Highlights
-
•
Stomoxys calcitrans (stable fly) is a potential mechanical vector for the lumpy skin disease virus.
-
•
Lumpy skin disease virus could be isolated from regurgitated blood and faeces of stable flies exposed to virus-spiked blood.
-
•
Lumpy skin disease virus could survive in the stable flies’ abdomens up to two days without losing its infectivity.
-
•
Stomoxys calcitrans was present in cattle farms also at higher altitudes (2128 m asl) in Switzerland.
Keywords: Artificial feeding, Biting flies, Cattle infectious disease, Sticky traps, Virus excretion
Abstract
Lumpy skin disease (LSD) is a viral disorder of cattle caused by the lumpy skin disease virus (LSDV) which can induce severe infections leading to high economic losses. Being of African origin, the first LSD outbreaks in Europe occurred in Greece and later in the Balkan region. Little is known about the mode of transmission, especially in relation to the potential role of arthropods vectors. The purpose of our study was to investigate the role of Stomoxys calcitrans in the transmission of LSDV and their presence at different farms in Switzerland. Laboratory-reared flies were exposed to LSDV spiked-blood and incubated under a realistic fluctuating temperature regime. Body parts, regurgitated blood, and faecal samples were analysed by qPCR for the presence of viral DNA and infectious virus at different time points post-feeding (p.f.). LSDV DNA was detected in heads, bodies, and regurgitated blood up to three days p.f. and up to two days p.f. in the faeces. Infectious virus was isolated from bodies and faeces up to two days and in the regurgitated blood up to 12 h p.f. There was no increase in viral load, consolidating the role of S. calcitrans as mechanical vectors for LSDV. Stomoxys flies were present at all eight farms investigated, including a farm located at 2128 m asl. The persistence of LSDV in S. calcitrans in combination with the long flight ranges of this abundant and widespread fly might have implications on LSD epidemiology and on implementing control measures during disease outbreaks.
Graphical abstract
1. Introduction
Lumpy skin disease (LSD) is an OIE-listed viral disorder affecting mostly cattle. The causative agent is a virus in the genus Capripoxvirus belonging to the subfamily Chordopoxvirinae from the Poxviridae family (Buller et al., 2005). The disease is characterised by pyrexia, the presence of firm, circumscribed skin nodules, internal lesions in the skeletal muscles and the mucosa of the digestive and respiratory tracts, and generalised lymphadenopathy (Davies, 1991). LSD is endemic in Africa (Carn and Kitching, 1995). However, since 2012 the virus has been reported in the Middle East and more recently in south-eastern Europe, where it spread rapidly and was eventually controlled by culling and vaccination campaigns (Calistri et al., 2020) as well as in Asia, including Taiwan, China, Bangladesh and Russia (5). Though several modes of transmission of the virus have been identified (seminal, intra-uterine, direct contact, by ticks) (Sprygin et al., 2019), it is generally accepted that LSDV is mainly mechanically transmitted via flying, blood-feeding insects, based on circumstantial evidence (Sprygin et al., 2019) and mathematical models (Magori-Cohen et al., 2012). However, which insect species and to what extent they contribute to LSDV transmission is unclear.
In a series of experiments, Chihota and colleagues showed that female Aedes aegypti mosquitoes could mechanically transmit LSDV to susceptible cattle up to two to six days post‐feeding on experimentally infected animals. However, other mosquito species (Culex quinquefasciatus and Anopheles stephensi), the biting midge species Culicoides nubeculosus and the stable fly Stomoxys calcitrans were unable to do so (Chihota et al., 2001; Chihota et al., 2003).
Stomoxys calcitrans is a widely distributed nuisance fly; both sexes feed on blood two-to-three times per day (Baldacchino et al., 2013). This species is known as a mechanical vector under experimental conditions of, e.g. African swine fever (Olesen et al., 2018) and sheep and goat poxviruses (Kitching and Mellor, 1986). Transmission of parasites like Theilaria orientalis (Hornok et al., 2020) or Besnoitia besnoiti (Sharif et al., 2019) by this fly have also been reported. High abundances of S. calcitrans in Israel were associated with LSDV outbreaks (Kahana-Sutin et al., 2017). Recent reports have presented data that support a role of S. calcitrans, other Stomoxys spp., and Tabanids (Haematopota spp.) in the mechanical transmission of LSDV (Sohier et al., 2019; Issimov et al., 2020).
The purpose of our study was to investigate stable flies for their capability of mechanical transmission of LSDV under laboratory conditions. Virus stability in different body parts of the flies, and at different time points after viral exposure, was here analysed. Laboratory reared S. calcitrans were exposed to LSDV-spiked blood through an artificial membrane. Engorged flies were incubated in a climatic chamber at a realistic fluctuating temperature regime, and body parts (heads, thorax and abdomens) were tested for the presence of LSDV DNA for up to 72 h post-feeding. Also, the presence of the virus in regurgitated blood and in faecal samples of the flies was investigated. To confirm the capability of S. calcitrans in transmitting LSDV in the field, the isolation of infectious virus particles from samples containing viral DNA was here carried out. In the likelihood of an LSDV outbreak in Switzerland, and in consideration of the potential role of this fly in transmitting the pathogen, we have investigated the presence of S. calcitrans on different farms and at different high elevation (over 2128 m above sea level). For the collection, sticky traps, an inexpensive and straightforward tool, were here deployed. Though S. calcitrans is globally distributed and few studies on its population dynamics are available from Europe, such as (Skovgard and Nachman, 2012; Lempereur et al., 2018), no reports are available for Switzerland.
