Summary
Background
The Omicron BA.2 sublineage has replaced BA.1 worldwide and has comparable levels of immune evasion to BA.1. These observations suggest that the increased transmissibility of BA.2 cannot be explained by the antibody evasion.
Methods
Here, we characterized the replication competence and respiratory tissue tropism of three Omicron variants (BA.1, BA.1.1, BA.2), and compared these with the wild-type virus and Delta variant, in human nasal, bronchial and lung tissues cultured ex vivo.
Findings
BA.2 replicated more efficiently in nasal and bronchial tissues at 33°C than wild-type, Delta and BA.1. Both BA.2 and BA.1 had higher replication competence than wild-type and Delta viruses in bronchial tissues at 37°C. BA.1, BA.1.1 and BA.2 replicated at a lower level in lung parenchymal tissues compared to wild-type and Delta viruses.
Interpretation
Higher replication competence of Omicron BA.2 in the human upper airway at 33°C than BA.1 may be one of the reasons to explain the current advantage of BA.2 over BA.1. A lower replication level of the tested Omicron variants in human lung tissues is in line with the clinical manifestations of decreased disease severity of patients infected with the Omicron strains compared with other ancestral strains.
Funding
This work was supported by US National Institute of Allergy and Infectious Diseases and the Theme-Based Research Scheme under University Grants Committee of Hong Kong Special Administrative Region, China.
Keywords: SARS-CoV-2, Omicron BA.2, Nasal tissue, Bronchial tissue, Transmission, Pathogenicity
Research in context.
Evidence before this study
SARS-CoV-2 Omicron variant has become the dominant variant circulating worldwide. Epidemiological data shows that the BA.2 has now replaced BA.1. The main aim of this study is to assess the viral replication and tissue tropism of Omicron BA.2 in comparison with previous SARS-CoV-2 strains in human respiratory tissue explants. We searched PubMed without language restriction on 25th May 2022 for articles using the terms “Omicron BA.2” or “SARS-CoV-2 BA.2” and “ex vivo” or “human respiratory tract” or “human respiratory tissue” or “human explant”, and found no relevant articles.
Added value of this study
We report that Omicron BA.2 replicated more efficiently in nasal and bronchial tissues at 33°C than wild-type, Delta and BA.1. Both BA.2 and BA.1 replicated to higher titres than wild-type and Delta viruses in bronchi at 37°C while the Omicron variants replicated less efficiently than wild-type and Delta viruses in lung parenchymal tissues.
Implications of all the available evidence
Omicron BA.2 replicates better than the BA.1 and previous strains in the upper respiratory tract but less efficiently in the lung parenchyma than wild-type and Delta. These findings are relevant to the understanding of the rapid expansion of BA.2 over BA.1 and the decreased disease severity of patients infected with the Omicron strain compared with other ancestral strains.
Alt-text: Unlabelled box
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of COVID-19 and the B.1.1.529 (omicron) variant has been classified as a variant of concern (VOC).1 Three Omicron sublineages were initially recognized: BA.1 (including BA.1.1, which has R346K), BA.2, and BA.3. Among Omicron, BA.2 is the dominant variant circulating worldwide.2
The ability of a virus to replicate in the human upper and conducting airways is an important parameter to evaluate the transmissibility of the virus among humans and its pandemic threat. Therefore, assessing viral replication in human respiratory tract provides direct evidence on the virulence of the viruses. Ex vivo human explants of the respiratory tract are highly physiologically relevant models to study respiratory viruses and their viral tropism. Complex and multicellular responses can be obtained in ex vivo models but not in cell lines. However, this model is highly dependent on the availability of fresh human respiratory tissues, which is not available in many research laboratories worldwide. Besides, the experimental observation time is relatively short (up to 96 hours post-infection) due to the viability of explant tissue. In addition, since the tissues cannot be expanded like cell cultures, the scale of experimental settings is limited.
