|---|
| Number of Unique Participants, N | 3281 | 90 | 123 | 849 |
| Age, years | N (%) | n (%) | n (%) | n (%) |
| Overall, median (range) | 37 (0·3-85) | 40 (0·9-81) | 41 (0·5-83) | 30 (0·3-85) |
| <5 | 202 (6·2) | 6 (6·7) | 8 (6·5) | 102 (12·0) |
| 5-11 | 208 (6·3) | 4 (4·4) | 10 (8·1) | 86 (10·1) |
| 12-17 | 109 (3·3) | 1 (1·1) | 6 (4·9) | 40 (4·7) |
| 18-49 | 1776 (54·1) | 45 (50·0) | 57 (46·3) | 420 (49·5) |
| 50-64 | 812 (24·8) | 28 (31·1) | 32 (26·0) | 174 (20·5) |
| ≥65 | 174 (5·3) | 6 (6·7) | 10 (8·1) | 27 (3·2) |
| Sex | ||||
| Male | 1979 (60·3) | 56 (62·2) | 74 (60·2) | 489 (57·6) |
| Female | 1243 (37·9) | 34 (37·8) | 46 (37·4) | 347 (40·9) |
| Other | 16 (0·5) | 0 | 0 | 2 (0·2) |
| Prefer not to say | 43 (1·3) | 0 | 3 (2·4) | 11 (1·3) |
| Race | ||||
| White | 1326 (40·4) | 41 (45·6) | 37 (30·1) | 355 (41·8) |
| Black | 1040 (31·7) | 33 (36·7) | 52 (42·3) | 275 (32·4) |
| Asian | 116 (3·5) | 1 (1·1) | 1 (0·8) | 15 (1·8) |
| American Indian or Alaskan Native | 129 (3·9) | 5 (5·6) | 4 (3·3) | 24 (2·8) |
| Native Hawaiian or Pacific Islander | 150 (4·6) | 1 (1·1) | 6 (4·9) | 59 (7·0) |
| Other | 292 (8·9) | 5 (5·6) | 13 (10·6) | 50 (5·9) |
| Prefer not to say | 228 (7·0) | 4 (4·4) | 10 (8·1) | 71 (8·4) |
| Ethnicity | ||||
| Hispanic | 495 (15·0) | 13 (14·4) | 16 (13·0) | 135 (15·9) |
| Non-Hispanic | 2728 (83·2) | 76 (84·4) | 103 (83·7) | 696 (82·0) |
| Unknown | 58 (1·8) | 1 (1·1) | 4 (3·3) | 18 (2·1) |
| Pregnancy Status Among Women of Child-Bearing Age | n = 851 | n = 19 | n = 33 | n = 226 |
| Pregnant | 17 (2·0) | 1 (5·3) | 0 | 5 (2·2) |
| Not Pregnant | 177 (20·8) | 16 (84·2) | 5 915·2) | 59 (26·1) |
| Prefer not to say | 657 (77·2) | 2 (10·5) | 28 (85·9) | 162 (71·7) |
| Smoking Status | ||||
| Current tobacco use | 1493 (45·5) | 44 (48·9) | 43 (35·0) | 354 (41·7) |
| E-cigarette use/Vape | 235 (15·7) | 3 (6·8) | 7 (16·3) | 63 (17·8) |
| Underlying Medical Conditions | ||||
| None | 2276 (69·4) | 56 (62·2) | 92 (74·8) | 615 (72·4) |
| At least one underlying medical condition | 1005 (30·6) | 34 (37·8) | 31 (25·2) | 234 (27·6) |
| Neurological disease | 76 (2·9) | 4 (5·0) | 2 (2·1) | 18 (2·5) |
| Cardiovascular disease | 108 (3·3) | 2 (2·2) | 2 (1·6) | 22 (2·6) |
| Asthma | 431 (13·1) | 15 (16·7) | 9 (7·3) | 103 (12·1) |
| Bronchitis | 101 (3·1) | 3 (3·3) | 1 (0·8) | 24 (2·8) |
| Chronic obstructive pulmonary disease | 126 (3·8) | 2 (2·2) | 2 (1·6) | 36 (4·2) |
| Hepatic disease | 92 (2·8) | 1 (1·1) | 3 (2·4) | 18 (2·1) |
| Diabetes mellitus | 214 (6·5) | 11 (12·2) | 7 (5·7) | 53 (6·2) |
| Immunosuppression | 45 (1·4) | 2 (2·2) | 1 (0·8) | 13 (1·5) |
| Cancer | 64 (2·0) | 1 (1·1) | 1 (0·8) | 17 (2·0) |
| Other | 38 (1·2) | 1 (1·1) | 0 | 10 (1·2) |
| Shelter Staff | 567 (17·3) | 5 (5·6) | 17 (13·8) | 91 (30·9) |
| Number of Encounters | 14,464 | 107 | 133 | 1329 |
Other respiratory viruses include adenovirus, human bocavirus, enterovirus, influenza (A, B, C), metapneumovirus, human parainfluenza virus (1-4), human parechovirus, rhinovirus, and respiratory syncytial virus (A and B).
