Abstract
Experimental and epidemiological studies suggest that maternal nutritional status during early pregnancy, including the period around the time of conception, may induce long-lasting epigenetic changes in the offspring. However, this remains largely unexplored in livestock. Therefore, the objective of this study was to evaluate if modification of the maternal diet of sheep (CTR: control; UND: 50% undernutrition) during the periconceptional period (42 d in total: −14/+28 from mating), would impact CpG methylation in muscle tissue (Longissimus dorsi) of adult offspring (11.5 mo old). Reduced representation bisulfite sequencing identified 262 (Edge-R, FDR < 0.05) and 686 (logistic regression, FDR < 0.001) differentially methylated regions (DMRs) between the UND and CTR groups. Gene ontology analysis identified genes related to development, functions of the muscular system, and steroid hormone receptor activity within the DMRs. The data reported here show that nutritional stress during early pregnancy leads to epigenetic modifications in the muscle of the resulting offspring, with possible implications for cardiac dysfunction, muscle physiology, and meat production.
Keywords: diet, epigenetics, methylation, muscle, undernutrition
Alteration of the maternal diet of sheep impacts the CpG methylation of genes related to muscular tissue development in muscle tissue of adult offspring.
Introduction
In utero fetal programming is well documented and supported by extensive epidemiological and experimental literature. Maternal undernutrition, specifically during early gestation, can result in permanent changes in the physiology and metabolism of the offspring (Godfrey and Barker, 2000; McMillen and Robinson, 2005), which in turn lead to an increased risk of cardiovascular and metabolic disorders in adulthood (Ravelli et al., 1998; Roseboom et al., 2000; Yan et al., 2013).
The epigenome constitutes the interface between the external environment and the genome, and suboptimal maternal nutrition may have long-term consequences for gene expression in the resulting offspring. The early developmental window, especially the periconceptional period, seems to be particularly sensitive to nutrient deficiencies (Van Soom and Fazeli, 2015; Sun et al., 2016). In this period, extensive epigenetic reorganization of the genome occurs (Reik et al., 2001). Shortly after fertilization, the mammalian genome undergoes extensive demethylation, then in preimplantation embryos, lineage-based methylation differences are established (Santos et al., 2002). As a result, different germ layers display distinct genome-wide DNA methylation patterns, which affect gene expression in the resulting tissues (Slieker et al., 2015). Therefore, the time around conception is crucial for the establishment of the adult epigenotype, and nutritional-induced epigenetic alterations can be maintained throughout life, influencing tissue-specific gene expression patterns (Lee, 2015).
Maternal undernutrition is frequently reported in livestock, due to seasonal and environmental variations in food quality and availability (Dunlap et al., 2015). Skeletal muscle has a low priority for nutrient allocation during fetal development (Redmer et al., 2004) and is vulnerable to reduced nutrient availability compared with other organs such as the brain or liver (Zhu et al., 2006; Yan et al., 2013). Skeletal muscle is therefore a good candidate tissue for assessing whether undernutrition induces long-lasting epigenetic changes.
There are several reports of altered methylation of the genome in muscle tissues of fetuses resulting from maternal undernutrition (Lan et al., 2013; Yan et al., 2013; Lie et al., 2014; Peñagaricano et al., 2014), while there are limited data regarding the epigenetic status after birth. Experimental evidence in sheep has shown that maternal undernutrition during the periconceptional period leads to poor metabolic outcomes in the resulting offspring due to altered regulation of the glucose–insulin axis (Yan et al., 2013; Dunlap et al., 2015; Oliver et al., 2020). Similarly in pigs, maternal undernutrition during pregnancy decreases GLUT4 expression in the muscle of adult offspring, which is most likely due to the increased methylation of the muscle GLUT4 promoter (Wang et al., 2016).
We explored the effect of the reduced diet (50% of the standard daily food ration) imposed on sheep during periconceptional period on the epigenetic programming of the skeletal muscle of adult offspring. Genome-wide methylation status of adult muscle tissues was explored using reduced representation bisulfite sequencing (RRBS) of adult offspring that had experienced periconceptional maternal undernourishment (UND) and normal feeding (CTR).
Material and Methods
All experimental procedures involving animals were conducted in accordance with Animal Protection Regulations of Italy (DPR 27/1/1992) in conformity with European Community regulation 86/609. All animal experiments were performed with the approval of the local ethical committee (CEISA-comitato etico interistituzionale per la sperimentazione animale No PROT.UNCHD12-222/2014). All chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Animal nutrition and breeding management
A commercial flock provided 12 multiparous Sarda breed sheep that were randomly assigned to 2 groups of 6 ewes and administered 1 of the 2 diets during the periconceptional period, from 14 d before mating (oocyte maturation) until 28 d after mating (early organogenesis), 42 d in total which corresponds to Carnegie stage 19 (Butler and Juurlink, 1987). CTR ewes were fed ad libitum with a balanced diet satisfying the nutritional requirements of pregnant ewes (300 g per animal per d). UND ewes were fed a restricted diet (50% of the requirements: 150 g per animal per d) of the same diet. The nutritional stress imposed on the animals was sufficient to induce a gross caloric restriction without affecting the health and well-being of the animals. All animals had a full clinical assessment each week (mucosal score, temperature, heart rate, and respiration). To optimize the pregnancy rate, estrus was synchronized by treating all ewes with intravaginal progesterone sponges (25 mg Chronogest; Intervet Ireland Ltd, Dublin) for 14 d. At the time of sponge removal, a ram of proven fertility was used to mate the two nutritional treatment groups, temporarily kept in a common paddock. From the time of mating onward, ewes were group-housed in pens under natural day length conditions with ad libitum access to water. On day 28 of gestation, all ewes were returned to an ad libitum diet, to meet daily energy and nutritional needs and ensure that all animals had regained sufficient fat reserves to maintain the pregnancy for the 149.7 d average gestation period for the Sarda sheep breed and for the onset and maintenance of lactation. Following parturition and birth-weight recording, all animals derived from a singleton pregnancy; six CTR (four males and two females) and six UND (three males and three females) were managed under standard farm conditions until 11.5 mo of age. A nonparametric one-way ANOVA (Kruskal–Wallis test) was used to analyze weight data using Graph Pad Prism software. Data are presented as mean ± SEM.