The BA.1 and BA.2 variants share 21 amino acid substitutions in the S protein while BA.1 and BA.2 variants have an additional 12 and 11 mutations and deletions which are not found in each other, respectively.3 This suggests that BA.2 variant may differ in its infectivity, transmissibility, pathogenicity and antigenicity from the BA.1 variant. Epidemiological data shows that the BA.2 has now replaced BA.1 globally with the majority of sequences belonging to BA.2.2,3 However, there appear to be no epidemiological or clinical differences observed between BA.1 versus BA.2 subvariants.4
Reduced antibody neutralizing activity against BA.1, BA.1.1 and BA.2 was reported when compared to the ancestral virus strain.5, 6, 7 The extent of evasion from neutralizing antibody elicited by vaccine immunity by BA.1 and BA.2 were comparable.5, 6, 7, 8 These findings suggest that increased antibody evasion may not be the sole reason for the current expansion of BA.2. Here, we characterized the viral replication competence of the BA.1, BA.1.1 and BA.2 variants and compared these with the wild-type (WT) virus and Delta variant in ex vivo explant cultures of human nasal, bronchial and lung tissues.
Methods
SARS-CoV-2 isolation
Vero E6 (E6) cells were used for virus isolation and propagation of wild-type virus and Vero E6-TMPRSS2 (T2) overexpressed cells which were developed and provided by Dr. Makoto Takeda,9 were used for Delta and Omicron variants. Both cell-lines were cultured in DMEM with 10% FBS and viruses were isolated as previously described.10 Viruses were isolated from clinical specimen of the nasopharyngeal and throat swab from patients infected with SARS-CoV-2 in virus transport medium as previously described.10 The virus stock was aliquoted and stored frozen at −80°C. Aliquots were titrated to determine plaque forming unit (pfu) in respective E6 or T2 cells. The experiments were carried out in a Bio-safety level 3 (BSL-3) facility at the School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong. In addition to the Omicron variants (BA.1, BA.1.1 and BA.2.3), we used a wild-type (WT) virus and a Delta variant for comparison. The information of the virus isolates was listed in Supplementary Table 1.
We deduced near full-length genomes and variants from the samples using a Illumina sequencing protocol previously described by us (see Supplementary Materials).11,12
Ex vivo cultures of human respiratory tract and its infection
Fresh non-tumour nasal turbinate (n=4), bronchus (n=5) and lung (n=6) tissues were obtained from patients aged 47-74 years undergoing elective surgery in Department of Surgery at Queen Mary Hospital (Pok Fu Lam, Hong Kong SAR, China) from January to March 2022 and were removed as part of routine clinical care but surplus for routine diagnostic requirements as detailed previously.10 The donor information is listed in the Supplementary Table 4. The virus infection procedures were performed as previously described.10 Briefly, similar sized pieces of human nasal, bronchus and lung tissues were infected with each virus at 5×105 pfu/mL for 1 h at 33°C (nasal and bronchus tissues) or 37°C (bronchus and lung tissues). Each tissue fragment was washed three times in culture medium to remove residual virus inoculum, topped up with fresh medium and incubated at 37°C as indicated. Mock-infected tissues served as negative controls. Aliquots of culture medium were removed at times indicated and stored at −80°C until titration. Infectious viral titres in culture supernatants were assayed by TCID50 in Vero E6-TMPRSS2 cells. Infected tissues were fixed in 10% formalin and processed for immuno-staining at 48 hours post infection (hpi) for nasal tissues or 72 hpi for bronchi and lungs. Viral titres were determined by using TCID50 assay (see Supplementary Materials). Immunohistochemistry staining of paraffin-embedded tissues was performed to visualize the SARS-CoV-2 infected cells (see Supplementary Materials).
Thermal inactivation
Virus cultures in tissue culture wells without tissues or cells were incubated at 33˚C, for human nasal and bronchus tissues or at 37˚C for human bronchus and lung tissues were incubated. Virus supernatants were harvested at indicated time-points for titration by TCID50 assay to define the thermal inactivation of the virus in the absence of replication.
Biosafety and ethics
All experiments were carried out in a Bio-safety Level 3 (BSL-3) facility. Informed consent was obtained from all subjects and approval was granted by the Institutional Review Board (IRB) of the University of Hong Kong and the Hospital Authority (Hong Kong West) (IRB approval no: UW 20-862 and UW 20-588).