HCoVs were detected in 107 encounters from 90 unique participants (median age 40 years, range: 0·9-81 years; 38% female). This represented 0·7% (95% CI[0·6%-0·8%]) of all 14, 464 samples and 7% (95% CI[6-8%]) of 1569 virus-positive samples. Virus-positive specimens obtained from adults aged ≥50 years had a significantly higher proportion of HCoV detected than those aged <50 years (11% vs 6%, respectively; p=0·0046; Supplemental Table 5). On March 23, 2020, Washington State ordered a COVID-19 pandemic stay-at-home ordinance19 including school closure. Before this date, HCoV was detected in 21% and 14% of virus-positive specimens from adults and children, respectively. After this date, HCoV represented 3% and 0·5% of virus-positive samples in adults and children, respectively. No HCoV-positive samples were identified between May 2020-December 2020 (Supplemental Figure 3). Most HCoV-positive specimens were collected during routine surveillance testing with the highest percentage of HCoV in shelters for residents aged ≥18 years (50%; Table 2). Among virus-positive samples, shelters for residents aged ≥50 years had a higher percentage of HCoV detection relative to other shelter sites (Supplemental Table 6). HCoV co-detection was common (18%) with rhinovirus (12%) and influenza B virus (3%) as the most common.
Table 2.
Seasonal human coronavirus species among all participant encounters by shelter types.a
| Type of shelter | Number of total encounters | Number of encounters with viral pathogen detection | Number of seasonal human coronavirus encounters | Seasonal human coronavirus encounters with species identification (n = 100 encounters) |
|||
|---|---|---|---|---|---|---|---|
| HCoV-229E | HCoV-NL63 | HCoV-HKU1 | HCoV-OC43 | ||||
| N | 14,464 | 1569 | 107 | 6 | 24 | 64 | 6 |
| Surveillance Testing | N (%) | N (%) | N (%) | n (%) | n (%) | n (%) | n (%) |
| Shelters: Adults and Children | 4761 (32·9) | 756 (48·2) | 29 (27·1) | 4 (66·7) | 4 (16·0) | 18 (28·1) | 2 (33·3) |
| Shelters: Adults ≥18 years old | 6241 (43·1) | 467 (29·8) | 53 (49·5) | 0 | 15 (60·0) | 32 (50·0) | 3 (50·0) |
| Shelters: Adults 18-25 years old | 1179 (8·1) | 120 (7·7) | 6 (5·6) | 2 (33·3) | 0 | 4 (6·3) | 0 |
| Shelters: Adults ≥ 50 years old | 849 (5·9) | 103 (6·5) | 18 (16·8) | 0 | 6 (24·0) | 10 (15·6) | 1 (16·7) |
| Surge Testing | |||||||
| Shelters: Adults and Children | 318 (2·2) | 30 (1·9) | 0 | 0 | 0 | 0 | 0 |
| Shelters: Adults ≥18 years old | 704 (4·9) | 39 (2·5) | 0 | 0 | 0 | 0 | 0 |
| Shelters: Adults 18-25 years old | 143 (1·0) | 11 (0·7) | 0 | 0 | 0 | 0 | 0 |
| Shelters: Adults ≥ 50 years old | 269 (1·9) | 43 (2·7) | 1 (0·9) | 0 | 0 | 0 | 0 |
Percentages listed here are column percentages.
