ABSTRACT
Catecholamine-resistant postoperative vasoplegic syndrome (PVS) lacks effective treatment modalities. Synthetic angiotensin II was recently approved for the treatment of vasodilatory shock; however, its use in PVS is not well described. We report outcomes in six patients receiving angiotensin II for the treatment of isolated PVS. All patients achieved their MAP goal and the majority showed improvement in lactate and background catecholamine dose; however, variables of perfusion changed discordantly. Three of six patients survived to hospital discharge.
Keywords: Angiotensin II, cardiac vasoplegia, postoperative vasoplegic syndrome, shock
INTRODUCTION
Postoperative vasoplegic syndrome (PVS) is a distinct life-threatening complication that most commonly presents following cardiac surgery requiring cardiopulmonary bypass (CPB) support.[1] It is described as a distributive shock that lacks other established identifiable factors. The prevalence of PVS following cardiac surgery is most commonly reported in the range of 8–26% of postoperative patients and its underlying pathophysiological mechanisms are complex.[1] PVS refractory to catecholamine and arginine vasopressin therapies presents a challenging clinical scenario that lacks a well-validated treatment modality. Therapeutic options that have received attention include methylene blue, ascorbic acid, thiamine, hydroxocobalamin, and corticosteroids. Each of these agents has a limited level of evidence to support their use. Their benefit is limited to hemodynamic improvement without a clear effect on mortality or other patient-centered outcomes.[2]
Angiotensin II (ATII) is one of the final products of the renin–angiotensin–aldosterone system and elicits a profound effect on regulating vascular tone.[3] Angiotensin II for the Treatment of High-Output Shock (ATHOS-3), the randomized controlled trial that led to Federal Drug Agency approval of ATII for treatment of vasodilatory shock in the United States, demonstrated a positive effect on blood pressure in septic shock.[4] However, the direct effect on mortality and other patient-centered outcomes remains unanswered. The PVS population was largely underrepresented in ATHOS-3; however, a post-hoc analysis of ATHOS-3 demonstrated a predominantly robust response to ATII in the PVS patient population. Eighty-nine percent (8/9) of patients reached the desired mean arterial pressure (MAP) goal compared to 0% (0/7) in the control arm.[5] Several case reports demonstrate substantial effects on MAP, cumulative vasopressor exposure, and renin levels with the use of ATII in intra/postoperative vasoplegia.[6,7,8] There remains a deficiency in reporting clinical outcomes associated with the use of ATII in treating PVS To address the lack of data involving ATII use in the PVS population, we conducted a retrospective review of patients that received ATII at our institution. In this case series, we provide an analysis of six patients who received ATII (Giapreza) for PVS following cardiac surgery at our institution.
CASE HISTORY
At our institution, the use of ATII is restricted to patients with high-output vasodilatory shock unable to meet MAP goals while receiving ≥3 high dose vasopressors [Table 1]. Between June 2019 and November 2020, ATII was utilized in 33 patient cases with distributive shock at our institution. Six of these patients had a clinical picture consistent with PVS defined as Systemic Vascular Resistance (SVR) <820 dyn s/cm5 and cardiac index (CI) >2.2 L/min/m2 in the setting of recent (<24 h) exposure to CPB. Cases were omitted from the analysis if the presence of hemorrhage, under-resuscitation, or a component of sepsis was apparent. Three primary clinical criteria were chosen to assess the effect of ATII on tissue perfusion and cumulative vasopressor exposure: Change in serum creatinine (SCr) over 72 h, change in lactate over 12 h, and a change in background of vasopressor agents (standardized to norepinephrine equivalents) over 48 h following ATII initiation.
Table 1.
Institutional criteria for use of angiotensin II
| Criteria for use | Value |
|---|---|
| High-output vasodilatory shock | CI >2.3 or |
| ScvO2 >70% and CVP >8 | |
| Refractory hypotension despite adequate fluid resuscitation and receiving ≥3 high-dose vasopressors | Norepinephrine >20 mcg/min Epinephrine >20 mcg/min Phenylephrine >300 mcg/min Vasopressin >0.04 units/min Methylene blue |
| Initial rate | 10-20 ng/kg/min |
| Max dose initial 3 h | 80 ng/kg/min |
| Max maintenance dose | 40 ng/kg/min |
| Max duration before attempt to wean | 48 h |
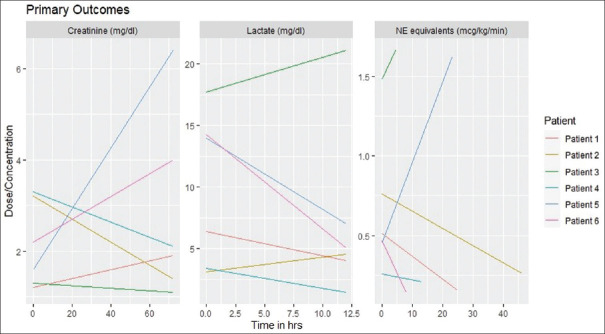
Results were mixed across the study population [Table 2]. ATII was initiated at 10–20 ng/kg/min and titrated to a MAP goal of >65 mmHg in each case per institutional protocols. The maximum dose of ATII required to achieve MAP goal and duration of infusion varied across the study population. Two cases had variables that followed distinctly divergent paths. Patient 6, for example, experienced a rapid improvement in serum lactate and NE equivalents allowing discontinuation of ATII following 8 h of treatment with an increase in serum creatinine. Patient 4 demonstrated a consistent unidirectional change across all three variables. Taken as a whole, lactate levels and cumulative vasopressor dose decreased in the majority of patients while serum creatinine levels improved in half of the patients [Figure 1]. All patients successfully achieved their MAP goal within 6 h. Half of the population survived to hospital discharge.