2. Materials & methods
2.1. Flies
Stable flies (S. calcitrans) at the pupal stage were provided by The Pirbright Institute (Pirbright, UK). About 400 pupae were transferred into a BugDorm-1 insect rearing cage (17.5 × 17.5 × 17.5 cm; BugDorm-42222F; MegaView Science, Taichung, Taiwan) and maintained in a climatic chamber at 26 °C with 60–70% relative humidity and a light regime of 12L:12D until adults emerged (7–10 days). Freshly emerged adults were kept for 24 h without access to blood or sugar. After this starvation period, the flies were aspirated from the rearing cage, transferred into dispensing boxes (all-purpose dispensing boxes, f 5 cm high and 6 cm width, Adelphi Healthcare Packaging, Haywards Heath, UK) (50 flies/box), covered with a net on the top, and transported to a biosafety level 3 (BSL3) laboratory for oral exposure to LSDV.
2.2. In vitro feeding
The LSDV strain used originated from Macedonia (2016, field strain) (Möller et al., 2019). The virus was propagated five times on Madin-Darby bovine kidney epithelial cells (MDBK NBL-1; CCL-22 line) with Glasgow minimum essential medium (GMEM) (Gibco, Thermo Fisher Scientific, Reinach, Switzerland) and 2.5% horse serum (Horse serum Biowest S0910, Lubio Science GmbH, Zürich, Switzerland) under BSL3 conditions, reaching a final titre of 6.25 log10TCID50/ml.
Flies were exposed to heparinised bovine blood, obtained from a local abattoir in Zürich, spiked with LSDV at a final titre of 5.75 log10TCID50/ml. Flies were fed using a hemotek membrane feeding system, PS6 Power Unit (Hemotek Ltd., Lancashire, UK), layered with ParafilmⓇ M (Sigma-Aldrich, Buchs, Switzerland) serving as a feeding membrane, at 37 °C for one hour. The dispensing box, containing the flies, was positioned on the feeder with the net facing the membrane; hence the flies were feeding from above. After the one h exposure, the flies were anaesthetised by placing the boxes at −20 °C for a few mins. (max. five). Fully engorged flies were sorted on an ice-cold Petri dish under a stereomicroscope and individually transferred into 50 ml Falcon conical centrifuge tubes (Thermo Fischer Scientific, Zürich, Switzerland) containing a small piece of cotton soaked with non-infectious blood as a food source. The flies were incubated under a fluctuating temperature regime simulating an average summer day in Switzerland (14–28 °C, mean of 22 °C) with 80% relative humidity and a light regime of 12L:12D. At different time intervals (0, 3, 6, 12, 24, 36, 48 and 72 h) post-feeding (p.f.), seven-to-ten individuals were collected and euthanised by freezing at −80 °C and stored at −80 °C until further investigation.
2.3. Samples for LSDV detection
Virus presence in the flies was assessed from heads and bodies (thorax and abdomen) of flies collected at the p.f time intervals as described above by qPCR assay for LSDV viral DNA (see below).
Heads and bodies of the flies were separated by using sterile needles (26 G x ½”; Fine-Ject, Tuttlingen, Germany) and placed individually in 1.5 ml sterile Eppendorf tubes containing 400 μl Glasgow minimum essential medium (GMEM) (Gibco, Thermo Fisher Scientific, Reinach, Switzerland) supplemented with 2% antibiotics and fungizone (1000 IU/ml penicillin/streptomycin; 4 μg/ml amphotericin; Gibco). Samples were manually homogenised for 30 s using sterilised polypropylene pestles (Sigma-Aldrich, Gillingham, Dorset, UK) mounted to a motorised grinder (Micro Handrührer, Carl Roth, Karlsruhe, Germany) then stored at −80 °C until furthered use.
Blood saturated cotton feeding pads (containing regurgitated blood) present at the bottom of each Falcon tube from flies incubated from 3 to 72 h post-oral exposure were also analysed for the presence of virus.
Another way to confirm virus presence in the engorged flies, and a putative route of mechanical transmission by contamination through shedding was by the screening of faecal samples of Stomoxys flies present in the Falcon tubes. Samples were collected using a small piece of cotton. Then the cotton pads with blood and those with faeces were incubated individually for 20 min at room temperature in 2 ml sterile Eppendorf tubes containing 400 ml PBS (Dulbecco`s phosphate-buffered saline; Sigma-Aldrich, Buchs, Switzerland). After incubation, the cotton samples were manually squashed using a sterile polypropylene pestle, vortexed for 15 s, centrifuged for 1 min (Fontaine et al., 2016), and transferred into new tubes. The homogenates obtained were stored at −80 °C until subjection to DNA extraction or cell culture inoculation.
2.4. Detection of LSDV viral DNA
Total DNA from each 200 µl of sample (homogenates of the head, bodies; supernatant from cotton with regurgitated blood and from cotton with faeces) was extracted using the QIAamp viral DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions using the DNA tissue or body fluids protocols (elution volume 100 μl). Detection of LSDV DNA was performed using a quantitative polymerase chain reaction (qPCR) specific for Capripoxviruses (Stubbs et al., 2012) by targeting an 89 bp region within LSDV ORF074 encoding the intracellular mature virion envelope protein P32. The assay was done with EXPRESS qPCR Supermix Universal (Invitrogen, Thermo Fischer, Zug, Switzerland) and was performed with 1x reaction mix, 20 µM of PCR primers (forward: CaPV-074F1 5`-AAA ACG GTA TAT GGA ATA GAG TTG GAA-3; reverse: CaPV-074R1 5`-AAA TGA AAC CAA TGG ATG GGA TA-3`(Microsynth, Balgach, Switzerland), and five µM of TaqMan probe CaPV (CaPV-074P1 5`−6FAM-TGG CTC ATA GAT TTC CT-MGB/NFQ-3`) (Applied Biosystems, Inchinnan, United Kingdom). Amplification was done in a Thermocycler (QuantiStudioFlex 7, Applied Biosystems, Thermo Fischer, Zug, Switzerland) using the following program: 50 °C for 2 min, 95 °C for 2 min followed by 50 cycles of 15 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C.
