Highlights
-
•
We contrasted nutrient selection by wild grasshoppers at two time points.
-
•
Both times Oedaleus senegalensis selected a carbohydrate-biased intake.
-
•
Contrasting with lab studies, grasshoppers collected later did not regulate tightly.
-
•
In the field few plants were near O. senegalensis optimal protein:carbohydrate ratio.
-
•
Mobile herbivores must rely on post ingestive regulation in the field.
Keywords: Carbohydrates, Locust, Protein, Sex, Temporal variation
Abstract
There is generally a close relationship between a consumer's food and its optimal nutrients. When there is a mismatch, it is hypothesized that mobile herbivores switch between food items to balance nutrients, however, there are limited data for field populations. In this study, we measured ambient plant nutrient content at two time points and contrasted our results with the nutrient ratio selected by wild female and male grasshoppers (Oedaleus senegalensis). Few plants were near O. senegalensis’ optimal protein:carbohydrate ratio (P:C), nor were plants complementary. Grasshoppers collected earlier all regulated for a carbohydrate-biased ratio but females ate slightly more protein. We hypothesized that the long migration undertaken by this species may explain its carbohydrate needs. In contrast to most laboratory studies, grasshoppers collected later did not tightly regulate their P:C. These results suggest that field populations are not shifting their P:C to match seasonal plant nutrient shifts and that mobile herbivores rely on post-ingestive mechanisms in the face of environmental variation. Because this is among the first studies to examine the relationship between ambient nutrient landscape and physiological state our data are a key step in bridging knowledge acquired from lab studies to hypotheses regarding the role ecological factors play in foraging strategies.
1. Introduction
There is generally a close relationship between a consumer's food and its optimal nutrient intake (Lee et al., 2003). For instance, the optimal diet for an aphid that feeds on sugary plant sap low in nitrogen is not the same as the optimal diet for a carnivore that feeds on animals rich in lipid and protein but low in carbohydrates (Simpson and Raubenheimer, 2012). This pattern suggests that consumers have adapted to their host foods through post-ingestive regulation, or by adjusting physiological demands through behaviors such as decreased activity, or both. Consumers can also shift their nutrient intake by eating different foods based on their physiological state or environmental factors. For instance, adult honeybees select an increasingly carbohydrate-biased diet as they age (Paoli et al., 2014), and females and males of a given species may have different nutrient requirements (Jensen et al., 2015; Reddiex et al., 2013; Solon-Biet et al., 2015; South et al., 2011). Because there are limited data for free-living populations, the relative importance of nutrient availability versus physiological state on nutrient selection is largely unknown. This is particularly true for herbivores living in highly stochastic environments where the relationships between nutrient supplies and demands will change rapidly.
There is substantial evidence that the relative amounts of dietary macronutrients (proteins, carbohydrates, lipids) consumers eat is particularly important for fitness correlates in a wide range of organisms (Harrison et al., 2014; Le Couteur et al., 2016; Simpson et al., 2015; Simpson and Raubenheimer, 2012). When given the opportunity, consumers select a ratio of macronutrients based on various external and internal factors such as toxins and pathogens (Deans et al., 2016; Le Gall, 2014; Lee et al., 2006; Povey et al., 2009; Tessnow et al., 2018), predators (Schmitz et al., 2016, 2010), activity level (Clark et al., 2013; Guglielmo, 2010), previous dietary experience (Behmer et al., 2001; Tews et al., 1992), temperature (Clissold et al., 2013; Miller et al., 2009; Musten et al., 1974), sex (Jensen et al., 2015; Maklakov et al., 2008; Reddiex et al., 2013; Solon-Biet et al., 2015; South et al., 2011), and age (Paoli et al., 2014; Roeder and Behmer, 2014). However, much of this work on consumer behavioral nutrient regulation is done in laboratories with colony-reared animals. In contrast, field-based studies tracking foraging behavior usually focus on total energy consumption (Benoit-Bird, 2004; Davies, 1977; Lobel and Ogden, 1981; Nagy et al., 1984; Werner and Mittelbach, 1981) based on the assumption that all consumers are aiming to maximize energy gain (MacArthur and Pianka, 1966; Perry and Pianka, 1997), which is often not the case (Deans et al., 2015; Harrison et al., 2014; Le Couteur et al., 2016; Le Gall and Behmer, 2014; Reddiex et al., 2013; Solon-Biet et al., 2014).
It has often been hypothesized that consumers that do not have the possibility to switch between food items are more reliant on post-ingestive regulation, as shown by aphids excreting excess sugars as honeydew. However, some mobile herbivores such has grasshoppers exhibit substantial post-ingestive regulatory capacities (Behmer, 2009; Clissold et al., 2010; Le Gall and Behmer, 2014), despite their well-known capacity to adjust nutrient intake by switching between food items (Behmer, 2009). For example, grasshoppers can differentially secrete enzymes to compensate for protein and carbohydrate deficiencies (Clissold et al., 2010), and post-ingestively regulate phosphorus (Cease et al., 2016; Zhang et al., 2014). This physiological flexibility is thought to be an evolved response to the high level of nutritional heterogeneity in the environment, although functional data describing the multidimensional nutrient landscape of plant protein and carbohydrate content through space and time are scarce (Le Gall et al., 2020b; Lenhart et al., 2015). Thus, we still know comparatively little about how the relative macronutrient availability and demands of field populations change (for exceptions see (Casas et al., 2005, 2003; Lenhart et al., 2015)).
To test the potential for behavioral vs. post-ingestive regulation, we first measured plant nutrient content at two time points and contrasted our results with the nutrient ratio that field-caught grasshoppers selected when they were offered artificial food. Grasshoppers are a good model because they are usually very good at nutrient regulation (Behmer, 2009). This allowed us to create a framework to evaluate which strategies free-ranging animals are adopting in the field. For behavioral regulation to supersede post-ingestive regulation, the animal should be able to acquire plants that are 1) close to its optimal nutrient intake, or 2) unbalanced but complementary to facilitate diet mixing. If these conditions are not met in the environment, then the animal must rely on post-ingestive regulation and/or face decreased performance outcomes.
Second, we measured the plasticity of nutrient regulation for field-caught animals collected at different times and of different sexes to determine which factors influenced nutrient selection. We hypothesize that if a specific blend of nutrients is optimal for most physiological needs, then the insects would select a consistent nutrient ratio. If instead there is a trade-off between nutrient availability and physiological demands, then the ratio of nutrients selected by the herbivores would be plastic to match the shift in plant nutrient content. Finally, we hypothesize that if the nutrient ratio selected is influenced more by physiological needs than nutrient availability, the ratio of nutrients ingested should correlate with physiological indicators well, but poorly with changes in the nutritional landscape.
