Abstract
Epidermolysis bullosa (EB) is a group of rare, inherited diseases characterized by skin fragility and multiorgan system involvement that presents many anesthetic challenges. Although the literature regarding anesthetic management focuses primarily on the pediatric population, as life expectancy improves, adult patients with EB are more frequently undergoing anesthesia in nonpediatric hospital settings. Safe anesthetic management of adult patients with EB requires familiarity with the complex and heterogeneous nature of this disease, especially with regard to complications that may worsen during adulthood. General, neuraxial, and regional anesthetics have all been used safely in patients with EB. A thorough preoperative evaluation is essential. Preoperative testing should be guided by EB subtype, clinical manifestations, and extracutaneous complications. Advanced planning and multidisciplinary coordination are necessary with regard to timing and operative plan. Meticulous preparation of the operating room and education of all perioperative staff members is critical. Intraoperatively, utmost care must be taken to avoid all adhesives, shear forces, and friction to the skin and mucosa. Special precautions must be taken with patient positioning, and standard anesthesia monitors must be modified. Airway management is often difficult, and progressive airway deterioration can occur in adults with EB over time. A smooth induction, emergence, and postoperative course are necessary to minimize blister formation from excess patient movement. With careful planning, preparation, and precautions, adult patients with EB can safely undergo anesthesia.
Epidermolysis bullosa (EB) is a group of rare inherited skin disorders marked by extreme skin fragility. The hallmark of EB is blistering of the skin and mucosa in response to minor mechanical trauma.1 The perioperative management of adult patients with EB presents many challenges, yet the literature regarding safe anesthetic care is limited mostly to the pediatric population. Although EB remains incurable, modern therapies have helped to significantly extend life expectancy. As such, what is typically thought of as a pediatric disease is now being encountered more frequently in adult centers. Adult patients with EB may present for various types of procedures, and their care requires unique considerations. Therefore, a review of the perioperative management of adult patients with EB is warranted.2
PATHOPHYSIOLOGY
EB is a heterogeneous group of genetic disorders, ranging in phenotypic severity from mild to severe. Mutations in at least 16 different genes have been identified, which cause structural anomalies at the dermoepidermal junction.1,3 Various proteins such as keratin, plectin, laminin, collagen, and integrin may be affected, compromising the structural integrity and function of epithelial tissue.1 When affected skin and mucosa are subjected to mechanical stress, cleavage occurs at the site of the abnormal protein. Common cutaneous manifestations include blisters, peeling, ulcerations, chronic inflammation, nonhealing wounds, and scarring.1,3 Since any epithelial layer can be affected, multiple organ systems may be involved, depending on the type and severity.3 Based on data from the National EB Registry, the most comprehensive registry to date, the overall prevalence of EB is 11.1 per million, and the incidence is 19.6 per million live births in the United States.3 A similar prevalence of EB has been reported in Australia (10.3 per million), Norway (9.7 per million), Canada (9.9 per million), Italy (10.1 per million), and Croatia (9.6 per million).3–6 A lower prevalence has been reported in Japan and Romania (between 4 and 5 per million).7,8 A notably higher prevalence of EB has been reported in the Netherlands (22.4 per million), Northern Ireland (32 per million), and Scotland (49 per million).9–11
TYPES OF EPIDERMOLYSIS BULLOSA
EB is divided into 4 major types based on the location of blister formation within the dermoepidermal junction: simplex, junctional, dystrophic, and Kindler (Figure 1). Further classification into over 30 different subtypes is based on clinical features, inheritance pattern, and molecular findings.1
Figure 1.
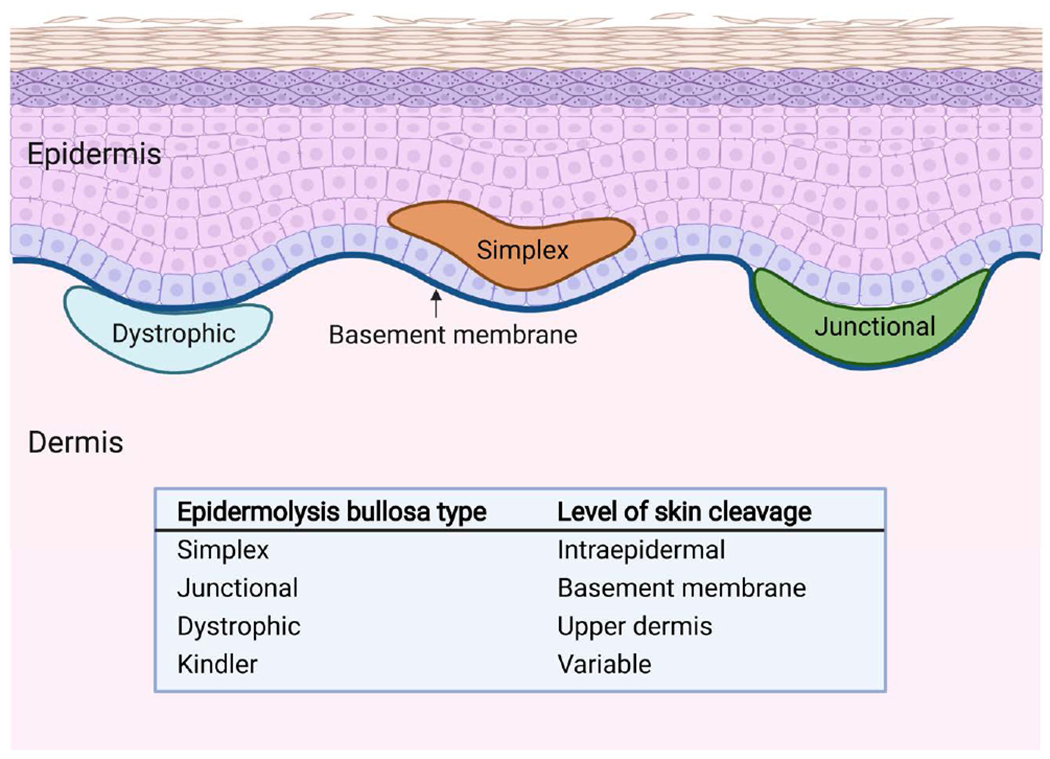
Types of epidermolysis bullosa. Epidermolysis bullosa is divided into 4 major types based on the location of blister formation within the dermoepidermal junction: simplex, junctional, dystrophic, and Kindler.
