Abstract
Respiratory diseases remain a major cause of morbidity and mortality worldwide. An imbalance of zinc, an essential trace element, is associated with a variety of lung diseases. We reviewed and summarized recent research (human subjects, animal studies, in vitro studies) on zinc in respiratory diseases to explore the protective mechanism of zinc from the perspective of regulation of oxidative stress, inflammation, lipid metabolism, and apoptosis. In the lungs, zinc has anti-inflammatory, antioxidant, and antiviral effects; can inhibit cancer cell migration; can regulate lipid metabolism and immune cells; and exerts other protective effects. Our comprehensive evaluation highlights the clinical and experimental effects of zinc in the pathogenesis of respiratory diseases. Our analysis also provides insight into the clinical application of zinc-targeted therapy for respiratory diseases.
Subject terms: Drug development, Nutrition
Introduction
The 2017 Global Burden of Disease Study, a comprehensive and up-to-date analysis of respiratory diseases from 1990 to 2017, revealed a 39.8% increase in the prevalence of chronic respiratory diseases and an 18% increase in mortality from these diseases in 2017 compared to that in 1990 [1]. Chronic respiratory diseases are the third leading cause of death globally after cardiovascular diseases and tumors [1]. The course of chronic respiratory diseases is relatively long, and the clinical manifestations are diverse, ranging from mild cough, expectoration, and other symptoms to varying degrees of respiratory failure. The pathology of lung biopsy ranges from mild inflammatory cell infiltration to the formation of diffuse nodular tumors or fibrosis in both lungs [2, 3]. In recent years, various antibiotics, hormones, immunosuppressants, and targeted drugs have been used to treat certain respiratory diseases, and some of these drugs have shown clinical success [4, 5]. However, adverse reactions such as abnormal blood pressure, dyslipidemia, and infection can occur following extensive application of these drugs [6]. Related studies showed that the incidence of asthma, chronic obstructive pulmonary disease (COPD), lung cancer, and other diseases are high and gradually increasing globally [7–9]. Horvata1 et al. summarized the protective effect of iron, a major nutrient in the human body, on respiratory diseases [10]. Zinc has important effects on human health and protects natural tissue barriers in the body, such as the respiratory epithelium, prevents the invasion of pathogens, balances the function of the immune and redox systems, and affects a variety of respiratory diseases [11]. Sources of zinc include beans, red meat (beef, veal, lamb, and mutton) and biofortified eggs and wheat [12–15]. Phytate in beans, unrefined grains, and nuts is the main inhibitor of dietary zinc absorption [16–18]. However, the chelation of phytate and zinc can be disrupted by improving food processing methods (soaking, heating, and fermentation) to improve the bioavailability of zinc [19, 20]. Therefore, dietary adjustment or nutritional supplement therapy can regulate zinc homeostasis which has clinical application value for preventing and treating respiratory diseases.
Zinc homeostasis and imbalance
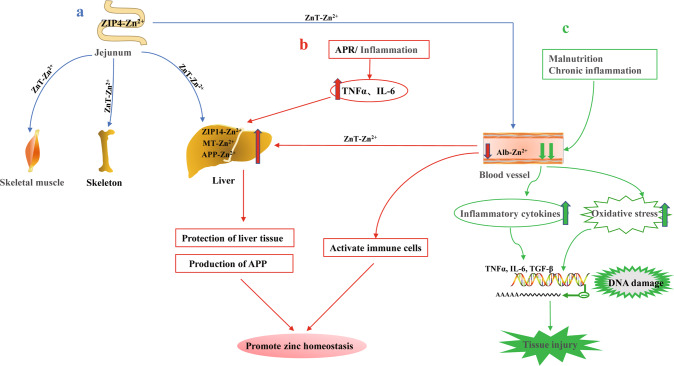
Zinc is among the most abundant trace metals (next to iron) in the human body and is present in a total amount of approximately 2–4 g [21]. In healthy people, homeostatic mechanisms maintain serum zinc concentrations within a narrow range (approximately 10–15 μmol/L; 70–98 μg/dL) [22–24]. When dietary zinc intake was maintained (around 5–20 mg/day), plasma zinc concentrations remain stable [23, 25]. However, zinc deficiency is a global problem affecting approximately two billion (approximately 20%) people [26]. Zinc is an important cofactor of more than 2000 transcription factors and more than 300 enzymes that regulate cell proliferation, differentiation, and basic metabolic functions [27]. Zinc cannot be stored in the body for long periods and must be ingested daily through food to ensure an adequate supply [28]. Zinc is mainly absorbed in the jejunum through intestinal cells and is stored in the skeletal muscle, bones, and liver. Although plasma zinc accounts for only 0.1% of the total zinc storage, it increases the rate of intercellular signaling and promotes zinc homeostasis [28] (Fig. 1a). This homeostasis is regulated by zinc transporters (ZnT1-10 and ZIP1-14) and zinc-binding proteins, some of which span the plasma membrane, whereas others are in the mitochondria, Golgi apparatus, and endoplasmic reticulum [29, 30]. ZnT transporters reduce the cytosolic zinc concentration by promoting zinc efflux from cells or zinc entry into organelles, whereas ZIP transporters increase the cytosolic zinc concentration by promoting zinc influx into cells or releasing zinc from organelles [29, 31]. During the acute phase of response, zinc is redistributed in vivo, mainly from the serum to the liver; during this response, serum tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) are significantly increased [32]. These proinflammatory factors upregulate zinc transporter ZIP-14 on the liver membrane and metallothionein (MT) and acute phase reactive protein in liver cells, resulting in transient serum hypozincemia and elevated liver zinc concentrations [30, 32]. High zinc levels in the liver tissue can upregulate and combine with acute phase reactive protein to achieve zinc homeostasis in the liver, protect the liver, and promote re-establishment of the zinc balance in the body [33, 34] (Fig. 1b). However, long-term malnutrition and severe inflammatory diseases occur simultaneously with serum hypozincemia [35]. This disrupts zinc’s protective physiological response, leads to the release of proinflammatory cytokines, enhances oxidative stress induced-oxidative damage to DNA, affects gene expression, and eventually damages tissues or organs [35, 36] (Fig. 1c).
Fig. 1. Regulation of zinc homeostasis and imbalance in humans.
a Absorption and transport of zinc (blue arrow) b Regulation of zinc levels during acute response (red arrow) c Severe malnutrition and chronic inflammation lead to zinc imbalance (green arrow). Zn Zinc, APR acute phase of response, TNF-α tumor necrosis factor α, IL-6 interleukin-6, MT metallothionein, APP acute phase reactive protein, ZIP, ZnT zinc transporters.
