Abstract
The reports on adverse drug reactions(ADR) to self-medication or over-the-counter medication are common across medical literature. However, the occurrence of oral fixed drug eruptions (FDE) to mefenamic acid is unique. We report a case of FDE to a drug obtained over the counter for menstrual pain. The essential findings from history, oral/dental examination, diagnostic approach, and treatment are briefly discussed along with a note on time and dosing for the oral drug provocation tests. The occurrence of ADR may be avoided by spreading awareness against OTC medication and labeling the specific drug for patients with established ADR.
Keywords: adverse drug reaction, fixed drug eruptions, mefenamic acid, oral cavity, over the counter medication, menstrual pain
Introduction
The adverse drug reactions (ADR) to self-medication and over-the-counter (OTC) medication are global issues encountered in clinical practice [1]. The menstrual pain following the absence of menstruation, is referred to as “normal menstrual pain” when not associated with dysmenorrhea (absence of menstruation for more than 35 days) [2]. The primary treatment of this pain is by non-steroidal anti-inflammatory drugs(NSAIDs) [3]. A survey had reported that self-medication for dysmenorrhea is common practice. The most efficacious drugs for this condition were reported to be mefenamic acid (alone or with dicyclomine as a combination) which are obtained often over-the-counter [4]. The NSAIDs are commonly implicated in ADR in remote settings with a lack of awareness. The advice on self-medication for menstrual pain comes majorly from non-medical sources (peers/mother). The ADR occurs due to failure to recognize drug interactions or adversities in such settings [1]. Fixed drug eruptions (FDE) are amongst these drug reactions, which occur when site-specific lesions characteristically recur each time due to the same offending drug [5]. The drug that led to an acute reaction may be often reported in past medical history with a note or label on that specific patient. In the absence of such evidence, confirmation of the reported drug is needed before labeling it to cause an ADR. The oral provocation tests, in this regard, are reported to be the ‘gold standard’ [6]. We report a case of oral FDE to mefenamic acid in a patient who had taken the said medication over the counter for an episode of menstrual pain.
Case report
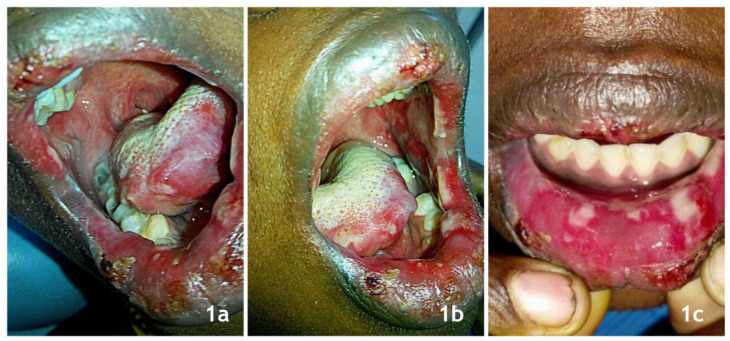
A 24 year-old female patient had presented to the dental outpatient department with complaints of ulcers in her mouth and lips, associated with pain, burning sensation, and difficulty in mouth opening for three days. The ulcers were reported to occur after medication taken for menstrual pain, obtained without a prescription from the local pharmaceutical supply. The drug was identified as mefenamic acid 500 mg. The medical history revealed an episode of lower abdominal pain radiating to her right thigh 3 days ago. The history of absence of menstruation for 40 calendar days was noted additionally. The patient reported previous episodes of painful menstruations which were not brought to the notice of medical specialists. Dental history was not contributory. On examination, multiple ulcerative lesions were noted bilaterally in the buccal mucosae extending from the commissure of the mouth to the retromolar regions. About 2 × 2 mm solitary ulcer was noted on tip of the tongue surrounded by diffused erythema extending laterally towards the left side. The upper and lower labial mucosae also showed similar lesions extending extraorally to the vermilion borders of the lip. The lip lesions were also surrounded by diffused erythema and some areas of crusting. On palpation, all the intraoral ulcerative sites were tender with no secondary changes (Figure 1a, 1b, 1c). Skin and other mucosal sites were free from lesions. A provisional diagnosis of ‘stomatitis medicamentosa’ was considered. Oral pemphigus vulagris, herpes gingivostomatitis, and fixed drug eruptions (FDE) were kept as differential diagnoses. Absences of prodromal symptoms and negative tzank smear helped to rule out the herpes infection, while history and negative nikolsky sign favored an acute drug reaction. An oral biopsy was planned; however, the apprehensive nature of the patient prevented us from carrying out the procedure. Considering the acute nature of symptoms, the patient was started on 20 mg of oral prednisone per day which was tapered to 5 mg per day over a period of one week. The tapering followed was 20 mg (1,2nd days) → 10 mg (3,4th days) → 5 mg (5,6th day) in two equally divided daily doses. Finally a 2.5 mg dose (7th day) was given once followed by cessation on steroid on 8th day. A topical application of Clobetasol propionate 0.05% three times a day and oral ranitidine 300 mg/day was prescribed additionally. The oral mucosal lesions were scored 9/10 on the visual analogue scale for pain/burning sensation induced by the patient. The lesions completely regressed after a week (Figure 2a, 2b, 2c). The patient was counseled against over-the-counter medication and, to stop the usage of an offending drug reported in the history. The patient was referred to a gynecologist (with caution on mefenamic acid) and was diagnosed with dysmenorrhea. Oral tramadol (50 mg) was prescribed for her abdominal pain and, she is currently on follow-up under a gynecologist.
