Abstract
Agroforestry systems (AFS) for cocoa production combine traditional land-use practices with local biodiversity conservation, resulting in both ecological and agricultural benefits. The cacao-cabruca AFS model is widely implemented in regions of the Brazilian Atlantic Forest. Carpotroche brasiliensis (Raddi) A. Gray (Achariaceae) is a tree found in cabruca landscapes that is often used for reforestation and biotechnological applications. Despite its importance, we still lack information about viruses circulating in C. brasiliensis, particularly considering the possibility of spillover that could affect cocoa production. In our study, we analyzed the Carpotroche brasiliensis virome from Atlantic Forest and cacao-cabruca AFS regions using metatranscriptomics from several vegetative and reproductive organs. Our results revealed a diverse virome detecting near-complete or partial coding sequences of single- and double-stranded DNA and RNA viruses classified into at least six families (Botourmiaviridae, Bromoviridae, Caulimoviridae, Genomoviridae, Mitoviridae, and Rhabdoviridae) plus unclassified elements. We described with high confidence the near-complete and the partial genomes of two tentative novel viruses: Carpotroche-associated ilarvirus and Carpotroche-associated genomovirus, respectively. Interestingly, we also described sequences likely derived from a rhabdovirus, which could represent a novel member of the genus Gammanucleorhabdovirus. We observed higher viral diversity in cacao-cabruca AFS and reproductive organs of C. brasiliensis with preferential tropism to fruits, which could directly affect production. Altogether, our results provide data to better understand the virome in this unexplored agroecological interface, such as cacao-cabruca AFS and forest ecosystem, providing information on the aspects of virus–plant interactions.
Keywords: virome, agroforestry, metatranscriptomics, rainforest tree, Carpotroche brasiliensis
Introduction
The agroforestry-based management systems (agroforestry systems, AFS) aggregate types of traditional land-use practices involving the deliberate combination of crops, animals, and tree vegetation for agricultural commodities production, and are often used by small and large agricultural producers around the world (Nair, 1993, 2012). AFS usually provide the greatest agricultural gain in ecosystem services that are beneficial to productivity, and, in turn, biodiversity is maintained in the environment (Bhagwat et al., 2008; Teixeira et al., 2021). Nevertheless, plantation management must occur in areas close to the conserved forests for increased benefits (Faria et al., 2007). In Brazil, pastureland, cropland, monoculture tree plantations, and mosaics of AFS have been used in regions of younger native forests or remnants of old-growth forests to maintain the ecosystem services (Joly et al., 2014; Rosa et al., 2021).
One example of AFS use in Brazil is the cacao-AFS. Cocoa fruits are produced by the Theobroma cacao L. (Malvaceae) and are cultivated in over 606,794 hectares (ha) of Brazilian territory, responsible for producing 5% of the world’s cocoa (Gama-Rodrigues et al., 2021). Of this area, 430,051 ha are part of the Atlantic forest in the States of Bahia and Espírito Santo (Gama-Rodrigues et al., 2021). The cacao-cabruca AFS model is practiced in about 250,000 ha of Atlantic Forest in southern Bahia state, an area that holds most of the forest in northeastern Brazil (Martini et al., 2007; Sambuichi et al., 2012; Gama-Rodrigues et al., 2021). This AFS model contributes to 44% of the 259,425 tons of national cocoa produced in 2019 (Gama-Rodrigues et al., 2021). The cabruca system consists of random intercropping of cocoa in native forest strata, exploiting native or exotic trees to provide shade. This ecosystem environment is characterized by a high diversity of trees that may vary according to age, environmental conditions, and management of the areas (Sambuichi et al., 2009, 2012; Gama-Rodrigues et al., 2021).
Carpotroche brasiliensis (Raddi) A. Gray (Achariaceae) is a plant species native to the Atlantic Forest that is found in cabruca landscapes (Sambuichi and Haridasan, 2007). The species, whose vernacular name is sapucainha, is widely used in reforestation programs, environmental restoration, and agroforestry systems due to shade tolerance (Brito-Rocha et al., 2017; Cerqueira et al., 2018) and its capacity to provide resources for wildlife (Marangon et al., 2010; Zucaratto et al., 2010). The fruits of C. brasiliensis are consumable by wild animals, mainly rodents, and marketed to the cosmetics industry that makes use of its oil in esthetics products (Lima et al., 2020). The Chaulmoogra oil can be extracted from C. brasiliensis seeds, and bioactive fatty acids like chaulmoogric and hydnocarpic acids have anti-inflammatory, analgesic, and antiparasitic pharmacological properties (Sharma and Hall, 1991; Parascandola, 2003; Lima et al., 2005; dos Santos et al., 2008; Krist, 2020). Therefore, the valorization of C. brasiliensis is fundamental to adding economic and ecological value to the cacao-AFS, and the evaluation of aspects of the microbial biodiversity, such as the viral diversity, results in a greater understanding of these unexplored ecosystems of tree species, to avoid the reduction of native biodiversity in agroforestry systems (Alexander et al., 2014; Piasentin et al., 2014).
Advances in plant virology after the emergence of high-throughput sequencing (HTS) have accelerated the identification of novel virus species from crops and fruit trees in agricultural ecosystems, expanding the knowledge of viral epidemiology in intensive and diverse production systems such as AFS models (Maclot et al., 2020). In Brazil, due to recurrent virus outbreaks in crops of economic importance, more than 200 virus species infecting plants were cataloged and officially recognized by the International Committee on Taxonomy of Viruses (ICTV) until 2018 (Kitajima, 2020). However, little is known about the ecology of viruses infecting wild hosts in native ecosystems adjacent to the crop fields, and the latter is known as an agroecological interface (Alexander et al., 2014; Roossinck and García-Arenal, 2015; Rodríguez-Nevado et al., 2020). Moreover, there is a real risk of spillover of virus infection between plants, which is common among plant viruses and emergent species, such as cacao swollen shoot virus (CSSV) (Muller, 2016). This virus seems to have originated from indigenous forest trees that work as alternative hosts for pathogens (Posnette et al., 1950; Dzahini-Obiatey et al., 2010; Topolovec-Pintaric, 2020). Interestingly, other groups of viruses are undergoing evolutionary radiation, adapting to infect new plant species in different environments (Pagán, 2018; Moury and Desbiez, 2020).
Plant viruses may act in different ecological contexts along the evolutionary relationships established with the host. These viral interactions may lead to asymptomatic infections in latent or persistent viral cycles, through the integration of fragments derived from the viral genome into the host DNA as endogenous viral elements (EVEs) (Lefeuvre et al., 2019). EVEs may have a beneficial relationship for adaptation in environments or promote competition in plant communities by affecting virulence and host tolerance/susceptibility, and subsequently affect the epidemiological profile of viral pathology in the ecosystem (Engering et al., 2013; Lefeuvre et al., 2019; Takahashi et al., 2019). Understanding how evolutionary forces act in distinct environments, together with the anthropic influence on the emergence of diseases, can provide knowledge to forecast and avert alterations in natural ecosystems that cause crop damage (Lefeuvre et al., 2019). The advances in plant virology are needed mainly for forest tree species that have a current few viral diversity data in their respective forest ecosystems (Rumbou et al., 2021).
In our study, we analyzed the virome of C. brasiliensis from Atlantic Forest regions and private properties of southern Bahia using cacao-cabruca AFS through HTS of the RNA samples obtained from vegetative and reproductive organs. Our results showed that the C. brasiliensis virome is composed of members spanning almost all members of the Baltimore classification of viruses [+ssRNA, double-stranded (ds)RNA, –ssRNA, reverse-transcribing (RT)-DNA, –ssDNA, dsDNA] that could be classified into at least six distinct families (Botourmiaviridae, Bromoviridae, Caulimoviridae, Genomoviridae, Mitoviridae, and Rhabdoviridae) together with three different unclassified elements. Of note, we successfully reconstituted with high confidence the complete coding sequence of two novel viruses (Carpotroche-associated ilarvirus and Carpotroche-associated genomovirus) and partial sequences from a virus likely representing a novel member of the genus Gammanucleorhabdovirus. Proteomics assay from Carpotroche seeds detected peptides from many viral sequences, further confirming the virus presence. Finally, we show the restricted occurrence of viruses from distinct families in exclusive ecosystems and specific organs of C. brasiliensis, as well as discrepant abundance among samples. Therefore, our results provide background for a better understanding of the viral diversity in the context of the agroecological interface, such as cacao-cabruca AFS and forest ecosystem.
Materials and methods
Sampling design
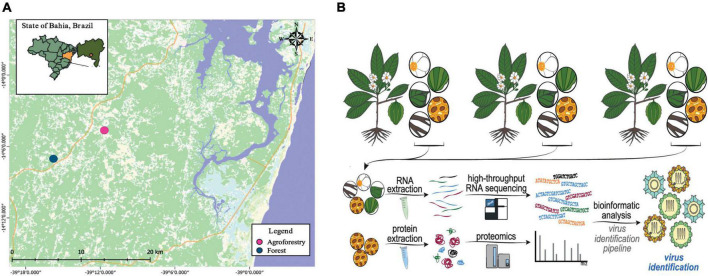
Samples from six different organs of C. brasiliensis (leaf, flower, flower bud, root, fruit, and seed) were obtained from each individual in June 2014 in Camamu-Maraú Country, State of Bahia, Brazil. All samples were extracted from asymptomatic individuals with no discoloration of any kind, wilting, or necrotic lesions. Sixteen adult trees were randomly selected to compose two sampling groups: eight from agroforestry systems and eight from natural Atlantic Forest ecosystems (Figure 1A; Supplementary Table 1). All samples were immediately frozen in liquid nitrogen and stored at −80°C for RNA extraction at the Center of Biotechnology and Genetics, Laboratory of Molecular Markers, Universidade Estadual de Santa Cruz (UESC), Brazil.
FIGURE 1.
Experimental design of the study. (A) Geographic location in the Brazilian Atlantic forest (blue spots) and agroforestry systems (pink spots) where the Carpotroche brasiliensis samples were collected. (B) Simplified scheme showing the strategy applied in our work, including tissue collection, RNA or protein extraction, RNA deep sequencing, mass spectrometry, and bioinformatics analysis.