Protective and prevention effect of zinc homeostasis on lung injury
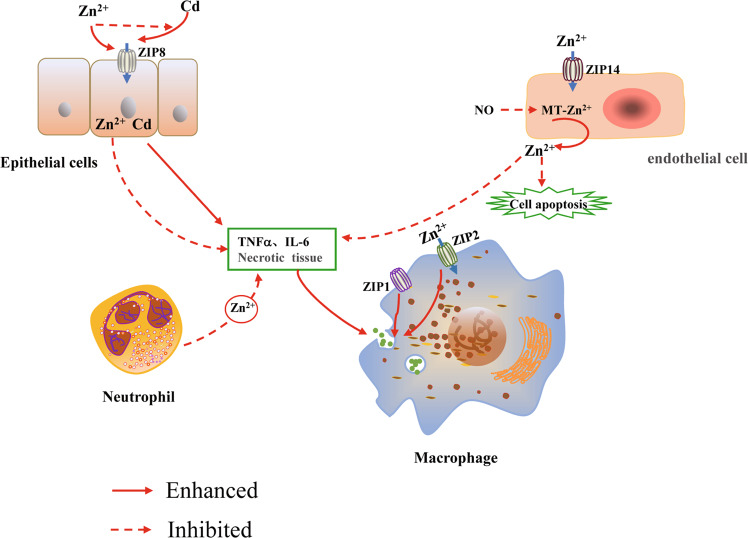
Deficiencies in micronutrients (vitamins A, D, E, selenium, zinc) not only affect lung development, but also may lead to lifelong impairment of lung function [37]. Zinc deficiency has become a global problem affecting people’s health [26]. Therefore, it is particularly important to investigate the effect of zinc homeostasis on the stability of lung function. The lung is the gateway for gas exchange between the body and outside world. Following exposure to harmful substances (cadmium, cigarette smoke), zinc levels in the lungs are increased to protect the lung tissue by affecting the functions of certain cells (macrophages, T lymphocytes and neutrophils) [38–40]. ZIP8 is a zinc transporter that is highly expressed in alveolar epithelial cells and is the main entry point for Cd, a toxic heavy metal and carcinogen that exists in large quantities in cigarette smoke [41]. When the body inhales Cd-containing gas, zinc competes with Cd to bind to ZIP8 protein to prevent Cd from entering cells and reduces the cytotoxicity of Cd to alveolar epithelial cells [38] (Fig. 2). Analysis of rat lung epithelial cells showed that progressive silencing of ZIP8 expression during chronic Cd exposure is involved in the acquisition of resistance against Cd in lung cells, representing an adaptive survival mechanism to resist Cd-induced cytotoxicity [42]. Both ZIP1 and ZIP2 proteins are found on the membranes of normal alveolar macrophages. ZIP2 receives the extracellular low zinc signal and rapidly balances the extracellular and intracellular zinc concentration [39]. ZIP1 does not respond to the low zinc signal but can assist in the efferocytosis of macrophages. Coordinated action of the ZIP1 and ZIP2 transporters promotes rapid clearance of apoptotic cells by macrophages, thereby limiting secondary necrosis and inflammation in the lung [39] (Fig. 2). In addition, T lymphocytes are important factors that regulate immune responses in allergic diseases, with airway allergic reactions mainly mediated by helper T cells 2 (Th2) differentiated from CD4+ T cells [43]. Th2 cells mainly secrete the cytokines IL-4, IL-5 and IL-13, which promote eosinophilia and airway remodeling [44, 45]. Studies have shown that T cell development, differentiation, and activation are zinc-dependent processes [46]. In clinical trials, mild zinc deficiency in humans (3–5 mg/day) caused dysfunctions in Th1 and Th2, leading to a decline in cell-mediated immunity [47] However, in vitro and in vivo experiments demonstrated that therapeutic zinc supplements can mediate Th1 and Th2 immune functions and reduce Th2-mediated chronic airway inflammation by activating regulatory T cells [40, 48]. Zinc in the lung tissue is regulated by zinc transporters and mainly binds to MT. MT is an intracellular metal-binding protein with antioxidant properties, and its zinc-thiolate moieties are key targets of nitric oxide (NO) [49]. The S-nitrosylation of MT by NO can lead to an increase in the unstable zinc concentration in pulmonary artery endothelial cells, which can directly inhibit the activity of caspase-3, thus inhibiting apoptosis of pulmonary endothelial cells [49, 50]. Several studies showed that the NO-MT-Zn signaling pathway is physiologically important for protecting lung endothelial cells and regulating vascular tension [49, 51, 52]. In addition, KLF4 is a transcription factor with a conserved zinc finger domain, and A20 is a zinc finger protein associated with hypoxia. As important modulators of vascular homeostasis, these molecules play important protective roles in pulmonary artery endothelial cells [53, 54]. Neutrophil recruitment disorders and excessive neutrophil activation are signs of acute lung injury (ALI). In lipopolysaccharide-induced mouse ALI models, Wessels et al. found that preventive zinc supplementation (30 µg, intraperitoneal injection) significantly reduced neutrophil recruitment and prevented its hyperactivity in the lungs, thereby reducing lung injury [55]. Using human cells (20 or 50 µM zinc sulfate) in vitro, the findings from a mouse ALI model were shown to be applicable to human diseases [55] (Fig. 2). Therefore, zinc levels in the lung can regulate various cell functions (macrophages and neutrophils), and zinc homeostasis plays an important protective role in the lung tissue.
Fig. 2. Regulation of zinc homeostasis in the lung.
In lung epithelial cells, Zn2+ competes with Cd to bind ZIP8 protein, preventing Cd from entering cells and inhibiting cell apoptosis and necrosis. In the hypoxic environment of pulmonary endothelial cells, NO can nitrosize the S-nitrosization of zinc-binding protein metallothionein (MT), causing it to release Zn2+, which contributes to regulating the pulmonary vascular tone; zinc significantly reduces neutrophil recruitment to the lungs and prevents their overactivity, thereby reducing inflammation. In macrophages, the zinc transporters ZIP1 and ZIP2 coordinate regulation of the zinc ion concentration, promote macrophage endocytosis, and remove necrotic tissue and inflammatory factors in the lung in a timely manner.