We conducted our study in the West African Sahel. The Sahel is a zone of ecoclimatic and biogeographic transition between the Sahara to the north and the Sudanian Savanna to the south. It undergoes a fast and dramatic shift in plant growth during the rainy season, so we expect plant nutrient content to change rapidly, as we have already observed in cultivated fields of millet (Le Gall et al., 2020a), which should allow us to test our hypotheses. Among herbivores living in the Sahel, the Senegalese grasshopper (Oedaleus senegalensis) is described as the main pest of cereal crops in the region and is a grass specialist. To our knowledge, this is the first study to examine the relationship between nutrient availability in the field, physiological state, and nutrient regulation. This a critical step in bridging advancements in nutritional regulation research from lab studies and hypotheses regarding the role ecological factors play in shaping ingestive trade-offs.
2. Material and methods
2.1. O. senegalensis nutritional preferences and physiological variables over time
2.1.1. The Senegalese grasshopper
Oedaleus senegalensis is a grass-feeder and a major pest of millet and other cereal crops of subsistence agriculture in the Sahel zone of West Africa. The field station where we ran the experiment belongs to the Direction de la Protection des Vegetaux (DPV), 13°49′23.8″N 15°24′19.4″W. It is located in the village of Nganda, in the West Central Agricultural Region of Senegal where most millet and groundnut production takes place.
We estimated grasshopper abundance in various agricultural fields (millet, groundnut, and fallow fields) with sweep net surveys within 20 m of each plot where vegetation samples were taken. The same researcher conducted all surveys by evenly sweeping 20 times, each a 180° arc approximately one m apart along a straight line.
2.1.2. Nutrient selection, mass gain, and body lipid content
We collected 5th instar grasshoppers at two time points in August 2017 around the DPV field station. We initiated the experiments on 04-Aug-2017 (early) and 17-Aug-2017, 2017 (late). We weighed and put grasshoppers in individual aerated plastic containers (15 × 10 cm). Each cage contained a water tube, a perch for roosting, and two dishes containing artificial diet. We prepared and dried the food in our laboratory at Arizona State University (United States) following the method developed by Simpson and Abisgold (1985). In total, we made three diets varying in protein to carbohydrate ratios but otherwise isocaloric: p35:c7, p28:c14, and p7:c35. These diets were chosen because they span extreme protein:carbohydrate ratios, therefore ensuring that the IT is not outside of the range offered to the grasshoppers. For each diet, “p” stands for percent of protein in the diet, and “c” stands for percent of carbohydrates in the diet. The protein component of all foods was a 3:1:1 mix of casein, peptone, and albumen, while the digestible carbohydrate (henceforth carbohydrates) component was a 1:1 mix of sucrose and dextrin. All foods contained similar amounts of Wesson's salt (2.4%), cholesterol (0.5%), linoleic acid (0.5%), ascorbic acid (0.3%) and vitamin mix (0.2%) (Dadd, 1961). The remainder of the diet was cellulose, a non-nutritive bulking agent.
We gave the grasshoppers one of two treatments of pre-weighed diet pairings: p7:c35 & p35:c7 or p7:c35 & p28:c14 and used 20–25 grasshoppers per treatment (approximately half males and half females). After three days, the diets were removed and dried for 24–36 h at 60 °C and then re-weighed to record consumption at the nearest 0.1 mg. Grasshoppers were weighed at the beginning and the end of the experiment to calculate mass gain. We extracted lipids from each individual grasshopper by using a series of three 24 h chloroform washes on the dried carcasses. Lipid content was calculated by subtracting post-chloroform wash dry mass from pre-chloroform wash dry mass.
2.2. Plant survey and chemical analysis
2.2.1. Plant survey
Plant surveys and collection took place on 5-Aug-2017 (early) and on 17-Aug-2017 (late). We randomly selected 5 quadrats of 10 m by 10 m within a fallow and millet field. We selected fallow and millet fields because these are where grasshoppers have been shown to be the most abundant (Le Gall et al., 2020b; Toure et al., 2013; Word et al., 2019). For each quadrat, we recorded the three most abundant plant species and presented the data for grasses (O. senegalensis is a grass-feeder). For each plant species, we collected enough plant material to obtain 20 mg of dry sample (different specimens of the same species were pooled together) (Fig. ESM2). We chose the most abundant species because grass diversity and overall plant biomass were low (Fig ESM2) but grasshopper density high (Fig. ESM1), therefore the most abundant grass were likely to be representative of the plants consumed by the grasshoppers.
2.2.2. Chemical analysis
Upon collection, we dried the plants in an oven at 60 °C for 48 h and brought back the samples to our laboratory at Arizona State University (United States). We ground plant samples for 30 s at 200 rpm using a Retsch MM 400 ball mill. We measured plant protein content with a Bradford assay and the non-structural carbohydrate content using the phenol-sulfuric acid method (Deans et al., 2018).
2.3. Contrasting nutrient regulation with nutrient availability
For animals to meet their optimal nutrient intake without relying on post-ingestive mechanisms there are two possibilities: 1) plant protein:carbohydrate ratio closely resembles the optimal protein:carbohydrate ratio (aka Intake Target, thereafter IT) measured in the choice experiment or 2) unbalanced but complementary foods are available so that by mixing among plants to achieve an optimal ratio. To test the first hypothesis, we plotted the self-selected protein:carbohydrate ratios from the choice experiment as nutritional rails (y = mx, m=Intake Target) and calculated the Euclidian distance separating plant nutritional content from that optimal rail in the nutrient space for the two time points. The longer the Euclidian Distance, the further an animal is from the optimal P:C ratio.
Euclidian distance = |mxplantprotein - yplantcarbohydrates|/√(1 + m2) where (xplantprotein, ycarbohydrates) are the coordinates of each plant in the nutrient landscape.
To test the second hypothesis, we compared the median protein:carbohydrate ratio of plants (50% of plants fall on either side of this number) with the self-selected protein:carbohydrate ratio measured in the choice experiment. If the median plant protein:carbohydrate ratio and the self-selected number of plants are different, it means there are more plants on one side of the nutrient space relative to the IT, making diet mixing for an optimal balance challenging.
Methodological consideration: all the plants presented are acceptable host plants for O. senegalensis. We acknowledge that we did not account for non-nutritional factors (e.g., plant structure or plant defenses) that could skew host plant selection. However, measuring and ranking these non-nutritional factors was not feasible in a single study, so for the purpose of this study, we only compare variation in total plant nutrients.
2.4. Statistical analysis
2.4.1. Grasshopper density in the field
Grasshopper density in fallow, millet, and groundnut fields at the two time points was compared using ANOVA.
2.4.2. Choice experiment
The amount of protein and carbohydrate eaten for each treatment was compared using MANCOVA techniques with start mass as a covariate to correct for size differences among individuals. We used a Pillai's test statistic. In all grasshopper-related analyses, we included sex as an independent variable. Protein, carbohydrate, and total food intakes were compared using ANCOVA techniques. Grasshopper wet start masses were compared using ANOVA techniques. Mass gain and lipid content were analyzed with ANCOVA techniques. We reported the effects of protein and carbohydrate intake on mass gain and lipid content. To account for size differences protein and carbohydrate intakes were plotted as mass corrected ratios. Temperatures were analyzed using an ANOVA.
