Abstract
Currently, exosomes (EXOs) are being explored as novel drug delivery carriers with greater advantages, including crossing the blood-brain-barrier and loading drugs. The present study utilized EXOs derived from neural stem cells (NSCs) for the delivery of molecular drugs to treat gliomas. miR-124-3p was selected according to previous studies by the authors, and the effects of the delivery of miR-124-3p to glioma cells by NSC-EXOs in vitro and in vivo were evaluated. It was found that NSC-EXOs successfully delivered miR-124-3p into glioma cells, and NSC-EXOs loaded with miR-124-3p significantly inhibited glioma cell proliferation, invasion and migration. Furthermore, the delivery of miR-124-3p by NSC-EXOs suppressed flotillin 2 (FLOT2) expression by specifically binding to the 3' untranslated region of the FLOT2 gene in gliomas; subsequently, AKT1 was found to be associated with the EXO-miR-124-3p/FLOT2 pathway. Moreover, the therapeutic effects of the delivery of miR-124-3p by NSC-EXOs were confirmed in a mouse tumor xenograft model of glioma. Thus, bio-carrier NSC-EXOs loaded with miR-124-3p suppressed glioma growth via the EXO-miR-124-3p/FLOT2/AKT1 pathway. On the whole, the present study provides insight into stem cell-free molecular-targeted therapy based on bio-carrier NSC-EXOs and provides a potential strategy for the treatment of glioma.
Keywords: drug carrier, neural stem cells, exosomes, glioma, miR-124-3p
Introduction
Glioblastoma multiforme (GBM) is the most invasive and malignant tumor of the central nervous system (CNS) with a median survival rate of <14.6 months (1,2). Currently, in clinical practice, the common treatment strategy for patients with GBM is combined neurosurgical resection with chemotherapy or radiotherapy; however, the recurrence rate of GBM in patients remains very high. With the development of molecular-targeted therapies, there have been numerous pre-clinical studies on novel targeted drugs (3-5); however, an appropriate drug carrier has not yet been discovered in order to solve certain key issues, particularly the restrictive ability of the blood-brain-barrier (BBB) and drug maldistribution (4,5).
Neural stem cells (NSCs) are CNS stem cells and reside in two major neurogenic niches of the brain: the subventricular and subgranular zone. They have self-renewal properties and multipotential differentiation abilities (they can differentiate into neurons, astrocytes and oligodendrocytes) (6). Previous studies have demonstrated that NSCs can migrate to brain lesions, including tumors, which is closely associated with the cell paracrine mechanism (7-10). Exosomes (EXOs) secreted by cells are the main functional paracrine molecules (11,12). EXOs have a lipid bilayer structure with a diameter of 30-200 nm. They contain proteins, lipids, mRNAs and microRNAs (miRNAs/miRs), and are critically involve in intercellular communications through the transfer of exosomal cargo from the source cells to targeted cells. Moreover, EXOs have greater advantages, including the presence of active molecules from source cells, the ability to easily cross the BBB, immune privilege, the ability to deliver drugs and they can be used in targeted therapy (11-13). In addition, when EXOs are taken up by cells, such as tumor cells, they regulate the immune response, tumor occurrence and metastasis by transferring exosomal cargos (14-16). Of note, EXOs can be modified to carry small molecular drugs, including miRNAs, for individual-based targeted therapy.
miRNAs, as small non-coding RNAs, modulate gene expression at the post-transcription level by binding to ≥1 gene in their specific 3'-untranslated region (UTR). Furthermore, miRNAs are crucially involved in the occurrence and development of GBM (17). Previous studies, including a previous study by the authors, have demonstrated that miR-124-3p overexpression significantly inhibits GBM cell proliferation and migration, as well as angiogenesis and enhances glioma chemosensitivity (18-20). The small molecule miR-124-3p can be considered as a tumor suppressor gene in gliomas.
Thus, the present study aimed to use natural biological carrier EXOs derived from NSCs to load miR-124-3p as a molecular drug for GBM treatment, as well as to further explore the potential mechanisms underlying the regulation of GBM progression by EXOs carrying miR-124-3p.
Materials and methods
Glioma cells and NSC culture
The U87MG (glioblastoma of unknown origin) and U251MG (glioblastoma) cell lines were preserved in the authors' laboratory. U87MG cells were matched with the ATCC database by short tandem repeat (STR) analysis. These cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The medium was changed at 2-day intervals and the cells were passaged on the 4th or 5th day through trypsinization (Trypsin; Gibco; Thermo Fisher Scientific, Inc.).
NSCs were generously provided by Professor Lukui Chen (Southern Medical University, Guangzhou, China); moreover, details regarding the cells have been previously described (21,22). NSCs were maintained in serum-free DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2% B27 (Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml basic fibroblast growth factor (PeproTech, Inc.), 20 ng/ml epidermal growth factor (PeproTech, Inc.) and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The medium was changed at 3-day intervals and the cells were passaged on the 6th or 7th day with dissociation using Accutase® dissociation reagent (MilliporeSigma).
EXO isolation and characterization
EXOs were isolated from NSC culture supernatants using the Exo-spin™ Exosome Isolation and Purification kit (Cell Guidance Systems) as per the manufacturer's instructions. Briefly, conditioned medium (CM) was collected, centrifuged at 2,000 × g, 4°C for 10 min and filtered to remove cell debris using a filter unit (MilliporeSigma) with a 0.22-µm membrane. As regards EXO isolation and purification, 1/2 volume of Exo-spin™ Buffer was added and mixed overnight, followed by centrifugation at 16,000 × g, 4°C for 1 h. EXO pellets were resuspended in 100 µl cold PBS. The purified EXOs were then collected using the Exo-spin™ column through careful centrifugation at 50 × g, 4°C for 1 min.
For transmission electron microscopy (TEM), a drop of EXOs was placed on a carbon-coated copper grid followed by the addition of a drop of 2% phosphotungstic acid to the stain for 5 min at room temperature. Subsequently, it was allowed to dry with a final examination using TEM (H-7650, Hitachi, Inc.). For nanoparticle tracking analysis (NTA), EXOs were added to the NanoSight instrument (Zetaview®, Particle Metrix) to measure the nanoparticle size. The EXOs were then separated and examined using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% SDS-PAGE), followed by immunoreaction with primary antibodies, anti-Alix (1:1,000, cat. no. 12422-1-AP), anti-Hsp90 (1:1,000, cat. no. 13171-1-AP), anti-Tsg101 (1:1,000, cat. no. 28283-1-AP), anti-GM130 (1:1,000, cat. no. 11308-1-AP) (all from ProteinTech Group, Inc.) at 4°C for overnight. Following incubation with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit, 1:10,000, cat. no. 111-035-003; goat anti-mouse, 1:10,000, cat. no. 115-035-003; both from Jackson ImmunoResearch Laboratories, Inc.) at room temperature for 1 h, the proteins were reacted with enhanced chemiluminescence reagent (ECL, Pierce; Thermo Fisher Scientific, Inc.).