Muscle tissue collection
Muscle tissue (50 mg) from Longissimus dorsi were surgically sampled, between the 12th and 13th rib, under general anesthesia (acethyl-promazin IM followed by Thyopental Sodium) from all CTR and UND lambs at around 11.5 mo of age and stored at −80 °C for subsequent molecular analysis.
RRBS library preparation and sequencing
Genomic DNA from each muscle sample (n = 12; 6 CTR and 6 UND) was isolated using the NucleoSpin Tissue kit (Macherey-Nagel, Düren, Germany), following the manufacturer’s instructions. DNA concentration was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). About 1 μg of DNA was digested with MspI (New England Biolabs, Ipswich, MA, USA) by overnight incubation at 37 °C, following the manufacturer’s instructions. Libraries were generated using a TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, CA, USA). After adapter ligation, samples were converted using an EpiTect Bisulfite Kit (Qiagen, Venlo, The Netherlands) and finally PCR amplified with KAPA HiFi Uracil+ kit (Kapa Biosystems, Potters Bar, UK). RRBS libraries were sequenced on an Illumina Hiseq 3000 (San Diego, CA, USA) to generate 150-base paired-end reads.
Bioinformatic analysis
The preliminary quality control of raw sequence reads was carried out with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Illumina raw sequences were then filtered with Trimmomatic software to remove adapters and low-quality bases at the ends of reads, using a sliding window approach (Bolger et al., 2014). Data are available in the Sequence Reads Archive (SRA), BioProject accession number, PRJNA757927. Bismark software v.0.17.0 was used to align each read to a bisulfite-converted sheep genome (Oar_v4.0) with option-N 1. Methylation calls were extracted using the Bismark methylation_extractor function. Seqmonk software (version 1.47.1) (http://www.bioinformatics.babraham.ac.uk/projects/seqmonk/) was used for the visualization and analysis of the Bismark output. Only cytosines present in all 12 animals (6 CTR and 6 UND) with coverage of 10X were imported and analyzed by splitting the reference genome into 200 bp sliding windows with a 100 bp offset. The methylation percentage was calculated for each window if at least three cytosines were detected. Differentially methylated regions (DMRs) were identified by comparing CTR with UND groups. DMRs were calculated using both Edge-R (FDR < 0.05) and a logistic regression (LR) filter in R (FDR < 0.001) with differences in methylation level between CTR and UND groups above 10%. LR uses a binomial distribution and is a simple yet efficient method for the estimation of differential methylation (Huh et al., 2019). Edge-R uses empirical Bayesian methods to model gene-specific biological variation between biological replicates through the negative binomial dispersion parameter. Edge-R linear models fit the total read count (methylated + unmethylated) along loci. At each locus, the ratio of methylated reads is modeled indirectly as an over-dispersed binomial-like distribution. This has been demonstrated to be an efficient solution for methylation studies even when only a few biological replicates are available (Chen et al., 2017). To compute shared DMRs and DMR-associated genes across comparisons, commonality analysis was run and visualized through Venn diagrams, using the Venn Diagrams software (http://bioinformatics.psb.ugent.be/webtools/Venn/). DMR-associated genes were classified according to classical Gene Ontology (GO) categories, using the Cytoscape plug-in ClueGO which integrates GO and enhances the biological interpretation of large lists of genes (Bindea et al., 2009). In ClueGO, the P-value was calculated with the Fisher exact test corrected using the Bonferroni step-down method.
Reverse transcription-quantitative real-time PCR
Total RNA was extracted from muscle tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) and cleaned up with a NucleoSpin miRNA kit (Macherey-Nagel, Dueren, Germany), following the protocol which gives small and large RNA in one fraction (total RNA). RNA was reverse transcribed into cDNA in a total reaction volume of 18 μL. About 9 μL of RNA was added to 0.5 μL of random hexamers, 0.5μL oligodT, 0.5μL dNTPs, and incubated at 65 °C for 5 min, then placed at 4 °C. About 4 μL RT buffer (5X), 1 μL of DTT (0.1 M), 0.5 μL RNase inhibitor, and 1 μL of SuperScript II Reverse Transcriptase (Thermo Fisher, Waltham, MA, USA) were added. Reverse transcription was carried out at 25 °C for 5 min, 42 °C for 1 h, and 70 °C for 15 min. The expression level of PPARGC1α PPARδ, PTP1N, and IRS2 was assessed by reverse transcription-quantitative real-time PCR (RT-qPCR) using the expression of GADPH, ACTB genes as reference. Primers were designed from specific exon–exon junctions to avoid amplifying genomic DNA (Supplementary Table S1). RT-qPCR was performed with three technical replicates, using 5 μL of the Power SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA, USA), 0.5 μL of forward and reverse primers (final concentration 400 nM), and 4 μL of diluted cDNA (1:20 Vol), with QuantStudio 6 Flex Real-Time PCR Systems (Applied Biosystems, Carlsbad, CA, USA). Relative expression of genes between CTR and UND groups and P-value (t-test) were calculated using the PCR R package (Ahmed and Deok, 2018).
Results
Offspring weight
Lamb birth weights were similar between maternal dietary groups (CTR: 3.5 ± 0.32 kg; UND: 3.6 ± 0.28 kg) and were appropriate, given the size of the ewe and plane of nutrition during pregnancy. At around 1 yr of age, at the time of muscle tissue collection, there was no difference in body weight between the two groups (11.5 mo; CTR: 44.20 ± 2.93 kg; UND: 40.63 ± 2.10 kg).
