ABSTRACT
Intravascular ventricular assist system (iVAS) is an investigative device in clinical trials for the management of advanced heart failure. It works on the principle of counterpulsation, similar to the classic intra-aortic balloon counterpulsation (IABP). We present a case of a 66-year-old man with iVAS in situ who required emergency laparotomy for a strangulated umbilical hernia. Patients with mechanical circulatory devices (MCD) are presenting more frequently for emergency and even elective noncardiac operations. Managing such patients poses significant challenges to the perioperative team due to its novelty and paucity of management recommendations.
Keywords: Counterpulsation, i VAS, mechanical circulatory device, noncardiac surgeries, patient safety
INTRODUCTION
Heart failure is a global health issue affecting millions of people and inflicts a significant economic burden on society.[1] Despite the improvement in available treatment modalities, most patients continue to have a poor quality of life.[2] Mechanical circulatory devices (MCD) were developed for the management of advanced heart failure as a bridge to overcome the problem of limited organ availability and prolonged wait times for heart transplantation.[3] The intravascular ventricular assist system (iVAS) (NuPulseCV, Raleigh, North Carolina) is an investigational ambulatory mechanical circulatory device that relies on counter pulsation to assist the failing heart.[4] It is designed to provide short- or long-term hemodynamic support, as bridging therapy for transplant, recovery, or even destination therapy.[5,6]
As the number of patients with MCD is increasing, it is more common for them to present for emergency or even elective noncardiac surgical procedures.[7] Perioperative considerations in patients with ventricular assist devices and other MCD for noncardiac surgical procedures have been discussed in the literature.[7] However, anesthetic considerations for patients with the iVAS device undergoing noncardiac surgical procedures are lacking. Therefore, we report a patient with iVAS implanted for ischemic cardiomyopathy with heart failure who required emergency laparotomy for a strangulated umbilical hernia.
CASE REPORT
A 66-year-old man, American Society of Anesthesiologist Physical Status 4E, with a history of advanced coronary artery disease, type II diabetes mellitus, hypertension, dyslipidemia, stage IV chronic kidney disease, gastric esophageal reflux, and peripheral vascular disease presented with an acute abdomen secondary to a strangulated umbilical hernia. His cardiac history was noteworthy for a prior triple vessel coronary artery bypass graft 20 years prior, with placement 6 years later of a dual-chamber pacemaker and internal cardiac defibrillator implant for symptomatic atrioventricular (AV) conduction delay. Most noteworthy, progressive heart failure secondary to ischemic cardiomyopathy led to the placement of iVAS system via the right subclavian artery in February 2019 [see Figure 1]. He had his implantable cardiac device checkup, done on April 2019, which showed that it is a Medtronic dual lead ICD with intrinsic rhythm predominantly atrial paced up to 97%, no arrhythmias, normal ICD function, and battery longevity of 5.2 years. His medications included amlodipine 5 mg PO, simvastatin 20 mg PO daily, long-acting insulin glargine 5 units at bedtime, and anticoagulation with warfarin 1–2 mg PO daily with institutional, international normalized ratio (INR) goal of 2 (range 1.7 to 2.2).
Figure 1.
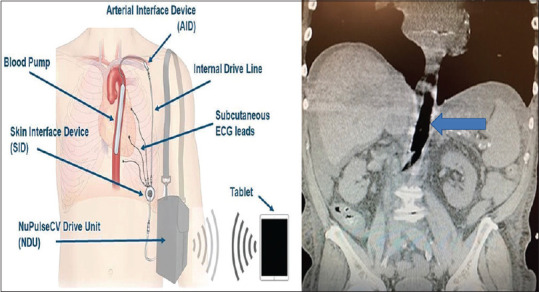
Schematic (left) and CT abdomen and pelvis (right) demonstrating the iVAS device positioned in the thoracic and abdominal aorta. The solid blue arrow indicated the iVAS balloon in the inflated position. Schematic mage available at http://www.nupulsecv.com
On examination, the patient had a nonreducible swelling in the umbilical region, with stable hemodynamics and respiration. His blood workup revealed mild anemia (10.1 g/dL), with an elevated creatinine close to his baseline (4.6 mg/dL), hyperlactatemia (2.0 mmol/L), and an INR of 1.8. Computerized tomography (CT) scan of the abdomen and pelvis showed an umbilical hernia with partial upstream obstruction but without pneumatosis, portal venous gas, nor perforation.
