Highlights
Climate change can affect insect performance and bees are particularly vulnerable.
Heat shock decreases bee activity, starvation resistance, and longevity.
This effect is stronger in males, as female bees are more stress tolerant
We also find a possible link between stress tolerance and daily rhythms.
Keywords: heat shock, bee health, circadian rhythm, alfalfa leafcutting bee
Abstract
The pollination services provided by insects have been a crucial part of evolution and survival for many species, including humans. For bees to be efficient pollinators they must survive the environmental insults they face daily. Thus, looking into the short- and long-term effects of heat exposure on bee performance provides us with a foundation for investigating how stress can affect insect pollination. Solitary bees are a great model for investigating the effects of environmental stress on pollinators because the vast majority of insect pollinator species are solitary rather than social. One of the most pervasive environmental stressors to insects is temperature. Here we investigated how a one-hour heat shock affected multiple metrics of performance in the alfalfa leafcutting bee, Megachile rotundata. We found that a short heat shock (1hr at 45°C) can delay adult emergence in males but not females. Bee pupae were rather resilient to a range of high temperature exposures that larvae did not survive. Following heat shock (1hr at 50°C), adult bees were drastically less active than untreated bees, and this reduction in activity was evident over several days. Heat shock also led to a decrease in bee survival and longevity. Additionally, we found a connection between starvation survival after heat shock and time of exposure, where bees exposed in the morning survived longer than those exposed in the afternoon, when they would normally experience heat shock in the field. These data suggest that there is an unexplored daily/circadian component to the stress response in bees likely similar to that seen in flies, nematodes, and plants which is constitutive or preemptive rather than restorative. Taken together our data indicate that single heat shock events have strong potential to negatively impact multiple life history traits correlated with reproduction and fitness.
Introduction
Insect pollinators spend most of their day foraging and experience varying environmental conditions. Insects, being poikilothermic ectotherms, do not actively regulate their internal body temperature using their metabolism (May 1979), and instead are heavily influenced by their behavior and external environmental temperature. Wing movements and flight are major contributors to internal temperature changes in insects (May 1979, Heinrich 1993), and the temperature of the internal thorax can rapidly exceed that of the external thorax during flight (Feller and Nachtigall 1989). Aside from dehydration, temperature may be the most influential abiotic environmental factor that insect pollinators face daily (Hadley 1994, Chown and Terblanche 2006). Some insects decrease their daytime activity when the temperature is too high, others tend to hide (Bodenheimer 1934), avoid (Barbagallo and Garrity 2015), or use evaporative cooling (Prange 1996, González-Tokman et al. 2020) to cope with those temperatures. Other insects remain active as long as there is daylight present (Bradshaw and Holzapfel 2001), as is the case of the alfalfa leafcutting bee, Megachile rotundata, where individuals are still active in days with higher temperatures (44 to 47˚C; Barthell et al. 2002), but they have shorter flights (Klostermeyer and Gerber 1969).
Megachile rotundata is a solitary, cavity nester. Females spend large periods of time, from mid-morning to mid-afternoon, collecting pollen and building brood cells (Klostermeyer and Gerber 1969). These bees use leaves, and flowers, to line cavities where they lay an egg and add a food provision. Additional leaf pieces are used to cap individual brood cells for development and overwintering. These cells are composed of 9 to 14 leaf pieces each depending on the diameter of the hole (Klostermeyer et al. 1973), and females spend anywhere between 81 and 150 minutes completing a single brood cell (Klostermeyer and Gerber 1969, Maeta and Adachi 2005, Pitts-Singer and Cane 2011). This translates into 6-hour foraging days in varying environmental conditions. Most of these female bee trips do not involve the acquisition of food, but rather the collection and transportation of leaf pieces used for cell construction. During these hours spent outside, the bees experience the warmest temperatures of the day (CaraDonna et al., 2018, López-Martínez and Denlinger, 2008, Wilson et al., 2020). At night, the bees will routinely enter empty cavities to spend the inactive nighttime period (Klostermeyer et al. 1973). This daily marathon of cell building, food/leaf collection and transportation, and temperature exposure falls strongly on the females as males are not involved in brood cell building. Consequently, fitness is tightly connected to the female's ability to build as many cells as possible under the prevailing environmental conditions.
Environmental stress has short- and long-term effects that go beyond the individual experiencing the stress and into future generations (Ho and Burggren 2010, Ayyanath et al. 2013, López-Martínez et al. 2014, 2016a). But the traditional timescale of investigation for stress responses falls between hours and days, excluding months (outside of overwintering work) and multiple generations. Comprehensive experiments into environmental stress exposure are important because they can address the multifarious effects of stress. Environmental stress effects are pervasive because they, in part, stem from mitochondria-induced free radical damage. Free radicals attack nucleic acids, lipids, and proteins leading to oxidative stress and potentially affecting multiple life history traits (Dowling and Simmons 2009). These free radical-mediated effects of stress can affect performance (López-Martínez and Hahn 2012, Margotta et al. 2018), mating (Ohinata et al. 1979, Nestel et al. 2007, López-Martínez and Hahn 2012, López-Martínez and Hahn 2014), reproduction (López-Martínez et al. 2014), longevity (López-Martínez and Hahn 2014, López-Martínez et al. 2014, Berry and López-Martínez 2020), and F1 progeny performance (Ayyanath et al. 2013, López-Martínez et al. 2014, López-Martínez et al. 2016a). Every life history trait that has been measured in response to stress has shown some level of responsiveness, displaying the plasticity of these traits.
Abiotic environmental factors predate the existence of animals, and evolution has produced strong and persistent responses to stress that allow insects to survive in their current climates (Williams et al. 2014, Marshall et al. 2020). These abiotic factors can have stimulatory/protective effects that are termed hormesis (Calabrese et al. 2007), or detrimental effects that negatively affect performance and fitness referred to as environmental stress (Berry and López-Martínez 2020). It is these latter negative effects that we refer to as being stressful or stress. It is conventionally thought that most stress responses are restorative in nature, where an insect elevates their defenses in response to a stressor (Ashburner and Bonner 1979, Feder and Hoffman 1999) therefore lowering the damage while restoring homeostasis. The bulk of the research into stress physiology focuses on these types of stress responses. However, when considering complex physiological responses like those involved in stress, one-sided restorative upregulation is only a part of the process. Strong constitutive responses are present in insects (Parsell and Lindquist 1993, Feder and Hofmann 1999, Rinehart et al. 2006), and complex physiological traits like reproduction and overwintering (i.e., diapause) have stress gene upregulation as part of their programming (Denlinger 2002, Sinclair et al. 2013). Heat shock proteins (HSPs), antioxidant enzymes, and late embryogenesis abundant (LEA) proteins are some of the proteins encoded by stress genes that are upregulated as an integral part of overwintering and reproduction in insects (Neuer et al. 2000, López-Martínez et al. 2009, Sim and Denlinger 2011), and some of them may also be involved in a daily anticipatory response to stress as well. Diapause, and likely other types of dormancy, are also accompanied by a suite of protective genes and pathways that are upregulated as part of the diapause programming (Robich et al. 2007, Rinehart et al. 2010, Sim and Denlinger 2011, Ragland et al. 2011). But those complex physiological syndromes involve multiple cues and seasonal programming which are thought to be connected to the overall stress response (Denlinger 2002, Michaud and Denlinger 2007, King and MacRae 2015). Still, outside of commonly studied complex physiology, is there a place in our understanding of stress regulation that accounts for daily cycles?