Plant material, RNA extraction, cDNA library preparation, and high-throughput sequencing
Total RNA was extracted from each sample using RNAqueous® Total RNA Isolation Kit (AM1912, Thermo Fisher Scientific), following the manufacturer’s recommendations. The integrity and quantity of RNA were confirmed using the TapeStation Agilent 2200 instrument (Agilent Technologies Co., Santa Clara, CA, United States), considering an RNA Integrity Number (RIN) value above 5. Only RNA samples of vegetative and reproductive organs with integrity and quantity acceptable were used to generate the 20 cDNA libraries. The cDNA libraries were constructed using 10 ng of total RNA and using the NEBNext Ultra RNA Library Prep Kit for Illumina (E7530S, New England Biolabs, Inc., Ipswich, MA, United States) and the NEBNext Multiplex (E7335, New England Biolabs, Inc., Ipswich, MA, United States) Oligos, following the manufacturer’s protocols (Illumina, San Diego, CA, United States). The libraries were quantified with KAPA Library Quantification Kit for Illumina Platforms (KAPA Biosystems, Wilmington, MA, United States) and Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, DE, United States). A total of 20 cDNA libraries (11 from samples of agroforestry systems and 9 samples of Atlantic Forest ecosystems) were sequenced on Illumina MiSeq 2 × 250 bp pair-end sequencing (Supplementary Table 1) to provide biological replicates. Raw sequencing data were deposited at NCBI Sequence Read Archive (SRA)1 under project number PRJNA858666.
Pre-processing, quality control, and transcriptome de novo assembly
Trimmomatic software v.0.32 was used to filter out sequences not fitting in following criteria: minimal average Phred score: 33, Leading: 30, Trailing: 30, Sliding window 4: 30, and Minlen: 50 (Bolger et al., 2014). Pre-processed libraries obtained from different plant tissues were used as input for the transcriptome assembly in the SPAdes tool (Bankevich et al., 2012) in two steps: initially, an assembly of the 20 libraries was made using the -rna parameter, with all other parameters kept as default; subsequently, contigs that showed sequence similarity to viral sequences in the previous step were used as an anchor for the new assembly, setting the –trusted-contigs parameter. An overview of the methods is shown in Figure 1B. We also performed a metagenomic analysis to determine the presence of possible contaminations using the Kaiju platform (Menzel et al., 2016).
Virome analysis
Assembled contigs were used as queries in the Diamond tool (Buchfink et al., 2015) against amino acid sequence databases to identify sequences of possible viral origin. In this analysis, we considered only the best hit for each contig and with an e-value < 1e-3. The result was further filtered using regular expression and manually inspected to select only hits of viral origin. The contigs with putative viral origin were submitted to CAP3 (Huang and Madan, 1999) and CD-HIT (Fu et al., 2012) tools to remove redundancy and extend assembled contigs. For CD-HIT clustering, sequences with 90% coverage and 90% identity were merged, and a representative sequence was defined. The putative viral contigs were analyzed by the NCBI BLAST online tool (Johnson et al., 2008) to guarantee the last updated version of nucleic acid databases. The sequences were submitted to BLASTN against the nt database and to BLASTX against the nr database to obtain the best hit, to confirm the results. The contigs that showed characteristics of viral sequences (contig length and hit with viral sequences) were subsequently analyzed in the ORFfinder tool2 to perform its structural annotation. Finally, conserved domains were annotated in each contig using the PHMMER tool (Potter et al., 2018) under the following parameters: sequence e-value 0.01 and hit e-value 0.03. For the quantification of viral sequences, we built the transcriptome index using the putative viral contigs that were compared to each library using Salmon (Patro et al., 2017) with standard parameters. Counts were used to estimate the relative abundance at the transcript level through transcripts per million (TPM) and plotted as heatmap using the ComplexHeatmap package in R (Gu et al., 2016). Reconstituted high-quality viral sequences used in the phylogeny were deposited at the NCBI nucleotide database (GenBank) under accessions OL964097–OL964101. All viral sequences assembled in our work are also available in Supplementary Data Sheet 1.
Phylogenetic analyses
The assembled contigs were translated into amino acid sequences and aligned with the closest viral sequences available in the NCBI protein database (Geer et al., 2010) using the MAFFT program (Katoh and Standley, 2013). The best-fit model was selected for each alignment using the ProtTest 3.2 program considering the Akaike Information Criterion (AIC) (Akaike, 1974; Abascal et al., 2005). Maximum likelihood (ML) trees were constructed in MEGA X (Kumar et al., 2018), considering 1,000 bootstrap replicates. The trees generated were mid-term rooted and edited in Geneious Prime 2021.3 The sequences from the viruses common bean curly stunt virus (unclassified, tentative member of the family Geminiviridae), peanut clump virus (Pecluvirus genus) (NP_620047), and ampivirus A1 (YP_00913521) were used as outgroup for the phylogenies produced.
Protein analysis and mass spectrometry
Sample preparation
Total proteins were extracted from separate pools of whole seeds derived from staminated individuals using a protocol developed by Pirovani et al. (2008). Briefly, seeds were macerated in the presence of liquid nitrogen and 7% polyvinylpolypyrrolidone. In total, 0.1 g of seeds were used for three replications for stages S1 and S2. The samples were resuspended in 500 μL of 1-butanol: chloroform (1:9), and the mixture was vortexed and centrifuged for 5 min at 13,400 xg at 4°C. This procedure was repeated two times. Subsequently, the precipitate generated by centrifugation was washed two times with 500 μL of 100% acetone and centrifuged as in the previous step. Finally, the precipitate was washed with 500 μL of petroleum ether and centrifuged for 5 min at 13,400 xg at 4°C. After completion of delipidation, the samples were subjected to precipitation and protein extraction (Pirovani et al., 2008), with the modification being the incubation of the samples overnight at −20°C in the washing step with 10% trichloroacetic acid (TCA) in water. The final precipitate was resuspended in 400 μL of urea at 8 M. At the end, the proteins from the samples were stored in a freezer at −20°C until further use. The protein extracts obtained were quantified with the 2D Quant Kit (GE Healthcare Life Sciences, Chalfont, United Kingdom) following the manufacturer’s recommendations. A standard curve of bovine serum albumin (BSA) was constructed, which served as a basis for the quantification of samples of C. brasiliensis seeds.
Mass spectrometry
The solution containing the digested peptides was desalted using tips with C18 resin (10 μL; Millipore® Ziptips C18). The peptides were eluted in 50 μL of a solution containing 75% acetonitrile, 25% water, and 0.1% formic acid. The peptides were analyzed in a liquid chromatography system (Agilent 1290 Infinity II HPLC) coupled to a quadrupole/time-of-flight mass spectrometer (Agilent 6545 LC/QTOF) (Agilent Technologies, Santa Clara, CA, United States). Samples were separated using a reversed-phase column (C18; AdvanceBio Peptide Mapping 2.1 × 250 mm; Agilent), maintaining a temperature of 55°C. A 20-min gradient was applied with mobile phases A (water and 0.1% formic acid) and B (acetonitrile and 0.1% formic acid). The percentages of phase B along the grid were 5–35% (1–10 min.), 35–70% (11–14 min.), 70–100% (1618 min.), and 100% (16–20 min.). In addition, a final period of 5 min was programmed for column stabilization. Then, the samples were injected into three technical replicates. The samples were injected into the QTOF through an electrospray source, using the Auto MS/MS acquisition mode, with a maximum selection of 10 precursors per cycle. The parameters for the selection of precursors were as follows: threshold of 1,000, 10,000 counts/spectrum, the stringency of 100% purity, a cut-off of 30% purity, peptide isotopic model, charge preference of 2, 3, >3, and unknown. The instrument parameters were set as follows: gas temperature of 325°C, the gas flow of 13 L/min, a capillary voltage of 4,000 V, and skimmer voltage of 56 V. Nitrogen gas was used for the induced dissociation collision. Instrument control (HPLC and QTOF) and parameter configuration were performed using the Agilent MassHunter Acquisition software.
Identification of virus-derived peptides
The data integration of transcriptomic and proteomic analyses was implemented through mass spectrometry (MS) analysis to discover proteomics from C. brasiliensis. The identification of peptides derived from viral proteins was performed from spectral extraction and merging through analysis with Spectrum Mill Proteomics Workbench (Agilent Technologies, Santa Clara, CA, United States) against proteomic data from C. brasiliensis seeds. The static modification to carbamidomethylation was set to default, with a mass (MH +) range of 200–6,000 mass–charge ratio (m/z). Retention time tolerance was ± 60 s, m/z tolerance was ± 1.4 m/z, MS noise threshold was set to 10 counts, and charge general was set to default. The data searched were filtered for validation by score threshold with a false discovery rate (FDR) > 1%. The spectral intensity of identified proteins was searched against the viral ORFs analyzed. The MS/MS spectral comparison included four miss-cleavage sites and fixed modifications: carbamidomethylation on cysteine residues (C), differential modifications for oxidized methionine (M), pyroglutamic acid (N-termQ), deamidated (N), phosphorylated S (S), phosphorylated T (T), and phosphorylated Y (Y). To determine the combined score of minimal intensity, 10% with a mass tolerance of 20 ppm was validated and filtered by false discovery rate (FDR) > 1%. All MS files produced in this study were deposited at MassIVE4 with the identifier MSV000089145 and can be accessed at ftp://massive.ucsd.edu/MSV000089145/.
Results
Metavirome analysis
We deep sequenced 20 cDNA libraries derived from the tissues of C. brasiliensis from forest and agroforestry ecosystems (cacao-cabruca AFS), totalizing 28,177,426 raw reads (Figure 1). After pre-processing steps, including quality and length filters, 24,689,370 (87.62%) reads were kept (Supplementary Table 1). Transcriptome assembly produced 281,643 transcripts with N50 of 481 bp and a total number of bases in transcripts of 160,909,318 (Supplementary Table 2). From the total, 184 sequences showed sequence similarity to viral sequences stored in NCBI public databases, of which 136 (75%) showed hits related to elements with non-retroviral origin. Retroviral sequences were discarded from the analysis since they were of low reliability and often are misidentified with transposable elements. As quality control, we performed metagenomic analyses that did not detect sequences from animals, indicating a low chance of contamination from external sources (Supplementary Figure 1 and Supplementary Table 3). After removal of redundancy and manual curation, we selected 30 sequences larger than 400 nt for further characterization. The length of putative viral contigs ranged from 503 nt to 8,695 nt.
