ABSTRACT
Information on the mosquito species that transmit canine filariosis is scanty. Hence, an experimental study was conducted to identify the potential vectors responsible for the transmission of D. immitis Leidy and B. pahangi Buckley & Edeson. A total of 367 mosquitoes belonging to six species containing both laboratory and field strains (i.e. Aedes togoi Theobald, Aedes aegypti Linnaeus, Aedes albopictus Skuse, Culex quinquefasciatus Say, Culex vishnui Theobald and Anopheles dirus Peyton & Harrison) were used in this study. All mosquitoes were artificially fed on either D. immitis or B. pahangi microfilariae (mfs) infected blood by using the Hemotek™ membrane feeding system. Out of 367 mosquitoes, 228 (64.9%) were fully engorged. After feeding on D. immitis (20%) and B. pahangi (33%) mfs positive blood, the mortality rates for Cx. quinquefasciatus were found to be slightly lower than that of other species of mosquitoes. On the other hand, majority of An. dirus were found to be incapable to withstand the infection of mfs as the mortality rates were relatively high (D. immitis = 71.4%; B. pahangi = 100.0%). Brugia pahangi was detected in Ae. togoi and Cx. quinquefasciatus with infection rates of 50% and 25%, respectively. Aedes togoi was the only species infected with D. immitis with an infection rate of 69%. Our results showed that Ae. togoi was an excellent experimental vector for both D. immitis and B. pahangi. This study also documented the observation of B. pahangi, for the first time in the head region of Cx. quinquefasciatus under a laboratory setting.
KEYWORDS: Brugia pahangi, Dirofilaria immitis, filaria, mosquitoes, vector
1.0. Introduction
Mosquito-borne filarioid nematodes such as Dirofilaria immitis and Brugia pahangi cause diseases to mammals, especially in domestic dogs and cats [1,2]. Dirofilaria immitis has a wide geographical distribution whereas B. pahangi is endemic in Southeast Asia (i.e. Malaysia, Thailand and Indonesia) [3,4]. Both parasites principally infect canine host, but the increasing reports of human filariosis highlight their potential as emerging zoonosis globally [2,5]. Transmission of filarioid parasites depends on the availability of microfilaraemic hosts, vectors, and favorable temperatures for the growth of the infectious stages in mosquitoes [6]. Mosquitoes of the genera Aedes, Culex, and Anopheles are the main vectors of filarioid parasites and they transmit L3 infective larvae of these parasites to potential hosts through their bites [7].
The climate and weather in Malaysia provide a conducive environment for all these species of mosquitoes and consequently, their proliferation can increase the risk of transmission [8]. In Malaysia, Mansonia indiana Edwards was identified as the vector of D. immitis for the first time in 1937 [9]. Subsequently, Mansonia dives Schiner, Mansonia bonneae Edwards, Mansonia annulata Leicester, Mansonia uniformis Theobald, Anopheles campestris Reid, Anopheles lesteri Baisas, Anopheles nigerrimus Giles were also identified as the vectors in Malaysia [10–12]. On the other hand, infection of B. pahangi was observed in mosquitoes of five genera, Mansonia, Aedes, Anopheles, Armigeres and Culex. Notably, Armigeres obturbans Walker was reported as an efficient vector in transmitting B. pahangi in various studies [13–15].
Experimental studies on different mosquito species in Southeast Asia have been conducted to identify the potential vectors of D. immitis and Brugia spp [16–18]. However, most experimental studies were on Brugia malayi Brug and information regarding in vitro trial of potential vectors of B. pahangi is scarce. Indeed, an experimental study on different mosquito species or different populations is important for incriminating additional vectors responsible for transmission. Accordingly, this study was conducted to identify the potential vectors of D. immitis and B. pahangi through an in vitro trial using field and laboratory strains of various mosquito species.
2.0. Methods
2.1. Ethics statement
This study was approved by the Medical Ethics Committee of Department Veterinary Services Malaysia [JPV: BPI/500-4/1/2 (18)] and the Institutional Biosafety and Biosecurity Committee, Universiti Malaya [UMIBBC/NOI/R/TNC/TIDREC-014/13,062,019]. Written informed consents were obtained from the owners of the animal shelters.
2.2. Microfilariae inoculums
Microfilariaemic blood samples (D. immitis and B. pahangi) were collected from infected dogs in an animal shelter located in Semenyih, Kuala Lumpur, Malaysia. Dog blood samples were collected randomly from the animal shelters and screened for filarial parasites and other pathogens by using serological test and microscopic Giemsa stain technique prior to selecting dogs for the present study [19–21]. After examination, a one-time blood collection was performed on two dogs; one was positive with single infection of B. pahangi and another one with single infection of D. immitis. Ten milliliters of blood samples were collected in Citrate Phosphate Dextrose Adenine (CDPA) anticoagulated blood tubes by a trained veterinarian and stored at 4°C until further processing. No medications were given to these animals prior to blood collections. The presence, viability, and number of mfs in all samples obtained were confirmed by microscopic technique. Approximately, 100 µl of blood was covered with a cover slide, and mfs were counted by microscopic examination under 40x and 100x magnifications. Steps were repeated thrice, and an average was taken to determine the intensity of the infection. Further species identification of filarioid parasites was performed using a molecular technique [21]. In addition, a dog blood sample that was negative with all the detection techniques was taken and used as a control (control blood). Subsequently, blood was immediately fed to the mosquitoes by using the Hemotek™ system.