The notion that stress tolerance has a daily, or circadian, component is supported in plants where stress genes associated with temperature and osmotic stress have their peak expression early in the day (∼9am; Grundy et al. 2015). In animals, the majority of the work indicating that certain physiological parameters connected to stress and immunity are regulated in a circadian fashion comes from the nematode, C elegans (Migliori et al. 2011). Here the adults have a daily stress tolerance rhythm where they have varying responses to oxidative stress and osmotic shock throughout the day (Simonetta et al. 2008), and these responses are stronger in the morning than the evenings. In insects, the apple maggot Rhagoletis pomonella, expresses multiple inducible heat shock proteins (Hsp70 and Hsp90) at mild temperatures (23°C), well below the established expression inducibility threshold for these genes (36°C; Ashburner and Bonner 1979). These maggots living inside of apples upregulate these genes in preparation for the harsher temperatures (45 to 47°C) experienced in late afternoon due, in part, to solar irradiance (López-Martínez and Denlinger 2008). In order to accumulate enough protective gene products to survive the afternoon heat, the maggots must begin upregulation early in the day. This preventive expression pattern was only seen in the field, but not in lab colonies, indicating that other daily environmental cues (photoperiod or plant cues in addition to temperature) might be involved. And even though the maggots started their gene upregulation in the morning, it was not tested whether they dealt better with stress in the morning versus the afternoon.
In this study we hypothesized that a single heat shock event would negatively impact bee development and adult performance, and the effect on adults would be sex-specific with males being more vulnerable. We focused on a single maximum temperature exposure because these events have the potential to affect multiple life history traits in insects (Ma et al., 2015a, Ma et al., 2015b). We chose the alfalfa leafcutting bee, Megachile rotundata, because it is a cavity nester pollinator that is active during the warmer months of the year. Rather than a thermal regime, we choose a single pulse of high temperature to determine a baseline for future work combining multiple heat pulses and eventually thermal regime changes. We targeted developmental stages because they are immobile inside a closed brood cell and therefore more vulnerable to the effect of the stress. In this species, development resumes after overwintering (diapause and quiescence) and stress challenges at this stage could force the animals to shift resources meant for development towards repair and recovery as measured following anoxia exposure (Cervantes and Lopez-Martinez, 2022). We targeted newly emerged adults to test the potential long-lasting effects of stress on performance . We expected that a single heat shock event would increase the amount of time needed for bees to complete their development due to potential stress recovery costs. For adults, we focused on sex differences because we expected females to be more tolerant due to the disproportional amount of time they spend building brood cells for the developing and overwintering larvae in the hot afternoons and based on previous work indicating female insects are more stress tolerant than males (Lin et al. 1998, Matzkin et al. 2009, López-Martínez and Hahn 2012, López-Martínez et al. 2014, 2016, Niveditha et al. 2017, Kristensen et al. 2019). Additionally, we investigated whether tolerance to heat shock events differs throughout the day by comparing morning and afternoon heat shock responses. Our prediction was that bees experiencing stress in the afternoon will be better protected than those experiencing the heat shock in the morning due to the additional time needed to accumulate defenses (i.e., heat shock proteins, López-Martínez and Denlinger 2008). We report significant effects of age (stage), sex, and time of treatment on stress responses in leafcutting bees.
Methods
Bee maintenance
Alfalfa leafcutting bees, Megachile rotundata, were purchased from JWM Leafcutters (Nampa, ID) as post-diapause prepupae that were quiescent. To maintain that quiescence until needed, brood cells containing prepupa were stored in an incubator at 6°C in the dark (Percival Scientific, Perry, IA, USA). In preparation for experiments, bees were moved to a 29°C Percival incubator under long day (15L:9D) photoperiod and 50 ±5% relative humidity. Under these conditions it took ∼20 days for the prepupae to complete their development and emerge as adults. Bees were treated at different life stages and afterwards kept in the same 29°C incubator under the same conditions, a standard temperature for this bee species (Tepedino and Parker 1986).
Temperature treatments
Bees were treated to a single 1-hour heat shock. This heat shock occurred at one of three life stages tested (prepupa, pupa, or adult). Prepupae were treated 19 days prior to adult emergence, a day after the transfer from 6°C to 29°C, while still in the prepupal stage that just finished overwintering. Pupae were treated two days prior to adult emergence to target the pharate adult stage and make it comparable to previous work. Adults were treated within 24 hrs. of emergence from the brood cells. The treatments consisted of a one-hour exposure to 29 (control), 35, 40, 45, or 50°C. Exposures were carried out in EchoTherm (Torrey Pines Scientific, Carlsbad, CA, USA) bench top incubators. A 473 ml cup containing only water was added to the incubator chamber to maintain humidity during treatment but was not a drinking water source for the bees. All bees were treated in 473 ml cups lined with moist paper towels. This water was added to prevent dehydration-induced mortality during the temperature treatment as seen in preliminary studies. Prepupae and pupae were left inside their brood cells during treatment. Once emerged, all adult bees were fed Pro-sweet liquid feed ad libitum (Mann Lake Ltd., Hackensack, MN, USA). Pollen was not provided because in preliminary studies only certain females consumed the pollen provided and it was deemed as a nutritional advantage over males and females that did not consume pollen. Treated bees were only used for one experiment (emergence, starvation resistance, daily activity, or longevity).
Emergence
To monitor the effect of temperature on emergence, brood cells containing one treated bee (prepupa or pupa) were placed individually in 24-well plates (Corning Life Sciences, Corning, NY, USA). Five replicate plates were filled for each treatment (29, 35, 40, 45, and 50°C), and the experiment was performed at least two separate times (10 replicates/treatment). 24-well plates were monitored daily for emergence which normally occurred over an eight-day period with the males emerging in the first few days, a mix of sexes in days 4 and 5, and mostly females towards the end of the emergence period (, (Pitts-Singer and Cane, 2011, Pitts-Singer and James, 2005)). Data are presented as percent bee emergence per day by sex.
Starvation resistance
Because overwintering bees might not have immediate access to food upon emergence, we wanted to test the response to temperature in newly emerged bees without access to food. To determine the appropriate number of bees to use and whether density affected bee performance, a series of density experiments were carried out. Three replicates of 15 or 20 bees were place in 946 ml cages and starvation until death was recorded. The experiment was repeated four times. We found no effect of density on bee performance (Χ2 = 1.535, p = 0.2153; Fig. S1C). Three to five groups of adult bees (15 bees/group) were placed in cages in an incubator as described above. Exactly every 24 hrs. mortality was assessed, and dead bees were removed, sexed, and counted. To investigate the effect of time of treatment, an identical experiment was carried out, but bees were treated either in the morning (10 am) or the afternoon (3 pm). Both experiments were performed three separate times using different cohorts of bees. Data are presented as percent survival by day and by sex.