Muscle methylation
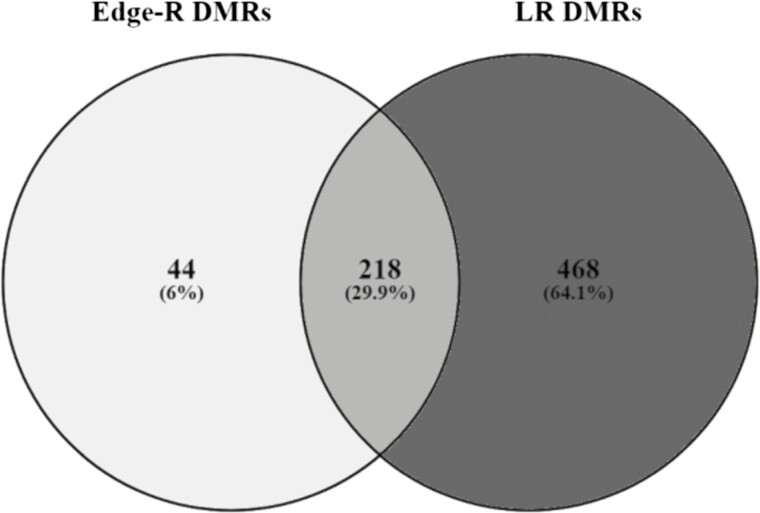
The average number of sequence reads per muscle sample was 20.4 M (ranging from 12.9 to 31.3 M) with a mapping efficiency of about 60%. RRBS had an average level of cytosine methylation in the CpG context of 59.2% across all muscle samples in both CTR and UND animals (Supplementary Table S2). A total of 112,852 methylated regions were detected across all samples using SeqMonk software. All these regions were evaluated using two different statistical methods to identify methylation variation between the two groups greater than 10% (Figure 1). Pairwise comparison of muscle methylation between experimental groups identified 262 and 686 DMRs in CTR vs. UND calculated using Edge-R (FDR < 0.05) and LR (FDR < 0.001), respectively; annotation of which identified 99 and 253 associated genes for Edge-R and LR, respectively.
Figure 1.
Venn diagram of shared differentially methylated regions identified with Edge-R and logistic regression methods.
Both statistical approaches identified genes that were hyper- and hypomethylated between CTR and UND groups. We investigated their function by GO analysis (Table 1). The GO classes showed enrichment for genes that had functions predominantly related to muscle development, growth, and maintenance, including actin cytoskeleton organization, myoblast proliferation, muscle cell formation and contraction, regulation of carbohydrate metabolic process, steroid hormone activity, and negative regulation of the ERK1 and ERK2 cascade (Table 1).
Table 1.
Gene ontology analysis of annotated genes found to be differentially methylated between the UND and CTR group using two Edge-R and LR approaches and considering the regions that were hyper-methylated in UND and CTR group
| GO-ID | Associated genes found | GO-term | P-value* |
|---|---|---|---|
| Total DMRs (Edge-R) | |||
| 2000291 | [MEIS2, PPARD, ZNF609] | Regulation of myoblast proliferation | 5.97E-04 |
| 2027 | [CACNA1C, CACNA1G, DMD, DSP, IRX5] | Regulation of heart rate | 1.30E-03 |
| 51450 | [MEIS2, PPARD, ZNF609] | Myoblast proliferation | 1.52E-03 |
| 31532 | [EZR, NTF3, PARVB, PHACTR1, PTPN1] | Actin cytoskeleton reorganization | 1.94E-03 |
| 14909 | [BCL2, ITGB3, PPARD, PPARGC1A] | Smooth muscle cell migration | 3.91E-03 |
| 70373 | [DMD, EZR, PTPN1] | Negative regulation of ERK1 and ERK2 cascade | 3.94E-03 |
| 86003 | [CACNA1C, CACNA1G, DSP] | Cardiac muscle cell contraction | 3.94E-03 |
| 86001 | [CACNA1C, CACNA1G, DMD, DSP] | Cardiac muscle cell action potential | 4.00E-03 |
| 10518 | [ADCYAP1R1, ESR1, NTF3] | Positive regulation of phospholipase activity | 6.74E-03 |
| 14812 | [BCL2, ITGB3, PPARD, PPARGC1A] | Muscle cell migration | 6.81E-03 |
| 86091 | [CACNA1C, CACNA1G, DSP] | Regulation of heart rate by cardiac conduction | 7.33E-03 |
| 51899 | [ADORA2A, BCL2, CACNA1C, CACNA1G] | Membrane depolarization | 7.85E-03 |
| 14910 | [BCL2, PPARD, PPARGC1A] | Regulation of smooth muscle cell migration | 8.93E-03 |
| 86065 | [CACNA1C, CACNA1G, DSP] | Cell communication involved in cardiac conduction | 1.09E-02 |
| 1900274 | [ADCYAP1R1, ESR1, NTF3] | Regulation of phospholipase C activity | 1.09E-02 |
| 10863 | [ADCYAP1R1, ESR1, NTF3] | Positive regulation of phospholipase C activity | 1.09E-02 |
| 3707 | [ESR1, NR2C2, PPARD] | Steroid hormone receptor activity | 1.12E-02 |
| 10676 | [ADCYAP1R1, KAT2B, PPARGC1A] | Positive regulation of cellular carbohydrate metabolic process | 1.14E-02 |
| 86002 | [CACNA1C, CACNA1G, DSP] | Cardiac muscle cell action potential involved in contraction | 1.25E-02 |
| Hyper-methylated UND (Edge-R) | |||
| 14909 | [BCL2, ITGB3, PPARD, PPARGC1A] | Smooth muscle cell migration | 7.31E-04 |
| 86001 | [CACNA1C, CACNA1G, DMD, DSP] | Cardiac muscle cell action potential | 7.70E-04 |
| 14812 | [BCL2, ITGB3, PPARD, PPARGC1A] | Muscle cell migration | 1.50E-03 |