PKH67-labeled EXOs and their endocytosis
Briefly, regarding PKH67 fluorescently-labeled EXOs, the EXOs were initially mixed with Diluent C (MIDI67, MilliporeSigma). Subsequently, PKH67-Diluent C dye solution was rapidly added, mixed and incubated at room temperature for 15 min, with subsequent termination using 0.5% BSA, which was followed by centrifugation at 12,000 × g, 4°C for 30 min and re-extraction as per the manufacturer's instructions. In vitro, PKH67-labeled EXOs were added to the U87MG and U251MG cells for 12 h. Subsequently, they were stained using DAPI (DA0001, Leagene, Inc.) at room temperature for 5 min and observed under a fluorescence microscope (IX73, Olympus Corporation).
Loading of EXOs with miRNA-124-3p via electroporation
EXOs were loaded with miR-124-3p through electroporation using a Gene Pulser Xcell (Bio-Rad Laboratories, Inc.) electroporation system. First, miR-124-3p mimics (Guangzhou RiboBio Co., Ltd.) were mixed with EXOs in an electroporation buffer at a final concentration of 200 nM. The mixtures were then cultured at 4°C for 10 min and transferred to ice-cold 0.2-cm cuvettes for electroporation at 400 V and 125 µF capacitance with a single pulse. Subsequently, the EXOs were maintained on ice for ≥15 min following electroporation. Negative control (NC) mimics (Guangzhou RiboBio Co., Ltd.) mixed with EXOs were electroporated to serve as a control.
Immunofluorescence
The NSCs (including neurospheres and monoplasts) and their differentiated cells (neurons, astrocytes and oligodendrocytes) (cell differentiation was induced using differentiation medium as follows: DMEM/F12 supplemented with 1% B27, 2% FBS and 1% penicillin-streptomycin, cultured for 7-10 days) were washed and fixed using 4% paraformaldehyde at room temperature for 30 min. After washing and fixation, the cells were incubated with primary rabbit anti-Nestin (1:200, cat. no. ab221660, Abcam), anti-SOX2 (1:200, cat. no. ab92494, Abcam), anti-βIII τubulin (Tuj1, 1:200, cat. no. ab18207, Abcam), anti-glial fibrillary acidic protein (GFAP; 1:200, cat. no. GB11096, Wuhan Servicebio Technology Co., Ltd.), anti-myelin oligodendrocyte glycoprotein (MOG; 1:200, cat. no. 12690-1-AP, ProteinTech Group, Inc.) and mouse anti-Musashi1 (1:200, cat. no. ab129819, Abcam), antibodies at 4°C overnight. Following incubation with secondary antibodies [goat anti-rabbit 488 (1:600, cat. no. 4412, Cell Signaling Technology, Inc.), anti-rabbit 555 (1:600, cat. no. 4413, Cell Signaling Technology, Inc.) and goat anti-mouse 555 (1:600, cat. no. 4409, Cell Signaling Technology, Inc.) at room temperature for 1 h, the slides were mounted with DAPI (Beyotime Institute of Biotechnology), followed by examination under an inverted fluorescence microscope (IX73, Olympus Corporation) with light incubation being avoided.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from all samples (glioma cells) using the Total RNA kit (Omega Bio-Tek, Inc.) as per the manufacturer's instructions. cDNA synthesis was performed using the Transcriptor First Strand cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.) and qPCR was performed using the ABI Detection System with FastStart Universal SYBR-Green Master (Roche Diagnostics) following the manufacturer's instructions. The primers used were as follows: miR-124-3p forward, 5′-ACA CTC CAG CTG GGT AAG GCA CGC GGT GAA-3′ and reverse, 5′-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG TTG GCA TT-3′; flotillin 2 (FLOT2) forward, 5′-TTG CTG ACT CTA AGC GAG CC-3′ and reverse, 5′-TCC ACG GCA ATC TGT TTC TTG-3′; U6 forward, 5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′; GAPDH forward, 5′-GTC TCC TCT GAC TTC AAC AGC G-3′ and reverse, 5′-ACC ACC CTG TTG CTG TAG CCA A-3′; ANXA5 forward, AAC CCT CTC GGC TTT ATG ATG C-3′ and reverse, 5′-CGC TGG TAG TAC CCT GAA GTG-3′; SUB1 forward, 5′-GGT GAG ACT TCG AGA GCC CT-3′ and reverse, 5′-GCG AAC ACT AAC GTA CCT CAT TT-3′; MYH9 forward, 5′-CCT CAA GGA GCG TTA CTA CTC A-3′ and reverse, 5′-CTG TAG GCG GTG TCT GTG AT; RHOG forward, 5′-ACT AAC GCT TTC CCC AAA GAG and reverse, 5′-GTG TAC GGA GGC GGT CAT AC-3′; and SMAD forward, 5′-CCA GCA GTA AAG CGA TTG TTG G-3′ and reverse, 5′-GGG GTA AGC CTT TTC TGT GAG-3′. RT-qPCR was performed under the following conditions: 95°C for 5 sec, followed by 45 cycles at 95°C for 10 sec, 60°C for 20 sec, and 72°C for 20 sec. At least three biological replicates were used. The results were then analyzed using the 2−ΔΔCq method (23), ΔCq=Cq (gene)-Cq (U6/GAPDH), and the 2−ΔΔCq indicated the difference in mRNA.
Western blot analysis
To analyze the protein levels, protein (glioma cells and tissues) was isolated using the Minute™ Total Protein Extraction kit (SD-001, Invent Biotechnologies), and the quantification of protein was performed using the Pierce BCA Protein Assay kit (cat. no. 23223, Thermo Fisher Scientific, Inc.). Equal amounts of total proteins (20-60 µg) from each cell group were subjected to SDS-PAGE (10%) and transferred onto polyvinylidene difluoride membranes (MilliporeSigma). The membranes were then blocked using 5% milk at room temperature for 1 h and probed with primary rabbit anti-FLOT2 (1:1,000, cat. no. 66881-1-Ig.), anti-PI3K (1:1,000, cat. no. bs-0128R, BIOSS), anti-AKT1 (1:1,000, cat. no. bs-0115R, BIOSS), anti-phosphorylated (p-)AKT1 (1:1,000, cat. no. bs-10996R, BIOSS), anti-GAPDH (1:1,000, cat. no. 10494-1-AP, ProteinTech Group, Inc.) and anti-β-actin antibodies (1:1,000, cat. no. bs-0061R, BIOSS) at 4°C for overnight. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit, 1:10,000, cat. no. 111-035-003; goat anti-mouse, 1:10,000, cat. no. 115-035-003; both from Jackson ImmunoResearch Laboratories, Inc.); subsequently, they were reacted with an enhanced ECL substrate (32106, Pierce; Thermo Fisher Scientific, Inc.), imaged using a Chemiluminescence imager (ChemiScope 6300, Clinx Science Instruments Co., Ltd.), and analyzed using ImageJ software (version 1.50i; National Institutes of Health).