2.3. Mosquitoes
2.3.1. Field strains
Host-seeking mosquitoes were collected from urban and sub-urban areas in Klang Valley by using a human-landing catch technique. Briefly, human landing catch was conducted by sitting on the chairs with legs exposed. Collectors caught the landing mosquitoes with a transparent glass tube. Aedes aegypti and Ae. albopictus were collected from urban areas (GPS coordinate: 3.1162287831250723, 101.65313602754688), whereas Cx. vishnui was collected from a sub-urban area (GPS coordinate: 3.5623536415921504; 101.09889656993488). The age of the adult mosquitoes collected from field was unknown. Mosquitoes were transferred into plastic cups with moist cotton wool and transported to the laboratory for oral inoculation to the filarial infected blood.
2.3.2. Laboratory strains
Laboratory strains of Ae. aegypti, Ae. albopictus, Ae. togoi, Cx. quinquefasciatus originated from Institute for Medical Research Malaysia, and An. dirus originated from Malaria Research and Reference Reagent Resource Center (MR4/BEI resources) were reared and maintained in an insectarium under standard conditions (i.e. 26 ± 2°C) and a relative humidity (RH) of 80 ± 10% under a L12:D12 h light: dark cycle. In addition, 10% of sugar solution was provided daily to the mosquitoes as food source. Female mosquitoes aged 5–7 days were chosen for the inoculation experiments.
2.4. Infection of mosquitoes with microfilariae
Mosquitoes were starved overnight before the inoculation experiments. Three study groups were included: D. immitis-inoculated group, B. pahangi-inoculated group and negative control. Number of mosquitoes were recorded and separated into several cups according to species and placed inside a glove box approximately 127 cm × 254 cm × 95 cm in dimensions. Laboratory and field strains of mosquitoes were allowed to feed through Hemotek™ system (Discovery Workshop, UK) which had a surface area of 9.62cm2 with blood containing single infection of D. immitis or B. pahangi mfs and negative control blood (negative control: Ae. togoi laboratory strain only) in an Arthropod Containment Level 2 (ACL2) laboratory located at Department of Parasitology, Universiti Malaya. Firstly, rubber rings were used to keep five aluminum holders in place after coated with parafilm. Two milliliters of blood were injected into the ports, which were then sealed with plastic stoppers. The temperature of the blood meal was maintained at 37°C using an electric heating element in the Hemotek™ system throughout the blood feeding process. The blood was fed to the mosquitoes by placing the cups (each carrying different species of mosquitoes) underneath the feeder, with the cup’s nylon netting in contact with the feeder’s membrane. The microfilaraemic counts of D. immitis and B. pahangi in the blood samples were 1000mfs/ml and 9000mfs/ml, respectively. All mosquitoes were sorted after inoculation. The cups containing mosquitoes were placed in a − 20°C freezer for 30 seconds to induce a cold anesthesia. The cups which contained anaesthetised mosquitoes were then placed inside an ice box. Each mosquito that had been fed with either D. immitis or B. pahangi infected blood was inspected under the Olympus SZ51 stereomicroscope to examine if it had a full meal; and to determine the proportion of engorged females with blood bloated abdomens. Fully engorged female mosquitoes were transferred to a new cup according to species. Mosquitoes that did not take a full blood meal or did not take any meal were discarded.
2.5. Maintenance of the mosquitoes after inoculation
Mosquitoes were kept for 14 days [22] in a double containment (inner containment: sterile polystyrene cup, outer containment: small cage of 24 cm × 18.5 cm × 13 cm dimensions). The mosquitoes were maintained in ACL2 at 26°C with relative humidity of 85% and provided with 10% sugar solution. All mosquitoes were dissected individually after 14 days.
2.6. Assessment of mosquito mortality and detection of filarioid larvae in mosquitoes
Mortality of mosquitoes was recorded daily for 14 days, and dead mosquitoes were dissected to examine the presence of filarioid parasites. Mosquitoes were anaesthetized briefly by placing them in a freezer at −20°C for 30 sec. The mosquitoes were immobilized by removing their legs and wings on a chill table. Subsequently, they were kept at 4°C and dissected individually in a drop of buffered saline on a slide before microscopic examination. The head, thorax and abdomen of the mosquitoes were separated. Each body part was transferred to separate drops of buffered saline and macerated to liberate larvae of the filarioid parasites. Clean entomological forceps and needles were used for each dissection. All mosquitoes were dissected under a dissecting microscope. The number and location of larvae, and the stage of development were recorded.
2.7. Data analysis
Cumulative mortality rates were calculated based on the total number of mosquitoes that died naturally on 1-, 7- and 14-day post infection (dpi) divided with the total number of blood-fed mosquitoes. In addition, mortality rates were calculated by dividing the numbers of mosquitoes surviving for 14 days after the infective blood meal by the number of mosquitoes that took complete blood meals [4].
The accumulative numbers of dead mosquitoes were plotted against time, and the correlation co-efficient were calculated and compared by using IBM SPSS V21 [23]. Infection rate (IR) was calculated based on the equation below [4].
3.0. Results
3.1. Feeding rates
The feeding rates were calculated as shown in Table 1. Three groups of mosquitoes had 100% feeding rate [i.e; An. dirus laboratory strain (14/14), Ae. albopictus field strain (13/13) feeding on D. immitis microfilaraemic blood and Ae. aegypti field strain (3/3) feeding on B. pahangi microfilaraemic blood]. On the other hand, there were lower feedings rates noted in Cx. quinquefasciatus laboratory strain [D. immitis = 5/15 (33.3%) and B. pahangi = 5/15 (31.6%)] and Cx. vishnui field strain [D. immitis = 10/24 (41.7%) and B. pahangi = 11/26 (42.3%)].
