Abstract
Background:
Unintentional drug overdoses have reached epidemic levels in the U.S. This study tests the hypothesis that people who have used non-prescribed buprenorphine more frequently in the past six months were less likely to experience a drug overdose during that same time period.
Methods:
Participants age 18 years or older with opioid use disorder who reported use of non-prescribed buprenorphine in the last six months were recruited from the Dayton, Ohio, area using a combination of targeted and modified respondent-driven sampling. Participants completed a structured interview, including six-month timeline follow-back, after informed consent. Logistic regression was used to test the association between (log-transformed) frequency of non-prescribed buprenorphine use and overdose in the previous six months, adjusted for confounding due to sex, homelessness, incarceration, substance use treatment, previous overdose, heroin/fentanyl injection, psychiatric comorbidity, and (log-transformed) frequencies of other (non-opioid) drug use.
Results:
Almost 89% of 356 participants were white, 50.3% were male, and 78.1% had high school or greater education. Over 27% (n=98) reported experiencing an overdose in the past six months. After adjusting for confounding, greater frequency of non-prescribed buprenorphine use was significantly associated with lower risk of overdose (AOR=0.81, 95% CI=0.66, 0.98; p=.0286). Experiencing an overdose more than six months ago (AOR=2.19, 95% CI=1.24, 3.97); injection as the most common route of administration of heroin/fentanyl (AOR=2.49, 95% CI=1.36, 4.71); and frequency of methamphetamine use (AOR=1.13, 95% CI=1.02, 1.27) were strongly associated with increased risk of recent overdose in multivariable analysis.
Discussion:
The findings support our hypothesis that higher frequency of non-prescribed buprenorphine use is associated with lower risk of drug overdose, a potential harm reduction consequence of diversion. Improving the availability of buprenorphine though standard substance use disorder treatment, primary care, and other innovative methods is urgently needed.
Keywords: Unintentional Drug Overdose, Buprenorphine Diversion, Heroin, Non-Pharmaceutical Fentanyl
Introduction
The opioid epidemic in the U.S. shows little signs of diminishing, with unintentional overdose deaths reaching unsurpassed levels, although a small decrease is indicated in 2018 (mainly due to decreases in overdoses in which prescription opioids were involved) (CDCNVSS, 2019; Gladden, O’Donnell, Mattson, & Seth, 2018; Goodnough, Katz, & Sangor-Katz, 2019; Hedegaard, Minino, & Warner, 2018; Rudd, Aleshire, Zibbell, & Gladden, 2016). Drug overdose deaths increased from 47,055 people in 2014 to 70,237 people in 2017 (Hedegaard et al., 2018; Rudd et al., 2016). The recent escalation in the number of unintentional overdose deaths has been linked to the increased presence of non-pharmaceutical fentanyl and its analogues, such as carfentanil, acryl fentanyl, and others (Centers for Disease Control and Prevention, 2015; Ciccarone, 2017; Jones, Einstein, & Compton, 2018; O’Donnell, Gladden, Mattson, & Kariisa, 2018).
A range of risk factors for opioid overdose have been identified. One of the strongest correlates of overdose risk is having experienced a previous overdose (Coffin et al., 2007; Najman, Mcllwraith, Kemp, & Smirnov, 2017; Stoove, Dietze, & Jolley, 2009). Reduced tolerance, as a result of release after forced abstinence resulting from incarceration and abstinence resulting from substance abuse treatment, including detoxification, have also been associated with increased risk of overdose (Binswanger et al., 2007; Kinner et al., 2012; Leach & Oliver, 2011; Murphy et al., 2018; Strang et al., 2003; Wines, Saitz, Horton, Loyd-Travaglini, & Samet, 2007; Maughan and Becker, 2019). Quantity of opioids consumed (Rowe, Wheeler, Vittinghoff, Santos, & Behar, 2018), changes in heroin purity (Darke, Hall, Weatherburn, & Lind, 1999), increased availability of non-prescribed fentanyl analogues with varying levels of potency (Ciccarone, Ondocsin, & Mars, 2017; Daniulaityte et al., 2019-a; Fairborn, Coffin, & Walley, 2018), and injection route of administration increase risk of overdose (Brugal et al., 2002). Combining multiple CNS depressants (Coffin et al., 2003), such as alcohol (Ruttenber & Luke, 1984), use of non-prescribed benzodiazepines (McClure, Niles, Kaufman, & Gudin, 2017; Zedler et al., 2015) and prescribed tranquilizers (Silva, Schrager, Kecojevic, & Lankenau, 2013) have been associated with increased risk of overdose. Finally, use of stimulants, including cocaine and methamphetamine, often in combination with heroin (Brugal et al., 2002; Coffin et al., 2003; Jalal et al., 2018; Kinner et al., 2012; Ochoa et al., 2005; Warner, Trinidad, Bastian, Minino, & Hedegaard, 2016; Werb et al., 2008) have also been associated with increased overdose risk.
Three primary public health interventions have been used to address the increase in overdose deaths, including wide-scale distribution of naloxone to reverse overdose (Bennett, Holloway, & Bird, 2014; Centers for Disease Control and Prevention, 2012; Kinner et al., 2012), and substance use disorder treatment, most often involving the use of medication assisted therapy, including methadone, naltrexone, naltrexone injectable suspension, and buprenorphine (Amato et al., 2005; Degenhardt et al., 2010; C. Jones et al., 2018; Mattick, Breen, Kimber, & Davoli, 2014; Soyka et al., 2011). Supervised injection facilities, although not yet widely adopted in the U.S., are also potentially important public health interventions to address overdose deaths (Irvine, et al., 2019; Tyndall, 2006; Marshall, Milloy, Wood, Montaner, Kerr, 2011).
Approved for treating opioid use disorder in 2002 in the United States, buprenorphine products have filled an exceptionally important role in responding to the opioid epidemics (Yokell, Zaller, Green, & Rich, 2011b). Significantly, buprenorphine was approved for use by private physicians after completing training in its use, thus providing a potentially new means of treating opioid use disorder in primary care settings (Amass et al., 2004; Hser et al., 2015; Johnson et al., 1995; Saloner, Stoller, Alexander, 2018; Zoorob, Kowalchuk, & Mejia de Grubb, 2018).
Not surprisingly, and similar to other pharmaceutical opioids, diversion of buprenorphine products in communities of people who use illicit drugs has become of some concern both in the U.S. (Bazazi, Yokell, Fu, Rich, & Zaller, 2011; Daniulaityte, Falck, & Carlson, 2012; Furst, 2013, 2014; Johanson, Arfken, di Menza, & Schuster, 2012; Kenney, Anderson, Bailey, & Stein, 2017; Wish et al., 2012) and in many other countries (Carrieri et al., 2006; Lofwall & Walsh, 2014; Maria Patrizia et al., 2006; Winstock, Lea, & Sheridan, 2008; Yokell, Zaller, Green, & Rich, 2011a). In the U.S., there are reports that some patients are disregarding physician’s directions for the intended use of the drug, using it to make money, often to buy and use heroin and/or other illicit drugs (Lin, Lofwall, Walsh, Gordon, & Knudsen, 2018); others who have prescriptions give buprenorphine to people to help them avoid withdrawal symptoms (Havnes, Clausen, & Middelthon, 2013).
In contrast to the negative sequelae associated with the non-prescribed use of buprenorphine, it has also been suggested that diversion is a sign of the inability of people who use opioids to access buprenorphine treatment or barriers to participation in treatment (Carroll, Rich, & Green, 2018; Cicero, Ellis, & Chilcoat, 2018; Lofwall & Havens, 2012; McLean & Kavanaugh 2019). As such, the use of non-prescribed buprenorphine products may have some unintended public health benefits, such as not (generally) using other opioids when using buprenorphine, perhaps as a way to bridge waiting time to get into treatment without using other opioids, and even, more importantly, as a means to achieve some form of self-recovery, for some degree of time (Allen & Harocopos, 2016; Mitchell et al., 2009; Monico et al., 2015). Other harm-reduction benefits may also exist.
There is a dearth of research examining the relationship between use of non-prescribed buprenorphine use and unintentional drug overdose (Bretteville-Jensen, Lillehagen, Gjersing, & Andreas, 2015). In the current study, we test the hypothesis that, among individuals with opioid use disorder who have used non-prescribed buprenorphine in the previous six months, those who use non-prescribed buprenorphine more frequently are less likely to experience an unintentional drug overdose. Variables controlled for in the analysis were selected based on previous research as cited above.
