Abstract
Background:
Perioperative pediatric anxiety is common and can have a negative psychological impact on children undergoing surgery and anesthesia. Studies have shown an incidence of anxiety at induction of up to 50%. Audiovisual distraction, including virtual reality (VR), is a non-invasive, non-pharmacological modality that may reduce perioperative anxiety. The goal of this study was to determine whether immersive audiovisual distraction with a VR headset during induction of general anesthesia (GA) in pediatric patients reduced preoperative anxiety.
Methods:
In this randomized, controlled, parallel-group study, 71 children aged 5 to 12 years scheduled for elective surgery with GA were randomly allocated to virtual reality (VR group) or a non-virtual reality control (No VR group). VR group patients underwent audiovisual distraction with a VR headset during induction in the operating room, whereas the control group received no audiovisual distraction. The primary outcome was the Modified Yale Preoperative Anxiety Scale (mYPAS), which was measured at three time points to assess patient anxiety: in the preoperative holding area before randomization, on entering the operating room, and during induction of GA. The primary outcome was analyzed using univariate analysis and a linear mixed-effects model. Secondary outcomes included post-induction parental anxiety measured by the State-Trait Anxiety Inventory, pediatric induction compliance, and parental satisfaction.
Results:
Average patient age was 8.0 ± 2.3 years (mean ± SD) and 51.4% of patients were female. Baseline variables were not substantially different between the VR group (33 patients) and No VR group (37 patients). No patients received preoperative anxiolytic medication. Baseline mYPAS scores were not different between the groups, with scores of 28.3 [23.3–28.3] (median [IQR]) in both. The change in mYPAS scores from baseline to time of induction was significantly lower in the VR group vs. control (0.0 [0.0–5.0] vs. 13.3 [5.0–26.7], p<0.0001). In the mixed-effects model, the VR group had an estimated 6.0-point lower mYPAS score (95% CI, 0.7–11.3, p=0.03) at room entry than the No VR group, and 14.5-point lower score (95% CI, 9.3–19.8, p<0.0001) at induction vs. control. Randomization to VR did not alter parental anxiety (0 [−2 to 2]), pediatric induction compliance (0 [0 to 0]), or parental satisfaction (−3 [−8 to 2]) (difference in medians [95% CI]).
Conclusions:
This study demonstrates a reduction in pediatric preoperative anxiety with the use of VR. Preoperative VR may be an effective non-invasive modality for anxiolysis during induction of anesthesia in children.
Introduction
Perioperative pediatric anxiety is common and can have a negative psychological impact on children undergoing anesthesia and surgery. Prior research has shown that induction of general anesthesia is the most stressful event for children before surgery and the incidence of distressing anxiety at induction can be as high as 50% in pediatric patients.1–3 The negative psychological impact of perioperative anxiety can stretch beyond the perioperative period and includes maladaptive postoperative behaviors such as separation anxiety, nightmares, and eating disorders.1,4–8 Reasons for pediatric anxiety include fear of the unknown, loss of control, potential pain, and separation from parents. Common approaches to pediatric anxiety in the perioperative period include premedication with oral midazolam and parental presence during induction of anesthesia. These methods have their own challenges and limitations such as difficulty in delivery of medication to an anxious child and overall limited anxiolysis with parental presence.9
Several studies have explored audiovisual technology and distraction as novel alternative strategies and demonstrated their effectiveness in alleviating perioperative pediatric anxiety.10–14 Children today are increasingly familiar with technological devices, including smart phones, tablets, and even virtual reality. Virtual reality (VR) is the use of a headset to display a fully immersive three-dimensional environment.15 Distraction using VR has been shown to have beneficial effects on anxiety and distress in children. A recent systematic review and meta-analysis demonstrated the effectiveness of VR as a distraction mechanism to reduce pain and anxiety in pediatric patients undergoing medical procedures.15 A randomized clinical trial showed that children who underwent a preoperative VR tour of the operating room with a well-known animated character had significantly less preoperative anxiety and increased compliance during induction of anesthesia.16 In addition, VR has been applied to pediatric settings outside of the operating room, including during vascular access procedures and radiation therapy.17,18 Studies have also shown high patient, parent, and provider satisfaction with audiovisual distraction during induction of anesthesia.11,19 Notably, however, there have been no studies examining the use of VR during induction of anesthesia in children.
The goal of this study was to evaluate whether immersive distraction utilizing a VR mobile application and headset would reduce preoperative anxiety in pediatric patients undergoing elective surgery and induction of general anesthesia.
Methods
In this pragmatic, randomized controlled, parallel-group study, children aged 5 to 12 years scheduled for elective surgery with general anesthesia were randomly allocated to a virtual reality group (VR group) or a non-virtual reality control group (No VR group). The study was approved by the institutional review board at the University of California, San Francisco and was conducted at a single institution (University of California, San Francisco Benioff Children’s Hospital) between August 2018 and March 2019. Parental written informed consent and, for children aged 7 years or greater, pediatric assent were obtained prior to enrollment. Parental written informed consent was also obtained before any media, such as demonstrative photos, were collected. The study was prospectively registered on Clinicaltrials.gov with the identifier NCT03583450 (Principal Investigator: Michael Jung, registered July 11, 2018) and adheres to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Figure 1).