2.4.3. Plant nutrient analyses and relationship to the ITs
Plant P:C ratio was analyzed with a MANOVA and plant protein and carbohydrate content with ANOVAs. Finally, we compared the Euclidian distances of plant p:c to the ITs using ANOVA techniques; median plant p:c ratio and median ITs were compared using a median test score.
3. Results
3.1. O. senegalensis density in the field
We found few O. senegalensis nymphs during our surveys as many had already molted, thus we presented the data for adults only. Grasshoppers were a lot more abundant in fallow fields at the beginning of the rainy season than in other field types at either time points (≈39 grasshoppers per transect vs. 0–4 grasshoppers per transect, Electronic Supplementary Material (Fig. S1)).
3.2. O. senegalensis nutritional preferences over time
3.2.1. Early: IT, protein intake, carbohydrate intake, and food intake
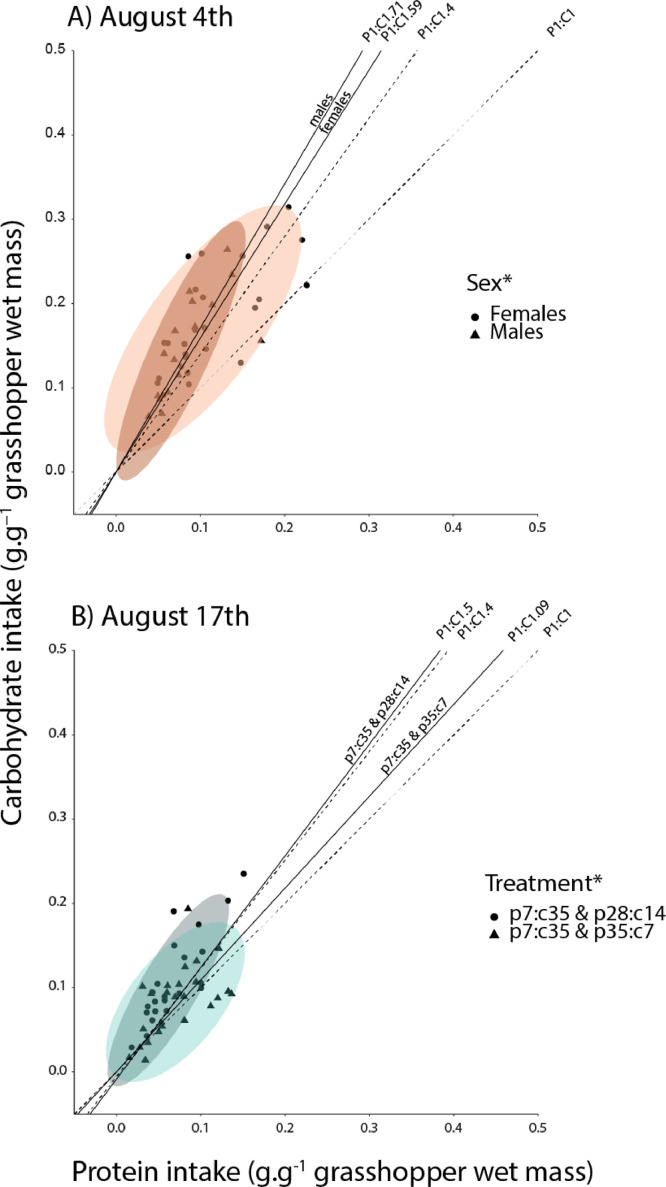
At the beginning of the rainy season (August 5th, early), grasshoppers (5th instar nymphs) actively regulated for a specific protein:carbohydrate ratio (Table S2). There were significant differences between males and females, with males regulating for a more carbohydrate-biased ratio than females (P1:C1.71 vs. P1:c1.59) (Fig. 1, Table S2). As a result, females ingested more protein, about twice more than males, and males more carbohydrates, about 25% more than females (Fig. 1, Table 1). Females also consumed proportionally 7% more food than males (Fig. 1, Table 1).
Fig. 1.
Panel A: mass corrected protein:carbohydrate intakes by grasshoppers early (warm color) and panel B late (cold color). Straight lines represent the nutritional rails defined by the average protein:carbohydrate ratio intake. Dotted lines represent the average protein:carbohydrates ratio defined by the food pairings if consumption was random. Ellipses represent 95% confidence intervals for males and females (panel A) and food pairing treatments (panel B). For each treatment we used 20–25 grasshoppers. *<0.05.
Table 1.
Results from the ANCOVA for protein intake, carbohydrate intake, and total food intake at two time points.
| Variable | Source | df | F-ratio | P-value |
|---|---|---|---|---|
| Early | ||||
| Protein intake (g) | Treatment | 1,65 | 0.1928 | 0.6621 |
| Wet start mass | 1,65 | 0.0140 | 0.9060 | |
| Sex | 1,65 | 29.3025 | <0.0001* | |
| Treatment | 1,65 | 0.0395 | 0.8430 | |
| Carbohydrate intake (g) | Wet start mass | 1,65 | 0.0095 | 0.9228 |
| Sex | 1,65 | 24.4067 | <0.0001* | |
| Treatment | 1,65 | 0.0085 | 0.9268 | |
| Food intake (g) | Wet start mass | 1,65 | 0.0141 | 0.9060 |
| Sex | 1,65 | 32.7863 | <0.0001* | |
| Late | ||||
| Treatment | 1,47 | 0.9858 | 0.3262 | |
| Protein intake (g) | Wet start mass | 1,47 | 3.6039 | 0.0642 |
| Sex | 1,47 | 0.0731 | 0.7882 | |
| Treatment | 1,47 | 2.2871 | 0.1376 | |
| Carbohydrate intake (g) | Wet start mass | 1,47 | 4.3067 | 0.0438* |
| Sex | 1,47 | 0.3938 | 0.5335 | |
| Treatment | 1,47 | 0.0512 | 0.8221 | |
| Food intake (g) | Wet start mass | 1,47 | 4.6047 | 0.0374* |
| Sex | 1,47 | 0.2284 | 0.6351 | |
| Whole model | ||||
| Time point | 1113 | 0.0363 | 0.8492 | |
| Protein intake (g) | Treatment | 1113 | 1.5559 | 0.2149 |
| Protein intake (g) | Wet start mass | 1113 | 1.9669 | 0.1636 |
| Sex | 1113 | 13.4224 | 0.0004* | |
| Time point | 1113 | 5.8485 | 0.0172* | |
| Carbohydrate intake (g) | Treatment | 1113 | 1.0840 | 0.3001 |
| Wet start mass | 1113 | 0.2703 | 0.6042 | |
| Sex | 1113 | 15.2377 | 0.0002* | |
| Time point | 1113 | 1.6032 | 0.2082 | |
| Food intake (g) | Treatment | 1113 | 0.0056 | 0.9406 |
| Wet start mass | 1113 | 1.0814 | 0.3007 | |
| Sex | 1113 | 17.2722 | <0.0001* |
Notes: Time point refers to the time of the testing (early or late August), Treatment refers to the food pairing (p7:c35 & p35:c7 and p7:c35 & p28:c14); wet start mass was used as a covariate to adjust for size differences among insects, and sex was included as a cofactor. For each treatment we used 20–35 grasshoppers. * P < 0.05.