Plasmid construction and dual-luciferase reporter assay
The target sequences of the FLOT2 wild-type (WT) 3'-UTR containing the predicted miR-124-3p binding sites and its mutant (MUT) 3'-UTR were synthesized. The fragments were sub-cloned into the pmirGLO dual-luciferase reporter vector (Promega Corporation) as per the manufacturer's instructions. For the luciferase reporter assay, glioma cells were cultured in 24-well plates and transfected with a complex of 50 nM miR-124-3p or NC mimics (Ribobio, Inc.) and the WT or MUT luciferase reporter vectors (OBiO Technology Corp., Ltd.) at 37°C for 48 h. Normalization was performed using the Renilla luciferase construct. Following 48 h of incubation at 37°C, the luciferase activity was measured using a dual-luciferase reporter system (E1960, Promega Corporation).
RNA interference and transfection
Duplex siRNA targeting human FLOT2 were purchased from GengPharma. Co., Ltd. Following a 24-h incubation at 37°C with an antibiotic-free medium, the glioma cells were transfected with anti-FLOT2 small interfering RNA (siFLOT2, 20 µM) using Lipofectamine 2000® Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 3 days prior to the start of the subsequent experiments. The FLOT2-siRNA sequences were as follows: siRNA-1 sense, 5′-GCU GUU GUG GUU CCG ACU A-3′ and antisense, 3′-CGA CAA CAC CAA GGC UGA U-5′; siRNA-2 sense, 5′-GCA GUU UCU GGG UAA GAA U-3′ and antisense, 3′-CGU CAA AGA CCC AUU CUU A-5′; siRNA-3 sense, 5′-CCA AGA UUG CUG ACU CUA A-3′ and antisense, 3′-GGU UCU AAC GAC UGA GAU U-5′. For the experiment using the AKT1 agonist, Sc79 (S7863, Selleck Chemicals LLC.), Sc79 (10 µg/ml) was used to treat the cells for 3 days following transfection with siRNA FLOT2; total protein was then extracted for western blot analysis.
Cell Counting Kit-8 (CCK-8) assay for cell viability assessment
The glioma cells were seeded in 96-well plates for cell viability analysis using the CCK-8 assay. After 24 h, EXOs or small molecules were added to the co-culture for 1-4 days; subsequently, 10 µl CCK-8 (Nanjing KeyGen Biotech Co., Ltd.) was added into each pore. After 2-4 h of incubation at 37°C, the absorbance value (OD 450 nm) of each pore was colorimetrically determined (Multiskan MK3, Thermo Fisher Scientific, Inc.).
Transwell invasion assays
Briefly, as regards the Transwell invasion assay, the U87MG and U251MG cells were added into 24-well plates with an 8-mm-pore polycarbonate membrane coated with 20 mg of Matrigel (BD Biosciences). The 2×104 cells were cultured in the upper chamber in serum-free medium; additionally, medium containing 10% FBS was added to the lower chamber. Following a 24-h incubation at room temperature, the invasive cells were fixed using 4% paraformaldehyde, stained using 0.1% crystal violet (Beyotime Institute of Biotechnology) at room temperature for 30 min, and photographed using an Inverted Microscope (IX73, Olympus Corporation). Three independent experiments were performed.
Wound healing assays
Briefly, for the wound healing assays, the U87MG and U251MG cells were seeded in complete medium of 6-well plates until at least 80% confluency was reached. Subsequently, a wound was created through scraper manual scraping. After removing the floating cells by washing twice using serum-free medium, the cells were incubated at room temperature for 24 h. Migrated cells were observed using an inverted microscope (IX73, Olympus Corporation). The values obtained were expressed as the cell migration percentage.
Glioma xenograft model
A total of 12 4-week-old BALB/c male nude mice were purchased from the Guangdong Animal Center. The present study was reviewed and approved (permit no. A2020-015) by the Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Guangzhou Medical University, and was performed according to the guidelines of the Committee on Animal Research and Ethics. All mice were kept in an aseptic environment with a constant humidity (40-60%) and temperature (24±2°C) with a 12-h light/dark cycle. The animals were also provided with free access to food and water, and the padding was replaced twice a week. To establish the glioma model, U87MG cells (1×107 cells in 100 µl PBS for each mouse) were subcutaneously injected into the right axilla of nude mice for 10 days; subsequently, EXOs loaded with miR-124-3p (concentration 4×108 particles in 20 µl PBS/each time; untreated cells with 20 µl PBS were used as a control, twice per week, n=6 mice/group) were used for tumor treatment by intratumoral injection, and the anesthesia method used was gas isoflurane 3-4% induction, 1-2% maintenance, flow 0.6-0.8 l/min. The mice were monitored daily for tumor development and sacrificed on day 28 after the injection of the glioma cells. No toxic side-effects were observed in the mice. The mice were euthanized by carbon dioxide inhalation (30% vol/min), and death was verified by the loss of consciousness, apnea and the fading of eye color. Subsequently, the tumors were removed and photographed, and the tumor volume and weight were recorded. The tumors were fixed in formalin and embedded in paraffin or snap-frozen in liquid nitrogen.
Bioinformatics analysis
Predicted miRNA targets or predicted genes were detected using the miRWalk (http://zmf.umm.uniheidelberg.de/apps/zmf/mirwalk2/), TargetScan (http://www.targetscan.org), and miRTar-Base (https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php) databases. Furthermore, the miRCancer database (http://mircancer.ecu.edu/index.jsp) and GEPIA database (http://gepia.cancer-pku.cn/index.html) were used to evaluate the expression of FLOT2 and its association with the overall survival (Kaplan-Meier method with the log-rank test) of patients with GBM derived from the database.
Statistical analysis
Statistical analyses were performed using GraphPad 8.0 software (GraphPad Software Inc.). Data were analyzed using a two-tailed Student's t-test and are presented as the mean ± standard deviation (mean ± SD). The significance of the differences between different groups was examined using one-way ANOVA followed by the Tukey-Kramer post hoc test. Values of P<0.05 and P<0.01 were considered to indicate statistically significant and highly statistically significant differences, respectively).