For relative quantification, a standard curve was generated with 10-fold serial dilutions of the viral inoculum used in the experiments. According to the standard curve, a cut-off of Cq ≤ 34.6 was established (Fig. S1) to exclude samples which tested positive for viral DNA but not for infectious virus particles (Veronesi et al., 2013).
2.5. Virus detection by virus isolation in cell culture
Cell-based assays to confirm the presence of infectious virus particles were performed for qPCR-positive body homogenates, supernatants of regurgitated blood, and faeces. Cell culturing flasks CorningⓇ Zellkulturkolben (Merck, Darmstadt, Germany), with a surface area of twenty-five cm2 were seeded with 7 × 105 cells (MDBK NBL-1; CCL-22 line) 24 h prior to their inoculations with 200 μl of homogenates or supernatants. Flasks were incubated for one h (at 37 °C with 5% CO2) after which growth media (GMEM with 2.5% horse serum; Horse serum Biowest S0910, Lubio Science GmbH, Zürich, Switzerland) was added and the incubation continued at the same conditions for seven days. The presence of cytopathic effect (CPE) in the cells, identified under an inverted microscope by observation of dead cells, was recorded daily.
2.6. Monitoring for the presence of S. calcitrans at Swiss farms
The presence of S. calcitrans was monitored at eight farms with different livestock animals in Switzerland. Flies were collected using commercially available white traps (Rebell bianco, Andermatt, Grossdietwil, Switzerland) covered with a thin layer of liquid glue (Tangle-Trap; Andermatt, Grossdietwil, Biocontrol, Grossdietwil, Switzerland) which was replaced after each collection. Traps were placed inside the barns where the animals were resting and replaced after one-to-three weeks in 2018 (June-November) and 2019 (August-November).
2.7. Statistics
We used a generalised linear model (glm) with a Tweedie (compound Poisson) distribution to compare (Cq values) viral loads on cotton, in the bodies or the heads with time: that is three glms with a single dependant and independent variable. There was a log link function in each case. P values were based on the t distribution of the parameter values. Correlations between Cq values (viral loads) in the head, body and cotton were compared in 3 glms testing each two way association. The analyses were performed using R (https://www.r-project.org/) using the cplm package (Zhang, 2013; Zhu et al., 2016).
3. Results
3.1. Detection of LSDV viral DNA in fly samples regurgitated blood and faeces
Overall, 500 flies were exposed to LSDV-spiked blood, 246 (49%) took a blood meal of which 155 did not survive the three days incubation period. Seventy-six individuals were dissected, and the remaining 15 were used for faecal sample collection.
A total of 76 bodies (100%), 74 heads (97%), ten faecal samples (66%), and 20 blood regurgitants (26%) were “positive” for viral LSDV DNA exhibiting Cq values ranging between 19.5 and 42 (Suppl. Table S1).
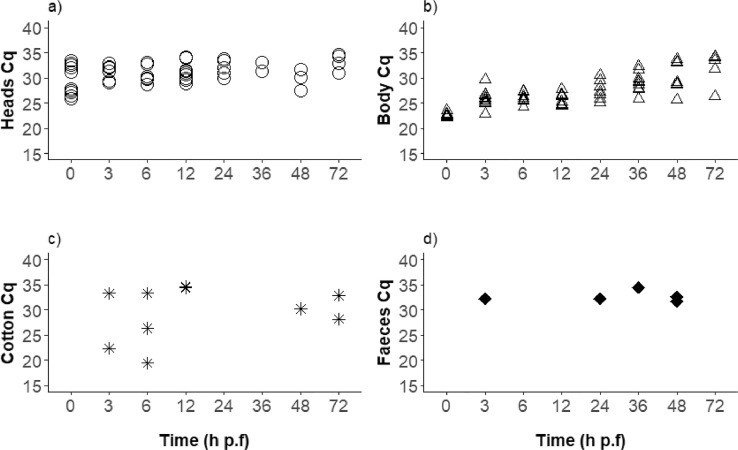
By applying a cut-off of Cq ≤ 34.6 as generated from the standard curve of the virus inoculum used for oral fly exposure (Suppl. Fig. S1), the overall number of positive samples was 73 (96%) bodies and 47 (62%) heads (Fig. 1a, b; Suppl. Table S1), five faecal samples (33%), and ten cotton pads (15%). All the bodies between 0 and 36 h p.f. were PCR positive, whereas, at 48 and 72 h p.f., 80% and 86% of the bodies, respectively, were positive (Fig. 1b). There was a statistically significant decrease in viral load in the bodies with time (P<0.001). The heads of all flies tested immediately after feeding were positive. Until 12 h p.f., 60% or more of the tested heads were positive, and lower values between 20 and 44% were observed with the samples from 24 to 48 h p.f. However, at the end of the experiment (72 h p.f.), 4/7 heads (57%) tested had Cq values above the threshold (Fig. 1a).
Fig. 1.
Stomoxys calcitrans qPCRs positive (Cq ≤34.6) for LSDV DNA over time after exposure to virus-spiked blood meals. (A) S. calcitrans heads, (B) S. calcitrans bodies (thorax and abdomen, C) cotton pads with regurgitated blood or (D) faecal samples. Seven to 10 flies were tested per time point, and the percentages of qPCR-positive flies are also given (A, B). A statistically significant decrease in viral load with time was only identified in bodies.
h p.f. = hours post-feeding.