In this study, 11 participants had repeated HCoV-positive samples; five had new HCoV infections with a different species and one had a possible repeat infection with the same HCoV species. Prolonged shedding in the latter participant could not be excluded (Supplemental Table 7). Among the five participants with new species detection, four had an infection with another HCoV genus (i.e., alphacoronavirus vs betacoronavirus) with a time to reinfection range between 5-415 days. One participant had both HCoV-229E and HCoV-NL63 infections (both alphacoronaviruses) over the study period which were separated by 142 days. Among the nine participants with multiple positive samples for the same HCoV infection course, the range of total days of detection was 3-20 days.
There were 76 encounters among unique participants with only HCoV detected, of which 15 (20%) were asymptomatic (Table 3). The most common symptoms overall were runny nose or congestion, cough and sore throat. Fever or shortness of breath were each only reported in 16 (29%) symptomatic adult encounters, with 25% of adult participants reporting shortness of breath also reporting a history of lung disease. Both ILI [13 (24%)] and CLI [13 (24%)] symptoms were reported among symptomatic adult encounters where only HCoV was detected with 11 (20%) encounters fulfilling definitions for both ILI and CLI. Although these percentages were higher than unique symptomatic adult participants without any virus detected, the difference was not statistically significant (ILI p = 0·24; CLI p = 0·12). HCoV was the only virus detected in 11% of virus-positive samples from participants reporting ILI and 11% of those reporting CLI symptoms.
Table 3.
Symptoms among unique participants with only seasonal human coronavirus detection.a
| Encounters with seasonal human coronavirus only among unique participants |
Encounters where no viral pathogen was detected among unique participants | |||
|---|---|---|---|---|
| Adults ≥18 years | Children <18 years | Total | Total | |
| Number of Unique Participants, N | 68 | 8 | 76 | 2996 |
| n (%) | n (%) | N (%) | n (%) | |
| Asymptomatic | 13 (19·1) | 2 (25·0) | 15 (19·7) | 2155 (71·9) |
| Symptomaticb | 55 (80·8) | 6 | 61 | 841 |
| Runny nose or congestion | 44 (80·0) | 6 (100) | 50 (82·0) | 533 (63·4) |
| Cough | 39 (70·9) | 4 (66·7) | 43 (70·5) | 465 (55·3) |
| Sore Throat | 30 (54·6) | 4 (66·7) | 34 (55·7) | 288 (34·2) |
| Fatigue | 25 (45·5) | 1 (16·7) | 26 (42·6) | 311 (37·0) |
| Myalgias | 23 (41·8) | 0 | 23 (37·7) | 298 (35·4) |
| Headaches | 21 (38·2) | 1 (16·7) | 22 (36·1) | 293 (34·8) |
| Subjective Fevers | 16 (29·1) | 0 | 16 (26·2) | 193 (23·0) |
| Shortness of breath | 16 (29·1) | 0 | 16 (26·2) | 141 (16·8) |
| Sweats | 13 (23·6) | 0 | 13 (21·3) | 163 (19·4) |
| Nausea or vomiting | 15 (27·3) | 0 | 15 (24·6) | 182 (21·6) |
| Chills | 10 (18·2) | 0 | 10 (16·4) | 190 (22·6) |
| Diarrhoea | 6 (10·9) | 0 | 6 (9·8) | 126 (15·0) |
| Rash | 5 (9·1) | 0 | 5 (8·2) | 56 (6·7) |
| Ear pain or discharge | 3 (5·5) | 0 | 3 (4·9) | 53 (6·3) |
| Loss of taste or smell | 0 of 42 people surveyed | 0 of 4 people surveyed | 0 of 52 people surveyed | 6 of 628 people surveyed (1·0) |
| Influenza-like Illness | 13 (23·6) | 0 | 13 (21·3) | 148 (17·6) |
| COVID-19-like Illness | 13 (23·6) | 0 | 13 (21·3) | 133 (15·8) |
Symptoms calculated among n = 76 encounters among unique participants where seasonal human coronavirus was the only virus detected in the sample; instances in which an individual was enrolled with multiple encounters, symptoms reported from their first positive seasonal human coronavirus was included in this table.