Results
Characterization of EXOs derived from NSCs
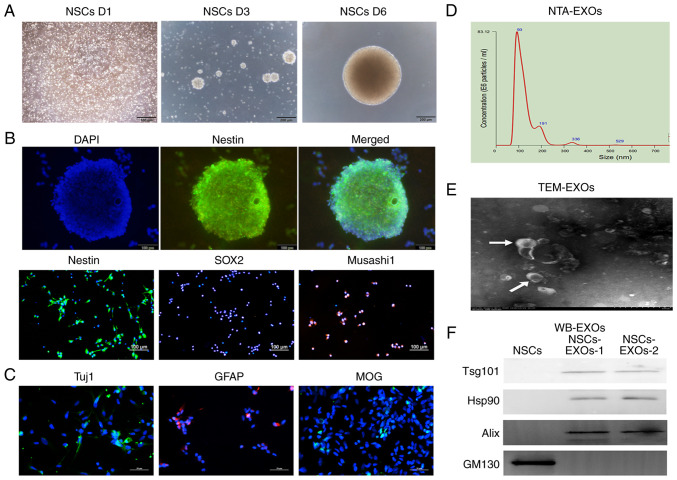
NSCs have self-renewal properties. When they were cultured in complete medium, the NSCs gradually formed increasingly large neurospheres over time (Fig. 1A). As shown in Fig. 1B, the NSCs expressed the typical NESTIN neurosphere marker, as well as the stem cell markers, SOX2 and Musashi1. Furthermore, NSCs are capable of multipotential differentiation; specifically, following induction in differentiation medium (DMEM/F12 supplemented with 1% B27, 2% FBS and 1% penicillin-streptomycin), they gradually differentiated into neurons, astrocytes and oligodendrocytes, which expressed Tuj1, GFAP and MOG, respectively, as revealed by immunofluorescence analysis (Fig. 1C).
Figure 1.
Characterization of exosomes derived from NSCs. (A) The morphology of NSCs was recorded on day 1, 3 and 6 after cell thawing. Scale bar, 200 µm. (B) Immunofluorescence detected the stem cell markers, Nestin, SOX2 and Musashi1. Scale bar, 100 µm. (C) Immunofluorescence detected neurons (Tuj1), astrocytes (GFAP) and oligodendrocytes (MOG). Scale bar, 50 µm. (D) NTA analysis revealed the particle distribution of exosomes derived from NSCs. (E) The exosomes was detected using TEM. Scale bar, 100 nm. (F) Western blot analysis of the exosomal markers, Tsg101, Hsp90 and Alix, and the Golgi marker, GM130. NSCs, neural stem cells; NTA, nanoparticle tracking analysis; MOG, myelin oligodendrocyte glycoprotein; GFAP, glial fibrillary acidic protein; TEM, transmission electron microscopy; EXOs, exosomes.
To evaluate NSC-derived EXOs, secreted vesicles were extracted from the NSC medium through standard super-centrifugation and SEC methods. EXO characterization was performed using NTA, TEM and western blot analysis methods based on the minimal information from research on extracellular vesicles 2018 (24). NTA revealed that the vesicle diameter mainly ranged from 30 to 200 nm (Fig. 1D). TEM revealed that the EXOs had a concave-cup morphology (Fig. 1E). Western blot analysis revealed that the EXOs significantly expressed exosomal classical markers, including Alix, Hsp90 and Tsg101; however, they did not express the cell protein (Golgi marker), GM130 (Fig. 1F). Taken together, these findings represented features of NSC-secreted EXOs; accordingly, we termed them as NSC-EXOs.
NSC-EXOs loaded with miR-124-3p suppress glioma cell proliferation, invasion and migration
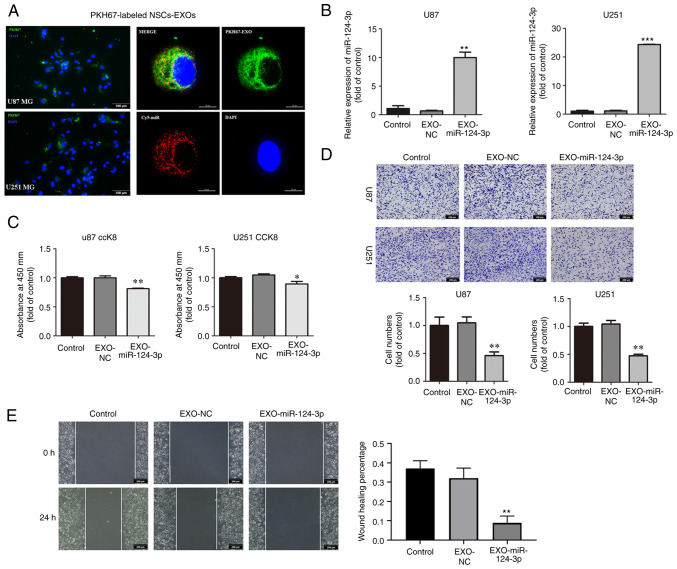
To assess NSC-EXO uptake by the glioma cells, NSC-EXOs were labeled using PKH-67 followed by co-incubation with the U87MG and U251MG cells, and loaded miR-124-3p mimics into NSC-EXOs through electroporation. Following a 24-h co-incubation, PKH67-labeled NSC-EXOs and carrier Cy5-miR were significantly detected in the cytoplasm and nucleus of the glioma cells (Fig. 2A). It was found that NSC-EXOs can freely enter glioma cells. Subsequently, following the addition of the EXOs loaded with miR-124-3p to glioma cells, RT-qPCR revealed a markedly higher miR-124-3p expression in the EXO-miR-124-3p mimics group than in the control and NC groups for both the U87MG and U251MG cells (Fig. 2B).
Figure 2.
NSC-EXOs loaded with miR-124-3p suppress glioma cell proliferation, invasion and migration. (A) EXOs were isolated from neural stem cell supernatant, dyed with PKH67 (green), and co-cultured with glioma cells for 24 h. Subsequently, they were dyed with DAPI (blue) and examined (EXOs labeled with PKH67, miR labeled with Cy5; original magnifications, ×100 (left panel) and ×400 (right panel). (B) miR-124-3p expression was detected using reverse transcription-quantitative PCR following incubation of the glioma cells for 48 h. (C) The proliferative ability of U87 and U251MG cells was tested using CCK-8 assay. (D) Transwell invasion assays in glioma cells were used to determine cell invasion. (E) Cell migration was detected through a wound-healing assay in glioma cells (original magnifications, ×100) Data represent the mean ± SD from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001, statistically significant differences between the NC and EXO-miR-124-3p group. NC, miR negative control; NSC, neural stem cell; EXOs, exosomes.
Subsequently, the effects of NSC-EXOs loaded with miR-124-3p on glioma cell proliferation, invasion and migration were evaluated using CCK-8, Transwell and wound healing assays. Compared with the control and NC groups, the EXO-miR-124-3p group exhibited a significantly decreased cell proliferation, particularly at 48 h following the addition of EXOs to glioma cells (Fig. 2C). Compared with the control and NC groups, the EXO-miR-124-3p group exhibited a significantly lower number of invaded and migrated cells in the Transwell and wound healing assays, respectively (Fig. 2D and E). These results indicated that the NSC-EXOs could successfully transfer miR-124-3p into glioma cells to suppress tumor cell proliferation, invasion and migration.