Sixty-six blood regurgitant soaked cotton pads were analysed, and ten (15%) were positive by PCR.
Specifically, 20–30% positivity was observed in the first 3–12 h of incubation, 0% between 24 and 36 h, whereas between 48 and 72 h 10–30% of the samples, respectively, were positive (Fig. 1c). The Cq values had a considerable variation (between 19.5 and 34.4).
Overall, five (33%) of the 15 faecal samples collected (approx. 30 faecal dots/tube) tested positive as follows: 1 positive amongst 1 tested from 3 h p.f., 0/5 from 6 h p.f., 1/2 from 24 h p.f., 1/5 from 36 h p.f., and 2/2 from 48 h p.f. (Fig. 1d).
3.2. Virus isolation in cell culture
Twenty-nine out of 35 body homogenates tested on MDBK cell assay were positive. Infectious virus particles causing a cytopathic effect (CPE) were observed starting with the second-day p. f. in all the tested body samples and CPE was detected in samples from up to 48 h p.f. The results were inconclusive for the remaining six samples, including all four samples investigated from 72 h p.f. (Supp. Table S1) because of the contamination of the cell cultures during the seven days incubation time. Amongst the ten samples with regurgitated blood that were positive by qPCR, five (50%) samples taken up to 12 h p.f. gave CPE on the cell-based assay, and two samples (from 72 h p.f.) were inconclusive due to contamination. (Suppl. Table S1). From the five PCR-positives faecal samples, four showed CPE (1 each from 3 h p.f., 24 h p.f., 36 h p.f., 48 h p.f.) and one (48 h p.f.) was contaminated.
3.3. Stomoxys calcitrans presence and monitoring at different farms in Switzerland
Stomoxys calcitrans were collected at all the monitored farms, although the average number of flies calculated per week varied between 0.3 ± 0.14 (mean ± standard error) and 156.0 ± 43.3 (Table 1). The lowest number of flies was collected at the two farms with only horses. Although only two collections were carried out at the high-altitude site (2128 m) on the 8th and the 23rd of August 2019, they were both positive for S. calcitrans with 47 and 15 individuals, respectively. Five out of the eight farms were monitored for a more extended period in 2018. While there was a considerable variation in the number of S. calcitrans collected between the farms, the abundance of S. calcitrans at the beginning of the collections in June 2018 was very low (4) at all farms (Supp. Fig. S2). In November 2018, several farms still showed a high peak in S. calcitrans abundance and one site (Hittnau) in particular recorded the highest number of S. calcitrans of the season in that month (Supp. Fig. S2). The closest farms were 850 m apart (Bennau1 and Bennau2), while the most distantly separated were Juf and Bennau2 (102 km), and Juf and Zürich (135 km, Table 1, Suppl. Fig. S3).
Table 1.
Features of Stomoxys calcitrans trapping sites and results of fly collections.
| Farm§ (altitude m asl) | GPS coordinates | Livestock, horses present | No1 collections | Mean/trap/week ± SE* | Fly numbers Max./trap/week | Min./trap/week |
|---|---|---|---|---|---|---|
| Hittnau† (685) |
47°22′12.05″N 8°50′17.40″E |
Cattle | 15 | 39.0 ± 7.8 | 97.0 | 1.0 |
| Wetzikon† (552) |
47°19′26.32″N 8°48′40.94″E |
Cattle | 15 | 12.5 ± 2.6 | 39.9 | 1.2 |
| Zürich (546) |
47°24`10.1″N 8°30`30.1″E |
Cattle and horses | 15 | 37.0 ± 7.7 | 109.2 | 0 |
| Hinwil (734) |
47°18′24.08″N 8°50′50.72″E |
Horses | 15 | 4.0 ± 1.3 | 19.6 | 0 |
| Fehraltorf† (543) |
47°22′54.65″N 8°45′42.10″E |
Horses | 15 | 0.3 ± 0.1 | 1.9 | 0 |
| Bennau 1‡ (885) |
47° 9′1.90″N 8°43′15.25″E |
Cattle and horses | 6 | 107.4 ± 44.6 | 220.0 | 7 |
| Bennau 2‡ (850) |
47° 8′44.17″N 8°43′45.47″E |
Cattle and goats | 6 | 156.0 ± 43.3 | 283.0 | 16 |
| Juf‡ (2128) |
46°26`40.6″N 9°34`50.5″E |
Cattle | 2 | 31.0 ± 16.0 | 47.0 | 15 |
White sticky traps were placed inside barns in 2018† (June-November) and 2019‡ (August-November) with a frequency of 1–3 weeks.
SE= standard error.
Distance between farms: Bennau2-Juf: 102 km, Bennau1-Bennau2: 850 m; Bennau2-Zürich: 33.02 km; Bennau2-Hinwil: 20 km; Hinwil-Wetzikon: 3.3 km; Wetzikon-Hittnau: 5.5 km; Hittnau-Fehraltorf: 6 km; Fehraltorf-Zürich: 19.2 km; Zürich-Juf:135 km.
3.4. Factors determining virus amplification in stable flies
The Cq values in the bodies were increasing (decrease of the viral loads) with time (t74=11.91, P<0.001). At time 0, the mean Cq value was 24.5 cq (confidence intervals 23.9–25.1). These increased by 0.13 Cq (confidence intervals 0.11–0.16) per hour. There was no variation in Cq values in heads (t74=−0.031, P = 0.97) or on cotton (t64=0.109, P = 0.914) with time. There was no correlation of Cq values, in bodies (t64=1.4, P = 0.16), heads (t64=0.6, P = 0.56) or cotton (t74=0.62, P = 0.54).
