ABSTRACT
Free-living amoebae (FLA) are considered environmental pathogens and thus pose a public health threat. Their ubiquity in natural sources may magnify the potential severity of health outcomes in the future. However, less attention was given despite several probable public health risks that arise from the presence of pathogenic strains in the environment. Here, we provide epidemiological data based on investigations involving the distribution and occurrence of free-living amoebae in the Republic of the Philippines. This aims to connect data of fragmented studies of these organisms and provide potential roadmaps in FLA research in the country. The majority of the reviewed articles (n = 19) focused on characterization studies (36.8%; 7/19) while environmental isolation and isolation from biological samples had an equal frequency of 31.6% (6/19) each. There is a great disparity between the established ubiquity in environmental sources and the number of cases of FLA infections in the country. FLA-related research in the Philippines is still in its inceptive stage with several gaps to fill, which can be used to formulate policy briefs in the future regarding its isolation, identification, diagnosis, therapeutic management, and control of FLA infections in the country.
KEYWORDS: Acanthamoeba, epidemiology, free-living amoeba, Naegleria, Philippines
Introduction
Studies on free-living amoebae (FLA) have in recent decades gained traction in the field of protozoology and parasitology. Known for their ubiquity, FLAs have been isolated from various environmental matrices, such as soil, fresh and brackish water, wastewater, hot springs, dust, and others [1–6]. Aside from environmental sources, FLAs were also reported from cooling towers, air conditioning systems, bromeliad plants, dental water supplies, and are known to contaminate contact lens and lens fluids [7]. In addition, FLA can harbor pathogenic microorganisms within its cytoplasm, enabling them to persist and eventually be transmitted to a broad spectrum of biological hosts via multiple transmission routes. Due to this, FLA is dubbed as ‘Trojan horses’ of the microbial community [8,9]. Prokaryotes persisting within FLA have been called amoebae-resistant bacteria (ARB) by some researchers [10], while most recently, the collective term ‘endocytobionts’ has been used to describe the variety of microorganisms that multiply within an FLA host [11]. This term may be more appropriate considering that FLA, in general, can also internalize viruses [12], fungi [13], and protozoa [4,14,15].
Among the FLAs, the genera belonging to Naegleria, Acanthamoeba, Balamuthia, and Sappinia are considered by the World Health Organization (WHO) as medically important due to the morbidity or mortality reports in humans [16]. The route of cerebral infections for pathogenic FLA, in particular Naegleria spp., is initiated by the entry of the amoeba via the nasal cavity usually upon inhalation of contaminated water [17]. Upon reaching the brain via the cribriform plate, FLAs can mediate cytopathic effects resulting in the inflammation of the brain known as meningitis [18]. Depending on the FLA species or genotype and type of infection, conditions have been referred to as Primary Amoebic Meningoencephalitis (PAM) for Naegleria spp. infections [19], Granulomatous Amoebic Meningoencephalitis (GAE) for Acanthamoeba spp. infection [20], Balamuthia Amoebic Encephalitis (BAE) for Balamuthia mandrillaris infection, and Sappinia Amoebic Encephalitis (SAE) for Sappinia spp. infections [21]. Clinical conditions have almost equal morbidity to mortality ratio due to the rapid progression of the disease following the onset of symptoms [22]. Further, the symptoms presented by FLA-related meningitis mimic viral and bacterial forms, thus, making diagnosis and management of the disease challenging for clinicians and almost always leads to death [23]. Among the FLAs, pathogenic genotypes of Acanthamoeba spp. can inflict extra-cerebral infections like Acanthamoeba keratitis, and in rare cases, disseminated cutaneous infection [24–27].
The tenacity of FLA to proliferate in harsh ecosystems, and survive desiccation or disinfection make these organisms an important emerging waterborne, foodborne, and airborne protozoan pathogen of our time [11]. Considering that the overall global burden of FLA-related human infections is relatively low and infections from FLA are still rare [28,29], the almost equal proportion of morbidity to mortality ratio of reported cases, especially those causing a meningitis-like condition and the lack of effective therapeutic regimen, necessitates the consideration of FLAs as serious threats to human health.
In the Philippines, the worsening problem with freshwater pollution causes detrimental effects to both its utility for human use, consumption, and aquaculture productivity [30]. The uncontrolled pollution of freshwaters systems in the country traced to indiscriminate dumping of industrial and domestic wastewater to rivers and other freshwater systems, the rapidly changing and expanding urban landscape causing soil runoff, and challenges brought upon by extreme weather conditions and climate change are contributory factors for the spread of waterborne protozoan pathogens [31,32]. FLA research in the Philippines is still in its infancy. To date, there are only two cases of fatal FLA-related encephalitis reported in the country [33,34] while 12 cases of non-fatal eye infections were documented [3,35,36]. This data, however, contradicts the high FLA isolation rate from environmental reservoirs frequented by people as documented by several recent studies [37–44]. The present study aimed to provide data on the current status of FLA epidemiology in the Republic of the Philippines, discuss the implications of environmental factors to human cases, and provide an inceptive roadmap for the progression of FLA investigation in the country.
Methods
Literature search strategy
Studies involving the biological and environmental isolation of Naegleria spp., Acanthamoeba spp., Balamuthia spp., Sappinia spp., and Vermamoeba spp., in the Republic of the Philippines, were searched systematically in PubMed and Google Scholar databases without years restriction. The search terms used to obtain the relevant studies were: ‘Acanthamoeba’, ‘Naegleria’, ‘Balamuthia’, ‘Sappinia’, ‘Vermamoeba’, and ‘Philippines’. To maximize the number of included studies and to prevent missing any relevant studies, the reference lists of included studies were searched for literature that can be included. This systematic review followed preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [45].