NSC-EXO-miR-124-3p inhibits glioma growth by targeting FLOT2
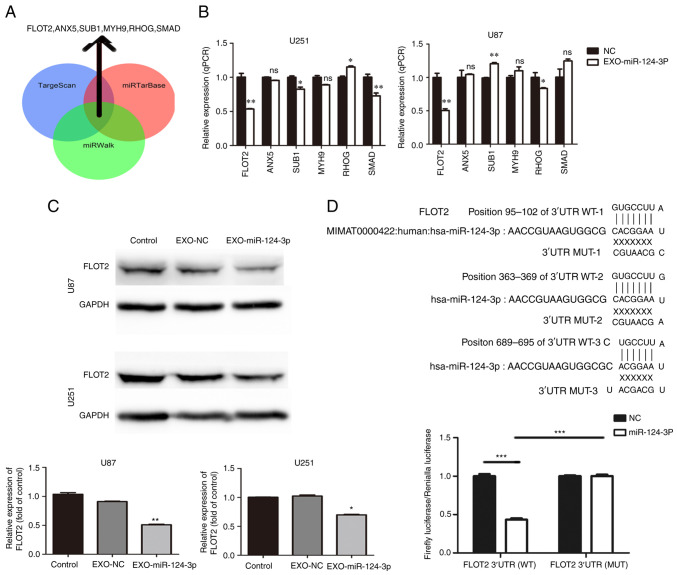
Three miRNA bioinformatics databases (TargetScan, miRTarBase and miRWalk) were used to predict the potential target genes of miR-124-3p; subsequently, six key candidate genes were selected (Fig. 3A). Subsequently, the NSC-EXOs loaded with miR-124-3p were incubated with the glioma cells for 48 h; moreover, RT-qPCR was used to determine the mRNA expression of the six candidate genes in the glioma cells. Compared with the control and NC groups, the EXO-miR-124-3p group exhibited a significantly lower FLOT2 expression (Fig. 3B). However, relative to the control/NC group, the expression of SUB1 was inconsistent in the EXO-miR-124-3p group of U87 and U251 cells (higher in U87 and lower in U251 cells), SMAD expression was only significantly lower in the U251 cells, but not in the U87 cells; and RHOG expression was higher in the U251 cells, and lower in the U87 cells (vs. the control). Thus, FLOT2 protein expression was then examined. Similarly, compared with the control and NC groups, the EXO-miR-124-3p group exhibited a significantly lower FLOT2 protein expression (Fig. 3C).
Figure 3.
miR-124-3p delivery through NSC-EXOs is involved in glioma by targeting FLOT2. (A) Venn diagrams illustrating the six potential miR-124-3p targets identified from TargetScan, miRTarBase and miRWalk. (B) Reverse transcription-quantitative PCR analysis of six potential miR-124-3p target genes in glioma cells. (C) FLOT2 protein levels were detected using western blot analysis in glioma cells transfected with control and NSC-EXOs loaded with miR-124-3p mimic or negative control. (D) The potential binding sites of miR-124-3p within the FLOT2 WT and MUT 3'-UTR. Luciferase reporter gene assays were used to analyze the miR-124-3p effect on luciferase activity. *P<0.05, **P<0.01 and ***P<0.001, statistically significant differences between the NC and EXO-miR-124-3p group. NC, miR negative control; NSC, neural stem cell; EXOs, exosomes; FLOT2, flotillin 2; siFLOT2, siRNA FLOT2; WT, wild-type; MUT, mutant.
Consequently, the present study further investigated whether FLOT2 was the direct miR-124-3p target in glioma cells. A luciferase reporter plasmid containing the functional 3'-UTR and mutant 3'-UTR site of FLOT2 (termed pMIR-REPORT Luciferase-FLOT2 3'-UTR WT and MUT) was constructed (Fig. 3D). Following co-transfection of FLOT2 WT or MUT plasmid with either miR-124-3p mimics or NC in glioma cells for 48 h, a significantly decreased luciferase activity by ~57% was observed in the group co-transfected with miR-124-3p mimics and luc-FLOT2 3'-UTR WT than in the other groups (Fig. 3D). These findings demonstrated that EXOs-miR-124-3p directly targeted the 3'-UTR of FLOT2 and mediated glioma cell progression mainly by regulating FLOT2 expression.
NSC-EXOs loaded with miR-124-3p suppress glioma cell progression via the FLOT2/AKT pathway
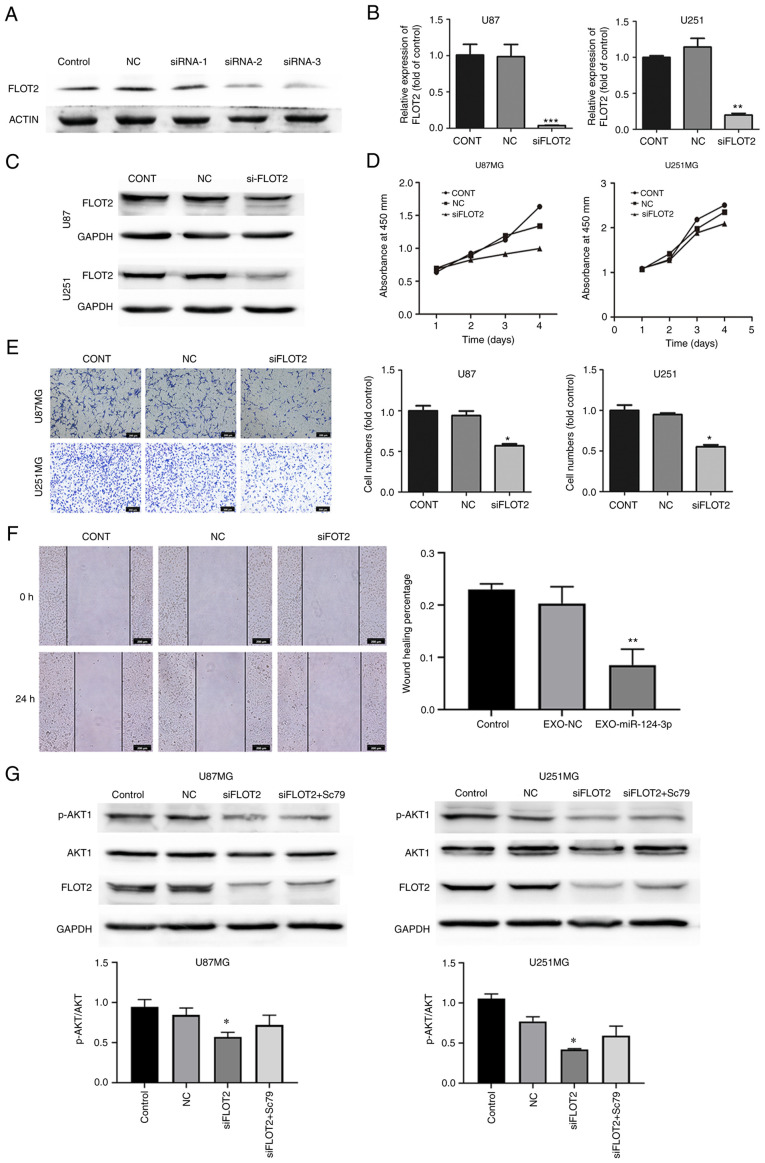
siRNA targeting FLOT2 (siFLOT2) was constructed to knockdown FLOT2 expression in glioma cells and siRNA-2 was screened as the most efficient siFLOT2 (Fig. 4A), with the findings confirming a marked decrease in both the mRNA and protein expression of FLOT2 in glioma cells (Fig. 4B and C). Subsequently, the effects of siFLOT2 on glioma cell proliferation, invasion and migration were evaluated. CCK-8, Transwell and wound healing assays confirmed that siFLOT2 significantly suppressed glioma cell proliferation, invasion and migration, respectively (Fig. 4D-F). Taken together, these findings suggested that NSC-EXOs loaded with miR-124-3p regulated glioma cell progression mainly through the direct suppression of FLOT2 expression.