| 70373 | [DMD, EZR, PTPN1] | Negative regulation of ERK1 and ERK2 cascade | 1.51E-03 |
| 86003 | [CACNA1C, CACNA1G, DSP] | Cardiac muscle cell contraction | 1.51E-03 |
| 86091 | [CACNA1C, CACNA1G, DSP] | Regulation of heart rate by cardiac conduction | 1.73E-03 |
| 14910 | [BCL2, PPARD, PPARGC1A] | Regulation of smooth muscle cell migration | 2.26E-03 |
| 10676 | [ADCYAP1R1, KAT2B, PPARGC1A] | Positive regulation of cellular carbohydrate metabolic process | 3.25E-03 |
| 86065 | [CACNA1C, CACNA1G, DSP] | Cell communication involved in cardiac conduction | 3.28E-03 |
| 3707 | [ESR1, NR2C2, PPARD] | Steroid hormone receptor activity | 3.53E-03 |
| 86002 | [CACNA1C, CACNA1G, DSP] | Cardiac muscle cell action potential involved in contraction | 4.02E-03 |
| Total DMRs (LR) | |||
| 2000291 | [GDNF, MEIS2, PPARD, ZNF609] | Regulation of myoblast proliferation | 1.19E-03 |
| 51450 | [GDNF, MEIS2, PPARD, ZNF609] | Myoblast proliferation | 4.90E-03 |
| 2000288 | [GDNF, MEIS2, PPARD] | Positive regulation of myoblast proliferation | 9.70E-03 |
| 31532 | [BAIAP2, EZR, FARP2, NTF3, PARVB, PHACTR1, PTPN1] | Actin cytoskeleton reorganization | 1.75E-02 |
| 7422 | [ALDH3A2, DMD, GDNF, NTF3, PLXNA4, RUNX1] | Peripheral nervous system development | 1.84E-02 |
| 48813 | [BAIAP2, CUX1, KIF1A, MAP2, NSMF, PHACTR1, SEMA4D, SHANK1] | Dendrite morphogenesis | 2.54E-02 |
| 86001 | [CACNA1C, CACNA1G, DMD] | Cardiac muscle cell action potential | 4.89E-02 |
| Hyper-methylated UND (LR) | |||
| 86001 | [CACNA1C, CACNA1G, DMD] | Cardiac muscle cell action potential | 1.55E-02 |
| 36037 | [BCL2, RUNX1, SH3RF1] | CD8-positive, alpha-beta T cell activation | 2.14E-02 |
| 50855 | [FOXP1, LYN, RUNX1] | Regulation of B cell receptor signaling pathway | 2.28E-02 |
| 30521 | [FOXP1, NCOR2, PMEPA1, PPARGC1A] | Androgen receptor signaling pathway | 2.29E-02 |
| 10522 | [ADCYAP1R1, BCL2, CACNA1C, DMD, LYN] | Regulation of calcium ion transport into cytosol | 2.33E-02 |
| 50854 | [EZR, FOXP1, LYN, RUNX1] | Regulation of antigen receptor-mediated signaling pathway | 2.46E-02 |
| 19395 | [ACOXL, ALDH3A2, PPARD, PPARGC1A, SOAT2] | Fatty acid oxidation | 2.55E-02 |
| 31532 | [BAIAP2, EZR, FARP2, PHACTR1, PTPN1] | Actin cytoskeleton reorganization | 2.59E-02 |
| 34440 | [ACOXL, ALDH3A2, PPARD, PPARGC1A, SOAT2] | Lipid oxidation | 2.76E-02 |
| 2548 | [CCL16, CCL17, LYN] | Monocyte chemotaxis | 2.78E-02 |
| 40014 | [BCL2, EZR, FTO, RAI1] | Regulation of multicellular organism growth | 2.88E-02 |
| 14909 | [BCL2, ITGB3, PPARD, PPARGC1A] | Smooth muscle cell migration | 2.94E-02 |
| 70373 | [DMD, EZR, LYN, PTPN1] | Negative regulation of ERK1 and ERK2 cascade | 2.99E-02 |
| 7422 | [ALDH3A2, DMD, PLXNA4, RUNX1] | Peripheral nervous system development | 3.53E-02 |
| 60612 | [FTO, PPARD, PPARGC1A] | Adipose tissue development | 4.01E-02 |
| 32623 | [EZR, HDAC7, RUNX1] | Interleukin-2 production | 4.01E-02 |
| 14812 | [BCL2, ITGB3, PPARD, PPARGC1A] | Muscle cell migration | 4.28E-02 |
| 71526 | [FARP2, PLXNA4, SEMA4D] | Semaphorin-plexin signaling pathway | 4.43E-02 |
| 1901505 | [SLC25A48, SLC29A3, SLC35A2] | Carbohydrate derivative transmembrane transporter activity | 4.60E-02 |
| 50919 | [ITGB3, PLXNA4, SEMA4D] | Negative chemotaxis | 4.61E-02 |
| 5089 | [ARHGEF10L, ARHGEF28, FARP2] | Rho guanyl-nucleotide exchange factor activity | 4.75E-02 |
| 14910 | [BCL2, PPARD, PPARGC1A] | Regulation of smooth muscle cell migration | 4.75E-02 |
| Hyper-methylated CTR (LR) | |||
| 2000291 | [GDNF, MEIS2, ZNF609] | Regulation of myoblast proliferation | 1.38E-04 |
| 51450 | [GDNF, MEIS2, ZNF609] | Myoblast proliferation | 2.97E-04 |
| 50885 | [CAMTA1, RBFOX1, SHANK1] | Neuromuscular process controlling balance | 2.59E-03 |
| 48512 | [ADORA2A, BTBD9, ZFHX3] | Circadian behavior | 2.82E-03 |
Corrected with Bonferroni step down.