Protective role of zinc in respiratory diseases
Antiviral and immune activity of zinc in COVID-19
In December 2019, a series of pneumonia cases of unknown cause was reported. The causal agent was identified as a novel coronavirus (2019–nCoV) [56]. This pathogen is mainly transmitted through droplets, physical contact, and aerosols at a high speed with high pathogenicity, and predominately invades the respiratory system [57]. Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can result in complications such as acute respiratory distress syndrome (ARDS), sepsis, septic shock, and multiple organ failure [56]. The World Health Organization declared SARS-CoV-2 (COVID-19) an international public health emergency [58]. Currently, there are no approved specific treatments for COVID-19. Recent in vitro studies showed that the master protease (Mpro) in SARS-CoV-2 is a key component of the viral replication, and Zn2+ can bind to its enzyme active site to form a stable Zn2+-Mpro complex that inhibits virus replication [59]. Severe infection with SARS-CoV-2 can lead to significant hematopoietic changes, particularly lymphocytopenia and lymphocyte immune regulatory failure [60]. Elevated levels of classic serum proinflammatory cytokines such as TNF-α, IL-6, IL-7, IL-8, granulocyte-colony-stimulating factor, and C-reactive protein have been associated with increased mortality in patients with SARS-CoV-2 infection [61, 62]. Zinc deficiency can enhance the release of these inflammatory factors, which can then combine with the inflammatory response of pathogens and aggravate lung damage [55]. Given the increased incidence of acute pulmonary embolism in patients with severe SARS-CoV-2 infection, zinc homeostasis is essential for all aspects of physiological coagulation and affects thrombosis and fibrinolysis [63, 64]. A pilot case-control study showed that a low serum level of zinc (approximately 56 ng/mL) is a risk factor for COVID-19 susceptibility [65]. Recent clinical studies showed that disease severity and mortality in patients with COVID-19 were inversely correlated with the serum zinc concentration, with more complications, longer hospital stays, and higher mortality observed in patients with zinc deficiency [66, 67]. Another study showed that a high-dose oral zinc salt treatment administered to SARS-CoV-2-infected patients significantly improved their clinical symptoms and objective indicators [68]. Vaccination is highly effective for preventing severe disease courses. Several vaccines (Sputnik V, AZD1222, and BBIBP‐CorV) have entered phase-III clinical trials [69]. Recent studies demonstrated that Zn-chitosan can induce the generation of effective and durable neutralizing antibodies against SARS-CoV-2 as a promising vaccine candidate; however, additional immunological and structural studies at the monoclonal antibody level are required [70]. Zinc blocks the replication of 2019-nCoV by inhibiting viral replication and regulating immunity, and zinc supplements have clinical value for preventing and treating COVID-19.
Zinc reduces the progression of harmful gas-induced COPD
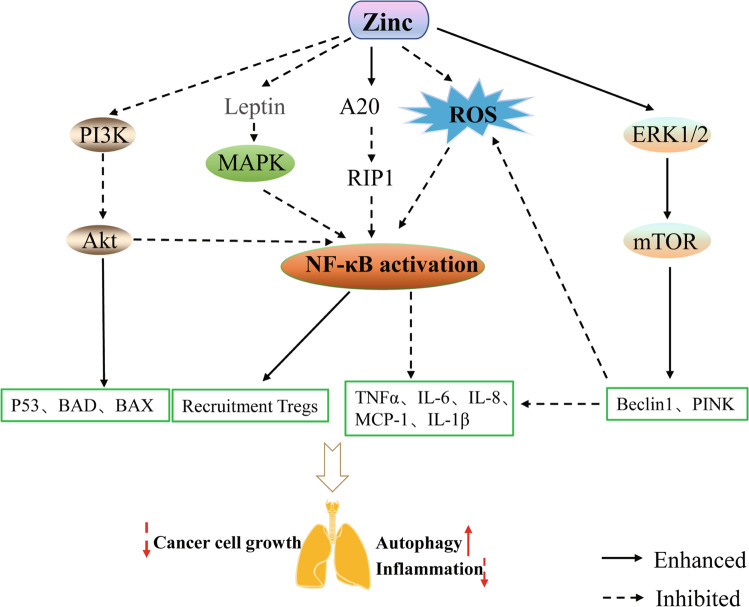
COPD occurs when exposure to chronic inflammation restricts the airflow and is often associated with pulmonary hypertension, hypoxemia, and hypercapnia, all of which substantially increase the incidence of cardiopulmonary complications. Numerous studies have shown that cigarette smoke exposure is a major cause of the onset and progression of COPD, which leads to excessive inflammatory responses in the airway, alveoli, and microvascular system [71, 72]. Cd is a toxic heavy metal present in large quantities in cigarette smoke. Inhalation of Cd can increase oxidative stress, lung inflammation, and emphysema; reduce lung function; and induce epithelial-mesenchymal transition [73]. As described above, zinc and Cd competitively bind ZIP8; the expression of ZIP8 is progressively silenced in the presence of excessive Cd, which can reduce the toxic effect of Cd on lung epithelial cells [38, 42] (Fig. 2). In addition, inhaled toxic gases or environmental pollutants (automobile exhaust, occupational dust exposure, and smoking) induce mitochondrial autophagy dysfunction to cause oxidative overload, which has been identified as a major cause of refractory inflammation in COPD [72, 74]. Mitochondrial autophagy disorders lead to increased production of reactive oxygen species and enhanced inflammatory responses in small airway epithelial cells in patients with COPD, exacerbating the disease progression [75]. Extracellular signal-regulated kinase 1/2 (ERK1/2) induces autophagy by inactivating the mammalian target of rapamycin (mTOR; Fig. 3) [76]. Harmful gases promote reactive oxygen species production, leading to oxidative release of zinc from MTs. An increased zinc concentration can activate ERK1/2-mediated autophagy, remove peroxides, and downregulate excessive inflammatory responses [77]. Cell experiments showed that zinc increased ERK activity and Beclin1 expression, as well as activated the mitochondrial PINK autophagy signaling pathway, thereby removing damaged mitochondria and reducing mitochondrial superoxide production [78]. Zinc-induced activation of the mitochondrial autophagy pathway exhibited a significant protective effect on bronchial epithelial cells in patients with COPD [72]. Therefore, the anti-inflammatory and antioxidant effects of zinc are important for improving the condition of patients with COPD and delaying disease progression.
Fig. 3. Zinc delays the progression of lung disease by affecting a variety of signaling pathways.
Zinc targets receptor-interacting protein 1 degradation by increasing the activity of zinc finger protein A20, thereby inhibiting the inflammatory cascade of downstream NF-κB activation. Zinc supplementation inhibits activation of the PI3K/AKT pathway in cancer cells, thereby promoting inhibition of tumor cells by the BAD and BAX, which are members of the pro-apoptotic family, enhancing p53-mediated apoptosis, and blocking activation of the NF-κB pathway for regulatory T lymphocyte recruitment. In patients with obesity, zinc blocks inflammatory lung damage by inhibiting the production of leptin, an adipocytokine, thereby blocking activation of the MAPK/NF-κB pathways. Zinc removes peroxide and inflammatory factors by activating the ERK/mTOR autophagy pathway.