Table 1.
Feeding and mortality rates of mosquitoes during infection trials with D. immitis and B. pahangi.
| Species of mosquito | No. of mosquitoes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total mosquitoes | No. of engorged mosquitoes with blood meal N (%) | Average no. of mortality daily (range) | Mortality rates (%) |
No. of surviving more than 14 days (N) | ||||
| 1 dpi N (%) |
7 dpi N (%) |
14 dpi N (%) |
||||||
| D. immitis | ||||||||
| Laboratory strain | ||||||||
| Ae. aegypti | 13 | 10 (76.7) | 0.35 (0–1) | 0 | 1 (10.0) | 5 (50.0) | 5 | |
| Cx. quinquefasciatus | 15 | 5 (33.3) | 0.07 (0–1) | 0 | 1 (20.0) | 1 (20.0) | 4 | |
| Ae. togoi | 29 | 24(82.6) | 0.71 (0–4) | 0 | 5 (20.8) | 10 (41.7) | 14 | |
| An. dirus | 14 | 14(100) | 1.00 (0–2) | 0 | 3 (21.4) | 10 (71.4) | 4 | |
| Field strain | ||||||||
| Ae. albopictus | 13 | 13(100) | 0.79 (0–3) | 1 (0.08) | 6 (46.2) | 11 (84.6) | 2 | |
| Cx. vishnui | 24 | 10(41.7) | 0.36 (0–1) | 1 (10.0) | 1 (10.0) | 5 (50.0) | 5 | |
| B. pahangi | ||||||||
| Laboratory strain | ||||||||
| Ae. albopictus | 18 | 17(94.4) | 0.43 (0–1) | 2 (11.8) | 4 (23.5) | 6 (35.3) | 11 | |
| Ae. aegypti | 14 | 12(81.0) | 0.57 (0–1) | 1 (8.3) | 5 (41.7) | 8 (66.7) | 4 | |
| Cx. quinquefasciatus | 19 | 6(31.6) | 0.14 (0–1) | 1 (1.7) | 1 (1.7) | 2 (33.3) | 4 | |
| Ae. togoi | 36 | 30(83.3) | 1.29 (0–4) | 1 (3.3) | 8 (26.6) | 18 (60.0) | 12 | |
| An. dirus | 19 | 12(63.2) | 0.57 (0–5) | 0 | 4 (33.3) | 12 (100.0) | 0 | |
| Field strain | ||||||||
| Ae. albopictus | 22 | 21(95.5) | 1.0 (0–7) | 4 (19.0) | 6 (28.6) | 14 (66.6) | 7 | |
| Cx. vishnui | 26 | 11(42.3) | 0.64 (0–3) | 0 | 4 (36.4) | 9 (81.8) | 2 | |
| Ae. aegypti | 3 | 3(100) | 0.07 (0–1) | 0 | 1 (33.3) | 1 (33.3) | 2 | |
| Negative control | ||||||||
| Laboratory strain | ||||||||
| Ae. togoi | 50 | 40(80) | 0.2 (0–2) | 0 | 3(7.5) | 8(20.0) | 32 | |
3.2. Survivability rates of mosquitoes
Mortality and survivability rates of mosquitoes are summarized in Table 1. At 1dpi, B. pahangi-inoculated group (0.8%) had a higher overall mortality rate (for both laboratory and field strains) compared to D. immitis-inoculated group (0.2%). No mortality was observed in all laboratory strains in D. immitis-inoculated group. However, mortality was observed in all field strains in D. immitis-inoculated group (Ae. albopictus = 7.69%; Cx. vishnui = 10%). On the other hand, mortality was observed in all laboratory strains (Ae. albopictus = 11.8%; Ae aegypti = 8.3%; Cx. quinquefasciatus = 1.7%; Ae. togoi = 3.3%) in B. pahangi-inoculated group except An. dirus. Besides, except Ae. albopictus (19%) which had the highest mortality rate among all other groups, no mortality was observed in field strains of Cx. vishnui and Ae. aegypti in B. pahangi-inoculated group.
On 7 dpi, all mosquito species in both microfilaraemic-inoculated groups had mortality rates ranging from 10 to 46.2%. Aedes albopictus (6/13; 46.2%) laboratory strain in D. immitis-inoculated group and Ae. aegypti (5/12; 41.7%) laboratory strain in B. pahangi-inoculated group had the highest cumulative mortality rates.
Of all mosquito species, laboratory strain of Cx. quinquefasciatus which was blood-fed on D. immitis and B. pahangi mfs had the lowest mortality rates of 20% (1/5) and 33.3% (2/6), respectively. On the other hand, An. dirus was shown to be incapable to withstand the infection of mfs as the mortality rates were relatively high (D. immitis = 71.4% (10/14); B. pahangi = 100% (12/12)).
