Abstract
Oral administration of indigestible markers and subsequent urine collection is a useful method to determine in vivo gastrointestinal tract (GIT) permeability in cattle for research purposes. However, urine sampling techniques often rely on total waste collection, which reduces the ability to perform more frequent sampling and obtain accurate volumes and sterile samples. An alternative is urethral catheterization, though the feasibility of this technique has not been thoroughly tested in preweaned Holstein heifer calves. The study objective was to develop a urethral catheter placement procedure in preweaned Holstein heifer calves for continuous and accurate urine collection to evaluate GIT permeability using an indigestible marker. Fifteen Holstein heifer calves had catheters placed at approximately 1 wk (8.0 ± 1.5 d) and 6 wk (40.0 ± 1.5 d) of age. During the procedure, calves were individually housed and restrained. The vulva was sterilized and then a sterile, lubricated speculum was inserted into the vagina. A sterile 0.09 cm diameter guidewire was guided into a lubricated, sterile 10 French Foley catheter. The catheter was inserted at approximately 5 through 7 cm into the urethral opening, guided into the bladder, and the catheter balloon was filled with 10 mL of water. The guidewire was removed, and urine flow confirmed correct placement before a 4-L urinary drainage bag was attached to the catheter. After catheterization (24 h), 1 L of chromium (Cr)-ethylenediaminetetraacetic acid was orally dosed to the calves. Calf health observations were made six times over a 48-h period, and any occurrence of vaginal discharge, tissue discharge in catheter, bleeding, inflammation, or abnormal urine was considered a localized reaction. The proportion of localized reactions for each age group was determined using Microsoft Excel, and the total Cr output was analyzed using PROC GLIMMIX. Localized reactions occurred for 20.0% of the 1-wk-old calves and 13.3% of the 6-wk-old calves. In the first 4 h, urine was collected every 15 min, and there were no overall Cr output differences (P = 0.38; 10.28 ± 3.21 mg Cr) when comparing 1- and 6-wk-old calves. However, 1-wk-old calves tended (P = 0.08) to have greater overall Cr output at 480 min (19.2%) and 1,440 min (41.9%) when compared with 6-wk-old calves. In summary, urinary catheterization is a viable urinary collection method for the determination of in vivo GIT permeability in preweaned Holstein heifer calves.
Keywords: calves, gastrointestinal permeability, heifers, preweaned, urine collection
Urethral catheterization can be successfully applied to evaluate gastrointestinal tract permeability in 1- and 6-wk-old Holstein heifer calves.
Introduction
Enteric disease is the leading cause of morbidity and mortality in neonatal Holstein heifer calves with a majority of calf deaths occurring as a result of diarrhea or other digestive problems (USDA, 2018). Preweaned calves are most susceptible to enteric disease from their first day of life to 3 wk old due to immature immunity, which can lead to greater gastrointestinal tract (GIT) permeability in calves (Araujo et al., 2015), and the effects on performance and health can be long-lasting. Increased GIT permeability commonly results from damage to the intestinal mucosa caused by pathogenic infections as a result of enteric disease (Meale et al., 2017; Filho et al., 2019). This disruption of small intestine barrier function can also result in reduced absorptive capacity and maldigestion, as well as neonatal diarrhea that can negatively affect the performance and nutritional status of the infected calf (Roy and Ternouth, 1972; Klein et al., 2008). Consequently, several efforts have been made to assess the impacts of GIT permeability on enteric disease in calves (Patt, 1977; Wilms et al., 2019).
Current methods to evaluate GIT permeability in calves primarily rely on blood or tissue collection. Specifically, the use of histomorphology and immunohistochemistry are often used as indirect indicators of intestinal damage that may lead to increased permeability (Yang et al., 2015; Pyo et al., 2020). In addition, some researchers have relied on the use of tight junction protein gene expression and/or total tight junction protein abundance to evaluate whether calves have increased GIT permeability (Walker et al., 2015; Ghaffari et al., 2021). Although these methods are important and provide a greater understanding of how environmental stressors, management practices, and nutrition can influence calf intestinal function, they rely on a localized section of the intestinal tract at a single point in time and do not allow for repeated measures on an individual. Tissue collection methods also inherently require the sacrifice of the study subject, which presents ethical and welfare concerns. Furthermore, blood has been used to evaluate in vivo intestinal permeability in calves via a lactulose:mannitol test (Araujo et al., 2015). However, blood sampling can increase calf stress due to restraint, and this method only allows for the determination of in vivo intestinal permeability at individual timepoints. Therefore, the application of these methods may be insufficient for understanding the total GIT permeability of individual animals over time.