Figure 1:
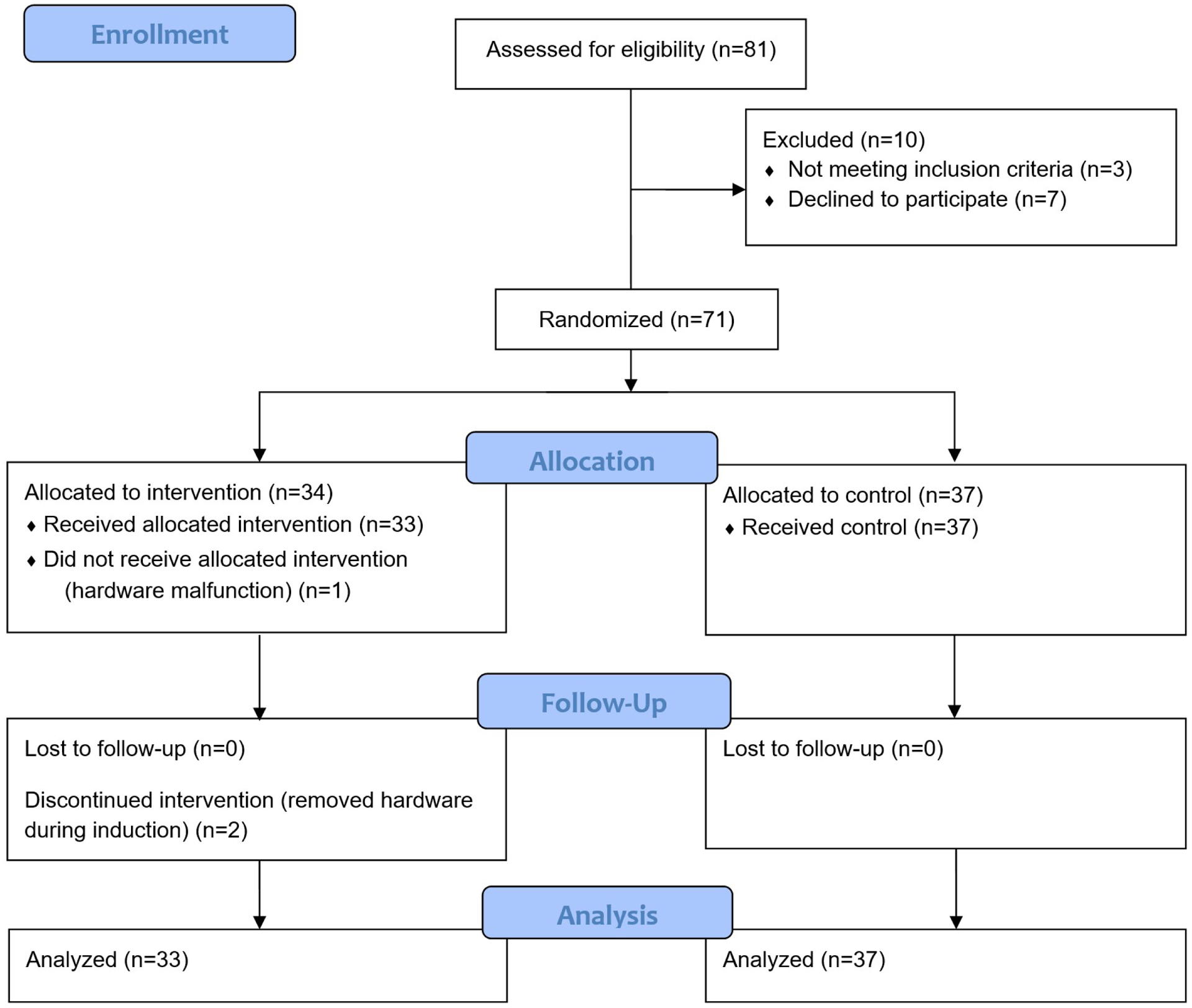
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participants.
Inclusion criteria were patients aged 5 to 12 years who presented for an elective surgery or procedure requiring general anesthesia via inhaled induction. Due to the size of the VR headset and VR software content, it was determined prior to study enrollment that it would not be feasible to include patients under the age of 5. Exclusion criteria were patients with injuries to the head/face that would prohibit wearing of the headsets; loss of consciousness, altered mental status, life-threatening injuries/illness or multi-trauma; open skin, lice, scabies, or other infectious skin conditions on the head/face; symptoms of vertigo; blindness (best-corrected visual acuity < 20/200 in the better-seeing eye; determined via parental report and confirmed with written prescription if deemed necessary); significant developmental or cognitive delays; patients on whom the VR headset did not fit appropriately; and non-English speaking patients due to limited availability of non-English study documents and consents.
Parental consent and, for children aged 7 years or greater, pediatric assent, followed by baseline assessment were performed in the preoperative area prior to randomization. Following baseline measurement, participants were randomized using a secure, web-based application (Research Electronic Data Capture, REDCap, Vanderbilt University, Nashville, TN) and the aid of a statistician.20,21 The simple randomization sequence was concealed from study staff until completion of baseline assessment. After consent and assent, if indicated, were obtained and baseline assessment performed, patient information was entered into the secure web-based application which then revealed a time-stamped randomized treatment assignment. Patients in the VR group received audiovisual distraction with a VR headset during inhaled mask induction of general anesthesia in the operating room. The No VR group served as the control group and received standard medical care without any audiovisual devices.