Research setting
The study was conducted in the Dayton (Montgomery County), Ohio, area, an epicenter of the opioid overdose crisis. In 2016, Ohio had one of the highest overdose death rates (39.1) per 100,000 in the nation (Hedegaard et al., 2018), and in 2017, Montgomery County had the highest per capita overdose death rate in the state of Ohio, with 521 overdoses, and an age-adjusted death rate of 95.24 per 100,000 (Rossen, Bastian, Warner, Khan, & Chong, 2019). Our study on trends in non-prescribed fentanyl-related overdose deaths in Montgomery County, Ohio, found that overdose deaths involving non-prescribed fentanyl increased 377% from the second half of 2015 to the first half of 2017 (Daniulaityte et al., 2019a). Over that same time period, we found a high presence of fentanyl and variety of fentanyl analogues. Over 75% of all non-prescribed fentanyl positive cases tested positive for fentanyl analogues in the first six months of 2017 (Daniulaityte et al., 2019a).
Methods
Subject recruitment
Targeted recruitment (Korf, van Ginkel, & Benschop, 2010; Sifaneck & Neaigus, 2001) and modified respondent-driven sampling (RDS) (Biernacki & Waldorf, 1981; Daniulaityte, Falck, Li, Nahhas, & Carlson, 2012; Heckathorn, 1997) methods were used to recruit people in the community who met the following eligibility criteria: 1) at least 18 years of age; 2) current moderate or severe opioid use disorder 3) report use of non-prescribed buprenorphine at least once in the past six months; and 4) residence in the Dayton, Ohio, metropolitan area, including surrounding counties. Recruitment began May 2017 and ended October 2018. Initial sample seeds were identified by recruitment fliers posted in locations such as laundromats, pawn shops, gas stations, and convenience store, and ads on Craigslist, Facebook, and a local paper. Modifications to RDS included having a flexible limit on the number of referrals to improve recruitment. Participants were compensated $15 for each person he/she referred to the project.
A two-phase process was used to determine eligibility. In Phase I, participants were briefly screened for eligibility by phone. In Phase II, those who were potentially eligible were scheduled for an office interview in which they were screened in more detail after completing an informed consent approved by the Wright State University and Columbia University Institutional Review Boards. If eligible, participants completed a baseline structured interview, lasting 1.5–2 hours, administered by trained interviewers in private offices. Participants were compensated $40 for their time participating in research, and $10 for transportation, by gift card or check. Urine screening was used to enhance the veracity of self-report. Further details regarding recruitment and data collection are available elsewhere (Daniulaityte et al., 2019b).
Variables
Sociodemographic and drug use characteristics were captured using questions developed by the research team, some of which had been used in previous studies (Carlson, Wang, Falck, & Siegal, 2005; Falck, Siegal, Wang, Carlson, & Draus, 2005). Questions covered the following domains: 1) Time since first use of illicit opioids (“How old were you the very first time you used [drug]”); 2) Mode of administration (“In the past six months, how did you most often administer [drug]”); and 3) Lifetime history of unintentional drug overdose (“How many times in your life have you experienced an unintentional drug-related overdose?”).
Past twelve-month psychiatric comorbidity, including post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), and major depressive disorder (MDD) were assessed by using the computerized version of the Diagnostic Interview Schedule-V (Compton, Dawson, Goldstein, & Grant, 2013). Severity of lifetime opioid use disorder was assessed using the DSM-IV Checklist, modified for DSM-V (Forman, Svikis, Montoya, & Blaine, 2004; Hudziak et al., 1993).
The time-line follow-back method was used to assess frequency of non-prescribed buprenorphine use, heroin/non-prescribed fentanyl use, cocaine/crack use, methamphetamine use, prescribed and non-prescribed pharmaceutical opioids (excluding illicit buprenorphine), non-prescribed benzodiazepine use, past six-month drug overdose (“did you overdose?”), incarceration, and use of substance use disorder treatment services in the past six months (Ehrman & Robbins, 1994; Epstein-Ngo et al., 2013; Robinson, Sobell, Sobell, & Leo, 2012). Due to the difficulty of participants to distinguish between heroin and fentanyl, these drugs were combined into one category (Daniulaityte et al., 2019b). Data were captured by interviewers who entered responses on laptop computers using REDCap, an electronic data capture system maintained by Wright State University (Harris et al., 2009). The variable “previous overdose” was derived by comparing the number of overdoses reported in the previous six months with the lifetime number of unintentional drug overdoses reported; if the latter was greater, then we concluded the participant had an overdose experience more than six months prior to the interview.
Statistical analysis
Frequencies and proportions were computed for all categorical variables, and means, standard deviations, and medians for all continuous variables, overall and by past six-month overdose status.
Logistic regression was used to test the association between (log-transformed) frequency of non-prescribed buprenorphine use and overdose in the previous six months, adjusted for confounding due to the following covariates: sex, homelessness, incarceration, substance use treatment, previous overdose, heroin/fentanyl injection, psychiatric comorbidity (PTSD, GAD, or MDD), and (log-transformed) frequencies of use of cocaine/crack, methamphetamine, and non-prescribed benzodiazepines. This test was two-sided and carried out at the α = 0.05 level of significance. Data processing was carried out in SAS 9.4 (SAS Institute Inc, 2012) and data analysis was carried out in R 3.6.2 (R Core Team, 2019).
We chose the covariates that we considered a priori, based on previous research, to have the most potential for confounding the relationship between exposure (non-prescribed buprenorphine use) and outcome (overdose), without respect to their relationship with the outcome. Frequency of use of other opioids were not included due to their potential role as mediators of the non-prescribed buprenorphine-overdose relationship. While there was additional covariate information we could have included, we limited this list to approximately the number of events divided by 10 to assure reliable parameter estimates (Harrell, 2015). Other methods of variable selection, such as stepwise selection, were not used due to their leading to invalid p-values (Ferenci, 2017; Harrell, 2015; Rothman, Greenland, and Lash, 2008).
Both univariate and adjusted (for all other variables) odds ratios (OR, AOR) and their 95% confidence intervals are presented for all variables listed above. Since our primary analysis (association between non-prescribed buprenorphine use and overdose) consisted of a single test, we did not carry out an adjustment for multiple testing. While 95% confidence intervals and p-values are reported for all variables, only the primary analysis can be considered confirmatory; tests of association for all other variables are considered secondary (exploratory) analyses.
Some variables (listed in Table 3) were skewed and were therefore natural-log transformed to reduce the influence of large values; other than non-prescribed buprenorphine use, which had no zeros, 0.001 was added to each variable prior to transformation.
Table 3.
Correlates of overdose (yes/no) in the previous 6 months (98 of 356 (27.5%) individuals overdosed, 97 of 345 (28.2%) for the multivariable model; OR = unadjusted odds ratio; AOR = adjusted odds ratio, all variables in the model simultaneously.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Correlate (# of missing values, if any) | OR | 95% CI | AOR | 95% CI |
| Primary Exposure | ||||
| NP buprenorphine frequency of use (LN of % of days, past 6m)a | 0.82 | (0.70, 0.97) | 0.81 | (0.66, 0.98) |
| Confounders | ||||
| Sex = Male | 1.10 | (0.69, 1.76) | 1.18 | (0.70, 2.00) |
| Homeless (past 6m) | 1.53 | (0.95, 2.47) | 0.94 | (0.54, 1.63) |
| Incarceration (past 6m) | 2.23 | (1.37, 3.63) | 1.65 | (0.96, 2.82) |
| Substance use treatment (past 6m) | 1.53 | (0.96, 2.45) | 1.22 | (0.72, 2.07) |
| Previous overdose (more than 6m ago) (1) | 2.62 | (1.55, 4.55) | 2.19 | (1.24, 3.97) |
| Heroin or fentanyl injection (most common route, past 6m) (10) | 3.16 | (1.82, 5.72) | 2.49 | (1.36, 4.71) |
| Psychiatric comorbidity (past 12m) | 1.56 | (0.96, 2.55) | 1.62 | (0.94, 2.84) |
| Cocaine/crack frequency of use (LN of % of days + 0.001, past 6m)a | 1.13 | (1.02, 1.25) | 1.05 | (0.93, 1.18) |
| Methamphetamine frequency of use (LN of % of days + 0.001, past 6m)a | 1.21 | (1.10, 1.34) | 1.13 | (1.02, 1.27) |
| NP benzodiazepine frequency of use (LN of % of days + 0.001, past 6m)a | 1.05 | (0.96, 1.16) | 1.05 | (0.94, 1.18) |
These variables were skewed and were therefore natural-log transformed to reduce the influence of large values. Other than NP buprenorphine, which had no zeros, 0.001 was added to each variable prior to transformation.