Symptoms are calculated out of total symptomatic participants.
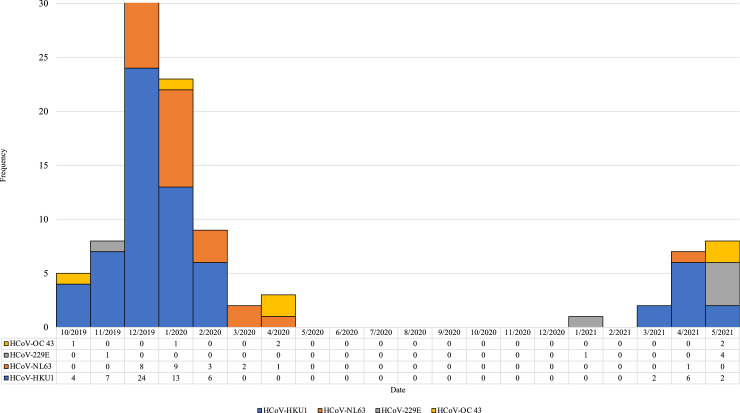
Coronavirus species was determined for 100 (93%) of 107 HCoV-positive specimens: 63 HCoV-HKU1 only, 24 HCoV-NL63 only, six HCoV-229E only, and six HCoV-OC43 only (Figure 1). Co-detection of HCoV-HKU1 and HCoV-NL63 was identified in one sample. Genome sequencing was completed on 54 of 62 HCoV-positive specimens (Supplemental Figure 4 A-E). Eight specimens had insufficient amplification; one had insufficient RNA and seven generated poor quality genomes (>50% missing data), one sample had missing data at about 25% of genomic sites, while the remaining genomes all had <15% missing data (50 had <10% missing data). Coronavirus species was determined for all 54 samples with genome sequence data: 33 (52%) HCoV-HKU1, 12 (22%) HCoV-NL63, five (9%) HCoV-OC43, and four (7%) HCoV-229E. HCoV-HKU1 viruses were sub-divided into three genotypes, A, B, and C.20 Of the 33 sequenced HCoV-HKU1 specimens, five were genotype A (HCoV-HKU1A) and 28 were genotype B (HCoV-HKU1B). A maximum likelihood phylogenetic tree including these 54 genomes and the four HCoV species reference genomes is shown in Figure 2.
Figure 1.
Seasonal human coronavirus species among homeless shelter participants – October 2019 to May 2021.1
1Among the 107 seasonal human coronavirus samples, the species was determined for n = 100 samples including one sample with both HCoV-HKU1 and HCoV-NL63.
Figure 2.
Phylogenetic tree of seasonal human coronaviruses in homeless shelters – King County, Washington.
Six participants had >1 sequenced specimen (15 genomes total); the remaining 39 genomes are from unique participants. We used a subset of 47 unique genomes originating from ten different shelters. For the HCoV-HKU1A, HCoV-HKU1B, and HCoV-OC43 species, all shelter genomes formed a single clade within their respective trees with good bootstrap support (100%, 100%, and 98%, respectively). For species HCoV-NL63 and HCoV-229E, the majority of shelter genomes (nine of 11 and two of three, respectively) clustered together with high bootstrap support (both 100%).
For HCoV-HKU1B, there were eight genomes from shelter L, six from E, three from M, three from O, and two from C. Two samples from shelter L cluster together with good bootstrap support (97%, Supplemental Figure 4A). Otherwise, there was no clear clustering by shelter among the HCoV-HKU1B samples. Among the nine HCoV-NL63 shelter genomes that form the main cluster, there were three from shelter M and two from O. The three shelter M genomes clustered together with one O genome (98% support) (Supplemental Figure 4B). Similarly, the four HCoV-HKU1A genomes from shelter F clustered together with good support (only four single nucleotide changes distinguish these four genomes and two were identical, Supplemental Figure 4C) as do the two HCoV-OC43 genomes from shelter G (Supplemental Figure 4D).