3.2.2. Late: IT, protein intake, carbohydrate intake, and food intake
Later in the rainy season (August 17th, late), insects (5th instar nymphs) did not regulate their nutrient intake as there was no convergence to a statistically indistinguishable point in nutrient space and there were no differences between sexes (Fig. 1, Table S2). Insect start mass was significant for carbohydrate and total food intakes later in the season (Fig. 1, Table 1). In summary, later in the season, larger insects consumed more food which resulted in greater nutrient intakes.
3.2.3. Whole model: ITs, protein intake, carbohydrate intake, and food intake
When we compared the two time points, we found that grasshoppers selected different optimal protein:carbohydrate ratios. The ratio selected became less carbohydrate-biased later in the rainy season (P1: C1.55 vs. P1:C1.39) (Fig. 2. Table S2). The effect of time superseded both the effect of sex observed early in the season and of treatment later in the season (Table S2). For the whole model, females ate more protein, more carbohydrates, and overall more food than males (Table 1). Insects caught earlier ate more carbohydrates than insects caught later (Fig. 2, Table 1).
Fig. 2.
Mass corrected protein:carbohydrate intakes by grasshoppers on August 4th (early = warm color) and August 17th (late = cold color). Straight lines represent the nutritional rails defined by the average protein:carbohydrate ratio calculated from the MANCOVA analysis (see Table 1). Dotted lines represent the average protein:carbohydrates ratio defined by the food pairings if consumption is random. Ellipses represent 95% confidence intervals for each time point. For each treatment we used 20–25 grasshoppers. *<0.05.
3.3. Physiological variables over time
3.3.1. Start mass
Initially (August 4th) there were no differences between male's and female's weights. However, the female grasshoppers we collected later were significantly heavier, they were about twice the mass of early females, and twice the mass of males at either time point, roughly 0.4 g vs. 0.2 g (Fig. 3, panel A, Table 2).
Fig. 3.
Panel A: start wet mass (± SE); panel B: mass corrected wet mass gain (± SE); panel C: lipid content (± SE) for female (dark color) and male (light color) grasshoppers early (warm color) and late (cold color). For each treatment we used 20–25 grasshoppers. Different letters indicate statistical differences. * <0.05.
Table 2.
Results of ANOVA for wet start mass, and ANCOVAs for mass gain and lipid content.
| Variable | Source | df | F-ratio | P-value |
|---|---|---|---|---|
| Wet start mass (g) | Time point | 1118 | 77.7183 | <0.0001* |
| Sex | 1118 | 128.1881 | <0.0001* | |
| Time point * Sex | 1118 | 74.9377 | <0.0001* | |
| Time point | 1112 | 13.0561 | 0.0005* | |
| Wet start mass | 1112 | 83.7876 | <0.0001* | |
| Mass gain (g) | Sex | 1112 | 0.9801 | 0.3244 |
| Protein intake | 1112 | 14.7505 | 0.0002* | |
| Carbohydrate intake | 1112 | 3.7335 | 0.0560 | |
| Time point | 1111 | 1.4740 | 0.2274 | |
| Lipids (mg) | Wet start mass | 1111 | 17.6075 | <0.0001* |
| Sex | 1111 | 0.0751 | 0.7845 | |
| Protein intake | 1111 | 1.1578 | 0.2844 | |
| Carbohydrate intake | 1111 | 1.6167 | 0.2063 |
Notes: Time point refers to the time of the testing (early or late August); wet start mass was used as a covariate to adjust for size differences among insects, and sex was included as a cofactor. Interactive terms are presented only when significant. For each treatment we used 20–35 grasshoppers. * P < 0.05.
3.3.2. Mass gain
Over the course of the three-day feeding experiment, we observed that insects captured later gained about three times more mass than the grasshoppers captured at the beginning of the month. We found that protein intake had a significant negative effect on mass gain, but carbohydrate intake did not (Fig. 3, panel B, Table 2).
3.3.3. Lipid content
Lipid content was proportional to insect mass and was not affected by time point, sex, or nutrient intake over the course of three days (Fig. 3, panel C, Table 2).
3.4. Relationship between plant nutrient content and ITs
3.4.1. Plant protein: carbohydrate ratio, protein and carbohydrate content
We found that most grasses sampled were carbohydrate biased. Over the course of two weeks, their protein and carbohydrate shifted significantly and became increasingly less carbohydrate biased (from P1:C2.26 to P1:C1.30). We found that protein content increased over time and carbohydrate content decreased (Fig. 4, Table 3).
Fig. 4.
Protein and carbohydrate content of grasses in fallow and millet fields at two time points (early and late). Straight lines represent the nutritional rails defined by the average protein:carbohydrate ratio calculated from the choice experiment (see Table 1). Dotted lines represent the average grass protein:carbohydrate ratio. Ellipses represent the 95% confidence intervals for each time point. *<0.05.
Table 3.
Results of MANOVA for grass protein and carbohydrate ratios at two time points and ANOVAs for grass protein and carbohydrate content at two time points.
| Variable | Source | df | F-ratio | P-value |
|---|---|---|---|---|
| Grass P:C ratio | Time point | 1,32 | 25.13 | <0.0001* |
| Land-use | 1,32 | 43.67 | <0.0001* | |
| Grass protein (%) | Time point | 1,33 | 10.29 | <0.01* |
| Land-use | 1,33 | 70.22 | <0.0001* | |
| Grass carbohydrates (%) | Time point | 1,32 | 30.57 | <0.0001* |
| Land-use | 1,32 | 0.61 | 0.44 |
Notes: Time point refers to the time of the testing (early or late August), and land-use to the type of field where grasses were collected (millet or fallow). * P < 0.05.
3.4.2. Euclidian distance between ITs and plant P:C ratio
We found no differences in Euclidian distances to the ITs over time, meaning that at no time points were the insects statistically closer to their respective IT (Fig. 4, Table S3). However, the p-value was smaller later compared to earlier in the rainy season, suggesting “early” grasshoppers were best able to find plants that contained their optimal nutrient balance (Table S3).
3.4.3. Plant distribution in regard to ITs: median test
The median protein:carbohydrate ratio selected by the insects was significantly different from the median plant protein:carbohydrate ratio, meaning that at no point was there an equal number of plants on either side of the optimal protein:carbohydrate ratio. That difference was smaller later compared to earlier in the rainy season (fewer plants were skewed toward a specific nutrient), suggesting that even if grasshoppers caught later were unable to find plants containing their optimal nutrient ratio, they could attain nutrient balance by eating from among different plants (Fig. 4, Table S4).
