Abstract
Pharmaceutical companies market to physicians through individual detailing accompanied by monetary or in-kind transfers. Large compensation payments to a small number of physicians account for most of this promotional spending. Studying US promotional payments and prescriptions for anticoagulant drugs, we investigate how peer influence broadens the payments’ reach. Following a compensation payment, prescriptions for the marketed drug increase by both the paid physician and the paid physician’s peers. Payments increase prescriptions to both recommended and contraindicated patients. Over three years, marketed anticoagulant prescriptions rose 23 percent due to payments, with peer spillovers contributing a quarter of the increase.
Keywords: health care, technology diffusion, peer effects, networks, pharmaceutical advertising
JEL Codes: I11, O33, L14
Introduction
Drug and medical device companies spend the majority of their promotional budgets, over $20 billion annually, on marketing to health care providers. Much of this spending is on individual detailing efforts to encourage adoption of new clinical products. Recent evidence suggests this marketing affects prescription behavior, and an ongoing public debate centers on the influence of drug manufacturers’ promotional efforts (Schwartz and Woloshin 2019). While pharmaceutical companies’ interactions with physicians may educate doctors about new drugs, such engagement may also increase the prescribing volume of higher cost, brand name products marketed by the industry, not necessarily in the best interests of patients or payers (Thomas et al. 2014; Elliot 2016).
Large payments to select few physicians constitute the majority of promotional spending on provider payments.1 These large payments reportedly target thought leaders, i.e. physicians who may be highly influential on the practice of their peers. Supported by a burgeoning commercial intelligence industry that identifies Key Opinion Leaders in different locations and therapy areas, pharmaceutical marketing increasingly leverages indirect influence (Campbell 2008). While influencer marketing and viral marketing are common promotional strategies in consumer goods markets (Goldenberg et al. 2009), understanding their scope in medicine, where information asymmetries leave a large potential for over- and under-adoption of new technologies, is of particular policy importance.
In this paper, we study how pharmaceutical detailing payments affect drug diffusion through the peer networks of targeted doctors. We combine administrative data on prescription claims from Medicare Part D with two other data sources: (1) the universe of payments and value transfers to US physicians by drug manufacturers and distributors, and (2) data on physician networks, where physicians are considered connected if they share patients in the baseline year or, alternatively, if they share a group practice. With these data, we observe pharmaceutical payments and prescriptions over time and across the entire network.
We focus on anticoagulants (commonly referred to as “blood thinners”), a widely used therapeutic class to which a new generation of drugs was introduced shortly before we begin observing payments and prescriptions. During our study period, 2014–2016, over $100 million was spent on marketing payments for doctors to promote new anticoagulant drugs. This class includes Xarelto, the single drug with the highest spending on doctor payments over our study period, and Eliquis, which had the third-highest spending (Ornstein et al. 2019).
For each drug in our sample, roughly one-third of practicing primary care and cardiologist physicians receive small in-kind transfers of food and beverages (typically under $40) associated with detailing interactions with marketing salespersons; we refer to these as “food payments.” In contrast, fewer than 1 percent of physicians receive large payments associated with speaking, consulting, and other services; we refer to these as “compensation payments.” Despite the vastly lower penetration, compensation payments account for two-thirds of the total dollar value transferred. The median compensation payment is over $2,000, and most recipients receive repeated payments for the same drug. We show that compensation payments disproportionately target specialist physicians with many peers.
Our empirical strategy investigates whether physicians increase their prescription volume after their peer receives a pharmaceutical payment. The quasi-experiment applies the insight from Angrist (2014) that compelling peer effect designs manipulate peer characteristics in a way that is unrelated to individual outcomes. Specifically, we test whether physicians increase their prescription volume immediately following changes in peer payment exposure. The framework allows physicians who engage with pharmaceutical companies to differ in both their baseline propensity to prescribe and their speed of new drug adoption. One advantage of our focus on peer effects is that we are studying payment influence on doctors who were not themselves directly targeted or selected by the pharmaceutical company, which mitigates endogeneity concerns around payment timing.