Figure 1.
1a: Initial lesions on right buccal mucosa; 1b: Initial lesions on left buccal mucosa; 1c: Initial lesions on lower labial mucosa.
Figure 2.
Figure 2a, 2b, 2c: Completely healed lesions- upper and lower lips and oral mucosal sites.
The assumption of drug identified during interrogation, needed a sound conformation before labeling patient allergic to mefenamic acid or related prototypes. A skin prick test (SPT) was performed during initial patient visit, but was reported negative. Thus, oral drug provocation tests (DPT) was performed 2 months later after obtaining the patient’s consent. The test was conducted with an initial 50 mg of mefenamic acid (1/10th original dose) given orally. The second dose was given after 30 minutes, following which the patient reported a burning sensation of mouth and throat (one of the initial presenting symptoms). The patient had developed notable erythema of lower lip and right buccal mucosa, followed by irregular oral ulcers in initial sites following 1 hour of DPT (Figure 3a, 3b). The patient scored 7/10 on the visual analogue scale for burning/pain associated with new lesions post-DPT. A score of P1 (positive) was marked for DPT, as per standard criteria given by Girard et al [7]. The test was performed under medical supervision simultaneously monitoring vitals and any detectable drug reaction-based signs. The topical steroid was successfully reemployed for the management of ulcers induced after provocation tests. After DPT, a score of ‘11’ was obtained, as per the Naranjo probability scale [World Health Organization-Uppsala Monitoring Centre (WHO-UMC)] [8] criteria for evaluating the causal relationship between drugs and the FDE. The patient was labeled “allergic to mefenamic acid” and sent home with a letter stating the diagnosis of ‘oral fixed drug eruptions’ to the same drug. She was informed to present this evidence in case of future medical/surgical appointments.
Figure 3.
Figure 3a, 3b: Site specific oral ulcers to provocation tests.
Discussion
The terms “menstrual pain” and “dysmenorrhea” are mostly synonymous in medical literature, while some authors suggest “normal menstrual pain” when not associated with dysmenorrhea [3]. Dysmenorrhea is the absence of menstruation for more than 35 days and, that menstrual pain where there is “the need for medication and the inability to function normally” [9]. The clinical presentation of dysmenorrhea is in 90% of women, often as a kind of colicky suprapubic pain which may be accompanied by lumbosacral backache, pain radiating down the anterior thigh associated with nausea, vomiting, diarrhea, and rarely syncope attacks [4]. The current patient had similar described pain radiating to the right thigh region. The choice of treatment is NSAIDs followed by oral contraceptives, beta-blockers, or in some rare cases by surgical corrections [3]. Amongst the NSAIDs, mefenamic acid, dicyclomine was used as self-medication as per an Indian study. The combination (mefenamic acid with dicyclomine) was the most efficacious in comparison to other drugs in moderate to severe dysmenorrhea as per the same study [4]. The practice of self-medication for dysmenorrhea is reported with an incidence of 38–80%, mostly obtained over-the-counter. The lack of awareness regarding the appropriate choice of drugs, therapeutic doses, and their associated side effects were noted in such instances [4].