Patients assigned to the VR group received a customized Samsung Gear VR headset (Samsung Electronics, Suwon, South Korea) that displayed a pre-selected, interactive game (Mighty Immersion, Palo Alto, CA) designed for pediatric perioperative use featuring an animated animal character moving through a landscape. In preliminary testing, the headsets were confirmed not to interfere with anesthesia face mask fit. The headsets had built-in speakers with adjustable volume that allowed patients to listen to parents and providers while engaging with the VR headset. In the preoperative area, patients in the VR group received a five-minute orientation to the headset that included instructions, an opportunity to temporarily wear the headset, adjust headset fit to the patient, and operate the controller. The VR headsets were then administered immediately before induction of GA. The headsets were non-disposable and cleaned between uses with disposable antimicrobial wipes.
Anesthesia providers conducted routine anesthetic care at their medical discretion independent of VR use, including the use of pre-medication for anxiolysis or parental presence during induction. At our institution, nearly all patients have parents present in the operating room during induction and patients rarely receive oral midazolam preoperatively. The standard inhaled mask induction of general anesthesia consists of using an age appropriate mask and circle system to administer oxygen (3 L/min) and nitrous oxide (7 L/min) for approximately one minute followed by an incremental increase in sevoflurane to a maximum vaporizer setting of 8%. An intravenous catheter was placed after inhaled induction of anesthesia and the elective surgery or procedure was performed.
The primary outcome was preoperative pediatric anxiety as measured by the Modified Yale Preoperative Anxiety Scale (mYPAS). The mYPAS is a validated perioperative pediatric anxiety instrument with observational measurements of anxiety in five categories (activity, emotional expressivity, state of arousal, vocalization, and use of parents) with a range of 23.3 to 100, with higher scores indicating greater anxiety.22 The mYPAS was administered at three time points: in the preoperative holding area (baseline, or T0), on entering the operating room (T1), and during induction of general anesthesia (T2). An independent anesthesiologist who was not part of the anesthesia team delivering care recorded all measurements. Secondary outcomes were perioperative parental anxiety, pediatric induction compliance, and parental and patient satisfaction. Perioperative parental anxiety was measured using the State-Trait Anxiety Inventory (STAI) at two time points: in the preoperative holding area (baseline, or T0) and after induction of general anesthesia (T3). The STAI is a validated self-reported anxiety instrument containing two separate two-item subscales that measure trait (baseline) and state (situational) anxiety, with a range of 20 to 80 with higher scores indicating greater anxiety.23 Pediatric induction compliance was measured by the Induction Compliance Checklist (ICC) during induction of general anesthesia (T2). The ICC is a previously reported 11-item observational scale used to describe the compliance of a child during induction of general anesthesia, with a range of 0 to 11 with higher scores indicating poorer compliance.24 Parental and patient satisfaction with the perioperative experience was measured with a previously reported 21-item parental and patient satisfaction questionnaire after induction of general anesthesia (T3).25
Statistical Analysis
For analysis, data were visualized using histograms and the Shapiro-Wilk test was used to determine whether continuous data were normally distributed. Baseline variables are reported with standardized differences, which are the difference in means or proportions divided by the standard deviation and used to assess imbalance. For standardized differences, established guidelines were used to interpret the magnitude of difference: 0.2 = small, 0.5 = medium, 0.8 = large.26 The Wilcoxon rank sum test was used for univariate comparisons for all continuous and ordinal data between the VR and No VR groups, and Fisher’s exact test was used to compare categorical data because expected cell frequencies were small.
The mYPAS score had three repeated measurements over time (T0, T1, and T2) and the between-group effect for the use of VR. The mYPAS score did not follow a normal distribution, and log transformation was also tested. Results from transformed data were not appreciably different, therefore raw scores are reported.
The primary analysis was a univariate comparison of mYPAS scores from T0 to T1 and T0 to T2 using Wilcoxon rank sum tests. The effect size corresponding to the primary analysis was the difference in medians, with the confidence limits calculated by bootstrapping with replacement with 200,000 repetitions. Bootstrapping has been found to provide better confidence limits with asymmetric data, such as the mYPAS score in our case.27 Confidence limits are 95% for raw mYPAS scores, and 97.5% for changes in mYPAS scores to account for multiple comparisons.
We also modeled the mYPAS scores using linear mixed-effects regression to address the within-subject correlation of mYPAS scores and the two repeated-measures time points (T1 and T2). Given the two time points, a linear model was deemed adequate. The model incorporated random slopes (i.e., trend over time from T1 to T2) and intercepts for each patient, and adjusted for baseline mYPAS score (T0), which was obtained prior to randomization. Baseline adjustment can increase the statistical power to improve the estimation of the treatment effect for studies with a small sample size.28 An interaction term between group and time point was used to remove the assumption that the impact of the intervention was the same at room entry (T1) vs. induction (T2).