RT-qPCR evaluation of PPARGC1α, PPARδ, PTP1N, and IRS2 expression in muscle
The expression level of PPARGC1α, PPARδ, and PTP1N was lower in the UND vs. CTL group while IRS2 higher in UND animals. PPARGC1α showed the greatest variation in gene expression (Supplementary Figure S1).
Interestingly, the expression of these genes was inversely correlated with the methylation level of DMRs in which they were found (Supplementary Table S3). PPARGC1α and PPARδ DMRs had higher methylation and IRS2 lower methylation in UND animals compared with CTL. Conversely, PTPN1 had both a higher methylation level and a slightly higher expression in UND animals (Supplementary Table S3).
Discussion
To date, diet-induced effects on the development of muscle have been assessed using morphological parameters, such as size, weight, fiber number, and composition (Fahey et al., 2005; Quigley et al., 2005; Sharples et al., 2016), however, the molecular mechanisms controlling differences remain largely unexplored. This study found that the 50% diet restriction imposed on sheep around the time of conception lead to epigenetic alterations in the muscle of the adult offspring. This is consistent with other reports that have shown that reduced nutrition during early fetal life is associated with abnormal methylation status in fetal muscle tissues (Lan et al., 2013; Namous et al., 2018).
In the present study, the methylation frequency of CpG sites of Longissimus dorsi muscle tissues from 11.5 mo old lambs ranged between 56% and 62% with no difference in overall methylation levels between UND and CTR groups. Similar results have been reported for sheep Longissimus dorsi muscles of 8-mo old lambs with about 50% to 55% of the total CpG sites methylated (Couldrey et al., 2014). A small increase of 5-mC content in muscle DNA in pigs receiving a methyl supplemented diet has been suggested, although the difference was not significant (Braunschweig, 2009).
In the present study, analysis of differential methylation between UND and CTR groups found several DMRs which were consistently identified using two different statistical methods. The two methods identified alterations in the methylation status of specific genes, the functions of which are mainly related to muscular development and activity. GO analysis of annotated DMRs identified the “negative regulation of the ERK1 and ERK2 cascade” pathway, which has recently been associated with the switch between fast-to-slow muscular fiber types (Boyer et al., 2019). We found several genes containing DMRs that have also been identified previously in diet-restriction studies. These include the peroxisome proliferator-activated receptor-γ coactivator-1α (PPARGC1α), which is involved in glucose and fatty acid metabolism in the liver and muscle (Liang and Ward, 2006). A rat model of intrauterine growth restriction showed an increase in the DNA methylation of specific CpG sites in PPARGC1α, and a relative decrease in the transcriptional level in muscle tissues with a positive correlation with fasting insulin concentration (Xie et al., 2015). We also found increased methylation in PPARδ and PPARGC1α, and a lower, although not significant, expression of these two genes in UND ewes. Interestingly, both PPARGC1α and PPARδ have been previously reported to regulate muscle fiber composition. PPARGC1a is a principal factor regulating muscle fiber type determination by activating genes implicated in slow fiber gene expression (Lin et al., 2002). Endurance exercise training promotes PPARδ expression by inducing an increase in the number of type I muscle fibers (Wang et al., 2004). Fiber composition within a muscle is important for the growth potential of skeletal muscle, endurance fitness, and adaptability to environmental stress. Maternal nutrition during pregnancy may influence the type and amount of adult muscle fibers which are determined in utero (Zhu et al., 2004; Fahey et al. 2005; Quigley et al., 2005). Interestingly, despite the lack of muscle structural analysis, in this work, PPARGC1α and PPARδ both showed changes in methylation level and expression in lambs born to ewes that had undergone diet restriction.
PPARGC1and PPARδ also impact the aerobic physical fitness and insulin sensitivity in humans (Stefan et al., 2007). The long-term effects of undernutrition of sheep during the periconceptional period have been reported to impair glucose–insulin axis function (Todd et al., 2009; Dunlap et al., 2015), affect the insulin-signaling pathway (Nicholas et al., 2013) as well as glucose uptake (Dunlap et al., 2015) in skeletal muscle of adult offspring. This suggests diet affects the metabolic health of the next generation.
Maternal undernutrition is known to have a transgenerational effect, causing fetal hyperinsulinemia, increased diabetic risk, and obesity in both first- and second-generation offspring of rats (Aerts and Van Assche, 2006). Transgenerational transmission requires alterations in the DNA of the germ cell line. Although general demethylation occurs during early embryo cleavage, a proportion of genomic domains are resistant to early embryo methylation reprogramming (Li et al., 2018). In mice, the intergenerational transmission of glucose intolerance is the result of altered expression of ATP-binding cassette subfamily C member 8 (ABCC8) coding for the sulfonylurea receptor 1 (SUR1) protein in β-cell islets (Jimenez-Chillaron et al., 2009). Sperm of in utero undernourished offspring has been shown to have specific DMRs in ABCC8 that are retained in the F2 population (Radford et al., 2014). In our study, specific alterations in ABCC8 methylation were also observed in lamb muscle tissue. In addition, methylation patterns of other key genes related to glucose metabolism including insulin receptor substrate 2 (IRS2), fat mass and obesity-associated protein or (FTO), ATPase phospholipid transporting (ATP11A and ATP13A5), and protein tyrosine phosphatase, non-receptor type 1 (PTP1N) differed between CTR and UND progeny.
Whole-genome bisulfite sequencing of human sperm revealed methylation differences in PPARGC1α and FTO and other family members including insulin receptor substrate 1 (IRS1) and ATPase phospholipid transporting 10B (ATP10) in patients with type 2 diabetes mellitus (Chen et al., 2019). PTPN1 also showed variations in methylation in peripheral blood mononuclear cells in patients with type 2 diabetes mellitus (Huang et al., 2017). The effect of maternal undernutrition is known to cause insulin resistance in skeletal muscle by reducing glucose uptake stimulated by insulin and promoting lipid accumulation in muscle cells, which may be ascribed to methylation changes in lamb muscle (Phielix and Mensink, 2008).