Eligibility criteria
Titles of original research articles that reported FLA in the Philippines were screened. The inclusion criteria were: (1) studies with an abstract, (2) studies with full-text, and (3) studies written in the English or Filipino language. The exclusion criteria were: (1) Reviews and mini-reviews, (2) books and book chapters (3) method evaluation studies, and (4) intervention studies. Initial screening of retrieved records was performed by three authors (GDM, MFZH, and JP). Any disagreement with screening was resolved by consensus of three authors (GLM, EPM, and FRM). The final screening was performed by two authors (GDM and PK).
Data extraction
Data such as year of study, study site, sources or types of samples, clinical manifestations, isolated FLA, genotypes, prevalence, methods used for identification, title, and references were extracted from the included studies. Data extraction was performed by five authors (GDM, MFZH, GLM, EPM, and JP) and was checked by three authors (GM, FRM, and PK) for accuracy.
Results
Search results
A total of 40 articles were retrieved from two databases (20 from PubMed and 20 from Google scholar). After the screening of abstracts and titles and retrieval of full-texts, 19 duplicate records were removed. Two out of 21 articles were excluded for the following reasons: Review paper (1), and no full text (1). Finally, a total of 19 articles were included in the present review (Figure 1).
Figure 1.
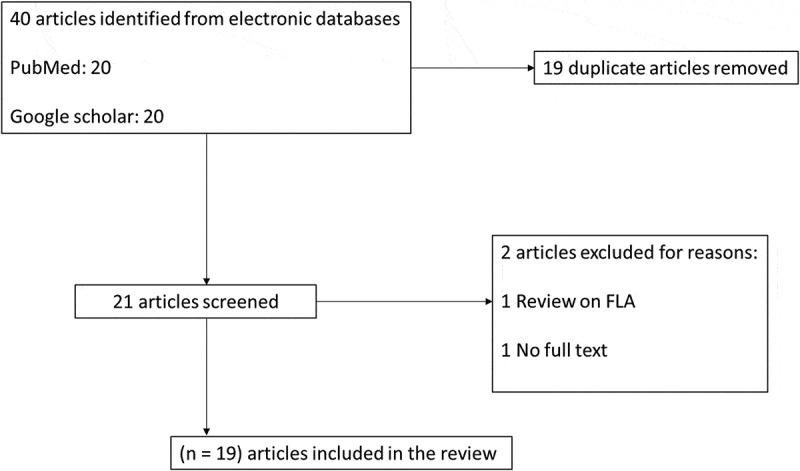
Flow diagram of article search, screening, and selection.
Characteristics of included articles
Among the included studies, 31.6% (6/19) focused on the isolation of FLA from environmental sources, such as soil, rivers, swimming pools, pond waters, lakes, and major water reservoirs in the Philippines. Biological samples composed 31.6% (6/19) of the included studies where 83.3% (5/6) reported FLA from clinical (human) samples while 16.7% (1/6) reported FLA from non-clinical samples, that is, fishes. FLA characterization comprised 36.8% (7/19) of the included studies and focused on the investigation of pathogenicity, thermo-tolerance, and chlorine resistance (Figure 2). Geographically, the majority of FLA detection was concentrated in the Island of Luzon where two genera of Acanthamoeba and Naegleria were identified from environmental sources, while only Acanthamoeba spp. was reported from the two other major Islands of Visayas and Mindanao (Figure 3 and Table 1). Only the genus of Vermamoeba, previously known as Hartmanella, was reported from a non-clinical sample, that is, fish, while the genera of Naegleria and Acanthamoeba have been implicated in human infection cases in the country (Table 2).
Figure 2.
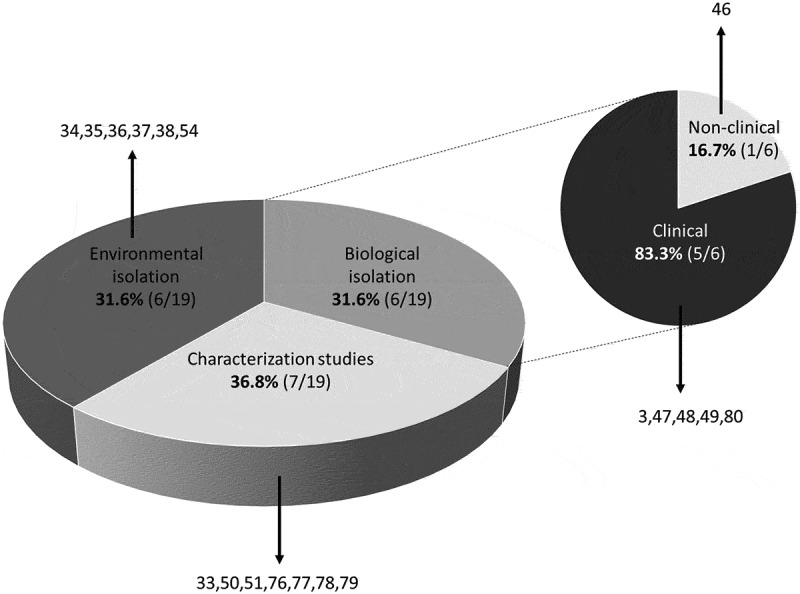
Distribution of FLA studies in the Philippines according to study type. Numbers refer to included studies in the reference list.
Figure 3.
