Abstract
The transcription factor FIS has been implicated in the regulation of several stable RNA promoters, including that for the major tRNALeu species in Escherichia coli, tRNA1Leu. However, no evidence for direct involvement of FIS in tRNA1Leu expression has been reported. We show here that FIS binds to a site upstream of the leuV promoter (centered at −71) and that it directly stimulates leuV transcription in vitro. A mutation in the FIS binding site reduces transcription from a leuV promoter in strains containing FIS but has no effect on transcription in strains lacking FIS, indicating that FIS contributes to leuV expression in vivo. We also find that RNA polymerase forms an unusual heparin-sensitive complex with the leuV promoter, having a downstream protection boundary of ∼−7, and that the first two nucleotides of the transcript, GTP and UTP, are required for formation of a heparin-stable complex that extends downstream of the transcription start site. These studies have implications for the regulation of leuV transcription.
The leuV operon encodes three of the four genes for tRNA1Leu, one of the most abundant Escherichia coli tRNA species (12, 21). The promoter for leuV is strong, with activity similar to that of the rRNA promoter rrnB P1 (6, 7); like many other rRNA and tRNA promoters, it is regulated in response to growth rate and amino acid starvation (6, 37). The leuV promoter has several features similar to those of rRNA promoters, including near-consensus −10 and −35 hexamers spaced at the nonconsensus distance of 16 bp, a G+C-rich sequence (the discriminator region) between the −10 element and the transcription start site, and an upstream sequence that contributes to promoter activity (Fig. 1). However, the effect of upstream sequence at leuV is smaller than that at rrnB P1 (∼10- to 40-fold versus ∼300-fold) (6, 7, 34), and the mechanism(s) responsible for its effects has not been fully characterized.
FIG. 1.
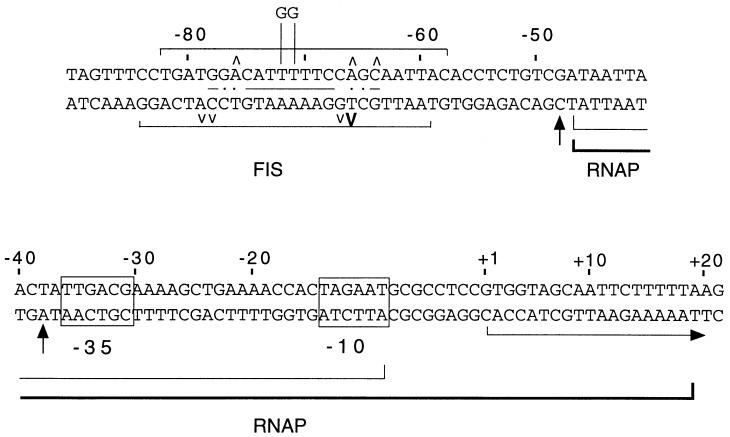
Sequence of the leuV promoter region. Positions protected by FIS in DNase I footprints and positions of enhanced DNase I cleavage within the FIS site (carets) are indicated. Enhanced DNase I cleavage at −38 and −48 in the presence of RNAP is indicated by vertical arrows. The 2-bp substitution mutant T−71G T−72G reduces FIS binding (Fig. 2). Boundaries of protection by RNAP in the absence (thin underline, −47 to −7) or the presence (thick underline, −47 to +20) of the initiating nucleotides GTP and UTP are indicated. Similarity of the FIS site to a consensus derived from information in references 15 and 20 [Gnn(c/t)(A/g)(a/t)(a/t)(T/A)(t/a)(t/a)(T/c)(g/a)nnC] is indicated by lines between the top and bottom strands. Dots between strands in the FIS site indicate poorly conserved positions in different FIS sites.
The leuV upstream sequence has two components, and their contributions to promoter strength are similar (6, 7). The region just upstream of the −35 hexamer (−39 to −47) is likely to increase transcription by interacting with the C-terminal domain of the α subunit of RNA polymerase (RNAP), since it is quite similar to the promoter-proximal region of the UP element consensus (13) and since in vitro transcription of leuV in the absence of proteins other than RNAP is reduced by an RNAP α-subunit mutation that abolishes UP element recognition (αΔ235) (35). The second region, between −47 and −107, affects transcription by a previously uncharacterized mechanism. It was originally suggested that a T tract in this region (at −69 to −73) influences leuV promoter activity through its effects on DNA bending (7). However, a 2-bp substitution within this T tract (T−71G T−72G) abolished the upstream effect on transcription without affecting the anomalous electrophoretic mobility (bending) of the promoter fragment (7).