After a physician receives a compensation payment, each of his peers increases use of the target drug by 2 percent on average. This result is shown graphically in Figure 1. Peer spillover effects of compensation payments are stronger when the compensated physician and his peer share more patients in common, and these effects persist when peers are not affiliated with the same group practice as the compensated physician. The indirect effect of compensation payments on each of the recipient’s peers’ prescriptions is roughly 1/20 the size of the direct effect of the compensation payment on the paid recipient himself. However, physicians targeted with these compensation payments have more than 60 peers on average, and so the total estimated impact of a compensation payment on all first-degree peers eclipses the estimated impact of a compensation payment on the paid physician’s own prescription volume.
Figure 1:
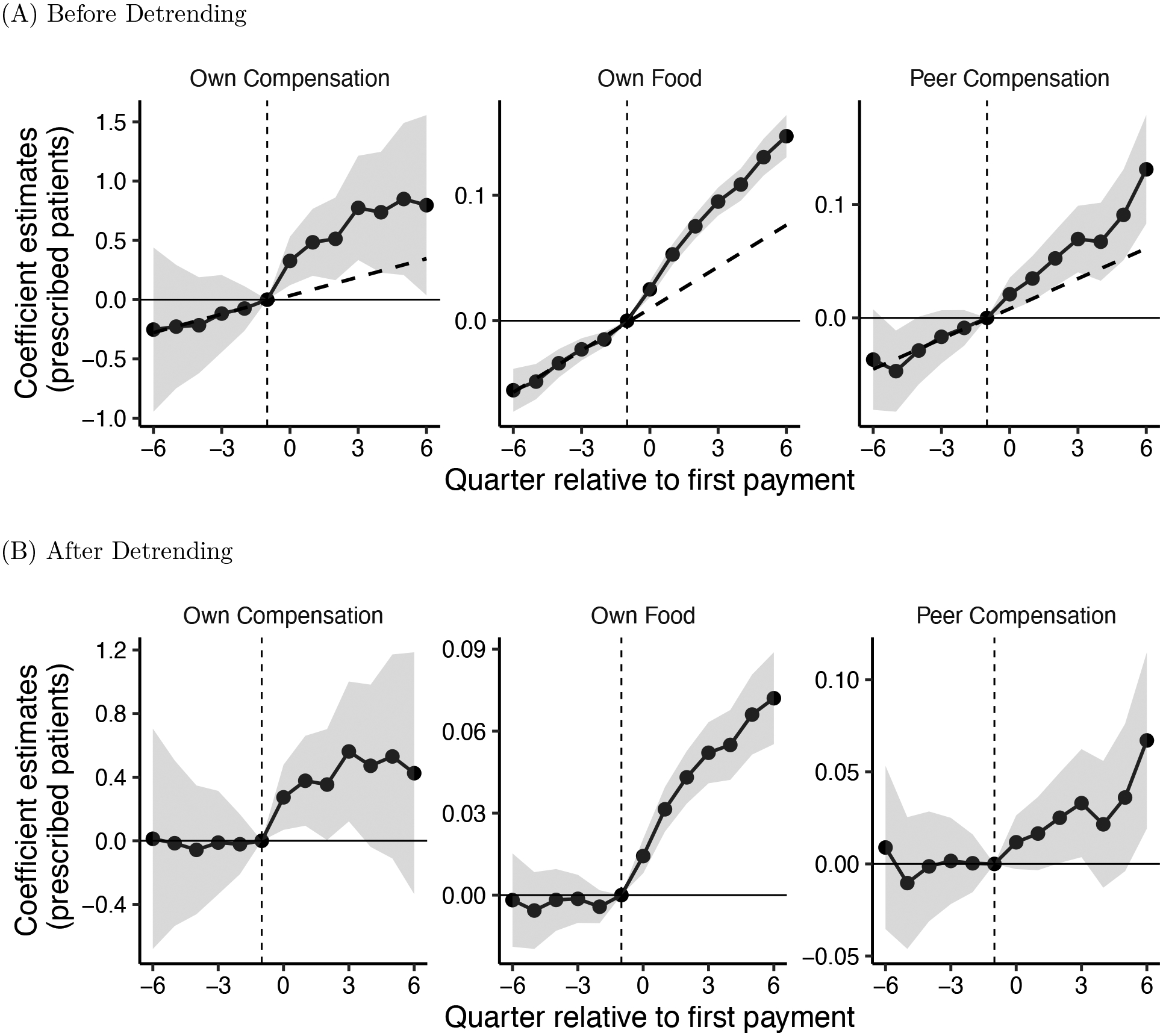
Event Study: The Impact of Payments on Prescription Volume
Notes: Figure shows event study coefficients estimated from equation (1), showing the response of physicians to own and peer payments of different types. The facets show coefficients for different payment types—own food, own compensation, and peer compensation—that were all jointly estimated using 5,467,536 doctor-drug-quarter observations. Panel (A) reports coefficients from a single regression that excludes a differential pre-trend for paid physicians; a dashed line is fitted to the pre-trend for illustration. Panel (B) reports coefficients from a single regression after detrending, using the two-step procedure described in Section 2. All regressions also include variables for peer food, own travel, and peer travel, alongside fixed effects for doctor-drug and drug-specialty-quarter. Quarter 0 indicates the quarter of the first payment of each type. Shaded areas show 95 percent confidence intervals. Note that facet vertical axes have different scales.