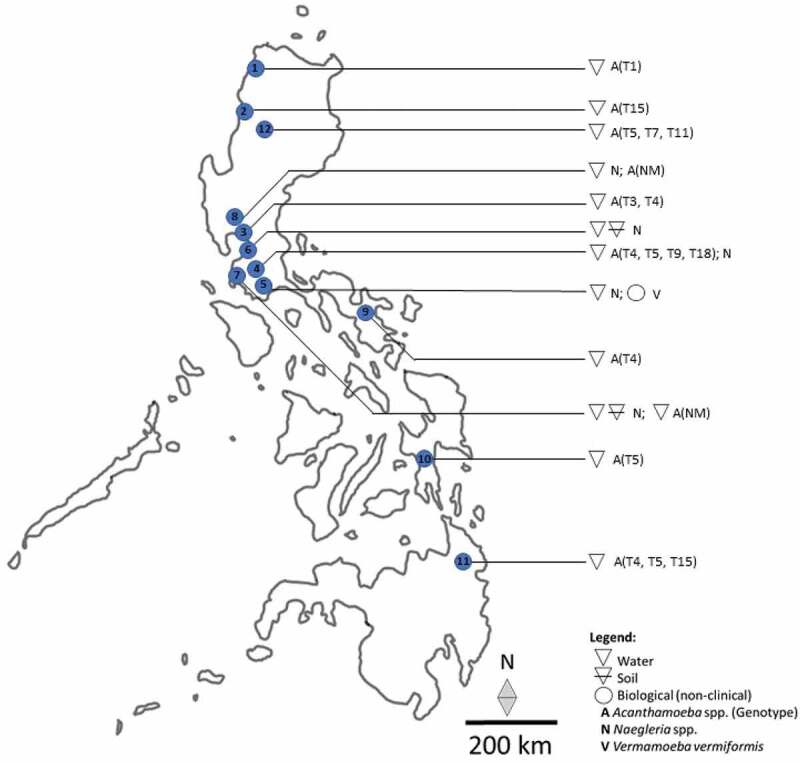
Geographical distribution of FLA from environmental and biological (non-clinical) samples in the Philippines. 1 Ilocos Norte; 2 Ilocos Sur; 3 Bulacan; 4 Laguna; 5 Batangas; 6 Metro Manila; 7 Cavite; 8 Pampanga; 9 Camarines Sur; 10 Leyte; 11 Agusan del Sur; 12 Cordillera Autonomous Region; A Acanthamoeba; (Genotype); N Naegleria; V Vermamoeba; NM genotype not mentioned. Note that FLA isolation in humans could not be mapped as the point sources were either not mentioned or speculative only.
Table 1.
Environmental isolates of FLA in the Philippines based on molecular identification.
| Island | Regions | Province/City | Sources | Species | Genotypes | References |
|---|---|---|---|---|---|---|
| Luzon | Region 1 | Ilocos Sur | Pond water | Acanthamoeba spp. | T15 | [40] |
| Luzon | Region 1 | Ilocos Norte | Lake surface water | Acanthamoeba spp. | T1 | [40] |
| Luzon | Region 3 | Bulacan | Reservoir surface water | Acanthamoeba graffini | T3 | [39] |
| Luzon | Region 3 | Bulacan | Reservoir surface water | Acanthamoeba castellanii | T4 | [39] |
| Luzon | Region 4a | Laguna | Lake surface water | Acanthamoeba spp. | T4 | [42] |
| Luzon | Region 4a | Laguna | Lake surface water | Acanthamoeba spp. | T5 | [42] |
| Luzon | Region 4a | Laguna | Lake surface water | Acanthamoeba spp. | T9 | [42] |
| Luzon | Region 4a | Laguna | Lake surface water | Acanthamoeba spp. | T18 | [42] |
| Luzon | NCR | Manila | Lake surface water | N. australiensis | NA | [44] |
| Luzon | Regions 3 & 4a | Cavite, Batangas, Pampanga | Lake surface water, Tap water, bottled water | Naegleria spp. | NA | [38] |
| Luzon | NCR, Region 4a | Manila, Cavite, Laguna | Soil and water | N. philippinensis | NA | [37] |
| Luzon | NCR, Regions 3 & 4a | Manila, Cavite, Batangas, Pampanga | River, Swimming pool, Pond water, Lake surface water | Acanthamoeba spp. | NM | [38] |
| Luzon | Region 5 | Camarines Sur | Lake surface water | Acanthamoeba spp. | T4 | [41] |
| Luzon | CAR | Ifugao | Reservoir surface water | Acanthamoeba spp. | T5 | [39] |
| Luzon | CAR | Ifugao | Reservoir surface water | Acanthamoeba spp. | T7 | [39] |
| Luzon | CAR | Ifugao | Reservoir surface water | Acanthamoeba spp. | T11 | [39] |
| Visayas | Region 8 | Leyte | Lake surface water | Acanthamoeba spp. | T5 | [40] |
| Mindanao | Region 13 | Agusan del Sur | Lake surface water | Acanthamoeba spp. | T4, T5, T15 | [40] |
NA Not applicable; NM Not mentioned; NCR National Capital Region; CAR Cordillera Administrative Region.
Table 2.
FLA isolated from biological samples in the Philippines.
| Year of isolation | FLAs | Genotypes | Sample | Clinical manifestations | Prevalence (%) |
Methods | Suspected source | References |
|---|---|---|---|---|---|---|---|---|
| Non-clinical (animal) sample | ||||||||
| 2017 |
Vermamoeba vermiformis |
NA |
Fish intestine |
NA |
100% (1/1) |
Culture/Microscopy/PCR/Sequencing |
Freshwater |
[43] |
| Clinical (human) samples | ||||||||
| 1984 | Naegleria philippinensis | NA | CSF | PAM | 100% (1/1) | Culture/Microscopy | Thermally polluted stream | [34] |
| 1999 | Acanthamoeba spp. | NM | CSF | AK | 100% (1/1) | NM | NM | [33] |
| 2009 | Acanthamoeba spp. | NM | Corneal scraping | AK | 100% (3/3) | Microscopy | NM | [35] |
| 2014 | Acanthamoeba spp. | T4 | Corneal scraping | AK | 100% (1/1) | PCR | Tap water | [36] |
| 2014 | Acanthamoeba spp. | T4 and T5 | Nasal swab | None | 4.44% (8/180) | PCR/Sequencing | Garbage, soil, and dust | [3] |
NA not applicable; NM not mentioned; PAM primary amoebic encephalitis; AK Acanthamoeba Keratitis; PCR polymerase chain reaction.
The predominant Acanthamoeba genotype from environmental sources in descending order was: T4 and T5 (four reports each) > T15 (two reports) > T1, T3, T7, T9, T11, and T18 (one report each). Naegleria was reported in three studies where Naegleria spp., N. australiensis, and N. philippinensis were isolated from water samples while N. philippinensis was also isolated from soil and water samples.