FIS is a 12-kDa DNA binding protein that directly activates transcription from a number of promoters by binding to sites upstream of the core promoter (e.g., rrnB P1, thrU/tufB, tyrT, proP, and mar [26, 29, 31, 32, 36, 40]). FIS also plays a role in other cellular processes, including repression of transcription (41), site-specific recombination (15), transposition (39), and DNA replication (14). It was suggested that FIS contributes to tRNA1Leu transcription, since at higher growth rates in fis mutant strains the concentration of tRNA1Leu (as well as of some other tRNAs) is reduced relative to that of 16S rRNA (30). However, it was not known whether this effect of fis was direct or indirect.
The concentration of FIS in the cell varies dramatically as a function of growth rate and growth phase (2, 3), and the extent of activation by FIS at some promoters varies as a function of growth rate (1, 11). However, regulation of rrnB P1 with growth rate appears to involve a different mechanism that involves sensing of the initiating nucleotide concentration (16). The extent of activation of rrnB P1 by FIS does not vary substantially with growth rate in wild-type strains (1), although FIS is responsible for growth rate-dependent regulation of rrnB P1 in strains with RNAP mutations that alter the nucleoside triphosphate (NTP)-sensing mechanism (4). Thus, the contribution of FIS to promoter activity and regulation can vary, depending on the specific kinetic properties of a promoter and other regulatory mechanisms that affect it.
In this work, we have identified a FIS binding site in the leuV promoter upstream region and we have examined the effects of FIS on leuV expression both in vivo and in vitro by using promoter derivatives with mutant or wild-type FIS binding sites. We have also identified an unusual heparin-sensitive RNAP complex with the leuV promoter. These studies support the proposal that multiple mechanisms, including activation by FIS and NTP sensing, contribute to the transcription and regulation of leuV.
Identification of a FIS binding site upstream of the leuV promoter.
FIS binds to the upstream region in several stable RNA promoters (rrnB P1, tyrT, tufB, and valU [10, 31, 36, 38]), and putative FIS binding sites have been identified upstream of many others, including leuV (24, 25, 30). The proposed leuV FIS site contains a one-base mismatch from the consensus (Fig. 1) (15). However, the degeneracy of the FIS consensus sequence has limited its predictive value, and not all consensus sequences actually bind purified FIS (14, 15). We therefore determined the location of FIS binding sites in the leuV promoter region experimentally.
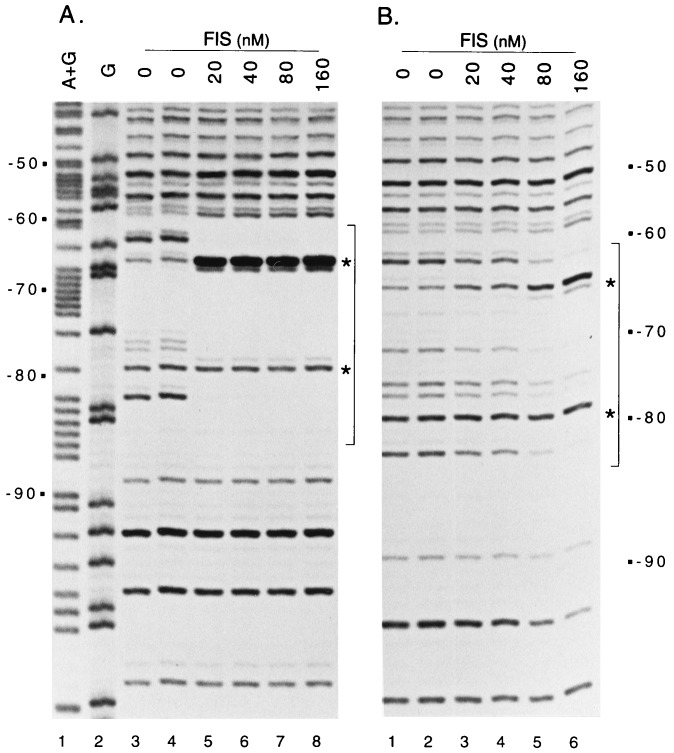
In a DNase I footprinting experiment, FIS protected a site in the leuV promoter centered at −71 (Fig. 1 and 2A). The position of the site and the apparent Kd, approximately 2 to 4 nM (determined from additional DNase I footprint titrations [not shown]), were similar to that of FIS site I in the rrnB P1 promoter (17, 36). Sites of enhanced DNase I cleavage within the leuV site (−66, −67, −78, and −79 on the bottom strand; −64, −66, and −76 on the top strand [Fig. 1 and 2A]) are likely to reflect FIS-induced DNA distortion or kinking, as noted for other FIS sites (15). At much higher FIS concentrations (∼160 nM), there was partial occupancy of a second site, overlapping the core promoter region (data not shown). Similar weak binding sites for FIS also occur in the rrnB P1 core promoter (34), but no function has been ascribed to these sites.