Data are displayed as n (%), mean ± standard deviation (SD), median [interquartile range], and differences in median with confidence intervals. The significance level was 0.05 for each hypothesis. All statistical tests were two-tailed. Data were analyzed using Stata 14.2 (StataCorp, College Station, TX) and JMP Pro 14.0 (SAS Institute, Cary, NC). Patients were analyzed according to modified intention-to-treat, defined as analysis of patients who were randomized and received at least a portion of the study intervention. Mean and standard deviation used in the power analysis for the mYPAS scores were 30.1 and 8.4, for the control group per a study examining preoperative anxiety in pediatric patients aged 5 to 12 years.29 A power analysis indicated that 31 participants per group would yield a power of 80% (β=0.20) to detect a 20% (6 point) decrease in mYPAS scores. A significance level of 0.05 (α=0.05) was used. To account for potential drop-out, a recruitment goal of at least 70 patients was determined prior to the start of the study.
Results
81 patients were assessed for eligibility and 10 patients were excluded (Figure 1). Of the 10 excluded patients, 3 patients declined to be in the study and 7 patients did not meet eligibility, as they were non-English speakers. 71 patients underwent randomization, with 34 in the VR group and 37 in the No VR group. There was one unanticipated hardware malfunction due to battery depletion before intervention for one patient in the VR group, thus the patient did not receive the allocated intervention, and was not included in the analysis. No patients were lost to follow up. Two patients in the VR group discontinued the intervention midway through mask induction of anesthesia but were included in the analysis per modified intention-to-treat. These two patients discontinued the intervention as they wished to see their parents during the induction of anesthesia. A total of 70 patients (33 in the VR group and 37 in the No VR group) underwent analysis.
Basic demographic information is shown in Table 1. Baseline variables were not substantially different between the VR group (33 patients) and No VR group (37 patients). Parents were present during 87.9% of inductions in the VR group and 94.6% of inductions in the No VR group. No patients received preoperative anxiolytic medication.
Table 1.
Demographic and Perioperative Characteristics
| No Virtual Reality | Virtual Reality | Standardized Difference | |
|---|---|---|---|
| n | 37 (52.9%) | 33 (47.1%) | |
| Age (years) | 7.8 ± 2.3 | 8.2 ± 2.2 | 0.16 |
| Gender | 0.12 | ||
| Female | 18 (48.6%) | 18 (54.5%) | |
| Male | 19 (51.4%) | 15 (45.5%) | |
| Height (cm) | 130 ± 16 | 131 ± 12 | 0.07 |
| Weight (kg) | 31.9 ± 15.2 | 32.4 ± 11.6 | 0.03 |
| BMI (kg/m2) | 18.0 ± 4.8 | 18.3 ± 4.0 | 0.07 |
| Caucasian | 19 (51.4%) | 19 (57.6%) | 0.13 |
| ASA Class | 0.29 | ||
| 1 | 15 (40.5%) | 11 (33.3%) | |
| 2 | 18 (48.6%) | 15 (45.5%) | |
| 3 | 4 (10.8%) | 7 (21.2%) | |
| Prior General Anesthesia | 18 (48.6%) | 18 (54.5%) | 0.12 |
| Number of Prior GA’s | 2.7 ± 5.2 | 3.3 ± 4.2 | 0.13 |
| NPO length (minutes) | 842 ± 185 (33) | 855 ± 163 (25) | 0.07 |
| Anesthesia length (minutes) | 101 ± 119 | 82 ± 92 | 0.19 |
| Surgery length (minutes) | 77 ± 106 | 63 ± 86 | 0.15 |
| Parent Present? | 35 (94.6%) | 29 (87.9%) | 0.24 |
| mYPAS T0 | 28.3 [23.3, 28.3] | 28.3 [23.3, 28.3] | 0.14 |
| STAI State T0 | 38.0 [31.0, 45.0] | 36.0 [26.0, 43.0] | 0.08 |
Data are n (%), mean ± SD, mean ± SD (n) if n differs from total, or median [interquartile range]
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiology; GA, general anesthesia; NPO, nil per os; T0, baseline time point in preoperative area; mYPAS, Modified Yale Preoperative Anxiety Scale, scored 23.3–100; STAI, parental State-Trait Anxiety Inventory, scored 20–80.
Standardized difference is the difference in means or proportions divided by the standard deviation and calculates potential imbalance in groups; 0.2 = small, 0.5 = medium, 0.8 = large imbalance.26
Outcomes
The mYPAS scores at each time point in the VR and No VR groups are displayed in Figure 2. The change in mYPAS scores from baseline (T0) to time of induction (T2) were significantly lower in the VR group vs. control (0.0 [0.0, 5.0] vs. 13.3 [5.0, 26.7], difference in medians 13.3 (97.5% CI, 3.7–23.0, p<0.0001), and are shown in Table 2 and Figure 3. Twenty-seven patients (73.0%) in the No VR group had an increase in anxiety from T0 to T1, while only 6 patients (18.2%) had increased anxiety in the VR group (p<0.0001). 32 (86.5%) patients in the No VR group had increased anxiety at T2, while only 11 (33.3%) patient in the VR group had an increase in anxiety (p<0.0001).