In conclusion, the present study shows that maternal periconceptional undernourishment of sheep impacts the muscle methylome in adult offspring. Due to the limited number of samples and an unbalanced sex ratio, we are not able to define gender-related differences as reported in a previous work analyzing the periconceptional undernutrition effect on the phenotype of adult sheep (Todd et al., 2009; Jaquiery et al., 2012). However, specific genes involved in muscle fiber structure and muscular energy metabolism are affected, suggesting that early life events have long-term consequences for adult life. Interestingly, in the most DMRs, many genes responsible for muscle development were identified, suggesting that undernutrition may increase the risk of metabolic disease. The investigation of the expression of genes associated with metabolism in DMRs showed a decreased expression in UND. A follow-up of muscle methylation status vs. metabolic profiles and structural differences of muscle in the progeny of ewes with different nutritional diets are needed, to shed light on the biological mechanisms linking undernutrition, epigenomics, and muscle physiology.
Supplementary Material
Acknowledgments
We acknowledge the support of the following projects: SCALA-MEDI, Improving sustainability and quality of Sheep and Chicken production by leveraging the Adaptation potential of LocAl breeds in the MEDIterranean area, funded by the European Union’s Horizon 2020 Partnership for Research and Innovation in the Mediterranean Area Programme (PRIMA) Grant 2012; LEO, Livestock Environment Opendata, 16.2 – PSRN 2014–2020 financed through Fondo Europeo Agricolo per lo Sviluppo Rurale (FEASR); NOVALSELPROV, InNOVAzioni nelle PROduzioni casearie OVine di sicilia e sardegna, PON project n. ARS01_00580. The datasets presented in this study can be found in online repositories. Raw data were deposited to the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under BioProject accession number PRJNA757927.
Glossary
Abbreviations
- 5-mC
5-methylcytosine
- ABCC8
ATP-binding cassette subfamily C member 8
- ATP10B
ATPase phospholipid transporting 10B
- ATP11A
ATPase phospholipid transporting 11A
- ATP13A5
ATPase phospholipid transporting 13A5
- CTR
control
- DMRs
differentially methylated regions
- ERK1
extracellular signal-regulated kinase 1
- ERK2
extracellular signal-regulated kinase 2
- FDR
false discovery rate
- FTO
fat mass and obesity-associated protein
- GLUT4
glucose transporter type 4
- GO
gene ontology
- IRS1
insulin receptor substrate 1
- IRS2
insulin receptor substrate 2
- LR
logistic regression
- PBMCs
peripheral blood mononuclear cells
- PPARGC1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- PPARδ
peroxisome proliferator-activated receptor-δ
- PTP1N
protein tyrosine phosphatase non-receptor type 1
- RRBS
reduced representation bisulfite sequencing
- SLC2A4
solute carrier family 2 member 4
- SUR1
sulfonylurea receptor 1
- UND
undernutrition
Contributor Information
Emanuele Capra, Institute of Agricultural Biology and Biotechnology (IBBA), National Research Council (CNR), Lodi 26900, Italy.
Paola Toschi, Department of Veterinary Sciences, University of Turin, Grugliasco 10095, TO, Italy.
Marcello Del Corvo, Department of Animal Science, Food and Technology – DIANA, and Nutrigenomics and Proteomics Research Center – PRONUTRIGEN, Università Cattolica del Sacro Cuore, Piacenza 29122, Italy.
Barbara Lazzari, Institute of Agricultural Biology and Biotechnology (IBBA), National Research Council (CNR), Lodi 26900, Italy.
Alessandra Stella, Institute of Agricultural Biology and Biotechnology (IBBA), National Research Council (CNR), Lodi 26900, Italy.
John Lewis Williams, Institute of Agricultural Biology and Biotechnology (IBBA), National Research Council (CNR), Lodi 26900, Italy; Davies Research Centre, School of Animal and Veterinary Sciences, University of Adelaide, Roseworthy, SA 5371, Australia; Department of Animal Science, Food and Technology – DIANA, and Nutrigenomics and Proteomics Research Center – PRONUTRIGEN, Università Cattolica del Sacro Cuore, Piacenza 29122, Italy.
Pasqualino Loi, Laboratory of Experimental Embryology, Faculty of Veterinary Medicine, University of Teramo, Teramo 64100, Italy.
Paolo Ajmone Marsan, Institute of Agricultural Biology and Biotechnology (IBBA), National Research Council (CNR), Lodi 26900, Italy; Department of Animal Science, Food and Technology – DIANA, and Nutrigenomics and Proteomics Research Center – PRONUTRIGEN, Università Cattolica del Sacro Cuore, Piacenza 29122, Italy.
Conflict of interest statement
The authors declare no conflict of interest.