All but one sequenced HCoV-HKU1 specimen identified prior to the pandemic were HCoV-HKU1B, while all identified during the pandemic were HCoV-HKU1A (although the latter are the four closely related genomes from shelter F). Among four HCoV-HKU1A genomes identified during the pandemic, all were distinct from the one “pre-pandemic” genome. Two identical HCoV-HKU1A shelter F genomes from April 2021 differed by two and four single nucleotide changes, respectively, from the two collected in May 2021. Two HCoV-OC43 genomes from May 1, 2021, and May 20, 2021, formed their own clade within the HCoV-OC43 shelter group (from the same shelter) and the two HCoV-229E genomes (from different shelters) both collected on May 28, 2021, clustered separately from the one pre-pandemic genome.
Discussion
Our study of HCoV epidemiology is among the few studies on respiratory viral epidemiology in homeless shelters.21 We detected HCoV in 0·7% of all specimens and 7% of virus-positive specimens, with a marked decrease in HCoV during the spring of 2020, coinciding with COVID-19 pandemic community-wide mitigation measures. There was a higher proportion of HCoV detection among adults than children throughout the study period. HCoV-HKU1, a betacoronavirus in the same genus as SARS-CoV-2, remained the predominant species before and after the start of the COVID-19 pandemic. Our findings highlight that HCoV species circulate among people of all ages residing in congregate settings such as homeless shelters and that pandemic-associated mitigation measures may have had an effect on HCoV circulation. These findings may provide epidemiologic data to guide control measures for HCoVs.
Older adults, especially those who reside in congregate settings, constitute an underappreciated high-risk demographic with a disproportionate burden of HCoV infections. In a study of symptomatic nursing home residents, HCoV was the most common infection detected over three influenza seasons.22 Another study in Belgium, HCoV was most commonly found in the ≥65 year age-group and children aged <5 years among hospitalised patients.1 Of hospitalised adults, 34% experienced in-hospital complications or died; many of whom had underlying medical risk factors.1 The burden of HCoV infections in children has been well-studied23 and our study adds to the growing body of adult HCoV literature. We found that shelter age-group differences of HCoV proportions were most pronounced when calculated as a percentage of virus-positive samples and less so when these proportions are calculated with samples from all encounters. This may be due to a greater burden of non-infectious causes of symptoms in the older shelter participants that prompted individual enrolment in the study. As such, the burden of HCoV infections in older shelter participants was more apparent when virus-negative samples were excluded.
HCoV symptoms were mild in the shelter participants we studied; however, in other studies where the focus was on patients seeking medical care, symptom profiles have varied. In one multi-year study of hospitalised patients with HCoV infection, fever, cough and dyspnoea were the most common symptoms in adults.24 Our study found that rhinorrhoea, cough and sore throat were the most commonly reported symptoms in either children or adults, although direct comparison of other studies is limited given the differences in study population,4,25 differences in inclusion criteria26 and a lack of age-group symptoms differentiation.4,26 Our expanded symptom inclusion criteria allowed for a more complete characterisation of symptoms including those who are pauci-symptomatic and asymptomatic. For example, we found that one in five participants with HCoV reported no symptoms at the time of enrolment. Whether these individuals were asymptomatic, pre-symptomatic or post-symptomatic is not known as participants were not longitudinally followed. These individuals also illustrate a possible transmission risk in congregate settings especially if mitigation efforts are based solely on symptoms.
The recognition of HCoV burden across the age spectrum and the use of molecular testing in community respiratory viral surveillance has facilitated an improved understanding of HCoV burden at the local,26,27 regional28 and national levels.29 Our study offers a unique look at HCoV detection before and during the COVID-19 pandemic in homeless shelters. Notably, the detection of HCoV in the shelter population peaked before the detection of the first SARS-CoV-2 infection in the US and quickly tapered coinciding with community-wide mitigation interventions with HCoV-HKU1 predominating throughout the study period. Decreasing cases may be because of multiple factors including seasonality, viral interference30 and non-pharmaceutical public health interventions. These patterns of respiratory virus circulation from 2019-2021 mirror the trends in HCoV observed in SFS, a community-wide surveillance study of respiratory pathogens27 and a county level surveillance study in California.28 Despite consistent participant enrolment throughout the summer months, HCoV was not detected again until January 2021, a delay compared to the previous year. The US National Respiratory and Enteric Virus Surveillance System (NREVSS), a passive surveillance network, provides regional and national surveillance of HCoV.29 NREVSS data similarly showed a decline of HCoV from April 2020 onward but steady increases in HCoV detection the following year varied by region and HCoV species.29 These combined surveillance efforts help characterize HCoV circulation and may inform nuanced local timing of public health interventions in specific settings of high-risk individuals.