For clinical (human) samples, two genera of FLA, Acanthamoeba, and Naegleria were identified with Acanthamoeba having a total of 13 cases while Naegleria has only one case reported. The point sources of clinical (human) isolates could not be mapped as the detection of FLA, that is, Acanthamoeba and Naegleria were all performed in the city of Manila with the suspected source of FLA either not mentioned or being speculative only (Table 2). The predominant genotype of Acanthamoeba isolated from human samples was T4 (two reports) followed by T5 (one report) while N. philippinensis was only reported once from a PAM patient (Table 2).
No Philippine reports on Balamuthia and Sappinia were retrieved.
Discussion
Distribution and diversity of FLA in the Philippines
Isolation and identification of FLA in different environmental sources, especially those considered pathogenic, at present, is rare in the Philippines where matrices, such as soil, water sediments, biofilms, dust, and air, require exploration. It is hypothesized by the authors that the archipelagic profile and abundance of freshwater systems in the Philippines support a wide spectrum of FLA biodiversity that remains unexplored. This warrants the promotion of environmental surveys for FLA biodiversity in the country. Although there are defined surveillance studies conducted for other parasites like soil-transmitted helminths [46,47], there appears to be none for FLA despite the molecular identification of Acanthamoeba, Naegleria, and Vermamoeba from the soil, freshwater, and fishes in the country [37–43]. Surveillance studies are important not only in identifying the existence of pathogenic species and genotypes but also in defining multiple transmission routes involving the environment, animals, and humans. This in turn will lead to the formulation of strategies in the mitigation and management of FLA infections. National surveillance studies for FLA exist in countries like the United States, which enables the health-care sector to monitor outbreaks [48]. In Luzon Island, water reservoirs that are vital freshwater sources have demonstrated the presence of Acanthamoeba genotypes T3, T4, T5, T7, and T11 [39] while Naegleria spp. were reported from environmental sources as well as bottled water [37,38,44]. In the Visayas Island, Lakes Danao, and Bito in Ormoc, Leyte were positive with Acanthamoeba genotype T5 while three genotypes T4, T5, and T15 were isolated in Lake Mainit in the Island of Mindanao [40].
FLA infections in the Philippines
Despite the reported diversity of FLA and pathogenic genotypes in various environmental sources in the country (Figure 3), reports on morbidity and/or mortality cases of FLA-related infections remain largely unknown. Acanthamoeba keratitis (AK) was first reported in the country in 1992 [33]. Between 2002 and 2004, three more cases of AK were reported [35]. In 2009, a 76-years-old male reported pain, redness, and visual impairment of the right eye where molecular testing confirmed Acanthamoeba genotype T4 [36]. It is important to note that all these AK cases involved non-contact lens wearers and that the latter case used tap water to cleanse the face before the onset of symptoms. Surprisingly, in the study of Cruz and Rivera (2014), 4.4% (8/180) of nasal swabs from asymptomatic volunteers were positive with Acanthamoeba spp. T4 and T5 [3]. The proposed transmission route was obviously from the chronic exposure of the volunteers to garbage, soil, and dust as part of their everyday activity. In this case, it is interesting to speculate on the potential health outcomes in the event of depression or suppression of the immune system of the Acanthamoeba-positive volunteers. Also, it opens up perspectives into the health hazards on FLA-related infections faced by high-exposure groups of laborers working in unsanitary conditions and environments. Also, the predominance of Acanthamoeba genotype T4 and T5 in local human cases was potentially influenced by the propensity of the same genotypes reported in the highest frequencies in local environmental sources.
To date, there is only one report of Naegleria causing PAM infection in the Philippines [34]. This 1984 isolate was later identified as a new species of Naegleria and was given the name N. philippinensis after a series of molecular characterization and pathogenicity assays and was suspected to have been contracted from a thermally polluted stream [49]. Although N. philippinensis was not reported from environmental sources in the Philippines, the isolation of N. australiensis from a Lake surface water in the National Capital Region (NCR) poses a health threat to the general public [44]. In addition, the isolation of Vermamoeba vermiformis from a freshwater fish (Oreochromis niloticus) in a volcanic lake (Lake Taal) in Luzon Island [43] strengthened the perspective of the role of fishes as biological reservoirs for FLAs [50–53].
Methods of isolation and identification of FLA
From the first local isolation and characterization of Naegleria and Acanthamoeba from human samples [33,34], the use of light microscopy in the detection and description of FLA has proven valuable in aiding clinicians in the course of disease management. However, with the advent of new techniques and the introduction of molecular testing, there was an improvement in the specificity and accuracy of detection and identification of FLAs [3,36].
In the case of point source isolation from aquatic systems, modifications in sample collection have been introduced for the convenient, fast, and cost-effective detection of waterborne protozoan pathogens [54,55]. From obtaining at least 10 L water samples [37,38], the introduction of low-volume water sampling (50 mL for microcopy and 1000 mL for molecular assay) and manual filtration using a 1.2 µm pore-size filter has proven reliable for detecting waterborne protozoan pathogens from surface water, sediments, and substrate-associated biofilms [41,42,44,56,57].
Previous identification of FLAs was solely based on phenotypic characteristics with the aid of light microscopy and the use of an enflagellation test to differentiate Naegleria from Acanthamoeba [33,34]. However, some studies have demonstrated the limitations of the enflagellation test [58]. The use of polymerase-chain reaction (PCR) for the detection and identification of FLAs enabled researchers to accurately identify interspecies differences [39–42]. Also, in-vitro analysis such as osmotolerance and thermotolerance assay, encystment rate, scanning electron microscopy, and molecular techniques in combination helped to further characterize the species, genotype, and pathogenicity of isolates [40]. Regardless of the identification technique, culture was key in the successful isolation of FLAs from environmental and biological samples.