EB simplex is the most common type of EB with a reported prevalence of 6 per million in the United States.1 The most common proteins affected are keratin 5 and keratin 14, which are found in the cytoskeleton of basal keratinocytes.3 In EB simplex, mechanical stress causes cleavage to occur within the basal layer of keratinocytes in the epidermis.1 Clinical severity of EB simplex varies widely. Many patients may exhibit only mild palmoplantar blistering, while others have generalized blistering and early mortality.1,3 Blistering tends to improve progressively over time and is often limited to acral surfaces in many adult patients.1
Junctional EB is characterized by skin cleavage within the lamina lucida of the basement membrane.3 All subtypes are inherited in an autosomal recessive pattern. Commonly affected proteins include type XVII collagen, laminin 332, integrin α6β4, and the integrin α3 subunit, which play a critical role in anchoring basal keratinocytes to the basement membrane and cell signaling.3 Dental enamel defects occur in all forms of junctional EB due to impaired adhesion of ameloblasts required for mineralization of the dental enamel matrix.3 Although phenotypic severity varies widely, severe junctional EB is a major subtype marked by widespread blistering in infancy and is usually fatal by 2 years old.1,3 This likely explains the low prevalence of 0.49 per million in the United States.1,3
Dystrophic EB is defined by cleavage in the upper dermis caused by aberrant type VII collagen.3 All subtypes result from a mutation in the gene COL7A1.1 Type VII collagen is the major component of anchoring fibrils that provide dermal-epidermal adhesion.12 Depending on the subtype of dystrophic EB, anchoring fibrils may be structurally abnormal, reduced in number, or absent.12 The prevalence of dystrophic EB in the United States is approximately 6 per million, with higher figures reported in other countries such as Scotland.1 Due to the deep level of blistering and chronic nonhealing wounds, extensive scarring and fibrosis are common and worsen with age.1,13 Patients may develop microstomia, joint contractures, and pseudosyndactyly.3 Recessive dystrophic EB is generally more severe than dominant dystrophic EB, and extracutaneous complications are common.1 Adult patients with dystrophic EB frequently require general anesthesia to undergo treatment of complications such as esophageal stenosis, pseudosyndactyly, and cutaneous malignancies.
Kindler EB is a very rare type of EB with approximately 250 total cases reported worldwide.1 In Kindler EB, blister formation can occur at various levels within the dermoepidermal junction simultaneously.1 The affected protein is kindlin 1, which is involved in integrin activation, cell adhesion, migration, and proliferation, and the extracellular matrix.3,14 Clinical features include generalized blistering, photosensitivity, mucosal fragility, and gingivitis.3
EXTRACUTANEOUS COMPLICATIONS
Ocular
Eye involvement is reported across all types of EB and may include corneal erosions, corneal scarring, keratitis, and blepharitis.3,15 Scarring or adhesion of the eyelid to the globe (symblepharon) may prevent complete closure of the eyelids, necessitating the use of lubricating eye drops and ointments.3 Ocular complications may develop early in life and lead to progressive visual impairment.15
Oral Cavity
Patients with severe types of junctional and dystrophic EB often exhibit extensive blistering and scarring in the oral cavity as a result of trauma from normal consistency foods.3 Blisters may occur on any oral mucosal surface and are often blood or fluid-filled.16 Repeated blistering and scarring frequently lead to microstomia and trismus.3,16 In severe recessive dystrophic EB, tethering of the tongue (ankyloglossia) and loss of the vestibular sulcus also occur.3 Dental hygiene is often challenging, and patients may present with dental caries and dentoalveolar infections.3
Nasal Cavity
Blisters, erosions, and crusting of the nares are common. Some patients, especially those with junctional EB, may develop granulation tissue that can cause partial or complete obstruction of the nares.15
Larynx
Laryngeal disease can occur in any type of EB and may be supraglottic, glottic, or subglottic.15 Blisters, erosions, edema, webbing, scarring, and granulation tissue may occur, which can ultimately lead to upper airway occlusion.15 Patients with severe forms of junctional EB are at highest risk, and early childhood death from airway complications is common.15 By age 6, cumulative risk of laryngeal stricture or stenosis is as high as 40%.17 In patients with severe recessive dystrophic EB, the risk of laryngeal stenosis or obstruction is approximately 5% by 30 years old.17
Cardiovascular
Dilated cardiomyopathy is a serious complication associated with certain subtypes of EB.3 Based on data from the National Epidermolysis Bullosa Registry, cardiomyopathy or congestive heart failure is most common in patients with severe recessive dystrophic EB (cumulative risk 18.86% by age 35)18 and may account for up to 30% of deaths in this subtype of EB.3 Suggested causes include iron overload from repeated blood transfusions, chronic anemia, carnitine, or other micronutrient deficiencies.18 Cardiomyopathy also occurs in other subtypes of dystrophic EB, junctional EB, and certain subtypes of EB simplex, some of which have minimal skin involvement.1,3,19 In particular, EB simplex caused by KHLH24 mutations is associated with a high risk of dilated cardiomyopathy, which may be missed since skin manifestations improve with age in this subtype.19
Gastrointestinal
Involvement of the gastrointestinal tract is common, resulting in complications such as esophageal strictures, webs, and gastroesophageal reflux disease. Almost all patients with severe recessive dystrophic EB have difficulty in eating and swallowing by the time they reach adulthood.3 Inadequate nutritional intake and malabsorption from small intestinal involvement often lead to anemia, decreased bone mineral density, failure to thrive, pubertal delay, and vitamin and mineral deficiencies.3,15,20 Chronic constipation is common and made worse by anal blistering, anal fissures, opioid therapy, and iron supplementation.3
Genitourinary
Recurrent blistering of the urethra, ureterovesicular junction, and ureters may lead to hydronephrosis and hydroureter resulting in renal impairment.15 Chronic kidney disease may also occur from poststreptococcal glomerulonephritis, IgA nephropathy, and renal amyloidosis.3,21 End-stage renal disease is more common in the more severe forms of EB and is reported among the common causes of mortality in severe recessive dystrophic EB.3,22 Successful kidney transplantation has been described in 2 adult patients with EB.21,23
Musculoskeletal
Osteopenia and osteoporosis arise early in patients with EB as a result of vitamin D deficiency, delayed puberty, reduced exercise, and chronic inflammation.3 Fractures are common, and skeletal growth may be impacted. A study of skull radiographs in patients with severe recessive dystrophic EB reported reduced maxillary and mandibular size, likely a result of malnutrition and growth restriction from scarring.24
Progressive scarring and fibrosis of the extremities are common in recessive dystrophic EB, leading to digital webbing, flexion contractures, and mitten deformity (pseudosyndactyly).3 Gait abnormalities are common due to lower extremity and plantar involvement. A rare form of EB simplex is also associated with progressive muscular dystrophy that is usually apparent during childhood but may present later in adulthood.3,25
Anemia
Anemia is common in patients with severe EB as a result of reduced dietary iron intake, losses from skin and gastrointestinal erosions, and chronic inflammation.3 Many patients require blood transfusion and intravenous iron infusions.20
Cancer
Aggressive cutaneous cancers are common in adults with EB. Most notably, patients with severe junctional and recessive dystrophic EB are at significantly increased risk for lethal squamous cell carcinoma, owing to chronic inflammation and a microenvironment that promotes tumor growth and spread.3 Metastatic squamous cell carcinoma is the leading cause of mortality in severe recessive dystrophic EB, with the risk of death approaching 80% by 55 years of age despite aggressive treatment.26 Basal cell carcinoma is common in severe EB simplex and tends to occur mostly in sun-exposed sites.26 Rates of internal malignancies are comparable with those of the general population.26
TREATMENT
There is currently no curative treatment for EB. Supportive treatment consists of wound care, infection prevention, and pain management. There are many active clinical trials investigating a wide range of translational therapies such as gene therapy, in which a corrected gene is delivered through skin grafts or viral vectors.27 Cellular therapies under investigation include intradermal injection of allogenic or genetically modified fibroblasts.27 Disease-modifying medications such as gentamicin, which induces read through of nonsense mutations to increase protein production, and losartan, which interrupts inflammatory signaling to improve fibrosis and slow tumor progression, are also being studied in clinical trials.3,27
PREOPERATIVE EVALUATION
The preoperative meeting with EB patients should be scheduled well in advance to facilitate multidisciplinary coordination and completion of preoperative testing. In addition to the patient’s dermatologist, the procedural and anesthesia teams should be in communication regarding timing and operative plan. All staff who will interact with the patient, including perioperative nurses, trainees, and surgical technicians, should be made aware of the patient’s extreme vulnerability. It is important to increase the scheduled surgical time to allow for the extra precautions and care required for EB patients. A first start case is preferable to minimize time spent waiting in the preoperative area. EB patients within the same family may prefer to schedule procedures on the same day for ease of travel and access to teams familiar with EB. A list of common surgical procedures in adult EB patients is shown in Table 1.28,29
Table 1.