3.5. Discussion
We have studied the role of S. calcitrans in the transmission of lumpy skin disease virus (LSDV) after feeding on virus-spiked blood under laboratory conditions. The presence of infectious virus particles was not only confirmed in bodies (thorax and abdomen) of the examined flies up to 48 h p.f., but also in regurgitated blood and faecal droplets up to 12 and 48 h p.f., respectively.
Based on our results, the virus most likely persists without amplification in the Stomoxys body for at least three days. This would be of great consequence if the virus continues to be infectious during this time period. These findings are essential in the context of the vector competence of stable flies which are known to feed in intervals from four to 72 h (Baldacchino et al., 2013) but also concerning LSDV epidemiology. It has been shown that stable flies can fly up to 28 km in 24 h (Bailey et al., 1973; Eddy et al., 1962) and thus could initiate new outbreaks in distant farms.
Similar feeding experiments have been published, but the Stomoxys flies were allowed to feed to repletion on experimentally infected cattle rather than through a membrane (Chihota et al., 2003; Sohier et al., 2019; Issimov et al., 2020). LSDV was detected by PCR on whole fly homogenates only up to 1 or 2 days, and virus isolation was successful only in samples processed immediately after feeding or from six h p.f. Two aspects might have contributed to these discrepancies in virus detection sensitivity: 1) The virus inoculum when offered in the blood through membranes was putatively higher than in the infected cattle. In our study, all the membrane fed flies tested positive for the virus immediately after the blood meal whereas only a few were positive at this time point when fed on cattle; 2) The methods for virus detection used might differ in sensitivity, i.e. qPCR vs classical PCR; established cell line (MDBK) vs primary cells (lamb testis).
Results highly similar to ours, although using a different virus, were obtained with S. calcitrans that were membrane-fed with blood spiked with African swine fever virus (ASFV), with qPCR of flies’ bodies (thorax and abdomen) positive for up to 72 h p.f. Virus isolation was possible until 12 h p.f. Nevertheless, samples from later time points were inconclusive due to microbial contamination (Olesen et al., 2018). As with LSDV, ASFV DNA decreased over time, indicating that S. calcitrans do not act as biological vectors. Recent studies have elucidated the mechanical mode of transmission for LSDV by S. calcitrans in cattle (Sohier et al., 2019; Issimov et al., 2020). Viral infection in cattle was observed after exposure to flies previously fed on viraemic donor cattle. In these studies, high numbers (> 200) of flies were experimentally infected by donor animals, and immediately (or one h later) exposed to the acceptor animals, mimicking the interrupted feeding behaviour of S. calcitrans flies (Schofield and Torr, 2002). Both acceptor animals became infected in one of the studies (Issimov et al., 2020) whereas, in the other study (Sohier et al., 2019), only 5/14 (36%) of the tested animals exposed to S. calcitrans presented clinical signs of LSDV. One explanation is that the number of flies which continued their feeding on the acceptor animals could not be estimated, and it was assumed that some of the animals were bitten by more infected flies than others. Another assumption was that higher infection doses could have been inoculated by flies through regurgitation during blood-feeding (Butler et al., 1977). Our data support such differences in individual flies with regard to regurgitation.
In our work, we describe a technique that enables us to investigate the presence of the virus or viral DNA in the flies’ mouthpart or their regurgitation. After exposure to LSDV-spiked blood, the flies were individually kept and allowed to feed on small cotton pads soaked with blood, simulating the feeding on animals. Indeed, infectious virus and/or viral DNA were confirmed in cotton pads (Fig. 1C, Supp. Table 1). Interestingly, no LSDV DNA could be observed in the regurgitated blood of the flies at 24 h to 36 h p.f possibly because the flies did not feed in this time frame. Likewise, in the study of (Chihota et al., 2003), infected S. calcitrans flies failed to mechanically transmit LSDV when exposed to naïve cattle after 24 h p. f. on an infected animal. It was then hypothesised (Sohier et al., 2019) that the length of time might have interfered with mechanical transmission by the flies through losing the infectivity or clearing the pathogen from the mouthparts.
It remains to be determined whether virus present at the fly's mouthpart suffices to trigger an animal infection. In the study of Issimov and colleagues (Issimov et al., 2020) infectious LSDV could be recovered from 70% of the proboscis within one h after the feeding process on diseased animals. Moreover, the virus titre was 10−4 TCID50/ml which seems to be a low potential inoculum. The amount of virus that a fly could pick up from an animal at the peak of its viraemia (105.6 TCID50/ml) (Babiuk et al., 2008) is calculated at 10−0.8 TCID50/ml (Sohier et al., 2019). A viral dose of 101.4 TCID50/ml was estimated to induce viraemia in an acceptor animal (Sohier et al., 2019). In our study, two cotton pads with regurgitated blood that were positive in virus isolation had Cq values similar to the abdomens of the corresponding flies (Suppl. Table S1) whereas most of the heads of flies, from which virus had been isolated from the abdomen, had lower amounts of viral DNA (high Cq). This would mean that these flies fed on the virus-free blood supplied and they regurgitated the previous infectious blood.
However, it is not clear if the amount of regurgitated blood per fly can intervene in the transmission of the pathogen. Probably, the volume of regurgitated blood varies from fly to fly; three to five interrupted feedings are necessary to complete a full blood meal (Schofield and Torr, 2002). Although we observed blood in the abdomens of most of the flies post-incubation, we cannot be certain if this was repletion from the infectious blood meal or from the continuation of feeding on the cotton pads with virus-free blood during the incubation time. The regurgitation process could differ at the individual level. This might explain the discrepancy between the 92% positive bodies and only 15% positive cotton pads with blood and the Cq variability amongst samples that showed CPE. This behaviour concerning regurgitation of flies should be studied more in detail because it may play a significant role in pathogen transmission.