An accepted method to assess total GIT permeability in calves is the use of orally administered chromium–ethylenediaminetetraacetic acid (Cr–EDTA; an indigestible marker) that is measured in the urine and allows for total GIT permeability (Branco Pardal et al., 1995) and repeated sampling on an individual animal (Amado et al., 2019). Some efforts have been made to develop noninvasive urine collection devices specifically for mature heifers (Magner et al., 1988; Deliberto and Urness, 1995); however, their use has not been validated in preweaned heifer calves. Metabolism crates have been used for urine collection in preweaned bull calves (Bascom et al., 2007; Wood et al., 2015), but their use does not allow for sterile or isolated urine collection, and their applicability for use with heifers may be limited due to anatomical incompatibility (i.e., urine does not flow straight down in heifer calves). Urinary catheterization is the most effective method for continuous, sterile urine collection in more mature heifers and has been effectively used in beef heifers greater than 380 kg (Zhang et al., 2013; Briggs et al., 2021). However, despite the successful use of urinary catheters in mature heifers, to our knowledge, they have not been used in preweaned heifers. Therefore, the study objective was to develop and validate a urinary catheterization procedure for continuous urine collection in 1- and 6-wk-old Holstein heifer calves to evaluate GIT permeability through the oral administration of Cr–EDTA. We hypothesized that the urinary catheterization procedure would allow for the accurate and precise measurement of Cr–EDTA output in the urine of 1- and 6-wk-old Holstein heifer calves for the in vivo evaluation of GIT permeability.
Material and Methods
Animals and housing
The Purdue University Animal Care and Use Committee approved all animal procedures (protocol #2006002047), and animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010).
In September 2020, a total of 15 Holstein heifer calves were procured from a commercial dairy farm at 1.5 ± 0.5 d of age (37.1 ± 0.86 kg body weight [BW]) and then transported in a livestock trailer (7.3 × 2.1 m; Model # 8127; Featherlite Trailers; Cresco IA, USA) for approximately 1 h and 15 min (101 km) to the Purdue University Animal Sciences Research and Education Center (ASREC) Dairy Farm in West Lafayette, IN, USA. Upon arrival, all calves were housed in individual calf hutches (Calf-Tel, 20|64 [ECO]; Germantown, WI, USA), which provided 2 m2 of interior space and 4.2 m2 of fenced-in outside space (3.5 × 1.2 m). All calves were provided milk replacer (3.8 L/calf/day, 24% crude protein [CP], 17% fat, and 12.5% solids) as well as ad libitum water and commercially available, non-medicated starter grain (17% CP, 2% fat). Ambient temperature and relative humidity (RH) were monitored using a data logger (Hobo model #MX1101; data logger temperature/RH; accuracy ± 0.20 °C and ±2% RH; Onset; Bourne, MA, USA) mounted within an empty hutch approximately 15 m from the calves on study. Throughout the course of the trial, the ambient temperature was 12.2 ± 7.5 °C, and the relative humidity was 69.0 ± 23.9%.
Catheter placement
Catheters were placed in three groups of calves (n = 5 calves per group) at approximately 1 wk (8.0 ± 1.5 d old; 37.5 ± 3.38 kg BW) and again at 6 wk (40.0 ± 1.5 d old; 59.3 ± 5.38 kg BW) of age. Calves were tested in three groups based upon the availability from the commercial dairy farm to ensure similar ages at the time of catheterization. Calves were moved approximately 400 m from their outdoor hutches into individual indoor stalls (1.87 m2) at the Purdue University ASREC Dairy Farm using the same livestock trailer previously described. All calves had ad libitum starter grain and water access for the duration of the trial. Urinary catheter placement occurred within 1 h of the calves being placed within the stalls, and the procedure lasted approximately 10 to 30 min per calf depending on calf resistance. Prior to catheterization, the calves’ tails were wrapped with bandaging tape (Vetrap Bandaging Tape; 3M; St. Paul, MN, USA), and their vulvas were shaved (Size 10 Blade, Clipper Pulse Andis 79015 DBLC-2 ZRII; Andis Company; Sturtevant, WI, USA) and cleaned with three alternating rounds of povidone-iodine (Betadine solution; 5% povidone-iodine; Purdue Pharma L.P.; Stamford, CT, USA) and 70% ethanol. Following cleaning, calves were physically restrained by two individuals, and a sterile, lubricated 13.97 × 5.72 × 9.53 cm speculum (Killian K-9 Vaginal Speculum; Jorgensen Laboratories Inc.; Loveland, CO, USA) was inserted into the vagina and opened to an approximately 3 cm diameter to assist with visualization of the urethral meatus. A sterile metal guidewire (0.09 cm width × 80 cm length; Starter Guidewire; Boston