Zinc relieves asthma attacks by regulating the immune system
Asthma is a chronic inflammatory disease of the airway and is characterized by hyperresponsiveness to common allergens as well as airway remodeling. The type 2 immune response modulated by Th2 cells is considered as the core molecular mechanism of asthma [43]. The airway type 2 immune response is primarily mediated by inflammatory cells such as TH2 cells, eosinophils, mast cells, basophils, and IgE-producing B cells. Th2 cells primarily secrete cytokines IL-4, IL-5, and IL-13 to promote the increase of airway eosinophils and airway remodeling and to stimulate the occurrence of the type 2 immune response [44, 45]. After intraperitoneal injection of zinc aspartate (30 µg) in mice with allergic asthma, the ability of CD4 + T cells to proliferate and differentiate in the lungs was significantly reduced [40]. In cell experiments, zinc aspartate dose-dependently (200 > 150 > 100 μM) inhibited the activation, proliferation, and Th1 and Th2 differentiation of murine CD4+ T cells [40]. In a mouse model of allergic airway inflammation, Morgan et al. found that zinc supplementation (10 mg/kg, intraperitoneal injection) enhanced the activity of the zinc finger protein A20, which inhibits the NF-κB pathway by degrading receptor-interacting protein 1, inhibits the inflammatory cascade caused by downstream NF-κB activation, reduces inflammatory cell infiltration and TNF-α cytokine release in the airway of mice, and considerably reduces airway hyperresponsiveness and serum IgE levels [79] (Fig. 3). The zinc transporters ZIP1 and ZIP2 on the cell membrane of human alveolar macrophages assist in the endocytosis of macrophages and promote in clearing inflammatory mediators in chronic inflammatory diseases (asthma, COPD) upon perceiving changes in the zinc concentration inside and outside of the cell [39]. Zinc homeostasis in the lung can balance the role of Th1 and Th2 cytokines and avoid the relative dominance of Th2, reduce Th1 cytokine (such as interferon-γ) production, impair the immune defense, and induce allergic diseases caused by zinc deficiency [80]. In allergen-activated peripheral blood mononuclear cells, zinc sulfate (50 μM) inhibited cell proliferation by activating regulatory T cells, enhancing the Th1 cytokine response, and ameliorating chronic allergic reactions mediated by Th2 cells [48]. Additionally, a zinc-supplemented diet (95 mg/kg) was demonstrated to inhibit the production of eosinophil chemokines and monocyte chemotactic protein-1 in the airway of asthmatic rats, increase the expression of lung interferon-γ mRNA, and reduce airway inflammation [81]. In summary, in the immune system, zinc plays an important role in reducing the severity of asthma and frequency of acute asthma by modifying NF-κB and other signaling pathways as well as by regulating the function of inflammatory cells (T cells, macrophages, and B cells).
Adjuvant therapeutic effect of zinc on non-small cell lung cancer
Non-small cell lung cancer (NSCLC) accounts for approximately 85–90% of all respiratory malignancies. The disease is insidious and progresses rapidly, with most patients at the middle and advanced stages at the time of diagnosis, thus posing a serious health threat [82]. The NF-κB inflammatory pathway is unregulated in both tumor cells and the tumor microenvironment [83]. A study of drug-induced lung tumor models in mice showed that activation of the NF-κB pathway in the airway epithelium led to persistent chronic airway inflammation and recruitment of functional regulatory T cells, thereby promoting lung tumor development [83] (Fig. 3).In addition, continued activation of the NF-κB inflammatory pathway led to the resistance of lung tumor cells to cisplatin and other chemotherapy drugs, reducing the treatment effect [84, 85]. Phosphoinositide 3-kinase (PI3K), another key molecule in lung cancer, is involved in the PI3K/Akt signaling pathway and is commonly mutated in NSCLC [86]. NF-κB is among the most important downstream signaling molecule of Akt. Following activation of the PI3K/Akt pathway, IKKα is phosphorylated and then phosphorylates the IκB protein and promotes proteasome degradation, resulting in NF-κB nuclear translocation and transcription of anti-apoptotic genes (Fig. 3) [87].Studies have shown that zinc combined with CuO nanocomposites inhibited tumor growth in vitro and in vivo through ROS-dependent apoptosis and autophagy via the NF-κB pathway [88]. Makorin RING Zinc finger 2 (MKRN2) of the Makorin RING zinc finger family encodes ribonucleic proteins with a unique array of zinc finger domains [89]. MKRN2 is a ubiquitin E3 ligase that targets the P65 subunit of NF-κB to negatively regulate inflammatory responses [90]. Other studies showed that MKRN2 inhibits the migration and invasion of NSCLC cells by negatively regulating the PI3K/Akt pathway [91]. In addition, zinc supplements can be used as drug delivery platforms to kill tumor cells for treating NSCLC and also be combined with other chemotherapy drugs to enhance anti-tumor activity [92, 93]. Therefore, zinc supplements play an important role in inhibiting the progression and invasion of NSCLC.
Zinc may reduce obesity-related lung injury by regulating lipid metabolism
Obesity (body mass index ≥30 kg/m2) has become one of the most serious public health problems worldwide. Severe obesity not only leads to a group of metabolic disorders including dyslipidemia, hyperglycemia, hypertension, and atherosclerosis [94, 95], but also increases the body’s susceptibility to lung injury through the above-mentioned metabolic disorders. This affects the pathological occurrence and clinical outcomes of lung injury and pulmonary fibrosis [96, 97]. Obesity is an independent risk factor for lung damage, and a multicenter clinical study showed that obesity is strongly associated with an increased incidence of moderate to severe COPD [98]. Other studies revealed that the incidence of ALI in patients with obesity was significantly higher than that in patients with a normal weight [99]. Leptin, a product of the obesity gene, is a 16-kDa adipocytokine derived from fat cells. Some studies of obesity and lung inflammation showed that leptin activates the MAPK/NF-κB/ P300 cascade, and then promotes the expression of proinflammatory protein cytosolic phospholipase A2-α in human alveolar type II A549 cells, leading to the release of inflammatory factors in the lung [100]. Leptin also promotes the production and secretion of mucin 5AC in the human bronchial epithelial cell line 16 by activating the JAK2-STAT3 pathway through IL-13, causing airway obstruction [101]. Visceral fat infiltration first results in the recruitment of activated M1 macrophages into adipose tissue, where macrophages and adipocytes co-produce a variety of proinflammatory and anti-inflammatory factors, including leptin, adiponectin, TNF-α, IL-6, and monocyte chemotactic protein-1 [102]. In addition, fat cells release saturated fatty acids that bind to macrophage Toll-like receptor 4 and activate inhibitors of κB kinase to activate the NF-κB pathway and induce inflammation [103]. Liu et al. found that a zinc-deficient diet (0.5–1.5 mg/kg) increased leptin production and increased macrophage infiltration into adipose tissue in obese mice, suggesting that zinc is important in the metabolic and immune dysregulation of obesity [104] (Fig. 3). The late adipogenic factor peroxisome proliferator-activated receptor γ is a core participant in adipogenesis, mediating adipocyte hypertrophy and insulin resistance induced by a high-fat diet [105]. Zinc has an insulin-like effect on 3T3-L1 preadipocytes and adipocytes and induces glucose transport into cells while enhancing insulin-induced glucose transport, possibly through insulin signaling pathways [106]. A clinical study revealed a significantly reduced serum zinc concentration (<70 μg/dL) in individuals with obesity compared to in normal individuals [107]. In addition, Zn supplementation (30 mg/day) has favorable effects on reducing anthropometric measurements, inflammatory markers, insulin resistance and appetite in individuals with obesity [108]. Based on these previous findings, zinc supplementation may reduce lung injury caused by obesity by inhibiting adipokine production, alleviating macrophage infiltration, and coordinating peroxisome proliferator-activated receptor γ-mediated lipid metabolism.
Protective role of zinc in other lung diseases
In critically ill patients with ARDS, the zinc concentration was significantly lower than the normal range, and C-reactive protein and IL-6 levels were significantly elevated at admission [109]. Following sufficient zinc supplementation in patients with ARDS, the recovery of zinc homeostasis was associated with decreased inflammatory factors, increased lymphocyte counts, and clinical recovery [109]. Excess mucus production and fluid imbalance in the airways are important markers of cystic fibrosis. One study showed that loss of the zinc transporter ZIP2 led to intracellular zinc deficiency and induced the secretion of mucin 5AC, which is highly expressed in the airways. This effect led to cystic fibrosis of airway epithelial cells. Zinc homeostasis plays an important protective role in airway epithelial cells [110] and is critical for inhibiting the development of various respiratory diseases.