Since the primary exposure was log-transformed, for explanatory purposes, we carried out the following two secondary analyses to estimate odds ratios that are more easily interpretable: 1) We dichotomized the exposure at the median value (use of non-prescribed buprenorphine on 5.4% of days (10 of 184) in the previous six months) and computed the AOR in the multivariable model for this binary exposure instead of the continuous version; 2) We computed AORs from the primary analysis over a range of exposure values on the original scale. Since this assumes linearity, we also checked the linearity assumption by cutting the exposure into six categories based on equal-length intervals on the log-scale (these correspond to, on the original scale, less than 0.7% (1 day in 184), 0.7% to 1.8% (2–3 days), 1.9% to 5.0% (4–9 days), (5.1% to 13.5% (10–24 days), 13.6% to 36.8% (25–67 days), and 36.9% or more (68 days or more) and computed the corresponding AORs relative to the first group (1 day of use). The resulting AORs and 95% confidence intervals are illustrated, along with the corresponding estimates from the primary analysis.
We hypothesize that at least part of the mechanism by which increased use of non-prescribed buprenorphine lowers risk of overdose is through a corresponding decrease in use of heroin and/or fentanyl. While a full mediation analysis is beyond the scope of this paper, as a secondary analysis to begin to investigate this hypothesis, we used linear regression to test the association between (log-transformed) frequency of use of non-prescribed buprenorphine and (log-transformed) frequency of use of heroin/fentanyl, and logistic regression to test the association between (log-transformed) frequency of use of heroin/fentanyl and overdose.
Results
Sample characteristics
Overall, 50.3% of the sample of 356 participants were male, almost 89% were non-Hispanic white, and only 23% were married or living with a partner. About 78% had a secondary school/GED or higher education, approximately one-fourth (25.8%) were employed part or full-time, and about half (54.8%) considered themselves as being homeless in the past six months (Table 1). The mean age was 39.2 years (Table 2).
Table 1.
Descriptive statistics for categorical variables, overall and by overdose in the previous 6 months (n=356)
| Overall (n=356) | Did not overdose (n=258, 72.5%) | Overdosed (n=98, 27.5%) | ||||
|---|---|---|---|---|---|---|
| Characteristic (# of missing values, if any) | N | % | N | % | N | % |
| Sex = Male | 179 | 50.3 | 128 | 49.6 | 51 | 52.0 |
| Race/Ethnicity = White/Non-Hispanic | 316 | 88.8 | 226 | 87.6 | 90 | 91.8 |
| Education = Secondary school or greater | 278 | 78.1 | 197 | 76.4 | 81 | 82.7 |
| Employed | 92 | 25.8 | 67 | 26.0 | 25 | 25.5 |
| Married/living with partner | 82 | 23.0 | 61 | 23.6 | 21 | 21.4 |
| Homeless (past 6m) | 195 | 54.8 | 134 | 51.9 | 61 | 62.2 |
| Incarceration (past 6m) | 110 | 30.9 | 67 | 26.0 | 43 | 43.9 |
| Substance use treatment (ever past 6m) | 180 | 50.6 | 123 | 47.7 | 57 | 58.2 |
| Previous overdose (more than 6m ago) (1) | 221 | 62.3 | 146 | 56.6 | 75 | 77.3 |
| Heroin or fentanyl injection (most common route, past 6m) (10) | 225 | 65.0 | 145 | 58.5 | 80 | 81.6 |
| Psychiatric comorbidity (GAD, MDD, or PTSD) past 12m) | 209 | 58.7 | 144 | 55.8 | 65 | 66.3 |
Table 2.
Descriptive statistics for continuous variables, overall and by overdose in the previous 6 months (n=356)
| Overall (n=356) | Did not overdose (n=258, 72.5%) | Overdosed (n=98, 27.5%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median |
| Age (years) | 39.2 | 9.5 | 37.8 | 39.4 | 10.0 | 38.3 | 38.5 | 8.2 | 37.0 |
| Time since first illicit use of opioids (years) | 18.5 | 9.6 | 17.3 | 18.4 | 10.1 | 17.1 | 18.5 | 8.2 | 18.3 |
| NP buprenorphine frequency of use (% of days, past 6m) | 14.6 | 20.4 | 5.4 | 16.4 | 22.3 | 6.8 | 10.0 | 13.5 | 4.3 |
| Heroin/fentanyl frequency of use (% of days, past 6m) | 56.4 | 36.5 | 67.4 | 52.5 | 37.9 | 60.1 | 66.6 | 30.8 | 76.1 |
| Prescribed pain pill frequency of use (% of days, past 6m) | 2.7 | 10.3 | 0.0 | 2.9 | 11.1 | 0.0 | 2.3 | 8.1 | 0.0 |
| NP pain pill frequency of use (% of days, past 6m) | 11.6 | 22.5 | 0.5 | 12.8 | 24.2 | 1.1 | 8.4 | 16.9 | 0.0 |
| Cocaine/crack frequency of use (% of days, past 6m) | 19.0 | 27.8 | 4.3 | 17.6 | 27.1 | 3.3 | 22.6 | 29.4 | 6.3 |
| Methamphetamine frequency of use (% of days, past 6m) | 11.9 | 23.0 | 0.5 | 10.8 | 22.8 | 0.0 | 14.8 | 23.5 | 4.3 |
| NP benzodiazepine frequency of use (% of days, past 6m) | 12.9 | 23.1 | 1.6 | 11.6 | 20.8 | 1.1 | 16.6 | 28.3 | 2.2 |
Overdose experience
Almost 28% (27.5%, n=98) of the sample experienced at least one drug overdose in the past six months, and 221 (62.3%) reported at least one prior overdose (more than six months ago) (Table 1). Of those who overdosed in the past six months, 77.3% (75/97) had experienced a prior overdose. In the previous six months, participants experienced 0–10 unintentional overdoses (median = 0, mean = 0.48, SD = 1.02). Over their lifetime, participants experienced 0–200 unintentional overdoses (median = 2, mean = 3.56, SD = 11.5), with 31.8% never experiencing an overdose, 17.5% with one overdose, and 50.7% reporting 2 or more overdoses. Over 95% (95.5%) of lifetime overdoses were related to the use of heroin/non-prescribed fentanyl or non-prescribed pharmaceutical opioids. The mean number of people the participant was personally acquainted with who died from an unintentional overdose was 11.7 (SD=16.0).
Drug use characteristics
Over the previous six months (184 days), participants used non-prescribed buprenorphine 14.6% (26.9 days) of the days on average (SD = 20.4%) (Table 2). Close to 90% of participants reported using non-prescribed buprenorphine for self-treatment of opioid use withdrawal symptoms. Over 50% (56.9%) of the participants reported having ever sold buprenorphine to someone, and 47.9% said they had given buprenorphine prescribed to them, to others.
Participants used heroin/non-prescribed fentanyl a mean of 56.4% of the days in the past six months, cocaine/crack 19% of days, and methamphetamine 11.9% of days (Table 2). 65% reported injection as their most frequent route of heroin/non-prescribed fentanyl use (Table 1). About half (50.6%) had been in substance use treatment in the past six months, and almost 31% had been incarcerated (Table 1). Among the psychiatric comorbidities we assessed, generalized anxiety disorder (45.5%) was most common, followed by major depressive disorder (34.3%), and PTSD (27.5%); overall, almost 59% had at least one of these three in the past year (Table 1).
Association between frequency of non-prescribed buprenorphine use and unintentional drug overdose (primary hypothesis)
Higher mean percentage of days of non-prescribed buprenorphine use in the past six months was significantly associated with a decreased risk of an overdose in the past six months in both unadjusted (OR 0.82, 95% CI=0.70, 0.97, p = .0180) and adjusted analyses (AOR = 0.81; 95% CI=0.66, 0.98, p =.0286) (Table 3), strongly supporting our hypothesis.