Diversity and distribution of viral sequences
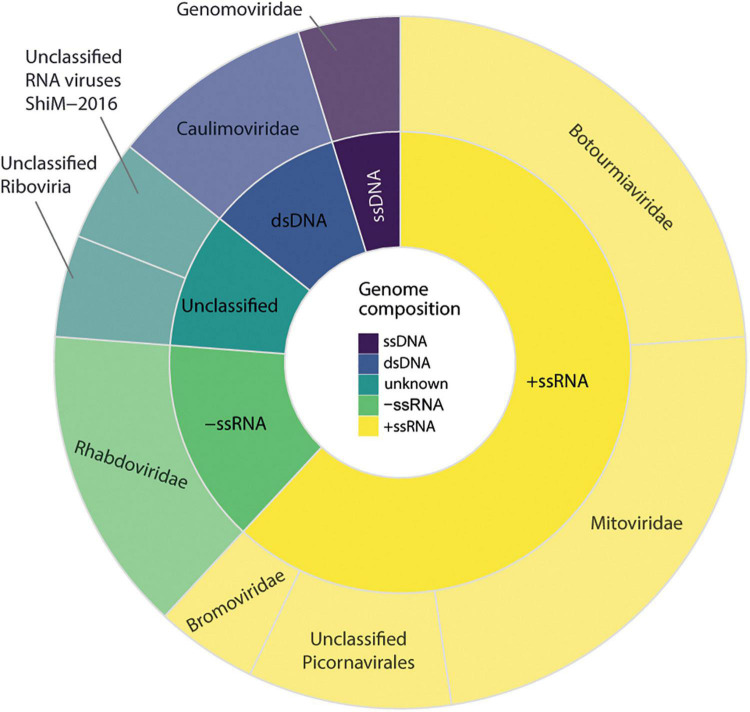
We observed that 30 sequences of possible viral origin are related to viruses from at least six different viral families. Of these, 17 sequences showed similarity to either RNA-dependent RNA polymerases, replicases, or polyproteins, referred hereafter as key sequences, and were considered in the diversity analysis (Figure 2). From the total of non-retroviral viral sequences identified, 16 sequences could be identified by nucleotide similarity, suggesting they are possibly strains of known viruses, and 14 were classified only at the amino acid level (Supplementary Table 4). Overall, sequences showed similarity to closely related viruses from at least six different families or unclassified. Eight transcripts showed similarity to Maize fine streak virus genus Gammanucleorhabdovirus (Rhabdoviridae, −ssRNA); four transcripts to viruses of the genus Ourmiavirus, three to an unclassified virus of the family Botourmiaviridae (+ssRNA), five transcripts to viruses from genus Mitovirus (Mitoviridae, +ssRNA), three transcripts to viruses of the genus Ilarvirus (Bromoviridae, +ssRNA), and two unclassified sequences showing similarity with picornavirus (+ssRNA); one transcript to viruses of the genus Caulimovirus, and one to viruses of the genus Solendovirus (Caulimoviridae, +dsDNA); one transcript to viruses of the genus Genomovirus (Genomoviridae, −ssDNA) and other two unclassified transcripts (Supplementary Table 4).
FIGURE 2.
Diversity of viral sequences identified in Carpotroche brasiliensis samples. Virus classification was performed based on the information of the closest related sequence present in NCBI databases classified by genome structure and viral family. The diversity analysis only included considered sequences derived from RNA-dependent RNA polymerases, replicase, or polyproteins.
These sequences were detected in the sum of all 20 C. brasiliensis libraries analyzed. Co-occurrence analyses indicated that all three segments of a tentative new virus from the genus Ilarvirus were detected in 16 samples, while at least two sequences appeared together in 18 different samples. The second most abundant family was Rhabdoviridae, genus Gammanucleorhabdovirus. In total, the sequences showing similarity to the viruses of the genus Gammanucleorhabdovirus were detected in nine libraries of C. brasiliensis with a maximum number of seven different contigs in a single library. Then, in decreasing order of abundance sequences presenting similarity with viruses from the families, Mitoviridae was detected in 12 libraries, Botourmiaviridae was found in 4 libraries, Caulimoviridae was found in 3 libraries, and elements of Picornavirales, Genomoviridae, and unclassified Riboviria were detected in two different libraries. Only one contig showing similarity with unclassified RNA viruses was restricted to a single library.
Characterization of near-complete viral genomes
To increase certainty for high-quality viral sequences, we performed an extra assembly step with Cap3. Four out of the 30 transcripts had their lengths extended, of which three sequences showed similarity to ilaviruses and one was likely derived from an unclassified picornavirus. Through manual curation via BLAST (Supplementary Table 4), it was possible to confirm the similarity of these elements to previously identified viruses available in the NCBI sequence databases.
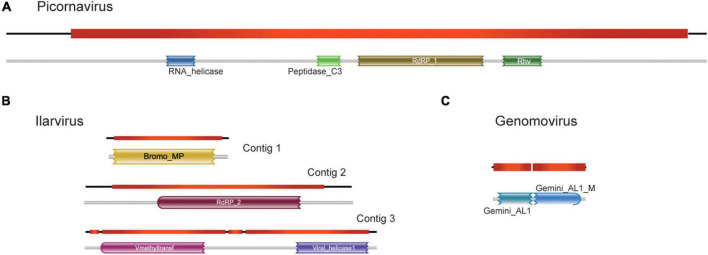
The largest transcript containing 8,636 nt was closely related to Skokie picorna-like virus (SPV), with a size 8,401 bp, 94% nucleotide sequence identity, and >97% of amino acid sequence identity, which is an unclassified picornavirus that infects the mite Dermatophagoides pteronyssinus. Remarkably, we observed very similar patterns of ORF distribution and domain organization when comparing this assembled transcript with SPV (Figure 3A; Supplementary Figure 2), suggesting a close evolutionary relationship. Indeed, they share conserved domains encoded by RNA-dependent RNA polymerase (PFAM PF00680) and capsid protein (PFAM PF00073) of viruses from the order Picornavirales (Figure 3A). For sequences presenting similarity with the elements of the family Bromoviridae, the Contig1 (2,442 nt) was closely related to the phytopathogenic virus Lilac ring mottle virus (LRMV), with 2,287 nt and 73% identity, which belongs to the genus Ilarvirus. Both sequences also share the same pattern of ORFs, although the main ORF in Contig1 is 11 amino acids longer than the main ORF of LMRV (Figure 3B; Supplementary Figure 3). Domain analysis indicates that this major ORF encodes for a Bromovirus movement protein (PFAM PF01573), increasingly the possibility of functionality (Figure 3B; Supplementary Figure 3). Contig2 (2,954 nt), on the other hand, was most similar to another phytopathogenic ilarvirus Citrus leaf rugose virus, with 2,990 nt and 72% identity. Besides having little difference in length, they also had similar ORF patterns (Figure 3B; Supplementary Figure 3). The major ORF differs from one other by only 36 bp, and both code for the RNA-dependent RNA polymerase enzyme (PFAM PF00978) (Figure 3B; Supplementary Figure 3). Contig3 (3,467 nt) was closely related to a phytopathogenic ilarvirus, Tomato necrotic streak virus (with 3,378 nt and 68% identity with segment RNA 1). Structural annotation shows that the longest ORF in both sequences differs only by 4 nt, which was only identified at the amino acid level as the replicase protein. Domain analysis also shows that both longest ORFs share methyltransferase (PFAM PF01660) and helicase (PFAM PF01443) conserved domains (Figure 3B; Supplementary Figure 3).
FIGURE 3.
Structural and functional annotation of high-confident viral sequences. For sequences showing similarity to viruses of the families (A) Picornavirus, (B) Ilarvirus, and (C) Genomovirus, the detected open reading frames (ORF) and the presence of conserved domain are shown.
We also selected one transcript showing similarity to that of the family Genomoviridae to undergo further structural and functional annotations. This transcript was chosen because the sequence recovered corresponds to an intact replicase with a length consistent with genomoviruses. This transcript (943 nt) showed the highest identity to chicken genomovirus mg4_1247 (complete genome: 2,142 nt; replicase: 1,008 nt, 62% identity) (Figure 3C; Supplementary Figure 4). Domain analysis revealed that both sequences present a conserved Gemini_AL1 domain (PFAM00799) (Figure 3C; Supplementary Figure 4).
Phylogenetic analysis of Carpotroche-associated viruses
We selected putative viral sequences with near-complete or complete coding sequences according to the closest relative virus in public databases to perform further phylogenetic characterization. Therefore, here we assessed the phylogenetic relationship of the three viral transcripts with their closely related viruses from the genera Picornavirus, Ilarvirus, and Genomovirus, respectively (Figure 4). The phylogeny of the sequence corresponding to a virus of the family Picornaviridae confirmed the similarity observed by local alignment with the Skokie picorna-like virus with 100% of bootstrap. This sequence was also related to the Bat posalivirus, suggesting that the assembled transcript belongs to the genus Picornavirus (family Picornaviridae) (Figure 4A). This virus was named Skokie picorna-like virus Carpotroche isolate. The transcript showing similarity to viruses of the genus Ilarvirus was close to the clade of plant ilarviruses containing viruses of the species Citrus leaf rugose virus, Tulare apple mosaic virus, Tomato necrotic streak virus, Spinach latent virus, Asparagus virus 2, Citrus variegation virus, and Elm mottle virus (Figure 4B), thus we can infer that this sequence is from the genus Ilarvirus (Bromoviridae) and the putative virus was named Carpotroche-associated ilarvirus. In the phylogeny of the element previously associated to Genomovirus, we observed that the sequence grouped with the virus chicken genomovirus mg4, and Gemycircularvirus sp. with a bootstrap value of 97.4%, indicating that this species belongs to the Genomoviridae (Figure 4C). This putative new virus was named Carpotroche-associated genomovirus.