4. Discussion
Our data support the hypothesis that mobile herbivores rely on post-ingestive mechanisms, in addition to behavioral selection, to regulate nutrient acquisition in the face of environmental variation. While diet mixing is possible in this environment, it would require extensive foraging efforts since when insects were regulating and abundant (early season), few plants were more protein-biased than their optimal nutrient intake (IT) (Fig. 4). ITs correlated poorly with grass nutrient content but varied with age and sex, highlighting that behavioral nutrient regulation is dynamic and likely based on physiological state rather than short-term shifts in nutrient availability.
When given the choice, male and female grasshoppers collected early actively regulated for a specific IT. Males selected a slightly more carbohydrate-biased ratio than females: P1:C1.71 vs. P1:C1.59 (Fig. 1, panel A). The difference between males and females may be because acquisition and consumption of protein by females is a critical factor for egg production (Clark et al., 2013; Lee, 2010; Maklakov et al., 2008; Reddiex et al., 2013; Solon-Biet et al., 2015; South et al., 2011). Nevertheless, for both sexes, the ITs were carbohydrate biased. In a separate study on adults of the same species, we found that females preferred leaves of plants that were more carbohydrate-biased but, contrasting with the results obtained here with artificial diets, adult males did not differentiate between leaves of different nutrient content (Le Gall et al., accepted). Later in the season, there were no differences between males and females, and insects ate randomly (Fig. 1, panel B). We found that females collected later were heavier than females collected earlier, as well as males at either time point (Fig. 3), perhaps because of ovariole development (Smith, 1964). To understand these sex-dependent shifts in nutritional regulation, longer-term studies are needed (Roeder and Behmer, 2014), particularly since for hemimetabolous insects, nutrient acquisition during both juvenile and adult stages fuels major biological processes like reproduction and migration.
Migration has substantial impacts on nutritional demands for all animals. This is particularly true for insects as they have the highest flight metabolism known (Kammer and Heinrich, 1978). Insect migrations are usually fueled by lipid reserves acquired largely by increased carbohydrate consumption (Blem, 1980; Rankin and Burchsted, 1992; Uvarov, 1928; Weis-Fogh, 1956). Oedaleus senegalensis adults are highly migratory: at the beginning of the rainy season, the first generation usually migrate from South to North following the Intertropical Convergence Zone (Elamin et al., 2013; Holt and Colvin, 1997; Maiga et al., 2008). O. senegalensis can fly up to 350 km in a night (Cheke, 1990; Riley and Reynolds, 1990, 1983). Despite differences in nutrient selection between sexes and collection date, all nutrient ratios selected were carbohydrate biased, highlighting the key role of this nutrient for this species. However, we found no difference in body lipid content for the two food pairings (p7:c35 & p28:c14 and p7:c35 & p35:c7), over time, or between sexes. In other grasshopper species (non-migratory) wide variations in lipid levels (2 to 5.8%) are recorded when fed different foods in no-choice experiments (Le Gall et al., 2020b). We expect this species would have varying body lipid content if constrained to diets that vary in protein:carbohydrate ratios in a no-choice experiment. A potential explanation for the lack of difference in lipid proportion between males and females is that flight energetics could be solely dictated by overall mass, thus maintaining approximately 2% body lipid may be sufficient for migration in this species (Table 2). We also found a negative correlation between mass gain and protein intake, perhaps because of the strong effect that protein has on satiety (Abisgold and Simpson, 1988; Gosby et al., 2014; Simpson and Raubenheimer, 2005). In another study, it appeared that carbohydrates had a positive effect on mass gain via fat deposition (Le Gall and Behmer, 2014); we did not observe an effect of carbohydrate consumption on fat deposition, likely due to the shorter experimental time period (3 days vs. whole instar duration – roughly two weeks).
Besides migration, another striking feature of locust density-dependent polyphenism is a release on diet breadth constraints. Gregarious locusts are notorious for eating more plant species than solitarious locusts and they will more readily eat plant toxins (COPR, 1982; Despland and Simpson, 2005a). In model locusts, the change in food choices is thought to be, in part, an antipredator strategy where eating plant toxins make the conspicuous swarms unpalatable to predators (Despland, 2005; Despland and Simpson, 2005a, 2005b; Sword, 2002; Sword et al., 2000). However, in lab colonies of the desert locust, this does not translate in changes in nutrient regulation and similar ITs were recorded for gregarious and solitarious nymphs (Simpson et al., 2002). Oedaleus senegalensis is a non-model locust (Song, 2010), and its density-dependent polyphenism is poorly understood but appears to be less pronounced than for model species. For instance, the nymphs do not appear to form large marching bands, however, the adults migrate large distances and populations densities are highly variables with regular outbreaks (Abdelmanan, 2010; Bal et al., 2015; Cheke, 1990; El Naim et al., 2014; Elamin et al., 2013; Riley and Reynolds, 1983). There was a locust upsurge the summer we ran the experiments for the current study. Insects were found at high densities in the study area, as shown by the sweep net survey early in the season (Fig. ESM1). The adult density was lower when we recorded density the second time, likely because most had already migrated north. Thus, grasshoppers collected later in the season were potentially shifting into migratory status, which may explain their increased diet breadth and lack of nutrient regulation. Unlike more sessile herbivores such as caterpillars, grasshoppers have been shown to be tight nutrient regulators, at least during nymphal development (Behmer, 2009). Our new data suggest that this might not consistently be the case for field populations and that there may be some sex-specific differences, although more studies are needed to demonstrate whether this is a general phenomenon or field or species-specific.
Beyond changes in the nutritional landscape, other environmental factors can influence feeding habits and nutrient demands. Temperature variation can be important in determining consumption in Orthoptera (Clissold et al., 2013; Miller et al., 2009) but there was only ∼3 °C variation in temperatures during the period of our study (data not shown). Differences in predator (Hawlena et al., 2012) and pathogen (Graham et al., 2014) exposure can also induce a shift in foraging behavior. Foraging is a dangerous exercise due to the increased risk for predation (Bernays, 1997) and herbivores may rely more heavily on post-ingestive regulation when predators and pathogens are abundant. In the current study, insects that were found later may have been exposed to more diseases due to the advancement of the rainy season supporting more pathogens and predators. Such increased predator and pathogen exposure may have contributed to the herbivores having relaxed nutrient selection later in the rainy season.