Scientific; Marlborough, MA, USA) was guided into a lubricated (VetOne OB Lube Non-Spermicidal Sterile Lubricating Jelly; MWI Animal Health; Boise, ID, USA) sterile 10 French Foley catheter (100% Silicone 2-Way Foley Catheter, 10 Fr, 3 mL Size, DYND11554; Select Silicone, Medline Industries; Northfield, IL, USA), and the catheter was guided along the floor of the vagina and inserted into the urethral meatus. If resistance to catheter insertion occurred, then it was assumed that the catheter had been placed into the suburethral diverticulum, which sits posterior to the urethral opening and can act as a small blind sac that may obstruct correct catheter placement (Cunningham et al., 1955). As such, the catheter would be repositioned to deflect the suburethral diverticulum. Once the catheter was inserted, a 12-mL syringe (Model # 1972, Ideal Syringe; Ideal Instruments Inc., USA) was used to fill the catheter inflation bulb with 10 mL of water, and a secure placement was confirmed by gently pulling the catheter against the urethral opening. Following confirmation of secure placement, the guide wire was removed, and correct placement was reconfirmed by the presence of urine flow from the catheter. The catheter was then attached to a 4-L urinary drainage bag (Drainage Bag, 4L, A/R Tower, Metal Clamp, DYND15405; Medline Industries; Northfield, IL, USA), bags were secured to the pen railing, and the calves were maintained in the individual stalls for approximately 24 h prior to Cr–EDTA testing. No calves became ill (e.g., enteric disease and respiratory disease) throughout the course of the experiment. Health observations to describe localized reactions to catheter placement were recorded by six times one individual over a 48-h period (n = 3 times per 24-h period). Observations included local inflammation (any swelling and redness around the vulva during the collection period), bladder/urethral discharge (e.g., tissue particles released in the urine and observed in the catheter line, urine bag, and/or the collection bucket), vaginal discharge (e.g., white or yellow, thick discharge released from the vagina), or vaginal bleeding (e.g., any blood visualized in the vagina due to catheter insertion). In addition, urine was monitored for normal volume, color, and consistency according to the guidelines outlined in the Veterinary Technicians Handbook of Laboratory Procedures (Bellwood and Andrasik-Catton, 2013). If any one of the aforementioned abnormal health observations were recorded within the 48-h period, then calves were assigned to a localized reaction group, and a veterinarian was consulted.
Urine collection system
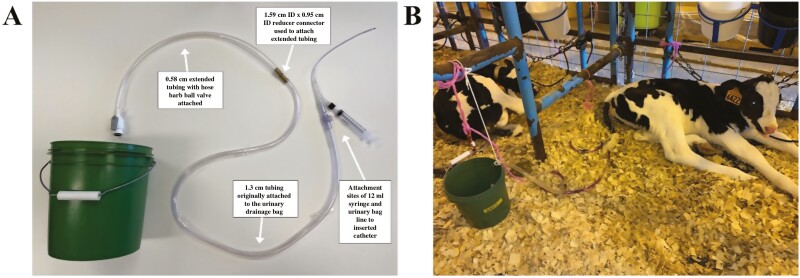
A urine collection system was designed and constructed to allow for time course collection of urine in 15 min intervals (Figure 1A). For construction, the outlet tube of the 4 L urinary drainage bag was attached to the end of the catheter and cut at approximately 10 cm from the base of the tube that meets the bag. A 1.59 cm ID × 0.95 cm ID brass reducer connector (Thogus Products; Avon Lake, OH, USA) was used to attach a 0.58-m section of Tygon tubing (0.95 cm ID × 1.27 cm OD; US Plastics Corp.; Lima, Ohio, USA) to the end of the drainage bag tubing. The tygon tubing length was based upon a preliminary study, which determined the maximum length of tubing required to accommodate urine expulsion from 1- and 6-wk-old Holstein heifer calves within a 15-min period (data not presented). A 0.95 cm OD × 0.95 cm hose barb ball valve (Part #: LFPPBVUCPB6; Parker Hannifin Corporation; Cleveland, OH, USA) was attached to the extended tube to allow the tubing to be opened and closed for urine collection (Figure 1A). The tygon tubing was placed into a plastic 3.8-L collection bucket, which was used to collect urine (Figure 1A and B). Urine was collected by draining the tygon tubing into the collection bucket, and then a 60-mL syringe (Model #: S60C01944T; Medtronic; Minneapolis, MN, USA) was used to measure total urine volume and collect a urine sample from the bucket for storage in a 15-mL tube (Product #: 91115; TPP Techno Plastic Products AG; Trasadingen, Switzerland). Urine was collected every 15 min for the first 240 min and then collected once at 480 and 1,440 min similar to previous research (Briggs et al., 2021). In between each collection timepoint, the hose barb valve was closed to prevent contamination, and the collection bucket was rinsed with water and dried with a clean towel to prevent cross-contamination. For longer collection times (over 15 min in this study) and overnight, the extended tubing with the hose barb ball valve was removed, and the urinary bag was reattached using the same reducer connector.
Figure 1.
Urine collection system (A) and urine collection system inserted into a preweaned Holstein heifer calf (B).