FIGURE 4.
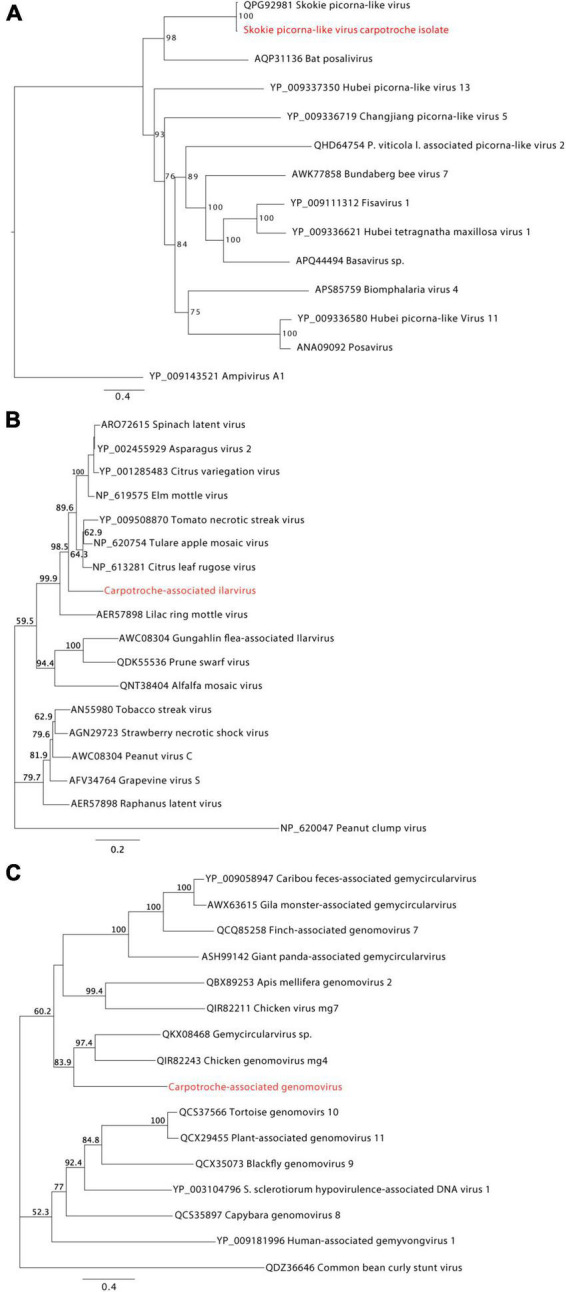
Phylogenetic analyses of high-confident viral sequences identified in Carpotroche brasiliensis. Phylogenetic trees inferred by maximum likelihood for transcripts associated with C. brasiliensis and showing similarity to (A) picornaviruses, (B) ilarviruses, and (C) genomoviruses species. RNA-dependent RNA polymerase or polyprotein aminoacid sequences were used in the analysis. Branch support was calculated using the bootstrap method, requiring 1,000 pseudoreplicates. Highlighted regions in red correspond to the assembled sequences obtained in our work.
Characterization of a tentative new virus from the genus Gammanucleorhabdovirus
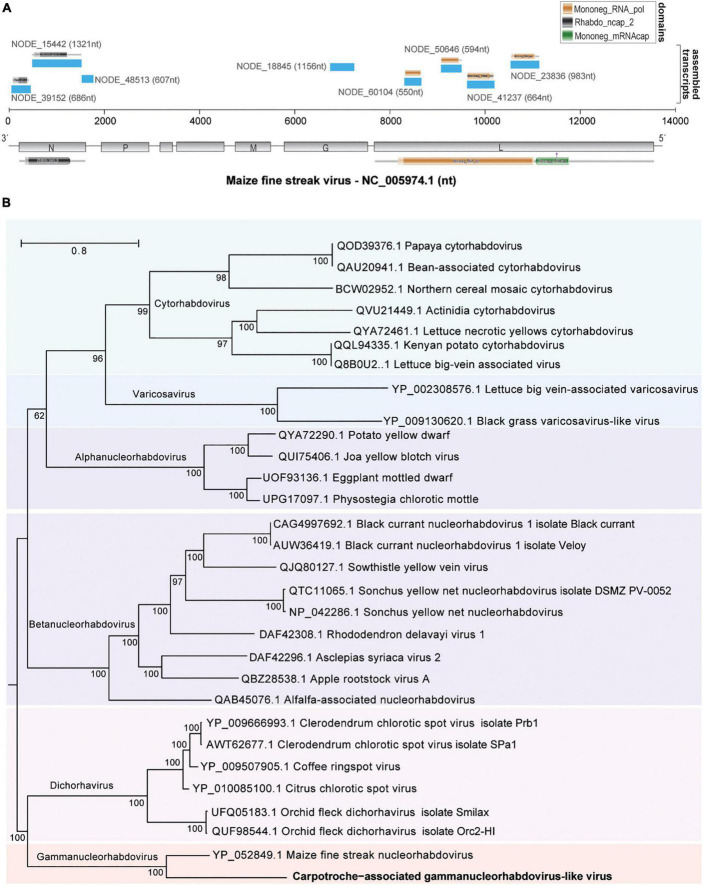
Metavirome analysis revealed a considerable number of viral transcripts likely derived from a plant-infecting rhabdovirus related to the viruses of the Gammanucleorhabidovirus genus. Indeed, 8 out of 30 viral transcripts presented similarity identity values at the protein level, ranging from 32.54 to 71.88%, with Maize fine streak virus (MFSV), the unique known member of the genus Gammanucleorhabdovirus genus accepted by ICTV (Figure 5A). These transcripts ranged from 550 to 1,321 nt totalizing 6,561 nt with an average size of ∼820 nt and were distributed along the MFSV genome. Six out of the eight contigs presented conserved domains commonly identified in rhabdoviruses, including MFSV (Figure 5A). Of note, we identified one transcript of 983 nt showing similarity to the RdRp gene, containing a Mononeg_RNA_pol (PF00946) domain. We took advantage of this transcript likely derived from viral polymerase to assess the phylogenetic relationship of the tentative virus with MFSV and other rhabdoviruses. According to our maximum likelihood tree, we observed the presence of six main clades, which refer to different genera of the subfamily Betarhabdovirinae—Rhabdoviridae (Cytorhabdovirus, Varicosavirus, Alphanucleorhabdovirus, Betanucleorhabdovirus, Dichorhabdovirus, and Gammanucleorhabdovirus) (Figure 5B). The transcript assembled clustered with Maize fine streak virus in a clade-specific manner with a bootstrap of 100, suggesting that the sequence probably belongs to a species from this genus (Figure 5B). However, since we were not able to reconstitute the complete genome of the virus, we named the viral species as a Carpotroche-associated gammanucleorhabdovirus-like virus.
FIGURE 5.
Characterization of a putative gammanucleorhabdovirus. (A) Distribution of assembled transcripts along the genome of the closest relative virus, Maize fine streak virus (MFSV). Conserved domains are indicated above each assembled transcript or below the MFSV genome. (B) Maximum likelihood tree containing sequences from viruses of the subfamily Betarhabdovirinae (Rhabdoviridae) based on the largest fragment, 983 nt, derived from L (polymerase) gene.
Ecosystem distribution and organ tropism
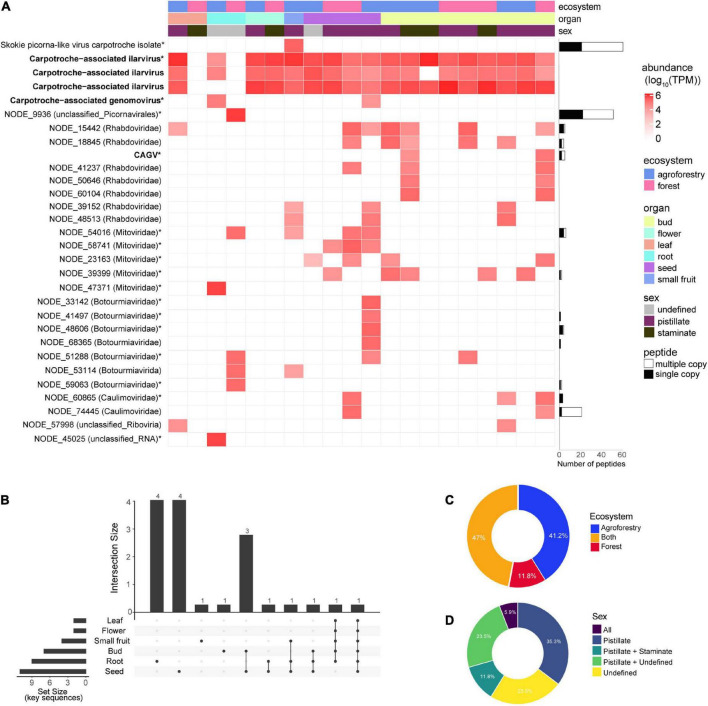
Since we deep sequenced the RNA of several tissues from vegetative and reproductive organs of C. brasiliensis originated from forestal ecosystems and agroforestry, we decided to explore the tropism of the identified viral sequences among the individuals assessed in this study. We also analyzed the relative abundance of viral elements detected in organ samples for each ecosystem. We noticed that the seed was the tissue with the greatest diversity of viral sequences, with the presence of 20 (11 key sequences) out of the 30 viral sequences identified in our study (Figures 6A,B). The libraries of floral bud, root, and small fruit also showed high percentages of viral diversity, with 18 (eight key sequences), 10 (eight key sequences), and 8 (three key sequences) viral sequences, respectively (Figures 6A,B). However, we acknowledge that these data have to be interpreted carefully, since we analyzed only one library of small fruit while we assessed nine libraries for floral buds. Besides, in one of the libraries from C. brasiliensis leaves, we were not able to detect any sequence of viral origin (Figure 6A). Of note, over half of the key sequences (10) were organ-specific, while approximately 22% were found in at least three different organs (Figure 6B). Surprisingly, only one viral sequence of Carpotroche-associated ilarvirus was identified in all tissues assessed (Figure 6B).