Our data show that the extraordinary capacity for behavioral nutrient regulation that laboratory grasshoppers and locusts exhibit is not the only tool used by field populations to meet their nutritional requirements. Post-ingestive regulation (e.g., limiting nutrients can be preferentially extracted by increasing the production of digestive enzymes (Clissold et al., 2010); excess carbohydrate can be respired (Zanotto et al., 1997) or stored as lipid; excess protein can be eliminated as uric acid and in volatile form (Harrison, 1995; Zanotto et al., 1993) or used to produce energy via gluconeogenesis (Thompson, 2000)) is likely necessary for field-caught O. senegalensis grasshoppers to meet their optimal nutrient needs, which are carbohydrate-biased. The capacity to be flexible by incorporating varying degrees of nutrient selection during foraging and post-ingestive regulation may be particularly important for animals inhabiting highly dynamic xeric habitats with short growing seasons.
Funding
This work was supported by the National Science Foundation, DEB-1313693 to AJC.
Author contributions
MLG and AC conceived the ideas and designed methodology; MLG, MW, AB, and AC collected the data in the field; MLG analyzed the data; MLG and AC led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Data will be available from the Dryad Digital Repository.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank La Direction de la Protection des Végétaux (DPV) for their collaboration and assistance on this project, in particular Emile Victor Coly, Ousmane Diene, Elhadji Omar Dieng, Aliou Badji, and Ansou Kamara. The Nganda DPV Phytosanitary base: Mamadou Diallo, Idrissa Biaye, Sidy Kaerou Badiane, Ismaela Thiow, Ibrahim Sadio, Makam Cisse, Manga, and Papa Ndiaye; Mamour Toure for advice working on O. senegalensis and field equipment support, Natalia Thompson for running the chemical assays, and Rick Overson for helpful discussions.
References
- Abdelmanan E.H.A. University of Kordofan; 2010. Bio-Ecology of the Senegalese Grasshopper Oedaleus Senegalensis (Krauss, 1877) (Orthoptera: Acrididae) in Kordofan Region, Sudan. PhD Thesis. [Google Scholar]
- Abisgold J.D., Simpson S.J. The effect of dietary protein levels and haemolymph composition on the sensitivity of the maxillary palp chemoreceptors of locusts. J. Exp. Biol. 1988;135:215–229. [Google Scholar]
- Bal A.B., Ouambama Z., Moumouni A., Dieng I., Maiga I.H., Gagare S., Axelsen J.A. A simple tentative model of the losses caused by the Senegalese grasshopper, Oedaleus senegalensis (Krauss 1877) to millet in the Sahel. Int. J. Pest Manag. 2015;61:198–203. doi: 10.1080/09670874.2015.1031201. [DOI] [Google Scholar]
- Behmer S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Behmer S.T., Raubenheimer D., Simpson S.J. Frequency-dependent food selection in locusts: a geometric analysis of the role of nutrient balancing. Anim. Behav. 2001;61:995–1005. [Google Scholar]
- Benoit-Bird K.J. Prey caloric value and predator energy needs: foraging predictions for wild spinner dolphins. Mar. Biol. 2004;145:435–444. [Google Scholar]
- Bernays E.A. Feeding by lepidopteran larvae is dangerous. Ecol. Entomol. 1997;22:121–123. [Google Scholar]
- Blem C.R. Academic Press, Inc. 1980. The energetics of migration, Animal Migration, Orientation, and Navigation; pp. 175–224. [Google Scholar]
- Casas J., Driessen G., Mandon N., Wielaard S., Desouhant E., Van Alphen J., Lapchin L., Rivero A., Christides J.P., Bernstein C. Energy dynamics in a parasitoid foraging in the wild. J. Anim. Ecol. 2003;72:691–697. doi: 10.1046/j.1365-2656.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- Casas J., Pincebourde S., Mandon N., Vannier F., Poujol R., Giron D. Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology. 2005;86:545–554. [Google Scholar]
- Cease A.J., Fay M., Elser J.J., Harrison J.F. Dietary phosphate affects food selection, post-ingestive phosphorus fate, and performance of a polyphagous herbivore. J. Exp. Biol. 2016;219:64–72. doi: 10.1242/jeb.126847. [DOI] [PubMed] [Google Scholar]
- Cheke R.A. A migrant pest in the Sahel: the Senegalese grasshopper Oedaleus senegalensis. Phil. Trans. R. Soc. Lond. B. 1990;328:539–553. doi: 10.1098/rstb.1990.0126. [DOI] [Google Scholar]
- Clark R.M., McConnell A., Zera A.J., Behmer S.T. Nutrient regulation strategies differ between cricket morphs that trade-off dispersal and reproduction. Funct. Ecol. 2013;27:1126–1133. doi: 10.1111/1365-2435.12103. [DOI] [Google Scholar]
- Clissold F., Coggan N., Simpson S. Insect herbivores can choose microclimates to achieve nutritional homeostasis. J. Exp. Biol. 2013;216:2089–2096. doi: 10.1242/jeb.078782. [DOI] [PubMed] [Google Scholar]
- Clissold F.J., Tedder B.J., Conigrave A.D., Simpson S.J. The gastrointestinal tract as a nutrient-balancing organ. Proc. R. Soc. B. 2010;277:1751–1759. doi: 10.1098/rspb.2009.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPR . Overseas Pest Research; London: 1982. The Locust and Grasshopper Agricultural Manual; p. 690. [Google Scholar]
- Dadd R.H. The nutritional requirements of locusts—IV. Requirements for vitamins of the B complex. Journal of Insect Physiology 6.1. 1961:1–12. [Google Scholar]
- Davies N.B. Prey selection and the search strategy of the spotted flycatcher (Muscicapa striata): a field study on optimal foraging. Anim. Behav. 1977;25:1016–1033. doi: 10.1016/0003-3472(77)90053-7. [DOI] [Google Scholar]
- Deans C.A., Sword G.A., Behmer S.T. Nutrition as a neglected factor in insect herbivore susceptibility to Bt toxins. Curr. Opin. Insect Sci. 2016;15:97–103. doi: 10.1016/j.cois.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Deans C.A., Sword G.A., Behmer S.T. Revisiting macronutrient regulation in the polyphagous herbivore Helicoverpa zea (Lepidoptera: Noctuidae): new insights via nutritional geometry. J. Insect Physiol. 2015;81:21–27. doi: 10.1016/j.jinsphys.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Deans C.A., Sword G.A., Lenhart P.A., Burkness E., Hutchison W.D., Behmer S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. J. Vis. Exp. 2018 doi: 10.3791/58164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despland E. Diet breadth and anti-predator strategies in desert locusts and other Orthopterans. J. Orthoptera Res. 2005;14:227–233. doi:10.1665/1082-6467(2005)14[227:DBAASI]2.0.CO;2. [Google Scholar]
- Despland E., Simpson S.J. Food choices of solitarious and gregarious locusts reflect cryptic and aposematic antipredator strategies. Anim. Behav. 2005;69:471–479. doi: 10.1016/j.anbehav.2004.04.018. [DOI] [Google Scholar]
- Despland E., Simpson S.J. Surviving the change to warning colouration: density-dependent polyphenism suggests a route for the evolution of aposematism. Chemoecology. 2005;15:69–75. [Google Scholar]
- El Naim A, Elamin A, Abdalla M: The biology of Senegalese grasshopper Oedaleus senegalensis, Krauss, 1977 (Orthoptera: Acrididae). Int J Adv Life Sci Technol 2014, 1:6–15.