A limitation of our work could be the possible contamination of the cotton pads containing the blood with faecal samples. Indeed, LSDV infectious virus was detected in faecal samples up to 48 h p.f. and thus S. calcitrans excretes LSDV both by regurgitation and defecation. Previous studies done on mosquitoes described the faecal shedding of different viruses like West Nile, dengue or hepatitis B (Fontaine et al., 2016; Ramirez et al., 2018). Faecal dots have a distinctive dark brown colour and can easily be observed on the walls of the Falcon tubes. No such specific dots were obvious as possible contamination when visually checking the cotton pads, although faecal samples could have been dissolved in the blood of the cotton pads. However, because the cotton pad samples from 24 to 36 h p.f. were PCR-negative while some samples of bodies tested from these specific incubating times were positive, we consider the risk of contamination minimal.
In Switzerland, little is known about the presence and abundance of stable flies. In our study, S. calcitrans were recorded at all farms investigated, even on a farm situated at 2128 m asl. Abundances were higher at farms with cattle, and the lowest numbers were at the two farms with only horses. This may be because these farms were relatively clean and free from manure and thus devoid of breeding sites. Different types of traps are utilised to catch S. calcitrans (Gilles et al., 2007; Solorzano et al., 2015; Hogsette and Kline, 2017). Traps based on the attraction of translucent fibreglass (Alsynite) and other materials to S. calcitrans are considered the standard, and they are commercially available in the USA (Hogsette and Kline, 2017). However, it was shown that sticky white traps were more efficient in catching these flies (Zhu et al., 2016). In our study, we used locally purchased white sticky traps which are used for pest control in orchards. They are made of polypropylene, low-priced (around 4 €), collapsible, and reusable after removing the included insect glue by a bio-degradable solvent. The number of flies collected with this white sticky trap cannot be compared to similar studies because of differences in trap type, location etc. However, considerable numbers were collected at some sites, up to 283 per trap per week. This is in the range of trappings in other regions with a temperate climate (Kahana-Sutin et al., 2017; Parravani et al., 2019) but lower than collections in tropical climate (Gilles et al., 2007; Solorzano et al., 2015). LSDV outbreaks in Israel have been linked to S. calcitrans (Kahana-Sutin et al., 2017), and the number of flies collected, with white sticky traps, was in the range of the numbers collected in our study at all farms except the pure horse farms. Thus, LSDV transmission by S. calcitrans seems possible also in Switzerland.
No attempts were made in our study to correlate the fly catches on the white sticky traps with fly counts on the animals, i.e. with the economic threshold which is considered at 10 S. calcitrans per animal (Gerry et al., 2007). It is conceivable that at the locations of our study with high fly collections, this threshold has been exceeded. Though sanitation of larval breeding sites is the best option to reduce stable flies, traps are adequate to limit them, particularly in smaller premises (Gerry et al., 2007).
We registered two peaks during our collection time (July and November), in accordance with other studies (Kahana-Sutin et al., 2017; Jacquiet et al., 2014), with the highest abundance in late autumn (November) as previously observed (Kahana-Sutin et al., 2017).
Conclusion
From our findings, we have consolidated the mechanical means of LSDV transmission for S. calcitrans. By using sensitive detection methods, we could show that the virus, which is excreted both by regurgitation and defecation, persists for at least three days in the flies. Future work should address the question on the location of the virus in different body parts and mechanisms of its persistence. This is the first work describing the presence of the stable fly S. calcitrans in Switzerland. Flies of this species were detected in all the monitored farms showing that they are not restricted to specific environments or altitudes since we collected them up to 2128 m asl.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors declare that they have no conflicts of interest. We kindly thank Dr Simon Carpenter and Dr Adrian Zagrajek (The Pirbright Institute, UK) for providing Stomoxys pupae via the Infravec2 project (this publication was supported by the project, Research Infrastructures for the control of vector-borne diseases (Infravec2), which has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 731060.); Dr Kurt Tobler (Institute of Virology, University of Zürich, Switzerland) for molecular analysis consulting; Jeannine Hauri and Jasmin Varga (Institute of Parasitology, University of Zürich, Switzerland) for fly maintenances and helping in the lab. We would also like to thank the farmers for allowing us to set the traps at their premises and for their kind support. We highly acknowledge the Swiss Federal Food Safety and Veterinary Office (FSVO) for financial support of the National Centre for Vector Entomology and for financing the project “Lumpy skin disease: improvement of diagnostic methods and assessment of the vector potential of insects of Switzerland“ (Project number Aramis 1.18.08).
Author Statement
Anca I. Paslaru: investigation, flies infection, sample collection and processing, methodology, data analyses, original draft. Niels. O. Verhulst: writing. Lena M. Maurer: technical assistance. Alexsandra Brendle: data analyses. Nicole Pauli: field sample collection. Andrea Vögtlin: funding acquisition. Sandra Renzullo: technical assistance. Yelena Ruedin: technical assistance. Bernd Hoffmann: virus supply. Paul R. Torgerson: statistical analyses, writing. Alexander Mathis: funding acquisition, writing, experimental design. Eva Veronesi: funding acquisition, conceptualization, experimental design, methodology, data analyses, writing. All authors edited, read and approved the final manuscript.
This work was supported by the Swiss Federal Food Safety and Veterinary Office (FSVO) [grant number Aramis 1.18.08].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cris.2020.100007.
Contributor Information
Anca I. Paslaru, Email: ancaiona.paslaru@uzh.ch.
Niels O. Verhulst, Email: niels.verhulst@uzh.ch.
Lena M. Maurer, Email: lenamaria.maurer@uzh.ch.
Nicole Pauli, Email: nicole.pauli@uzh.ch.