The continuous evolution of FLAs through their adaptability to different environmental conditions warrants its exploration in a wide variety of environmental, infrastructural, biological, and clinical regimes, as with the recent isolation of Acanthamoeba from seawater and hospital ward dust [59] and from the urinary tract of humans [60]. These are suggestive of the role of FLAs as latent carriers for resistant bacteria and biofilm formation as well as the introduction of FLA stages during invasive and operational procedures.
Current status of FLA study in the Philippines
FLA-related research in the Philippines is still in its inceptive stage. To date, only a fraction of the 101 lakes scattered across the country [61] has been explored for FLA biodiversity. Although the first nationwide study on FLA diversity was successful in defining previously unreported species and genotypes [40], more research is necessary to further establish its biodiversity from a wide range of environmental, infrastructural, and biological regimes.
The presence and proliferation of FLA in aquatic systems may, in part, be due to rapid urbanization. The increased disturbance caused by informal human settlements near lakes and other freshwater bodies thereby increases domestic waste pollution, soil runoff, human and animal waste pollution, and thermal pollution from industries that dump wastes directly to freshwater bodies [44,62]. Also, the increasing temperature of waterbodies due to climate change potentially enhances the thermotolerance of the FLA fauna [40]. Further, meteorological events like flooding facilitate the widespread dispersal of waterborne protozoan pathogens including FLA from terrestrial to aquatic environments and back [55].
Despite the environmental ubiquity of FLA, FLA–related infections in the Philippines remain low. In the last 36 years, only 13 cases of Acanthamoeba and 1 case of Naegleria infection were documented in the country, two of which led to fatal outcomes [33,34]. The current hygienic status of public hospitals and health-care centers in the country is appalling [63]. The poor distribution and accessibility of health-care facilities especially in rural settings, the general lack of knowledge on FLA, the limited use of existent international protocols for identification of FLA-related infections, and resource limitations may be contributory factors for the rarity of reports on FLA-related infections or the lack of it in the Philippines. This was observed in a five-year report (2016–2020) of the Epidemiology Bureau on the distribution of Acute Meningitis-Encephalitis Syndrome (AMES) cases in the Philippines, which shows a very high number of unclassified encephalitis cases from different regions of the country (see supplemental data). Similarly, this was observed in the United States where the high occurrence of unspecified causes of meningitis was observed [64]. It is also important to consider the possibility of an acquired immunity or tolerance among subpopulations to certain strains of potentially pathogenic FLAs [3]. Some studies have suggested the presence of mammalian antibodies against AK causing Acanthamoeba genotypes and those that cause fatal brain infections [65,66]. Although there is no direct evidence on this, it should be taken into perspective. In-vitro pathogenicity assays demonstrated variations in FLA pathogenicity and suggest that pathogenic and nonpathogenic genotypes exist within previously established pathogenic groups [40,67]. These factors may rationalize the uneven proportion of morbidity and/or mortality cases to high isolation rates of FLAs from environmental sources.
The recent isolation of Vermamoeba vermiformis from the intestine of an edible freshwater fish (O. niloticus) opened a new direction in FLA studies in the Philippines [43]. Although no evidence of pathogenicity to its fish host has been documented, this FLA is capable of hosting endocytobionts/endosymbionts within its cytoplasm, which may induce pathogenicity to humans [68,69]. This is suggestive of the role of fishes as vectors or biological reservoirs for FLAs to proliferate and persist in aquatic environments and potentially infect fishermen, fish handlers, and those consuming raw fish, in particular, small fishes that are not eviscerated and eaten whole. Acanthamoeba and Naegleria have demonstrated propensity in aquatic sources in the Philippines. With evidence of the pathogenicity of these isolates [40], there is a potential risk of infection for those in direct contact with contaminated water sources. The proliferation of these pathogenic FLAs in various environmental settings can be mitigated by focusing on the implementation of control efforts on different factors mentioned previously.
Challenges and inceptive roadmap for FLA research in the Philippines
The Philippines is an archipelago of more than 7,100 Islands rich in freshwater ecosystems hypothesized to be teeming with FLA biodiversity and potentially pathogenic species and genotypes. However, a case definition for FLA-related or suspected infection is not included in the country’s Acute Meningitis-Encephalitis Syndrome (AMES) surveillance program of the Epidemiology Bureau Public Health Surveillance Division of the Department of Health. Although a nationwide study on FLA distribution was recently conducted in the Philippines [40], a plethora of study sites and biological sources remains unexplored.
A 2021 Thailand study surveyed public freshwater sources across 28 provinces and offered a treasure trove of information in the development of FLA study in a country. It demonstrated the value of defining FLA distribution and biodiversity. The survey of 100 sources demonstrated the isolation of nine previously reported (T2/6, T3-T5, T9, T11, T12, and T18) and one novel genotype (T23) proposed to be Acanthamoeba bangkokensis. Also, a study of this scale and depth established the following: (1) the predominant species or genotype, (2) the reproductive success of FLA in diverse ecological niche, (3) perspectives on cryptic subtypes within defined genotypes, (4) potential discovery of novel species/genotypes, (5) evolution, (6) data on genetic and morphological characteristics useful for identification, and (7) the establishment of a library of sequences and isolates for in-vivo and in-vitro studies [70]. Such information is valuable for widespread information dissemination to the public, scientific, and medical community.
Success in the progression of FLA study in the Philippines entails the exploration of a wide variety of sources, which aside from the natural environment, also includes infrastructure/gray structures [59], unexplored or less explored sample matrices like sediments and biofilms [71,72], and anatomic sites/clinical regimes not previously thought to be colonized by FLAs [60].
Collaborations with local government units as well as members of the local communities for information dissemination on safety protocols against contracting FLA infections like wearing goggles and nose clips should be strongly advocated among fishermen and other stakeholders. This may also help in building a local industry on locally made goggles and nose clips using recyclable materials that will not only aid in health promotion but a sustainable livelihood among locals. Likewise, establishing the distribution and identity of FLAs and its possible endosymbionts [73] in various sources will help to form perspectives on potential outcomes for the general human population as well as its subpopulations, primarily, immunocompromised individuals who are at the highest risk of developing progressive and fatal infections [74].