Clinical application of zinc supplements in patients with respiratory diseases
Up to 30% of common respiratory infections, including the common cold, are caused by viruses (rhinoviruses, adenoviruses, and parainfluenza viruses) [111]. However, zinc appears to inhibit the activity of RNA polymerase from influenza virus and leads to a decreased viral titer [112]. In addition, zinc supplementation was shown to reduce the frequency and duration of common cold symptoms [111, 113, 114]. Moreover, zinc supplements induced apoptosis and enhanced the anti-tumor efficacy of cisplatin and paclitaxel in NSCLC by inhibiting the NF-κB signaling pathway and reducing or reversing drug resistance [83, 92]. Asthma is characterized by chronic inflammation and airway remodeling, and this remodeling is resistant to drug therapy [115]. Some clinical studies showed that zinc salicylate inhibited the mTOR signaling pathway and induced the expression of the asthma-related cell cycle inhibitor P21(Waf1/Cip1), thus preventing airway remodeling in asthma [115]. Recent in vitro studies showed that Zn2+ interacts with SARS-CoV-2 Mpro to form a stable Zn2+-Mpro complex, which interferes with coronavirus replication [59]. Oral administration of large doses of zinc salts in patients with SARS-CoV-2 infection significantly improved the clinical symptoms of COVID-19 [68, 116]. In patients with COPD, zinc supplementation reduced cytotoxicity, cell necrosis, and apoptosis by antagonizing the inhalation of harmful heavy metals [38]. In patients with cystic fibrosis and respiratory tract infection, zinc supplementation significantly reduced the time of antibiotic use and incidence of infection, particularly the frequency of respiratory tract infection in children [117–120]. In conclusion, zinc has many biological functions and acts as an anti-inflammatory, antioxidant, antiviral agent, and immunomodulator. Supplementing with zinc has important clinical value for treating various respiratory diseases (Table 1).
Table 1.
Clinical application of zinc supplements.
| Zinc supplementation | Disease | Study object | Mechanism | Range of supplementation | Effect | Refs. |
|---|---|---|---|---|---|---|
|
Zinc gluconate Zinc acetate Zinc sulfate |
Common cold |
Adults Children |
Immune regulation, inhibition of proinflammatory cytokines |
13.3 mg/2 h (while awake) 80 mg/day 30 mg/day (children) |
Shorten the duration of cold symptoms; Reduce the frequency of colds | [111, 113, 114] |
|
Zinc Zinc oxide nanoparticles |
NSCLC |
A549 cells H1299 cells |
Induce apoptosis and cytotoxicity |
50 μM or 100 μM Nanoparticles |
Reduce or reverse drug resistance and inhibit tumor growth | [92, 93] |
| Zinc salicylate | Asthma | Human airway smooth muscle cells (ASMC) | Block remodeling pathway Akt-mTOR and upregulate cell cycle inhibitor P21(Waf1/Cip1) | 10 µg/mL | Prevent airway wall remodeling | [115] |
|
Zinc citrate Zinc gluconate Zinc acetate |
nCoV pneumonia | Adults | Zn2+ can bind to SARS-CoV-2 Mpro to form stable Zn2+-Mpro complex |
23–200 mg/day 23–200 mg/day 15–200 mg/day |
Inhibit virus replication, enhance clinical efficacy of other drugs, and improve clinical symptoms | [59, 68] |
| Zinc gluconate | Cystic fibrosis | Children | Reduce plasma levels of inflammatory cytokines such as IL-6 and IL-8 | 30 mg/day | Reduce respiratory tract infection and reduce antibiotic use time | [117] |
| Zinc oxide | Upper RTI | Children | Blocking ICAM-1 receptor, antiviral | 5 mg/day | Reduce infection rates | [120] |
|
Zinc gluconate Zinc |
Lower RTI | Children | Regulate the immune system, anti-inflammatory, antiviral |
10 mg/day ≥70 mg/week |
Reduce infection rates | [118, 119] |
NSCLC non-small-cell lung cancer, RTI respiratory tract infection, ICAM-1 intercellular adhesion molecule-1.
Adverse effects of excessive zinc in humans
Zinc is a transition metal that is essential for regulating vital activities in the body. However, zinc is toxic to cells at high concentrations. Exposure to smoke grenades during military training or combat can cause zinc chloride (ZnCl2) smoke inhalation injury, which can lead to fatal progressive diffuse lung injury [121]. Workers in galvanizing plants experience occupational asthma due to long-term exposure to high levels of zinc, and baseline spirometry results were normal three months after they left the work environment [122]. However, the tumor cell toxicity of zinc can be used for anti-tumor therapy. ZnO nanoparticles provide a drug delivery platform that preferentially kills tumor cells in vitro and have been proposed as anti-tumor therapy for NSCLC [92, 93]. Therefore, further studies of the therapeutic effects of high concentrations of zinc on diseases can translate its toxicity into valuable clinical applications.
Conclusions
Zinc, as an intracellular signaling molecule, plays important roles in cell-mediated immune function and oxidative stress and, as a stable divalent cation, participates in regulating the oxidant/antioxidant balance. Zinc supplements inhibit viral replication and inflammatory cytokines in cell cultures. Zinc and its specific transporters are potential targets of respiratory diseases, and zinc supplements have some therapeutic effects in asthma, COPD, lung cancer, COVID-19, and other respiratory diseases. Through dietary adjustment or nutritional supplement therapy, the essential trace element zinc can be supplemented to the body where it exerts anti-inflammatory, antioxidant, antiviral, and immunomodulatory functions and reduces the adverse reactions of hormones, immunosuppressants, and other drugs. These properties can control various health conditions and improve the quality of life of patients. However, excessive levels of zinc in the body can cause cytotoxicity. To demonstrate the preventative effects and treatment potential of zinc, clinical controlled trials of zinc supplementation for the diseases described above and the development standards for assessing the zinc status are needed for COVID-19 research and treatment.
Author contributions
RML: literature reading, writing of the original draft; DYD: reviewing and sorting literature; QFX: reading and revising the article structure; HL and YJW: literature search and data analysis; JLY: creating the structure of the article, supervision.
Funding
This work was supported by the Department of Science and Technology of Jilin Province, China [grant number 20190201279JC]; and the Department of Finance of Jilin Province, China [grant number 20106145].