Figure 4.
NSC-EXOs loaded with miR-124-3p suppress glioma cell progression via the FLOT2/AKT pathway. (A) Western blot analysis was used to examine FLOT2 expression following siRNA knockdown. (B) The efficiency of si-FLOT2 knockdown was determined using reverse transcription-quantitative PCR. (C) FLOT2 protein expression in glioma cells was detected using western blot analysis. (D) The proliferative ability of glioma cells transfected with si-FLOT2 or NC was examined using CCK-8 assay; the x axis represents the days. (E) Cell invasion was determined using Transwell invasion assay in glioma cells transfected with si-FLOT2 (original magnification, ×100). (F) Cell migration was detected using wound healing assay in glioma cells transfected with si-FLOT2 (original magnification, ×100). *P<0.05, **P<0.01 and ***P<0.001, statistically significant differences between the NC and siFLOT2 group. (G) FLOT2 and AKT1 expression was detected following the use of Sc79. Data are presented as the mean ± SD of three independent experiments. *P<0.05, statistically significant difference the siFLOT2 and siFLOT2 + Sc79 group. NC, siRNA negative control; NSC, neural stem cell; EXOs, exosomes; FLOT2, flotillin 2.
A previous study by the authors demonstrated that miR-124-3p promoted glioma progression mainly via the PI3K/AKT pathway (20). As shown in Fig. 4G, PI3K/AKT protein expression was examined and it was found that compared with the control and NC groups, there was a decreased p-AKT1/AKT1 expression in the siFLOT2 group; however, PI3K expression was unaltered (data not shown). Subsequently, the AKT1 agonist, Sc79, was used to rescue AKT1 expression in the siFLOT2 group. Compared with the si-FLOT2 group, the combined treatment group exhibited a higher AKT1 expression (Fig. 4G). These findings suggested that EXO-miRNA-124-3p/FLOT2 mediated glioma cell progression via the AKT1 signaling pathway. Thus, NSC-EXOs loaded with miR-124-3p suppressed glioma cell progression mainly via the FLOT2/AKT1 pathway.
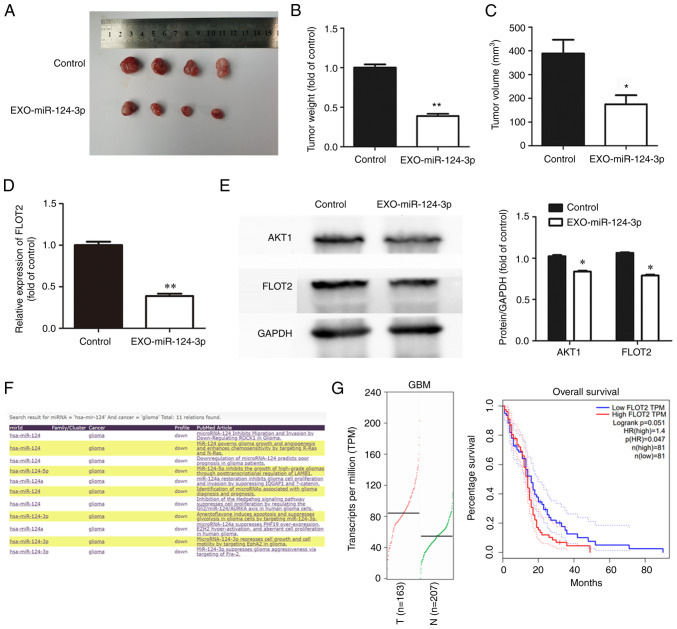
NSC-EXOs loading with miR-124-3p inhibits tumor growth in vivo
To explore the effects of NSC-EXOs loaded with miR-124-3p on glioma cell growth in vivo, glioma cells were initially injected into the subcutaneous tissue of nude mice after 10 days to allow tumor growth at an appropriate volume. Subsequently, NSC-EXOs loaded with miR-124-3p were used for treatment, with PBS as a control. Compared with the control group, treatment using NSC-EXOs-miR-124-3p markedly suppressed tumor growth (Fig. 5A). Additionally, compared with the control group, the tumor-bearing mice in the treatment group exhibited a significantly smaller tumor volume and weight in (Fig. 5B and C). This demonstrated that NSC-EXOs loaded with miR-124-3p also had a marked inhibitory effect on glioma cells in vivo with no toxic side-effects observed in the mice. The present study further examined FLOT2 expression in tumor tissue using RT-qPCR and western blot analysis. The results revealed the significant inhibition of FLOT2 and AKT1 expression (Fig. 5D and E) in the group treated with the NSC-EXOs loaded with miR-124-3p. In addition, the miRCancer and GEPIA databases were used to evaluate gene expression, as well as the association between FLOT2 expression and the overall survival of patients with GBM (Fig. 5F and G). The expression of hsa-miR-124 was found to be decreased in gliomas, which was consistent with the findings from a previous study (20) by the authors. The expression of FLOT2 was significantly increased in the tissues of patients with GBM. Furthermore, the overall survival of patients with GBM with a high FLOT2 expression was significantly lower than that of patients with a low expression. There was a negative association between miR-124-3p and FLOT2 in gliomas. Thus, NSC-EXOs loading with miR-124-3p also inhibited glioma growth in vivo, which was consistent with the results obtained in vitro; moreover, NSC-EXO-miR-124-3p suppressed glioma cell progression mainly via the FLOT2/AKT1 pathway.
Figure 5.
NSC-EXOs loaded with miR-124-3p inhibit tumor growth in vivo. (A) Nude mice were injected with subcutaneous xenografts of U87 glioma cells, followed by euthanization after 3-week treatment using NSC-EXOs-miR-124-3p or PBS (twice per week). (B and C) Tumor weight and volume were measured after the 3-week treatment. (D) The expression of FLOT2 was measured in mouse tumors using reverse transcription-quantitative PCR. (E) FLOT2 and AKT1 protein expression in mouse tumors were detected using western blot analysis. Data are presented as the mean ± SD of three independent experiments. *P<0.05 and **P<0.01, vs. control orEXO-miR-124-3p group. (F) The expression of hsa-miR-124 was significantly decreased in gliomas according to data from miRCancer (http://mircancer.ecu.edu/index.jsp). (G) The expression of FLOT2 was significantly increased in GBM tissues, and the overall survival of patients with GBM with a high FLOT2 expression was significantly decreased (data were from the GEPIA database; http://gepia.cancer-pku.cn/index.html). NSC, neural stem cell; EXOs, exosomes; FLOT2, flotillin 2; GBM, glioblastoma multiforme.