Given the availability of high number of Ae. togoi to test against all inoculated groups, a graph was plotted to determine the vector competency of Ae. togoi toward the infection of both B. pahangi and D. immitis. The cumulative mortality of Ae. togoi infected with D. immitis and B. pahangi, and Ae. togoi fed with negative microfilaraemic blood was plotted against time with correlation coefficient of 0.979, 0.942 and 0.980, respectively for each curve (Figure 1). In addition, the mortality rates for both microfilaraemic-inoculated groups had significant differences (D. immitis and B. pahangi p = 0.02) compared to control group. However, there was no significant difference (p = 0.901) when compared between both microfilaraemic-inoculated groups.
Figure 1.
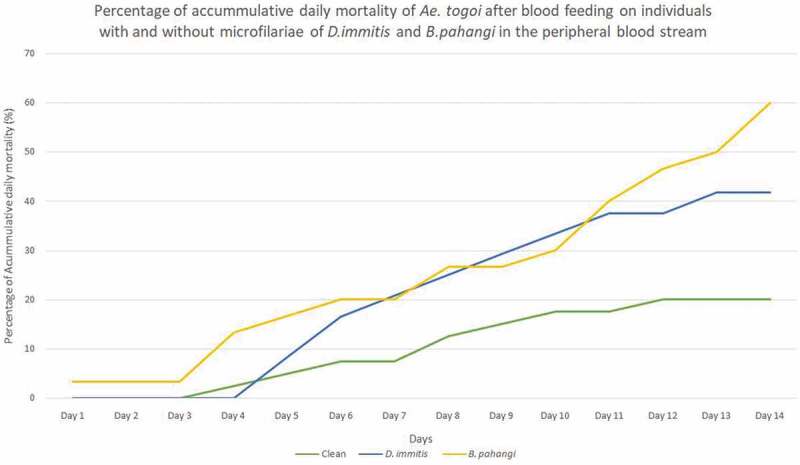
Percentage of accumulative daily mortality of Ae. togoi after blood feeding on individuals with and without microfilariae of D. immitis and B. pahangi in the peripheral blood stream.
3.3. Infection rate of mosquitoes
Out of six mosquito species, L3 larvae could only be found in two species of mosquitoes (Table 2). The infection rates for Ae. togoi in both microfilaraemic (D. immitis and B. pahangi) groups and Cx. quinquefasciatus in B. pahangi group were 69%, 50%, and 25%, respectively. For D. immitis-inoculated group, only Ae. togoi was found to be infected with L3 larvae at the head (71.1%) and thorax (28.9%) regions of mosquitoes with infection rate of 69%. Figure 2 shows the picture of L3 larva of B. pahangi in the head region of Aedes togoi. In addition, Ae. togoi and Cx. quinquefasciatus were also found to be infected with L3 larvae of B. pahangi at the head (Ae. togoi = 39.5%; Cx. quinquefasciatus = 100%), thorax (Ae. togoi = 55.3%) and abdomen (Ae. togoi = 5.3%) regions of mosquitoes with infection rates of 50% and 25%, respectively. However, only one larva of B. pahangi was found in a Cx. quinquefasciatus. There was no significance difference (p = 0.296) between Ae. togoi infected with D. immitis and B. pahangi.
Table 2.
Infective rates and parasite load of Ae. togoi and Cx. quinquefasciatus after feeding on blood containing D. immitis(microfilarial density = 1000mf/100 μl) and B. pahangi microfilariae (microfilarial density = 9000 mf/100 μl), with all mosquitoes dissected 14 days after feeding
| Mosquito Species | Infection rate % (N) |
Average No L3 per infected mosquito (range) | No. (%) of L3 found |
||
|---|---|---|---|---|---|
| % Head (No.) |
% Thorax (No.) | % Abdomen (No.) | |||
| D. immitis | |||||
| Ae. togoi | 69 (9/13)* | 5 (1–12) | 71.1 (32) | 28.9 (13) | 0 (0) |
| B. pahangi | |||||
| Ae. togoi | 50 (8/16)* | 4.75 (1–9) | 39.5 (15) | 55.3 (21) | 5.3 (2) |
| Cx. quinquefasciatus | 25 (1/4) * | 1 (1) | 100 (1) | 0 (0) | 0 (0) |
* Number of mosquitoes infected/Total mosquitoes dissected after 14 days
Figure 2.
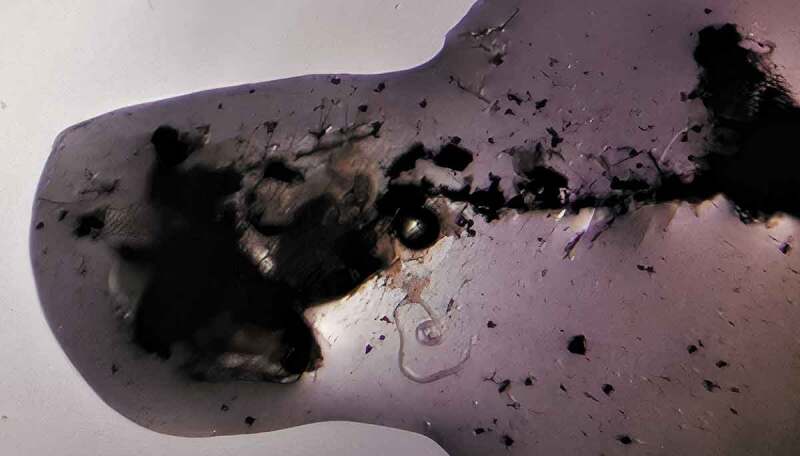
L3 larva of B. pahangi at the head region of Ae. togoi.