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respiratory Med. 2020;8:585–96. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. The Lancet. Respiratory Med. 2018;6:138–53. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal GM, Bunn PA, Jr, Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2018;13:1818–31. doi: 10.1016/j.jtho.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respiratory Med. 2018;6:747–58. doi: 10.1016/S2213-2600(18)30327-8. [DOI] [PubMed] [Google Scholar]
- 5.Vettorazzi S, Bode C, Dejager L, Frappart L, Shelest E, Klaßen C, et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun. 2015;6:7796. doi: 10.1038/ncomms8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respiratory Med. 2013;1:210–23. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 7.Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J. 2020;56. 10.1183/13993003.02094-2020. [DOI] [PubMed]
- 8.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet (Lond, Engl) 2018;391:1706–17. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 9.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer J clinicians. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 10.Ali MK, Kim RY, Karim R, Mayall JR, Martin KL, Shahandeh A, et al. Role of iron in the pathogenesis of respiratory disease. Int J Biochem cell Biol. 2017;88:181–95. doi: 10.1016/j.biocel.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab care. 2009;12:646–52. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 12.Yu Q, Liu H, Yang K, Tang X, Chen S, Ajuwon KM, et al. Effect of the level and source of supplementary dietary zinc on egg production, quality, and zinc content and on serum antioxidant parameters and zinc concentration in laying hens. Poult Sci. 2020;99:6233–8. doi: 10.1016/j.psj.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Signorell C, Zimmermann MB, Cakmak I, Wegmüller R, Zeder C, Hurrell R, et al. Zinc absorption from agronomically biofortified wheat is similar to post-harvest fortified wheat and is a substantial source of bioavailable zinc in humans. The. J Nutr. 2019;149:840–6. doi: 10.1093/jn/nxy328. [DOI] [PubMed] [Google Scholar]
- 14.Donangelo CM, Woodhouse LR, King SM, Toffolo G, Shames DM, Viteri FE, et al. Iron and zinc absorption from two bean (Phaseolus vulgaris L.) genotypes in young women. J Agric food Chem. 2003;51:5137–43. doi: 10.1021/jf030151w. [DOI] [PubMed] [Google Scholar]
- 15.Lafarga T, Hayes M. Bioactive peptides from meat muscle and by-products: generation, functionality and application as functional ingredients. Meat Sci. 2014;98:227–39. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Adams CL, Hambidge M, Raboy V, Dorsch JA, Sian L, Westcott JL, et al. Zinc absorption from a low-phytic acid maize. Am J Clin Nutr. 2002;76:556–9. doi: 10.1093/ajcn/76.3.556. [DOI] [PubMed] [Google Scholar]
- 17.Ma G, Li Y, Jin Y, Zhai F, Kok FJ, Yang X. Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. Eur J Clin Nutr. 2007;61:368–74. doi: 10.1038/sj.ejcn.1602513. [DOI] [PubMed] [Google Scholar]
- 18.Brnić M, Hurrell RF, Songré-Ouattara LT, Diawara B, Kalmogho-Zan A, Tapsoba C, et al. Effect of phytase on zinc absorption from a millet-based porridge fed to young Burkinabe children. Eur J Clin Nutr. 2017;71:137–41. doi: 10.1038/ejcn.2016.199. [DOI] [PubMed] [Google Scholar]
- 19.Saunders AV, Craig WJ, Baines SK. Zinc and vegetarian diets. Med J Aust. 2013;199:S17–21. doi: 10.5694/mja11.11493. [DOI] [PubMed] [Google Scholar]
- 20.Hemalatha S, Platel K, Srinivasan K. Influence of germination and fermentation on bioaccessibility of zinc and iron from food grains. Eur J Clin Nutr. 2007;61:342–8. doi: 10.1038/sj.ejcn.1602524. [DOI] [PubMed] [Google Scholar]
- 21.Norouzi S, Adulcikas J, Sohal SS, Myers S. Zinc transporters and insulin resistance: therapeutic implications for type 2 diabetes and metabolic disease. J Biomed Sci. 2017;24:87. doi: 10.1186/s12929-017-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008;99:S14–23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- 23.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol: organ Soc Miner Trace Elem (GMS) 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980) Am J Clin Nutr. 2003;78:756–64. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 25.Tran CD, Miller LV, Krebs NF, Lei S, Hambidge KM. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am J Clin Nutr. 2004;80:1570–3. doi: 10.1093/ajcn/80.6.1570. [DOI] [PubMed] [Google Scholar]
- 26.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr (Bethesda, Md) 2013;4:176–90. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mammadova-Bach E, Braun A. Zinc homeostasis in platelet-related diseases. Int J of Mol Sci. 2019;20. 10.3390/ijms20215258. [DOI] [PMC free article] [PubMed]
- 28.Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J of Mol Sci. 2017;18. 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed]
- 29.Kimura T, Kambe T. The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int J Mol Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Florea D, Molina-López J, Hogstrand C, Lengyel I, de la Cruz AP, Rodríguez-Elvira M, et al. Changes in zinc status and zinc transporters expression in whole blood of patients with Systemic Inflammatory Response Syndrome (SIRS) J Trace Elem Med Biol: organ Soc Miner Trace Elem (GMS) 2018;49:202–9. doi: 10.1016/j.jtemb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiological Rev. 2015;95:749–84. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 32.Wessels I, Cousins RJ. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am J Physiol Gastrointest liver Physiol. 2015;309:G768–78. doi: 10.1152/ajpgi.00179.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–37. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed]
- 35.Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. The. J nutritional Biochem. 2013;24:289–97. doi: 10.1016/j.jnutbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Kloubert V, Rink L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015;6:3195–204. doi: 10.1039/C5FO00630A. [DOI] [PubMed] [Google Scholar]
- 37.Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and lung growth. Nutrients. 2018;10. 10.3390/nu10070919. [DOI] [PMC free article] [PubMed]
- 38.Napolitano JR, Liu MJ, Bao S, Crawford M, Nana-Sinkam P, Cormet-Boyaka E, et al. Cadmium-mediated toxicity of lung epithelia is enhanced through NF-κB-mediated transcriptional activation of the human zinc transporter ZIP8. Am J Physiol Lung Cell Mol Physiol. 2012;302:L909–18. doi: 10.1152/ajplung.00351.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamon R, Homan CC, Tran HB, Mukaro VR, Lester SE, Roscioli E, et al. Zinc and zinc transporters in macrophages and their roles in efferocytosis in COPD. PloS one. 2014;9:e110056. doi: 10.1371/journal.pone.0110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krone A, Fu Y, Schreiber S, Kotrba J, Borde L, Nötzold A, et al. Ionic mitigation of CD4(+) T cell metabolic fitness, Th1 central nervous system autoimmunity and Th2 asthmatic airway inflammation by therapeutic zinc. Sci Rep. 2022;12:1943. doi: 10.1038/s41598-022-04827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoell DL, Smith D, Bao S, Sapkota M, Wyatt TA, Zweier JL, et al. Imbalance in zinc homeostasis enhances lung Tissue Loss following cigarette smoke exposure. J Trace Elem Med Biol: organ Soc Miner Trace Elem (GMS) 2020;60:126483. doi: 10.1016/j.jtemb.2020.126483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Xu Y, Wu D, Yu F, Yang L, Yao Y, et al. Progressive silencing of the zinc transporter Zip8 (Slc39a8) in chronic cadmium-exposed lung epithelial cells. Acta biochimica et biophysica Sin. 2017;49:444–9. doi: 10.1093/abbs/gmx022. [DOI] [PubMed] [Google Scholar]