Relative to a counterfactual without pharmaceutical payments of any type, our partial equilibrium analysis estimates that pharmaceutical payments have increased the US market for new oral anticoagulants from $6.2 billion to 7.6 billion. While much of this impact is driven by the direct influence of widespread food payments on recipient doctors, about a quarter of the increase is due to peer spillover effects of infrequent (but large) compensation payments. This estimate of spillovers is likely conservative because it fails to account for other peer relationships besides measured patient-sharing ties.
Industry payments may simply shift patients from similar drugs to the promoted one, or they may expand prescriptions to new patients spurring market growth. Exploring the effects of promotional payments on the prescription of competitor drugs, we find that on net, compensation payments expand the market for the new oral anticoagulant drug class and increase total anticoagulant prescribing. Food payments and exposures to a compensated peer do not induce significant business-stealing across drugs, although physicians who receive compensation payments reduce their prescribing of rival anticoagulants.
The welfare implications of pharmaceutical payments are not immediately obvious. One avenue through which payments may affect welfare is if they propagate useful information on evidence-based care. Studying prescription decisions for patients with atrial fibrillation, common candidates for anticoagulation, we find no evidence that detailing interactions increased concordance with clinical guidelines either among directly paid physicians or their peers. This evidence falls short of a comprehensive welfare assessment, but it does contrast with a common framing of detailing as simply a means for educating physicians about evidence-based standards of care.
Several recent papers have analyzed the effect of pharmaceutical marketing on prescribing decisions (David et al. 2010; DeJong et al. 2016; Larkin et al. 2017; Shapiro 2018a; Sinkinson and Starc 2019; Grennan et al. 2018; Carey et al. 2021). Our project extends this work by providing the first evidence on peer spillover effects of pharmaceutical payments, providing a new lens for understanding the large consulting and compensation payments that comprise a majority of promotional spending on physician payments.
Although our focus is on peer effects, we also estimate direct effects of payments on paid physicians. It is instructive to compare our approach and findings on these direct effects to other recent work. Most closely related is Carey et al. (2021), which applies a similar fixed effect design and finds each payment is associated with a 4% increase in prescription volume, which is very similar to 5% increase we estimate for the most common payment type (i.e. food and beverage payments). Grennan et al. (2018) estimate the effect of the cumulative relationship between pharmaceutical companies and physicians rather than the marginal effect of an additional payment, and thus recover larger effect sizes. Using cross-sectional variation in hospital bans on promotional meals, they find that payments increase statin prescribing by 73%.
Our findings are consistent with prior evidence on the importance of peer influence in health care decisions (Chan 2018; Navathe and David 2009; Oster and Thornton 2012; Silver 2020) as well as in other technology adoption settings (Banerjee et al. 2013; Golub and Sadler 2016; Galeotti et al. 2017). Prior work suggests that peer spillovers may be successful at increasing the use of new drugs (Coleman et al. 1957; Donohue et al. 2018; Agha and Molitor 2018), but may not help curb low-value or risky prescribing (Sacarny et al. 2019). Our paper brings a new focus to this area of inquiry, showing that private firms effectively leverage peer influence for marketing purposes.2
The paper proceeds as follows. Section 1 describes the data and contextual information about the class of anticoagulants. Section 2 explains our empirical strategy. Section 3 discusses our main estimates of the influence of pharmaceutical payments on prescription volume. Section 4 shows estimated effects of payments on rival drugs. Section 5 analyzes whether drug detailing promotes guideline-concordant anticoagulant use for patients with atrial fibrillation. Section 6 quantifies the impact of payments on the aggregate increase and spatial dispersion of prescription volumes. Section 7 concludes.