FIGURE 6.
Abundance and distribution of Carpotroche-associated viruses. (A) Heatmap was constructed based on the relative abundance of each viral transcript identified classified by ecosystem, tissue, and sex. Quantification was computed using transcripts per million (TPM). Horizontal bars indicate the abundance and uniqueness of peptides derived for each of the assembled transcript key sequences and are indicated with *. CAGV, Carpotroche-associated gammanucleorhabdovirus-like virus. The presence of key viral sequences by plant tissue (B), ecosystem (C), or sex (D).
Since tissue tropism analysis indicated seeds as containing the major number of viral sequences, we decided to perform a proteomics assay for the sample derived from whole seeds collected from staminated individuals to provide an additional level of confidence. From the 17 putative viral family elements identified in seeds, we were able to detect peptides for nine of them. We also detected peptides from NODE_15442 (Rhabdoviridae), NODE_18845 (Rhabdoviridae), NODE_68365 (Botourmiaviridae), and NODE_74445 (Caulimoviridae) sequences previously identified in other organs (Figure 6A—horizontal bars; Supplementary Table 5).
Relative to ecosystems, the abundance of viral elements in equivalent tissue samples is slightly higher in specimens originating from the cacao-cabruca AFS (41.2% of total sequences) when compared to the forest ecosystem. Viral sequences detected exclusively in the cacao-cabruca AFS were Skokie picorna-like virus isolate Carpotroche, Carpotroche-associated genomovirus, botourmiavirus (3), non-classified sequence (1), and mitovirus (1). In contrast, one sequence related to element from botourmiavirus and one related to unclassified picornavirus were identified exclusively in samples obtained from the forestal ecosystem (Figures 6A–C).
Regarding the reproductive organs, in both ecosystems, samples obtained from stamen tissues show a lower abundance and diversity in viral sequences compared to those obtained from pistil tissues (Figure 6D). While we noticed species that were organ-specific, none of the viral sequences were exclusively found in samples derived from staminate plants (Figure 6D).
Discussion
In this study, we identified viral sequences representing the C. brasiliensis-associated virome in native forest and cacao-cabruca AFS. Through HTS technologies associated with proteomics assay, it was possible to perform an in-depth analysis of viruses putatively associated with this plant, expanding our knowledge about environmental virology and describing the profile of viral infection among samples and ecosystems. Also, we enlightened the abundance and distribution of virus infection per vegetative and reproductive organs of C. brasiliensis and the composition of viral communities per sampled landscape.
The patterns of diversity, abundance, and virus tropism between vegetative and reproductive organs observed in this study can be related to the host preference of a virus, as well as to the immunological capacity in different cell types and tissues of the host (Navarro et al., 2019; Keesing and Ostfeld, 2021). Therefore, this can impact the amplification effects arising from increased transmissibility among hosts or dilution effects of infections in agricultural environments or in natural ecosystems (Keesing et al., 2006; Keesing and Ostfeld, 2021; Susi and Laine, 2021). Interestingly, we identified C. brasiliensis putative viruses related to two different families that have species described to infect different plant hosts of forest trees or crops, i.e., Ilarvirus and Mitovirus (Pallas et al., 2013; Rumbou et al., 2021). Of note, the highest diversity of these virus sequences was observed in reproductive organs. It is likely that viral exchange exists at the agroecological interface through the transmissibility of viruses detected in the reproductive structures of C. brasiliensis during pollination processes and dispersal over longer distances due to animal intermediation (Marangon et al., 2010; Zucaratto et al., 2010). Viruses with these characteristics can also mutate acquiring to the possibility to infect unrelated alternative hosts in the same ecological community, such as detected in cacao-cabruca AFS or forest ecosystem (Elena et al., 2009).
We observed widespread infection in C. brasiliensis by a tentative new virus of the genus Ilarvirus. Viruses of this genus can infect woody plants, such as C. brasiliensis, as well as fruits, vegetables, and forages of economic interest, such as peach, apricot, tomato, apple, vine, cucurbitaceae, banana, and alfalfa (Pallas et al., 2013). Ilarvirus infections range from no observable symptoms to the occurrence of leaf mosaics, structural malformations, abortive flowers, and atrophy in fruits and seeds, and in some cases, may lead to host death (Girgis et al., 2009). Contigs belonging to detected ilavirus (Carpotroche-associated ilarvirus 1, 2, 3) presented homology with three different viruses: citrus leaf rugose virus (CLRV), lilac ring mottle virus (LiRMoV), and tomato necrotic streak virus (TomNSV). CLRV can infect a wide range of citrus hosts with induction of milder symptoms, while LiRMoV can induce leaf deformation and reduce leaf size, ring spots, and line patterns (Sharma-Poudyal et al., 2016; Zhou et al., 2020). The infection with LiRMoV was observed in herbaceous plants in the experimental conditions, although it is possible that cryptic infection may exist in crop plants for many years (Sharma-Poudyal et al., 2016). On the other hand, TomNSV infects the Solanaceae and Chenopodiaceae families, producing serious damage to the leaves (Badillo-Vargas et al., 2016). However, we did not find any symptoms in C. brasiliensis individuals sampled.
Virus diversity observed in organs suggests an established viral community in C. brasiliensis in situ, with possible transmission between ecosystems via arthropod vectors, such as for the virus alfalfa mosaic virus, where thrips and aphid contamination is mediated by feeding on pollen grains or via direct contact with infected pollen, seeds, or fruits (Pallas et al., 2013; Silvestre et al., 2020). The arthropod vector-mediated contamination may also be related to the transmission of virus of the genus Gammanucleorhabdovirus (Rhabdoviridae), with different sequences detected in the samples of leaves, fruits, seed, and flower buds obtained from C. brasiliensis, showing similarity to the segments of MFSV, such as nucleocapsid protein (N), glycoprotein (G), and polymerase (L) (Dietzgen et al., 2020). Plant viruses of the family Rhabdoviridae have global circulation with damage to diverse commodities, including maize and wheat crops in South American countries, such as Argentina and Peru (Willie and Stewart, 2017; Maurino et al., 2018; Dietzgen et al., 2020). Infection symptoms by these viruses include yellowing, chlorosis, and streak formation on leaves, as well as dwarfism and leaf deformation. Although the specific vector of MFSV, the leafhopper Graminella nigrifrons (Cicadellidae) has been reported only in the United States and the Caribbean. These insects belong to the families Cicadellidae and Delphacidae, which have the potential to vector rhabdoviruses that are commonly found infecting grasses in Brazil. Although only partial sequence assembled, after further validation, this find could represent the first report of a virus from the genus Gammanucleorhabdovirus in South America (Todd et al., 2010; Oliveira et al., 2013; Dietzgen et al., 2020).
Viral sequences found in the root of C. brasiliensis may be associated with variation in soil biodiversity between forest and agricultural ecosystems (Pacchioni et al., 2014). Soil particularities between ecosystems (chemical composition, temperature, oxygenation, and organic matter) may influence the diversity and abundance of the microbiome, with a potential to be vectors or virus hosts in the soil, making the root susceptible to infections from different sources (Rodelo-Urrego et al., 2013; Souza et al., 2016; Tripathi et al., 2016). We identified sequences from viruses of the families Mitoviridae and Botourmiaviridae in seeds, floral buds, and roots. Mitoviruses have genomes containing a single ORF that encodes to an RdRp that presents genetic code specific for mitochondrial genomes, and possibiy the virus uses exclusively the mitochondrial machinery of the host in their replication cycle (Hillman and Cai, 2013; Nibert et al., 2018). Mitoviruses can cause latent infection in hosts and are thought to be transmitted by cell division or through the dispersal of spores (Nibert et al., 2018). In phytopathogenic fungi, the infection can result in fungal hypovirulence, revealing the potential of mitoviruses for use as biocontrol agents (Wu et al., 2010; Xu et al., 2015). Evidence shows that mitoviruses possibly adapted their genome for a cross-kingdom transmission due to the co-evolutive relationship between fungi and other organisms, such as flowering plants (Nibert et al., 2018; Fonseca et al., 2021; Wang et al., 2022). Viruses of the family Botourmiaviridae can be grouped into six genera. The first one presents three genomic segments and infects the cytoplasm of plants, whereas some other members infect filamentous fungi (Ourmiavirus). The second genus comprises viruses with a single segment and able to infect fungi and plants (Scleroulivirus), and the viruses of the four other genera (Botoulivirus, Magoulivirus, Penoulivirus, and Rhizoulivirus) infect mainly fungal hosts (Ayllón et al., 2020). Recently, representatives of the families Mitoviridae and Botourmiaviridae have also been detected in mycorrhizae, which are responsible for forming inter- or intracellular structures in roots of more than 90% of plant species, which assist in nutrient cycling, water uptake, and disease resistance (Bonfante and Genre, 2010; Sutela et al., 2020). The results found in our study indicate that the presence of viral genomes from this family can be ubiquitous in plant samples and may indicate a possible interaction among virus, plant, and fungal cells.
We also observed the presence of picornaviruses in the vegetative organs of C. brasiliensis, which had the closest hit with a polyprotein of Biomphalaria virus 2 (coverage of 95% and identity of 54.3%). In the phylogenetic analysis, the studied virus was grouped with Pittsburgh sewage-associated virus 1, a virus that was isolated from an urban sewage sample with an undetermined host (Cantalupo and Pipas, 2018). Biomphalaria virus 2 was originally identified in the microbiota of health snails Biomphalaria glabrata and B. pfeifferi, vectors of protozoa from the genus Schistosoma (Palasio et al., 2021). Previous studies using RdRp indicated the phylogenetic relationship of Biomphalaria virus 2 with viruses of the family Secoviridae, the only one from the order Picornavirales that has been identified to be infecting plants to date. Therefore, the presence of a picorna-like sequence only in the C. brasiliensis root sample obtained in the forest ecosystem could be driven by the presence of snails in this region. A second virus showing similarity to picornavirus (Skokie picorna-like virus carpotroche isolate), specifically to Skokie picorna-like virus, was found in our analysis. Another virus close to the studied picornavirus in the phylogenetic tree is Bat posalivirus, originally isolated from bat feces in Cameroon (Yinda et al., 2017). Coupled with the fact that the isolate of Skokie picorna-like virus is present only in fruit samples, this information indicates the possibility of this virus infecting C. brasiliensis to be transmitted by a vector. Considering the pathogenicity of picornaviruses in several species and the economic impact of severe vector-mediated plant diseases, the identification of this viral sequence is of central importance in the investigation of the C. brasiliensis virome (Jia et al., 2018).