- 43.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–83. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao B, Prasad AS, Beck FW, Bao GW, Singh T, Ali S, et al. Intracellular free zinc up-regulates IFN-γ and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochemical biophysical Res Commun. 2011;407:703–7. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad AS. Lessons learned from experimental human model of zinc deficiency. J Immunol Res. 2020;2020:9207279. doi: 10.1155/2020/9207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenkranz E, Hilgers RD, Uciechowski P, Petersen A, Plümäkers B, Rink L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur J Nutr. 2017;56:557–67. doi: 10.1007/s00394-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Cao R, Wasserloos KJ, Bernal P, Liu ZQ, Pitt BR, et al. Nitric oxide and zinc homeostasis in pulmonary endothelium. Ann N. Y Acad Sci. 2010;1203:73–8. doi: 10.1111/j.1749-6632.2010.05558.x. [DOI] [PubMed] [Google Scholar]
- 50.Thambiayya K, Kaynar AM, St Croix CM, Pitt BR. Functional role of intracellular labile zinc in pulmonary endothelium. Pulm circulation. 2012;2:443–51. doi: 10.4103/2045-8932.105032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St Croix CM, Leelavaninchkul K, Watkins SC, Kagan VE, Pitt BR. Nitric oxide and zinc homeostasis in acute lung injury. Proc Am Thorac Soc. 2005;2:236–42. doi: 10.1513/pats.200501-007AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, et al. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circulation Res. 2008;102:1575–83. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ban Y, Liu Y, Li Y, Zhang Y, Xiao L, Gu Y, et al. S-nitrosation impairs KLF4 activity and instigates endothelial dysfunction in pulmonary arterial hypertension. Redox Biol. 2019;21:101099. doi: 10.1016/j.redox.2019.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Zhang L, Zhang Y, Liu Y, Zhang H, Wei L, et al. A20 deficiency leads to angiogenesis of pulmonary artery endothelial cells through stronger NF-κB activation under hypoxia. J Cell Mol Med. 2016;20:1319–28. doi: 10.1111/jcmm.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wessels I, Pupke JT, von Trotha KT, Gombert A, Himmelsbach A, Fischer HJ, et al. Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax. 2020;75:253–61. doi: 10.1136/thoraxjnl-2019-213357. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. The N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet (Lond, Engl) 2020;395:1014–5. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panchariya L, Khan WA, Kuila S, Sonkar K, Sahoo S, Ghoshal A, et al. Zinc(2+) ion inhibits SARS-CoV-2 main protease and viral replication in vitro. Chem Commun (Camb, Engl) 2021;57:10083–6. doi: 10.1039/D1CC03563K. [DOI] [PubMed] [Google Scholar]
- 60.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol regulators Homeost agents. 2020;34:327–31. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 62.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283–5. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin AI, Rao G. COVID-19: a potential risk factor for acute pulmonary embolism. Methodist DeBakey cardiovascular J. 2020;16:155–7. doi: 10.14797/mdcj-16-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wessels I, Rolles B, Slusarenko AJ, Rink L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br J Nutr. 2022;127:214–32. doi: 10.1017/S0007114521000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghanei E, Baghani M, Moravvej H, Talebi A, Bahmanjahromi A, Abdollahimajd F. Low serum levels of zinc and 25-hydroxyvitmain D as potential risk factors for COVID-19 susceptibility: a pilot case-control study. European journal of clinical nutrition. 2022;1–6. 10.1038/s41430-022-01095-5. [DOI] [PMC free article] [PubMed]
- 66.Heller RA, Sun Q, Hackler J, Seelig J, Seibert L, Cherkezov A, et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38:101764. doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2020;100:343–9. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2020;99:307–9. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sampath V, Rabinowitz G, Shah M, Jain S, Diamant Z, Jesenak M, et al. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76:1640–60. doi: 10.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srivastava V, Niu L, Phadke KS, Bellaire BH, Cho MW. Induction of potent and durable neutralizing antibodies against SARS-CoV-2 using a receptor binding domain-based immunogen. Front Immunol. 2021;12:637982. doi: 10.3389/fimmu.2021.637982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J respiratory Crit care Med. 2017;195:557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 72.Yao RQ, Ren C, Xia ZF, Yao YM. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385–401. doi: 10.1080/15548627.2020.1725377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanwar VS, Zhang X, Jagannathan L, Jose CC, Cuddapah S. Cadmium exposure upregulates SNAIL through miR-30 repression in human lung epithelial cells. Toxicol Appl Pharmacol. 2019;373:1–9. doi: 10.1016/j.taap.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Y, Wang X, Hu D. Mitochondrial alterations during oxidative stress in chronic obstructive pulmonary disease. Int J chronic Obstr Pulm Dis. 2017;12:1153–62. doi: 10.2147/COPD.S130168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11:547–59. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, et al. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284:21412–24. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nuttall JR, Oteiza PI. Zinc and the ERK kinases in the developing brain. Neurotox Res. 2012;21:128–41. doi: 10.1007/s12640-011-9291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bian X, Teng T, Zhao H, Qin J, Qiao Z, Sun Y, et al. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radic Res. 2018;52:80–91. doi: 10.1080/10715762.2017.1414949. [DOI] [PubMed] [Google Scholar]
- 79.Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. J Inflamm (Lond, Engl) 2011;8:36. doi: 10.1186/1476-9255-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hönscheid A, Rink L, Haase H. T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocr, Metab immune Disord drug targets. 2009;9:132–44. doi: 10.2174/187153009788452390. [DOI] [PubMed] [Google Scholar]
- 81.Lu H, Xin Y, Tang Y, Shao G. Zinc suppressed the airway inflammation in asthmatic rats: effects of zinc on generation of eotaxin, MCP-1, IL-8, IL-4, and IFN-γ. Biol trace Elem Res. 2012;150:314–21. doi: 10.1007/s12011-012-9493-7. [DOI] [PubMed] [Google Scholar]