Daily activity
Density experiments were conducted in the activity monitors to determine the effect of density on bee activity and the appropriate number of bees. Five, ten, or twenty bees were placed in each activity monitor and their activity was recorded for three hours during the afternoon, two hours after treatment recovery. There was no effect of density on bee activity (p = 0.3482: Fig. S1B). Groups of ten bees (5 females and 5 males) were placed in drosophila vials (Genesee Scientific, San Diego, CA, USA) after determining that these conditions were neither crowding the bees nor negatively affecting their performance. The open end of the vial was closed with micro mesh and a 3D printed fitted lid that prevented bee escape while allowing adequate airflow. These vials were placed in Drosophila Population Monitors (DPMs; Trikinetics, Inc., MA, USA). Six DPMs ran simultaneously for three control (29°C) and three heat shocked (1hr @ 50°C) replicates. This temperature was chosen based on previous work showing that performance in this species decreases above 45°C (Barthell et al. 2002) and recent temperature data from our nest boxes (Wilson et al. 2020). Activity was tracked for 24 and 48 hours without food as there was no effect of food availability in activity within the first 48 hours following treatment (p = 0.774; Fig. S1A), and at that point a new batch of freshly treated bees was placed into the DPMs. There was no mortality recorded in the activity experiments within the first 48 hrs. The experiment was repeated three times using different bee cohorts. Data are presented as total activity/minute/.
Longevity
Groups of ∼20 treated (29 or 50°C) bees were place in 2.37 L containers with food (Pro-sweet soaked cotton in 60mm petri dish bottoms). Three times per week, the bee containers were checked for mortality and the dead bees were removed, sexed, and counted. The food was replaced as needed to ensure a surplus of fresh food. Data are presented as percent survivorship over time by sex.
Statistical analysis
Emergence data was analyzed using multivariable (one, two, or three-way) ANOVAs with temperature, time, sex, and their interaction as variables where applicable. Bee cohort was used as a random block term in the analysis. ANOVAs were followed with a Tukey's post hoc analysis. General linear models (GLMs) were used to analyze daily activity, followed by linear contrast analysis to tease out treatment effects. Starvation survivorship and longevity data were analyzed using a proportional hazards model. All statistical analysis were carried out in JMP15.
Results
Emergence
Adult alfalfa leafcutting bees emerged after treatment over the course of eight days, as expected (Richards and Whitfield 1988, Pitts-Singer and Cane 2011). Pupae treated at the highest survivable temperature (45°C) experienced a delay in emergence not seen in the other groups (Fig. 1A; F31,248 = 8.824, p model < 0.0001; F3,3 = 6.764, p temperature = 0.0002; F7,7 = 23.591, p day < 0.0001; F21,21 = 3.018, p temperature*day < 0.0001). The proportion of total bees that emerged was not different among treatments (F3,31 = 0.443, p = 0.7243), except for the 50°C treatment where all treated pupae died (Fig. 2A; F4,40 = 472.82, p model < 0.0001). Neither immature stage, prepupa or pupa, were able to survive one hour at 50°C; the temperature used in the adult experiments (Fig. 2B; F5,46 = 86.769, p model < 0.0001; F1,1 = 298.936, p temperature < 0.0001; F2,2 = 35.986, p stage < 0.0001; F2,2 = 23.499, p temperature*stage < 0.0001). Daily emergence patterns for pupae treated at 40 and 45°C were altered for males but not females (Fig. 1BC; F47,432 = 13.937, p model < 0.0001; F2,2 = 5.681, p temperature = 0.0037; F1,1 = 18.569, p sex < 0.0001; F7,7 = 48.001, p day < 0.0001; F2,2 = 5.681, p temperature*sex = 0.0037; F7,7 = 34.154, p day*sex < 0.0001; F14,14 = 2.396, p temperature*day = 0.0031; F14,14 = 1.95, p temperature*sex*day = 0.0202). Male emergence was affected by temperature at 40°C (Fig. 1B; F15,144 = 20.011, p model < 0.0001; F1,1 = 4.932, p temperature = 0.0279; F7,7 = 40.765, p day < 0.0001; F7,7 = 2.03, p temperature*day = 0.0551) and 45°C (Fig. 1B; F15,144 = 26.266, p model < 0.0001; F1,1 = 10.774, p temperature = 0.0013; F7,7 = 52.181, p day < 0.0001; F7,7 = 3.328, p temperature*day = 0.0026). Additionally, the interaction of temperature and emergence day implies that a delay in development was marginal at 40°C but significant in 45°C males. There was no effect of temperature on females, and female emergence only varied over time (Fig. 1C; F23,216 = 9.764, p model < 0.0001; F2,2 = 0, p temperature = 1; F7,7 = 28.588, p day < 0.0001; F14,14 = 1.505, p temperature*day = 0.111).
Fig. 1.
A) Bee emergence is delayed at the highest survivable temperature (45°C) within the range of temperatures the pupae experienced but only for males (B). Emergence for bees exposed to 35 and 40°C was not different from that seen in control bees (29°C). The lines indicate average emergence for control (black) vs 45°C (gray). C) Female development was not negatively affected by heat shock. Data presented as average percent emergence by day.
Fig. 2.
A) Pupae survive over a broad range of temperatures being more tolerant of heat shock pulses than larvae (B); except for an hour at 50°C which is deadly to both immature stages but not adults. Data presented as average percent emergence ± SE.
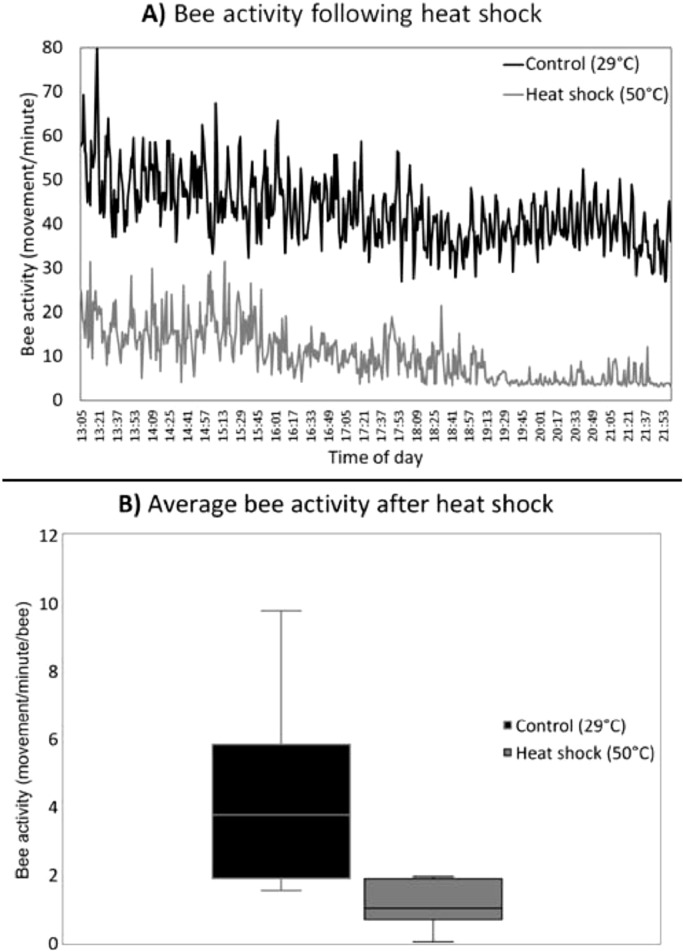
Daily activity
A strong decrease in bee activity was recorded in the first two days following temperature treatment (Fig. 3A). Heat shock treated bees had ∼25% of the activity recorded in the control bees (Fig. 3B; Χ2 = 11.513, p = 0.0007).