In the secondary analyses, to illustrate the magnitude of the exposure AOR on an understandable scale, the AOR for the binary exposure was 0.67 (95% CI=0.40, 1.13); that is, individuals who used non-prescribed buprenorphine on more than 5.4% of days (10 days; the median) had 33% lower odds of overdose. The AORs after cutting the exposure into six equal-length intervals on the log-scale were, for the upper five intervals, 0.80 (2–3 days; 95% CI=0.31, 2.10), 0.50 (4–9 days; 95% CI=0.20, 1.25), 0.39 (10–24 days; 95% CI=0.15, 1.00), 0.58 (25–67 days; 95% CI=0.22, 1.52), 0.20 (68 or more days; 95% CI=0.05, 0.70), respectively, relative to the first interval. In other words, taking buprenorphine on 2–3 days out of 184 reduced the odds of overdose by 20% compared to just 1 day, with an approximately linear trend of more use associated with even greater reduction in odds of overdose. These estimates are illustrated in Figure 1, which also demonstrates the adequacy of the linearity assumption of the primary analysis.
Figure 1.
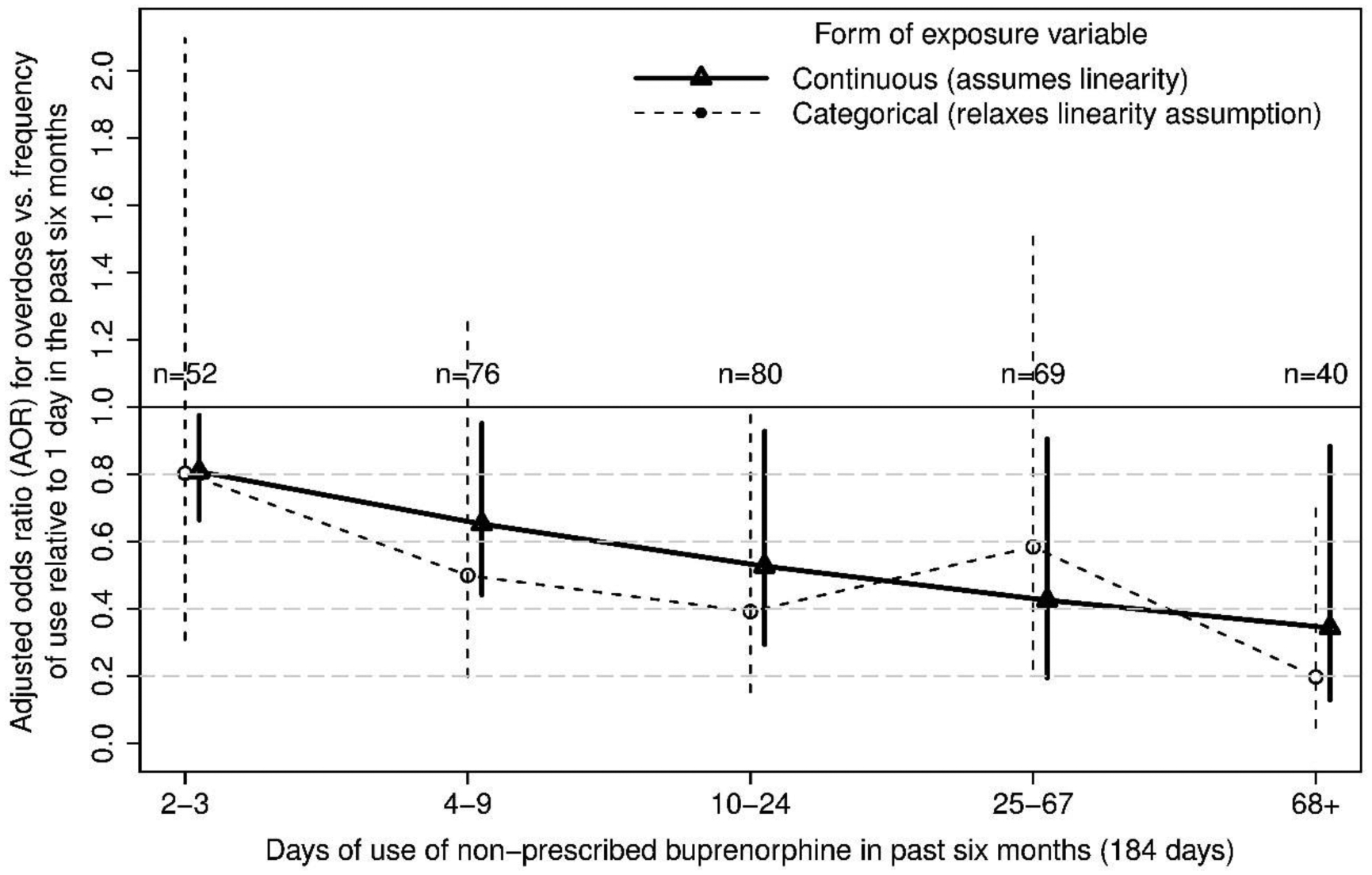
Adjusted odds ratios (AOR) and 95% confidence intervals for overdose vs. increasing frequency of use of non-prescribed buprenorphine in the past six months, relative to one day of use (n = 39).
Regarding our hypothesis that increased use of non-prescribed buprenorphine lowers risk of overdose at least in part through a corresponding decrease in the use of heroin and/or fentanyl, we found that (after adjusting for confounding) greater (log-transformed) frequency of use of non-prescribed buprenorphine is strongly associated with lower (log-transformed) frequency of use of heroin/fentanyl (beta = −0.076, 95% CI = −0.098, −0.053), and lower (log-transformed) frequency of use of heroin/fentanyl is strongly associated with lower risk of overdose (AOR = 2.77, 95% CI=1.19, 6.70).
Other correlates of unintentional drug overdose (secondary analyses)
In multivariable analysis, odds of past six-month overdose were more than two times greater for those with prior overdose experience (experiencing an overdose prior to the past six months) (AOR = 2.19; 95% CI = 1.24, 3.97; p < .01) (Table 3). Similarly, individuals who reported injection as the most common route of heroin/non-prescribed fentanyl administration in the past six months had almost 2.5 times the odds of experiencing an overdose, compared to those who reported a non-injection route (intranasal and/or smoking) (AOR = 2.49; 95% CI = 1.36, 4.71; p < .01). Higher frequency of methamphetamine use was also associated with greater odds of overdose in the past six months (AOR = 1.13; 95% CI = 1.02, 1.27; p < .05). Incarceration in the past six months and greater percentage of days of crack/cocaine HCl use in the past six months were each strongly associated with greater odds of overdose in unadjusted analyses, but after controlling for other variables these associations were attenuated.
Discussion
The results of this study confirmed our hypothesis that, among people with opioid use disorder who had used non-prescribed buprenorphine recently, more frequent use of non-prescribed buprenorphine is associated with lower risk of non-fatal unintentional drug overdose. Thus, non-prescribed buprenorphine use can potentially be serving harm-reduction purposes. The findings indicate that people who use non-prescribed buprenorphine more frequently are less likely to use heroin/non-prescribed fentanyl on those days and, therefore, reduce their chances of experiencing an overdose.
The relationship is illuminated in our secondary analysis indicating that people who used non-prescribed buprenorphine more than the median number of number of days (10 days) had 33% lower odds of overdose. After dividing non-prescribed buprenorphine use in six equal intervals, the AORs indicated a linear trend where more non-prescribed buprenorphine use was associated with even greater reduction in odds of overdose. This is consistent with the association between being in medication assisted therapy and reduced risk of overdose due to reduction in opioid use (Degenhardt et al., 2010; Soyka et al., 2010). The relationship between more frequent use of non-prescribed buprenorphine and lower risk of overdose is further supported by our findings that greater frequency of use of non-prescribed buprenorphine is strongly associated with lower frequency of use of heroin/fentanyl, and lower frequency of use of heroin/fentanyl is strongly associated with lower risk of overdose (Daniulaitye et al., 2019b).
To the best of our knowledge, this is one of the few studies examining the relationship between non-prescribed buprenorphine use and recent non-fatal unintentional overdose. Using latent class analysis to identify classes based on the underlying latent construct, “pattern of opioid use among individuals with current opioid use disorder,” our previous study using the same sample identified a salient subgroup that used diverted buprenorphine more frequently (Daniulaityte et al., 2019b). People in this class were significantly less likely to have experienced an overdose in the past six months.
Similar to prior research, we found the vast majority of participants used non-prescribed buprenorphine only to self-treat opioid use disorder withdrawal, and not to achieve euphoric effects (Allen & Harocopos, 2016; Chilcoat, Amick, Sherwood, & Dunn, 2019; Cicero et al., 2018). Our findings extend previous research indicating that non-medical use of medications such as buprenorphine is a lay harm-reduction strategy, resulting in short and longer-term abstinence from heroin/non-prescribed fentanyl use which may reduce injection-related risk behaviors and risk of overdose (Harris & Rhodes, 2013). Our qualitative data indicate that with increased proliferation of non-pharmaceutical fentanyl and other novel synthetic opioids in the Dayton area (Daniulaityte, 2019a; Daniulaityte, 2019c), some individuals sought non-prescribed buprenorphine to intentionally mitigate their risks for an unintentional overdose from highly potent and unpredictable novel synthetic opioid mixtures that were sold as and/or completely replaced heroin (Silverstein, Daniulaityte, Martins, Miller, & Carlson, 2019; Silverstein et al., in review).