Statistical analysis
Experiments with the ex vivo cultures were performed for individual donors with the donor number stated in the above section. Experiments with the in vitro cultures were performed independently at least three times. Results shown in figures are the calculated mean and standard deviation of mean. Area-under-curve (AUC) was calculated by integrating infectious virus titres at 24-48 or 24-72 hpi in ex vivo nasal, bronchial and lung tissues. The differences in log10 transformed viral titres between viruses and over time were compared using two-way analysis of variance (ANOVA) followed by a Tukey's multiple-comparison test. Statistical significances between groups were calculated using one-way ANOVA followed by a Tukey's multiple-comparison test or two-tailed t test. All the statistical significances were calculated using GraphPad Prism version 9.0. Differences were considered significant at a p < 0.05.
Role of the funding source
The funders had no role in study design, data collection, analysis, or interpretation of the data, or in the writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
BA.2 replicates efficiently in human upper airway at 33°C
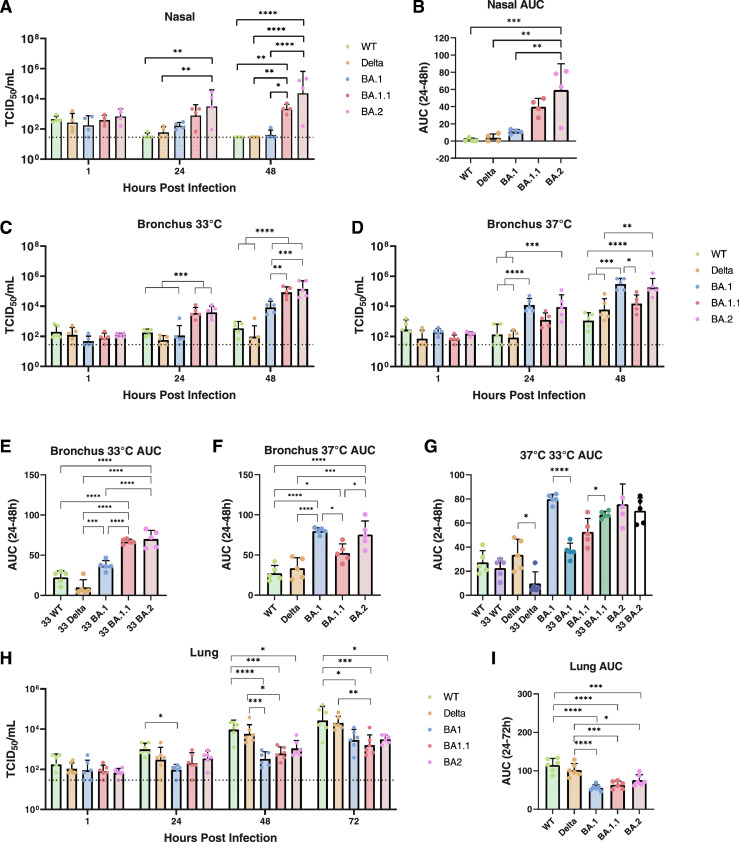
As the upper respiratory tract such as nasal tissues or nasopharynx is the initial site of SARS-CoV-2 infection following airborne or fomite mediated transmission, and is at a lower temperature to the conducting airway, we investigated and compared the viral replication kinetics of WT, Delta, Omicron BA.1, BA.1.1 and BA.2 in nasal turbinate tissue at 33°C by quantitating infectious virus using 50% tissue culture infectious dose (TCID50) titrations. BA.2 replicated to significantly higher titres than WT and Delta at 24 hpi and higher titres than WT, Delta and BA.1 at 48 hpi (Figure 1A). Similarly, BA.1.1 replicated to significantly higher titres than WT, Delta and BA.1 at 48 hpi (Figure 1A). Higher replication competence of BA.2 than WT, Delta and BA.1 was also observed in area under the curve (AUC) analysis aggregating virus titres at 24-48 hpi (Figure 1B). Although higher mean viral titres of BA.2 than that of BA.1.1 was observed at 24 and 48 hpi and also in AUC values, there was no statistical significances between them.
Figure 1.