- Elamin A.E., Abdalla A.M., El Naim A.M. Studies on population dynamics of Senegalese grasshopper (Oedaleus senegalensis) in Kordofan of Sudan. World J. Agric. Res. 2013;1:85–89. [Google Scholar]
- Gosby A.K., Conigrave A.D., Raubenheimer D., Simpson S.J. Protein leverage and energy intake. Obes. Rev. 2014;15:183–191. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- Graham R.I., Deacutis J.M., Pulpitel T., Ponton F., Simpson S.J., Wilson K. Locusts increase carbohydrate consumption to protect against a fungal biopesticide. J. Insect Physiol. 2014;69:27–34. doi: 10.1016/j.jinsphys.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Guglielmo C.G. Oxford University Press; 2010. Move That Fatty Acid: Fuel Selection and Transport in Migratory Birds and Bats. [DOI] [PubMed] [Google Scholar]
- Harrison J.F. CRC Press, Inc; Boca Raton: 1995. Nitrogen Metabolism and Excretion in Locusts. Nitrogen Metabolism and Excretion; pp. 119–131. [Google Scholar]
- Harrison S.J., Raubenheimer D., Simpson S.J., Godin J.-G.J., Bertram S.M. Towards a synthesis of frameworks in nutritional ecology: interacting effects of protein, carbohydrate and phosphorus on field cricket fitness. Proc. R. Soc. B. 2014;281 doi: 10.1098/rspb.2014.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlena D., Strickland M.S., Bradford M.A., Schmitz O.J. Fear of predation slows plant-litter decomposition. Science. 2012;336:1434–1438. doi: 10.1126/science.1220097. [DOI] [PubMed] [Google Scholar]
- Holt J., Colvin J. A differential equation model of the interaction between the migration of the Senegalese grasshopper, Oedaleus senegalensis, its predators, and a seasonal habitat. Ecol. Model. 1997;101:185–193. [Google Scholar]
- Jensen K., McClure C., Priest N.K., Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell. 2015;14:605–615. doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer A.E., Heinrich B. Advances in Insect Physiology. Elsevier; 1978. Insect flight metabolism; pp. 133–228. [Google Scholar]
- Le Couteur D.G., Solon-Biet S., Cogger V.C., Mitchell S.J., Senior A., de Cabo R., Raubenheimer D., Simpson S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell. Mol. Life Sci. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M. Texas A&M University; 2014. Diet-Mixing in a Generalist Herbivore: Trade-Offs Between Nutrient and Allelochemical Regulation. [Google Scholar]
- Le Gall M., Behmer S.T. Effects of protein and carbohydrate on an insect herbivore: the vista from a fitness landscape. Integr. Comp. Biol. 2014;54:942–954. doi: 10.1093/icb/icu102. [DOI] [PubMed] [Google Scholar]
- Le Gall M., Word M.L., Thompson N., Beye A., Cease A.J. Nitrogen fertilizer decreases survival and reproduction of female locusts by increasing plant protein to carbohydrate ratio. J. Anim. Ecol. 2020;89:2214–2221. doi: 10.1111/1365-2656.13288. [DOI] [PubMed] [Google Scholar]
- Le Gall M., Word M.L., Thompson N., Manneh B., Beye A., Cease A.J. Linking land use and the nutritional ecology of herbivores: a case study with the Senegalese locust. Funct. Ecol. 2020;34:167–181. [Google Scholar]
- Lee K.P. Sex-specific differences in nutrient regulation in a capital breeding caterpillar, Spodoptera litura (Fabricius) J. Insect Physiol. 2010;56:1685–1695. doi: 10.1016/j.jinsphys.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Cory J.S., Wilson K., Raubenheimer D., Simpson S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Raubenheimer D., Behmer S.T., Simpson S.J. A correlation between macronutrient balancing and insect host-plant range: evidence from the specialist caterpillar Spodoptera exempta (Walker) J. Insect Physiol. 2003;49:1161–1171. doi: 10.1016/j.jinsphys.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Lenhart P.A., Eubanks M.D., Behmer S.T. Water stress in grasslands: dynamic responses of plants and insect herbivores. Oikos. 2015;124:381–390. doi: 10.1111/oik.01370. [DOI] [Google Scholar]
- Lobel P.S., Ogden J.C. Foraging by the herbivorous parrotfish Sparisoma radians. Mar. Biol. 1981;64:173–183. doi: 10.1007/BF00397106. [DOI] [Google Scholar]
- MacArthur R.H., Pianka E.R. On optimal use of a patchy environment. Am. Nat. 1966;100:603–609. [Google Scholar]
- Maiga I.H., Lecoq M., Kooyman C. West Africa: Review and Prospects. Taylor & Francis; 2008. Ecology and management of the Senegalese grasshopper Oedaleus senegalensis (Krauss 1877) (Orthoptera: Acrididae) pp. 271–288. [Google Scholar]
- Maklakov A.A., Simpson S.J., Zajitschek F., Hall M.D., Dessmann J., Clissold F., Raubenheimer D., Bonduriansky R., Brooks R.C. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Clissold F.J., Mayntz D., Simpson S.J. Speed over efficiency: locusts select body temperatures that favour growth rate over efficient nutrient utilization. Proc. R. Soc. B. 2009;276:3581–3589. doi: 10.1098/rspb.2009.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musten B., Peace D., Anderson G.H. Food intake regulation in the weanling rat: self-selection of protein and energy. J. Nutr. 1974;104:563–572. doi: 10.1093/jn/104.5.563. [DOI] [PubMed] [Google Scholar]
- Nagy K.A., Huey R.B., Bennett A.F. Field energetics and foraging mode of kalahari lacertid lizards. Ecology. 1984;65:588–596. doi: 10.2307/1941421. [DOI] [Google Scholar]
- Paoli P.P., Donley D., Stabler D., Saseendranath A., Nicolson S.W., Simpson S.J., Wright G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids. 2014;46:1449–1458. doi: 10.1007/s00726-014-1706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Pianka E.R. Animal foraging: past, present and future. Trends Ecol. Evol. Amst. 1997;12:360–364. doi: 10.1016/s0169-5347(97)01097-5. [DOI] [PubMed] [Google Scholar]
- Povey S., Cotter S.C., Simpson S.J., Lee K.P., Wilson K. Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J. Anim. Ecol. 2009;78:437–446. doi: 10.1111/j.1365-2656.2008.01499.x. [DOI] [PubMed] [Google Scholar]
- Rankin M.A., Burchsted J.C.A. The cost of migration in insects. Annu. Rev. Entomol. 1992;37:533–559. [Google Scholar]