Common Procedures in Adults With Epidermolysis Bullosa
| Esophageal dilation |
| Dental extraction |
| Gastrostomy tube placement |
| Skin grafting |
| Squamous cell carcinoma resection |
| Contracture release |
| Vascular access |
| Cesarian section |
A physical examination and detailed review of systems are essential to the preanesthesia evaluation. The extent of cutaneous involvement should be documented, with particular attention to the oropharynx, eyes, and upper extremities. A careful airway examination should be performed. Adults with severe EB will likely have a very limited mouth opening, dental caries, and limited neck extension from contractures, which is important for difficult airway planning. The presence of hoarseness or inspiratory stridor warrants further evaluation for laryngeal involvement.17 When planning for regional or neuraxial anesthesia, examination of landmarks is useful to avoid puncture sites near open wounds that may pose an infection risk. Existing wounds and dressings should be noted, as well as patient preference for location of blood pressure cuff and intravenous access. Pertinent positives on review of systems may include gastroesophageal reflux disease, dysphagia, weakness, fatigue, heart failure symptoms, and urinary difficulties. A history of urethral stenosis should be noted in procedures where a foley catheter would normally be considered.
Many EB patients have chronic pain from blistering, denuded skin, and contractures that can result in immobility. A detailed medication reconciliation should be done to assess chronic pain requirements and antidepressant therapy. Patients on chronic opioid therapy should be advised to continue these medications preoperatively, including on the morning of surgery. Depending on the procedure, nonsteroidal anti-inflammatory drugs may be held the week before the procedure. It is important to identify treatments with anesthetic implications, such as systemic steroid therapy, which may lead to adrenal suppression.3 Patients may also be taking medications as part of a clinical trial, such as losartan or gentamicin, and corresponding hemodynamic effects and drug interactions must be anticipated.
Identifying the EB subtype of each patient is relevant not only to predict severity of disease but also to guide preoperative testing. As described in the previous section, there are several extracutaneous manifestations that warrant preoperative testing (Figure 2). A comprehensive metabolic panel is helpful to evaluate renal function, electrolyte disturbances, and hypoalbuminemia. It is important to evaluate serum potassium when considering the use of succinylcholine, particularly in patients with limited mobility or significant muscle atrophy. EB patients commonly present with anemia, thrombocytosis, and a history of prior transfusions, so a complete blood count and antibody screen should be ordered if warranted by the procedure. Leukocytosis and thrombocytosis may reflect chronic inflammation, but acute infection should also be ruled out. Transthoracic echocardiography is recommended to assess for cardiomyopathy in high-risk subtypes (severe recessive dystrophic EB, EB simplex with mutations in KHLH24) or when limited mobility makes assessment of functional status difficult.3,19
Figure 2.
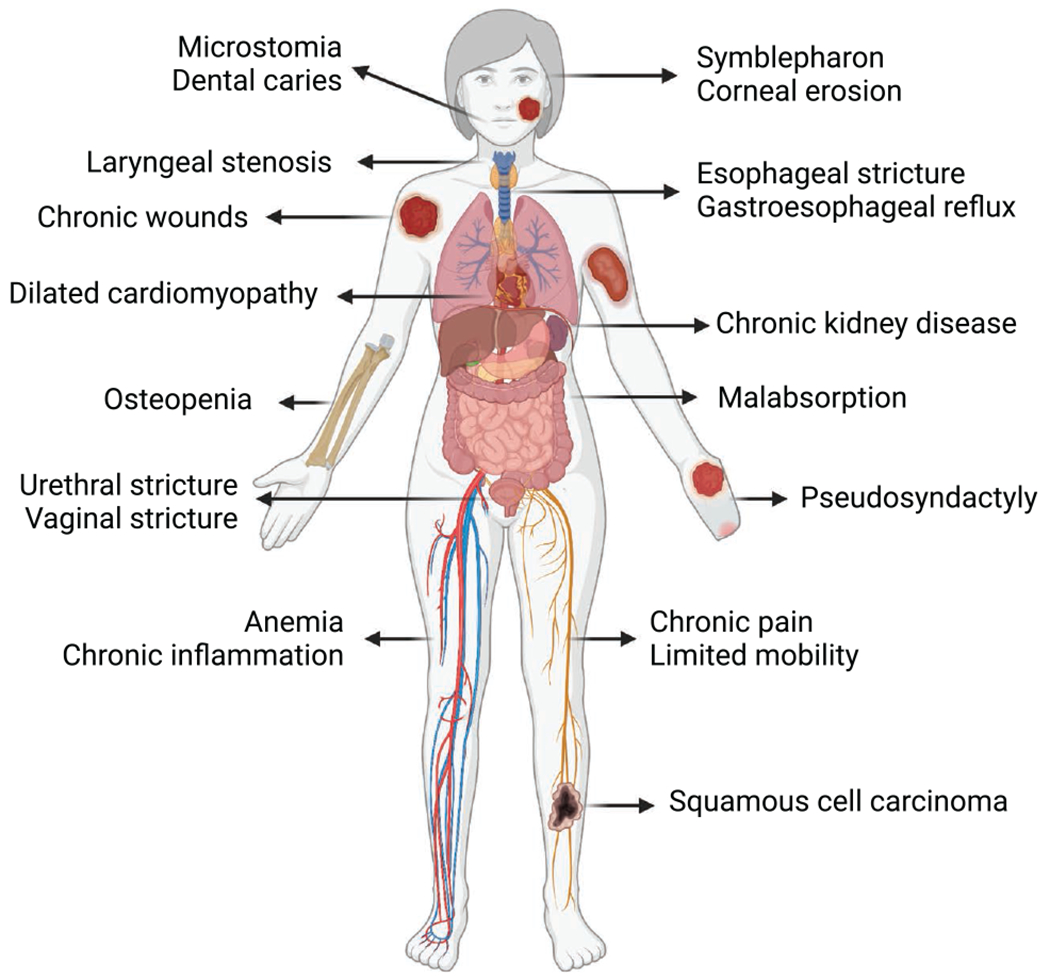
Clinical manifestations of epidermolysis bullosa. In addition to blistering and chronic wounds, there are several extracutaneous manifestations of epidermolysis bullosa that vary with subtype and severity and may worsen with age. Complications such as microstomia, laryngeal stenosis, cardiomyopathy, esophageal stricture, gastroesophageal reflux, anemia, and chronic pain warrant further preoperative investigation and planning.
The patient and their family often contribute valuable information about the success of prior anesthetics. Previous anesthetic records can be helpful during this conversation. A discussion about premedication with benzodiazepines should include the benefits of delaying administration since patient participation during transfer to the operating table and monitor placement can help prevent inadvertent trauma.
Special Considerations
For the laboring parturient with EB, early consultation is best to establish the extent of disease and the patient’s wishes. An early epidural may be indicated to avoid the risk of an emergent general anesthetic for cesarean delivery, and there are several reports of successful neuraxial anesthesia for both vaginal and cesarean deliveries.30–33 There are reports of successful breastfeeding in mothers with EB,30 and a consultation with a lactation specialist should be offered.