Our finding that previous unintentional drug overdose is associated with subsequent overdose is consistent with previous research (Coffin et al., 2007; Larochelle et al., 2018; Olfson, Wall, Wang, Crystal, & Blanco, 2018; Stoove et al., 2009). In a prospective study with 2,317 people who use drugs in Vancouver, Canada, Caudarella and colleagues (Caudarella, Dong, Milloy, Kerr, & Wood, 2016) found a dose response relationship where increases in cumulative non-fatal overdose were associated with future fatal overdose. As Coffin and colleagues (2007) conclude, one of the strongest predictors of a non-fatal overdose among people who use heroin is having experienced a previous overdose. Increased susceptibility to experiencing multiple overdoses may be related to comorbid medical conditions, especially those that impact the respiratory system (Coffin et al., 2007; Olfson et al., 2018), and psychiatric comorbidity (Bohnert & Ilgen, 2019; Fridell, Backstrom, Hesse, Krantz, & Nylen, 2019). Factors of socioeconomic disadvantage may also contribute to heightened risk of multiple overdoses (Nandi et al., 2006).
Our finding that increased risk of unintentional overdose is associated with injection drug use, rather than snorting or smoking, is also consistent with prior research. Injection route of administration increases the speed of onset and rate of drug absorption, thereby increasing overdose risk (Gossop, Griffiths, Power, & Strang, 2010; Samaha & Robinson, 2005; Strang et al., 1998; Strang, Des Jarlais, Griffiths, & Gossop, 1992). Several studies have suggested that harm reduction strategies to reduce overdose risk include switching from injection to smoking or inhaling heroin or non-prescribed fentanyl (Ciccarone et al., 2017; Fairborn et al., 2018; Harris, Forseth, & Rhodes, 2015).
Higher average percentage of days of methamphetamine use in the past six months was a strong correlate of overdose in multivariable analysis. This is also consistent with previous research (Brugal et al., 2002; Kinner et al., 2012; Werb et al., 2008). For example, in a study with 592 people who inject drugs in Denver, Colorado, Al-Tayyib and colleagues (Al-Tayyib, Koester, Langregger, & Raville, 2017) found that those who injected heroin and methamphetamine in the past 12 months were 2.8 times more likely to experience a non-fatal overdose in the same time period, compared to heroin only injectors in multivariable analyses. In addition, participants who injected heroin and methamphetamine at different times were more likely to report an overdose in the past 12 months, compared to people who injected a mixture of the drugs. In contrast, another study with 795 people who inject drugs recruited in San Francisco, Ochoa and colleagues (2005) found that those who injected a mixture of heroin and methamphetamine were more likely to experience an overdose in the previous 12 months, compared to those who did not report an overdose (Ochoa et al., 2005). In Dayton, Ohio, methamphetamine mentions in overdose deaths increased from 18% in 2017 to 26% in 2018, and methamphetamine seizures increased from 68,485 grams in 2017 to 256,766 grams in 2018 (Public Health Dayton and Montgomery County, 2018). A preliminary finding from our current research focusing on methamphetamine suggests the existence of lay beliefs among people who use drugs in Dayton that use of methamphetamine in conjunction with heroin/non-prescribed fentanyl may reduce overdose risk. This incorrect lay belief may contribute to increased methamphetamine use in conjunction with non-prescribed fentanyl, and may, in part, explain the significant role of methamphetamine use as a correlate of overdose.
Limitations
Limitations are noted. The sample was not randomly recruited. However, we used a combination of targeted recruitment and modified respondent-driven sampling to diversify recruitment networks. Nevertheless, the sample may be biased by income level, education, employment, and other factors. Second, the meaning of “experiencing an unintentional overdose” was not further defined; thus, there may be some variability in participants’ interpretations of what constitutes an “overdose.” It is also possible that some overdoses were intentional. However, as noted in previous research, a drug overdose may include elements of both intentionality and unintentionality, perceived intent may change among people with non-fatal overdose after recovery, and some people cannot distinguish whether an overdose was intentional or unintentional (Bohnert & Ilgen, 2019; Bohnert et al., 2018). The findings are based on self-report, and are subject to potential inaccurate recall. However, there is substantial support for the validity and reliability of self-report data among people who use drugs (Adair, Craddock, Miller, & Turner, 1995; Darke, 1998; Passik, Hays, Eisner, & Kirsh, 2006). In addition, we used urine sample testing to increase the veracity of self-report. Finally, although the TLFB has been used with people who use drugs successfully in other studies (Carey, Carey, Maisto, & Henson, 2004; Hjorthoj, Hjorthoj, & Nordentoft, 2012; Robinson et al., 2012), some degree of error in recall accuracy may have occurred.
Conclusions
Use of non-prescribed buprenorphine has been shown to be associated with subsequent substance use treatment entry and retention, and self-treatment, both for short-term detoxification and longer-term opioid abstinence (Allen & Harocopos, 2016; Mitchell et al., 2009; Monico et al., 2015). Our findings suggest that decreased risk of overdose associated with more frequent use of non-prescribed buprenorphine is a harm-reduction consequence of diversion. We cannot establish a causal relationship between higher frequency of non-prescribed buprenorphine use and reduced risk of overdose because of the cross-sectional nature of the study. Additional longitudinal studies are needed to further evaluate our findings.
It is difficult to imagine addressing the opioid epidemic in the U.S., now more than two decades in length, without buprenorphine becoming available for treating opioid use disorder in 2002. Medication assisted treatment reduces the risk of overdose deaths (Degenhardt et al., 2010; Larochelle et al., 2018; Morgan, Shackman, Weinstein, & Walley, 2019), and buprenorphine treatment has played a crucial role in providing a pathway to recovery among people with opioid use disorder. Nevertheless, only about 20% of people with opioid use disorder access treatment (Manhapra, Stefanovics, & Rosenheck, 2019; B. Saloner & Karthikeyan, 2015; Wu, Zhu, & Swartz, 2016). Use of diverted buprenorphine appears to fill needs that are unmet by access to medication assisted therapy and is clearly associated with harm reduction consequences among those who use it. Innovative methods to widely expand access to buprenorphine treatment, such as the use of mobile vans to recruit people into short-term buprenorphine treatment before transitioning to longer-term programs (Krawczyk, Buresh, Gordon, Blue, & Fingerhood, 2019), low barrier buprenorphine treatment among homeless on the streets and in shelters (Carter, Zevin, & Lum, 2019; Chatterjee et al., 2017), buprenorphine treatment provided through harm reduction programs (Fox, Chamberlain, Frost, & Cunningham, 2015), and buprenorphine induction in emergency departments (D’Onofrio et al., 2017) are urgently needed.
Role of funding source
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Acknowledgements
This study was supported by the National Institute on Drug Abuse, Grant No. R01DA040811. A previous version of the study was presented at the College on Problems of Drug Dependence annual meetings in San Antonio, Texas, June 19, 2019. We thank our participants for sharing their life experiences with us, as well as research assistant interviewers Angela Zaragoza, Kara Schaefer, Avery Moeller, and Kylie Getz for their dedicated work on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
CRediT author statement
Robert G. Carlson: Conceptualization, Investigation, Writing -Original Draft; Raminta Daniuaityte: Conceptualization, Investigation; Writing-Review & Editing; Project Administration, Funding Acquisition; Sydney M. Silverstein: Conceptualization, Writing—Review & Editing; Ramzi W. Nahhas: Conceptualization, Formal Analysis, Writing-Original Draft; Silvia S. Martins: Conceptualization, Writing-Review and Editing.