FIG. 2.
DNase I footprints of FIS bound to wild-type (A) or mutant (B) leuV promoter fragments. BglII-HindIII leuV promoter fragments were obtained from pleuD9 (leuV −109 to +33 [7]) or pHEB3 (leuV −109 to +11, T−71G T−72G [7]) and were 32P labeled in the bottom (template) strand at the BglII site, approximately 20 bp upstream from leuV position −109. Footprinting reactions were carried out at 22°C, essentially as described previously (35), in a solution of 10 mM Tris-Cl (pH 7.9), 10 mM MgCl2, 150 mM NaCl, 1 mM dithiothreitol, and 100 μg of bovine serum albumin per ml. FIS was present at the concentrations indicated. Sequence markers were prepared by the method of Maxam and Gilbert (27).
A previously constructed 2-bp substitution mutation in the leuV promoter is located within the FIS binding site (pHEB3, T−71G T−72G [Fig. 1] [7]). This mutation reduced the affinity for FIS by at least 10-fold (apparent Kd, ∼40 to 80 nM [Fig. 2B and data not shown]).
Multiple FIS binding sites contribute to activation at some other promoters (8, 29, 32, 36). However, at leuV only one FIS site was observed within the sequence extending to −109. Since the sequence upstream of ∼−76 was previously found not to contribute to leuV promoter activity (7), it seems unlikely that FIS sites upstream of −109 have a major effect on leuV transcription.
FIS activates transcription from the leuV promoter in vitro.
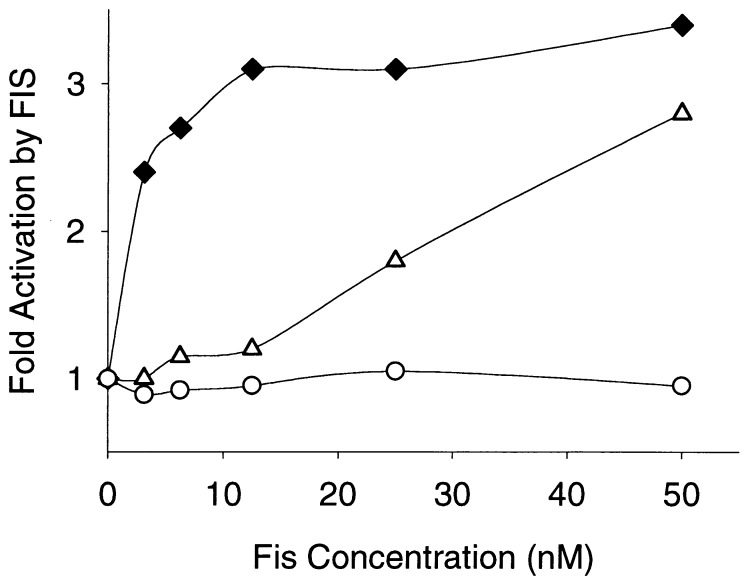
Transcription of the wild-type leuV promoter and of two mutant leuV promoters that lack a functional FIS binding site (T−71G T−72G and Δ−47, a leuV derivative with a deletion of sequences upstream of −47) was carried out in vitro in the presence of increasing concentrations of FIS. FIS stimulated transcription from the wild-type leuV promoter, with maximal activation (about threefold) observed at approximately 10 nM FIS (Fig. 3). Transcription from the promoter deleted for the FIS binding site (Δ−47) was not affected by FIS at any concentration tested, while that of the 2-bp substitution mutant promoter (T−71G T−72G) was stimulated only by much higher concentrations of FIS (>50 nM) than that required for the wild-type promoter (Fig. 3), consistent with the differences in affinity for FIS of the wild-type and mutant promoters observed by DNase I footprinting (Fig. 2).
FIG. 3.