INTRAOPERATIVE MANAGEMENT
Patients with EB should be cared for by a highly skilled anesthesiology team given the complexity of this patient population.20 Preventing the formation of new blisters as a result of friction and shear forces is of utmost importance.28 Careful planning and precautionary measures must be taken to minimize the risk of complications.
Choice of Anesthetic Technique
Several studies have demonstrated the safety of general, neuraxial, and regional anesthetics in patients with EB.34–39 Choice of anesthetic technique must be individualized depending on the patient and surgical procedure. For peripheral procedures, a combination of regional anesthesia and sedation may be chosen to avoid potential complications associated with airway management. Conversely, longer procedures or those with increased risk of aspiration such as esophageal dilation warrant general anesthesia with endotracheal intubation for airway protection.40 It is important to note that perioperative aspiration has been reported in EB patients with esophageal disease despite preoperative fasting.37 This may be due to abnormal esophageal peristalsis and stiffening of the lower esophageal sphincter due to recurrent scarring.15 Preoperative antacid administration should be considered to reduce the risk of acid aspiration.41 Additionally, prophylaxis of postoperative nausea and vomiting is important to minimize further esophageal trauma.42
Preparation of the Operating Room
Careful preparation of the operating room before patient arrival is important for safe anesthetic care. An EB anesthesia kit containing specialized supplies and a printed protocol is useful and can help streamline preparation (Table 2; Figure 3). The room should be warmed to reduce the risk of hypothermia since patients with EB tend to have a low body mass index and increased heat loss from skin lesions.43 The operating table should be well padded with foam and synthetic sheepskin padding. Patients with preserved mobility may transfer themselves to the operating table.44 For patients who require assistance with transfers, the synthetic sheepskin can be placed on the preoperative gurney and then used to lift and place the patient on the operating table, minimizing any sliding or shear force trauma. All members of the perioperative team should be familiar with EB and adhere to the “no-touch principle,” minimizing any unnecessary physical contact with the patient.35,45 In general, direct pressure is better tolerated than friction or shear forces.34
Table 2.
Contents of Adult EB Anesthesia Kit
| Printed EB anesthesia protocol | Petrolatum gauze |
| Wrap-around style pulse oximeter | Sterile scissors |
| Transparent film dressing | Sterile gauze |
| Soft silicone tape (Mepitaca) | Umbilical tape |
| Nonadherent mesh dressing (Mepitela) | Sterile saline vials |
| Absorbent foam dressing (Mepilexa) | Intravenous T-connector |
| Self-adherent wrap (Cobanb) | Petrolatum-based ointment |
| Cotton undercast padding | Methylcellulose artificial tears |
| Pediatric-size electrodes | Soft dental suction tips |
| Gel defibrillator pads | 5.0-mm ID endotracheal tube |
Abbreviations: EB, epidermolysis bullosa; ID, internal diameter.
Mepitac, Mepitel, and Mepilex are registered to Mölnlycke Health Care (Göteborg, Sweden).
Coban is trademark of 3M (St Paul, MN).
Figure 3.
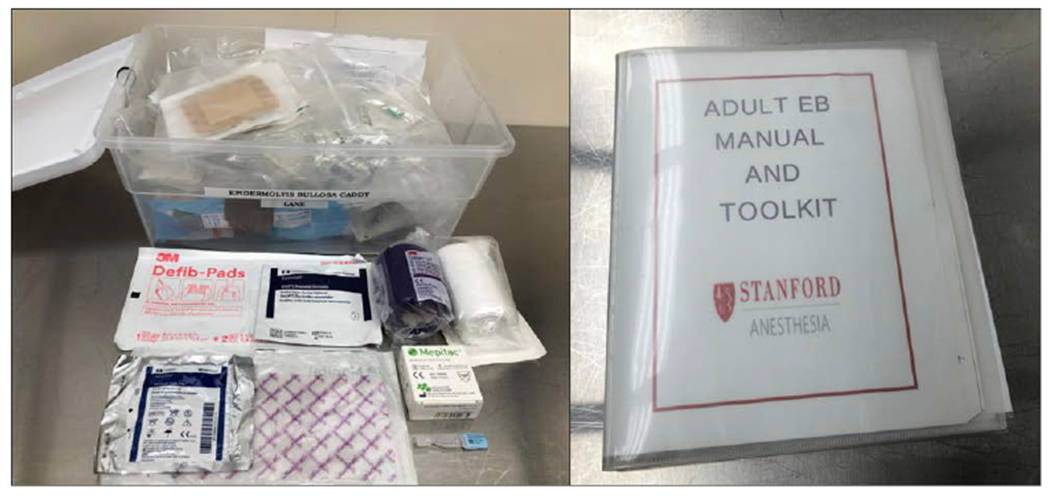
Epidermolysis bullosa anesthesia kit and manual.
Monitoring
Standard anesthesia monitors must be modified to be safely used in patients with EB. Any contact of the skin with adhesives must be strictly avoided. Disposable wrap-around style pulse oximeters should be covered with transparent film so that all adhesive surfaces are fully covered (Figure 4A). The pulse oximeter can then be lubricated and wrapped gently around a digit or stump and held in place by wrapping with self-adherent wrap such as Coban (3M) or gauze. Lubricated clip-on pulse oximeters may also be used on the ear lobe or on digits unaffected by webbing.28,46
Figure 4.
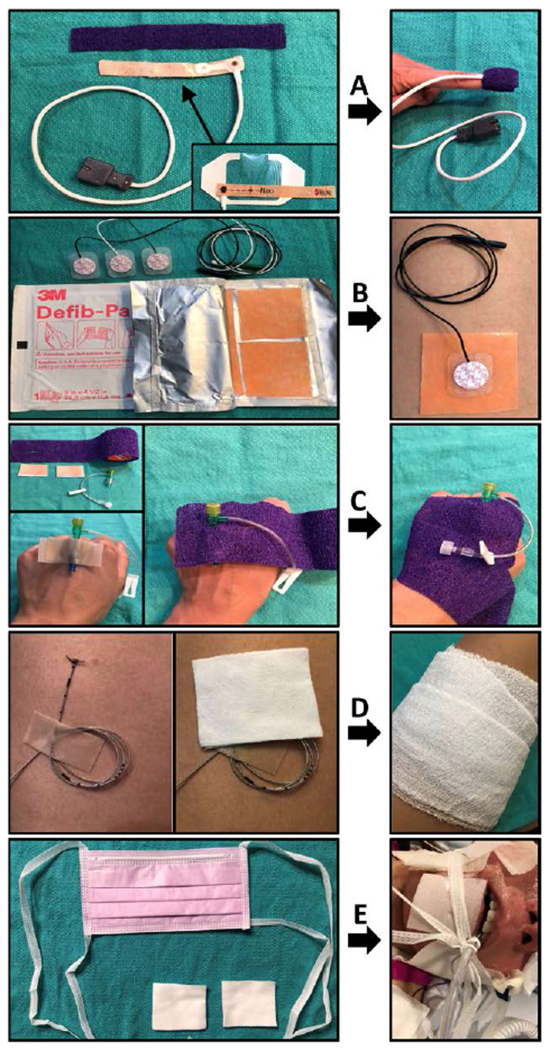
Modifications to avoid adhesives for patients with epidermolysis bullosa. A, The adhesive surface of a wrap-around style pulse oximeter sensor is covered in transparent film dressing. The sensor is then gently wrapped around the digit and held in place with self-adherent wrap. B, Pediatric-size electrodes are placed over nonadhesive gel defibrillator pad for cardiac monitoring. C, Intravenous catheter hub is placed between 2 strips of soft silicone tape and secured with self-adherent wrap. D, Epidural or peripheral nerve catheter is sutured in place, looped, and secured with soft silicone tape. Gauze may be wrapped around the extremity or trunk. E, Endotracheal tube may be secured by placing a surgical mask under the patient’s occiput, bringing the ties up past the cheeks (over padding), and knotting them securely around the endotracheal tube.