References
- Adair EB, Craddock SG, Miller HG, & Turner CF (1995). Assessing consistency of responses to questions on cocaine use. Addiction (Abingdon, England), 90(11), 1497–1502. [DOI] [PubMed] [Google Scholar]
- Al-Tayyib A, Koester S, Langregger S, & Raville L (2017). Heroin and methamphetamne injection: An emerging drug use pattern. Substance Use & Misuse, 50(8), 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, & Harocopos A (2016). Non-Prescribed Buprenorphine in New York City: Motivations for Use, Practices of Diversion, and Experiences of Stigma. Journal of Substance Abuse Treatment, 70, 81–86. doi: 10.1016/j.jsat.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, … Horton T (2004). Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions, 13 Suppl 1, S42–66. doi: 10.1080/10550490490440807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Perucci C, Ferri M, Faggiano F, & Mattick R (2005). An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment, 28, 321–329. [DOI] [PubMed] [Google Scholar]
- Bazazi AR, Yokell M, Fu JJ, Rich JD, & Zaller ND (2011). Illicit use of buprenorphine/naloxone among injecting and noninjecting opioid users. Journal of Addictive Medicine, 5(3), 175–180. doi: 10.1097/ADM.0b013e3182034e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Holloway K, & Bird SM (2014). Does take-home naloxone reduce non-fatal overdose? The Lancet, 383(9912), 124–125. doi: 10.1016/S0140-6736(14)60022-2 [doi] [DOI] [PubMed] [Google Scholar]
- Biernacki P, & Waldorf D (1981). Snowball sampling: problems and techniques of chain referral sampling. Sociological Methods & Research, 10(2), 141. [Google Scholar]
- Binswanger I, Stern M, Deyo R, Heagarty P, Cheadle A, Elmore J, & Koepsell T (2007). Release from Prison--A high risk of death for former inmates. New England Journal of Medicine, 356(2), 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, & Ilgen M (2019). Understanding links among opioid use, overdose, and suicide. The New England Journal of Medicine, 380, 71–79. [DOI] [PubMed] [Google Scholar]
- Bohnert ASB, Walton M, Cunningham RM, Ilgen M, Barry K, Chermack ST, & Blow F (2018). Overdose and adverse drug event experiences among patients in the emergency department. Addictive Behaviors, 86, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon DL, Milloy MJ, Wood E, Montaner JS, Kerr T (2011). Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. The Lancet, 377, 1429–1437. [DOI] [PubMed] [Google Scholar]
- Brugal M, Barrio G, La Fuente L, Regidor E, Royuela L, & Suelves J (2002). Factors associated with non-fatal heroin overdose: Assessing the effect of frequency and route of heroin administration. Addiction, 97, 319–327. [DOI] [PubMed] [Google Scholar]
- Carey KB, Carey MP, Maisto SA, & Henson JM (2004). Temporal stability of the timeline followback interview for alcohol and drug use with psychiatric outpatients. Journal of Studies on Alcohol, 65(6), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RG, Wang J, Falck RS, & Siegal HA (2005). Drug use practices among MDMA/ecstasy users in Ohio: a latent class analysis. Drug and Alcohol Dependence, 79(2), 167–179. doi: 10.1016/j.drugalcdep.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas G, Vlahov D, Wodak A, & Woody G (2006). Buprenorphine use: The internatonal experience. Clinical Infectious Diseases, 43(Supplement 4), S197–S215. [DOI] [PubMed] [Google Scholar]
- Carroll JJ, Rich JD, & Green TC (2018). The more things change: Buprenorphine/naloxone diversion continues while treatment remains inaccessible. Journal of Addiction Medicine, 12(6), 459–465. doi: 10.1097/ADM.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Zevin B, & Lum PJ (2019). Low barrier buprenorphine treatment for persons experiencing homelessness and injecting heroin in San Francisco. Addiction Science and Clinical Practice, 14, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, & Wood E (2016). Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug & Alcohol Dependence, 162, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDCNVSS. (2019). Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- Centers for Disease Control and Prevention. (2012). Community-based opioid overdose prevention programs providing naloxone---United States, 2010. Morbidity and Mortality Weekly Report, 61, 101–105. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. CDC; 2015. Retrieved from Atlanta, GA: http://emergency.cdc.gov/han/han00384.asp [Google Scholar]
- Chatterjee A, Obando A, Strickland E, Nestler A, Harrington-Levy R, Williams T, & LaCoursiere-Zucchero T (2017). Shelter-based opioid treatment: Increasing access to addiction treatment in a family shelter. American Journal of Public Health, 107, 1092–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Amick HR, Sherwood K, & Dunn E (2019). Buprenorphine in the United States: Motives for abuse, misuse, and diversion. Journal of Substance Abuse Treatment, 10.1016/j.jsat.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Ciccarone D (2017). Fentanyl in the US heroin supply: A rapidly changing risk environment. Internation Journal of Drug Policy, 46, 107–111. doi: 10.1016/j.drugpo.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D, Ondocsin J, & Mars SG (2017). Heroin uncertainties: Exploring users’ perceptions of fentanyl-adulterated and -substituted ‘heroin’. International Journal of Drug Policy, 46, 146–155. doi: 10.1016/j.drugpo.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, & Chilcoat HD (2018). Understanding the use of diverted buprenorphine. Drug and Alcohol Dependence, 193, 117–123. doi: 10.1016/j.drugalcdep.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, & Tardiff K (2003). Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–98. Addiction, 98(6), 739–747. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Bucciarelli TM, Ompad A, Vlahov D, & Galea S (2007). Identifying injection drug users at risk of nonfatal overdose. Academy of Emergency Medicine, 14, 616–623. [DOI] [PubMed] [Google Scholar]
- Compton WM, Dawson DA, Goldstein RB, & Grant BF (2013). Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug and Alcohol Dependence, 132(1–2), 387–390. doi: 10.1016/j.drugalcdep.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio GD, Chawarski MC, O’Connor PG, Pantalon MV, Busch SH, Owens PH, … Fiellin DA (2017). Emergency department-initiated buprenorphine for opioid depenence with continuation in primary care: Outcomes during and after intervention. Journal of General Internal Medicine, 32, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Falck R, & Carlson RG (2012). Illicit use of buprenorphine in a community sample of young adult non-medical users of pharmaceutical opioids. Drug and Alcohol Dependence, 122(3), 201–207. doi: 10.1016/j.drugalcdep.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Falck R, Li L, Nahhas RW, & Carlson RG (2012). Respondent-driven sampling to recruit young adult non-medical users of pharmaceutical opioids: problems and solutions. Drug and Alcohol Dependence, 121(1–2), 23–29. doi: 10.1016/j.drugalcdep.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Juhascik MP, Strayer KE, Sizemore IE, Zatreh M, Nahhas RW, Carlson RR (2019-a). Trends in fentanyl and fentanyl analogue-related overdose deaths – Montgomery County, Ohio, 2015–2017. Drug and Alcohol Dependence, 116–120. doi: 10.1016/j.drugalcdep.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Nahhas RW, Silverstein S, Martins SS, Zaragoza A, Moeller A, Carlson RG (2019-b). Patterns of non-prescribed buprenorphine and other opioid use among individuals with current opioid use disorder: a latent class analysis. Drug and Alcohol Dependence, 204, 107574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Carlson RR, Juhascik MP, Strayer KE, & Sizemore IE (2019c). Street fentanyl use: Experiences, preferences, and concordance between self-reports and urine toxicology. International Journal of Drug Policy, 71, 3–9. doi: 10.1016/j.drugpo.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S (1998). Self-report among Injecting Drug Users. Drug and Alcohol Dependence, 51, 253–263. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Weatherburn D, & Lind B (1999). Fluctuations in heroin purity and the incidence of fatal heroin overdose. Drug and Alcohol Dependence, 54(2), 155–161. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Brucello C, Mathers B, Briegleb C, Ali H, Hickman M, & McLaren J (2010). Mortality rates among users of dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction, 106, 32–51. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, & Robbins SJ (1994). Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology, 62(4), 843–850. [DOI] [PubMed] [Google Scholar]
- Epstein-Ngo QM, Cunningham RM, Whiteside LK, Chermack ST, Booth BM, Zimmerman MA, & Walton MA (2013). A daily calendar analysis of substance use and dating violence among high risk urban youth. Drug and Alcohol Dependence, 130(1–3), 194–200. doi: 10.1016/j.drugalcdep.2012.11.006; 10.1016/j.drugalcdep.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairborn N, Coffin PO, & Walley AY (2018). Naloxone for heroin, prescription opioids, and illicitly made fentanyl overdoses: Challenges and innovations responding to a dynamic epidemic. Internation Journal of Drug Policy, 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T (2017). Variable selection should be blinded to the outcome. International Journal of Epidemiology, 46(3), 1077–1079. 10.1093/ije/dyx048 [DOI] [PubMed] [Google Scholar]