4.0. Discussion
A broad range of mosquito species have been experimentally tested as the vectors for D. immitis and B. pahangi. For D. immitis, the vectors responsible for transmission include Ae. aegypti [24–26], Ae. albopictus [27], Ae. koreicus Edwards [28] and Cx. quinquefasciatus [25]. In addition, a recent study by 29,also found that Anopheles maculipennis Meigen, Aedes caspius Pallas, Aedes vexans Meigen, Culex theileri Theobald and Culex pipiens Linnaeus were the potential vectors of D. immitis with the detection of DNA in head-thorax pools. On the other hand, Ae. togoi [30], Ma. annulata and Ma. dives were found to be susceptible to B. pahangi.
Besides, a study was conducted to investigate the relationship between microfilarial load in human host and uptake and development of Wuchereria bancrofti Cobbold mfs by Cx. quinquefasciatus [31]. Out of 504 mosquitoes, 20% of the mosquitoes died before reaching 12 dpi and 64% of these dead mosquitoes harbored a parasite of any stage. The mean number of developing larvae per infected dead mosquitoes was significantly higher than the mosquitoes dissected after 12 days. This study is comparable with our study where lower mortality rate was observed in Ae. togoi (20%) which served as the negative control in the current study compared to mosquitoes infected with B. pahangi (60.0%) and D. immitis (41.7%). This could be an indication of higher mortality among mosquitoes with higher parasitic load [31].
Aedes aegypti, Ae. albopictus and Cx. quinquefasciatus were reported to be the vectors for D. immitis in various countries [32–35]. However, our study could not identify these species as the potential vector because none of the field-collected mosquitoes were found to be positive with D. immitis mfs. A previous study reported the development of D. immitis mfs in Cx. vishnui during the initial observation [36]. Unfortunately, there was no evidence that larvae of mfs could complete their development to the infective stage. This result is comparable with our study whereby none of the Cx. vishnui mosquitoes were tested positive for D. immitis. The variation of vector found across the world might be due to the differences of vector efficiency index based on rapid parasitic development, proper nursing and low parasitic damage or death across different populations [37]. In addition, a systemic review by 38,showed that different geographical properties, such as latitude, longitude, mean temperature, yearly rainfall, sea level, and humidity could influence the mosquito prevalence rates in field investigations. The parameter ‘country’ should be interpreted from various factors, including the respective national vector control strategies and the ecological setting [39]. In addition, biological variation of mosquitoes can be influenced by a variety of ecological and geographical factors. Climate change, in combination with altering weather patterns, could promote the growth of D. immitis and the development of larvae within the mosquitoes [40]. Collectively, climatic patterns could play important role in vector-borne disease transmission.
Culex quinquefasciatus and Ae. aegypti collected from Manila, the Philippines were found to be infected with D. immitis mfs. From the experiment, both species had equal capability to allow the development of D. immitis within the mosquitoes [41]. 42,collected Cx. quinquefasciatus from different habitats and infected them at different times during the year. The study revealed a variation in percentage of infection with D. immitis mfs. Furthermore, 15,discovered that Armigeres subalbatus Coquillet was positive with D. immitis after 14 days of inoculation and it was considered as the primary vector for D. immitis in Kuala Lumpur, Malaysia. In addition, Ar. subalbatus was also incriminated as a potential vector for B. pahangi in Malaysia [14]. Regrettably, Ar. subalbatus was not included in our study as we were unable to obtain this mosquito species from the sampling sites.
However, an experimental study using laboratory strain of mosquitoes in Singapore reported that Cx. quinquefasciatus, Ae. aegypti and Ae. albopictus were susceptible to D. immitis except Ar. subalbatus. In addition, Cx. quinquefasciatus, Ae. aegypti and Ae. albopictus were found to harbor more than 90% of L3 larvae in the head region [43]. A study done in 2005 by Vythilingam et al. observed only one Ae. aegypti which harbored L3 larvae in the head, thorax and abdomen regions. In addition, only one L1 larva was found in the malpighian tubes of Cx. quinquefasciatus [15]. Although 43 observed more than 90% of L3 of D. immitis larvae located in the head region of Cx. quinquefasciatus, 15,suggested that Cx. quinquefasciatus was refractory to D. immitis infection based on its poor feeding on infected dog, which is comparable to the feeding rate in our study (33.3%). Furthermore, previous study also showed that Cx. quinquefasciatus preferred avian blood compared to mammalian blood [44]. Although these mosquitoes were found to be infected with D. immitis under experimental settings in other studies, yet, none of these mosquito species (Cx quinqiefasciatus, Ae. aegypti and Ae. albopictus) were found to be susceptible to D. immitis in our study.
High infection rate of B. pahangi ranging from 95–100% in the laboratory strains of Mansonia crassipes Wulp, Ma. annulata and Anopheles barbirostris Wulp were observed in an earlier study [13]. However, Ae. albopictus showed no evidence of infection, whereas Ae. aegypti showed a small number of infective larvae. The results are comparable with our study in which none of the Ae. albopictus and Ae. aegypti were found positive. On the other hand, there were few experimental studies performed on other Brugia spp. against Ae. togoi. These studies found that Ae. togoi was a good experimental vector for both B. malayi and Brugia patei Buckley [16, 45; 17, 18, 46]. In addition, a study in Thailand [47] showed that Ae. togoi was a suitable laboratory vector for B. pahangi. Likewise, this finding parallels with our observation. Furthermore, Cx. quinquefasciatus was known to be resistant to B. pahangi in a study conducted in Kenya [48]. However, in our study, a L3 larva of B. pahangi was found in the head region of Cx. quinquefasciatus suggesting its potential as a vector for B. pahangi. It would be worthwhile to repeat these experiments by expanding the number of the mosquitoes in order to get a clearer picture on the infection rate and vector competency for B. pahangi in Cx. quinquefasciatus.