- 82.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer J clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 83.Zaynagetdinov R, Stathopoulos GT, Sherrill TP, Cheng DS, McLoed AG, Ausborn JA, et al. Epithelial nuclear factor-κB signaling promotes lung carcinogenesis via recruitment of regulatory T lymphocytes. Oncogene. 2012;31:3164–76. doi: 10.1038/onc.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao L, Lan X, Shi X, Zhao K, Wang D, Wang X, et al. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell death Dis. 2017;8:e2803. doi: 10.1038/cddis.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen PM, Cheng YW, Wu TC, Chen CY, Lee H. MnSOD overexpression confers cisplatin resistance in lung adenocarcinoma via the NF-κB/Snail/Bcl-2 pathway. Free Radic Biol Med. 2015;79:127–37. doi: 10.1016/j.freeradbiomed.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Scheffler M, Bos M, Gardizi M, König K, Michels S, Fassunke J, et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget. 2015;6:1315–26. doi: 10.18632/oncotarget.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heavey S, Godwin P, Baird AM, Barr MP, Umezawa K, Cuffe S, et al. Strategic targeting of the PI3K-NFκB axis in cisplatin-resistant NSCLC. Cancer Biol Ther. 2014;15:1367–77. doi: 10.4161/cbt.29841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu H, Yuan R, Liu X, Li X, Qiao G, Li C, et al. Zn-doped CuO nanocomposites inhibit tumor growth by NF-κB pathway cross-linked autophagy and apoptosis. Nanomed (Lond, Engl) 2019;14:131–49. doi: 10.2217/nnm-2018-0366. [DOI] [PubMed] [Google Scholar]
- 89.Jong MT, Gray TA, Ji Y, Glenn CC, Saitoh S, Driscoll DJ, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8:783–93. doi: 10.1093/hmg/8.5.783. [DOI] [PubMed] [Google Scholar]
- 90.Shin C, Ito Y, Ichikawa S, Tokunaga M, Sakata-Sogawa K, Tanaka T. MKRN2 is a novel ubiquitin E3 ligase for the p65 subunit of NF-κB and negatively regulates inflammatory responses. Sci Rep. 2017;7:46097. doi: 10.1038/srep46097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang J, Xu Y, Ren H, Wudu M, Wang Q, Song X, et al. MKRN2 inhibits migration and invasion of non-small-cell lung cancer by negatively regulating the PI3K/Akt pathway. J Exp Clin cancer Res: CR. 2018;37:189. doi: 10.1186/s13046-018-0855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kocdor H, Ates H, Aydin S, Cehreli R, Soyarat F, Kemanli P, et al. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des, Dev Ther. 2015;9:3899–909. doi: 10.2147/DDDT.S87662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19:295103. doi: 10.1088/0957-4484/19/29/295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur heart J. 2008;29:932–40. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 95.Al-Sulaiti H, Diboun I, Agha MV, Mohamed FFS, Atkin S, Dömling AS, et al. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J Transl Med. 2019;17:348. doi: 10.1186/s12967-019-2096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thais Fantozzi E, Rodrigues-Garbin S, Yamamoto Ricardo-da-Silva F, Oliveira-Filho RM, Spina D, Tavares-de-Lima W, et al. Acute lung injury induced by intestinal ischemia and reperfusion is altered in obese female mice. Pulm Pharmacol therapeutics. 2018;49:54–9. doi: 10.1016/j.pupt.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Manicone AM, Gong K, Johnston LK, Giannandrea M. Diet-induced obesity alters myeloid cell populations in naïve and injured lung. Respiratory Res. 2016;17:24. doi: 10.1186/s12931-016-0341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity Paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PloS one. 2016;11:e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsu PS, Wu CS, Chang JF, Lin WN. Leptin promotes cPLA2 gene expression through activation of the MAPK/NF-κB/p300 cascade. Int J Mol Sci. 2015;16:27640–58. doi: 10.3390/ijms161126045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao W, Wang J, Zhang Y, Wang Y, Sun L, Han W. Leptin positively regulates MUC5AC production and secretion induced by interleukin-13 in human bronchial epithelial cells. Biochemical biophysical Res Commun. 2017;493:979–84. doi: 10.1016/j.bbrc.2017.09.106. [DOI] [PubMed] [Google Scholar]
- 102.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arteriosclerosis, thrombosis, Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 104.Liu MJ, Bao S, Bolin ER, Burris DL, Xu X, Sun Q, et al. Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J Nutr. 2013;143:1036–45. doi: 10.3945/jn.113.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang X, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr. 2001;131:1414–20. doi: 10.1093/jn/131.5.1414. [DOI] [PubMed] [Google Scholar]
- 107.Rios-Lugo MJ, Madrigal-Arellano C, Gaytán-Hernández D, Hernández-Mendoza H, Romero-Guzmán ET. Association of serum zinc levels in overweight and obesity. Biol trace Elem Res. 2020;198:51–7. doi: 10.1007/s12011-020-02060-8. [DOI] [PubMed] [Google Scholar]
- 108.Khorsandi H, Nikpayam O, Yousefi R, Parandoosh M, Hosseinzadeh N, Saidpour A, et al. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: a randomized, placebo-controlled, double-blind trial. Diabetol Metab Syndr. 2019;11:101. doi: 10.1186/s13098-019-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Notz Q, Herrmann J, Schlesinger T, Helmer P, Sudowe S, Sun Q, et al. Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS. Nutrients. 2021;13. 10.3390/nu13062113. [DOI] [PMC free article] [PubMed]
- 110.Kamei S, Fujikawa H, Nohara H, Ueno-Shuto K, Maruta K, Nakashima R, et al. Zinc deficiency via a splice switch in zinc importer ZIP2/SLC39A2 causes cystic fibrosis-associated MUC5AC hypersecretion in airway epithelial cells. EBioMedicine. 2018;27:304–16. doi: 10.1016/j.ebiom.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mossad SB, Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med. 1996;125:81–8. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 112.Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan M, et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:245–52. doi: 10.7326/0003-4819-133-4-200008150-00006. [DOI] [PubMed] [Google Scholar]
- 114.Kurugöl Z, Akilli M, Bayram N, Koturoglu G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta paediatrica (Oslo, Nor: 1992) 2006;95:1175–81. doi: 10.1080/08035250600603024. [DOI] [PubMed] [Google Scholar]
- 115.Fang L, Roth M, S’Ng CT, Tamm M, Han B, Hoang BX. Zinc salicylate reduces airway smooth muscle cells remodelling by blocking mTOR and activating p21((Waf1/Cip1)) J nutritional Biochem. 2021;89:108563. doi: 10.1016/j.jnutbio.2020.108563. [DOI] [PubMed] [Google Scholar]
- 116.Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med hypotheses. 2020;142:109815. doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdulhamid I, Beck FW, Millard S, Chen X, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatr Pulmonol. 2008;43:281–7. doi: 10.1002/ppul.20771. [DOI] [PubMed] [Google Scholar]
- 118.Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 2010;39:795–808. doi: 10.1093/ije/dyp391. [DOI] [PubMed] [Google Scholar]
- 119.Shah UH, Abu-Shaheen AK, Malik MA, Alam S, Riaz M, Al-Tannir MA. The efficacy of zinc supplementation in young children with acute lower respiratory infections: a randomized double-blind controlled trial. Clin Nutr (Edinb, Scotl) 2013;32:193–9. doi: 10.1016/j.clnu.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 120.Martinez-Estevez NS, Alvarez-Guevara AN, Rodriguez-Martinez CE. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergologia et immunopathologia. 2016;44:368–75. doi: 10.1016/j.aller.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Hsu HH, Tzao C, Chang WC, Wu CP, Tung HJ, Chen CY, et al. Zinc chloride (smoke bomb) inhalation lung injury: clinical presentations, high-resolution CT findings, and pulmonary function test results. Chest. 2005;127:2064–71. doi: 10.1378/chest.127.6.2064. [DOI] [PubMed] [Google Scholar]
- 122.Malo JL, Cartier A, Dolovich J. Occupational asthma due to zinc. Eur respiratory J. 1993;6:447–50. doi: 10.1183/09031936.93.06030447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.