Fig. 3.
A) Bees that experienced a one-hour 50°C heat shock as adults had a strong reduction in activity throughout the entire day. Data presented as average movement for groups of 10 bees per minute. B) This reduction in activity was still evident on day three post treatment and represented roughly a 75% decrease. Data is presented as average movement per minute per bee.
Starvation resistance
48 hrs. after heat shock treatment, and in the absence of any food, survival in the heat shock group was lower (48% alive) compared to the controls (76% alive, Fig. 4A; F1,46 = 11.731, p = 0.0013). When survivorship under starvation was followed until all bees died, we found that heat shocked bees had a higher mortality risk than controls (Fig. 4B; Χ2 = 20.444, p = 0.0001). However, this survivorship was not attributed to temperature (Χ2 = 2.205, p temperature = 0.138), but rather an interaction of time and temperature (Χ2 = 17.298, p time < 0.0001; Χ2 = 7.436, p temperature * time = 0.0064). This indicates that heat shock bees living the longest under starvation were those that were treated in the morning rather than the afternoon (Fig. 4B). The fastest decline in survivorship recorded in all four treatments was in bees heat shocked in the afternoon.
Fig. 4.
A) Starvation resistance is lowest in the heat shock treatment, especially if treated in the afternoon. Forty-eight hours after treatment more than half the bees treated in the afternoon were already dead. B) Bees that experienced heat shock in the morning (10am) had higher starvation resistance than those that experienced it in the afternoon (3pm). Data presented as average percentage alive ± SE.
Longevity
Heat shocked bees experienced a shorter lifespan and faster initial mortality than controls (Fig. 5; Χ2 = 34.348, p model < 0.0001; Χ2 treatment = 13.083, p treatment = 0.0003; Χ2 sex = 12.656, p sex = 0.0004; Χ2 treatment*sex = 2.251, p treatment*sex = 0.1123). This overall effect is driven by males that died faster after heat shock (Fig. 5B; Χ2 = 33.336, p < 0.0001). There was no effect of heat shock on female longevity (Fig. 5C; Χ2 = 1.0192, p = 0.3127).
Fig. 5.
A) Heat stressed bees had a shorter lifespan and a higher risk of mortality than control bees. More than 80% of heat shock bees were dead three weeks after treatment, but control bees lived beyond two months. B) The shorter lifespan seen in bees in response to heat shock was characteristic of males due to their higher risk of death, and lower survivorship. C) Female bee risk of death and longevity were not negatively affected by heat shock at 50°C. Data presented as average percent survivorship.
Discussion
As climate continues to change, insect pollinators are exposed to greater daily variations in temperature and higher temperature extremes (Sinclair et al. 2013, Larson et al. 2019, Johansson et al. 2020, González-Tokman et al. 2020). To understand the multifarious effects of stress on insect performance we must engage on comprehensive studies that quantify multiple metrics of performance. The type of experiment that focuses on short-term survival (48 or 72 hrs. after exposure) following environmental insult is not rigorous enough to model the widescale ecological effects on keystone species just as bees (Easton-Calabria et al. 2019). Our experiments have highlighted that a single short bout (1hr) of high temperature (45 or 50°C) is enough to affect bee development, activity, short-term survival, and longevity; correlatives of fitness also affected by other stressors in insects (López-Martínez and Hahn 2012, Haeler et al. 2014, López-Martínez et al. 2016b). Insect pollinators spend most of their day foraging, making a one-hour heat shock at 50°C very relevant and while air temperatures may not reach 50°C yet, solar irradiation and conduction of nest surfaces, such as brood cavities, come close (47°C; López-Martínez and Denlinger 2008, (CaraDonna et al., 2018)Wilson et al. 2020). The nest boxes routinely used for bee management in this species experience daily temperature cycles reaching 46°C in the afternoon in the northern United States in the summer of 2018, more than 10 degrees above air temperatures (Wilson et al. 2020). Alfalfa leafcutting bees choose to build brood cells in cooler cavities and only used warmer cells as a last resort, indicating an avoidance of these threatening temperatures (Wilson et al. 2020). This behavior may be indicative of their sensitivity or vulnerability to high temperatures during the period (1 to 3 hrs.) necessary for females to complete a single brood cell and/or the vulnerability of immature stages to high temperatures. Developing bees confined to brood cells will experience daily short (minutes to an hour) heat shocks in the 40 to 46°C range for multiple days as development takes ∼three weeks in this species. Those bees that are destined to diapause, will also experience these daily insults, but must reach the prepupal phase before entering diapause. The impact of heat shock on diapause-fated bees might have broader consequences than on direct developers as seen in anoxia (Cervantes and Lopez-Martinez, 2022).
We focused on sex differences because of the growing collection of data showing stress responses vary by sex (Matzkin et al. 2009, Niveditha et al. 2017, Kristensen et al. 2019) and specifically the potential extensive exposure to heat that the females of this species endure, under natural conditions, during reproduction. Females return countless times to their cavities to deliver collected leaf pieces for brood cell construction while experiencing the temperature variations between the plants, the air, and the cavities. Within those cavities, the females will cut leaf pieces and “glue” them in place, taking additional time in the confined high temperature space. It is very likely that the number of trips is affected by exposure, and this may be a driving pressure for cell construction (81 to 180 minutes). If a one-hour heat shock can decrease the activity of bees for 2 days, then the days that follow the heat event would potentially be accompanied by a reduction in the number of trips for leaf collection/pollination (decreased ecological benefit) and the number of brood cells constructed (decreased fitness). And even though our data indicate that fully fed females have higher survivorship, this result is predicated on the ability of the bees successfully foraging following the stressful event. Our experiments raise the question of whether that decrease in activity is transient or whether it represent a permanent shift in activity and having focused on activity early in life, we cannot tell whether the effect is long lasting. One aspect of female bee behavior that we were not able to study was the influence of heat shock on leaf transportation. Flying while carrying leaf pieces is likely a stressor itself (i.e., increased load affecting internal temperature), and it will be important to understand the effect that temperature stress has on this behavior that is crucial for fitness in these bees. Specially because that increased physical activity involved in carrying nest materials could trigger protective mechanisms (i.e., hormesis) and help mitigate some of the effects of stress.