The lack of standardized management and effective drug for FLA-related human infections necessitates drug discovery. The Philippines is rich in natural products [75] that may show potential anti-protozoan activity against FLA-related morbidity. From a public health perspective, a multi/trans-disciplinary approach should be employed in the mitigation of FLA human infections, particularly in water-related occupations, tourism, and recreational sectors.
To the best of our knowledge, no report in FLA-related infections was ever included in the AMES national case reports of the DOH (see supplemental data). Also, no training and funding has been allocated to the identification of FLA in the Food and Water-Borne Disease Strategic plan for 2019–2023 in the country. This is strong evidence that FLA-related pathophysiology is either still relatively unknown or neglected and is a gaping hole in the health research agenda in the Philippines. The outcomes of the present review necessitate that a case definition for FLA-related infections be included in the country’s AMES surveillance program. Finally, studies on the potential of FLA to become a bioindicator of water pollution/contamination have been proposed by recent studies in selected lakes in the country [76 77 78 79 80 81].
Conclusions
FLA studies in the Philippines are fragmented and are still in their inceptive stage. Gathered literature highly suggests a severe lack of FLA research in the country. These gaps, however, can be translated to promising FLA-related studies following perspectives for a national FLA research road map. Despite the ubiquity of FLA in environmental sources in the country, documented human cases are rarely reported. The rarity of cases is most likely a result of underreporting due to the absence of a national case definition, lack of health facility, and/or the lack of diagnostic capacity to distinguish FLA infections from bacterial and viral-induced pathologies. FLA-related infections are a public health threat in the Philippines and demand further studies in identifying its epidemiologic aspect in both clinical cases and environmental sources to define the morbidity/mortality rates of this neglected parasitic infection.
Acknowledgments
The authors would like to thank Kimberly Anne Lipat, Vera Anne Perez, Crisher Wency Jaca, Daryl Caliwliw, Aiman Arabain, Seannon King Sebastian, and Kenneth Rodriguez for assisting in the collation of the literature. The Department of Health Epidemiological Bureau for providing the data for AMES.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Lasjerdi Z, Niyyati M, Haghighi A, et al. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res. 2011;109(3):575–580. [DOI] [PubMed] [Google Scholar]
- [2].Trabelsi H, Dendana F, Sellami A, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol. 2012;60(6):399–405. [DOI] [PubMed] [Google Scholar]
- [3].Cruz A, and Rivera W.. Genotype analysis of Acanthamoeba isolated from human nasal swabs in the Philippines. Asian Pac J Trop Med. 2014;7:S74–S78. [DOI] [PubMed] [Google Scholar]
- [4].Balczun C, Scheid PL.. Free-living amoebae as hosts for and vectors of intracellular microorganisms with public health significance. Virus. 2017;9(4):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gabriel S, Khan NA, Siddiqui R. Occurrence of free-living amoebae (Acanthamoeba, Balamuthia, Naegleria) in water samples in Peninsular Malaysia. J Water Health. 2019;17(1):160–171. [DOI] [PubMed] [Google Scholar]
- [6].Fabros MRL, Diesta XRS, Oronan JA, et al. Current report on the prevalence of free-living amoebae (FLA) in natural hot springs: a systematic review. J Water Health. 2021;19(4):563–574. [DOI] [PubMed] [Google Scholar]
- [7].Vaerewijck MJ, Baré J, Lambrecht E, et al. Interactions of foodborne pathogens with free-living protozoa: potential consequences for food safety. Compre Rev Food Sci Food Safe. 2014;13(5):924–944. [Google Scholar]
- [8].Barker J, Lambert P, Brown M. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1992;61(8):3503–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khan N. Acanthamoeba, biology and pathogenesis. Norfolk: Caister Academic Press; 2009. [Google Scholar]
- [10].Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17(2):413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scheid P. Free-living amoebae as human parasites and hosts for pathogenic microorganisms. Proceedings. 2018;2(11):692. [Google Scholar]
- [12].Gaëlle Reteno D, Benamar S, Khalil JB, et al. Faustovirus, an asfarvirus-related new lineage of giant viruses infecting amoebae. J Virol. 2015;89(13):6585–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maisonneuve E, Cateau E, and Kaaki S, et al. Vermamoeba vermiformis – Aspergillus fumigatus relationships and comparison with other phagocytic cells. Parasitol Res. 2016;115(11):4097–4105. [DOI] [PubMed] [Google Scholar]
- [14].Winiecka‐Krusnell J, Dellacasa‐Lindberg I, Dubey JP, et al. Toxoplasma gondii: uptake and survival of oocysts in free living amoebae. Exp Parasitol. 2009;121:124–131. [DOI] [PubMed] [Google Scholar]
- [15].Scheid P, Schwarzenberger R. Free living amoebae as vectors of cryptosporidia. Parasitol Res. 2011;109:499–504. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization . Guidelines for safe recreationalWater. Geneva: Switzerland: Coastal andFresh Waters WHO; 2003. p. 1. [Google Scholar]
- [17].Martinez A, Visvesvara G. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7(1):583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dando SJ, Mackay-Sim A, Norton R, et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 2014;27(4):691–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother. 2015;59(11):6677–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Salameh A, Bello N, Becker J, et al. Fatal granulomatous amoebic encephalitis caused by acanthamoeba in a patient with kidney transplant: a case report. Open Forum Infect Dis. 2015;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qvarnstrom Y, Silva AD, Schuster F, et al. Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J Infect Dis. 2009;199(8):1139–1142. [DOI] [PubMed] [Google Scholar]