Among the DNA viruses detected, a putative caulimovirus was detected in seed and floral buds. Viruses of the family Caulimoviridae have a circular dsDNA genome, with no envelope and a reverse transcription (RT) step in their life cycle, and are transmitted via arthropod vectors with several representatives considered important pathogens for a wide diversity of monocot and dicot plants, including apple, citrus, banana, cocoa, grape, cassava, rice, potato, corn, papaya, soybean, tomato, and others (Bhat et al., 2016; Teycheney et al., 2020). Some family members also possess the ability to integrate part of their genomes in the form of minichromosomes into the host genome during their replication cycle (Diop et al., 2018). Particularly for T. cacao, the studies mostly focused on symptomatic infections that affect the economic potential of the production with the genus Badnavirus, and correlate to the outbreak of Cacao Swollen Shoot Virus Disease (CSSVD) that started in 1922 in West Africa and is still devastating cacao production in Eastern African regions (Mondego et al., 2016). Thus, 10 species of viruses were described in West Africa or in some Caribbean islands, and most recently, one isolate of CaMMV-BR-like virus was identified in the Bahia state, Brazil, and also one species (Cacao bacilliform Sri Lanka virus) from Sri Lanka (Muller et al., 2021; Ramos-Sobrinho et al., 2021). It is likely that the caulimovirus found in C. brasiliensis is an EVE, given the sequence detected has a more likely link to the Aristotelia chilensis virus 1, an endogenous virus corresponding to the genus Petuvirus (Villacreses et al., 2015). DNA viruses of the family Genomoviridae were also detected in C. brasiliensis. This group of ssDNA viruses is considered a sister group of the family Geminiviridae and has the potential to cause fungal hypovirulence, identified in different environmental samples associated with plants and animals, including in Brazil infecting common beans and citrus (Lamas et al., 2016; Chabi-Jesus et al., 2020; Fontenele et al., 2020; Varsani and Krupovic, 2021). Phylogenetic analysis of the putative viral sequence suggests its association with viruses from the family Genomoviridae, indicating a close relationship to chicken genomovirus mg4 and other viruses of the genus Gemycircularvirus. While the first is pathogenic to birds, the latter can infect the phytopathogenic fungus Sclerotinia sclerotiorum and decrease its virulence (Varsani and Krupovic, 2017).
To corroborate the viral sequences present in our samples, we performed a proteomics assay to explore the presence of viral proteins. Mass spectrometry-based proteomics for viral infection analyses employed in this study is recognized for both structural virology and virus–host interaction studies (Dülfer et al., 2019). The detection of the charge state spectrum at high resolution allows the investigation of size, mass, stability, and shape of viral protein complexes (Uetrecht and Heck, 2011; Dülfer et al., 2019). Thus, the strategy to compare peptide sequences in different MS spectra readouts is ideal to detect the direct presence of viral proteins. In our analysis, we detected peptides derived from 13 viral sequences, eight of them representing key sequences from the variants of known viruses or viruses likely belonging to novel species. In seeds, the same organ for which we performed proteomics assay, we were able to detect viral peptides for 9 out of 17 sequences identified in RNA sequencing analysis.
Data availability statement
The datasets presented in this study can be found in online repositories. Specific repositories and accession codes for each dataset are specified at “Materials and methods” section.
Author contributions
EA and FG: conceptualization, methodology, and supervision. ÍL, PF, RO, LV, and FB: formal analysis. EA, FG, and CP: resources. AV, ÍL, PF, FG, and EA: writing of the original draft preparation. AV, ÍL, FG, and EA: reviewing and editing. FG: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank the ideas and suggestions raised during the scientific discussions at Conservation Genetics Group and Virus Bioinformatics Laboratory. We also acknowledge the editor and reviewers for the careful reading and suggestions in the manuscript, which substantially improved the final version of this manuscript.
Footnotes
Funding
This work was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 306160/2017-0 and # 403670/2020-9), Fundação de Apoio á Pesquisa do Estado da Bahia (Fapesb # TSC0017/2014), and Financiadora de Estudos e Projetos (FINEP # 01.18.0087.00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.874319/full#supplementary-material
References
- Abascal F., Zardoya R., Posada D. (2005). ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21 2104–2105. 10.1093/bioinformatics/bti263 [DOI] [PubMed] [Google Scholar]
- Akaike H. (1974). A new look at the statistical model identification. IEEE Trans. Automat. Control. 19 716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Alexander H. M., Mauck K. E., Whitfield A. E., Garrett K. A., Malmstrom C. M. (2014). Plant-virus interactions and the agro-ecological interface. Eur. J. Plant Pathol. 138 529–547. 10.1007/s10658-013-0317-1 [DOI] [Google Scholar]
- Ayllón M. A., Turina M., Xie J., Nerva L., Marzano S.-Y. L., Donaire L., et al. (2020). ICTV virus taxonomy profile: Botourmiaviridae. J. Gen. Virol. 101 454–455. 10.1099/jgv.0.001409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badillo-Vargas I. E., Baker C. A., Turechek W. W., Frantz G., Mellinger H. C., Funderburk J. E., et al. (2016). Genomic and biological characterization of tomato necrotic streak virus, a novel subgroup 2 Ilarvirus infecting tomato in Florida. Plant Dis. 100 1046–1053. 10.1094/PDIS-12-15-1437-RE [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Computat. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat S. A., Willis K. J., Birks H. J. B., Whittaker R. J. (2008). Agroforestry: a refuge for tropical biodiversity? Trends Ecol. Evol. 23 261–267. 10.1016/j.tree.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Bhat A. I., Hohn T., Selvarajan R. (2016). Badnaviruses: the current global scenario. Viruses 8:177. 10.3390/v8060177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P., Genre A. (2010). Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 1:48. 10.1038/ncomms1046 [DOI] [PubMed] [Google Scholar]
- Brito-Rocha E., dos Anjos L., Schilling A. C., Dalmolin ÂC., Mielke M. S. (2017). Individual leaf area estimations of a dioecious tropical tree species Carpotroche brasiliensis (Raddi) A. Gray, Achariaceae. Agroforest. Syst. 91 9–15. 10.1007/s10457-016-9927-x [DOI] [Google Scholar]
- Buchfink B., Xie C., Huson D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12 59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- Cantalupo P. G., Pipas J. M. (2018). Complete genome sequence of Pittsburgh sewage-associated virus 1. Genome Announc. 6:e01460–17. 10.1128/genomeA.01460-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira A. F., Dalmolin ÂC., dos Anjos L., da Silva Ledo C. A., da Costa Silva D., Mielke M. S. (2018). Photosynthetic plasticity of young plants of Carpotroche brasiliensis (Raddi) A. Gray, Achariaceae. Trees 32 191–202. 10.1007/s00468-017-1623-6 [DOI] [Google Scholar]
- Chabi-Jesus C., Najar A., Fontenele R. S., Kumari S. G., Ramos-González P. L., Freitas-Astúa J., et al. (2020). Viruses representing two new genomovirus species identified in citrus from Tunisia. Arch. Virol. 165 1225–1229. 10.1007/s00705-020-04569-8 [DOI] [PubMed] [Google Scholar]
- Dietzgen R. G., Bejerman N. E., Goodin M. M., Higgins C. M., Huot O. B., Kondo H., et al. (2020). Diversity and epidemiology of plant rhabdoviruses. Virus Res. 281:197942. 10.1016/j.virusres.2020.197942 [DOI] [PubMed] [Google Scholar]
- Diop S. I., Geering A. D. W., Alfama-Depauw F., Loaec M., Teycheney P.-Y., Maumus F. (2018). Tracheophyte genomes keep track of the deep evolution of the Caulimoviridae. Sci. Rep. 8:572. 10.1038/s41598-017-16399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos F. S. D., de Souza L. P. A., Siani A. C. (2008). [Chaulmoogra oil as scientific knowledge: the construction of a treatment for leprosy]. Hist Cienc Saude Manguinhos 15 29–47. 10.1590/s0104-59702008000100003 [DOI] [PubMed] [Google Scholar]
- Dülfer J., Kadek A., Kopicki J.-D., Krichel B., Uetrecht C. (2019). “Chapter seven – Structural mass spectrometry goes viral,” in Advances in Virus Research Complementary Strategies to Understand Virus Structure and Function, ed. Rey F. A. (Cambridge, MA: Academic Press; ), 189–238. 10.1016/bs.aivir.2019.07.003 [DOI] [PubMed] [Google Scholar]
- Dzahini-Obiatey H., Domfeh O., Amoah F. M. (2010). Over seventy years of a viral disease of cocoa in Ghana: from researchers perspective. AJAR 5 476–485. 10.5897/AJAR09.625 [DOI] [Google Scholar]
- Elena S. F., Agudelo-Romero P., Lalić J. (2009). The evolution of viruses in multi-host fitness landscapes. Open Virol. J. 3 1–6. 10.2174/1874357900903010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engering A., Hogerwerf L., Slingenbergh J. (2013). Pathogen–host–environment interplay and disease emergence. Emerg. Microbes Infect. 2 1–7. 10.1038/emi.2013.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria D., Paciencia M. L. B., Dixo M., Laps R. R., Baumgarten J. (2007). Ferns, frogs, lizards, birds and bats in forest fragments and shade cacao plantations in two contrasting landscapes in the Atlantic forest, Brazil. Biodivers. Conserv. 16 2335–2357. 10.1007/s10531-007-9189-z [DOI] [Google Scholar]