Our sex-specific data concurs with available data from other insect species, where the females are more tolerant of stress and males are the vulnerable sex (Lin et al. 1998, Matzkin et al. 2009, López-Martínez and Hahn 2012, 2014, Niveditha et al. 2017, Kristensen et al. 2019). A single heat shock event is enough to lead to early male death. While some male mortality is not as crucial to overall fitness of a species where parental care falls solely on the females, there are two ways that male vulnerability can impact insect pollinators. Given that males are more sensitive during development, the initial delay in emergence after a 45°C heat shock may decrease male mating success, as other stressors do (López-Martínez and Hahn 2014). The end result of this could be a larger number of lower quality males available for reproduction. A potentially larger problem is the negative correlation that exists between temperature and nest size in cavity nesting bees, where small cavities are made at the warmest temperatures (Kamm 1974). In alfalfa leafcutting bees this connection between temperature and size is not fully understood (Rothschild 1979), but males are smaller than females. At Higher temperatures, more males are produced, skewing the sex ratio towards the stress-vulnerable sex. Similar evidence exists from thrips where heat shock treatment in the parents altered the F1 generation's sex ratio (Sun et al. 2019), while in grain aphids the F1 progeny were smaller when the parents experience high temperatures (Jeffs and Leather 2014). This tipping of the sex ratio can have profound implications because the sex ratio in this species plays a delicate role in male courtship attempts and overall female reproductive success. Females complete fewer foraging trips, that tend to be longer in duration, when they are actively and aggressively pursued by males (Rossi et al. 2010). Because of this, offspring production is delayed which ultimately decreases fitness. But this decrease in fitness further exacerbates the potential sex ratio problem because females that travel further for resources produce more sons (Peterson and Roitberg 2006a,(Peterson and Roitberg, 2006b)). This second effect of heat translates into a potential swift in the sex ratio from the heat tolerant sex to the heat vulnerable one, therefore exacerbating the effects of heat on overall fitness. In the context of a warming climate, more males can be produced. These males are more sensitive to heat stress. And the decreased number of females could now face increased unwanted mating attempts by these males. On a broader ecological context, since females visit more plants, than males, to collect leaf pieces for brood cell construction, and unwanted mating aggression can decrease the number of trips, there is a potential for heat stress to reduce insect pollination effectiveness.
The bigger picture we present here is meant to emphasize how a single and short event can derail the performance of an insect for the rest of its life. If we look at males, we see that their final hours of pupal development are delayed at the highest survivable temperature (Fig. 1), but no phenotypic deformities were recorded from emerged males. They may also experience decreases in performance and longevity following that development exposure event; we did not test that specifically here as we tracked longevity of treated adults not immatures. Adult bees experience decreased activity for days following heat shock. This decrease likely affects foraging for resources crucial to overwintering and heat stress recovery. Decreased foraging (i.e., starvation) lowers survival (Fig. 4A) and is worsened by the timing of exposure (afternoon; Fig. 4B), which is ecologically relevant in this, and many pollinator species (Wilson et al. 2020). Nevertheless, the additional protection (López-Martínez and Denlinger 2008) garnered by experiencing stress in the morning still increases mortality (Fig. 4) and decreases longevity (Fig. 5). So, male bees accumulate enough damage from a single event to comprehensively impact their performance. We did not find much in the way of negative effects to female performance, other than decreased activity during recovery, which may be decreased male performance bringing down overall activity as is the case for longevity (Fig. 5). This could be related to the fact that female insects have strong defenses as documented in other insect models (Neuer et al. 2000, Sim and Denlinger 2011). This female effect may be more robust in Hymenopterans because of their haplodiploidy, where females have additional copies of stress tolerant genes and therefore can mount a stronger response as is the case in pesticide resistance (Carrière 2003). Additionally, our current experimental setup may represent some of the worst sublethal effects of heat stress because we applied our treatments during development and early adult emergence. It is possible that the effects might be lower in magnitude if the bees were treated at older ages and there may be a protective effect, or better recovery, associated with multiple bouts of heat shock as has been seen on other stressors like anoxia (Visser et al. 2018).
Our understanding of the relationship between daily cycles and the stress response is still in its infancy, but data continue to accumulate indicating that a connection exists (López-Martínez and Denlinger 2008, Lai et al. 2012, Kock et al. 2017, Xu et al., 2019), and our results agree with previous data from other systems. Here we find that survival under starvation, the type of survival that requires energy reallocation, thus likely some form of a tradeoff, varies depending on the time of day that the insulting event occurred. While the highest temperatures normally occur in the afternoon (López-Martínez and Denlinger 2008, Wilson et al. 2020), bees survive better when treated in the morning. This pattern of morning protection is evident in flies, nematodes, and plants (Simonetta et al. 2008, López-Martínez and Denlinger 2008, Migliori et al. 2011. Grundy et al. 2015). And this protection involves the upregulation of genes required for stress exposure recovery and survival (López-Martínez and Denlinger 2008). Ours bees were not prepared to better survive the afternoon exposure as we had expected and that may be an artifact of lab studies because a similar response was seen in the apple maggot flies, where exposure had to occur in the field to yield the strongest upregulation of heat shock proteins 70 and 90 (López-Martínez and Denlinger 2008). The results from the apple maggot lab vs field study indicate that the strong anticipatory response observed requires prior experience in the form of overnight exposure similarly to that seen in beetles (Bai et al. 2019). It is this prior experience that is missing in our experimental design and the likely reason why afternoon bees did not have higher survival while still having a strong morning response. Whether this daily pattern of stress preparation is connected to the circadian rhythm, or some other regulatory pathway, requires future investigation. It is possible that these bees have strong diurnal defenses similar to the strategy of desert insects; switching activity to morning and evenings to avoid the warmest parts of the day (Cloudsley-Thompson and Constantinou 1985). However, these bees are not active in the evening, having switched their activity towards early morning rather than midday, may have allowed them to have enhanced stress tolerance later in the day due to inducible stress protection (López-Martínez and Denlinger 2008). In species such as Megachile with short seasonal activity (a couple of months in northern North America), the impact of changing temperature can be dramatic and place this species in additional vulnerability (Johansson et al. 2020).
We conclude that a single short exposure to high temperature can have long-lasting consequences affecting multiple life history traits in a sex-specific manner. It is clear from previous work that these effects can vary from species to species and across habitats (Ma et al., 2015a(Ma et al., 2015b)), but the notion that temperature increases linked to climate change can potentially skew the sex ratio of a species affecting its overall fitness is alarming. Our current data continues to add evidence to the idea of daily regulation of stress responses and speaks to the dire need for additional work towards the understanding of daily variation driving anticipatory/preemptive stress responses. Studies looking into the multifarious effects of stress on insect performance are more informative when a wide temporal scope is applied, and our data confirms that looking at multiple times during the life on an individual provides a more accurate representation of the effect of stress. Whenever possible, we expect that transgenerational studies will reveal the effects of parental stress on offspring performance as seen in cactus moth caterpillars (López-Martínez et al. 2016) and other animals (Berry and López-Martínez 2020). Additionally, the impact of sex differences on fitness may be hard to predict because of this duality where males are more vulnerable to stress than females (i.e., at what point does a decrease in male numbers start to affect female reproductive output?) The unforeseen, and understudied, ways that sex-specific responses to high temperature can shift the sex ratio towards the vulnerable sex and the potential increased male courtship attempts, are just two of the indirect effects that stress can have on female fitness which were not measured in our experiments but merit future investigation. Intrinsically physiology is highly regulated, and the continued exploration of how circadian regulation affects the stress response is an area of stress physiology that will surely continue to bear fruit.