For cardiac monitoring, pediatric or adult-size electrodes that have been trimmed of excess adhesive should be placed on the top of a gel defibrillator pad that provides a conductive, nonadhesive barrier between the electrode and the skin (Figure 4B).35 Moistened gauze placed over the electrodes helps to prevent drying of the gel pads and maintain conductivity. Blood pressure cuffs should be placed over cotton undercast padding or existing dressings on the extremities. Compressive forces from noninvasive blood pressure monitoring or surgical tourniquets are generally well-tolerated.28 Lubricated temperature probes can be gently placed in the axilla to avoid trauma from esophageal or nasopharyngeal probes.38
Invasive monitoring with arterial and central lines may be required depending on patient comorbidities and surgical procedure. Site selection for arterial cannulation will differ from patient to patient. Significant scarring of the wrist may complicate cannulation of the radial artery, and an alternate site free of scarring, contractures, and open wounds should be chosen.34 The internal jugular vein is commonly used for central venous access.37 Peripheral insertion of central venous catheters through the antecubital fossa has also been described.47 Arterial and central lines should be sutured in place and padded with sterile, nonadhesive dressings.37,41,43 Since patients with EB are at high risk for infection, arterial and central lines should be removed as soon as they are no longer necessary.48,49
Intravenous Access
Intravenous access is often difficult and may require ultrasound guidance. Dressings can be cut away to expose areas of prior successful cannulation such as the antecubital fossa. The skin should be cleansed by gently dabbing with alcohol.28 Infiltration of the skin with local anesthetic should be avoided.28 An assistant can provide gentle manual pressure to distend the vein to avoid using a tourniquet.28,38 Once intravenous access is established, the catheter hub may be placed in between 2 strips of nonadhesive dressing or soft silicone tape such as Mepitac (Mölnlycke Health Care) and then wrapped with self-adherent wrap or gauze to secure it in place (Figure 4C).28,38
Regional and Neuraxial Catheters
Epidural and peripheral nerve catheters may be placed in patients with EB for continuous regional or neuraxial anesthesia. Catheters should be sutured in place, with the excess length looped and secured with Mepitac (Mölnlycke Health Care) (Figure 4D).43,46,50,51 Cotton padding or gauze may be wrapped around the extremity or trunk to provide additional stability. Alternatively, catheters may be tunneled under the skin.31,52 In EB patients, catheters are commonly removed on the first postoperative day, but use of a peripheral catheter for as long as 5 days without complication has been reported.21,53,54 Catheters should be removed as soon as feasible, balancing the benefits of analgesia with the risk of infection, which will vary depending on the location of catheter and proximity to chronic wounds.
Induction of Anesthesia
Intravenous induction is preferred in adults with EB to avoid facial trauma and an excitatory phase during inhalational induction.51 Propofol facilitates a smooth induction; ketamine is also commonly used.34,55 Both depolarizing and nondepolarizing neuromuscular blocking agents have been used without complications in patients with EB.34,35,37,55 Immediately after induction of anesthesia, care should be taken to protect the eyes. Methylcellulose artificial tears without preservatives should be applied to the eyes before closing the eyelids.43 A nonadherent protective layer such as Mepitel (Mölnlycke Health Care) can be used for eye protection and covered with moist gauze to prevent drying.
Airway Management
Airway management is regarded as one of the most challenging aspects of caring for patients with EB but can be accomplished safely with careful preparation.28,40,51,56 In the absence of symptomatic upper airway obstruction, mask ventilation is usually not difficult.40,43,57 However, difficult intubation is common and should be anticipated in all adult patients with EB.37,43 Direct laryngoscopy is often difficult or impossible due to microstomia, intraoral scarring, ankyloglossia, and a thickened, immobile epiglottis.2,34,43 Likewise, supraglottic airway insertion may be difficult for the same reasons.2,35 Shearing forces from both direct laryngoscopy and supraglottic airway placement may cause bullae formation and bleeding of friable tissue.2,40 In a case series of adult patients with dystrophic EB, progressive airway deterioration was reported over time, and difficulty was encountered in patients with prior uneventful airway management as children.2 In addition, the risk of edema, blistering, and bleeding of friable tissues caused by mucosal contact makes airway management hazardous.28 Difficult airway equipment including supplies for emergent surgical airway should be readily available.
All airway equipment and adjuncts should be well lubricated.28 Facemasks in smaller sizes should be available and generously lubricated with petroleum-based ointment and fully inflated to minimize friction and blister formation on the face. Additionally, petroleum gauze and padding should be placed on the mandible under the anesthesiologist’s fingers to minimize trauma during mask ventilation. Alternatively,Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) may be used for preoxygenation and apneic oxygenation.58 This technique employs use of high-flow nasal oxygen cannula placed over petrolatum gauze, reducing the risk of facial trauma from mask ventilation and prolonging the safe apneic time during difficult intubation.58,59
Careful fiber-optic intubation with a well-lubricated, small endotracheal tube (size 5.0- or 6.0-mm internal diameter) is the recommended technique for airway management and carries the least risk of trauma to the airway.2,40,51 Oral airways and other intubating guides should be avoided due to the risk of shear force trauma. Instead, careful placement of a bite block between the upper and lower teeth can provide adequate mouth opening for oral fiber-optic intubation. Meticulous technique by an experienced operator helps to minimize contact with oropharyngeal mucosa and associated trauma. Lubrication of the endotracheal tube and a small endotracheal tube diameter help to facilitate smooth passage of the tube over the fiber-optic scope into the trachea. Nasal fiber-optic intubation has also been described37,57,60 but may not be possible in patients with nasal obstruction or stenosis.