- Falck RS, Siegal HA, Wang J, Carlson RG, & Draus PJ (2005). Nonmedical drug use among stimulant-using adults in small towns in rural Ohio. Journal of Substance Abuse Treatment, 28(4), 341–349. doi: 10.1016/j.jsat.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Forman RF, Svikis D, Montoya ID, & Blaine J (2004). Selection of a substance use disorder diagnostic instrument by the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment, 27(1), 1–8. doi: 10.1016/j.jsat.2004.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AD, Chamberlain A, Frost T, & Cunningham CO (2015). Harm reduction agencies as a potential site for buprenorphine treatment. Substance Abuse, 36, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell M, Backstrom M, Hesse M, Krantz P, & Nylen A (2019). Prediction of psychiatric comorbidity on premature death in a cohort of patients with substance use disorders: a 42-year follow-up. BMC Psychiatry, 19, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst RT (2013). Suboxone misuse along the opiate maintenance treatment pathway. Journal of Addictive Diseases, 32(1), 53–67. doi: 10.1080/10550887.2012.759860 [doi] [DOI] [PubMed] [Google Scholar]
- Furst RT (2014). Diffusion and Diversion of Suboxone: An Exploration of Illicit Street Opioid Selling. Journal of Addictive Diseases, 33, 177–186. [DOI] [PubMed] [Google Scholar]
- Gladden RM, O’Donnell J, Mattson CL, & Seth P (2018). Chanegs in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamin--25 states, January-December 2017 to January-June 2018. MMWR Morbidity and Mortality Weekly Report, 68, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough A, Katz J, & Sangor-Katz M (2019). Drug overdose deaths drop in the U.S. for the first time since 1990. New York Times, July 17. https://www.nytimes.com/interactive/2019/07/17/upshot/drug-overdose-deaths-fall.html?rref=collection%2Fbyline%2Fabby-goodnough&action=click&contentCollection=undefined®ion=stream&module=stream_unit&version=latest&contentPlacement=7&pgtype=collection. Accessed 9/4/2019. [Google Scholar]
- Gossop M, Griffiths P, Power B, & Strang J (2010). Coaine: patterns of use, route of administration, and severity of dependence. British Journal of Psychiatry, 164, 1–9. [DOI] [PubMed] [Google Scholar]
- Harrell FE Jr. (2015). Regression modeling strategies (Second ed.). Switzerland: Springer. Pages 72–73. [Google Scholar]
- Harris M, Forseth K, & Rhodes T (2015). “It’s Russian roulette”: Adulteration, adverse effects and drug use transitions during the 2010/2011 United Kingdom heroin shortage. International Journal of Drug Policy, 26, 51–58. [DOI] [PubMed] [Google Scholar]
- Harris M, & Rhodes T (2013). Methadone diversion as a protective strategy: the harm reduction potential of ‘generous constraints’. The International Journal of Drug Policy, 24(6), e43–50. doi: 10.1016/j.drugpo.2012.10.003 [doi] [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havnes IA, Clausen T, & Middelthon AL (2013). ‘Diversion’ of methadone or buprenorphine: ‘harm’ versus ‘helping’. Harm Reduction Journal, 10, 24. doi: 10.1186/1477-7517-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn D (1997). Respondent-driven sampling: a new approach to the study of hidden populations. Social Problems, 44(2), 174–199. [Google Scholar]
- Hedegaard H, Minino A, & Warner M (2018). Drug Overdose Deaths in the United States, 1999 – 2017. NCHS Data Brief, 329, 1–7. [PubMed] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, & Nordentoft M (2012). Validity of timeline follow-back for self-reported use of cannabis and other illicit substances--systematic review and meta-analysis. Addictive Behaviors, 37(3), 225–233. doi: 10.1016/j.addbeh.2011.11.025; 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll K, … Ling W (2015). Long-term outcomes after randomization to buprenorphine versus methadone in a multi-site trail. Addiction, 111, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak JJ, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, & Przybeck T (1993). The use of the DSM-III-R checklist for initial diagnostic assessments. Comprehensive Psychiatry, 34(6), 375–383. [DOI] [PubMed] [Google Scholar]
- Irvine MA, Kuo M, Buxton JA, Balshaw R, Otterstatter M, Macdougall L, Milloy MJ, Bjarmel A, Henry B, Tyndall M, Coombs D, Gilbert M (2019). Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction, 114, 1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal H, Buchanich M, Roberts M, Balmert L, Zhang K, & Burke D (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science (New York, N.Y.), 361, 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Arfken CL, di Menza S, & Schuster CR (2012). Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug and Alcohol Dependence, 120(1–3), 190–195. doi: 10.1016/j.drugalcdep.2011.07.019 [doi] [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, & Bigelow GE (1995). A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence, 40(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Jones C, Christman Z, Smith C, Safferman M, Salzman M, Baston K, & Haroz R (2018). Comparison between buprenorphine provider availability and opioid deaths among US counties. Journal of Substance Abuse Treatment, 93, 19–25. [DOI] [PubMed] [Google Scholar]
- Jones CM, Einstein EB, & Compton WM (2018). Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. Journal of the American Medical Association, 319(17), 1819–1821. doi: 10.1001/jama.2018.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SR, Anderson BJ, Bailey GL, & Stein MD (2017). The relationship between diversion-related attitudes and sharing and selling buprenorphine. Journal of Substance Abuse Treatment, 78, 43–47. doi: 10.1016/j.jsat.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner SA, Milloy MJ, Wood E, Qi J, Zhang R, & Kerr T (2012). Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addictive Behaviors, 37(6), 691–696. doi: 10.1016/j.addbeh.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf DJ, van Ginkel P, & Benschop A (2010). How to find non-dependent opiate users: A comparison of sampling methods in a field study of opium and heroin users. International Journal of Drug Policy, 21(3), 215–221. doi: 10.1016/j.drugpo.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Krawczyk K, Buresh M, Gordon MS, Blue TR, & Fingerhood MI (2019). Expanding low-threshold buprenorphine to justice-involved individuals through mobile treatment: Addressing a critical gap. Journal of Substance Abuse Treatment, 103, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M, Bernson D, Land T, Stopka T, Wang N, Xuan Z, … Walley AY (2018). Medication for opioid use disorder after nonfatal overdose and association with mortality: a cohort study. Annals of Internal Medicine, 169, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D, & Oliver P (2011). Drug-related death following release from prison: a brief review of the literature with recommendations for practice. Current Drug Abuse Reviews, 4, 292–297. [DOI] [PubMed] [Google Scholar]
- Lin LA, Lofwall MR, Walsh SL, Gordon AJ, & Knudsen HK (2018). Perceptions and practices addressing diversion among US buprenorphine prescribers. Drug and Alcohol Dependence, 186, 147–153. doi: 10.1016/j.drugalcdep.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, & Havens JR (2012). Inability to access buprenorphine treatment as a risk factor for using diverted buprenorphine. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2012.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, & Walsh SL (2014). A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. Journal of Addiction Medicine, 8(5), 315–326. doi: 10.1097/ADM.0000000000000045 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhapra A, Stefanovics E, & Rosenheck R (2019). Initiating opioid agonist treatment for opioid use disorder nationally in the veterans health administration: who gets what? Substance Abuse, 10.1080/08897077.2019.1640831. [DOI] [PubMed] [Google Scholar]
- Maria Patrizia C, Leslie A, Gregory ML, Vlahov D, Alex W, & Woody GE (2006). Buprenorphine use: The international experience. Clinical Infectious Diseases, 43, S197–S215. [DOI] [PubMed] [Google Scholar]
- Mattick R, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence (review). Cochrane Database of Systematic Reviews (Online)(2), 1–85. [DOI] [PubMed] [Google Scholar]
- Maughan BC and Becker EA (2019). Drug-related mortality after discharge from treatment: A record-linkage study of substance abuse clients in Texas, 2006–2012. Drug and Alcohol Dependence, 204, 107473. [DOI] [PubMed] [Google Scholar]
- McClure F, Niles J, Kaufman H, & Gudin J (2017). Concurrent use of opioids and benzodiazepines: Evaluation of prescription drug monitoring by a United States laboratory. Journal of Addiction Medicine, 11, 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K, & Kavanaugh PR (2019). “They’re making it so hard for people to get help:” motivations for non-prescribed buprenorphine use in a time of treatment expansion. International Journal of Drug Policy, 71, 118–124. [DOI] [PubMed] [Google Scholar]