Data availability statement
Data for this study have been archived in Dryad and are available: https://doi.org/10.5061/dryad.c59zw3r7p.
Credit author statement
Tayia Hayes and Giancarlo López-Martínez: Conceptualization and design of experiments. Giancarlo López-Martínez: obtained the funding. Tayia Hayes and Giancarlo López-Martínez: carried out the experiments, data analysis, and wrote the manuscript. Tayia Giancarlo López-Martínez: revisions, additional experiments, and editing.
AUTHOR DECLARATION TEMPLATE
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from (giancarlo.lopez@ndsu.edu)
Signed by all authors as follows:
Tayia Hayes 12/30/2020
Giancarlo López-Martínez 12/30/2020
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
TH and GLM conceived the idea and designed the experiments. GLM obtained the funding. TH and GLM carried out the experiments. TH and GLM carried out the data analysis, and TH and GLM wrote the manuscript. Research reported in this publication was supported by
National Science Foundation Office of Integrative Actives RII Track-2 #1826834 and USDA NACA 58-3060-9-025 to GLM. The authors wish to thank multiple anonymous reviewers for making meaningful contributions to the thesis of our manuscript. Lastly, we thank Pollination Nation, where TH was a participant, and the Fargo ICE network for their assistance in the early stage of these experiments and for comments on the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cris.2021.100020.
Appendix. Supplementary materials
References
- Ashburner M., Bonner J.J. The induction of gene activity in Drosophila by heat shock. Cell. 1979;17:241–254. doi: 10.1016/0092-8674(79)90150-8. doi:10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ayyanath M-M., Cutler G.C., Scott-Dupree C.D., Sibley P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLOS ONE. 2013;8:e74532. doi: 10.1371/journal.pone.0074532. https://doi.org/10.1371/journal.pone.0074532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C-M., Ma G., Cai W-Z., Ma C-S. Independent and combined effects of daytime heat stress and night-time recovery determine thermal performance. Biol. Open. 2019;8 doi: 10.1242/bio.038141. doi:10.1242/bio.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo B., Garrity P.A. Temperature sensation in Drosophila. Curr. Opin. Neurobiol. 2015;34:8–13. doi: 10.1016/j.conb.2015.01.002. doi:10.1016/j.conb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthell J.F., Hranitz J.M., Thorp R.W., Shue M.K. High temperature responses in two exotic leafcutting bee species: Megachile apicalis and M. rotundata (Hymenoptera: Megachilidae) Pan-Pac Entomol. 2002;78:235–246. [Google Scholar]
- Berry R., III, López-Martínez G. A dose of experimental hormesis: when mild stress protects and improves animal performance. Comp. Biochem. Phys. A. 2020;242 doi: 10.1016/j.cbpa.2020.110658. https://doi.org/10.1016/j.cbpa.2020.110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenheimer F.S. Studies on the ecology of Palastinean Coleoptera. II. Seasonal and diurnal appearance and activity. Bull. Soc. R. Entomol. Egypte. 1934;17:211–241. https://doi.org/10.1007/BF01426857. [Google Scholar]
- Bradshaw W.E., Holzapfel C.M. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl. Acad. Sci. USA. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J., Bachmann K.A., Bailer A.J., Bolger P.M., Borak J., Cai L., et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol. Appl. Pharm. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. https://doi.org/10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- CaraDonna P.J., Cunningham J.L., Iler A.M. Experimental warming in the field delays phenology and reduces body mass, fat content, and survival: implications for the persistence of a pollinator under climate change. Funct. Ecol. 2018;32:2345–2356. https://doi.org/10.1111/1365-2435.13151. [Google Scholar]
- Carrière Y. Haplodiploidy, sex, and the evolution of pesticide resistance. J. Econ. Entomol. 2003:1626–1640. https://doi.org/10.1603/0022-0493-96.6.1626. [PubMed] [Google Scholar]
- Cervantes L, Lopez-Martinez G. Anoxia hormesis following overwintering diapause boosts bee survivorship and adult performance. Sci. Total Environ. 2022;802 doi: 10.1016/j.scitotenv.2021.149934. [DOI] [PubMed] [Google Scholar]
- Chown S.L., Terblanche J.S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 2006;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. doi: 10.1016/S0065-2806(06)33002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloudsley-Thompson J.L., Constantinou C. Biological clocks in desert beetles (Tenebrionidae) with special reference to Erodius octocostatus Peyerimhof in Kuwait. J. U. Kuwait-Sci. 1985;12:237–242. [Google Scholar]
- Denlinger D.L. Regulation of diapause. Annu. Rev. Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Dowling D.K., Simmons L.W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton-Calabria A., Demary K.C., Oner N.J. Beyond pollination: Honey Bees (Apis mellifera) as zootherapy keystone species. Front. Ecol. Evol. 2019;6:161. https://doi.org/10.3389/fevo.2018.00161. [Google Scholar]
- Feder M.E., Hofmann G.E. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feller P., Nachtigall W. Flight of the honeybee. II. Inner and surface thorax temperatures and energetic criteria, correlated to flight parameters. J. Comp. Physiol. B. 1989;158:719–727. https://doi.org/10.1007/BF00693010. [Google Scholar]
- González-Tokman D., Córdoba-Aguilar A., Dáttilo W., Lira-Noriega A., Sánchez-Guillén R.S., Villalobos F. Insect responses to heat: physiological mechanisms, Evolution and ecological implications in a warming world. Biol. Rev. 2020;95:802–821. doi: 10.1111/brv.12588. https://doi-org.ezproxy.lib.ndsu.nodak.edu/10.1111/brv.12588. [DOI] [PubMed] [Google Scholar]
- Grundy J., Stoker C., Carré I.A. Circadian regulation of abiotic stress tolerance in plants, Front. Plant Sci. 2015;6:648. doi: 10.3389/fpls.2015.00648. doi: 10.3389/fpls.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley N.F. Academic Press; California: 1994. Water Relations of Terrestrial Arthropods. first ed. [Google Scholar]
- Haeler E., Fiedler K., Grill A. What prolongs a butterfly's life?: Trade-offs between dormancy, fecundity, and body size. PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0111955. doi:10.1371/journal.pone.0111955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. Springer-Verlag; Berlin: 1993. The Hot-blooded Insects: Strategies and Mechanisms of Thermoregulation. first ed. [Google Scholar]