Alternative airway management strategies to fiber-optic intubation must be considered on a case-by-case basis. The presence of severe microstomia, fibrotic tissue, or ankyloglossia may preclude use of video laryngoscopes and supraglottic airways in certain patients.61 When mouth opening is sufficient, laryngeal mask airways have been used successfully in patients with EB but may be complicated by lingual bullae formation.2,40 There are insufficient data regarding the superiority of a particular type of supraglottic airway in patients with EB. However, devices with a flexible curvature and inflatable cuff, such as the LMA® ProSeal™a (Teleflex Inc), may be less traumatic during insertion and removal. Regardless of the type of supraglottic device, it is recommended to choose a small size, generously lubricate the cuff and shaft, inflate the cuff such that a leak remains, and consider removal before emergence from anesthesia to reduce airway trauma.40
Successful video laryngoscopy in adults with EB has been reported using the McGRATH MAC (Medtronic) and Pentax-AWS Airwayscope (Nihon Kohden). In children with EB, the C-MAC (Karl Storz) and GlideScope (Verathon Inc) have been used.46,58,62,63 Video laryngoscope blades should be well lubricated and inserted gently. If repositioning is required, the blade should be lifted to avoid trauma from sliding over vulnerable tissue.43 There are insufficient data to recommend a particular device; however, choice of a hyperangulated blade is likely to improve glottic view in patients who have limited cervical spine mobility and tissue mobility.64 It is also important to consider the width of the blade compared to the patient’s maximal mouth opening. Devices with an integrated channel may be advantageous when limited intraoral space makes maneuvering a stylet difficult. The combination of video laryngoscopy with a fiber-optic bronchoscope as a flexible video stylet should also be considered and has been shown to increase the success rate of intubation compared to video laryngoscopy alone in difficult airways.65 Combined techniques confer many benefits including improved glottic view, stylet maneuverability, and visualization of endotracheal tube passage into the trachea. This technique was used successfully in a 13-year-old patient with EB in whom fiber-optic intubation alone failed.63
After placement, the endotracheal tube must be carefully secured without the use of adhesives, usually by using well-lubricated soft tube ties or gauze anchored behind the patient’s head.28 A surgical mask placed under the patient’s occiput can also be used for this purpose by bringing the ties of the mask up past the cheeks (over padding) and knotting them securely around the endotracheal tube (Figure 4E). Care must be taken to prevent inadvertent endotracheal tube dislodgement, especially during procedures such as esophageal dilation or surgeries involving the head and neck.
Positioning
Before sterile draping, the patient should be carefully examined to ensure adequate padding of extremities and ensure minimal contact between the patient and any lines or equipment. Placement of extra foam padding in strategic locations may help create an extra barrier to protect the patient from friction from the drapes. Surgical staff should be reminded not to lean on or place unnecessary equipment on the patient after draping. If a grounding pad is required, it should be trimmed of all adhesive and placed on an area of skin free of blisters or wounds.57
Fluid Management
Although adults with EB have increased evaporative losses through skin wounds, significant intraoperative hypovolemia as a result of this has not been reported. Dehydration should be corrected preoperatively, and intraoperative fluid therapy should focus on maintaining euvolemia and replacing blood loss. Excessive crystalloid administration should be avoided to reduce the risk of complications such as hypervolemia, tissue edema that can further impair wound healing, and dilution of clotting factors.66 Patients with cardiomyopathy or renal failure, which is a common cause of mortality in adults with EB, are particularly susceptible to hypervolemia. To minimize metabolic derangements, a balanced electrolyte solution should be used until a transfusion threshold is met.67 When significant blood loss or fluid shifts are expected, a goal-directed approach to fluid management guided by invasive monitoring is recommended.68,69
Emergence
Emergence and tracheal extubation should take place in a controlled environment with difficult airway equipment readily available. A smooth emergence with minimal coughing and patient movement is ideal to prevent formation of new blisters or airway bleeding due to rupture of blisters.40 Although tracheal extubation during a deep plane of anesthesia has advantages, this must be weighed against the risk of aspiration and laryngospasm, especially in the setting of a difficult airway.43 A low-dose remifentanil infusion is useful to facilitate a smooth awake tracheal extubation.70 Soft dental suction tips work well for gentle suctioning of the oropharynx. After tracheal extubation, stiff nonrebreather masks are best avoided. Instead, high-flow nasal cannula or the lubricated anesthesia circuit facemask can be used to provide supplemental oxygen. Patients should be transferred from the operating table to the gurney by lifting the sheet or synthetic sheepskin underneath them, avoiding any rolling or sliding.51
POSTOPERATIVE MANAGEMENT
The staff in the postanesthesia care unit (PACU) should all be made aware of the “no touch” principle for monitoring and handling of the patient. Plastic oxygen masks and all adhesives should be removed from the patient’s PACU area. If supplemental oxygen is required, the nasal cannula or mask should be cushioned and well lubricated. It may be helpful to allow caregivers into the PACU to assist in the recovery and care of the patient.
A smooth postoperative course is important in EB patients to minimize agitation and excess patient movement. Pain management should be multimodal and aim to control the acute exacerbation of chronic pain typically found in EB patients. Intravenous opioids, while often necessary, may result in worsening baseline pruritis that is difficult to treat.51 Supplemental regional techniques should be considered when appropriate.
Before leaving the PACU, the patient should be assessed for possible perioperative complications (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D648). New bullae are most commonly found on the face or oropharynx.40 If an adherent dressing or tape has been inadvertently applied, silicone medical adhesive remover may help minimize the trauma of removal.3 Tense blisters may be pierced with a sterile needle and drained with the roof intact. An atraumatic, nonadhesive dressing should be placed on any broken skin.
SUMMARY
The perioperative management of adult patients with EB requires many special considerations (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D648). Awareness of the complex, systemic, and progressive nature of this disease is critical to providing safe anesthetic care. A thorough preoperative evaluation is necessary to identify extracutaneous complications, many of which may worsen in adulthood. A difficult airway is common and should be anticipated. Utmost care must be taken to avoid shear forces or friction on skin and mucosal surfaces throughout the perioperative period. With careful planning and vigilance, adult patients with EB can safely undergo anesthesia with minimal morbidity.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge David R. Drover, MD, PhD, for his mentorship and editorial assistance. Figures 1 and 2 were created with BioRender.com (Toronto, Canada) and are submitted for publication with permission.
GLOSSARY
- EB
epidermolysis bullosa
- ID
internal diameter
- PACU
postanesthesia care unit
- THRIVE
transnasal humidified rapid-insufflation ventilatory exchange
Footnotes
DISCLOSURES
Name: Brita M. Mittal, MD.
Contribution: This author helped design the work, provide content expertise, draft the manuscript, and approve the final manuscript.
Name: Candida L. Goodnough, MD, PhD.
Contribution: This author helped design the work, draft the manuscript, and approve the final manuscript.
Name: Erin Bushell, MD.
Contribution: This author helped design the work, provide content expertise, critically revise the manuscript, and approve the final manuscript.
Name: Sophia Turkmani-Bazzi, MD.
Contribution: This author helped design the work, provide content expertise, critically revise the manuscript, and approve the final manuscript.
Name: Kelly Sheppard, MD.
Contribution: This author helped design the work, provide content expertise, critically revise the manuscript, and approve the final manuscript.
This manuscript was handled by: Richard P. Dutton, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
LMA ProSeal is a registered trademark of Teleflex Incorporated or its affiliates.
The authors declare no conflicts of interest.