Viral replication kinetics of SARS-CoV-2 variants in ex vivo cultures of human respiratory tract. Viral replication of SARS-CoV-2 variants in human ex vivo nasal turbinate at 33°C (A and B), bronchus at 33°C (C, E, G) or 37°C (D, F, G) and lung at 37°C (H and I). The horizontal dotted line denotes the limit of detection in the TCID50 assay. Viral titres from A, C, D and H are depicted as area under the curve (AUC) (B, E, F, G and I). Data are presented as geometric mean values. Error bars indicate SD with N = 4 (nasal, individual donors); N = 5 (bronchus, individual donors); N = 6 (lung, individual donors). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, (A, C, D and H, Two-way ANOVA followed by Tukey's test; B, E, F and I, One-way ANOVA followed by Tukey's test; G, two-tailed T test).
At 33°C, BA.1.1 and BA.2 had very similar viral titres at both 24 and 48 hpi in bronchial tissues (Figure 1C). Besides, BA.1.1 and BA.2 had higher viral titres than BA.1, WT and Delta viruses at 24 and 48 hpi in bronchial tissues (Figure 1C). BA.1 replicated to higher virus titres than WT and Delta at 48 hpi. The higher replication competence of BA.1.1 and BA.2 than BA.1, WT and Delta was confirmed by AUC analysis (Figure 1E).
BA.2 and BA.1 have comparable replication in human bronchial tissues at 37°C
The replication of BA.1 in the bronchus was significantly enhanced at 37°C and BA.1 and BA.2 were comparable with each other and both were higher than WT and Delta at 24 and 48 hpi (Figure 1D). In addition, both BA.1 and BA.2 replicated to higher titres than BA.1.1 at 48 hpi. The higher replication competence of BA.1 and BA.2 than WT, Delta and BA.1.1 was confirmed in AUC analysis (Figure 1F). BA.1.1 had a higher value of AUC than WT. When AUC of each virus at 33°C and 37°C were compared, Delta and BA.1 replicated more efficiently at 37°C than 33°C, BA.1.1 replicated to higher titres at 33°C than 37°C and there was no significant differences in replication efficiencies at 33°C or 37°C for WT and BA.2 (Figure 1G).
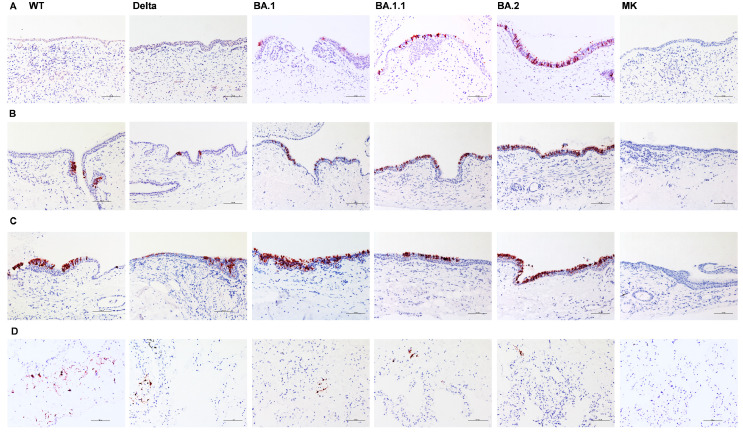
The tropism of the Omicron variants in the bronchial and nasal tissues was visualized by immunohistochemical staining of the SARS-CoV-2 nucleoprotein (Figure 2). There was no observable differences in cell tropism in the bronchus tissues.
Figure 2.
Tissue tropism of SARS-CoV-2 variants in ex vivo cultures of human respiratory tract. Immunohistochemical staining of SARS-CoV-2 nucleoprotein in ex vivo cultures of human nasal turbinate at 48 hpi (A), bronchus with infection at 33°C (B), 37°C at 72 hpi (C) and lung tissues at 72 hpi (D) infected with wild-type (WT), Delta and Omicron variants (BA.1, BA.1.1 and BA.2). Positive cells are red-brown. Scale bar, 100 μm. The images are representative of two individual donors.
Omicron variants have a lower replication competence in human lung tissues than previous strains
Similar to our previous results10 in ex vivo cultures of human lung tissues, WT replicated to higher titres than BA.1 as early as at 24 hpi, while significantly higher viral loads of WT infection were detected when compared to all three Omicron variants (BA.1, BA.1.1 and BA.2) at both 48 and 72 hpi (Figure 1H). The Delta variant replicated to higher titres than BA.1 and BA.1.1 at 48 hpi and higher titres than BA.1.1 at 72 hpi. AUC levels also show the higher replication capacity of WT and Delta than all three Omicron variants (Figure 1I).