The iVAS was set to the 1:1 mode with full augmentation. A recent electrocardiogram (EKG) and echocardiogram showed atrial paced rhythm with AV conduction delay, reduced left ventricular systolic function with estimated ejection fraction 20%–25%, and mild reduction in right ventricular function. Standard monitors along with transcutaneous defibrillator pads were attached in an anterior-posterior orientation. A magnet was used to deactivate the ICD function with no changes in pacemaker settings (AAI, 84/min). General anesthesia was induced with intravenous etomidate 18 mg, fentanyl 100 mcg, and rocuronium 100 mg after a preinduction arterial catheter placed in the left radial artery. The patient's airway was secured with an 8.0 endotracheal tube. Surgery was completed via an open laparotomy using only bipolar cautery, resulting in no interruption to pacemaker functionality. No changes were made in iVAS parameters throughout the procedure; however, an iVAS device nurse was present throughout the procedure for monitoring and diagnostic assessments. After the surgical procedure was completed, neuromuscular blockade was reversed, the magnet was removed, and the patient's trachea was extubated. He was transferred to the postanesthesia care unit breathing spontaneously. The patient recovery was uneventful. His oral warfarin was resumed on postoperative day # 2 without the need for any specific bridging therapy.
DISCUSSION
In this report, we share our experience with the anesthetic management of the patient with i VAS undergoing emergency umbilical hernia repair. i VAS, ambulatory MCD [Figure 1] is inserted surgically via the left or right subclavian artery and positioned within the descending thoracic aorta.[5] It works on the same principles of counterpulsation as that of a conventional IABP.[5,6] However, a major difference is that i VAS has a small console with a surgically implanted device and three subcutaneous EKG leads. Also, the iVAS uses air instead of helium as the drive gas, and the only source to trigger the device is the internal electrocardiogram. It has two groups of components – internal (intravascular and extravascular) and external as clearly depicted in Figure 1.[6,8] The skin interface device is a surgically implanted component that functions by communicating the captured EKG signals from the three subcutaneous EKG to the driver via a patient connector and external driveline and also act as an electromechanical and pneumatic conduit for allowing the flow of air between the pump and driver.[6,8] An external wearable drive unit is the source of compressed air for continuous inflation and deflation of the balloon.[6,8]
Management of patients with i VAS in the perioperative period is challenging and necessitates the anesthesia team applies specific management strategies unique to cardiac patients. Our preoperative evaluation and assessment were focused on concurrent organ dysfunction secondary to chronic heart failure, forming a multidisciplinary team with members having familiarity and understanding in regard to device function and its interaction with the patient's physiology that includes hemodynamic and hematological changes.[8,9] In addition, continuous diagnostics of the i VAS system mandated a trained expert should be part of the team for monitoring and potential adjustments to the device as conditions warrant during and after surgery.
Perioperative monitoring in patients with i VAS requires specific considerations in addition to the standard ASA monitoring. Maintaining a stable intrinsic or paced rhythm with a rate less than 100 bpm is ideal for the optimal functioning of iVAS. If the patient is pacemaker dependent, a plan should be established to avoid or minimize electrosurgical interference and/or preoperatively programming the pacemaker to an asynchronous mode to avoid suboptimal functioning of iVAS during the surgery.[10]
Unlike continuous flow MCD, patients with i VAS will have a palpable peripheral pulse.[8] Therefore, it is possible to use a continuous noninvasive blood pressure cuff on the arm opposite to which the device was implanted. Bispectral index monitoring may facilitate the titration of inhalational or iv anesthetics to avoid drug-induced hypotension. Additional monitors such as intra-arterial catheters, central venous or pulmonary artery catheters, and transesophageal echocardiography may be considered. Device dysfunction, interruption, or disconnection can occur temporarily during the perioperative period. However, clinicians should be reassured in those situations as hemodynamics were initially marginally disrupted,[6,8] as the i VAS balloon is always paused in a collapsed posture allowing uninterrupted high-velocity blood flow down the thoracic aorta.[6,8] Appropriately titrated standard anesthetic induction agents, drugs, and techniques can be safely employed for the induction and maintenance of anesthesia. Red blood cell shearing is minimal with the iVAS system, so the risk of bleeding and thromboembolic events is low.[10] Thus, anticoagulation for these patients should be maintained with warfarin and aspirin with the target INR goal of 1.5–2.0.[10] The decision to resume or continue to hold anticoagulation for any MCD in the perioperative period must weigh the risk and benefits of bleeding vs thrombosis.[7]