Genomic sequencing revealed that HCoV infections were due to a diverse virus population with HCoV-HKU1 predominating in this study. Whether this species-level pattern reflects community circulation is not known. Community-based studies suggest that species predominance changes by year.4,26 Most shelters where HCoV detection occurred experienced infections due to more than one species, suggesting that >1 introduction event occurred. However, among viruses of the same species, genomes from the same shelter were often more related to one another than to other shelter genomes. This may suggest some cases of intra-shelter viral spread rather than new introductions of closely related viruses. The exception to this observation was the prevalence of HCoV-HKU1B viruses. While HCoV-HKU1B viruses collected in this study could be distinguished from viruses with genomes in GenBank, there was very little clustering within this clade. This may be the result of recombination among HCoV-HKU1B viruses circulating locally.31
Finally, although few HCoVs were identified during the pandemic, there was limited evidence of a change in circulating HCoV species after the start of the COVID-19 pandemic. HCoV-HKU1B and HCoV-NL63 viruses were not observed among sequenced HCoV specimens detected after January 2021, although we do not have species or genome sequence level data for all cases. Also, HCoV viruses identified during the pandemic either formed their own clusters within clades formed by shelter samples (HCoV-HKU1A, HCoV-OC43) or pre and during pandemic shelter sequences formed their own separate clusters (HCoV-229E). From a genomic perspective, these shelter data suggest a greater similarity between viruses clustered by time rather than geographic origin. These observations further lend support to the fact that HCoV species predominance and genomic diversity may change by location and season.
Limitations
Our study was subject to several limitations. First, although attempts were made to make enrolment accessible to all residents at shelter sites, selection bias may have occurred as participation in the study was based on self-enrolment. Second, it is likely those who were asymptomatic or minimally symptomatic were underestimated earlier in the study until enrolment criteria expanded after April 1, 2020. Third, we were unable to describe illness course or clinical outcomes associated with HCoV. Fourth, the linking of multiple enrolment encounters by unique participant was limited despite associating encounters by participant name and birthdate. Fifth, we did not have community HCoV species-level or genomic data to make a direct comparison between shelter and surrounding community epidemiology. Sixth, our custom arrayed RT-PCR panel did not include human bocavirus or human parechovirus during the latter part of the study and may have missed detection of these viruses (Supplemental Table 2). Seventh, we did not collect data on the overall number of residents or staff at each shelter site. Eighth, we were unable to account for geographic clustering of individuals within individual shelters. Lastly, shelter specific mitigation efforts were not systemically collected, although masking, crowding reductions, and movement of residents to single rooms were part of the citywide mitigation strategy in congregate settings. Future studies evaluating non-pharmaceutical mitigation efforts in homeless shelters would provide important data for public health mitigation interventions.
Our study shows that HCoV circulated among homeless shelter residents before and during the COVID-19 pandemic and may be an underappreciated contributor of respiratory viral infections in older adults. Just as older age-groups are the focus of vaccination campaigns in other vaccine-preventable infections, including influenza and SARS-CoV-2, targeted public health surveillance and interventions where older adults reside may be required to mitigate infection in this vulnerable age-group. The implementation of community-wide mitigation measures was associated with a delayed detection of HCoV in the second year of the study. Additional congregate-setting-based community studies would help to understand the epidemiology of endemic respiratory viruses, especially among those who bear a significant burden of infection.
Contributors
Study Design: E.J.C., A.M.C., P.R., J.P.H., T.M.U., M.A.R., E.M., J.A.E., H.Y.C.
Data Collection: J.H.R., P.D.H., H.X., M.G.M., T.V.N., B.P., S.N.C., C.R.W., L.A.S.