Anopheles dirus, a well-known vector of Plasmodium parasites in Thailand, Cambodia, Laos and Vietnam [49–52] was found to be responsible for filariosis in western Pacific and Southeast Asia [53]. Notably, An. dirus from northern Peninsular Malaysia was found to have a certain level of susceptibility toward B. pahangi [54]. However, none of the An. dirus was susceptible to D. immitis and B. pahangi in our study and the mortality rate of An. dirus was found to be very high (D. immitis = 71.4%, B. pahangi = 100%). Anopheles dirus was previously known as a species complex comprising at least seven members [55]. Different members or species might have different susceptibility toward the infection; however, we could not confirm whether the true A. dirus was used in 54.
More than 360 individuals of mosquitoes were used in the present study. Unfortunately, the reduced numbers of blood-fed mosquitoes and mosquitoes that survived at 14 dpi have made the sample size smaller, which is the limitation of the present study. An increased sample size of mosquitoes in future study would be beneficial in understanding the infection rate and vector competency of the tested mosquito species for both D. immitis and B. pahangi.
5.0. Conclusion
Laboratory strain of Ae. togoi remains as a good experimental vector for D. immitis and B. pahangi. This study showed for the first time that the laboratory strain of Cx. quinquefasciatus has the potential to be a vector for B. pahangi. However, extensive studies by increasing the sample size of mosquitoes and dissecting them on different days to show the developmental stages of the parasites should be carried out. Natural infection of B. pahangi in the field populations of Cx. quinquefasciatus should also be investigated.
Acknowledgments
We would like to express our gratitude to the owners of the animal shelters and veterinarians for their assistance in sample collection. We are also grateful to Dr Jonathan Liew Wee Kent for his technical assistance in this study.
Funding Statement
This study was financially supported by the Higher Institution Centre of Excellence (HICoE) program (MO002-2019) and the Universiti Malaya research grant (PG098-2016A)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Sample credit author statement
Wei Yin Vinnie-Siow: Methodology, Investigation, Writing-Original Draft, Formal analysis, Van Lun Low: Conceptualization, Investigation, Writing-Review & Editing, Supervision, Tiong Kai Tan: Investigation, Writing-Review & Editing, Supervision, Meng Li Wong: Investigation, Cherng Shii Leong: Investigation, Nazni Wasi Ahmad: Resources, Writing- Review& Editing, Yvonne Ai Lian Lim: Writing-Review & Editing, Supervision.
References
- [1].Cringoli G, Rinaldi L, and Veneziano V, et al. A prevalence survey and risk analysis of filariasis in dogs from the Mt. Vesuvius area of southern Italy. Vet. Parasitol. 2001;102(3):243–252. [DOI] [PubMed] [Google Scholar]
- [2].Denham DA, and McGreevy PB.. Brugian filariasis: epidemiological and experimental studies. Adv. Parasitol. 1977;15:243–309. [DOI] [PubMed] [Google Scholar]
- [3].Mak JW, Yen PKF, and Lim KC, et al. Zoonotic implications of cats and dogs in filarial transmission in Peninsular Malaysia. Trop.Geogr. Med. 1980;32:259–264. [PubMed] [Google Scholar]
- [4].Silaghi C, Beck R, Capelli G, et al. Development of Dirofilaria immitis and Dirofilaria repens in Aedes japonicus and Aedes geniculatus. Parasit Vectors. 2017;10(1). DOI: 10.1186/s13071-017-2015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kini RG, Leena JB, and Shetty P, et al. Human dirofilariasis: an emerging zoonosis in India. J. Parasitic. Dis. 2015;39(2):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sassnau R, Daugschies A, and Lendner M, et al. Climate suitability for the transmission of Dirofilaria immitis and D. repens in Germany. Vet. Parasitol. 2014;205(1–2):239–245. [DOI] [PubMed] [Google Scholar]
- [7].Irwin PJ, and Jefferies R.. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004;20(1):27–34. [DOI] [PubMed] [Google Scholar]
- [8].Short EE, Caminade C, and Thomas BN.. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. (Auckl). 2017;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hodgkin E. Trapping of mosquitoes in malarial states. Federated Malay States. Ann. Rep. Inst. Med. Res. 1937;71–96.