Figure 2:
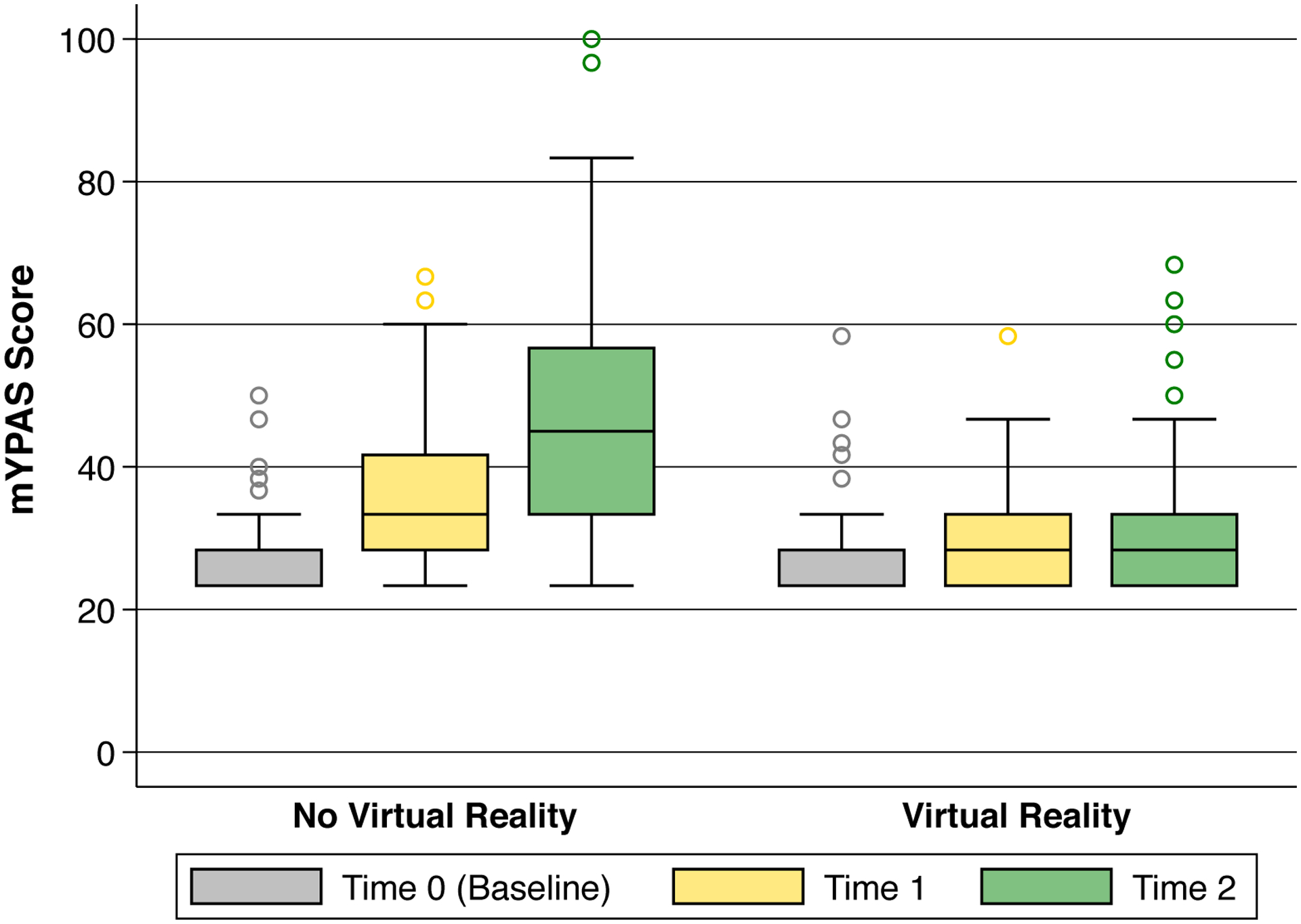
Box plot of raw mYPAS scores in the virtual reality (VR) and No VR groups. T0 = baseline in preoperative holding area (gray), T1 = entry into the operating room (yellow), T2 = during induction of general anesthesia (green). The mixed-effects model controlled for the baseline mYPAS score and demonstrated significant interaction between time and group assignment (p=0.003).
Table 2.
Modified Yale Preoperative Anxiety Scale (mYPAS) Outcomes
| No Virtual Reality | Virtual Reality | Difference (confidence interval) | P Value (between) | |
|---|---|---|---|---|
| n | 37 (52.9%) | 33 (47.1%) | ||
| Absolute mYPAS Score | ||||
| mYPAS T1 | 33.3 [28.3, 41.7] | 28.3 [23.3, 33.3] | 5.0 (0.3–9.7) | 0.03 |
| mYPAS T2 | 45.0 [33.3, 56.7] | 28.3 [23.3, 33.3] | 16.7 (6.0–27.4) | 0.0005 |
| Change in mYPAS Score | ||||
| mYPAS T1-T0 | 5.0 [0.0, 10.0] | 0.0 [0.0, 0.0] | 5.0 (2.0–8.0) | < 0.0001 |
| mYPAS T2-T0 | 13.3 [5.0, 26.7] | 0.0 [0.0, 5.0] | 13.3 (3.7–23.0) | < 0.0001 |
Data are n (%), median [interquartile range], or difference in median (confidence interval).
Univariate comparisons and p values were calculated using Wilcoxon rank sum.