- Mitchell SG, Kelly SM, Brown BS, Schacht Reisinger H, Peterson JA, Ruhf A, … Schwartz RP (2009). Uses of diverted methadone and buprenorphine by opioid-addicted individuals in Baltimore, Maryland. The American Journal on Addictions, 18(5), 346–355. doi: 10.3109/10550490903077820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monico LB, Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, & Jaffe JH (2015). Prior experience with non-prescribed buprenorphine: Role in treatment entry and retention. Journal of Substance Abuse Treatment, 57, 57–62. doi:S0740–5472(15)00109–9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Shackman BR, Weinstein ZM, & Walley AY (2019). Overdose following initiation of natrexone and buprenorphene medication treatment for opioid use disorder in a United States commercially insured cohort. Drug and Alcohol Dependence, 200, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P, Mohammed F, Wareing M, Cotton A, McNeill J, Irving P, … Elton P (2018). High drug related mortality rates following proson release: Assessing the acceptance of a naltrexone injection and related concerns. Journal of Substance Abuse Treatment, 92, 91–98. [DOI] [PubMed] [Google Scholar]
- Najman JM, Mcllwraith F, Kemp R, & Smirnov A (2017). When knowledge and experience do not help: A study of nonfatal drug overdoses. Journal of Addiction Medicine, 11(4), 280–285. [DOI] [PubMed] [Google Scholar]
- Nandi A, Galea S, Ahern J, Bucciarelli A, Vlahov D, & Tardiff K (2006). What explains the association between neighborhood-level income inequality and the risk of fatal overdose in New York City? Social Science and Medicine, 63, 662–674. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Gladden RM, Mattson CL, & Kariisa M (2018). Notes from the field: overdose deaths with carfentanil and other fentanyl analogs detected - 10 states, July 2016-June 2017. MMWR Morbidity and Mortality Weekly Report, 67(27), 767–768. doi: 10.15585/mmwr.mm6727a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa K, Davidson P, Evans J, Hahn J, Page-Shafer K, & Moss A (2005). Heroin overdose among young injection drug users in San Francisco. Drug and Alcohol Dependence, 80, 297–302. [DOI] [PubMed] [Google Scholar]
- Olfson M, Wall M, Wang s., Crystal S, & Blanco C (2018). Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug and Alcohol Dependence, 190, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik S, Hays L, Eisner N, & Kirsh K (2006). Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. Journal of Pain Palliative Care Pharmacotherapy, 20, 5–13. [PubMed] [Google Scholar]
- Public Health Dayton, & Montgomery County. (2019). Data Unit 2018 Annual Report, Community Overdose Action Team. https://www.phdmc.org/coat-documents/coat-data-unit/1109-2018-coat-data-unit-report-january-1-2018-through-december-31-2018/file accessed January 9, 2020. [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2012). Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors, 28, 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Rossen LM, Bastian B, Warner M, Khan D, & Chong Y (2019). Drug poisoning mortality: United States, 1999–2017. National Center for Health Statistics. Retrieved from https://data.cdc.gov/NCHS/NCHS-Drug-Poisoning-Mortality-by-County-United-Sta/rpvx-m2md (Last accessed 29 August 2019). [Google Scholar]
- Rothman KJ, Greenland S, & Lash TL (2008). Modern epidemiology (Third ed.). [Google Scholar]
- Philadelphia PA: Lippincott Williams & Wilkins. Pages 261–263. [Google Scholar]
- Rowe C, Wheeler E, Vittinghoff E, Santos GM, & Behar E (2018). Quantity fluctuations of illicitly used opioids and overdose risk. International Journal of Drug Policy, 58, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Gladden RM (2016). Increases in drug and opioid overdose deaths - United States, 2000–2014. MMWR Morbidity and Mortality Weekly Report, 64(50–51), 1378–1382. [DOI] [PubMed] [Google Scholar]
- Ruttenber A, & Luke J (1984). Heroin-related deaths: New epidemiologic insights. Science (New York, N.Y.), 226, 14–20. [DOI] [PubMed] [Google Scholar]
- Saloner B, & Karthikeyan S (2015). Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013. Journal of the Ameican Medical Association, 314(14), 1515–1517. [DOI] [PubMed] [Google Scholar]
- Saloner B, Stoller KB, Alexander GC (2018). Moving addiction care to the mainstream---improving the quality of buprenorphine treatment. New England Journal of Medicine, 379, 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, & Robinson TE (2005). Why does the rapid delivery of drugs to the brain promote addiction? Trends in Pharmacological Sciences, 26, 82–87. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2012). SAS Version 9.4. Cary, NC: SAS Institute Inc. [Google Scholar]
- Sifaneck SJ, & Neaigus A (2001). The ethnographic accessing, sampling and screening of hidden populations: heroin sniffers in New York City. Addiction Research & Theory, 9(6), 519–543. [Google Scholar]
- Silva K, Schrager SM, Kecojevic A, & Lankenau SE (2013). Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug and Alcohol Dependence, 128(1–2), 104–110. doi: 10.1016/j.drugalcdep.2012.08.014 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Daniulaityte R, Martins SS, Miller SC, & Carlson RG (2019). “Everything is not right anymore” Buprenorphine experiences in an era of street fentanyl. International Journal of Drug Policy, 74, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S, Daniulaityte R, Miller S, Martins S & Carlson RG (in review). On my own terms: Motivations for self-treating opioid-use disorder with non-prescribed buprenorphine. Drug and Alcohol Dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Träder A, Klotcsche J, Backmund M, Bühringer G, Rehm J, & Wittchen HU (2011). Six-year mortality rates of patients in methadone and buprenorphine maintenance therapy Results from a nationally representative cohort study. Journal of Clinical Psychopharmacology, 31(5), 678–680. [DOI] [PubMed] [Google Scholar]
- Stoove M, Dietze M, & Jolley D (2009). Overdose deaths following previous non-fatal heroin overdose: Record linkage of ambulance attendance and death registry data. Drug and Alcohol Review, 28, 347–352. [DOI] [PubMed] [Google Scholar]
- Strang J, Bearn J, Farrell M, Gossop M, Griffiths P, Marsden J, & Wolff K (1998). Route of drug use and its implications for drug effect, risk of dependence and health consequences. Drug and Alcohol Review, 197–211. [DOI] [PubMed] [Google Scholar]
- Strang J, Des Jarlais D, Griffiths P, & Gossop M (1992). The study of transitions in the route of drug use: the route from one route to another. British Journal of Addiction, 87, 473–483. [DOI] [PubMed] [Google Scholar]
- Strang J, McCambridge J, Best D, Beswick T, Bearn J, Rees S, & Gossop M (2003). Loss of tolerance and overdose mortality after inpatient detoxification: follow-up study. British Medical Journal, 326, 959–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall MW, Kerr T, Zhang R, King E, Montaner JG, Wood E (2006). Attendance, drug use patterns, and referrals made from North America’s first supervised injection facility. Drug and Alcohol Depencence, 83, 193–198. [DOI] [PubMed] [Google Scholar]
- Warner M, Trinidad J, Bastian B, Minino A, & Hedegaard H (2016). Drugs most frequently involved in drug overdose deaths: United States, 2010–2014. National Vital Statistics Reports, 65(10), 1–14. [PubMed] [Google Scholar]
- Werb D, Kerr T, Lai C, Math M, Montaner J, & Wood E (2008). Nonfatal overdose among a chort of street-involved youth. Journal of Adolescent Health, 42, 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines JD Jr., Saitz R, Horton N, Loyd-Travaglini C, & Samet J (2007). Overdose after detoxification: A prospective study. Drug and Alcohol Dependence, 89, 161–169. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lea T, & Sheridan J (2008). Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. The International Journal of Drug Policy, 19(6), 450–458. doi: 10.1016/j.drugpo.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Wish ED, Artigiani E, Billing A, Hauser W, Hemberg J, Shiplet M, & DuPont RL (2012). The emerging buprenorphine epidemic in the United States. Journal of Addictive Disease, 31(1), 3–7. doi: 10.1080/10550887.2011.642757 [DOI] [PubMed] [Google Scholar]
- Wu LT, Zhu H, & Swartz MS (2016). Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence, 169, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokell MA, Zaller ND, Green TC, & Rich JD (2011a). Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Current Drug Abuse Reviews, 4(1), 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedler B, Xie L, Wang L, Joyce A, Vick C, Brigham J, … Murrelle LE (2015). Development of a risk index for seroius prescription opioid-indiced respiratory depression or overdose in veteran’s health administration patients. Pain Meicine, 16, 1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoorob R, Kowalchuk A, & Mejia de Grubb M (2018). Buprenorphine therapy for opioid use disorder. American Family Physician, 97(5), 313–320. [PubMed] [Google Scholar]