Cr–EDTA preparation
For the Cr–EDTA clearance test, Cr–EDTA was prepared as described by Binnerts et al. (1968). Briefly, in separate beakers, 48.0 g chromium trichloride hexahydrate (98%) was dissolved in 400 mL of distilled water, and 67.0 g of disodium EDTA dihydrate was dissolved in 600 mL of distilled water. Both solutions were combined, boiling chips were added, and the solution was heated on a hot plate until boiling occurred. A watch glass was used to cover the combined solution, and it was heated for 45 min until the solution changed color to a deep purple hue. The solution was heated uncovered for an additional 15 min, and 15.0 mL of 1.0 M calcium chloride (110.98 g CaCl2 and 1.0 L nanopure water) was added. Finally, the solution was filtered, adjusted to a pH between 6 and 7 using a NaOH solution, and diluted with distilled water until the total volume reached 1.0 L.
Cr–EDTA clearance test
The Cr–EDTA solution was used to reconstitute the milk replacer powder at a ratio of 1 L Cr–EDTA solution (15.24% Cr; 180 mM) to 0.89 L water (1.89 L milk replacer). The Cr–EDTA solution dosing amount was based upon the procedures outlined by Wood et al. (2015) in which 1.0 L of Cr–EDTA was dosed to 2-, 4-, and 6-wk-old Holstein bull calves, respectively. At 0800 hours, the milk replacer was poured into bottles (1.89 L, Valley Vet Supply, Marysville, KS, USA) and fed to the calves. If any calves refused to drink any amount of their allotted milk replacer, they were intubated with an esophageal calf fluid tube feeder (Model #: 602028, MWI Animal Health, Boise, ID, USA) and fed the remainder. Only one 6-wk-old calf refused to finish its milk replacer (approximately 0.63 L) and had to be tubed during the study; however, this refusal was not illness-related. Although researchers generally collect urine over a 48-h period (Wood et al., 2015; Briggs et al., 2021), previous research (Briggs et al., 2021) has determined that peak Cr excretion occurs at approximately 15 h and returns to initial dosing levels by 24 h and then linearly returns to baseline levels at 48 h. Therefore, in the present study, following oral administration of Cr–ETDA, urine was collected from all calves over only a 24-h period in order to capture the peak Cr excretion and reduce the amount of time calves were subjected to the Cr–EDTA test in accordance with refinement to reduce potential distress based upon Russell and Burch’s 3Rs (Russell and Burch, 1959). For the first 4 h, urine samples were obtained in 15-min intervals using the urine collection system by draining the urine collection line into a bucket and then using a 60-mL syringe (Model #: S60C01944T; Medtronic; Minneapolis, MN, USA) to collect the urine from the bucket (Figure 1B). After the 4 h timepoint, the catheter was re-attached to the 4 L urinary drainage bag, and the total urine output was collected at 480 min (total output from 4 to 8 h) and 1,440 min (total output from 8 to 24 h) following administration. Total cumulative urine volume excreted within each period was calculated as follows 240 min (4 h) = total urine output from 0 to 240 min, 480 min (8 h) total urine output from 0 to 480 min, and 1,440 min (24 h) = total urine output from 0 to 1,440 min (24 h). Total output was recorded, and a 10-mL urine sample was collected and stored in a 15-mL tube at all timepoints. Samples were stored at −80 °C for later analysis (Figure 2).
Figure 2.
Visual demonstration of the progression of Cr output in the urine of preweaned Holstein heifer calves from 0 to 150 min.
Urinary Cr analysis
Prior to analysis, samples were thawed to room temperature and then diluted (1:100) with nanopure water. Chromium concentrations (mg) were analyzed using atomic absorbance spectroscopy (SpectraAA 220 FS; Varian, Inc.; Palo Alto, CA) at a wavelength of 425.4 nm as previously described (Vicente et al., 2004). Standards were prepared in 5% nitric acid to obtain concentrations of 0, 1, 2, 3, 4, and 5 ppm. Highly concentrated samples (>standard concentration values) were further diluted to 1:1,000 and reanalyzed. Three readings were performed for each sample, and mean concentrations (mg/L) were recorded as an average of the three runs. Standards were re-run every 50 samples for quality control. The intra- and inter-assay coefficients of variation were 9.2% and 12.5%, respectively.
Statistical analyses
All data related to the Cr–EDTA clearance test were analyzed using PROC GLIMMIX in SAS 9.4 (SAS Inst. Inc.; Cary, NC), and localized reaction data were summarized using Microsoft Excel (2008). The assumptions of normality, homogeneity of variance, and linearity were confirmed post hoc. Calf was considered the experimental unit for all analyses. Fixed effects included age (1 wk, 6 wk), sampling timepoint (samples 1 through 17 representing every 15 min until 240 min, 18 for 240 to 480 min, and 19 for 480 to 1,440 min), and their interactions. Group (1, 2, and 3) and batch (groups that samples were analyzed in) were used as random effects. Means of main effects and interactions were adjusted using a Tukey–Kramer adjustment. A Kenward–Rogers denominator degree of freedom correction was applied using the ddfm option. Sampling timepoint was used as a repeated effect, and covariance structure was selected based upon the Akaike Information Criterion goodness-of-fit criteria (Littell et al., 1998). The proportion of localized reactions by age was calculated. Chromium output data are presented as least squares means (LSmeans) ± SE. Statistical significance was defined as P ≤ 0.05, and a tendency was defined as 0.05 < P ≤ 0.10.