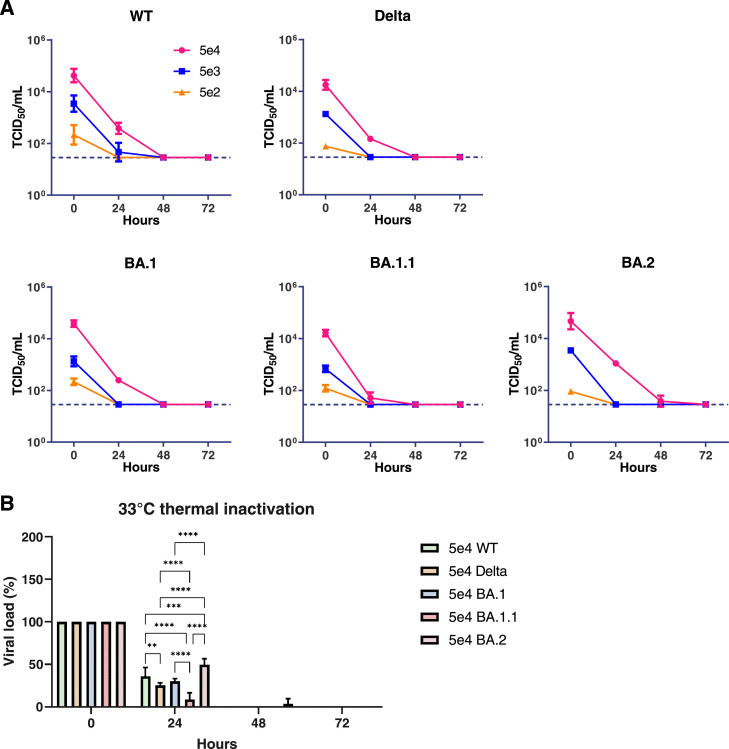
BA.2 has higher thermal stability than previous strains at 33°C
In order to investigate the stability of infectious virus particles at 33°C and 37°C, thermal inactivation of each virus was performed by incubating the virus at various concentrations at 33°C or 37°C. The infectious virus titres were monitored by TCID50 assay (Figure 3A). The infectious virus concentrations at or below 5×103 TCID50/mL reduced to the detection limit after 24 h incubation at 33°C while all viruses at 5×104 TCID50/mL reduced to the detection limit after 24 h incubation at 37°C except WT at 5×104 TCID50/mL remained 9% (Supplementary Figure 1). Using titres of 5×104 TCID50/mL at 0 h as reference, we found that BA.2 had the highest percentage of residual infectious titres (50%) after 24 h incubation at 33°C, followed by WT (36%), BA.1 (30%), Delta (25%) and BA.1.1 (9%) (Figure 3B).
Figure 3.
Thermal inactivation curves at 33°C. Infectious virus titres in cell culture wells at 33˚C (A). The horizontal dotted line denotes the limit of detection in the TCID50 assay. Percentages of infectious viral titre calculated with reference to the titres at 0 h (B). Graphs show the mean values. Error bars indicate SD with N = 3 independent experiments. **P < 0.01; ***P < 0.001; ****P < 0.0001, (Two-way ANOVA followed by Tukey's test).
To determine whether the difference in replication was due to host or intrinsic viral factors, we next explored the replication competence of the Omicron variants at 33°C and 37°C. Vero E6-TMPRSS2 cells were infected with Omicron BA.1, BA.1.1 and BA.2 at a MOI of 0.1 and incubated at 33°C or 37°C for 1 to 48 h. Virus titres in the culture supernatants were monitored by TCID50 assay at indicated time points (Supplementary Figure 2). All three Omicron variants replicated more efficiently at 37°C than 33°C at 24 hpi. The AUC analysis confirmed the higher replication competence at 37°C than 33°C. All three variants had similar replication efficiency at 33°C and 37°C and no significant differences was detected (Supplementary Figure 2). These findings suggest that there was no differences on the temperature sensitivity between the three Omicron variants in cell lines. The higher replication competence of BA.2 over BA.1 at 33°C in nasal and bronchi is probably specific to the virus-host interactions in the different tissues.