Effects of FIS on in vitro transcription of wild-type and mutant leuV promoters (wild type, diamonds; T−71G T−72G mutant, triangles; FIS site deletion [−47 endpoint], circles). Transcription was carried out in the absence of FIS or in the presence of the indicated concentrations of FIS by using supercoiled plasmid templates with leuV promoter fragments inserted into the EcoRI and HindIII sites of pRLG770, upstream of the rrnB T1 terminator (36). Plasmids used were pRLG927 (wild-type leuV −109 to +33, obtained from pleuD9 [7]), pRLG930 (leuV −109 to +11, T−71G T−72G, obtained from pHEB3 [7]), and pRLG931 (leuV −47 to +55, obtained from pLC118 [7]). Multiple-round transcription was carried out essentially as described previously (36) except that nucleotide concentrations were 100 μM (for ATP, CTP, and GTP) or 10 μM (for UTP, with [α-32P]UTP [DuPont, NEN]). Purified FIS was a gift from Reid Johnson (University of California at Los Angeles). leuV transcripts were analyzed on 6.5% acrylamide–7 M urea gels and quantified with a Molecular Dynamics PhosphorImager. The effect of FIS is shown as a ratio of transcript values in the presence and absence of FIS. Results from a representative experiment are shown.
FIS activates transcription from the leuV promoter in vivo.
The effect of FIS on leuV transcription in vivo was determined by comparing the activity of the wild-type promoter with that of the promoter containing the FIS site mutation T−71G T−72G. Promoter activities were determined in strains containing single-copy chromosomal promoter-lacZ fusions. The FIS site mutation reduced promoter activity to about 44% of its wild-type activity in a strain containing FIS (Table 1 [see also Table 2]), a result consistent with previous observations with similar constructs (7). However, this mutation did not reduce leuV promoter activity in a strain lacking FIS (a fis::kan strain [Table 1]). This result indicates that the effect of the mutation in the wild-type strain is attributable to loss of FIS binding and activation.
TABLE 1.
Effects of FIS site substitution or deletion mutations on leuV promoter activity in wild-type fis and fis::kan strains
| Strain | Promoter | fis allele | Activitya | % Activityb |
|---|---|---|---|---|
| RLG4043 | leuV (−105 to +11) | Wild type | 4,962 | 100 |
| RLG4045 | leuV (−105 to +11, T−71G T−72G) | Wild type | 2,173 | 44 |
| RLG4044 | leuV (−47 to +11) | Wild type | 1,393 | 28 |
| RLG3274 | leuV (−105 to +11) | fis::kan-767 | 6,869 | 100 |
| RLG3276 | leuV (−105 to +11, T−71G T−72G) | fis::kan-767 | 6,895 | 100 |
| RLG3285 | leuV (−47 to +11) | fis::kan-767 | 5,065 | 74 |
β-Galactosidase levels were determined in promoter-lacZ fusion-containing strains grown for several generations in Luria-Bertani medium (28) and are averages of duplicate determinations differing by less than 10%. Strains were monolysogenic for phage λ system I (34), carrying fusions of the indicated leuV promoters to lacZ (Table 2). Promoter-lacZ fusions were constructed from leuV promoter fragments obtained by PCR from the MG1655 chromosome. fis::kan-767 derivatives of these strains (RLG3274, RLG3276, and RLG3285 [Table 2]) were constructed by P1 transduction from strain RJ1617 (23).
Activity expressed as a percentage of that of the wild-type leuV promoter (−105 to +11) in the appropriate strain background (wild type or fis::kan-767).
TABLE 2.
Strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Strains | ||
| RLG4006 | MG1655 lacZΔ145 thi-39::Tn10 | 5 |
| RLG4043 | RLG4006 λleuV (−105 to +11)-lacZ | This work |
| RLG4044 | RLG4006 λleuV (−47 to +11)-lacZ | 5 |
| RLG4045 | RLG4006 λleuV (−105 to +11, T−72G T−71G)-lacZ | This work |
| RLG3274 | RLG4043 fis::kan-767 | This work |
| RLG3276 | RLG4045 fis::kan-767 | This work |
| RLG3285 | RLG4044 fis::kan-767 | This work |
| RJ1617 | MC1000 fis::kan-767 | 23 |
| Plasmids | ||
| pRLG927 | pleuV (−109 to +33) in pRLG770 | This work |
| pRLG930 | pleuV (−109 to +11, T−72G T−71G) in pRLG770 | This work |
| pRLG931 | pleuV (−47 to +33) in pRLG770 | This work |
Deletion of the entire FIS site (Δ−47) had a slightly larger effect on promoter activity than the 2-bp substitution, reducing it to 28% of wild-type activity (Table 1). This suggests either that the 2-bp substitution does not fully eliminate activation by FIS (consistent with the weak affinity of the 2-bp mutant DNA for FIS [Fig. 2B and 3]), that sequences upstream of −47 contribute slightly to the leuV UP element, or both. The latter possibility is consistent with the slight reduction in activity of the Δ−47 promoter (74% of the wild type) in a fis::kan strain (Table 1).