LITERATURE CITED
- Aerts, L., and Van Assche F. A... 2006. Animal evidence for the transgenerational development of diabetes mellitus. Int. J. Biochem. Cell Biol. 38:894–903. doi: 10.1016/j.biocel.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Ahmed, M., and Deok R. K... 2018. pcr: an R package for quality assessment, analysis and testing of qPCR data. PeerJ. 6:e4473. doi: 10.7717/peerj.4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea, G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A., Fridman W. H., Pagès F., Trajanoski Z., and Galon J... 2009. ClueGO, a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 25:1091–1093. doi: 10.1093/bioinformatics/btp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, A. M., Lohse M., and Usadel B... 2014. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 30:2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, J. G., Prasad V., Song T., Lee D., Fu X., Grimes K. M., Sargent M. A., Sadayappan S., and Molkentin J. D... 2019. ERK1/2 signaling induces skeletal muscle slow fiber-type switching and reduces muscular dystrophy disease severity. JCI Insight. 5:e127356. doi: 10.1172/jci.insight.127356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig, M. H. 2009. Quantification of global DNA methylation with infrared fluorescence in liver and muscle tissues of differentially fed boars. Luminescence. 24:213–216. doi: 10.1002/bio.1098 [DOI] [PubMed] [Google Scholar]
- Butler, H., and Juurlink, B. H. J.. 1987. An atlas for staging mammalian and chick embryos, 1st ed. Boca Raton (FL): CRC Press; p. 218. [Google Scholar]
- Chen, X., Lin Q., Wen J., Lin W., Liang J., Huang H., Li L., Huang J., Chen F., Liu D.,. et al. 2019. Whole genome bisulfite sequencing of human spermatozoa reveals differentially methylated patterns from type 2 diabetic patients. J. Diabetes Investig. 11:856–864. doi: 10.1111/jdi.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Pal B., Visvader J. E., and Smyth G. K... 2017. Differential methylation analysis of reduced representation bisulfite sequencing experiments using EdgeR. F1000Research 6:2055. doi: 10.12688/f1000research.13196.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couldrey, C., Brauning R., Bracegirdle J., Maclean P., Henderson H. V., and McEwan J. C... 2014. Genome-wide DNA methylation patterns and transcription analysis in sheep muscle. PLoS One 9:e101853. doi: 10.1371/journal.pone.0101853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap, K. A., Brown J. D., Keith A. B., and Satterfield M. C... 2015. Factors controlling nutrient availability to the developing fetus in ruminants. J. Anim. Sci. Biotechnol. 6:16. doi: 10.1186/s40104-015-0012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey, A. J., Brameld J. M., Parr T., and Buttery P. J... 2005. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J. Anim. Sci. 83:2564–2571. doi: 10.2527/2005.83112564x [DOI] [PubMed] [Google Scholar]
- Godfrey, K. M., and Barker D. J... 2000. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s [DOI] [PubMed] [Google Scholar]
- Huang, Q., Han L., Liu Y., Wang C., Duan D., Lu N., Wang K., Zhang L., Gu K., Duan S.,. et al. 2017. Elevation of PTPN1 promoter methylation is a significant risk factor of type 2 diabetes in the Chinese population. Exp. Ther. Med. 14:2976–2982. doi: 10.3892/etm.2017.4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, I., Wu X., Park T., and Yi S. V... 2019. Detecting differential DNA methylation from sequencing of bisulfite converted DNA of diverse species. Brief. Bioinform. 20:33–46. doi: 10.1093/bib/bbx077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquiery, A. L., Oliver M. H., Honeyfield-Ross M., Harding J. E., and Bloomfield F. H... 2012. Periconceptional undernutrition in sheep affects adult phenotype only in males. J. Nutr. Metab. 2012:123610. doi: 10.1155/2012/123610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Chillaron, J. C., Isganaitis E., Charalambous M., Gesta S., Pentinat-Pelegrin T., Faucette R. R., Otis J. P., Chow A., Diaz R., Ferguson-Smith A.,. et al. 2009. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 58:460–468. doi: 10.2337/db08-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, X., Cretney E. C., Kropp J., Khateeb K., Berg M. A., Peñagaricano F., Magness R., Radunz A. E., and Khatib H... 2013. Maternal diet during pregnancy induces gene expression and DNA methylation changes in fetal tissues in sheep. Front. Genet. 4:49. doi: 10.3389/fgene.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. 2015. Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients. 7:9492–9507. doi: 10.3390/nu7115467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Fan Y., Li G., Xu X., Duan J., Li R., Kang X., Ma X., Chen X., Ke Y.,. et al. 2018. DNA methylation reprogramming of functional elements during mammalian embryonic development. Cell Discov. 4:41. doi: 10.1038/s41421-018-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, H., and Ward W. F... 2006. PGC-1alpha, a key regulator of energy metabolism. Adv. Physiol. Educ. 30:145–151. doi: 10.1152/advan.00052.2006 [DOI] [PubMed] [Google Scholar]
- Lie, S., Morrison J. L., Williams-Wyss O., Suter C. M., Humphreys D. T., Ozanne S. E., Zhang S., Maclaughlin S. M., Kleemann D. O., Walker S. K.,. et al. 2014. Periconceptional undernutrition programs changes in insulin-signaling molecules and microRNAs in skeletal muscle in singleton and twin fetal sheep. Biol. Reprod. 90:5. doi: 10.1095/biolreprod.113.109751 [DOI] [PubMed] [Google Scholar]
- Lin, J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N.,. et al. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801. doi: 10.1038/nature00904 [DOI] [PubMed] [Google Scholar]
- McMillen, I. C., and Robinson J. S... 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85:571–633. doi: 10.1152/physrev.00053.2003 [DOI] [PubMed] [Google Scholar]