Andrea Vögtlin, Email: Andrea.Voegtlin@ivi.admin.ch.
Sandra Renzullo, Email: sandra.renzullo@ivi.admin.ch.
Yelena Ruedin, Email: yelena.ruedin@ivi.admin.ch.
Bernd Hoffmann, Email: Bernd.Hoffmann@fli.de.
Paul R. Torgerson, Email: paul.torgerson@uzh.ch.
Alexander Mathis, Email: alexander.mathis@uzh.ch.
Eva Veronesi, Email: eva.veronesi@uzh.ch.
Appendix. Supplementary materials
References
- Babiuk S., Bowden T.R., Parkyn G., Dalman B., Manning L., Neufeld J., Embury-Hyatt C., Copps J., Boyle D.B. Quantification of Lumpy skin disease virus following experimental infection in cattle. Transbound Emerg. Dis. 2008;55(7):299–307. doi: 10.1111/j.1865-1682.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- Bailey D.L., Whitfield T.L., Smittle B.J. Flight and dispersal of stable fly diptera-muscidae. J. Econ. Entomol. 1973;66:410–411. doi: 10.1093/jee/66.2.410. [DOI] [Google Scholar]
- Baldacchino F., Muenworn V., Desquesnes M., Desoli F., Charoenviriyaphap T., Duvallet G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite. 2013:20. doi: 10.1051/parasite/2013026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M., Arif B.M., Black D.N., Dumbell K.R., Esposito J.J., Lefkowitz E.J., Mcfadden G., Moss B., Mercer A.A., Moyer R.W., Skinner M.A., Tripathy D.N. In: Virus taxonomy: Classification and Nomenclature of viruses. Eighth report of the International Committee On Taxonomy of Viruses. Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. E.A. Press; San Diego: 2005. Family Poxviridae; pp. 117–133. Editor. Editor. [Google Scholar]
- Butler J.F., Kloft W.J., DuBose L.A., Kloft E.S. Recontamination of food after feeding a 32P food source to biting muscidae. J. Med. Entomol. 1977;13(4–5):567–571. doi: 10.1093/jmedent/13.4-5.567. [DOI] [PubMed] [Google Scholar]
- Calistri P., De Clercq K., Gubbins S., Klement E., Stegeman A., Abrahantes J.C., Marojevic D., Antoniou S.E., Broglia A., Efsa Lumpy skin disease epidemiological report IV: data collection and analysis. Efsa J. 2020;18(2) doi: 10.2903/j.efsa.2020.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carn V.M., Kitching R.P. An investigation of possible routes of transmission of Lumpy skin disease virus (Neethling) Epidemiol. Infect. 1995;114(1):219–226. doi: 10.1017/S0950268800052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. Mechanical transmission of Lumpy skin disease virus by Aedes aegypti (Diptera: culicidae) Epidemiol. Infect. 2001;126(2):317–321. doi: 10.1017/S0950268801005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. Attempted mechanical transmission of Lumpy skin disease virus by biting insects. Med. Vet. Entomol. 2003;17(3):294–300. doi: 10.1046/j.1365-2915.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- Davies F.G. Lumpy skin disease, an African capripox virus disease of cattle. Br. Vet. J. 1991;147(6):489–503. doi: 10.1016/0007-1935(91)90019-J. [DOI] [PubMed] [Google Scholar]
- Eddy G.W., Roth A.R., Plapp F.W. Studies on the flight habits of some marked insects. J. Econ. Entomol. 1962;55:603–607. [Google Scholar]
- Fontaine A., Jiolle D., Moltini-Conclois I., Lequime S., Lambrechts L. Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes. Sci. Rep. 2016;6:24885. doi: 10.1038/srep24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry, A.C., N.G. Peterson, and B.A. Mullens. Predicting and controlling stable flies on California dairies. 10.3733/ucanr.8258. Retrieved from https://escholarship.org/uc/item/93x282bt, 2007. [DOI]
- Gilles J., David J.F., Duvallet G., De La Rocque S., Tillard E. Efficiency of traps for Stomoxys calcitrans and Stomoxys niger niger on Reunion Island. Med. Vet. Entomol. 2007;21(1):65–69. doi: 10.1111/j.1365-2915.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasedistributionmap/index/newlang?disease_type_hidden=&disease_id_hidden=&selected_disease_name_hidden=&disease_type=0&disease_id_terrestrial=7&species_t=0&disease_id_aquatic=-999&species_a=0&sta_method=semesterly&selected_start_year=2020&selected_report_period=2&selected_start_month=1&date_submit=OK (Accessed 14th of November 2020).