- Ho D.H., Burggren W.W. Epigenetics and transgenerational transfer: a physiological perspective. J. Exp. Biol. 2010;213:3–16. doi: 10.1242/jeb.019752. doi: 10.1242/jeb.019752. [DOI] [PubMed] [Google Scholar]
- Jeffs C.T., Leather S.R. Effects of extreme, fluctuating temperature events on life history traits of the grain aphid, Sitobion avenae. Entomol. Exp. Appl. 2014;150:240–249. doi 10.1111/eea.12160. [Google Scholar]
- Johansson F., Orizaola G., Nilsson-Örtman V. Temperate insects with narrow seasonal activity periods can be as vulnerable to climate change as tropical insect species. Sci. Rep. 2020;10:8822. doi: 10.1038/s41598-020-65608-7. https://doi.org/10.1038/s41598-020-65608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm D.R. Effects of temperature, day length, and number of adults on the sizes of cells and offspring in a primitively social bee (Hymenoptera: Halictidae) J. Kansas Entomol. Soc. 1974;47:8–18. [Google Scholar]
- King A.M., MacRae T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- Klostermeyer E.C., Gerber H.S. Nesting behavior of Megachile rotundata (Hymenoptera: Megachilidae) monitored with an event recorder. Ann. Entomol. Soc. Am. 1969;62:1321–1326. https://doi.org/10.1093/aesa/62.6.1321. [Google Scholar]
- Klostermeyer E.C., Mech S.J., Jr., Rasmussen W.B. Sex and weight of Megachile rotundata (Hymenoptera: Megachilidae) progeny associated with provision weights. J. Kansas Entomol. Soc. 1973;46:536–548. [Google Scholar]
- Koch C.E., Leinweber B., Drengberg B.C., Blaum C., Oster H. Interaction between circadian rhythms and stress. Neurobiol. Stress. 2017;6:57–67. doi: 10.1016/j.ynstr.2016.09.001. http://dx.doi.org/10.1016/j.ynstr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T.N., Loeschcke V., Tan Q., Pertoldi C., Mengel-From J. Sex and age specific reduction on stress resistance and mitochondrial DNA copy number in Drosophila melanogaster. Sci. Rep. 2019;9:12305. doi: 10.1038/s41598-019-48752-7. https://doi.org/10.1038/s41598-019-48752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A.G., Doherty C.J., Mueller-Roeber B., Kay S.A., Schippers J.H.M., Dijkwel P.P. Circadian clock-associated 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17129–17134. doi: 10.1073/pnas.1209148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E.L., Tinghitella R.M., Taylor S.A. Insect hybridization and climate change. Front. Ecol. Evol. 2019;7:348. https://doi.org/10.3389/fevo.2019.00348. [Google Scholar]
- Lin Y.J., Seroude L., Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- López-Martínez G., Denlinger D.L. Regulation of heat shock proteins in the apple maggot Rhagoletis pomonella during hot summer days and overwintering diapause. Physiol. Ent. 2008;33:346–352. https://doi.org/10.1111/j.1365-3032.2008.00639.x. [Google Scholar]
- López-Martínez G., Benoit J.B., Rinehart J.P., Elnitsky M.A., Lee R.E., Jr., Denlinger D.L. Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. J. Comp. Physiol. B. 2009;179:481–491. doi: 10.1007/s00360-008-0334-0. doi: 10.1007/s00360-008-0334-0. [DOI] [PubMed] [Google Scholar]
- López-Martínez G., Hahn D.A. Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Biol. 2012;215:2150–2161. doi: 10.1242/jeb.065631. doi: 10.1242/jeb.065631. [DOI] [PubMed] [Google Scholar]
- López-Martínez G., Hahn D.A. Early life hormetic treatments decrease irradiation-induced oxidative damage, increase longevity, and enhance sexual performance during old age in the Caribbean fruit fly. PLOS ONE. 2014;9:e88128. doi: 10.1371/journal.pone.0088128. https://doi.org/10.1371/journal.pone.0088128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martínez G., Carpenter J.E., Hight S.D., Hahn D.A. Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. J. Econ. Ent. 2014;107:185–197. doi: 10.1603/ec13370. https://doi.org/10.1603/EC13370. [DOI] [PubMed] [Google Scholar]
- López-Martínez G., Carpenter J.E., Hight S.D., Hahn D.A. Anoxia-conditioning hormesis alters the relationship between irradiation doses for survival and sterility in the cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae) Fla. Entomol. 2016;99:95–104. https://doi.org/10.1653/024.099.sp113. [Google Scholar]
- López-Martínez G., Meagher R.L., Jeffers L.A., Bailey W.D., Hahn D.A. Low oxygen atmosphere enhances post-irradiation survival of Trichoplusia Ni (Lepidoptera: Noctuidae) Fla. Entomol. 2016;99:24–33. https://journals.flvc.org/flaent/article/view/88670. [Google Scholar]
- Ma G., Hoffmann A.A., Ma C-S. Daily temperature extremes play an important role in predicting thermal effects. J. Exp. Biol. 2015;218:2289–2296. doi: 10.1242/jeb.122127. doi:10.1242/jeb.122127. [DOI] [PubMed] [Google Scholar]
- Ma G., Rudolf V.H.W., Ma C-S. Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob. Change Biol. 2015;21:1794–1808. doi: 10.1111/gcb.12654. doi: 10.1111/gcb.12654. [DOI] [PubMed] [Google Scholar]
- Maeta Y., Adachi K. Nesting behaviors of the alfalfa leaf-cutting bee, Megachile (Eutricharaea) rotundata (Fabricius) (Hymenoptera, Megachilidae) J. Melittology. 2005;18:5–21. https://doi.org/10.17161/jom.v0i69.6532. [Google Scholar]
- Margotta J.W., Roberts S.P., Elekonich M.M. Effects of flights activity and age on oxidative damage in the honey bee, Apis mellifera. J. Exp. Bio. 2018:221. doi: 10.1242/jeb.183228. doi: 10.1242/jeb.183228. [DOI] [PubMed] [Google Scholar]
- Marshall K.E., Gotthard K., Williams C.M. Evolutionary impacts of winter climate change on insects. Curr. Opin. Insect Sci. 2020;41:54–62. doi: 10.1016/j.cois.2020.06.003. https://doi.org/10.1016/j.cois.2020.06.003. [DOI] [PubMed] [Google Scholar]
- Matzkin L.M., Watts T.D., Markow T.A. Evolution of stress resistance in Drosophila: interspecific variation in tolerance to desiccation and starvation. Funct. Ecol. 2009;23:521–527. doi: 10.1111/j.1365-2435.2008.01533.x. [Google Scholar]
- May M.L. Insect Thermoregulation. Ann. Rev. Entomol. 1979;24:313–349. https://doi.org/10.1146/annurev.en.24.010179.001525. [Google Scholar]
- Michaud M.R., Denlinger D.L. Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J. Comp. Physiol. B. 2007;177:753–763. doi: 10.1007/s00360-007-0172-5. doi: 10.1007/s00360-007-0172-5. [DOI] [PubMed] [Google Scholar]
- Migliori M.L., Simonetta S.H., Romanowski A., Golombek D.A. Circadian rhythms in metabolic variables in Caenorhabditis elegans. Physiol. Behav. 2011;103:315–320. doi: 10.1016/j.physbeh.2011.01.026. doi: 10.1016/j.physbeh.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Nestel D., Nemny-Lavy E., Islam S.M., Wornoayporn V., Caceres C. Effects of pre-irradiation conditioning of medfly pupae (Diptera: Tephritidae): hypoxia and quality of sterile males. Fla. Entomol. 2007;90:80–87. https://doi.org/10.1653/0015-4040(2007)90[80:EOPCOM]2.0.CO;2. [Google Scholar]