- Fonseca P., Ferreira F., da Silva F., Oliveira L. S., Marques J. T., Goes-Neto A., et al. (2021). Characterization of a novel Mitovirus of the sand fly Lutzomyia longipalpis using genomic and virus–host interaction signatures. Viruses 13:9. 10.3390/v13010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenele R. S., Roumagnac P., Richet C., Kraberger S., Stainton D., Aleamotu‘a M., et al. (2020). Diverse genomoviruses representing twenty-nine species identified associated with plants. Arch. Virol. 165 2891–2901. 10.1007/s00705-020-04801-5 [DOI] [PubMed] [Google Scholar]
- Fu L., Niu B., Zhu Z., Wu S., Li W. (2012). CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28 3150–3152. 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama-Rodrigues A. C., Müller M. W., Gama-Rodrigues E. F., Mendes F. A. T. (2021). Cacao-based agroforestry systems in the Atlantic forest and Amazon biomes: an ecoregional analysis of land use. Agric. Syst. 194:103270. 10.1016/j.agsy.2021.103270 [DOI] [Google Scholar]
- Geer L. Y., Marchler-Bauer A., Geer R. C., Han L., He J., He S., et al. (2010). The NCBI biosystems database. Nucleic Acids Res. 38 D492–D496. 10.1093/nar/gkp858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis S. M., Bem F. P., Dovas C. I., Sclavounos A., Avgelis A. D., Tsagris M., et al. (2009). Characterisation of a novel Ilarvirus causing grapevine angular mosaic disease. Eur. J. Plant Pathol. 125 203–211. 10.1007/s10658-009-9472-9 [DOI] [Google Scholar]
- Gu Z., Eils R., Schlesner M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32 2847–2849. 10.1093/bioinformatics/btw313 [DOI] [PubMed] [Google Scholar]
- Hillman B. I., Cai G. (2013). “Chapter six – The family Narnaviridae: simplest of RNA viruses,” in Advances in Virus Research Mycoviruses, ed. Ghabrial S. A. (Cambridge, MA: Academic Press; ), 149–176. 10.1016/B978-0-12-394315-6.00006-4 [DOI] [PubMed] [Google Scholar]
- Huang X., Madan A. (1999). CAP3: a DNA sequence assembly program. Genome Res. 9 868–877. 10.1101/gr.9.9.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Chen Q., Mao Q., Zhang X., Wu W., Chen H., et al. (2018). Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 28 127–132. 10.1016/j.coviro.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S., Madden T. L. (2008). NCBI BLAST: a better web interface. Nucleic Acids Res. 36 W5–W9. 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly C. A., Metzger J. P., Tabarelli M. (2014). Experiences from the Brazilian Atlantic forest: ecological findings and conservation initiatives. N. Phytol. 204 459–473. 10.1111/nph.12989 [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F., Ostfeld R. S. (2021). Dilution effects in disease ecology. Ecol. Lett. 24 2490–2505. 10.1111/ele.13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F., Holt R. D., Ostfeld R. S. (2006). Effects of species diversity on disease risk. Ecol. Lett. 9 485–498. 10.1111/j.1461-0248.2006.00885.x [DOI] [PubMed] [Google Scholar]
- Kitajima E. W. (2020). An annotated list of plant viruses and viroids described in Brazil (1926-2018). Biota Neotrop. 20 1–101. 10.1590/1676-0611-BN-2019-0932 [DOI] [Google Scholar]
- Krist S. (2020). “Chaulmoogra oil,” in Vegetable Fats and Oils, ed. Krist S. (Cham: Springer International Publishing; ), 223–226. 10.1007/978-3-030-30314-3_34 [DOI] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas N. S., Fontenele R. S., Melo F. L., Costa A. F., Varsani A., Ribeiro S. G. (2016). Complete genome sequence of a genomovirus associated with common bean plant leaves in Brazil. Genome Announc. 4:e01247–16. 10.1128/genomeA.01247-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeuvre P., Martin D. P., Elena S. F., Shepherd D. N., Roumagnac P., Varsani A. (2019). Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 17 632–644. 10.1038/s41579-019-0232-3 [DOI] [PubMed] [Google Scholar]
- Lima J. A., Oliveira A. S., de Miranda A. L. P., Rezende C. M., Pinto A. C. (2005). Anti-inflammatory and antinociceptive activities of an acid fraction of the seeds of Carpotroche brasiliensis (Raddi) (Flacourtiaceae). Braz. J. Med. Biol. Res. 38 1095–1103. 10.1590/S0100-879X2005000700013 [DOI] [PubMed] [Google Scholar]
- Lima T. M., Amaral E. S., Gaiotto F. A., dos Anjos L., Dalmolin ÂC., Santos A. S., et al. (2020). Fruit and seed biometry of Carpotroche brasiliensis (RB) A. Gray (Achariaceae), a tropical tree with great potential to provide natural forest products. Austral. J. Crop Sci. 14 1826–1833. 10.21475/ajcs.20.14.11.p2596 [DOI] [Google Scholar]
- Maclot F., Candresse T., Filloux D., Malmstrom C. M., Roumagnac P., van der Vlugt R., et al. (2020). Illuminating an ecological blackbox: using high throughput sequencing to characterize the plant virome across scales. Front. Microbiol. 11:578064. 10.3389/fmicb.2020.578064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon G. P., Cruz A. F., Barbosa W. B., Loureiro G. H., de Holanda A. C. (2010). Dispersão de sementes de uma comunidade arbórea em um remanescente de mata atlântica, município de Bonito, PE. Revista Verde Agroecologia Desenvolvimento Sustentável 5:19. [Google Scholar]
- Martini A. M. Z., Fiaschi P., Amorim A. M., da Paixão J. L. (2007). A hot-point within a hot-spot: a high diversity site in Brazil’s Atlantic forest. Biodivers. Conserv. 16 3111–3128. 10.1007/s10531-007-9166-6 [DOI] [Google Scholar]
- Maurino F., Dumón A. D., Llauger G., Alemandri V., de Haro L. A., Mattio M. F., et al. (2018). Complete genome sequence of maize yellow striate virus, a new Cytorhabdovirus infecting maize and wheat crops in Argentina. Arch. Virol. 163 291–295. 10.1007/s00705-017-3579-7 [DOI] [PubMed] [Google Scholar]
- Menzel P., Ng K. L., Krogh A. (2016). Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7:11257. 10.1038/ncomms11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondego J., Thomazella D., Teixeira P. J., Pereira G. (2016). “Genomics, transcriptomics, and beyond: the fifteen years of Cacao’s Witches’ broom disease genome project,” in Cacao Diseases, eds Bailey B., Meinhardt L. (Cham: Springer; ). 10.1007/978-3-319-24789-2_6 [DOI] [Google Scholar]
- Moury B., Desbiez C. (2020). Host range evolution of potyviruses: a global phylogenetic analysis. Viruses 12:111. 10.3390/v12010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E. (2016). “Cacao swollen shoot virus (CSSV): history, biology, and genome,” in Cacao Diseases: A History of Old Enemies and New Encounters, eds Bailey B. A., Meinhardt L. W. (Cham: Springer International Publishing; ), 337–358. 10.1007/978-3-319-24789-2_10 [DOI] [Google Scholar]
- Muller E., Ullah I., Dunwell J. M., Daymond A. J., Richardson M., Allainguillaume J., et al. (2021). Identification and distribution of novel badnaviral sequences integrated in the genome of cacao (Theobroma cacao). Sci. Rep. 11:8270. 10.1038/s41598-021-87690-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P. K. R. (1993). An Introduction to Agroforestry. Berlin: Springer Science & Business Media. [Google Scholar]
- Nair P. K. R. (2012). “Climate change mitigation: a low-hanging fruit of agroforestry,” in Agroforestry – The Future of Global Land Use Advances in Agroforestry, eds Nair P. K. R., Garrity D. (Dordrecht: Springer; ), 31–67. 10.1007/978-94-007-4676-3_7 [DOI] [Google Scholar]
- Navarro J. A., Sanchez-Navarro J. A., Pallas V. (2019). Key checkpoints in the movement of plant viruses through the host. Adv. Virus. Res. 104 1–64. 10.1016/bs.aivir.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Nibert M. L., Vong M., Fugate K. K., Debat H. J. (2018). Evidence for contemporary plant mitoviruses. Virology 518 14–24. 10.1016/j.virol.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C. M. D., Oliveira E. D., Souza I. R. P. D., Alves E., Dolezal W., Paradell S., et al. (2013). Abundance and species richness of leafhoppers and planthoppers (Hemiptera: Cicadellidae and Delphacidae) in Brazilian maize crops. Florida Entomol. 96 1470–1481. 10.1653/024.096.0427 [DOI] [Google Scholar]
- Pacchioni R. G., Carvalho F. M., Thompson C. E., Faustino A. L. F., Nicolini F., Pereira T. S., et al. (2014). Taxonomic and functional profiles of soil samples from Atlantic forest and Caatinga biomes in northeastern Brazil. Microbiol. Open 3 299–315. 10.1002/mbo3.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán I. (2018). The diversity, evolution and epidemiology of plant viruses: a phylogenetic view. Infect. Genet. Evol. 65 187–199. 10.1016/j.meegid.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Palasio R. G. S., de Azevedo T. S., Tuan R., Chiaravalloti-Neto F. (2021). Modelling the present and future distribution of Biomphalaria species along the watershed of the Middle Paranapanema region, São Paulo, Brazil. Acta Trop. 214:105764. 10.1016/j.actatropica.2020.105764 [DOI] [PubMed] [Google Scholar]
- Pallas V., Aparicio F., Herranz M. C., Sanchez-Navarro J. A., Scott S. W. (2013). “Chapter five – The molecular biology of Ilarviruses,” in Advances in Virus Research, eds Maramorosch K., Murphy F. A. (Cambridge, MA: Academic Press; ), 139–181. 10.1016/B978-0-12-407698-3.00005-3 [DOI] [PubMed] [Google Scholar]
- Parascandola J. (2003). Chaulmoogra oil and the treatment of leprosy. Pharm. Hist. 45 47–57. [PubMed] [Google Scholar]
- Patro R., Duggal G., Love M. I., Irizarry R. A., Kingsford C. (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14 417–419. 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasentin F. B., Saito C. H., Sambuichi R. H. R. (2014). Preferências locais quanto às árvores do sistema cacau-cabruca no sudeste da Bahia1. Ambiente Sociedade 17 55–78. [Google Scholar]