REFERENCES
- 1.Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614–627. [DOI] [PubMed] [Google Scholar]
- 2.Fröhlich S, O’Sullivan E. Airway management in adult patients with epidermolysis bullosa dystrophica: a case series. Anaesthesia. 2011;66:842–843. [DOI] [PubMed] [Google Scholar]
- 3.Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. [DOI] [PubMed] [Google Scholar]
- 4.Tadini G, Gualandri L, Colombi M, et al. II registre italiano delle epidermolisi bollose ereditarie. G Ital Dermatol Venereol. 2005;140:359–372. [Google Scholar]
- 5.Kho YC, Rhodes LM, Robertson SJ, et al. Epidemiology of epidermolysis bullosa in the antipodes: the Australasian Epidermolysis Bullosa Registry with a focus on Herlitz junctional epidermolysis bullosa. Arch Dermatol. 2010;146:635–640. [DOI] [PubMed] [Google Scholar]
- 6.Pavicić Z, Kmet-Vizintin P, Kansky A, Dobrić I. Occurrence of hereditary bullous epidermolyses in Croatia. Pediatr Dermatol. 1990;7:108–110. [DOI] [PubMed] [Google Scholar]
- 7.Shinkuma S, Natsuga K, Nishie W, Shimizu H. Epidermolysis bullosa in Japan. Dermatol Clin. 2010;28:431–432. [DOI] [PubMed] [Google Scholar]
- 8.Dănescu S, Has C, Senila S, Ungureanu L, Cosgarea R. Epidemiology of inherited epidermolysis bullosa in Romania and genotype-phenotype correlations in patients with dystrophic epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2015;29:899–903. [DOI] [PubMed] [Google Scholar]
- 9.Baardman R, Yenamandra VK, Duipmans JC, et al. Novel insights into the epidemiology of epidermolysis bullosa (EB) from the Dutch EB Registry: EB more common than previously assumed? J Eur Acad Dermatol Venereol. 2021;35:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna KE, Walsh MY, Bingham EA. Epidermolysis bullosa in Northern Ireland. Br J Dermatol. 1992;127:318–321. [DOI] [PubMed] [Google Scholar]
- 11.Horn HM, Priestley GC, Eady RA, Tidman MJ. The prevalence of epidermolysis bullosa in Scotland. Br J Dermatol. 1997;136:560–564. [PubMed] [Google Scholar]
- 12.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cianfarani F, Zambruno G, Castiglia D, Odorisio T. Pathomechanisms of altered wound healing in recessive dystrophic epidermolysis bullosa. Am J Pathol. 2017;187:1445–1453. [DOI] [PubMed] [Google Scholar]
- 14.Michael M, Begum R, Chan GK, et al. Kindlin-1 regulates epidermal growth factor receptor signaling. J Invest Dermatol. 2019;139:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part I. Epithelial associated tissues. J Am Acad Dermatol. 2009;61:367–384. [DOI] [PubMed] [Google Scholar]
- 16.Krämer S, Lucas J, Gamboa F, et al. Clinical practice guidelines: oral health care for children and adults living with epidermolysis bullosa. Spec Care Dentist. 2020;40(suppl 1):3–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine JD, Johnson LB, Weiner M, Suchindran C.Tracheolaryngeal complications of inherited epidermolysis bullosa: cumulative experience of the National Epidermolysis Bullosa Registry. Laryngoscope. 2007;117:1652–1660. [DOI] [PubMed] [Google Scholar]
- 18.Fine JD, Hall M, Weiner M, Li KP, Suchindran C. The risk of cardiomyopathy in inherited epidermolysis bullosa. Br J Dermatol. 2008;159:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwieger-Briel A, Fuentes I, Castiglia D, et al. Epidermolysis bullosa simplex with KLHL24 mutations is associated with dilated cardiomyopathy. J Invest Dermatol. 2019;139:244–249. [DOI] [PubMed] [Google Scholar]
- 20.Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. Other organs. J Am Acad Dermatol. 2009;61:387–402. [DOI] [PubMed] [Google Scholar]
- 21.Ceuppens SHE, Kimenai HJAN, Roodnat JI, et al. Living donor kidney transplantation in a patient with epidermolysis bullosa: a case report. Transplant Proc. 2019;51:3074–3076. [DOI] [PubMed] [Google Scholar]
- 22.Fine JD, Johnson LB, Weiner M, et al. ; National Epidermolysis Bullosa Registry. Inherited epidermolysis bullosa and the risk of death from renal disease: experience of the National Epidermolysis Bullosa Registry. Am J Kidney Dis. 2004;44:651–660. [PubMed] [Google Scholar]
- 23.Ungureanu S, Adni T, Brown T, Inston N, Heagerty A. Successful renal transplant in a patient with non-Herlitz junctional epidermolysis bullosa. Clin Exp Dermatol. 2014;39:330–332. [DOI] [PubMed] [Google Scholar]
- 24.Shah H, McDonald F, Lucas V, Ashley P, Roberts G. A cephalometric analysis of patients with recessive dystrophic epidermolysis bullosa. Angle Orthod. 2002;72:55–60. [DOI] [PubMed] [Google Scholar]
- 25.Kyrova J, Kopeckova L, Buckova H, et al. Epidermolysis bullosa simplex with muscular dystrophy. Review of the literature and a case report. J Dermatol Case Rep. 2016;10:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol. 2009;60:203–211. [DOI] [PubMed] [Google Scholar]
- 27.Welponer T, Prodinger C, Pinon-Hofbauer J, et al. Clinical perspectives of gene-targeted therapies for epidermolysis Bullosa. Dermatol Ther. Published online Jun 10, 2021. doi: 10.1007/s13555-021-00561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen L, Burtonwood MT. Anaesthetic management of children with epidermolysis bullosa. BJA Educ. 2018;18:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intong LRA, Choi SD, Shipman A, et al. Retrospective evidence on outcomes and experiences of pregnancy and childbirth in epidermolysis bullosa in Australia and New Zealand. Int J Womens Dermatol. 2017;3:S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanafusa T, Tamai K, Umegaki N, et al. The course of pregnancy and childbirth in three mothers with recessive dystrophic epidermolysis bullosa. Clin Exp Dermatol. 2012;37:10–14. [DOI] [PubMed] [Google Scholar]
- 31.Araújo M, Brás R, Frada R, Guedes-Martins L, Lemos P. Caesarean delivery in a pregnant woman with epidermolysis bullosa: anaesthetic challenges. Int J Obstet Anesth. 2017;30:68–72. [DOI] [PubMed] [Google Scholar]
- 32.Baloch MS, Fitzwilliams B, Mellerio J, Lakasing L, Bewley S, O’Sullivan G. Anaesthetic management of two different modes of delivery in patients with dystrophic epidermolysis bullosa. Int J Obstet Anesth. 2008;17:153–158. [DOI] [PubMed] [Google Scholar]
- 33.Boria F, Maseda R, Martín-Cameán M, De la Calle M, de Lucas R. Recessive dystrophic epidermolysis bullosa and pregnancy. Actas Dermosifiliogr (Engl Ed). 2019;110:50–52. [DOI] [PubMed] [Google Scholar]
- 34.Iohom G, Lyons B. Anaesthesia for children with epidermolysis bullosa: a review of 20 years’ experience. Eur J Anaesthesiol. 2001;18:745–754. [DOI] [PubMed] [Google Scholar]
- 35.Ames WA, Mayou BJ, Williams KN, Williams K. Anaesthetic management of epidermolysis bullosa. Br J Anaesth. 1999;82:746–751. [DOI] [PubMed] [Google Scholar]
- 36.Wright JT. Comprehensive dental care and general anesthetic management of hereditary epidermolysis bullosa. A review of fourteen cases. Oral Surg Oral Med Oral Pathol. 1990;70:573–578. [DOI] [PubMed] [Google Scholar]
- 37.Griffin RP, Mayou BJ. The anaesthetic management of patients with dystrophic epidermolysis bullosa. A review of 44 patients over a 10 year period. Anaesthesia. 1993;48:810–815. [DOI] [PubMed] [Google Scholar]