Data Analysis: E.J.C., A.M.C., J.H.R., P.R., S.N.C., J.P.H., T.M.U., M.A.R., E.M., M.M.S., J.S.D., N.S., L.A.S., J.A.E., H.Y.C.
Interpretation of Results: E.J.C., A.M.C., J.H.R., P.R., S.N.C., C.R.W., J.P.H., T.M.U., M.A.R., E.M., M.M.S., J.S.D., N.S., L.A.S., J.A.E., H.Y.C.
Manuscript Writing: All Co-authors.
Data sharing statement
The authors have indicated that a de-identified individual participant dataset can be shared upon email request to the corresponding author to researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals may be submitted up to 36 months following article publication.
Declaration of interests
Dr. Chow reported honoraria from Providence Health & Services, Renton, Washington for presentations on COVID-19. Ms. Cox reported honoraria from University of California, Berkeley for presentations on COVID-19. Dr. Roychoudhury reported honoraria from The Bill & Melinda Gates Foundation for presentations on COVID-19. Dr. Englund reported consulting with Sanofi Pasteur, AstraZeneca, and Meissa Vaccines, and has received research funding from AstraZeneca, GlaxoSmithKline, Merck, and Pfizer outside the submitted work. All other authors report no conflicts of interest. Dr. Chu reported consulting with Ellume, Pfizer, The Bill and Melinda Gates Foundation, Glaxo Smith Kline, and Merck. She has received research funding from Gates Ventures, Sanofi Pasteur, and support and reagents from Ellume and Cepheid outside of the submitted work.
Acknowledgements
We would like to acknowledge the homeless shelter staff, program managers, all the participants and research collaborators who contributed to this study.
Funding
This work was supported by a donation from Gates Ventures and a contract from the Centers for Disease Control and Prevention (Contract #: 75D30120C09322 AM002). This work was also supported in part by the National Institute of Health – National Institute of Allergy and Infectious Diseases (Grant #: T32 AI007044 to EJC).
Footnotes
Disclaimer: The views expressed are those of the authors and do not necessarily reflect the official policy of the Centers for Disease Control and Prevention.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100348.
Appendix. Supplementary materials
References
- 1.Fischer N, Dauby N, Bossuyt N, et al. Monitoring of human coronaviruses in Belgian primary care and hospitals, 2015-20: a surveillance study. Lancet Microbe. 2021;2(3):e105–e114. doi: 10.1016/S2666-5247(20)30221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S, Lee Y, Michelow IC, Choe YJ. Global seasonality of human coronaviruses: a systematic review. Open Forum Infect Dis. 2020;7(11):ofaa443. doi: 10.1093/ofid/ofaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng ZQ, Chen DH, Tan WP, et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37(2):363–369. doi: 10.1007/s10096-017-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48(8):2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283(4):499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Filho LV, Zerbinati RM, Tateno AF, et al. The differential clinical impact of human coronavirus species in children with cystic fibrosis. J Infect Dis. 2012;206(3):384–388. doi: 10.1093/infdis/jis274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung CS, Ho MM, Kiss A, Gundlapalli AV, Hwang SW. Homelessness and the response to emerging infectious disease outbreaks: lessons from SARS. J Urban Health. 2008;85(3):402–410. doi: 10.1007/s11524-008-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Department of Housing and Urban Development . 2021. The 2020 Annual Homeless Assessment Report (AHAR) to Congress.https://www.huduser.gov/portal/sites/default/files/pdf/2020-AHAR-Part-1.pdf Accessed 27 February 2022. [Google Scholar]
- 9.Rogers JH, Link AC, McCulloch D, et al. Characteristics of COVID-19 in homeless shelters: a community-based surveillance study. Ann Intern Med. 2021;174(1):42–49. doi: 10.7326/M20-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall CB. The nosocomial spread of respiratory syncytial viral infections. Annu Rev Med. 1983;34:311–319. doi: 10.1146/annurev.me.34.020183.001523. [DOI] [PubMed] [Google Scholar]