Discussion
The higher replication capacity of BA.2, and to a lesser extent of BA.1.1, compared with BA.1 and previous strains in nasal tissues provides laboratory evidence supporting epidemiological data that BA.2 has higher transmissibility than BA.1.13, 14 The body temperature of the upper airways (approximately 33°C) is lower than that in the lower airways (37°C). Therefore, infection of the nasal and bronchial tissues at these two temperatures may provide information reflecting the virus phenotype at the upper and lower airways, respectively. We found that BA.2 replicated equally well at both 33°C and 37°C in the bronchial tissues and more efficiently than the previous virus strains (WT, Delta and BA.1) while BA.1 replicated more efficiently at 37°C than 33°C. In addition, the higher viral load of BA.2 than BA.1 variants and previous virus strains in the upper respiratory explants of nasal turbinate is in line with the findings from the United Kingdom and Denmark that BA.2 variant may be more transmissible than the BA.1 variant13, 14 and our findings may explain the higher transmissibility of BA.2 than BA.1 and the replacement of BA.1 in many countries.
The enhanced replication of Omicron variants in the human bronchi compared to Delta and WT may be attributed to the mutations near the furin cleavage site in BA.1 and BA.2. The mutation of S:655Y in the spike, which is found in BA.1, BA.1.1 and BA.2, enhances viral replication, spike protein cleavage, transmissibility than its ancestor S:655H in a hamster model and outcompetes S:655H in both hamsters and a human primary airway model.15 Another hamster study reports that Omicron viral RNA lasts longer in the oral swab and the viral antigen is found mainly in the bronchioles in the infected animals when compared to Delta or ancestral virus infection,16 which may be a major source of excreted virus particles and hence may contribute to increased transmission. The combination of mutations S477N, Q498R and N501Y in the S protein of the Omicron variants increases the binding affinity to ACE2 in in-vitro evolution studies.17
Our findings of BA.2 and BA.1 having lower tropism in human lung tissues supports existing clinical data that that BA.2 may cause less severe disease than Delta and WT. Our findings are consistent with experimental studies on the pathogenicity in BA.1 and BA.2 in infected mice and hamsters which show that BA.1 and BA.2 viruses led to less pathogenicity than the early virus strains.18 The finding that Omicron replicated less efficiently in the human lungs is in line with a recent report that less viral antigen was stained in the hamster lung after Omicron infection compared to Delta and ancestral strain and Omicron has an attenuated pathogenicity in hamster models.16 It has been reported that Omicron is less fusogenic than Delta variant and ancestral strain since the spike protein of Omicron is less efficiently cleaved into two subunits when compared to that of Delta and ancestral SARS-CoV-2.16,19 These findings of low fusogenic potential of the Omicron spike could translate to impaired cell-cell spread, which may indicate less pathogenicity of Omicron when compared to variants with higher fusogenic potential such as Delta. Furthermore, by assessment of inflammatory responses in the lungs of wild-type and hACE2 transgenic mice, BA.2 had significantly higher levels of some pro-inflammatory cytokines and chemokines (such as IL-1β, IFNγ, and MIP-1β in wild-type mice and IL-1α, TNF and MIP-1α in hACE2 mice) compared with mice infected with BA.1.20 However, the levels of many pro-inflammatory cytokines and chemokines were lower in the lungs of both BA.1- and BA.2-infected mice than in those of ancestral- and Beta variant-infected mice, probably owing to the low replication of BA.2 in the lungs than the previous variants.20 Therefore, similar to BA.1, BA.2 induced a limited pro-inflammatory cytokine / chemokine responses in mouse lungs and it is less pathogenic than previous variants.20
Our findings are consistent with a previous publication showing that BA.1 omicron replicated more efficiently in human bronchial tissues than the previous variants including WT and Delta variants while less efficiently in human lung tissues than WT and Delta variants.10 Several recent studies report that BA.2 is sensitive to E64d, a cathepsin inhibitor that blocks the endocytosis-dependent entry of SARS-CoV-2, like BA.1 while Delta is resistant to E64d.10,19,21 The shift of cellular tropism away from TMPRSS2 expressing cells confers BA.1 and BA.2 to have altered pathogenesis from previous variants.