Results and Discussion
There are few published studies focused on evaluating total GIT intestinal permeability in 1- and 6-wk-old preweaned Holstein heifer calves. Because of the intricate structure of the urinary anatomy in preweaned heifers, methods for in vivo continuous and sterile urine collection that do not negatively affect the health of the animal have not been thoroughly tested. For that reason, some studies have collected urine ex vivo via metabolism crates in Holstein bull calves and used these data as a model for all calves regardless of sex (Wood et al., 2015) due to anatomical incompatibility issues between metabolism crate use and heifer calves. However, physiological sex differences that exist in mammals (Koch et al., 1973; Kolstad et al., 1996) may influence the applicability of results derived from bull calves to heifer calves. Therefore, a catheterization method in 1- and 6-wk-old Holstein heifer calves was developed and applied to collect urine continuously and accurately for the evaluation of in vivo GIT permeability.
In 1-wk-old calves, there was a 20.0% (n = 3 calves) proportion of calves with localized reactions (Table 1). While not quantified during the study, it was observed that the 1-wk-old calves had more adverse behavioral reactions (e.g., kicking, moving, and head shaking) in response to restraint when compared with the calves at 6 wk of age. To prevent injury, the speculum was removed from the vagina if the calves resisted catheter insertion, which prolonged the total duration of the catheter insertion process in the 1-wk-old calves (approximately 30 min per calf). Greater resistance and longer catheterization times for the 1-wk-old calves may have increased the contact between the speculum and the vaginal tissue lining and thus increased the likelihood of localized reactions. It is possible that procedural time could be reduced if the animal is immobilized and nonreactive. Therefore, calf restraint using a squeeze chute or application of an epidural block would likely be advantageous in future studies requiring urinary catheterization in preweaned heifer calves.
Table 1.
Incidence of localized reactions defined as bladder/urethral discharge1, vaginal bleeding2, vaginal discharge3, inflammation4, and abnormal urine appearance5 in 1-wk (8.0 ± 1.5 d) and 6-wk-old (40.0 ± 1.5 d) preweaned Holstein heifer calves recorded over a 48-h period
| Age | No reaction | Localized reaction |
|---|---|---|
| 1 wk | 80.0% | 20.0% |
| 6 wk | 86.7% | 13.3% |
Tissue particles released in the urine and observed in the catheter line or drainage bag.
Any incidence of internal bleeding during catheter insertion and throughout the collection period.
Any abnormal thick white or yellow discharge released visualized in the vaginal opening or any discharge that is not clear.
Any signs of redness or swelling at the site during catheter insertion and throughout the collection period.
Abnormal urine appearance as defined by the Veterinary Technicians Handbook of Laboratory Procedures (Bellwood and Andrasik-Catton, 2013).
There was a 13.3% (n = 2 calves) proportion of localized reactions for the 6-wk-old calves (Table 1). The reduced proportion for the 6-wk-old calves when compared with 1-wk-old calves could be due to reduced resistance and catheterization timing (approximately 10 min per calf) from the calves in response to catheter insertion as observed by handlers. A decreased frequency of resistant behaviors likely reduced overall insertion time in the 6-wk-old calves, thus reducing the likelihood of any localized reactions due to the catheter insertion. However, localized reactions still occurred for the 6-wk-old calves, and this effect could be due to a lack of sufficient time between catheterization rounds to allow for recovery (Tamura et al., 2014). This should be considered for future studies that require multiple urinary catheterizations in preweaned heifer calves. Additionally, vaginal fluid discharge (an indicator of localized reactions in the present study) is also associated with both catheter and non-catheter urine collection methods, and thus localized reactions based on discharge should not be a sole indicator of safety and efficacy (Fellner et al., 1988). Furthermore, it should be noted that these calves were a part of a longer study where no long-lasting localized or systemic reactions were observed as a result of this procedure.