1. Data and Context
Our analysis focuses on new oral anticoagulants (NOACs), studying the diffusion of three drugs: Eliquis (generic name apixaban), Pradaxa (dabigatran), and Xarelto (rivaroxaban). Eliquis and Xarelto are among the top five drugs with the most associated physician payments during every year of our sample (from 2014–2016) (Ornstein et al. 2019). Eliquis, Pradaxa, and Xarelto were introduced between 2010 and 2012, shortly before our sample period began in 2014. These drugs comprise a growing market for alternatives to the older anticoagulant warfarin (coumadin), as shown in Figure 2.3 All three NOACs had patent protection for the duration of our study period.
Figure 2:
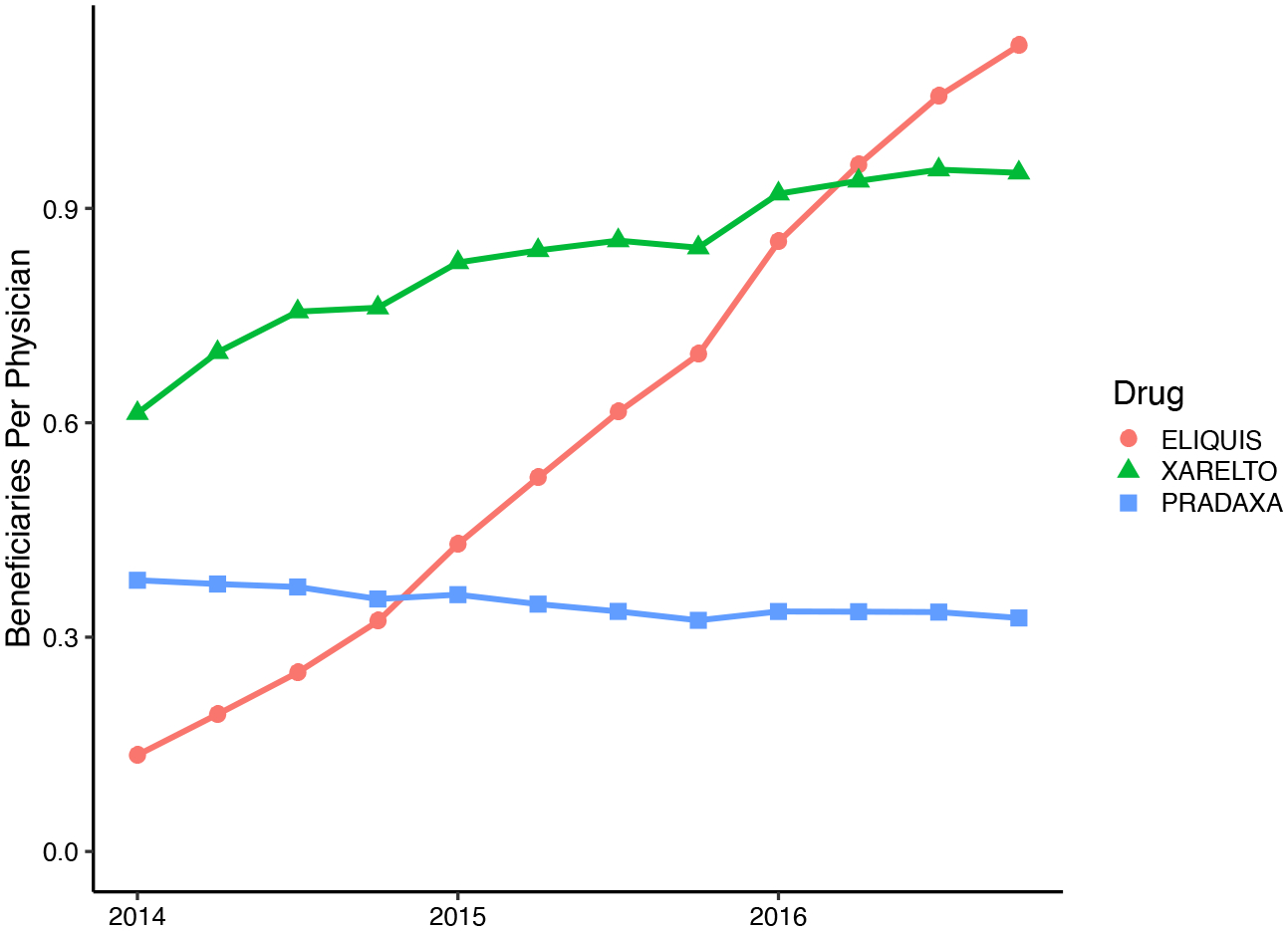
NOAC Prescription Volume over Time
Notes: For the three NOAC drugs we study, figure shows the average number of prescribed beneficiaries per quarter, per physician, in our sample. The average covers all physician-quarters in our sample, including those with zero prescriptions. The FDA first approved Pradaxa in 2010, Xarelto in 2011, and Eliquis in 2012. Data are from a 40 percent of Medicare Part D claims.