Differences are the differences in median values with confidence intervals calculated by bootstrapping; confidence intervals for absolute mYPAS scores are 95%, and those for changes in mYPAS scores are 97.5% to compensate for multiple comparison.
Abbreviations: mYPAS, Modified Yale Preoperative Anxiety Scale, scored 23.3–100; T0 is the baseline time point in the preoperative holding area; T1 is the time point on entering the operating room; T2 is the time point on induction of general anesthesia; T1-T0 is the difference between T1 and T0; T2-T0 is the difference between T2 and T0.
Figure 3:

Box plot of mYPAS individual score differences in the VR and No VR groups. T1-T0 (yellow) = difference between T1 (entry into the operating room) and T0 (baseline in preoperative holding area). T2-T0 (green) = difference between T2 (during induction of general anesthesia) and T0 (baseline in preoperative holding area). Both mYPAS score differences were significantly different between the VR and No VR groups (p<0.0001).
After adjustment for baseline mYPAS score, the mixed-effects model demonstrated a significant group-by-time interaction (p=0.003). The VR group had an estimated 6.0-point lower mYPAS score (95% CI, 0.7–11.3, p=0.03) at time of room entry than the No VR group, and a 14.5-point lower mYPAS score (95% CI, 9.3–19.8, p<0.0001) at time of induction compared with the No VR group. Inclusion of an interaction term better describes not just a difference in group assignment, but how the mYPAS score changed over time, whereas a model without interaction would force the trajectory to be parallel from T1 to T2. The mYPAS scores did not change in the VR group from room entry to induction, whereas anxiety increased in the No VR group (Table 2, Figure 2). Adjustment for baseline variables was evaluated for variables with standardized differences ≥0.2. No variable with standardized difference ≥0.2 had a statistically significant effect on the association with group assignment and mYPAS scores.
The secondary outcomes of pediatric induction compliance, perioperative parental anxiety as measured by the STAI, and parental satisfaction did not show statistically significant differences (Table 3). Parental satisfaction was high in both groups.
Table 3.
Secondary outcomes.
| No Virtual Reality | Virtual Reality | Difference (95% confidence interval) |
P Value | |
|---|---|---|---|---|
| n | 37 (52.9%) | 33 (47.1%) | ||
| Induction Compliance | 0 [0, 1] | 0 [0, 0] | 0 (0 to 0) | 0.12 |
| Parental Satisfaction | 80 [76, 83] | 83 [76, 84] | −3 (−8 to 2) | 0.15 |
| Parental Satisfaction (%) | 95% [90%, 99%] | 99% [90%, 100%] | −4% (−9% to 2%) | 0.15 |
| STAI State (T3) | 39 [28, 47] | 35 [26, 46] | 4 (−6 to 14) | 0.41 |
| STAI State (T3-T0) | 0 [−3, 3] | 0 [−3, 3] | 0 (−2 to 2) | 0.97 |
Data are n (%), median [interquartile range], or difference in medians (confidence interval).
Univariate comparisons and p values were calculated using Wilcoxon rank sum.
Differences are the differences in median values with 95% confidence intervals calculated by bootstrapping.
Induction compliance score is 0–10; Parental satisfaction score is 21–84.
Abbreviations: STAI, parental State-Trait Anxiety Inventory, scored 20–80. T0 is the baseline time point in the preoperative holding area; T3 is the time point after induction of general anesthesia.
Discussion
Our study demonstrated a strong benefit of VR by preventing the increase in anxiety that occurred in pediatric patients as they entered the operating room and began induction of anesthesia. These results were consistent in both a simple analysis looking at the change in anxiety and the mixed-effects model, including repeated-measures and comparison of groups with and without VR. As demonstrated in Figure 2, mYPAS scores remained nearly unchanged in the VR group, while rising at each stage in the group without VR. The study may not have been powered to fully demonstrate the association of other factors with anxiety, such as prior general anesthesia and length of surgery and anesthesia. In this study, these factors did not differ between the VR and No VR groups, had no impact in the multivariable analysis, and thus did not impact our conclusions. Finally, the results showed assignment to the VR group reduced anxiety at T1, which corresponds to entering the operating room and before any headset application or induction of anesthesia. This indicates that the brief preoperative VR headset orientation or simply the anticipation of the VR headset alone may have been sufficient to reduce anxiety during entry into the operating room.
Virtual reality for pediatric patients has been an emerging audiovisual distraction modality to reduce anxiety. Initial studies in audiovisual distraction during induction of anesthesia in pediatric patients utilized technology such as smartphones, video glasses, and even operating room video monitors traditionally used for laparoscopic surgery to demonstrate reductions in mYPAS scores.11,19,30 This is the first study to evaluate the effectiveness of VR as an audiovisual distraction technology during the induction of general anesthesia. Moreover, the recent systematic review and meta-analysis on VR in pediatrics found, through 17 studies, that VR was an effective distraction intervention to reduce pain and anxiety in pediatric patients undergoing a variety of medical procedures.15 These medical procedures predominantly involved venous access, dental procedures, burn care, and oncological care, such as port access or dressing changes. Notably, the review found only one study examining the effects of VR during the perioperative period and that study used a VR headset to show a video of a preoperative tour of the operating room before the actual anesthetic and not during the induction of anesthesia as in our study.16 Thus, the purpose and findings of this study complement existing literature examining VR in pediatric patients. This study examines the previously unexplored realm of the specific use of VR during the induction of general anesthesia, a particularly important time when pediatric anxiety is prevalent and at high levels.