As observed previously for other promoters (26, 36), the activity of each of the leuV promoters was greater in fis::kan strains than in wild-type fis strains (Table 1). This increase may reflect contributions from at least two factors. First, some of the increase in leuV activity is likely to result from a compensating effect of the rRNA feedback system acting on core promoter function, as described previously for the rrnB P1 and tufB promoters (32, 36). This feedback effect is thought to result from loss of activation of the rrn operons by FIS and may operate through the recently described NTP-sensing mechanism for growth rate regulation of rrn promoters (16). Transcription from growth rate-regulated rrn P1 promoters lacking FIS sites is increased to a greater extent (approximately four- to fivefold) than transcription from control promoters (see below) in fis::kan strains (34b, 36). An effect of the feedback system on the leuV promoter is consistent with previously described effects of altered rrn gene dosages on tRNA expression (19, 22).
In addition, some of the increase in leuV activity in fis::kan strains is likely to derive from a promoter-independent effect on the lacZ reporter system, since all promoter-lacZ fusions that we have tested (including non-growth-rate-regulated promoters such as lacUV5 and growth rate-defective mutant derivatives of rrnB P1) show some degree of increase in activity in fis::kan strains (∼1.5- to 2-fold) (34a). Since FIS has many roles in the cell and fis mutants have pleiotropic effects (14), this nonspecific effect is not surprising.
Although transcription of the leuV-lacZ fusion appears to be as active in fis::kan strains as in wild-type strains (Table 1), reduced levels of tRNA1Leu have been reported (relative to 16S rRNA) in fis mutant strains (30). These observations are consistent with the proposed contribution of a nonspecific increase in promoter-lacZ fusion activity in fis::kan strains, together with a feedback derepression of the leuV core promoter activity that may not be as great as the derepression observed for rrnB P1. This suggests that the leuV promoter may not be as responsive to the NTP-sensing mechanism as is rrnB P1 (see also references 5 and 33). Alternatively, the apparent discrepancy between pleuV-lacZ fusion activity and reduced tRNA1Leu levels in fis::kan strains may reflect either an overestimate of tRNA1Leu production from the leuV operon (which encodes three tandem tRNALeu genes) with the promoter-lacZ fusion or reduced tRNA production from the argT operon, which encodes the fourth tRNA1Leu gene.
Properties of RNAP-leuV promoter complexes.
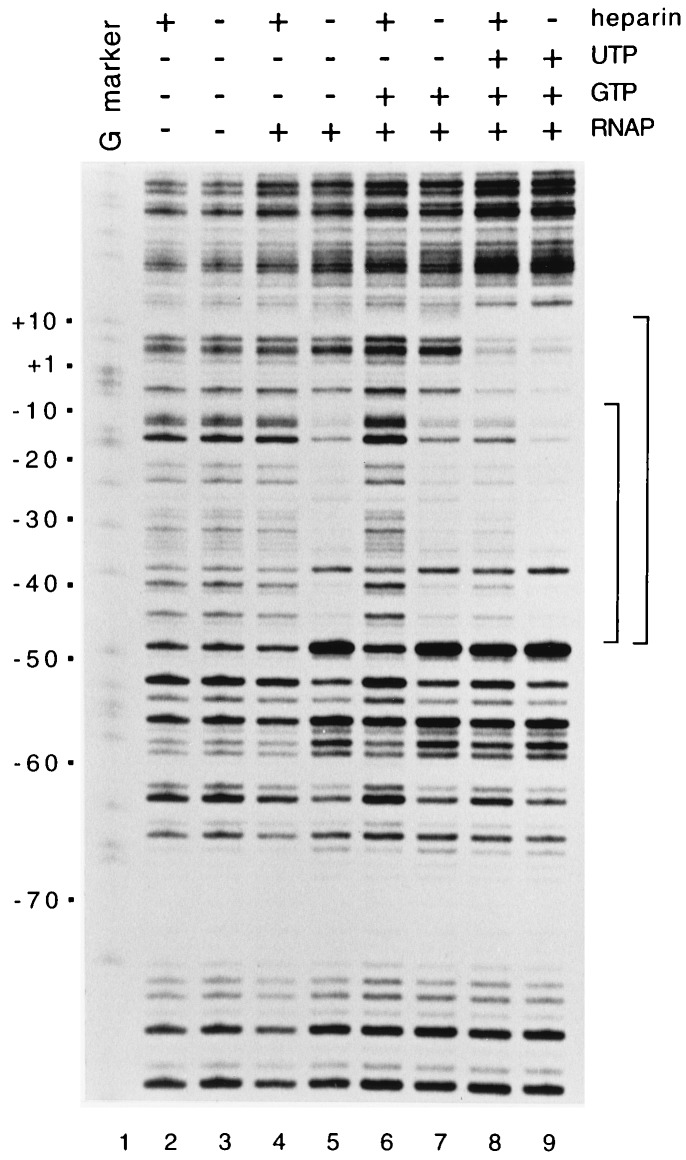
Since RNAP forms an unstable, heparin-sensitive complex with the rrnB P1 promoter, a feature responsible at least in part for its regulation by the NTP-sensing mechanism, we also characterized the properties of complexes formed between RNAP and the leuV promoter by using DNase I footprinting. RNAP formed a heparin-sensitive complex with the leuV promoter in the absence of NTPs (Fig. 4, lanes 4 and 5). The boundaries of this complex are somewhat unusual, extending from −47 to about −7, thus not including the transcription start site. At rrnB P1, a closed, heparin-sensitive complex with protection extending approximately to the transcription start site (−60 to +1) was observed under similar conditions (5, 8). These complexes differ from the open, heparin-stable complexes formed at most other promoters in the absence of nucleotides.