The OTC medication and self-medication are common practices in rural and urban settings respectively. An Indian study reported the prevalence of 18.72% for the use of OTC medication [10]. The NSAIDs drugs are commonly implicated in adverse drug reactions and often availed OTC. Fixed drug eruptions (FDE) occur when site-specific lesions are noted each time an offending drug is taken. This was noted in our patient after the DPT. The pathophysiology of such ADR is accounted to the specific binding between two groups of molecules i.e. CLA/alpha 4beta1/CD4a with E-selectin/vascular cellular adhesion molecule -2 favoring T cells mediated damage [5]. The reason for site-specific recurrence of lesions is due to persistence of in situ CD8 + memory T cells [11]. The oral provocation tests/DPTs are the ‘gold standard’ in conforming the ADR [6]. They were used as the patient had not consented to biopsy, and skin prick test (SPT) done with the same drug was negative. The dosing, time period between dosing, and interpretation of results for DPT were followed as per reported standards [6]. The replication of site-specific lesions and scores on analogue scale had further aided in diagnosis. Safety of DPT is relatively low as opposed to SPT or histopathology; however, clinicians can consider them with extreme caution at hospital settings. A DPT was done months after initial lesions allowing the plasma concentration of steroids not to impact the results. The SPT was done after 1 week of steroid treatment and the results could be biased. Also, the memory cells would be around the oral epithelium which would not favor a response from SPT done over the forearm.
The lesions occurring in the above case were very similar to ‘stomatitis medicamentosa’ or ‘erythema multiforme minor’, which was misleading. A similar scenario wherein mefenamic acid had induced ‘multifocal fixed drug eruptions much similar to erythema multifome’ was reported only once in historic literature [12]. FDE to mefenamic acid in dysmenorrhea cases were documented in historic case series while recent reports were not routinely cited [13]. Clinicians in general/dental practice may consider menstrual and OTC drug histories to be important when evaluating adverse oral reactions. The apt use of DPT in establishing ADR in biopsy contraindicated cases is noteworthy to mention. Labeling drugs that led to ADR for such cases and spreading awareness against OTC medication must be employed in clinical practice as a part of the routine treatment protocol.
References
- 1.De Sanctis V, Soliman AT, Daar S, Di Maio S, Elalaily R, Fiscina B, et al. Prevalence, attitude and practice of self-medication among adolescents and the paradigm of dysmenorrhea self-care management in different countries. Acta Biomed. 2020;91:182–192. doi: 10.23750/abm.v91i1.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandi G, Ferrari S, Xholli A, Cannoletta M, Palma F, Romani C, et al. Prevalence of menstrual pain in young women: what is dysmenorrhea? J Pain Res. 2012;5:169–174. doi: 10.2147/JPR.S30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omidvar S, Esmailzadeh S, Baradaran M, Basirat Z. Effect of fennel on pain intensity in dysmenorrhoea: A placebo-controlled trial. Ayu. 2012;33:311–313. doi: 10.4103/0974-8520.105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugumar R, Krishnaiah V, Channaveera GS, Mruthyunjaya S. Comparison of the pattern, efficacy, and tolerability of self-medicated drugs in primary dysmenorrhea: a questionnaire based survey. Indian J Pharmacol. 2013;45:180–183. doi: 10.4103/0253-7613.108312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teraki Y, Shiohara T. IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. J Allergy Clin Immunol. 2003;112:609–615. doi: 10.1016/s0091-6749(03)01624-5. [DOI] [PubMed] [Google Scholar]
- 6.Wahlang JB, Sangma KA, Marak MD, Brahma DK, Lynrah KG, Ksih A. Fixed drug eruption due to metronidazole: Review of literature and a case report. Int J Pharma Sci Res. 2012;3:331–334. [Google Scholar]
- 7.Girard M. Conclusiveness of rechallenge in the interpretation of adverse drug reactions. Br J Clin Pharmacol. 1987;23:73–79. doi: 10.1111/j.1365-2125.1987.tb03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwala MK, Mukhopadhyay S, Sekhar MR, Peter CD. Bullous Fixed Drug Eruption Probably Induced by Paracetamol. Indian J Dermatol. 2016;61:121. doi: 10.4103/0019-5154.174098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawood MY. Dysmenorrhoea and prostaglandins: pharmacological and therapeutic considerations. Drugs. 1981;22:42–56. doi: 10.2165/00003495-198122010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Panda A, Pradhan S, Mohapatro G, Kshatri JS. Predictors of over-the-counter medication: A cross-sectional Indian study. Perspect Clin Res. 2017;8:79–84. doi: 10.4103/2229-3485.203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–863. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 12.Sowden JM, Smith AG. Multifocal fixed drug eruption mimicking erythema multiforme. Clin Exp Dermatol. 1990;15:387–388. doi: 10.1111/j.1365-2230.1990.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 13.Long CC, Finlay AY, Marks R. Fixed drug eruption to mefenamic acid: a report of three cases. Br J Dermatol. 1992;126:409–411. doi: 10.1111/j.1365-2133.1992.tb00691.x. [DOI] [PubMed] [Google Scholar]