Our findings are in concordance with clinical reports showing that no epidemiological or clinical differences were observed between BA.1 and BA.2 patients4 and that both BA.1 and BA.2 infected patients were less likely to be hospitalized, required less intense respiratory support and had a reduced period of hospital stay.22
The three Omicron variants have amino acid substitutions in the nucleocapsid protein (R203K and G204R), which are not found in WT and Delta variants and these have been associated with enhanced virus replication.23 Our findings that BA.2 had a higher replication competence than BA.1 in the human nasal and bronchus at 33°C may be attributed by the additional mutations in BA.2. BA.2 has four unique amino acid substitutions (S:S371F, S:T376A, S:D405N, S:R408S) in the receptor binding domain (RBD) while it loses two mutations (SG446S, SG496S) in the ACE2 binding motif compared to BA.1 (Supplementary Table 3).24 The relationship between these mutations in the RBD and their replication capacity in the upper airway remains to be elucidated.
The replicase-transcriptase complex is mainly encoded from the sequences of ORF1a and ORF1b.25 Despite amino acid substitutions in the Spike, there are a number of unique mutations in ORF1a, ORF1b, ORF3a and ORF6 of BA.2, which are not in BA.1 (Supplementary Table 3). These mutations within the ORF1a and ORF1b may affect the replication and transcription efficiency of BA.1 and BA.2 and further investigation is needed.
While the genetic makeup of a virus could provide some information on the functions of a particular mutation site, biological study using authentic viruses in physiologically relevant models could provide more insights on the replication and tropism of the SARS-CoV-2 variants because individual mutation could have epistatic or independent effects that counteract the functions of each other. A recent report demonstrates that despite the presence of mutations predicted to facilitate the cleavage of spike S1/S2, the spike of Omicron variant is less efficiently cleaved when compared to Delta variant.19 Furthermore, host factors would also play a role in the differential replication capacity of the variants in terms of the expression of the receptors including ACE2 and TMPRSS2, and the activity of these enzymes and proteases in different tissues.
In this study, we used authentic viruses and ex vivo models of human respiratory tissues, which are highly physiologically relevant to the human body, and we believe that the ex vivo models can provide more accurate information reflecting the phenotype of the SARS-CoV-2 variants in the human respiratory tissues when compared to in vitro models. However, the number of donors is small (between 4 to 6) and this is due to the limitations of human respiratory tract tissue availability. Another limitation is only one virus strain from each variant was tested. Besides, several omicron subvariants have been detected—BA.2.12.1 was first identified in the United States and BA.4 and BA.5 were first detected in South Africa. BA.5 and BA.4 subvariants currently outcompeting the previous BA.1 and BA.2 subvariants and are circulating in 83 and 73 countries, respectively.26 While BA.4 and BA.5 share similar mutations in the S gene, BA.2.12.1 contains unique mutations.27 Several reports show that while immune evasion of BA.2.12.1 is only moderately enhanced when compared to BA.2, a more robust immune evasion by BA.4 and BA.5 is detected.27,28 Further investigations on the biological characteristics of these subvariants would provide insights on their transmission and pathogenicity.
In summary, our data demonstrate that BA.2 has intrinsically higher replication competence in the human upper respiratory tract (nasal and bronchi tissues) at 33°C compared with WT, Delta and BA.1. This may contribute to increased transmission of BA.2 in the human population compared to the early virus strains. Furthermore, the similar and lower tissue tropism of BA.1, BA.1.1 and BA.2 in lungs compared to previous strains is in line with the clinical manifestations of decreased disease severity of patients infected with the Omicron strain compared with other ancestral strains.