FIG. 4.
DNase I footprints of RNAP bound to the wild-type leuV promoter. Complexes were formed with RNAP (10 nM) and the leuV promoter fragment (described in the legend to Fig. 2) in the presence or absence of the initiating nucleotides (500 μM GTP or 500 μM GTP and 50 μM UTP) at 22°C in buffer described in the legend to Fig. 2, except that it contained 30 mM KCl rather than 150 mM NaCl. Where indicated, heparin (10 μg/ml) was added prior to DNase I digestion.
A heparin-stable leuV promoter-RNAP complex, in which protection extended downstream to ∼+20, was formed in the presence of the initiating nucleotides GTP and UTP but not with GTP alone (Fig. 4, lanes 6 to 9). These results are similar to those obtained with rrnB P1, where the initiating nucleotides ATP and CTP, generating a 5-mer slipped transcript (9, 18), were required for a heparin-stable complex. At leuV, the presence of GTP and UTP would be predicted to result in formation of a template-directed 5-mer transcript.
The proximal UP element region of the leuV promoter (∼−40 to −47) was protected by RNAP in both the heparin-sensitive (−47 to −7) and heparin-stable (−47 to +20) complexes, although the region upstream of −47 was not protected. This protection pattern is consistent with stimulation of transcription by the sequence between −39 and −47 (7). Sites of enhanced DNase I cleavage occurred at positions −38 and −48 (Fig. 1 and 4), suggesting that RNAP may bend or distort the DNA at these sites. Similar enhanced cleavage was observed at position −38 in the rrnB P1 promoter (35).
Implications of these findings for the regulation of leuV promoter activity.
The results presented here are consistent with the model that multiple mechanisms, including activation by FIS and an NTP concentration-sensing mechanism, may contribute to regulation of leuV transcription. We find that leuV transcription is directly activated by FIS and that, like rrn P1 promoters, it responds to a feedback regulation signal generated by mutation of the fis gene. However, the response of the leuV promoter to the feedback signal may not be as great as that observed for rrnB P1, since tRNA1Leu levels are somewhat reduced in fis::kan strains (30). Consistent with this hypothesis, RNAP mutations that alter the NTP-sensing mechanism at rrnB P1 also affect leuV transcription but to a lesser degree than rrnB P1 (5). Other findings are also consistent with the possibility that the NTP-sensing mechanism described for rrnB P1 affects leuV transcription. These include the formation of unusual heparin-sensitive complexes of the leuV promoter with RNAP (Fig. 4), the moderate level of growth rate-dependent regulation of leuV promoter derivatives lacking a FIS site (6, 33), and the dependence of leuV transcription in vitro on the concentration of the initiating nucleotides GTP and CTP (some transcripts were observed to initiate with CTP [33]). Thus, leuV transcription most likely reflects multiple regulatory inputs (33).