Age-related Cr output differences have been previously observed in Holstein bull calves (Wood et al., 2015), whereby 2-wk-old calves have greater Cr output when compared with 4- and 6-wk-old calves. Similarly, 1-wk-old Holstein heifer calves in the present study tended to have greater (P = 0.08) Cr output at 480 min (61.07 ± 17.33 mg Cr) and 1,440 min (102.20 ± 18.24 mg Cr) when compared with 6-wk-old calves at 480 min (41.85 ± 17.01 mg Cr) and 1,440 min (61.92 ± 17.59 mg Cr) of the Cr–EDTA clearance test (Figure 3). However, no overall Cr output differences were detected over the entire 24-h period (P = 0.19; 53.47 ± 15.66 mg Cr) when comparing 1- and 6-wk-old calves (Figure 4). Regardless, the observed tendency for an age by timepoint effect was likely related to differences in GIT maturation between 1- and 6-wk-old calves (Wood et al., 2015). As calves age, the introduction and increase in calf starter intake promote volatile fatty acid production, which improves ruminal structure and activity during the preweaning period (Malmuthuge et al., 2013; Meale et al., 2017). In addition, physical and chemical stimuli from consuming starter feed encourage GIT development through epithelial proliferation and improved barrier function (Diao et al., 2019), possibly indicating a reduction in GIT permeability as the digestive system matures. Therefore, we would expect to see greater GIT permeability of large markers such as Cr (approximately 340 kDa) in the 1-wk-old calves when compared with the Cr output in 6-wk-old calves with a more mature GIT (Wood et al., 2015; Wilms et al., 2019). However, it should be noted that, although there is a precedent in the literature for dosing calves with 1.0 L of Cr–EDTA solution regardless of age or BW (Zhang et al., 2013; Wood et al., 2015), it may be beneficial to consider dosing Cr–EDTA on a BW basis due to potential age-related differences in passage rate when evaluating treatment-related differences in GIT permeability.
Figure 3.
Total chromium output calculated from 0 min to 240, 480, and 1,440 min, respectively, after Cr–ETDA dosing in preweaned Holstein heifer calves at 1 wk of age (8.0 ± 1.5 d) and 6 wk of age (40.0 ± 1.5 d). Lettersa–c indicate a significant overall time effect (P < 0.01). Lettersx, y indicate an age by time tendency (P = 0.08). No overall age effect was detected (P = 0.19). Data are presented as LSmeans ± SE. Abbreviations: EDTA, ethylenediaminetetraacetic acid; LSmeans, least squares means.
Figure 4.
Total chromium output from Cr–ETDA dosing to 240 min (4 h) post-dosing in preweaned Holstein heifer calves at 1 wk of age (8.0 ± 1.5 d) and 6 wk of age (40.0 ± 1.5 d). Lettersa–g indicate a significant (P < 0.01) overall time effect. No age by time (P = 0.99) or overall age effects (P = 0.38) were detected. Data are presented as LSmeans ± SE. Abbreviations: EDTA, ethylenediaminetetraacetic acid; LSmeans, least squares means.
When considering the 4-h collection period, there was an overall timepoint effect for Cr output (P < 0.01); however, no age-related effects were observed (P = 0.38; 10.29 ± 3.19 mg Cr) when comparing 1- and 6-wk-old calves (Figure 4). Previous research (Briggs et al., 2021) indicates that treatment-related differences in Cr output may be observed between 0 and 9 h post Cr–EDTA dosing when collecting urine via catheters in heifers. However, this study (Briggs et al., 2021) used more mature heifers (> 400 kg) treated with aspirin to induce GIT permeability. Therefore, the differences in methodology between the present study and the study by Briggs et al. (2021) likely explain this discrepancy. Nevertheless, these results indicate that detection of age-related differences in GIT permeability via the use of Cr–EDTA may require at least a 24-h urine collection and that collecting urine in 15 min intervals during the first 4 h post-Cr–EDTA dosing may not be warranted.
Conclusions
A safe and effective urine collection system is important for the accurate and precise determination of in vivo GIT permeability using indigestible markers in preweaned Holstein heifer calves. Therefore, the study objective was to determine the efficacy of urethral catheters in 1- and 6-wk-old preweaned Holstein heifer calves to evaluate in vivo GIT permeability using Cr–EDTA, an accepted indigestible marker. It was determined that the urinary catheter placement resulted in minimal localized reactions and no severe illness, and it was a safe and effective method (e.g., no long-term negative consequences to calf health) to collect urine in preweaned heifer calves to evaluate in vivo GIT permeability. Additionally, Cr output tended to be greater in 1-wk-old when compared with 6-wk-old Holstein heifer calves, confirming previous reports in Holstein bull calves. This procedure may be a valuable method to assess in vivo GIT permeability in preweaned heifer calves.
Acknowledgments
We would like to thank the dairy farm staff at Purdue University for animal housing and management assistance. Additionally, we also thank the employees at the USDA-ARS Livestock Behavior Research Unit for assistance in daily animal care and data collection. The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Glossary
Abbreviations
- BW
body weight
- CP
crude protein
- EDTA
ethylenediaminetetraacetic acid
- GIT
gastrointestinal tract
- RH
relative humidity
Contributor Information
Guadalupe Ceja, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA; Livestock Behavior Research Unit, USDA-ARS, West Lafayette, IN 47907, USA.
Jacquelyn P Boerman, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA.