- 11.Fields VL, Kiphibane T, Eason JT, et al. Assessment of contact tracing for COVID-19 among people experiencing homelessness, Salt Lake County Health Department, March-May 2020. Ann Epidemiol. 2021;59:50–55. doi: 10.1016/j.annepidem.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowan SE, McCormick DW, Wendel KA, et al. Lower prevalence of SARS-CoV-2 infection among people experiencing homelessness tested in outdoor encampments compared with overnight shelters - Denver, Colorado, June - July 2020. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raoult D, Foucault C, Brouqui P. Infections in the homeless. Lancet Infect Dis. 2001;1(2):77–84. doi: 10.1016/S1473-3099(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 14.Tobolowsky FA, Gonzales E, Self JL, et al. COVID-19 outbreak among three affiliated homeless service sites - King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(17):523–526. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman KL, Rogers JH, McCulloch D, et al. Point-of-care molecular testing and antiviral treatment of influenza in residents of homeless shelters in Seattle, WA: study protocol for a stepped-wedge cluster-randomized controlled trial. Trials. 2020;21(1):956. doi: 10.1186/s13063-020-04871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCulloch DJ, Kim AE, Wilcox NC, et al. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peddu V, Shean RC, Xie H, et al. Metagenomic analysis reveals clinical SARS-CoV-2 infection and bacterial or viral superinfection and colonization. Clin Chem. 2020;66(7):966–972. doi: 10.1093/clinchem/hvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greninger AL, Zerr DM, Qin X, et al. Rapid metagenomic next-generation sequencing during an investigation of hospital-acquired human parainfluenza virus 3 infections. J Clin Microbiol. 2017;55(1):177–182. doi: 10.1128/JCM.01881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.State of Washington: Office of the Governor . 2020. Proclamation by the Governor Amending Proclamation 20-05: Stay Home-Stay Healthy.https://www.governor.wa.gov/sites/default/files/proclamations/20-25%20Coronovirus%20Stay%20Safe-Stay%20Healthy %20%28tmp%29%20%28002%29.pdf Accessed 27 January 2022. [Google Scholar]
- 20.Dominguez SR, Shrivastava S, Berglund A, et al. Isolation, propagation, genome analysis and epidemiology of HKU1 betacoronaviruses. J Gen Virol. 2014;95(Pt 4):836–848. doi: 10.1099/vir.0.059832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ly TDA, Edouard S, Badiaga S, et al. Epidemiology of respiratory pathogen carriage in the homeless population within two shelters in Marseille, France, 2015-2017: cross sectional 1-day surveys. Clin Microbiol Infect. 2019;25(2):249 e1-e6. doi: 10.1016/j.cmi.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone J, Parsons R, Botelho F, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogimi C, Kim YJ, Martin ET, Huh HJ, Chiu CH, Englund JA. What's new with the old coronaviruses? J Pediatric Infect Dis Soc. 2020;9(2):210–217. doi: 10.1093/jpids/piaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim T, Choi H, Shin TR, et al. Epidemiology and clinical features of common community human coronavirus disease. J Thorac Dis. 2021;13(4):2288–2299. doi: 10.21037/jtd-20-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masse S, Capai L, Villechenaud N, Blanchon T, Charrel R, Falchi A. Epidemiology and clinical symptoms related to seasonal coronavirus identified in patients with acute respiratory infections consulting in primary care over six influenza seasons (2014-2020) in France. Viruses. 2020;12(6) doi: 10.3390/v12060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the hive cohort of households in Michigan. J Infect Dis. 2020;222(1):9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.2022. Seattle Flu Study: Pathogens.https://seattleflu.org/pathogens Accessed 27 January 2022. [Google Scholar]
- 28.Cooksey GLS, Morales C, Linde L, et al. Severe acute respiratory syndrome Coronavirus 2 and respiratory virus sentinel surveillance, California, USA, May 10, 2020-June 12, 2021. Emerg Infect Dis. 2022;28(1):9–19. doi: 10.3201/eid2801.211682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piret J, Boivin G. Viral interference between respiratory viruses. Emerg Infect Dis. 2022;28(2):273–281. doi: 10.3201/eid2802.211727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo PC, Lau SK, Yip CC, et al. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80(14):7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.