Acknowledgments
This work was supported by grant GM37408 from the National Institutes of Health to R.L.G. and by grant GM50747 to W.M.H.
We thank Yanira O’Neill-Morales and Mike Bartlett for construction of leuV promoter-lacZ fusions.
REFERENCES
- 1.Appleman J A. Ph.D. thesis. University of Wisconsin—Madison; 1998. [Google Scholar]
- 2.Appleman J A, Ross W, Salomon J, Gourse R L. Activation of Escherichia colirRNA transcription by FIS during a growth cycle. J Bacteriol. 1998;180:1525–1532. doi: 10.1128/jb.180.6.1525-1532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball C A, Osuna R, Ferguson K C, Johnson R C. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett M S. Ph.D. thesis. University of Wisconsin—Madison; 1997. [Google Scholar]
- 5.Bartlett M S, Gaal T, Ross W, Gourse R L. RNA polymerase mutants that destabilize RNA polymerase-promoter complexes alter NTP-sensing by rrnP1 promoters. J Mol Biol. 1998;279:331–345. doi: 10.1006/jmbi.1998.1779. [DOI] [PubMed] [Google Scholar]
- 6.Bauer B F, Elford R M, Holmes W M. Mutagenesis and functional analysis of the Escherichia colitRNA(1Leu) promoter. Mol Microbiol. 1993;7:265–273. doi: 10.1111/j.1365-2958.1993.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 7.Bauer B F, Kar E G, Elford R M, Holmes W M. Sequence determinants for promoter strength in the leuV operon of Escherichia coli. Gene. 1988;63:123–134. doi: 10.1016/0378-1119(88)90551-3. [DOI] [PubMed] [Google Scholar]
- 8.Bokal A J, IV, Ross W, Gourse R L. The transcriptional activator protein FIS: DNA interactions and cooperative interactions with RNA polymerase at the Escherichia coli rrnBP1 promoter. J Mol Biol. 1995;245:197–207. doi: 10.1006/jmbi.1994.0016. [DOI] [PubMed] [Google Scholar]
- 9.Borukhov S, Sagitov V, Josaitis C A, Gourse R L, Goldfarb A. Two modes of transcription initiation in vitro at the rrnB P1 promoter of Escherichia coli. J Biol Chem. 1993;268:23477–23482. [PubMed] [Google Scholar]
- 10.Champagne N, Lapointe J. Influence of FIS on the transcription from closely spaced and non-overlapping divergent promoters for an aminoacyl-tRNA synthetase gene (gltX) and a tRNA operon (valU) in Escherichia coli. Mol Microbiol. 1998;27:1141–1156. doi: 10.1046/j.1365-2958.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Kirsebom L A, Nilsson L. Growth rate regulation of 4.5S RNA and M1 RNA the catalytic subunit of Escherichia coliRNase P. J Mol Biol. 1996;261:303–308. doi: 10.1006/jmbi.1996.0461. [DOI] [PubMed] [Google Scholar]
- 12.Duester G, Campen R K, Holmes W M. Nucleotide sequence of an Escherichia colitRNA (Leu 1) operon and identification of the transcription promoter signal. Nucleic Acids Res. 1981;9:2121–2139. doi: 10.1093/nar/9.9.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estrem S T, Gaal T, Ross W, Gourse R L. Identification of an UP element consensus sequence for bacterial promoters. Proc Natl Acad Sci USA. 1998;95:9761–9766. doi: 10.1073/pnas.95.17.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filutowicz M, Ross W, Wild J, Gourse R L. Involvement of Fis protein in replication of the Escherichia colichromosome. J Bacteriol. 1992;174:398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel S E, Johnson R C. The Fis protein: it’s not just for DNA inversion anymore. Mol Microbiol. 1992;6:3257–3265. doi: 10.1111/j.1365-2958.1992.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 16.Gaal T, Bartlett M S, Ross W, Turnbough C L, Jr, Gourse R L. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- 17.Gosink K K, Ross W, Leirmo S, Osuna R, Finkel S E, Johnson R C, Gourse R L. DNA binding and bending are necessary but not sufficient for Fis-dependent activation of rrnBP1. J Bacteriol. 1993;175:1580–1589. doi: 10.1128/jb.175.6.1580-1589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gourse R L. Visualization and quantitative analysis of complex formation between E. coliRNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988;16:9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourse R L, Nomura M. The level of rRNA, not tRNA, synthesis controls transcription of rRNA and tRNA operons in Escherichia coli. J Bacteriol. 1984;160:1022–1026. doi: 10.1128/jb.160.3.1022-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengen P N, Bartram S L, Stewart L E, Schneider T D. Information analysis of Fis binding sites. Nucleic Acids Res. 1997;25:4994–5002. doi: 10.1093/nar/25.24.