Rafael C Neves, Department of Veterinary Clinical Sciences, Purdue University, West Lafayette, IN 47907, USA.
Nicholas S Johnson, Mercy Hospital St. Louis, St. Louis, MO 63141, USA.
Jon P Schoonmaker, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA.
Matthew W Jorgensen, Livestock Behavior Research Unit, USDA-ARS, West Lafayette, IN 47907, USA.
Jay S Johnson, Livestock Behavior Research Unit, USDA-ARS, West Lafayette, IN 47907, USA.
Conflict of interest statement
No conflicts of interest, financial, or otherwise are declared by the authors.
Literature Cited
- Amado, L., Berends H., Leal L. N., Wilms J., Van Laar H., Gerrits W. J. J., and Martín-Tereso J.. . 2019. Effect of energy source in calf milk replacer on performance, digestibility, and gut permeability in rearing calves. J. Dairy Sci. 102:3994–4001. doi: 10.3168/jds.2018-15847 [DOI] [PubMed] [Google Scholar]
- Araujo, G., Yunta C., Terré M., Mereu A., Ipharraguerre I., and Bach A.. . 2015. Intestinal permeability and incidence of diarrhea in newborn calves. J. Dairy Sci. 98:7309–7317. doi: 10.3168/jds.2015-9666 [DOI] [PubMed] [Google Scholar]
- Bascom, S. A., James R. E., McGilliard M. L., and Van Amburgh M.. . 2007. Influence of dietary fat and protein on body composition of jersey bull calves. J. Dairy Sci. 90:5600–5609. doi: 10.3168/jds.2007-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwood, B., and Andrasik-Catton M.. . 2013. Veterinary technician's handbook of laboratory procedures. Ames, Iowa: John Wiley & Sons, Inc. [Google Scholar]
- Binnerts, W. T., Vanʹt Klooster A. T., and Frens A. M.. . 1968. Soluble chromium indicator measured by atomic absorption in digestion experiments. Vet. Rec. Open 82:470. [Google Scholar]
- Branco Pardal, P., Lallès J. P., André F., Delval E., and Toullec R.. . 1995. Assessment of gastrointestinal permeability to small marker probes in the preruminant calf. Reprod. Nut. Dev. 35:189–200. doi: 10.1051/rnd:19950207 [DOI] [PubMed] [Google Scholar]
- Briggs, N. G., Silva B. C., Godoi L. A., and Schoonmaker J. P.. . 2021. Effect of aspirin to intentionally induce leaky gut on performance, inflammation, and carcass characteristics of feedlot cattle. J. Anim. Sci. 99:1–9. doi: 10.1093/jas/skab328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, H. M., Frederick G. L., and Brisson G. J.. . 1955. Application of an inflatable urethral catheter for urine collection from cows. J. Dairy Sci. 38:997–999. doi: 10.3168/jds.S0022-0302(55)95068-2 [DOI] [Google Scholar]
- Deliberto, T., and Urness P.. . 1995. A total urine collection apparatus for female bison and cattle. J. Range Manag. 48:92–93. doi: 10.2307/4002511 [DOI] [Google Scholar]
- Diao, Q., Zhang R., and Fu T.. . 2019. Review of strategies to promote rumen development in calves. Animals 9:490. doi: 10.3390/ani9080490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation of Animal Science Societies. 2010. Chapter 7: Dairy Cattle. Guide for the care and use of agricultural animals in research and teaching (Ag Guide). 3rd ed. Champaign (IL): Federation of Animal Science Societies. [Google Scholar]
- Fellner, V., Weiss M. F., Belo A. T., Belyea R. L., Martz F. A., and Orma A. H.. . 1988. Urine cup for collection of urine from cows. J. Dairy Sci. 71:2250–2255. doi: 10.3168/jds.S0022-0302(88)79800-8 [DOI] [PubMed] [Google Scholar]
- Filho, L. C. F. B., Silva P. A., Buriti I. B., and Ramos T. R. R.. . 2019. Enteritis: still a problem in dairy calves. J. Dairy Vet. Sci. 13:1–5. doi: 10.19080/jdvs.2019.13.555862 [DOI] [Google Scholar]
- Ghaffari, M. H., Sadri H., Steinhoff-Wagner J., Hammon H. M., and Sauerwein H.. . 2021. Effects of colostrum feeding on the mRNA abundance of genes related to toll-like receptors, key antimicrobial defense molecules, and tight junctions in the small intestine of neonatal dairy calves. J. Dairy Sci. 104:10363–10373. doi: 10.3168/jds.2021-20386 [DOI] [PubMed] [Google Scholar]
- Klein, P., Kleinová T., Volek Z., and Šimůnek J.. . 2008. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet. Parasitol. 152:53–59. doi: 10.1016/j.vetpar.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, R. M., Cundiff L. V., Gregory K. E., and Dickerson G. E.. . 1973. Genetic and phenotypic relations associated with preweaning and postweaning growth of Hereford bulls and heifers. J. Anim. Sci. 36:235–239. doi: 10.2527/jas1973.362235x [DOI] [Google Scholar]