- [10].Reid JA, Wilson T, and Ganapathipillai V. Studies on Filariasis in Malaya: the mosquito vectors of periodic Brugia malayi in North-West Malaya. Ann. Trop. Med. Parasitol. 1962;56(3):323–336. [Google Scholar]
- [11].Wharton RH. Studies on filariasis in Malaya: field and laboratory investigations of the vectors of a rural strain of Wuchereria bancrofti. Ann. Trop. Med. Parasitol. 1960;54(1):78–91. [DOI] [PubMed] [Google Scholar]
- [12].Wharton RH. The biology of Mansonia mosquitoes in relation to the transmission of filariasis in Malaya. Bull. No. 11 Inst. Med. Res. 1962;114. [PubMed]
- [13].Edeson JF, Wharton RH, and Laing AB. A preliminary account of the transmission, maintenance and laboratory vectors of Brugia pahangi. Trans. R. Soc. Trop. Med. 1960;54(5):439–449. [DOI] [PubMed] [Google Scholar]
- [14].Muslim A, Fong MY, and Mahmud R, et al. Armigeres subalbatus incriminated as a vector of zoonotic Brugia pahangi filariasis in suburban Kuala Lumpur, Peninsular Malaysia. ParasitVectors. 2013;6(219). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vythilingam I, Mooto P, and Jeffery J, et al. (2005). Potential mosquito (Diptera: Culicidae) vectors of Dirofilaria immitis (Filariidae: onchocercidae) in two urban areas of Kuala Lumpur and its prevalence in stray dogs. Proceedings of the Fifth International Conference on Urban Pests Singapore: Lee CY, and Robinson WH, (eds.), 393–398. [Google Scholar]
- [16].Junkum A, Choochote W, and Jitpakdi A, et al. Comparative studies on the biology and filarial susceptibility of selected blood-feeding and autogenous Aedes togoi sub-colonies. Mem. Inst. Oswaldo Cruz. 2003;98(4):481–485. [DOI] [PubMed] [Google Scholar]
- [17].Kim HK, and Seo BS. Kisaengch’unghakchapchi. Korean J. Parasitol. 1968;6(1):1–13. [DOI] [PubMed] [Google Scholar]
- [18].Ramachandran CP, and Zaini MA. Studies on the transmission of sub-periodic Brugia malayi by Aedes (Finlaya) togoi in the laboratory. 3. The survival of infected mosquitoes under laboratory conditions. Med. J. Malaya. 1968;23(4):323–329. [PubMed] [Google Scholar]
- [19].Vinnie-Siow WY, Low VL, and Tan TK, et al. Observations of scrotal mass, liver mass, haemolytic jaundice, and central vestibular disorder in Brugia pahangi-infected dogs. Trop. Biomed. 2019;36(1):252–256. [PubMed] [Google Scholar]
- [20].Vinnie-Siow WY, Low VL, and Tan TK, et al. Serological survey of canine vector-borne diseases in two animal shelters in central Peninsular Malaysia. Trop. Biomed. 2021;38(1):145–149. [DOI] [PubMed] [Google Scholar]
- [21].Vinnie-Siow WY, Tan TK, Low VL, et al. Integration of microscopic, serologic and molecular techniques for detection of filarial parasites in dogs in Malaysia. Acta Parasitol. 2021;Advance online publication. doi: 10.1007/s11686-021-00490-5 [DOI] [PubMed] [Google Scholar]
- [22].Thanchomnang T, Intapan PM, and Lulitanond V, et al. Rapid detection of Dirofilaria immitis in mosquito vectors and dogs using a real-time fluorescence resonance energy transfer PCR and melting curve analysis. Vet. Parasitol. 2010;168(3–4):255–260. [DOI] [PubMed] [Google Scholar]
- [23].Calheiros CM, Fontes G, and Williams P, et al. Experimental infection of Culex (Culex) quinquefasciatus and Aedes (Stegomyia) aegypti with Wuchereria bancrofti. Mem. Inst. Oswaldo Cruz. 1998;93(6):855–860. [DOI] [PubMed] [Google Scholar]
- [24].Taylor AE. The development of Dirofilaria immitis in the mosquito aedes aegypti. J. Helminthol. 1960;34(1–2):27–38. [DOI] [PubMed] [Google Scholar]
- [25].Tiawsirisup S, and Nithiuthai S. Vector competence of Aedes aegypti (L.) and Culex quinquefasciatus (Say) for Dirofilaria immitis (Leidy). Southeast Asian J. Trop. Med. Public Health. 2006;37(3):110–114. [PubMed] [Google Scholar]
- [26].Webber WAF, and Hawking F. Experimental maintenance of Dirofilaria repens and D. immitis in dogs. Exp. Parasitol. 1955;4(2):143–164. [DOI] [PubMed] [Google Scholar]
- [27].Nayar JK, and Knight JW. Aedes albopictus (Diptera: culicidae): an Experimental and Natural Host of Dirofilaria immitis (Filarioidea: onchocercidae) in Florida, U.S.A. J. Med. Entomol. 1999;36(4):441–448. [DOI] [PubMed] [Google Scholar]
- [28].Montarsi F, Ciocchetta S, and Devine G, et al. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasite Vectors. 2015;8(1). DOI: 10.1186/s13071-015-0800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Demirci B, Bedir H, and Taskin Tasci G, et al. Potential mosquito vectors of Dirofilaria immitis and Dirofilaira repens (Spirurida: onchocercidae) in Aras Valley, Turkey. J. Med. Entomol. 2021;58(2):906–912. [DOI] [PubMed] [Google Scholar]
- [30].Lavoipierre M, and Ho B. Studies on Filariasis. I.—The Miģration of the infective larvae of Brugia pahangi in Aedes togoi and their loss from the mosquito under experimental conditions. J. Helminthol. 1966;40(3–4):343–362. [DOI] [PubMed] [Google Scholar]