- Reddiex A.J., Gosden T.P., Bonduriansky R., Chenoweth S.F. Sex-specific fitness consequences of nutrient intake and the evolvability of diet preferences. Am. Nat. 2013;182:91–102. doi: 10.1086/670649. [DOI] [PubMed] [Google Scholar]
- Riley J.R., Reynolds D.R. Nocturnal grasshopper migration in West Africa: transport and concentration by the wind, and the implications for air-to-air control. Philos. Trans. R. Soc. Lond. B. 1990;328:655–672. [Google Scholar]
- Riley J.R., Reynolds D.R. A long-range migration of grasshoppers observed in the Sahelian zone of Mali by two radars. J. Anim. Ecol. 1983;52:167–183. doi: 10.2307/4594. [DOI] [Google Scholar]
- Roeder K.A., Behmer S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014;28:1135–1143. doi: 10.1111/1365-2435.12262. [DOI] [Google Scholar]
- Schmitz O.J., Hawlena D., Trussell G.C. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 2010;13:1199–1209. doi: 10.1111/j.1461-0248.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- Schmitz O.J., Rosenblatt A.E., Smylie M. Temperature dependence of predation stress and the nutritional ecology of a generalist herbivore. Ecology. 2016;97:3119–3130. doi: 10.1002/ecy.1524. [DOI] [PubMed] [Google Scholar]
- Simpson S., Clissold F., Lihoreau M., Ponton F., Wilder S., Raubenheimer D. Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol. 2015;60:293–311. doi: 10.1146/annurev-ento-010814-020917. [DOI] [PubMed] [Google Scholar]
- Simpson S., Raubenheimer D. Princeton University Press; 2012. The Nature of Nutrition: A Unifying Framework From Animal Adaptation to Human Obesity. [Google Scholar]
- Simpson S.J., Abisgold J.D. Compensation by locusts for changes in dietary nutrients: behavioural mechanisms. Physiol. Entomol. 1985;10:443–452. [Google Scholar]
- Simpson S.J., Raubenheimer D. Obesity: the protein leverage hypothesis. Obes. Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Raubenheimer D., Behmer S.T., Whitworth A., Wright G.A. A comparison of nutritional regulation in solitarious- and gregarious-phase nymphs of the desert locust Schistocerca gregaria. J. Exp. Biol. 2002;205:121–129. doi: 10.1242/jeb.205.1.121. [DOI] [PubMed] [Google Scholar]
- Smith D.S. Ovarioles and developing eggs in grasshoppers. Can. Entomol. 1964;96:1255–1258. doi: 10.4039/Ent961255-9. [DOI] [Google Scholar]
- Solon-Biet S.M., McMahon A.C., Ballard J.W.O., Ruohonen K., Wu L.E., Cogger V.C., Warren A., Huang X., Pichaud N., Melvin R.G. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S.M., Walters K.A., Simanainen U.K., McMahon A.C., Ruohonen K., Ballard J.W.O., Raubenheimer D., Handelsman D.J., Couteur D.G.L., Simpson S.J. Macronutrient balance, reproductive function, and lifespan in aging mice. PNAS. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Density-dependent phase polyphenism in nonmodel locusts: a minireview. Psyche. 2010 doi: 10.1155/2011/741769. [DOI] [Google Scholar]
- South S.H., House C.M., Moore A.J., Simpson S.J., Hunt J. Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: implications for the study of condition dependence. Evol. N.Y. 2011;65:1594–1606. doi: 10.1111/j.1558-5646.2011.01233.x. [DOI] [PubMed] [Google Scholar]
- Sword G.A. A role for phenotypic plasticity in the evolution of aposematism. Proc. R. Soc. B. 2002;269:1639–1644. doi: 10.1098/rspb.2002.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword G.A., Simpson S.J., El Hadi O.T.M., Wilps H. Density–dependent aposematism in the desert locust. Proc. R. Soc. B. 2000;267:63–68. doi: 10.1098/rspb.2000.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessnow A.E., Behmer S.T., Walsh T.K., Sword G.A. Protein-carbohydrate regulation in Helicoverpa amigera and H. punctigera and how diet protein-carbohydrate content affects insect susceptibility to Bt toxins. J. Insect Physiol. 2018;106:88–95. doi: 10.1016/j.jinsphys.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Tews J.K., Repa J.J., Harper A.E. Protein selection by rats adapted to high or moderately low levels of dietary protein. Physiol. Behav. 1992;51:699–712. doi: 10.1016/0031-9384(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Thompson S.N. Pyruvate cycling and implications for regulation of gluconeogenesis in the insect, Manduca sexta L. Biochem. Biophys. Res. Commun. 2000;274:787–793. doi: 10.1006/bbrc.2000.3238. [DOI] [PubMed] [Google Scholar]
- Toure M., Ndiaye M., Diongue A. Effect of cultural techniques: rotation and fallow on the distribution of Oedaleus senegalensis (Krauss, 1877) (Orthoptera: Acrididae) in Senegal. Afr. J. Agric. Res. 2013;8:5634–5638. [Google Scholar]
- Uvarov B.P. Imperial Bureau of Entomology; London: 1928. Locusts and Grasshoppers. A Handbook for Their Study and Control. [Google Scholar]
- Weis-Fogh T. Biology and physics of locust flight II. Flight performance of the desert locust (Schistocerca gregaria) Phil. Trans. R. Soc. Lond. B. 1956;239:459–510. [Google Scholar]
- Werner E.E., Mittelbach G.G. Optimal foraging: field tests of diet choice and habitat switching. Integr. Comp. Biol. 1981;21:813–829. doi: 10.1093/icb/21.4.813. [DOI] [Google Scholar]
- Word M.L., Hall S.J., Robinson B., Manneh B., Beye A., Cease A.J. Soil-targeted interventions could alleviate locust and grasshopper pest pressure in West Africa. Sci. Total Environ. 2019;(663):632–643. doi: 10.1016/j.scitotenv.2019.01.313. [DOI] [PubMed] [Google Scholar]
- Zanotto F., Gouveia S.M., Simpson S., Calder D. Nutritional homeostasis in locusts: is there a mechanism for increased energy expenditure during carbohydrate overfeeding? J. Exp. Biol. 1997;200:2437–2448. doi: 10.1242/jeb.200.18.2437. [DOI] [PubMed] [Google Scholar]
- Zanotto F.P., Simpson S.J., Raubenheimer D. The regulation of growth by locusts through post‐ingestive compensation for variation in the levels of dietary protein and carbohydrate. Physiol. Entomol. 1993;18:425–434. doi: 10.1111/j.1365-3032.1993.tb00617.x. [DOI] [Google Scholar]
- Zhang Z., Elser J.J., Cease A.J., Zhang X., Yu Q., Han X., Zhang G. Grasshoppers regulate N: P stoichiometric homeostasis by changing phosphorus contents in their frass. PLoS ONE. 2014;9 doi: 10.1242/jeb.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]