Table 2.
Patient characteristics and outcomes
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age (years) | 86 | 75 | 71 | 67 | 61 | 58 |
| Sex | Male | Male | Male | Male | Male | Male |
| BMI | 20.88 | 27.31 | 25.25 | 39.2 | 26.82 | 37.25 |
| ACEi/ARB use in previous 72 h | No | No | No | No | No | No |
| Baseline vasopressor dose (NE equivalent mcg/kg/min) | 0.513 | 0.76 | 1.479 | 0.258 | 0.452 | 0.456 |
| Max ATII dose (ng/kg/min) | 40 | 80 | 40 | 20 | 10 | 20 |
| Duration of ATII infusion (h) | 25 | 46 | 5 | 13 | 23 | 8 |
| Change in SCr at 72 h (mg/dL) | 0.7 | −1.8 | −0.2 | −0.9 | 4.8 | 1.8 |
| Change in lactate at 12 h | −2.36 | 1.44 | 3.39 | −1.94 | −7 | −9.2 |
| Max change in cumulative vasopressor dose (NE equivalent mcg/kg/min) | −0.359 | −0.496 | 0.184 | −0.048 | 1.168 | −0.322 |
| MAP goal achieved (>65 mmHg) | Yes | Yes | Yes | Yes | Yes | Yes |
| Survival at hospital discharge | Yes | No | No | Yes | No | Yes |
| Thromboembolic event | No | Yes | No | No | No | No |
Figure 1.
Clinical outcomes following ATII initiation
DISCUSSION
Given the significant mortality associated with PVS, there remains a need to develop or repurpose agents targeting the reversal of its underlying physiological drivers. ATII has demonstrated its strength as a vasoactive agent in vasodilatory shock and is associated with improved mortality in patients requiring renal replacement.[4,9] Reports of its use outside of the ATHOS-3 trial are limited and include primarily case reports. Published reports describing ATII in PVS have focused primarily on the effect on MAP and background vasopressor doses. We sought to extend this analysis to include direct measurements of tissue perfusion.
Consistent with findings from the post-hoc analysis of PVS patients in ATHOS-3, a majority of our patients achieved a robust hemodynamic response while achieving hemodynamic goals with the introduction of ATII, although a variable effect on markers of perfusion was observed. Only three of the six patients survived to hospital discharge. A positive response in serum lactate occurred at 12 h post-ATII initiation in four patients. This appeared to correlate well with a positive ATII response defined as a reduction in cumulative background vasopressor dose. SCr improved at 72 h post-ATII initiation in three patients. SCr did not correlate uniformly with a reduction in lactate which may have been a result of the limitations of SCr as a delayed marker of true renal function. As opposed to the ATHOS-3 trial where venous thromboembolic events were described, one patient from our study experienced this complication. Given the small size of our reported patient population, the clinical significance of this finding is unclear.
A case series of this size is inevitably limited in its explanatory capacity. That being said, it is important that real-world examples of the use of ATII continue to be reported. In selecting patients for our analysis, it was difficult to capture all variables that contributed to refractory hypotension. It would be beneficial to have a well-defined, physiology-derived variable that could direct our use of ATII. Bellomo and colleagues recently performed a post-hoc analysis of the ATHOS-3 trial in an attempt to provide such an objective approach. They compared renin levels and angiotensin I/ATII ratios (ATI) and found that patients with a mean renin level >176 (pg/mL) at the time of ATII initiation had improved morality (50.9% vs. 69.9%, P = 0.012).[10] These results are encouraging and may prove beneficial in predicting positive responses. Future studies should focus on establishing the effect of ATII treatment on patient-centered outcomes such as ischemic injury, tachyarrhythmia, and thrombotic risk in a large PVS population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Busse LW, Barker N, Petersen C. Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care. 2020;24:36. doi: 10.1186/s13054-020-2743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA. 2020;323:423–31. doi: 10.1001/jama.2019.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrêa TD, Takala J, Jakob SM. Angiotensin II in septic shock. Crit Care. 2015;19:98. doi: 10.1186/s13054-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–30. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 5.Klijian A, Khanna AK, Reddy VS, Friedman B, Ortoleva J, Evans AS, et al. Treatment with angiotensin II is associated with rapid blood pressure response and vasopressor sparing in patients with vasoplegia after cardiac surgery: A post-hoc analysis of angiotensin II for the treatment of high-output shock (ATHOS-3) study. J Cardiothorac Vasc Anesth. 2021;35:51–8. doi: 10.1053/j.jvca.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Evans A, McCurdy MT, Weiner M, Zaku B, Chow JH. Use of angiotensin II for post cardiopulmonary bypass vasoplegic syndrome. Ann Thorac Surg. 2019;108:e5–7. doi: 10.1016/j.athoracsur.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Wieruszewski PM, Radosevich MA, Kashani KB, Daly RC, Wittwer ED. Synthetic human angiotensin II for postcardiopulmonary bypass vasoplegic shock. J Cardiothorac Vasc Anesth. 2019;33:3080–4. doi: 10.1053/j.jvca.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Trethowan B, Michaud CJ, Fifer S. Use of angiotensin II in severe vasoplegia after left pneumonectomy requiring cardiopulmonary bypass: A renin response analysis. Crit Care Med. 2020;48:e912–5. doi: 10.1097/CCM.0000000000004502. [DOI] [PubMed] [Google Scholar]
- 9.Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949–57. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellomo R, Forni LG, Busse LW, McCurdy MT, Ham KR, Boldt DW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. A clinical trial. Am J Respir Crit Care Med. 2020;202:1253–61. doi: 10.1164/rccm.201911-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]