- [31].Subramanian S, Krishnamoorthy K, and Ramaiah KD, et al. The relationship between microfilarial load in the human host and uptake and development of Wuchereria bancrofti microfilariae by Culex quinquefasciatus: a study under natural conditions. Parasitol. 1998;116(3):243–255. [DOI] [PubMed] [Google Scholar]
- [32].Cancrini G, Frangipane Di Regalbono A, and Ricci I, et al. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet. Parasitol. 2003;118(3–4):195–202. [DOI] [PubMed] [Google Scholar]
- [33].Hendrix CM, Brunner CJ, and Bellamy LK. Natural transmission of Dirofilaria immitis by Aedes aegypti. J. Am. Mosq. Control Assoc. 1986;2(1):48–51. [PubMed] [Google Scholar]
- [34].Todorovic S, and McKay T. Potential mosquito (Diptera: culicidae) vectors of Dirofilaria immitis from residential entryways in Northeast Arkansas. Vet. Parasitol. 2020;282:109105. [DOI] [PubMed] [Google Scholar]
- [35].Vezzani D, Mesplet M, and Eiras DF, et al. PCR detection of Dirofilaria immitis in Aedes aegypti and Culex pipiens from urban temperate Argentina. Parasitol. Res. 2010;108(4):985–989. [DOI] [PubMed] [Google Scholar]
- [36].Intermill RW, and Frederick RM. A study of potential mosquito vectors of Dirofilaria immitis Leidy, On Okinawa, Ryukyu Islands. J. Med. Entomol. 1970;7(4):455–461. [DOI] [PubMed] [Google Scholar]
- [37].Chandra G. Nature limits filarial transmission. Parasit Vectors. 2008;1(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Riahi SM, Yusuf MA, and Azari-Hamidian S, et al. Prevalence of Dirofilaria immitis in mosquitoes (Diptera) - systematic review and meta-analysis. J. Nematol. 2021;53:e2021–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Genchi C, Rinaldi L, and Mortarino M, et al. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009;163(4):286–292. [DOI] [PubMed] [Google Scholar]
- [40].Morchón R, Carretón E, and González-Miguel J, et al. Heartworm disease (Dirofilaria immitis) and their vectors in Europe - new distribution trends. Front. Physiol. 2012;3:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Del Rosario A. Studies on the biology of Philippine mosquitoes. II. Observations on the life and behavior of Aedes albopictus (Skuse) in the laboratory. Philip. J. Sci. 1963;92:89–103. [Google Scholar]
- [42].Hu SMK. Studies of host parasite relationships of Dirofilaria immitis Leidy and its Culicine intermediate hosts. Am. J. Trop. Med. Hyg. 1931;14:614–629. [Google Scholar]
- [43].Chellapah WT, and Chellapah GR. Susceptibility of Four Common Singapore Mosquitoes to Dirofilaria immitis Leidy. J. Med. Entomol. 1968;5(3):358–361. [DOI] [PubMed] [Google Scholar]
- [44].Vythilingam I, Mahadevan S, and Tan SK, et al. Host feeding and resting habits of Japanese encephalitis vectors found in Sepang, Selangor, Malaysia. Trop. Biomed. 1996;13:45–50. [Google Scholar]
- [45].Laurence B, and Pester F. Adaptation of a filarial worm, Brugia patei, to a new mosquito host, Aedes togoi. J. Helminthol. 1967;41(4):365–392. [DOI] [PubMed] [Google Scholar]
- [46].Ramachandran CP, Wharton RH, and Dunn FL, et al. Aedes (Finlaya) togoi Theobald, a Useful Laboratory Vector in Studies of Filariasis. Ann. Trop. Med. Parasitol. 1963;57(4):443–445. [DOI] [PubMed] [Google Scholar]
- [47].Choochote W, Abeyewickreme W, and Sucharit S, et al. Aedes togoi Koh Nom Soa, Chanthaburi, a laboratory vector for Brugia malayiand Brugia pahangi in Thailand. J. Trop. Med. Parasito. 1983;6:25–31. [Google Scholar]
- [48].Irungu LW. Factors influencing the development of Brugia pahangi microfilariae in Culex quinquefasciatus and Aedes aegypti mosquitoes. Insect Sci. Its App. 1989;10(5):661–675. [Google Scholar]
- [49].Chareonviriyaphap T, Bangs MJ, and Ratanatham S. Status of malaria in Thailand. Southeast Asian J. Trop. Med. Public Health. 2000;31:225–237. [PubMed] [Google Scholar]
- [50].Manguin S, Garros C, and Dusfour I, et al. Bionomics, taxonomy, and distribution of the major malaria vector taxa of (Anopheles subgenus Cellia in Southeast Asia: an updated review. Infect. Genet. Evol. 2008;8(4):489–503. [DOI] [PubMed] [Google Scholar]
- [51].Trung HD, Van Bortel W, and Sochantha T, et al. Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop. Med. Int. Health. 2004;9(2):230–237. [DOI] [PubMed] [Google Scholar]
- [52].Vythilingam I, Phetsouvanh R, and Keokenchanh K, et al. The prevalence of Anopheles (diptera: culicidae) mosquitoes in Sekong Province, Lao PDR in relation to malaria transmission. Trop. Med. Int. Health. 2003;8(6):525–535. [DOI] [PubMed] [Google Scholar]
- [53].Khanum H, Hossain I, and Sarkar F, et al. Lymphatic filariasis in Nilphamari district: an endemic area in Bangladesh. Bangladesh J. Zool. 2018;46(1):11–20. [Google Scholar]
- [54].Zahedi M, and White GB. Filaria vector competence of some Anopheles species. Trop. Med. Parasitol. 1994;45(1):27–32. [PubMed] [Google Scholar]
- [55].Obsomer V, Defourny P, and Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar. J. 2007;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]