- Neuer A., Spandorfer S.D., Giraldo P., Dieterle S., Rosenwaks Z., Witkin S.S. The role of heat shock proteins in reproduction. Hum. Reprod. Update. 2000;6:149–159. doi: 10.1093/humupd/6.2.149. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- Niveditha S., Deepashree S., Ramesh S.R., Shivanandappa T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B. 2017;187:899–909. doi: 10.1007/s00360-017-1061-1. https://doi.org/10.1007/s00360-017-1061-1. [DOI] [PubMed] [Google Scholar]
- Ohinata K., Ashraf M., Harris E.J. Mediterranean fruit flies: sterility and sexual competitiveness in the laboratory after treatment with gamma irradiation in air, carbon dioxide, helium, nitrogen, or partial vacuum. J. Econ. Entomol. 1979;70:165–168. doi: 10.1093/jee/70.2.165. doi: 10.1093/jee/70.2.165. [DOI] [PubMed] [Google Scholar]
- Parsell D.A., Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Peterson J.H., Roitberg B.D. Impacts of flight distance on sex ratio and resource allocation to offspring in the leafcutter bee, Megachile rotundata. Behav. Ecol. Sociobiol. 2006;59:589–596. https://doi.org/10.1007/s00265-005-0085-9. [Google Scholar]
- Peterson J.H., Roitberg B.D. Impact of resource levels on sex ratio and resource allocation in the solitary bee, Megachile rotundata. Environ. Entomol. 2006;35:1404–1410. https://doi.org/10.1093/ee/35.5.1404. [Google Scholar]
- Pitts-Singer T.L., James R.R. Emergence success and sex ratio of commercial alfalfa leafcutting bees from the United States and Canada. J. Econ. Entomol. 2005;98:1785–1790. doi: 10.1603/0022-0493-98.6.1785. https://doi.org/10.1093/jee/98.6.1785. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T.L., Cane J.H. The alfalfa leafcutting bee, Megachile rotundata: the world's most intensively managed solitary bee. Annu. Rev. Entomol. 2011;56:221–237. doi: 10.1146/annurev-ento-120709-144836. doi: 10.1146/annurev-ento-120709-144836. [DOI] [PubMed] [Google Scholar]
- Prange H.D. Evaporative cooling in insects. J. Insect Physiol. 1996;42:493–499. https://doi.org/10.1016/0022-1910(95)00126-3. [Google Scholar]
- Ragland G.J., Egan S.P., Feder J.L., Berlocher S.H., Hahn D.A. Developmental trajectories of gene expression reveal candidates for diapause termination: a key life-history transition in the apple maggot fly Rhagoletis pomonella. j. Exp. Bio. 2011;214:3948–3960. doi: 10.1242/jeb.061085. doi: 10.1242/jeb.061085. [DOI] [PubMed] [Google Scholar]
- Richards K.W., Whitfield G.H. Emergence and survival of leafcutter bees, Megachile rotundata, held at constant incubation temperatures (Hymenoptera: Megachilidae) J Apicult. Res. 1988;27(3) doi: 10.1080/00218839.1988.11100802. [DOI] [Google Scholar]
- Rinehart J.P., Hayward S.A.L., Elnitsky M.A., Sandro L.H., Lee R.E., Jr., Denlinger D.L. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc. Natl. Acad. Sci. USA. 2006;103:14223–14227. doi: 10.1073/pnas.0606840103. doi: 10.1073/pnas.0606840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J.P., R.M Robich, Denlinger D.L. Isolation of diapause-regulated genes from the flesh fly, Sarcophaga crassipalpis by suppressive subtractive hybridization. J. Insect Physiol. 2010;56:603–609. doi: 10.1016/j.jinsphys.2009.12.007. doi: 10.1016/j.jinsphys.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rossi B.H., Nonacs P., Pitts-Singer T.L. Sexual harassment by males reduces female fecundity in the alfalfa leafcutting bee, Megachile rotundata. Anim. Behav. 2010;79:165–171. https://doi.org/10.1016/j.anbehav.2009.10.023. [Google Scholar]
- Robich R.M., Rinehart J.P., Kitchen L.J., Denlinger D.L. Diapause-specific gene expression in the northern house mosquito, Culex pipiens L., identified by suppressive subtractive hybridization. J. Insect Physiol. 2007;53:235–245. doi: 10.1016/j.jinsphys.2006.08.008. doi: 10.1016/j.jinsphys.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild M. Factors influencing size and sex ratios of Megachile rotundata (Hymenoptera: Megachilidae) J. Kansas Entomol. Soc. 1979;52:392–401. [Google Scholar]
- Sim C., Denlinger D.L. Catalase and superoxide dismutase-2 enhance survival and protect ovaries during overwintering diapause in the mosquito Culex pipiens. J. Insect Physiol. 2011;57:628–634. doi: 10.1016/j.jinsphys.2011.01.012. doi: 10.1016/j.jinsphys.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta S.H., Romanowski A., Minniti A.N., Inestrosa N.C., Golombek D.A. Circadian stress tolerance in adult Caenorhabditis elegans. J. Comp. Physiol. A. 2008;194:821–828. doi: 10.1007/s00359-008-0353-z. doi: 10.1007/s00359-008-0353-z. [DOI] [PubMed] [Google Scholar]
- Sinclair B.J., Ferguson L.V., Salehipour-Shirazi G., MacMillan H.A. Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 2013;53:545–556. doi: 10.1093/icb/ict004. doi:10.1093/icb/ict004. [DOI] [PubMed] [Google Scholar]
- Sun L.J., Ma Y.B., Li H.G., Zheng C.Y. The maternal effects of heat shock on biological parameters and ovaries of Frankliniella occidentalis (Thysanoptera: Thripidae) Eur. J. Entomol. 2019;116:212–220. doi: 10.14411/eje.2019.023. [Google Scholar]
- Tepedino V.J., Parker F.D. Effect of rearing temperature on mortality, second-generation emergence, and size of adult in Megachile rotundata (Hymenoptera: Megachilidae) J Econ. Entomol. 1986;79:974–977. doi: 10.1093/jee/79.4.974. [DOI] [Google Scholar]
- Visser B., Williams C.M., Hahn D.A., Short C.A., López-Martínez G. Hormetic benefits of prior anoxia exposure in buffering anoxia stress in a soil-pupating insect. J. Exp. Biol. 2018:221. doi: 10.1242/jeb.167825. https://doi.org/10.1242/jeb.167825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.M., Henry H.A.L., Sinclair B.J. Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol. Rev. 2014:90. doi: 10.1111/brv.12105. doi: https://doi.org/10.1111/brv.12105. [DOI] [PubMed] [Google Scholar]
- Wilson E.S., Murphy C.E., Rinehart J.P., Yocum G., Bowsher J.H. Microclimate temperatures impact nesting preference in Megachile rotundata (Hymenoptera: Megachilidae) Environ. Entomol. 2020;49:296–303. doi: 10.1093/ee/nvaa012. https://doi.org/10.1093/ee/nvaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Kula-Eversole E., Iwanaszko M., Hutchison A.L., Dinner A., Allada R. Circadian clocks function in concert with heat shock organization protein to modulate mutant huntingtin aggregation and toxicity. Cell Rep. 2019;27:59–70. doi: 10.1016/j.celrep.2019.03.015. https://doi.org/10.1016/j.celrep.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study have been archived in Dryad and are available: https://doi.org/10.5061/dryad.c59zw3r7p.