Discussion
The present study mainly explored the ability of natural biological drug carrier EXOs to deliver small molecular miRNA into glioma cells, and found that NSC-EXO-miR-124-3p significantly inhibited glioma growth. This was achieved by specifically suppressing downstream FLOT2 expression and downregulating the AKT1 signal pathway in gliomas (Fig. 6). Currently, EXOs are being developed as diagnostic biomarkers for some diseases (25-27); however, the therapeutic advantages of EXOs remain to be completely exploited. Recent studies have confirmed that EXOs can be developed as new drug carriers, and can be used to deliver small molecules, including miRNAs, to target cells, which has provided new insight into disease treatment, particularly for numerous refractory diseases, including tumors (28,29). Furthermore, studies have confirmed that EXOs can successful transfer miRNAs to regulate the cell biological phenotype For example, Wu et al (30) utilized bone marrow mesenchymal stem cell-derived exosomal miR-193a to reduce drug resistance in non-small cell lung cancer; Huang et al (31) demonstrated that EXOs from plasma of patients with medulloblastoma could transfer miR-130b-3p to inhibit medulloblastoma tumorigenesis; Munson et al (32) discovered that exosomal miR-16-5P was a target suppressor miRNA for malignant mesothelioma. Furthermore, the present study used bio-carrier NSC-EXOs for loading miR-124-3p and found that they could successfully deliver miR-124-3p into glioma cells to suppress tumor progression.
Figure 6.
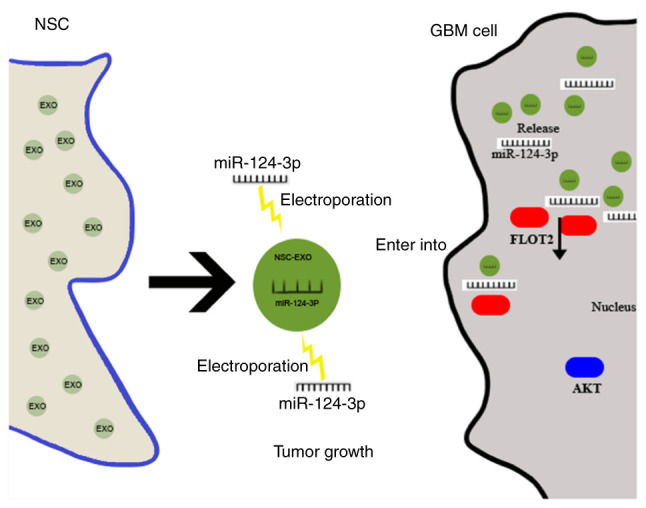
Schematic diagram of NSC-EXOs transferred miR-124-3p to inhibit glioma growth by targeting FLOT2. EXOs derived from NSCs were loaded with miR-124-3p mimics via electroporation, after deliver into glioma cells, the miR-124-3p was released by EXOs and specifically targeted the 3'-untranslated region of FLOT2 to inhibit the downstream FLOT2 expression, and then downregulated the expression of AKT1 signal pathway, finally suppressing glioma cell growth. NSC, neural stem cell; EXOs, exosomes; FLOT2, flotillin 2.
NSCs are exclusive brain stem cells involved in nerve tissue growth, development and repair. Moreover, NSCs are considered the most promising natural resource in the CNS and are capable of chemotaxis to lesions, including gliomas (7,8,10,33). The authors previously found that miR-124-3p expression was downregulated in glioma cells and tissues (20). Therefore, the present study used the advantages of NSCs and the capacities of carrier EXOs to load miR-124-3p into NSC-EXOs, named EXO-miR-124-3p. Subsequently, this molecular drug miRNA was successfully transferred into glioma cells to suppress tumor progression. The findings presented herein confirmed the hypothesis that carrier NSC-EXOs deliver miRNA-124-3p into glioma cells and are involved in inhibiting tumor cell proliferation, invasion and migration. Moreover, the effect of EXOs-miR-124-3p on glioma cell proliferation was about 20%, and the effects on cell invasion and migration were much more obvious, which was ~50%; therefore, EXO-miR-124-3p mediated the invasion and migration of glioma cells possibly via key downstream targets.
The present study then further investigated the potential mechanisms underlying the treatment effects of NSC-EXOs loaded with miR-124-3p on GBM progression. For this purpose, six potential target genes of miR-124-3p from three bioinformatics databases (TargetScan, miRTarBase and miRWalk) were selected, with FLOT2 being the key candidate. FLOT2 expression was significantly decreased by NSC-EXOs loaded with miR-124-3p and glioma cells. The FLOT2 gene is associated with numerous cancer types (34-36). FLOT2 is involved in the formation of caveolae or caveolae-like vesicles, has the functions of mediating cell adhesion, and mainly regulates the process of cell invasion and migration. Hazarika et al (34) revealed that the increased expression of FLOT2 was associated with melanoma progression. Wang et al (35) reported an association of a poor survival outcome of patients with breast cancer with a high FLOT2 expression level. Wang et al (36) also confirmed that FLOT2 promoted hepatocellular carcinoma by modulating the cell cycle and inducing epithelial-mesenchymal transition. Herein, when assessing whether FLOT2 was the direct miR-124-3p target in glioma cells, double luciferase reporter gene assay revealed that miR-124-3p directly targeted the 3'-UTR of FLOT2. The proliferation, invasion and migration of U87 and U251 glioma cells were significantly reduced by the knockdown of FLOT2 expression in tumor cells. Thus, bio-carrier NSC-EXOs loaded with miR-124-3p mediated glioma cell progression (particularly cell invasion and migration) mainly by inhibiting FLOT2.
Additionally, AKT, which is a classical signaling pathway, is involved in cancer progression (37,38). The present study found that NSC-EXOs loaded with miR-124-3p mimics downregulated FLOT2 expression and simultaneously inhibited p-AKT1 expression. This was reversed by the addition of the AKT1 agonist, Sc79. This suggested that NSC-EXOs loaded with miR-124-3p mimics suppressed glioma progression mainly by inhibiting FLOT2 and downregulating the AKT1 pathway. Notably, NSC-EXOs loaded with miR-124-3p significantly reduced glioma growth in mice; as PBS group was used as a control in vivo, the use of NSC-EXOs loaded with miR-124-3p demonstrated the inhibitory effects of these NSC-EXOs on tumor growth. Taken together, these findings confirmed that bio-carrier NSC-EXOs could be used to deliver molecular drug miR-124-3p into glioma cells, with subsequent EXO-miR-124-3p to target the downregulation of the FLOT2/AKT1 pathway. This eventually suppressed the proliferation, invasion and migration abilities of glioma.
However, the present study also has some limitations. These include the fact that the stimulatory effect of the BBB was not examined, multiple targets may be involved in in EXO-miR-124-3p treatment which need to be determined, and no in situ xenograft model of glioma was used. Furthermore, the contents in EXOs involved in the regulation of glioma cells need to be further investigated. The authors aim to further explore other applications of bio-carrier NSC-EXOs, including the key components of NSC-EXOs in the future.