- 38.Lin YC, Golianu B. Anesthesia and pain management for pediatric patients with dystrophic epidermolysis bullosa. J Clin Anesth. 2006;18:268–271. [DOI] [PubMed] [Google Scholar]
- 39.Van Den Heuvel I, Boschin M, Langer M, et al. Anesthetic management in pediatric patients with epidermolysis bullosa: a single center experience. Minerva Anestesiol. 2013;79:727–732. [PubMed] [Google Scholar]
- 40.James I, Wark H. Airway management during anesthesia in patients with epidermolysis bullosa dystrophica. Anesthesiology. 1982;56:323–326. [DOI] [PubMed] [Google Scholar]
- 41.Herod J, Denyer J, Goldman A, Howard R. Epidermolysis bullosa in children: pathophysiology, anaesthesia and pain management. Paediatr Anaesth. 2002;12:388–397. [DOI] [PubMed] [Google Scholar]
- 42.Chia WT, Liu YC, Wong TW. Optimize general anesthesia for a dystrophic epidermolysis bullosa patient that cannot be intubated. Asian J Anesthesiol. 2020;58:111–114. [DOI] [PubMed] [Google Scholar]
- 43.Goldschneider K, Lucky AW, Mellerio JE, Palisson F, Miranda MDCV, Azizkhan RG. Perioperative care of patients with epidermolysis bullosa: proceedings of the 5th international symposium on epidermolysis bullosa, Santiago Chile, December 4–6, 2008. Pediatr Anesth. 2010;20:797–804. [DOI] [PubMed] [Google Scholar]
- 44.Crowley KL, Shevchenko YO. Anesthetic management of a difficult airway in a patient with epidermolysis bullosa: a case report. AANA J. 2004;72:261–263. [PubMed] [Google Scholar]
- 45.Saraf SV, Mandawade NJ, Gore SK, Padhye UD, Pereira CS. Epidermolysis bullosa: careful monitoring and no touch principle for anesthesia management. J Anaesthesiol Clin Pharmacol. 2013;29:390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanobe I, Kida H, Sekine Y, et al. Safe skin management during open hepatectomy in a patient with recessive dystrophic congenital epidermolysis bullosa. Case Rep Surg. 2018;2018:1786786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milne B, Rosales JK. Anaesthesia for correction of oesophageal stricture in a patient with recessive epidermolysis bullosa dystrophica: case report. Can Anaesth Soc J. 1980;27:169–171. [DOI] [PubMed] [Google Scholar]
- 48.Sakan S, Basic-Jukic N, Tomasevic B, Kes P, Bandic Pavlovic D, Peric M. Vascular access for chronic hemodialysis in a patient with epidermolysis bullosa dystrophica Hallopeau-Siemens. Hemodial Int. 2013;17:126–129. [DOI] [PubMed] [Google Scholar]
- 49.Mellerio JE. Infection and colonization in epidermolysis bullosa. Dermatol Clin. 2010;28:267–269. [DOI] [PubMed] [Google Scholar]
- 50.Broster T, Placek R, Eggers GWNJ. Epidermolysis bullosa: anesthetic management for cesarean section. Obstet Anesth Dig. 1987;7:128. [PubMed] [Google Scholar]
- 51.Nandi R, Howard R. Anesthesia and epidermolysis bullosa. Dermatol Clin. 2010;28:319–324. [DOI] [PubMed] [Google Scholar]
- 52.Doi S, Horimoto Y. Subcutaneous tunnelling of an epidural catheter in a child with epidermolysis bullosa. Acta Anaesthesiol Scand. 2006;50:394–395. [DOI] [PubMed] [Google Scholar]
- 53.Meola S, Olivieri M, Mirabile C, Mastrandrea P. Anesthetic management for right upper extremity amputation due to recidivous cutaneous carcinoma and acute postoperative pain control in patients affected by epidermolysis bullosa. Minerva Anestesiol. 2010;76:144–147. [PubMed] [Google Scholar]
- 54.Nguyen L, Minville V, Riu B, Atallah F, Fourcade O. Anaesthetic management of a patient with epidermolysis bullosa undergoing percutaneous nephrolithotomy. Eur J Anaesthesiol. 2005;22:558–560. [DOI] [PubMed] [Google Scholar]
- 55.Lin AN, Lateef F, Kelly R, Rothaus KO, Carter DM. Anesthetic management in epidermolysis bullosa: review of 129 anesthetic episodes in 32 patients. J Am Acad Dermatol. 1994;30:412–416. [DOI] [PubMed] [Google Scholar]
- 56.Boughton R, Crawford MR, Vonwiller JB. Epidermolysis bullosa-a review of 15 years’ experience, including experience with combined general and regional anaesthetic techniques. Anaesth Intensive Care. 1988;16:260–264. [DOI] [PubMed] [Google Scholar]
- 57.Strupp KM, Zieg JA, Johnson B, Szolnoki JM. Anesthetic management of a patient with epidermolysis bullosa requiring major orthopedic surgery: a case report. A A Case Rep. 2017;9:73–76. [DOI] [PubMed] [Google Scholar]
- 58.Ng LY, Chan AKM, Lam TWY. The use of high-flow nasal oxygen during airway management in a child with epidermolysis bullosa dystrophica and a difficult airway. Anaesth Rep. 2019;7:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spence EA, Rajaleelan W, Wong J, Chung F, Wong DT. The effectiveness of high-flow nasal oxygen during the intraoperative period: a systematic review and meta-analysis. Anesth Analg. 2020;131:1102–1110. [DOI] [PubMed] [Google Scholar]
- 60.Lindemeyer R, Wadenya R, Maxwell L. Dental and anaesthetic management of children with dystrophic epidermolysis bullosa. Int J Paediatr Dent. 2009;19:127–134. [DOI] [PubMed] [Google Scholar]
- 61.Özkan AS, Kayhan GE, Akbaş S, Kaçmaz O, Durmuş M. Emergency difficult airway management in a patient with severe epidermolysis bullosa. Turk J Anaesthesiol Reanim. 2016;44:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noda Y, Komasawa N, Matsunami S, Minami T. Successful tracheal intubation using videolaryngoscope in Shwachman-Diamond syndrome patient combined with congenital epidermolysis bullosa. J Clin Anesth. 2019;56:27. [DOI] [PubMed] [Google Scholar]
- 63.Fitzmaurice BC, Lambert BG. Failed fiberoptic intubation in a child with epidermolysis bullosa, rescued with combined use of the Glidescope®. Paediatr Anaesth. 2016;26:455–456. [DOI] [PubMed] [Google Scholar]
- 64.Berkow LC, Morey TE, Urdaneta F. The technology of video laryngoscopy. Anesth Analg. 2018;126:1527–1534. [DOI] [PubMed] [Google Scholar]
- 65.Lenhardt R, Burkhart MT, Brock GN, Kanchi-Kandadai S, Sharma R, Akça O. Is video laryngoscope-assisted flexible tracheoscope intubation feasible for patients with predicted difficult airway? A prospective, randomized clinical trial. Anesth Analg. 2014;118:1259–1265. [DOI] [PubMed] [Google Scholar]
- 66.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632. [DOI] [PubMed] [Google Scholar]
- 67.Bampoe S, Odor PM, Dushianthan A, et al. Perioperative administration of buffered versus non-buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures. Cochrane Database Syst Rev. 2017;9:CD004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kendrick JB, Kaye AD, Tong Y, et al. Goal-directed fluid therapy in the perioperative setting. J Anaesthesiol Clin Pharmacol. 2019;35:S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boehm D, Menke H. A history of fluid management—from “one size fits all” to an individualized fluid therapy in burn resuscitation. Medicina (Mex). 2021;57:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong TH, Weber G, Abramowicz AE. Smooth extubation and smooth emergence techniques: a narrative review. Anesthesiol Res Pract. 2021;2021:8883257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