- [22].Matanock A, Mehal JM, Liu L, et al. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerging Infect Dis. 2018;24(1):162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma P, Visvesvara GS, Martinez AJ, et al. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990;12(3):490–513. [DOI] [PubMed] [Google Scholar]
- [24].Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paltiel M, Powell E, Lynch J, et al. Disseminated cutaneous acanthamebiasis: a case report and review of the literature. Cutis. 2004;73(4):241–248. [PubMed] [Google Scholar]
- [26].Walia R, Montoya JG, Visvesvara GC, et al. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl Infect Dis. 2007;9(1):51–54. [DOI] [PubMed] [Google Scholar]
- [27].Morrison AO, Morris R, Shannon A, et al. Disseminated Acanthamoeba infection presenting with cutaneous lesions in an immunocompromised patient: a case report. Am J of Clin Pathol. 2016;145(2):266–270. [DOI] [PubMed] [Google Scholar]
- [28].Rocha-Azevedo B, Tanowitz HB, and Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis. 2009;2009:251406 doi: 10.1155/2009/251406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Król-Turmińska K, Olender A. Human infections caused by free-living amoebae. Ann Agric Environ Med. 2017;24(2):254–260. [DOI] [PubMed] [Google Scholar]
- [30].Andrews G. Resolving the water pollution crisis in the Philippines: the implications of water pollution on public health and the economy. Pepperdine Policy Rev. 2018;10:2. [Google Scholar]
- [31].Ahmed SA, Flórez MG, Karanis P. The impact of water crises and climate changes on the transmission of protozoan parasites in Africa. Pathog Glob Health. 2018;112(6):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Semenza JC. Cascading risks of waterborne diseases from climate change. Nat Immunol. 2020;21(5):484–487. [DOI] [PubMed] [Google Scholar]
- [33].Lagmay JP, Matias RR, Natividad FF, et al. Cytopathogenicity of Acanthamoeba isolates on rat glial C6 cell line. Southeast Asian. J Trop Med Public Health. 1999;30(4):670–677. [PubMed] [Google Scholar]
- [34].Matias RR, Enriquez GL, and Natividad FF. Cell biology of the Philippine amoeboflagellate, Naegleria philippinensis. Trans Nat Acad Sci Tech. 1991;13:439–444. [Google Scholar]
- [35].Agahan ALD, Lim RBS, Valenton MJ. Successful treatment of Acanthamoeba keratitis without anti-amoebic agents. Ann Acad Med Singap. 2009;38:175–176. [PubMed] [Google Scholar]
- [36].Buerano CC, Trinidad AD, Fajardo LSN, et al. Isolation of Acanthamoeba genotype T4 from a non-contact lens wearer from the Philippines. Trop Med Health. 2014;42(4):145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fontanilla IKC, Matias RH, Enriquez GL. Morphological and physiological characteristics of Philippine Naegleria isolates. Phil J Sci. 2001;130(2):103–109. [Google Scholar]
- [38].Onichandran S, Kumar T, Salibay CC, et al. Waterborne parasites: a current status from the Philippines. Parasit Vectors. 2014;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Milanez G, Masangkay F, Hapan MF, et al. Detection of Acanthamoeba spp. in two major water reservoirs in the Philippines. J Water Health. 2020;18(2):118–126. [DOI] [PubMed] [Google Scholar]
- [40].Milanez GD, Masangkay FR, and Scheid P, et al. Acanthamoeba species isolated from Philippine freshwater systems: epidemiological and molecular aspects. Parasitol Res. 2020;119:3755–3761. [DOI] [PubMed] [Google Scholar]
- [41].Hagosojos B, Masangkay F, Fernandez J, et al. Molecular identification of Acanthamoeba sp. in Lake Buhi, Philippines. Ann Parasitol. 2020;66(1):111–114. [PubMed] [Google Scholar]
- [42].Ballares L, Masangkay F, Dionisio J, et al. Molecular detection of Acanthamoeba spp. in seven crater lakes of Laguna, Philippines. J Water Health. 2020;18(5):776–784. [DOI] [PubMed] [Google Scholar]
- [43].Milanez G, Masangkay F, Thomas R, et al. Molecular identification of Vermamoeba vermiformis from freshwater fish in lake Taal, Philippines. Exp Parasitol. 2017;183:201–206. [DOI] [PubMed] [Google Scholar]
- [44].Milanez GD, Masangkay FR, Somsak V, et al. Occurrence and the first report of Naegleria australiensis presence in a major lake in the Philippines. J Water Health. 2019;17(4):647–653. [DOI] [PubMed] [Google Scholar]
- [45].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Belizario VY, De Leon WU, Lumampao YF, et al. Sentinel surveillance of soil-transmitted helminthiasis in selected local government units in the Philippines. Asia Pac J Public Health. 2009;21(1):26–42. [DOI] [PubMed] [Google Scholar]
- [47].Leonardo L, Rivera P, and Saniel O, et al. A national baseline prevalence survey of schistosomiasis in the Philippines using stratified two-step systematic cluster sampling design. J Trop Med. 2012;2012:936128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc. 2014;4(4):68–75. [DOI] [PubMed] [Google Scholar]
- [49].Simeon EC, Natividad FF, Enriquez GL. The pathogenicity of a Philippine isolate of Naegleria sp. in mice: effects of dose levels and routes of infection. Southeast Asian. J Trop Med Public Health. 1990;21:598–606. [PubMed] [Google Scholar]
- [50].De Jonckheere JF. Occurrence of Naegleria and Acanthamoeba in aquaria. Appl Environ Microbiol. 1979;38(38):590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Franke ED, Mackiewicz JS. Isolation of Acanthamoeba and Naegleria from intestinal content of freshwater fishes and their potential pathogenicity. J Parasitol. 1982;68:164–166. [PubMed] [Google Scholar]