In conclusion, GBM is the most malignant and invasive tumor in the CNS, with a very poor prognosis. The present study exploited the ability of new biological carrier NSC-EXOs to deliver miR-124-3p mimics into gliomas. It was found that EXO-miR-124-3p could specifically target the 3'-UTR of FLOT2 to inhibit downstream FLOT2 expression. Furthermore, these EXOs downregulated the AKT1 signaling pathway and suppressed glioma cell growth. The findings of the present study provide evidence of stem cell-free molecular targeted therapy based on bio-carrier EXOs, and these findings may aid in the development of novel therapeutic strategies for neurological diseases in the future.
Acknowledgments
Not applicable.
Funding Statement
The present study was supported by the Natural Science Foundation of Guangdong Province (grant no. 2022A1515011328) and the China Postdoctoral Science Foundation (grant nos. 2019TQ0071 and 2020M672592).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GZ and YeW conceptualized the study. YoW, DC and CW were involved in the study methodology. CQ, YoW, YJ and GZ performed the experiments. CQ, YoW and YJ were involved in the writing of the original draft. GZ and YeW were involved in the writing, reviewing and editing of the manuscript, and supervised the study. GZ and YeW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was reviewed and approved (permit no. A2020-015) by the Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Guangzhou Medical University, and was performed according to the guidelines of the Committee on Animal Research and Ethics.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 4.Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas-implications for classification and therapy. Nat Rev Clin Oncol. 2017;14:434–452. doi: 10.1038/nrclinonc.2016.204. [DOI] [PubMed] [Google Scholar]
- 5.Ammirati M, Chotai S, Newton H, Lamki T, Wei L, Grecula J. Hypofractionated intensity modulated radiotherapy with temozolomide in newly diagnosed glioblastoma multiforme. J Clin Neurosci. 2014;21:633–637. doi: 10.1016/j.jocn.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagó JR, Alfonso-Pecchio A, Okolie O, Dumitru R, Rinkenbaugh A, Baldwin AS, Miller CR, Magness ST, Hingtgen SD. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun. 2016;7:10593. doi: 10.1038/ncomms10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutukula N, Elkabetz Y. 'Neural killer' cells: Autologous cytotoxic neural stem cells for fighting glioma. Cell Stem Cell. 2017;20:426–428. doi: 10.1016/j.stem.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Morshed R, Cheng SH, Tobias A, Auffinger B, Wainwright DA, Zhang L, Yunis C, Han Y, Chen CT, et al. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small. 2013;9:4123–4129. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portnow J, Synold TW, Badie B, Tirughana R, Lacey SF, D'Apuzzo M, Metz MZ, Najbauer J, Bedell V, Vo T, et al. Neural stem cell-based anticancer gene therapy: A first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23:2951–2960. doi: 10.1158/1078-0432.CCR-16-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marban E. The secret life of exosomes: What bees can teach us about next-generation therapeutics. J Am Coll Cardiol. 2018;71:193–200. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue X, Lan F, Xia T. Hypoxic glioma cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol Ther. 2019;27:1939–1949. doi: 10.1016/j.ymthe.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H, Zhao H, Xiao X, Huang Q, Zeng W, Tian B, Ma T, Lu D, Jin Y, Li Y. Long non-coding RNA MALAT1 upregulates ZEB2 expression to promote malignant progression of glioma by attenuating miR-124. Mol Neurobiol. 2021;58:1006–1016. doi: 10.1007/s12035-020-02165-0. [DOI] [PubMed] [Google Scholar]
- 19.Shi Z, Chen Q, Li C, Wang L, Qian X, Jiang C, Liu X, Wang X, Li H, Kang C, et al. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol. 2014;16:1341–1353. doi: 10.1093/neuonc/nou084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Chen L, Khan AA, Li B, Gu B, Lin F, Su X, Yan J. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int J Cancer. 2018;143:635–644. doi: 10.1002/ijc.31329. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Chen L, Guo X, Wang H, Chen W, Wu G, Gu B, Miao W, Kong J, Jin X, et al. Comparative analysis of microRNA expression profiles of exosomes derived from normal and hypoxic preconditioning human neural stem cells by next generation sequencing. J Biomed Nanotechnol. 2018;14:1075–1089. doi: 10.1166/jbn.2018.2567. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Zhu Z, Wang H, Yu Y, Chen W, Waqas A, Wang Y, Chen L. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J Adv Res. 2020;24:435–445. doi: 10.1016/j.jare.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extra-cellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saadatpour L, Fadaee E, Fadaei S, Nassiri Mansour R, Mohammadi M, Mousavi SM, Goodarzi M, Verdi J, Mirzaei H. Glioblastoma: Exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther. 2016;23:415–418. doi: 10.1038/cgt.2016.48. [DOI] [PubMed] [Google Scholar]
- 26.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Liu J, Sun G, Meng H, Wang J, Guan Y, Yin Y, Zhao Z, Dong X, Yin S, et al. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett. 2019;466:1–12. doi: 10.1016/j.canlet.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Katakowski M, Chopp M. Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. 2016;36:343–352. doi: 10.1007/s10571-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo S, Chen J, Chen F, Zeng Q, Liu WL, Zhang G. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. 2020 Nov 10; doi: 10.1136/gutjnl-2020-321187. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, Liu J, Mu Y, Yuan F, Liu W, Zhao Y. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11:801. doi: 10.1038/s41419-020-02962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Xue P, Han X, Zhang C, Yang L, Liu L, Wang X, Li H, Fu J, Zhou Y. Exosomal miR-130b-3p targets SIK1 to inhibit medulloblastoma tumorigenesis. Cell Death Dis. 2020;11:408. doi: 10.1038/s41419-020-2621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munson PB, Hall EM, Farina NH, Pass HI, Shukla A. Exosomal miR-16-5p as a target for malignant mesothelioma. Sci Rep. 2019;9:11688. doi: 10.1038/s41598-019-48133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin EY, Cooper DD, Abbott KL, Lennon J, Nagaraja S, Mackay A, Jones C, Vogel H, Jackson PK, Monje M. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170:845–859.e19. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazarika P, McCarty MF, Prieto VG, George S, Babu D, Koul D, Bar-Eli M, Duvic M. Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res. 2004;64:7361–7369. doi: 10.1158/0008-5472.CAN-04-0823. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Yang Q, Guo L, Li XH, Zhao XH, Song LB, Lin HX. Flotillin-2 is associated with breast cancer progression and poor survival outcomes. J Transl Med. 2013;11:190. doi: 10.1186/1479-5876-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM, Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al. Flot2 promotes tumor growth and metastasis through modulating cell cycle and inducing epithelial-mesenchymal transition of hepatocellular carcinoma. Am J Cancer Res. 2017;7:1068–1083. [PMC free article] [PubMed] [Google Scholar]
- 37.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 38.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.