- Namous, H., Peñagaricano F., Del Corvo M., Capra E., Thomas D. L., Stella A., Williams J. L., Marsan P. A., and Khatib H... 2018. Integrative analysis of methylomic and transcriptomic data in fetal sheep muscle tissues in response to maternal diet during pregnancy. BMC Genomics. 19:123. doi: 10.1186/s12864-018-4509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, L. M., Morrison J. L., Rattanatray L., Ozanne S. E., Kleemann D. O., Walker S. K., MacLaughlin S. M., Zhang S., Martin-Gronert M. S., and McMillen I. C... 2013. Differential effects of exposure to maternal obesity or maternal weight loss during the periconceptional period in the sheep on insulin signalling molecules in skeletal muscle of the offspring at 4 months of age. PLoS One 8:e84594. doi: 10.1371/journal.pone.0084594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, M. H., Bloomfield F. H., Bansal A., Phua H. H., Thorstensen E. B., Harding J. E., and Jaquiery A. A... 2020. Effects of maternal periconceptional undernutrition in sheep on offspring glucose–insulin axis function into adulthood. J. Dev. Orig. Health Dis.20:1–7. doi: 10.1017/S2040174420001063 [DOI] [PubMed] [Google Scholar]
- Peñagaricano, F., Wang X., Rosa G. J., Radunz A. E., and Khatib H... 2014. Maternal nutrition induces gene expression changes in fetal muscle and adipose tissues in sheep. BMC Genomics 15:1034. doi: 10.1186/1471-2164-15-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phielix, E., and Mensink M... 2008. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol. Behav. 94:252–258. doi: 10.1016/j.physbeh.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Quigley, S. P., Kleemann D. O., Kakar M. A., Owens J. A., Nattrass G. S., Maddocks S., and Walker S. K... 2005. Myogenesis in sheep is altered by maternal feed intake during the peri-conception period. Anim. Reprod. Sci. 87:241–251. doi: 10.1016/j.anireprosci.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Radford, E. J., Ito M., Shi H., Corish J. A., Yamazawa K., Isganaitis E., Seisenberger S., Hore T. A., Reik W., Erkek S.,. et al. 2014. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345:1255903. doi: 10.1126/science.1255903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli, A. C., van der Meulen J. H., Michels R. P., Osmond C., Barker D. J., Hales C. N., and Bleker O. P... 1998. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351:173–177. doi: 10.1016/s0140-6736(97)07244-9 [DOI] [PubMed] [Google Scholar]
- Redmer, D. A., Wallace J. M., and Reynolds L. P... 2004. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim. Endocrinol. 27:199–217. doi: 10.1016/j.domaniend.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Reik, W., Dean W., and Walter J... 2001. Epigenetic reprogramming in mammalian development. Science 293:1089–1093. doi: 10.1126/science.1063443 [DOI] [PubMed] [Google Scholar]
- Roseboom, T. J., van der Meulen J. H., Osmond C., Barker D. J., Ravelli A. C., Schroeder-Tanka J. M., van Montfrans G. A., Michels R. P., and Bleker O. P... 2000. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84:595–598. doi: 10.1136/heart.84.6.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, F., Hendrich B., Reik W., and Dean W... 2002. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 241:172–182. doi: 10.1006/dbio.2001.0501 [DOI] [PubMed] [Google Scholar]
- Sharples, A. P., Stewart C. E., and Seaborne R. A... 2016. Does skeletal muscle have an ‘epi’-memory? The role of epigenetics in nutritional programming, metabolic disease, aging and exercise. Aging Cell 15:603–616. doi: 10.1111/acel.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slieker, R. C., Roost M. S., van Iperen L., Suchiman H. E., Tobi E. W., Carlotti F., de Koning E. J., Slagboom P. E., Heijmans B. T., and Chuva de Sousa Lopes S. M... 2015. DNA methylation landscapes of human fetal development. PLoS Genetics 11: e1005583. doi: 10.1371/journal.pgen.1005583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan, N., Thamer C., Staiger H., Machicao F., Machann J., Schick F., Venter C., Niess A., Laakso M., Fritsche A.,. et al. 2007. Genetic variations in PPARD and PPARGC1A determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J. Clin. Endocrinol. Metab. 92:1827–1833. doi: 10.1210/jc.2006-1785 [DOI] [PubMed] [Google Scholar]
- Sun, C., Velazquez M. A., and Fleming T. P... 2016. DOHaD and the periconceptional period, a critical window in time. In: Rosenfeld, C. S., editor. The epigenome and developmental origins of health and disease. Boston, Massachusetts: Academic Press; p. 33–47. [Google Scholar]
- Todd, S. E., Oliver M. H., Jaquiery A. L., Bloomfield F. H., and Harding J. E... 2009. Periconceptional undernutrition of ewes impairs glucose tolerance in their adult offspring. Pediatr. Res. 65:409–413. doi: 10.1203/PDR.0b013e3181975efa [DOI] [PubMed] [Google Scholar]
- Van Soom, A., and Fazeli A... 2015. Epigenetics and periconception environment: an introduction. Reprod. Fertil. Dev. 27:iii–iv. doi: 10.1071/RDv27n5_IN [DOI] [PubMed] [Google Scholar]
- Wang, J., Cao M., Yang M., Lin Y., Che L., Fang Z., Xu S., Feng B., Li J., and Wu D... 2016. Intra-uterine undernutrition amplifies age-associated glucose intolerance in pigs via altered DNA methylation at muscle GLUT4 promoter. Br. J. Nutr. 116:390–401. doi: 10.1017/s0007114516002166 [DOI] [PubMed] [Google Scholar]
- Wang, Y. X., Zhang C. L., Yu R. T., Cho H. K., Nelson M. C., Bayuga-Ocampo C. R., Ham J., Kang H., and Evans R. M... 2004. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2:e294. doi: 10.1371/journal.pbio.0020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X., Lin T., Zhang M., Liao L., Yuan G., Gao H., Ning Q., and Luo X... 2015. IUGR with infantile overnutrition programs an insulin-resistant phenotype through DNA methylation of peroxisome proliferator-activated receptor-gamma coactivator-1alpha in rats. Pediatr. Res. 77:625–632. doi: 10.1038/pr.2015.32 [DOI] [PubMed] [Google Scholar]
- Yan, X., Zhu M. J., Dodson M. V., and Du M... 2013. Developmental programming of fetal skeletal muscle and adipose tissue development. J. Genomics. 8:29–38. doi: 10.7150/jgen.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. J., Ford S. P., Means W. J., Hess B. W., Nathanielsz P. W., and Du M... 2006. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 575:241–250. doi: 10.1113/jphysiol.2006.112110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. J., Ford S. P., Nathanielsz P. W., and Du M... 2004. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol. Reprod. 71:1968–1973. doi: 10.1095/biolreprod.104.034561 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.