There are several limitations to this study. One limitation is that outcomes were limited to the immediate perioperative period; further follow-up may have identified any continued maladaptive behaviors due to anxiety manifesting in the postoperative period. Although it has been established that perioperative anxiety can lead to postoperative maladaptive behavior, it is unclear whether measures to reduce preoperative anxiety translate to a decrease in postoperative maladaptive behaviors. A randomized controlled trial looking at video distraction with a smartphone in pediatric patients saw no difference in postoperative emergence delirium or newly developed negative behavior after 2 weeks despite lower mYPAS scores on induction of general anesthesia in patients receiving video distraction.11 Further studies are needed to determine whether preoperative anxiety reduction measures will lead to decreased postoperative maladaptive behaviors in pediatric patients. Additionally, our study was an open-label study and thus subject to potential bias, as blinding was not possible or considered to be potentially detrimental to the patient. For example, the use of a control headset with a blank or blacked out screen, essentially functioning as a blindfold, was considered to potentially cause more anxiety than no headset during study design. In addition, the most common practice for induction of anesthesia in children at our institution included parental presence during induction and no administration of anxiolytic premedication, which may differ from the practices of other institutions. A limitation of the study may relate to the control group, where a different distraction method such as headphones may have served as an effective control group and reduced possible placebo effect. In the study design, we believe that our standard practice induction of anesthesia represented the appropriate control group and contributed to the pragmatic trial design. Finally, technical issues may occur with the equipment, as occurred with one patient in the VR group who did not receive a VR headset due to battery depletion.
Strengths of the study include the randomized controlled design, pragmatic implementation, and the utilization of established outcome scales that are validated and behavior-based to reduce observer bias. Additionally, the broad inclusion criteria and diversity of cases represented increases the generalizability of the study, which included cases performed by clinicians from dentistry, diagnostic radiology, gastroenterology, general surgery, hematology-oncology, interventional radiology, neurosurgery, ophthalmology, orthopedic surgery, otolaryngology, and urology.
Future directions for further inquiry include longer postoperative follow-up, VR for procedures such as regional nerve blocks in pediatric patients, and emerging immersive audiovisual technologies such as augmented reality, which allows one to view both the virtual reality image and real-world environment simultaneously.
In conclusion, the results of this pragmatic, randomized controlled trial demonstrate that immersive audiovisual distraction with VR prevented an increase in anxiety in pediatric patients receiving general anesthesia. Perioperative VR was demonstrated to be an effective, non-invasive modality for anxiolysis during induction of anesthesia in pediatric patients.
Key Points.
Question:
Does audiovisual distraction using virtual reality (VR) reduce perioperative anxiety in pediatric patients at time of induction of general anesthesia?
Findings:
Pediatric patients who used VR were found to have significantly lower anxiety at time of induction of anesthesia compared to patients who did not use VR.
Meaning:
Preoperative VR is an effective non-invasive modality for anxiolysis during induction of anesthesia in pediatric patients.
Acknowledgments:
Recognition:
Sam Rodriguez, M.D. and Thomas Caruso, M.D., M.Ed., Clinical Associate Professors at the Stanford Childhood Anxiety Reduction through Innovation and Technology (CHARIOT) Program, Stanford University, Palo Alto, CA, U.S.A, for collaboration on virtual reality hardware and programming.
Funding:
The authors acknowledge financial support from the UCSF Department of Anesthesia and Perioperative Care, the UCSF Clinical & Translational Science Institute Resident Research Grant, the UCSF Clinical & Translational Science Institute Resident Grant Number UL1 TR001872, and the NIH Mentored Career Development Award KL2TR001870.
Glossary of Terms:
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- CI
Confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- GA
General anesthesia
- ICC
Induction Compliance Checklist
- IQR
Interquartile range
- mYPAS
Modified Yale Preoperative Anxiety Scale
- No VR
No Virtual Reality
- NPO
Nil per os
- PEDI-VR
Pediatric Distraction on Induction of Anesthesia with Virtual Reality
- SD
Standard deviation
- STAI
State-Trait Anxiety Inventory
- T0
Baseline time point in preoperative area
- T1
Time point on entering the operating room
- T2
Time point on induction of general anesthesia
- T3
Time point after induction of general anesthesia
- UCSF
University of California, San Francisco
- VR
Virtual Reality
Footnotes
Conflicts of Interest/Financial Disclosures: JLS is a clinical affiliate of the nonprofit
Chariot Kids Consortium (www.chariotkids.org) and has no financial or commercial interests.