Contributors
KPYH: study design, coordination, analysis, interpretation of results and writing of the manuscript; KCN, JCWH, HWY, RHHC: perform experiments, analysis and interpretation of results; HG: perform analysis and interpretation of sequencing results; JCKC, VLYC, KYS, MKYH, TWKA: provide human nasal, lung and bronchus tissue and critical review of the manuscript; LLMP: analysis and interpretation of sequencing results and critical review of the manuscript; MP: analysis and interpretation of results, writing and revision of the manuscript; JMN: concept design, analysis, co-ordination of sample collection, and interpretation of results from immunohistochemistry staining and critical review of the manuscript; MCWC: study design, overall coordination, interpretation of results and writing of the manuscript. All authors read and approved the final version of the manuscript. KPYH and MCWC verified the underlying data.
Data sharing statement
All the data pertaining to the study are available in the manuscript or in the supplementary materials.
Declaration of interests
The authors declare no competing financial interests.
Acknowledgements
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (Contract No. 75N93021C00016) and the Theme-Based Research Scheme (Ref: T11-705/21N and T11-712/19-N) under University Grants Committee of Hong Kong Special Administrative Region. Authors acknowledge the technical support of Timmy Shack, Tonia Tong Kam, Cassia Lin (School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China) and Kevin Fung (School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104232.
Appendix. Supplementary materials
References
- 1.Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2022. COVID-19 Weekly Epidemiological Update, 18 May 2022. [Google Scholar]
- 3.CoVariants. Overview of variants/mutations. https://covariants.org/variants. Accessed 22 April 2022.
- 4.Fonager J, Bennedbæk M, Bager P, et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Euro Surveillance. 2022;27(10) doi: 10.2807/1560-7917.ES.2022.27.10.2200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora P, Zhang L, Rocha C, et al. Comparable neutralisation evasion of SARS-CoV-2 omicron subvariants BA.1, BA.2, and BA.3. Lancet Infect Dis. 2022;22(6):766–767. doi: 10.1016/S1473-3099(22)00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386(16):1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng SM, Mok CKP, Chan KC, et al. SARS-CoV-2 Omicron variant BA.2 neutralisation in sera of people with Comirnaty or CoronaVac vaccination, infection or breakthrough infection, Hong Kong, 2020 to 2022. Euro Surveillance. 2022;27(18) doi: 10.2807/1560-7917.ES.2022.27.18.2200178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Nat Acad Sci USA. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 11.Choi EM, Chu DKW, Cheng PKC, et al. In-flight transmission of SARS-CoV-2. Emerg Infect Dis. 2020;26(11):2713–2716. doi: 10.3201/eid2611.203254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit THC, Brackman CJ, Ip SM, et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586(7831):776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyngse FP, Kirkeby CT, Denwood M, et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: Evidence from Danish Households. medRxiv (Preprint) 2022 doi: 10.1101/2022.01.28.22270044. [DOI] [Google Scholar]
- 14.Elliott P, Eales O, Bodinier B, et al. Dynamics of a national Omicron SARS-CoV-2 epidemic during January 2022 in England. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-32121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escalera A, Gonzalez-Reiche AS, Aslam S, et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe. 2022;30(3):373–387. doi: 10.1016/j.chom.2022.01.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603(7902):700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahradník J, Marciano S, Shemesh M, et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nature Microbiol. 2021;6(9):1188–1198. doi: 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- 18.Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607(7917):119–127. doi: 10.1038/s41586-022-04856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R. SARS-CoV-2 Omicron spike mediated immune escape and tropism shift. Res Sq., (rs.3.rs) 2022 doi: 10.21203/rs.3.rs-1191837/v1. [DOI] [Google Scholar]
- 20.Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607(7917):119–127. doi: 10.1038/s41586-022-04856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamasoba D, Kimura I, Nasser H, et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. Cell. 2022;185(12):2103–2115. doi: 10.1016/j.cell.2022.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Xing N, Meng K, et al. Nucleocapsid mutations R203K/G204R increase the infectivity, fitness, and virulence of SARS-CoV-2. Cell Host Microbe. 2021;29(12):1788–1801. doi: 10.1016/j.chom.2021.11.005. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 25.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . 2022. Weekly Epidemiological Update on COVID-19 - 6 July 2022. [Google Scholar]
- 27.Arora P, Kempf A, Nehlmeier I, et al. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. Lancet Infect Dis. 2022;22(8):1117–1118. doi: 10.1016/S1473-3099(22)00422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. Nature. 2022 doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.