- Pirovani C. P., Carvalho H. A. S., Machado R. C. R., Gomes D. S., Alvim F. C., Pomella A. W. V., et al. (2008). Protein extraction for proteome analysis from cacao leaves and meristems, organs infected by Moniliophthora perniciosa, the causal agent of the witches’ broom disease. Electrophoresis 29 2391–2401. 10.1002/elps.200700743 [DOI] [PubMed] [Google Scholar]
- Posnette A. F., Robertson N. F., McA Todd J. (1950). Virus diseases of cacao in West Africa. Ann. Appl. Biol. 37 229–240. 10.1111/j.1744-7348.1950.tb01041.x [DOI] [Google Scholar]
- Potter S. C., Luciani A., Eddy S. R., Park Y., Lopez R., Finn R. D. (2018). HMMER web server: 2018 update. Nucleic Acids Res. 46 W200–W204. 10.1093/nar/gky448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Sobrinho R., Ferro M. M. M., Nagata T., Puig A. S., Keith C. V., Britto D. S., et al. (2021). Complete genome sequences of three newly discovered cacao mild mosaic virus isolates from Theobroma cacao L. in Brazil and Puerto Rico and evidence for recombination. Arch. Virol. 166 2027–2031. 10.1007/s00705-021-05063-5 [DOI] [PubMed] [Google Scholar]
- Rodelo-Urrego M., Pagán I., González-Jara P., Betancourt M., Moreno-Letelier A., Ayllón M. A., et al. (2013). Landscape heterogeneity shapes host-parasite interactions and results in apparent plant–virus codivergence. Mol. Ecol. 22 2325–2340. 10.1111/mec.12232 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Nevado C., Gavilán R., Pagán I. (2020). Host abundance and identity determine the epidemiology and evolution of a generalist plant virus in a wild ecosystem. Phytopathology 110 94–105. 10.1094/PHYTO-07-19-0271-FI [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., García-Arenal F. (2015). Ecosystem simplification, biodiversity loss and plant virus emergence. Curr. Opin. Virol. 10 56–62. 10.1016/j.coviro.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. R., Brancalion P. H. S., Crouzeilles R., Tambosi L. R., Piffer P. R., Lenti F. E. B., et al. (2021). Hidden destruction of older forests threatens Brazil’s Atlantic forest and challenges restoration programs. Sci. Adv. 7:eabc4547. 10.1126/sciadv.abc4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbou A., Vainio E. J., Büttner C. (2021). Towards the forest virome: high-throughput sequencing drastically expands our understanding on virosphere in temperate forest ecosystems. Microorganisms 9:1730. 10.3390/microorganisms9081730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambuichi R. H. R., Mielke M. S., Pereira C. E. (2009). Nossas árvores: conservação, uso e manejo de árvores nativas no sul da Bahia. Ilhéus: Editus. [Google Scholar]
- Sambuichi R. H. R., Vidal D. B., Piasentin F. B., Jardim J. G., Viana T. G., Menezes A. A., et al. (2012). Cabruca agroforests in southern Bahia, Brazil: tree component, management practices and tree species conservation. Biodivers. Conserv. 21 1055–1077. 10.1007/s10531-012-0240-3 [DOI] [Google Scholar]
- Sambuichi R., Haridasan M. (2007). Recovery of species richness and conservation of native Atlantic forest trees in the cacao plantations of southern Bahia in Brazil. Biodivers. Conserv. 16 3681–3701. 10.1007/s10531-006-9017-x [DOI] [Google Scholar]
- Sharma D. K., Hall I. H. (1991). Hypolipidemic, anti-inflammatory, and antineoplastic activity and cytotoxicity of flavonolignans isolated from Hydnocarpus wightiana seeds. J. Nat. Prod. 54 1298–1302. 10.1021/np50077a010 [DOI] [PubMed] [Google Scholar]
- Sharma-Poudyal D., Osterbauer N. K., Putnam M. L., Scott S. W. (2016). First report of lilac ring mottle virus infecting lilac in the United States. Plant Health Prog. 17 158–159. 10.1094/PHP-BR-15-0055 [DOI] [Google Scholar]
- Silvestre R., Fuentes S., Risco R., Berrocal A., Adams I., Fox A., et al. (2020). Characterization of distinct strains of an aphid-transmitted Ilarvirus (Fam. Bromoviridae) infecting different hosts from South America. Virus Res. 282:197944. 10.1016/j.virusres.2020.197944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza R. C., Mendes I. C., Reis-Junior F. B., Carvalho F. M., Nogueira M. A., Vasconcelos A. T. R., et al. (2016). Shifts in taxonomic and functional microbial diversity with agriculture: how fragile is the Brazilian Cerrado? BMC Microbiol. 16:42. 10.1186/s12866-016-0657-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susi H., Laine A.-L. (2021). Agricultural land use disrupts biodiversity mediation of virus infections in wild plant populations. New Phytol. 230 2447–2458. 10.1111/nph.17156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutela S., Forgia M., Vainio E. J., Chiapello M., Daghino S., Vallino M., et al. (2020). The virome from a collection of endomycorrhizal fungi reveals new viral taxa with unprecedented genome organization. Virus Evol. 6:veaa076. 10.1093/ve/veaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Fukuhara T., Kitazawa H., Kormelink R. (2019). Virus latency and the impact on plants. Front. Microbiol. 10:2764. 10.3389/fmicb.2019.02764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira H. M., Bianchi F. J. J. A., Cardoso I. M., Tittonell P., Peña-Claros M. (2021). Impact of agroecological management on plant diversity and soil-based ecosystem services in pasture and coffee systems in the Atlantic forest of Brazil. Agric. Ecosyst. Environ. 305:107171. 10.1016/j.agee.2020.107171 [DOI] [Google Scholar]
- Teycheney P.-Y., Geering A. D. W., Dasgupta I., Hull R., Kreuze J. F., Lockhart B., et al. (2020). ICTV virus taxonomy profile: Caulimoviridae. J. Gen. Virol. 101 1025–1026. 10.1099/jgv.0.001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. C., Ammar E.-D., Redinbaugh M. G., Hoy C., Hogenhout S. A. (2010). Plant host range and leafhopper transmission of maize fine streak virus. Phytopathology 100 1138–1145. 10.1094/PHYTO-05-10-0144 [DOI] [PubMed] [Google Scholar]
- Topolovec-Pintaric S. (2020). Plant Diseases: Current Threats and Management Trends. Norderstedt: BoD – Books on Demand. [Google Scholar]
- Tripathi B. M., Song W., Slik J. W. F., Sukri R. S., Jaafar S., Dong K., et al. (2016). Distinctive tropical forest variants have unique soil microbial communities, but not always low microbial diversity. Front. Microbiol. 7:376. 10.3389/fmicb.2016.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht C., Heck A. J. R. (2011). Modern biomolecular mass spectrometry and its role in studying virus structure, dynamics, and assembly. Angewandte Chemie International Edition 50 8248–8262. 10.1002/anie.201008120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsani A., Krupovic M. (2017). Sequence-based taxonomic framework for the classification of uncultured single-stranded DNA viruses of the family Genomoviridae. Virus Evol. 3:vew037. 10.1093/ve/vew037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsani A., Krupovic M. (2021). Family Genomoviridae: 2021 taxonomy update. Arch. Virol. 166 2911–2926. 10.1007/s00705-021-05183-y [DOI] [PubMed] [Google Scholar]
- Villacreses J., Rojas-Herrera M., Sánchez C., Hewstone N., Undurraga S. F., Alzate J. F., et al. (2015). Deep sequencing reveals the complete genome and evidence for transcriptional activity of the first virus-like sequences identified in Aristotelia chilensis (Maqui Berry). Viruses 7 1685–1699. 10.3390/v7041685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zou Q., Dai Z., Hong N., Wang G., Wang L. (2022). Four novel mycoviruses from the hypovirulent Botrytis cinerea SZ-2-3y isolate from Paris polyphylla: molecular characterisation and mitoviral sequence transboundary entry into plants. Viruses 14:151. 10.3390/v14010151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie K., Stewart L. R. (2017). Complete genome sequence of a new maize-associated Cytorhabdovirus. Genome Announc. 5:e00591–17. 10.1128/genomeA.00591-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Zhang L., Li G., Jiang D., Ghabrial S. A. (2010). Genome characterization of a debilitation-associated mitovirus infecting the phytopathogenic fungus Botrytis cinerea. Virology 406 117–126. 10.1016/j.virol.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Xu Z., Wu S., Liu L., Cheng J., Fu Y., Jiang D., et al. (2015). A mitovirus related to plant mitochondrial gene confers hypovirulence on the phytopathogenic fungus Sclerotinia sclerotiorum. Virus Res. 197 127–136. 10.1016/j.virusres.2014.12.023 [DOI] [PubMed] [Google Scholar]
- Yinda C. K., Zell R., Deboutte W., Zeller M., Conceição-Neto N., Heylen E., et al. (2017). Highly diverse population of Picornaviridae and other members of the Picornavirales, in Cameroonian fruit bats. BMC Genomics 18:249. 10.1186/s12864-017-3632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., da Graça J. V., Freitas-Astúa J., Vidalakis G., Duran-Vila N., Lavagi I. (2020). “Chapter 19 – Citrus viruses and viroids,” in The Genus Citrus, eds Talon M., Caruso M., Gmitter F. G. (Sawston: Woodhead Publishing; ), 391–410. 10.1016/B978-0-12-812163-4.00019-X [DOI] [Google Scholar]
- Zucaratto R., Carrara R., Franco B. K. S. (2010). Dieta da paca (Cuniculus paca) usando métodos indiretos numa área de cultura agrícola na floresta Atlântica Brasileira. Biotemas 23 235–239. 10.5007/2175-7925.2010v23n1p235 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. Specific repositories and accession codes for each dataset are specified at “Materials and methods” section.