- Hogsette J.A., Kline D.L. The knight stick trap and knight stick sticky wraps: new tools for stable fly (diptera: muscidae) management. J. Econ. Entomol. 2017;110(3):1384–1389. doi: 10.1093/jee/tox042. [DOI] [PubMed] [Google Scholar]
- Hornok, S., N. Takacs, S. Szekeres, K. Szoke, J. Kontschan, G. Horvath, and L. Sugar. DNA of Theileria orientalis, T. equi and T. capreoli in stable flies (Stomoxys calcitrans). Parasites vectors, 2020. 13(1): p. 186. 10.1186/s13071-020-04041-1. [DOI] [PMC free article] [PubMed]
- Issimov A., Kutumbetov L., Orynbayev M.B., Khairullin B., Myrzakhmetova B., Sultankulova K., White P.J. Mechanical transmission of lumpy skin disease virus by Stomoxys Spp (Stomoxys calsitrans, Stomoxys sitiens, Stomoxys indica) Diptera: Muscidae. Anim. 2020;10(3) doi: 10.3390/ani10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquiet P., Rouet D., Bouhsira E., Salem A., Lienard E., Franc M. Population dynamics of Stomoxys calcitrans (L.) (Diptera: muscidae) in southwestern France. Rev. Med. Vet-Toulouse. 2014;165(9–10):267–271. [Google Scholar]
- Kahana-Sutin E., Klement E., Lensky I., Gottlieb Y. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Med. Vet. Entomol. 2017;31(2):150–160. doi: 10.1111/mve.12217. [DOI] [PubMed] [Google Scholar]
- Kitching R.P., Mellor P.S. Insect transmission of capripoxvirus. Res. Vet. Sci. 1986;40(2):255–258. doi: 10.1016/S0034-5288(18)30523-X. [DOI] [PubMed] [Google Scholar]
- Lempereur L., Sohier C., Smeets F., Marechal F., Berkvens D., Madder M., Francis F., Losson B. Dispersal capacity of haematopota spp. and stomoxys calcitrans using a mark-release-recapture approach in Belgium. Med. Vet. Entomol. 2018;32(3):298–303. doi: 10.1111/mve.12297. [DOI] [PubMed] [Google Scholar]
- Möller J., Moritz T., Schlottau K., Krstevski K., Hoffmann D., Beer M., Hoffmann B. Experimental Lumpy skin disease virus infection of cattle: comparison of a field strain and a vaccine strain. Arch. Virol. 2019;164(12):2931–2941. doi: 10.1007/s00705-019-04411-w. [DOI] [PubMed] [Google Scholar]
- Magori-Cohen R., Louzoun Y., Herziger Y., Oron E., Arazi A., Tuppurainen E., Shpigel N.Y., Klement E. Mathematical modelling and evaluation of the different routes of transmission of Lumpy skin disease virus. Vet. Res. 2012;43:1. doi: 10.1186/1297-9716-43-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen A.S., Hansen M.F., Rasmussen T.B., Belsham G.J., Bodker R., Botner A. Survival and localization of African swine fever virus in stable flies (Stomoxys calcitrans) after feeding on viremic blood using a membrane feeder. Vet. Microbiol. 2018;222:25–29. doi: 10.1016/j.vetmic.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Parravani A., Chivers C.A., Bell N., Long S., Burden F., Wall R. Seasonal abundance of the stable fly Stomoxys calcitrans in Southwest England. Med. Vet. Entomol. 2019;33(4):485–490. doi: 10.1111/mve.12386. [DOI] [PubMed] [Google Scholar]
- Ramirez A.L., Hall-Mendelin S., Doggett S.L., Hewitson G.R., McMahon J.L., Ritchie S.A., van den Hurk A.F. Mosquito excreta: a sample type with many potential applications for the investigation of Ross River virus and West Nile virus ecology. PLoS Negl. Trop. Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield S., Torr S.J. A comparison of the feeding behaviour of tsetse and stable flies. Med. Vet. Entomol. 2002;16(2):177–185. doi: 10.1046/j.1365-2915.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- Sharif S., Jacquiet P., Prevot F., Grisez C., Raymond-Letron I., Semin M.O., Geffre A., Trumel C., Franc M., Bouhsira E., Lienard E. Stomoxys calcitrans, mechanical vector of virulent Besnoitia besnoiti from chronically infected cattle to susceptible rabbit. Med. Vet. Entomol. 2019;33(2):247–255. doi: 10.1111/mve.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgard H., Nachman G. Population dynamics of stable flies Stomoxys calcitrans (Diptera: muscidae) at an organic dairy farm in Denmark based on mark-recapture with destructive sub-sampling. Environ. Entomol. 2012;41(1):20–29. doi: 10.1603/en11155. [DOI] [PubMed] [Google Scholar]
- Sohier C., Haegeman A., Mostin L., De Leeuw I., Campe W.V., De Vleeschauwer A., Tuppurainen E.S.M., van den Berg T., De Regge N., De Clercq K. Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. Horseflies. Sci. Rep. 2019;9(1):20076. doi: 10.1038/s41598-019-56605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano J.-.A., Gilles J., Bravo O., Vargas C., Gomez-Bonilla Y., Bingham G.V., Taylor D.B. Biology and trapping of stable flies (Diptera: muscidae) developing in pineapple residues (Ananas comosus) in Costa Rica. J. Insect Sci. 2015:15. doi: 10.1093/jisesa/iev127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprygin A., Pestova Y., Wallace D.B., Tuppurainen E., Kononov A.V. Transmission of Lumpy skin disease virus: a short review. Virus Res. 2019;269 doi: 10.1016/j.virusres.2019.05.015. [DOI] [PubMed] [Google Scholar]
- Stubbs S., Oura C.A.L., Henstock M., Bowden T.R., King D.P., Tuppurainen E.S.M. Validation of a high-throughput real-time polymerase chain reaction assay for the detection of capripoxviral DNA. J. Virol. Methods. 2012;179(2):419–422. doi: 10.1016/j.jviromet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Veronesi E., Antony F., Gubbins S., Golding N., Blackwell A., Mertens P.P.C., Brownlie J., Darpel K.E., Mellor P.S., Carpenter S. Measurement of the infection and dissemination of bluetongue virus in Culicoides biting midges using a semi-quantitative RT-PCR assay and isolation of infectious virus. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Likelihood-based and Bayesian methods for Tweedie compound Poisson linear mixed models. Stat. Comput. 2013;23:743–757. doi: 10.1007/s11222-012-9343-7. [DOI] [Google Scholar]
- Zhu J.J., Zhang Q.H., Taylor D.B., Friesen K.A. Visual and olfactory enhancement of stable fly trapping. Pest Manag. Sci. 2016;72(9):1765–1771. doi: 10.1002/ps.4207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.