Anticoagulants are primarily used to prevent strokes and other clotting events in patients with atrial fibrillation, deep vein thrombosis, and pulmonary embolism. These conditions are both common and serious, estimated to contribute to 240,000 deaths per year in the United States (Center for Disease Control 2019, 2020).
The NOAC global market was $23 billion in 2013, and is projected to double by 2025.4
NOACs were FDA approved on the basis of trials establishing them as noninferior to the warfarin. Cited advantages of NOACs relative to warfarin include improved safety, convenience of use, fewer interactions with other drugs, and no need for laboratory monitoring (Mekaj et al. 2015). These benefits come at a cost: NOACs had no direct generic substitutes over our study period, and were priced at more than $500 per month—many times the price of off-patent warfarin.5
1.1. Data Sources
To estimate peer effects in drug diffusion, we combine multiple databases on prescriptions, payments, and peer connections. Physician prescription volumes are derived from Medicare Part D administrative claims, from 2014–2016. Associated payments and in-kind transfers to physicians made by drug manufacturers are identified in the Open Payments database, from mid-2013 until the end of 2016. Physician shared-patients relationships are merged from the 2013 Referral Patterns database. Additional physician characteristics, including practice location and group practice affiliations are from Physician Compare.
Prescriptions
We analyze a 40 percent sample of Research Identifiable Medicare Part D claims in 2014–2016 (CMS 2013–2016a). To track the adoption and use of new anticoagulant drugs, we restrict attention to physicians of medical specialties that together comprise the majority of NOAC prescribers: primary care and cardiology.6
We construct a quarterly panel of prescription data for each doctor and anticoagulant, defining three outcome variables. Our primary outcome is the number of unique Medicare Part D beneficiaries filling prescriptions written by the index doctor, for the index drug in that quarter. Second, to distinguish new initiations of anticoagulation from prescription refills, we construct a count of newly prescribed patients, excluding prescription renewals or drug changes for patients already using anticoagulants. We define newly prescribed patients as those who did not fill any oral anticoagulant prescription for the prior 12 months. Finally, to measure the relative market share of each drug at the physician level, we calculate the fraction of the physician’s total anticoagulant prescription fills that are for the index NOAC.
Appendix Figure A1 shows the cumulative distribution of our primary outcome: prescription volume of the three NOACs under study at the doctor-drug-quarter level. 75% of doctors do not prescribe the index NOAC within a given quarter; prescribing the NOAC to more than 5 beneficiaries per quarter is rare, accounting for less than 2% of observations.
Peers
To study peer effects in prescription decisions, we combine prescription information with physician referral data from the Centers for Medicare and Medicaid Services Referral Patterns database (CMS 2013). In these data, two physicians have a shared patient if they both participated in the delivery of health services to the same Medicare patient within 30 days of one another. Two physicians are defined to be peers if they have 11 or more shared Medicare Fee-For-Service patients within a year. The threshold of 11 patients was chosen by CMS to protect patient privacy. According to survey evidence, this claims-based network definition aligns with physician self-reported professional networks (Barnett et al. 2011).7 Furthermore, a potential channel for peer influence is via observing peer prescription behavior for shared patients, so this definition of peer ties coincides with a potentially important mechanism for peer effects.