The goals of anesthetic care in patients with i VAS are to maintain optimal preload status, a normal intrinsic or paced rhythm with a rate <100 bpm, and avoidance of excess afterload for optimal functioning of i VAS. The hemodynamic effects of i VAS include a reduction in left ventricular wall stress.[10] The myocardial performance will be best optimized by a steady state of both preload and afterload. Judicious use of fluids, head down position, adjustment with ventilations, and vasopressors are useful strategies to counter the effect of anesthesia-induced hypotension.[6,8] Fluid overload and hypovolemia should be avoided, whereas any intraoperative blood loss should be promptly addressed.[11,12]
Device-related complications such as obstruction, rupture, and pump failure are extremely rare events with i VAS.[10] In case of rupture, there is a theoretical risk of air embolism due to air being used as a gas source. Given that the EKG signal is the sole trigger source, there is an increased possibility of suboptimal function or improper function of i VAS in case of sustained arrhythmias. For instance, in the event of cardiac arrest, the presence of i VAS can complicate the situation, especially with pulseless electrical activity or asystole with a functioning pacemaker and can lead to some arterial waveform even in the absence of adequate cardiac perfusion.[13] It is therefore recommended to switch the iVAS off in cases of cardiac arrest, whereas changing the trigger source to pressure mode in a 1:1 ratio, with full augmentation is possible in the classic IABP.[13,14]
In conclusion, patients with the implanted i VAS device can be successfully managed through focused preparation based on detailed knowledge regarding the factors that influence the function of an implanted counterpulsation device and by consulting a device representative during acute phases of perioperative care.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Richard Prielipp is a member of the Board of Directors of the Anesthesia Patient Safety Foundation (APSF). He also serves on the speakers’ bureau for Merck Co, Inc. and as an opinion leader for 3M.
The authors1,2 have no conflicts of interest to declare.
REFERENCES
- 1.Writing Group Members. Mozzafarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heo S, Lennie TA, Okoli C, Moser DK. Quality of life in patients with heart failure: Ask the patients. Heart Lung. 2009;38:100–8. doi: 10.1016/j.hrtlng.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schramm R, Morshuis M, Schoenbrodt M, Boergermann J, Hakim-Meibodi K, Hata M, et al. Current perspectives on mechanical circulatory support. Eur J Cardiothorac Surg. 2019;55(Suppl 1):i31–7. doi: 10.1093/ejcts/ezy444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Technology [Internet]. NuPulseCV. [Last accessed on 2019 Sep 14]. Available from: https://www.nupulsecv.com/technology/
- 5.New mechanical assist device for advanced heart failure shows promising results during initial evaluation in clinical trial-UChicago Medicine [Internet] [Last accessed on 2019 Sep 14]. Available from: https://www.uchicagomedicine.org/forefront/heart-and-vascular-articles/2016/september/nupulse .
- 6.Zehr L. NuPulseCV evaluating new intravascular ventricular assist system [Internet]. BioTuesdays. 2019. [Last accessed on 2019 Sep 14]. Available from: https://biotuesdays.com/2019/04/02/2019-4-2-nupulsecv-evaluating-new-intravascular-ventricular-assist-system/
- 7.Patel H, Madanieh R, Kosmas CE, Vatti SK, Vittorio TJ. Complications of continuous-flow mechanical circulatory support devices. Clin Med Insights Cardiol. 2015;9(Suppl 2):15–21. doi: 10.4137/CMC.S19708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeevanandam V, Song T, Onsager D, Ota T, LaBuhn CJ, Lammy T, et al. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J Heart Lung Transplant. 2018;37:1–6. doi: 10.1016/j.healun.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Feasibility Study of the Intravascular Ventricular Assist System (iVAS)-Full Text View-ClinicalTrials.gov. [Last accessed on 2019 Dec 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02645539 .
- 10.Crossley GH, Poole JE, Rozner MA, Asirvatham SJ, Cheng A, Chung MK, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: Facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS) Heart Rhythm. 2011;8:1114–54. doi: 10.1016/j.hrthm.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Stone M, Hinchey J, Sattler C, Evans A. Trends in the management of patients with left ventricular assist devices presenting for noncardiac surgery: A 10-year institutional experience. Semin Cardiothorac Vasc Anesth. 2016;20:197–204. doi: 10.1177/1089253215619759. [DOI] [PubMed] [Google Scholar]
- 12.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, et al. Clinical management of continuous flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Peberdy MA, Gluck JA, Ornato JP, Bermudez CA, Griffin RE, Kasirajan V, et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: A scientific statement from the American Heart Association. Circulation. 2017;135:e1115–34. doi: 10.1161/CIR.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 14.Gosev I, Nikolic I, Aranki S. Resuscitation practices in cardiac surgery. J Thorac Cardiovasc Surg. 2014;148:1152–6. doi: 10.1016/j.jtcvs.2014.06.033. [DOI] [PubMed] [Google Scholar]