- Kolstad, K., Jopson N. B., and Vangen O.. . 1996. Breed and sex differences in fat distribution and mobilization in growing pigs fed at maintenance. Livest. Prod. Sci. 47:33–41. doi: 10.1016/S0301-6226(96)01001-9 [DOI] [Google Scholar]
- Littell, R. C., Henry P. R., and Ammerman C. B.. . 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Magner, T., Sim W. D., and Bardsley D. H.. . 1988. Apparatus for urine collection from female cattle in metabolism crates. Aust. J. Exp. Agric. 28:725–727. doi: 10.1071/ea9880725 [DOI] [Google Scholar]
- Malmuthuge, N., Li M., Goonewardene L. A., Oba M., and Guan L. L.. . 2013. Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune responses and tight junctions in dairy calves during weaning transition. J. Dairy Sci. 96:3189–3200. doi: 10.3168/jds.2012-6200 [DOI] [PubMed] [Google Scholar]
- Meale, S. J., Chaucheyras-Durand F., Berends H., Guan L. L., and Steele M. A.. . 2017. From pre- to postweaning: transformation of the young calf’s gastrointestinal tract. J. Dairy Sci. 100:5984–5995. doi: 10.3168/jds.2016-12474 [DOI] [PubMed] [Google Scholar]
- Patt, Jr. J. A. 1977. Factors affecting the duration of intestinal permeability to macromolecules in newborn animals. Biol. Rev. 52:411–429. doi: 10.1111/j.1469-185X.1977.tb00855.x [DOI] [Google Scholar]
- Pyo, J., Hare K., Pletts S., Inabu Y., Haines D., Sugino T., Guan L. L., and Steele M.. . 2020. Feeding colostrum or a 1:1 colostrum:milk mixture for 3 days postnatal increases small intestinal development and minimally influences plasma glucagon-like peptide-2 and serum insulin-like growth factor-1 concentrations in Holstein bull calves. J. Dairy Sci. 103:4236–4251. doi: 10.3168/jds.2019-17219 [DOI] [PubMed] [Google Scholar]
- Roy, J. H. B., and Ternouth J. H.. . 1972. Nutrition and enteric diseases in calves. Proc. Nutr. Soc. 31:53–60. doi: 10.1079/PNS19720011 [DOI] [PubMed] [Google Scholar]
- Russell, W. M. S., and Burch R. L.. . 1959. The principles of humane experimental technique. Wheathampstead (UK): Universities Federation for Animal Welfare. [Google Scholar]
- Tamura, T., Nakamura H., Sato S., Seki M., and Nishiki H.. . 2014. A modified catheterization procedure to reduce bladder damage when collecting urine samples from Holstein cows. J. Vet. Med. Sci. 76:819–826. doi: 10.1292/jvms.13-0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. 2018. Dairy 2014: Health and management practices on U.S. dairy operations, 2014. 3rd ed. Fort Collins, CO: National Animal Health and Monitoring System, USDA. [Google Scholar]
- Vicente, F., Sarraseca A., de Vega A., and Guada J. A.. . 2004. Performance of several Cr and Yb analytical techniques applied to samples of different biological origin (digesta or faeces). J. Sci. Food Agric. 84:2035–2040. doi: 10.1002/jsfa.1908 [DOI] [Google Scholar]
- Walker, M. P., Evock-Clover C. M., Elsasser T. H., and Connor E. E.. . 2015. Short Communication: Glucagon-like peptide-2 and coccidiosis alter tight junction gene expression in the gastrointestinal tract of dairy calves. J. Dairy Sci. 98:3432–3437. doi: 10.3168/jds.2014-8919 [DOI] [PubMed] [Google Scholar]
- Wilms, J., Berends H., and Martín-Tereso J.. . 2019. Hypertonic milk replacers increase gastrointestinal permeability in healthy dairy calves. J. Dairy Sci. 102:1237–1246. doi: 10.3168/jds.2018-15265 [DOI] [PubMed] [Google Scholar]
- Wood, K. M., Palmer S. I., Steele M. A., Metcalf J. A., and Penner G. B.. . 2015. The influence of age and weaning on permeability of the gastrointestinal tract in Holstein bull calves. J. Dairy Sci. 98:7226–7237. doi: 10.3168/jds.2015-9393 [DOI] [PubMed] [Google Scholar]
- Yang, M., Zou Y., Wu Z. H., Li S. L., and Cao Z. J.. . 2015. Colostrum quality affects immune system establishment and intestinal development of neonatal calves. J. Dairy Sci. 98:7153–7163. doi: 10.3168/jds.2014-9238 [DOI] [PubMed] [Google Scholar]
- Zhang, S., Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. . 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barier function in beef cattle. J. Anim. Sci. 93:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]