- [52].Dykova I, Machackova B, Peckova H. Amoebae isolated from organs of farmed tilapias Oreochromis niloticus. F Parasitol. 1997;44(2):81–90. [Google Scholar]
- [53].Laoprasert T, Nualchan T, Chinabut S, et al. Amoebae isolated from fish and rearing water at Oscar asronatus ocellatus farm. Aquacult Sci. 2009;57:265–270. [Google Scholar]
- [54].Masangkay FR, Milanez GD, Chua NER, et al. Waterborne coccidians in Philippine water sheds: a national inceptive study. Asian J Biol Life Sci. 2016;5:112–119. [Google Scholar]
- [55].Masangkay FR, Milanez GD, and Somsak V, et al. Multi-spatial contamination of environmental aquatic matrices with Cryptosporidium: a climate, health, and regulatory framework for the Philippines. Environ Sci Eur. 2020;32(32):121. [Google Scholar]
- [56].Masangkay FR, Milanez GD, Tsiami A, et al. Waterborne protozoan pathogens in environmental aquatic biofilms: implications for water quality assessment strategies. Environ Poll. 2020;259:113903. [DOI] [PubMed] [Google Scholar]
- [57].Dela Peña LBRO, Vejano MRA, and Rivera WL. Molecular surveillance of Cryptosporidium spp. for microbial source tracking of fecal contamination in laguna lake, Philippines. J Water Health. 2021;19(3):534–544. [DOI] [PubMed] [Google Scholar]
- [58].De Jonckheere JF, Brown S, and Dobson PJ, et al. The amoeba to flagellate transformation test is not reliable for the diagnosis of the genus Naegleria. Description of three new Naegleria spp. Protist. 2001;152(152):115–121. [DOI] [PubMed] [Google Scholar]
- [59].Mahmoudi MR, Zebardast N, Masangkay FR, et al. Detection of potentially pathogenic free-living amoebae from the caspian sea and hospital ward dust of teaching hospitals in Guilan, Iran. J Water Health. 2021;19(2):278–287. [DOI] [PubMed] [Google Scholar]
- [60].Saberi R, Fakhar M, Makhlough A, et al. First evidence for colonizing of Acanthamoeba T4 genotype in urinary tracts of patients with recurrent urinary tract infections. Acta Parasitol. 2021;66(3):932–937. [DOI] [PubMed] [Google Scholar]
- [61].Guerrero III RD. Philippine lakes: status and strategies for sustainable development. Trans Nat Acad Sci Tech. 1999;21:278–286. [Google Scholar]
- [62].Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicol. 2004;198:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dela Cruz RZ, Ortega-Dela Cruz RA. Management of public healthcare facilities in the Philippines: issues and concerns. British J Health Man. 2019;25:10. [Google Scholar]
- [64].Matanock A, Mehal J, Liu L, et al. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg Infect Dis. 2018;24(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Clarke DW, Niederkorn JY. The immunobiology of Acanthamoeba keratitis. Microbes Infect. 2006;8:1400–1405. [DOI] [PubMed] [Google Scholar]
- [66].Hammersmith KM. (2006). Diagnosis and management of Acanthamoeba keratitis. Curr Opin Ophthal. 2006;17(4):327–331. [DOI] [PubMed] [Google Scholar]
- [67].Howe DK, Vodkin MH, Novak RJ, et al. Identification of two genetic markers that distinguish pathogenic and non-pathogenic strains of Acanthamoeba spp. Parasitol Res. 1997;83:345–348. [DOI] [PubMed] [Google Scholar]
- [68].Delafont V, Rodier M, Maisonneuve E, et al. Vermamoeba vermiformis: a free living amoeba of interest. Microb Ecol. 2018;76:991–1001. [DOI] [PubMed] [Google Scholar]
- [69].Masangkay F, Milanez G, and Karanis P, et al. Vermamoeba vermiformis – global trend and future perspective. Encycl Environ Health. 2019;2019:356–366. [Google Scholar]
- [70].Putaprontip C, Kuamsab N, Nuprasert W, et al. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. Sci Rep. 2021;11(1):17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].De Joncheere JF. Molecular identification of free-living amoebae of the Vahlkampfiidae and Acanthamoebidae isolated in Arizona (USA). Eur J Protistol. 2007;43:9–15. [DOI] [PubMed] [Google Scholar]
- [72].Martin H, Borlee GI, Wheat WH, et al. Busting biofilms: free-living amoebae disrupt preformed methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium bovis biofilms. Microbiol (Reading). 2020;166:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gomes TS, Vaccaro L, Magnet A, et al. Presence and interaction of free-living amoebae and amoeba-resisting bacteria in water from drinking water treatment plants. Sci Total Environ. 2020;719:137080. [DOI] [PubMed] [Google Scholar]
- [74].Damhorst GL, Watts A, and Hernandez-Romieu A, et al. Acanthamoeba castellanii encephalitis in a patient with AIDS: a case report and literature review. Lancet Infect Dis 2021;22:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Carag HM, and Buot IE Jr. A checklist of the orders and families of medicinal plants in the Philippines. Techn J Philippine Ecosys Nat Resources. 2017;27(1&2):40–49. [Google Scholar]
- [76].Paller VGV, Magcale-Macandog D, de Chavez ERC, et al. The seven lakes of San Pablo: assessment and monitoring strategies toward sustainable lake ecosystems. Phil Sci Letters. 2021;14(1):158–179. [Google Scholar]
- [77].Argayosa AM. Lectins and lectin receptors from Acanthamoeba sp. Trans Nat Acad Sci. 1993;15:143–151. [Google Scholar]
- [78].Rivera WL, and Adao DEV. Identification of the 18S-ribosomal-DNA genotypes of Acanthamoeba isolates from the Philippines. Ann Trop Med Parasitol. 2008;102(8):671–677. [DOI] [PubMed] [Google Scholar]
- [79].Gabriel AA, Panaligan DC. Heat and chlorine resistance of a soil Acanthamoeba sp. cysts in water. J App Microbiol. 2020;129(2):453–464. [DOI] [PubMed] [Google Scholar]
- [80].Yagita K, Matias RR, Yasuda T, et al. Acanthamoeba sp. from the Philippines: electron microscopy studies on naturally occurring bacterial symbionts. Parasitol Res. 1995;81:98–102. [DOI] [PubMed] [Google Scholar]
- [81].Rivera WL, Adao DEV. 18S ribosomal DNA genotypes of Acanthamoeba species isolated from contact lens cases in the Philippines. Parasitol Res. 2009;105(4):1119–1124. [DOI] [PubMed] [Google Scholar]