Clinical trial number and registry: Clinicaltrials.gov: NCT03583450, https://clinicaltrials.gov/ct2/show/NCT03583450
References
- 1.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–1245. [DOI] [PubMed] [Google Scholar]
- 2.Fortier MA, Kain ZN. Treating perioperative anxiety and pain in children: a tailored and innovative approach. Paediatr Anaesth. 2015;25:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson AJ, Shrivastava PP, Jamsen K, et al. Risk factors for anxiety at induction of anesthesia in children: a prospective cohort study. Paediatr Anaesth. 2006;16:919–927. [DOI] [PubMed] [Google Scholar]
- 4.Kain ZN, Wang SM, Mayes LC, Caramico LA, Hofstadter MB. Distress during the induction of anesthesia and postoperative behavioral outcomes. Anesth Analg. 1999;88:1042–1047. [DOI] [PubMed] [Google Scholar]
- 5.Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, McClain BC. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651–658. [DOI] [PubMed] [Google Scholar]
- 6.Fortier MA, Del Rosario AM, Rosenbaum A, Kain ZN. Beyond pain: predictors of postoperative maladaptive behavior change in children. Paediatr Anaesth. 2010;20:445–453. [DOI] [PubMed] [Google Scholar]
- 7.Watson AT, Visram A. Children’s preoperative anxiety and postoperative behaviour. Paediatr Anaesth. 2003;13:188–204. [DOI] [PubMed] [Google Scholar]
- 8.Fortier MA, Del Rosario AM, Martin SR, Kain ZN. Perioperative anxiety in children. Paediatr Anaesth. 2010;20:318–322. [DOI] [PubMed] [Google Scholar]
- 9.McCann ME, Kain ZN. The management of preoperative anxiety in children: an update. Anesth Analg. 2001;93:98–105. [DOI] [PubMed] [Google Scholar]
- 10.Kerimoglu B, Neuman A, Paul J, Stefanov DG, Twersky R. Anesthesia induction using video glasses as a distraction tool for the management of preoperative anxiety in children. Anesth Analg. 2013;117:1373–1379. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Jung SM, Yu H, Park SJ. Video Distraction and Parental Presence for the Management of Preoperative Anxiety and Postoperative Behavioral Disturbance in Children: A Randomized Controlled Trial. Anesth Analg. 2015;121:778–784. [DOI] [PubMed] [Google Scholar]
- 12.Won AS, Bailey J, Bailenson J, Tataru C, Yoon IA, Golianu B. Immersive Virtual Reality for Pediatric Pain. Children (Basel). 2017;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez S, Tsui JH, Jiang SY, Caruso TJ. Interactive video game built for mask induction in pediatric patients. Can J Anaesth. 2017;64:1073–1074. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez S, Caruso T, Tsui B. Bedside Entertainment and Relaxation Theater: size and novelty does matter when using video distraction for perioperative pediatric anxiety. Paediatr Anaesth. 2017;27:668–669. [DOI] [PubMed] [Google Scholar]
- 15.Eijlers R, Utens E, Staals LM, et al. Systematic Review and Meta-analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth Analg. 2019;129:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu JH, Park SJ, Park JW, et al. Randomized clinical trial of immersive virtual reality tour of the operating theatre in children before anaesthesia. Br J Surg. 2017;104:1628–1633. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JC, Rodriguez S, Caruso TJ, Tsui JH. Provider-controlled virtual reality experience may adjust for cognitive load during vascular access in pediatric patients. Can J Anaesth. 2017;64:1275–1276. [DOI] [PubMed] [Google Scholar]
- 18.Hiniker SM, Bush K, Fowler T, et al. Initial clinical outcomes of audiovisual-assisted therapeutic ambience in radiation therapy (AVATAR). Pract Radiat Oncol. 2017;7:311–318. [DOI] [PubMed] [Google Scholar]
- 19.Seiden SC, McMullan S, Sequera-Ramos L, et al. Tablet-based Interactive Distraction (TBID) vs oral midazolam to minimize perioperative anxiety in pediatric patients: a noninferiority randomized trial. Paediatr Anaesth. 2014;24:1217–1223. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomedl Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kain ZN, Mayes LC, Cicchetti DV, Bagnall AL, Finley JD, Hofstadter MB. The Yale Preoperative Anxiety Scale: how does it compare with a “gold standard”? Anesth Analg. 1997;85:783–788. [DOI] [PubMed] [Google Scholar]
- 23.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467–S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kain Zeev NMD, Mayes Linda CMD, Wang S-MMD, Caramico Lisa AMD, Hofstadter Maura BP. Parental Presence during Induction of Anesthesia versus Sedative Premedication: Which Intervention Is More Effective? Anesthesiology. 1998;89:1147–1156. [DOI] [PubMed] [Google Scholar]
- 25.Kain ZN, Mayes LC, Wang SM, Caramico LA, Krivutza DM, Hofstadter MB. Parental presence and a sedative premedicant for children undergoing surgery: a hierarchical study. Anesthesiology. 2000;92:939–946. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 27.Staffa SJ, Zurakowski D. Calculation of Confidence Intervals for Differences in Medians Between Groups and Comparison of Methods. Anesth Analg. 2020;130:542–546. [DOI] [PubMed] [Google Scholar]
- 28.Kahan BC, Jairath V, Dore CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moura LA, Dias IM, Pereira LV. Prevalence and factors associated with preoperative anxiety in children aged 5–12 years. Rev Lat Am Enfermagem. 2016;24:2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mifflin KA, Hackmann T, Chorney JM. Streamed video clips to reduce anxiety in children during inhaled induction of anesthesia. Anesth Analg. 2012;115:1162–1167. [DOI] [PubMed] [Google Scholar]