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikemura T. Correlation between the abundance of Escherichia colitransfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981;146:1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- 22.Jinks-Robertson S, Gourse R L, Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983;33:865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- 23.Johnson R C, Ball C A, Pfeffer D, Simon M I. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci USA. 1988;85:3484–3488. doi: 10.1073/pnas.85.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josaitis C A, Gaal T, Ross W, Gourse R L. Sequences upstream of the −35 hexamer of rrnBP1 affect promoter strength and upstream activation. Biochim Biophys Acta. 1990;1050:307–311. doi: 10.1016/0167-4781(90)90186-6. [DOI] [PubMed] [Google Scholar]
- 25.Keener J, Nomura M. Regulation of ribosome synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1417–1431. [Google Scholar]
- 26.Martin R G, Rosner J L. Fis, an accessorial factor for transcriptional activation of the mar (multiple antibiotic resistance) promoter of Escherichia coliin the presence of the activator MarA, SoxS, or Rob. J Bacteriol. 1997;179:7410–7419. doi: 10.1128/jb.179.23.7410-7419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 29.Muskhelishvili G, Buckle M, Heumann H, Kahmann R, Travers A A. FIS activates sequential steps during transcription initiation at a stable RNA promoter. EMBO J. 1997;16:3655–3665. doi: 10.1093/emboj/16.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson L, Emilsson V. Factor for inversion stimulation-dependent growth rate regulation of individual tRNA species in Escherichia coli. J Biol Chem. 1994;269:9460–9465. [PubMed] [Google Scholar]
- 31.Nilsson L, Vanet A, Vijgenboom E, Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990;9:727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson L, Verbeek H, Vijgenboom E, van Drunen C, Vanet A, Bosch L. FIS-dependent trans activation of stable RNA operons of Escherichia coliunder various growth conditions. J Bacteriol. 1992;174:921–929. doi: 10.1128/jb.174.3.921-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pokholok, D. K., M. Redlak, C. L. Turnbough, Jr., S. Dylla, and W. M. Holmes. The Escherichia coli leuV tRNA promoter utilizes multiple mechanisms for responding to growth rate dependent or stringent control. Submitted for publication.
- 34.Rao L, Ross W, Appleman J A, Gaal T, Leirmo S, Schlax P J, Record M T, Jr, Gourse R L. Factor independent activation of rrnBP1. An “extended” promoter with an upstream element that dramatically increases promoter strength. J Mol Biol. 1994;235:1421–1435. doi: 10.1006/jmbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- 34a.Ross, W. Unpublished observations.
- 34b.Ross, W., and V. Newburn. Unpublished observations.
- 35.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 36.Ross W, Thompson J F, Newlands J T, Gourse R L. E. coliFis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowley K B, Elford R M, Roberts I, Holmes W M. In vivo regulatory responses of four Escherichia colioperons which encode leucyl-tRNAs. J Bacteriol. 1993;175:1309–1315. doi: 10.1128/jb.175.5.1309-1315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbeek H, Nilsson L, Bosch L. The mechanism of trans-activation of the Escherichia coli operon thrU(tufB) by the protein FIS. A model. Nucleic Acids Res. 1992;20:4077–4081. doi: 10.1093/nar/20.15.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinreich M D, Reznikoff W S. FIS plays a role in Tn5 and IS50transposition. J Bacteriol. 1992;174:4530–4537. doi: 10.1128/jb.174.14.4530-4537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Johnson R C. Fis activates the RpoS-dependent stationary-phase expression of proP in Escherichia coli. J Bacteriol. 1995;177:5222–5231. doi: 10.1128/jb.177.18.5222-5231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Johnson R C. Identification of genes negatively regulated by Fis: Fis and RpoS comodulate growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1995;177:938–947. doi: 10.1128/jb.177.4.938-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]