We treat this network as static, undirected, and unweighted. One potential concern is that the network structure is itself endogenous, which could be the case if physicians adjusted their working relationships in response to payments received or prescriptions made by other physicians. We argue that such responses are likely small, as we measure shared-patient relationships using all services provided by sampled physicians to Medicare patients, of which anticoagulant prescriptions are just a small fraction. Furthermore, physician working relationships have been shown to be very persistent (Zeltzer 2020), so we define peers based on the observed network of shared-patient peers in 2013 (the year before our sample begins).
We supplement our baseline measure of peer linkages defined by shared patients with other definitions. First, we separate shared-patient peer relationships according to whether the two doctors have an above- or below-median count of shared patients, a proxy for relationship intensity (Barnett et al. 2011). Among reported links with at least 11 shared patients (our baseline measure), the median number of shared patients is 21. Second, we explore peer relationships based on common group practice affiliation. In our sample, the mean physician has 78 peers who share at least one group practice affiliation. Group-practice and shared-patients affiliations often overlap but do not subsume one other: on average, a physician shares a common group practice with only 43 percent of her shared-patient peers, and is a shared-patient peer with only 29 percent of her group practice peers.
Payments
We combine data on NOAC drug prescriptions with data on associated payments and value transfers to physicians by drug manufacturers and distributors. Industry payment data comes from the Open Payments database (CMS 2013–2016b) for the period from July 1, 2013 through December 31, 2016. This database is maintained by CMS as part of the Physician Financial Transparency Reports (Sunshine Act), a national disclosure program created by the Affordable Care Act. Since 2013, manufacturers have been required to submit data about all payments and other transfers of value made to physicians (which we refer to as payments). The reports include the amount paid (or value of nonmonetary transfer, such as food or travel expenses), the associated drug(s), and the nature of the transfer. Payment dates are recorded as the actual date of payment issue. We match doctors listed in Open Payments to National Provider Identifier codes based on physician name and address.8 We aggregate this data to construct a panel of physician payment amounts and payment types in each quarter and for each drug.
From 2014–2016, the reported payments total to $103 million for the three NOAC drugs we study. Table 1 shows the distribution of payment size by payment type. We group payment types into two main categories, based on average payment size: (1) food, beverage, and education; (2) consulting fees and compensation for services.
Table 1:
Summary Statistics for Different Types of Pharmaceutical Payments
| Assigned Category | Total Number of Payments | Mean Payment Size | Median Payment Size | Payment Total Amount (USD) | |
|---|---|---|---|---|---|
| Payment Type | (1) | (2) | (3) | (4) | (5) |
| Consulting Fee | Compensation | 2, 247 | 2, 370 | 2, 000 | 5, 325, 818 |
| Compensation for services | Compensation | 27, 426 | 2, 275 | 2, 400 | 62, 397, 361 |
| Travel and Lodging | Travel | 18,076 | 260 | 112 | 4, 695, 838 |
| Education | Food | 30, 208 | 36 | 9 | 1, 095, 886 |
| Food and Beverage | Food | 1, 759, 889 | 17 | 13 | 29, 295, 620 |
Notes: Payments for NOAC drugs to sampled physicians, 2014–2016. Rows are shown in descending order of mean payment size. The “Payment Type” column lists the payment category as reported in the Open Payments Database. The “Assigned Category” describes our groupings of these types into three categories based on payment size. We label these categories based on the most common payment type: Compensation, Travel, and Food.
The largest category of payments by both average size per payment and total expenditure is compensation for services and consulting fees. We observe nearly 30,000 and consulting payments, with each transaction averaging over $2,200. As we later show, these payments are concentrated among a small fraction of highly connected cardiac specialists. CMS defines compensation payments as “[p]ayments made to physicians for speaking, training, and education engagements that are not for continuing education.” Consulting payments are defined as “[p]ayments made to physicians for advice and expertise on a particular medical product or treatment, typically provided under a written agreement and in response to a particular business need” (CMS 2019). These payments suggest a deep relationship between the drug company and the physician; the physician’s professional reputation and expertise is being used either to directly market or to inform marketing strategy for the targeted drug.
The most common transfers are in the form of food, beverages, and educational materials provided by salespeople when discussing new drugs with physicians. Our sample includes 1.8 million transfers of this nature, most of them for food and beverages. These small payments, averaging below $40 per payment, are received by both generalists and specialists. Food and beverage transfers are often of modest value, with a median payment size of only $13; a typical interaction of this sort has the pharmaceutical sales representative providing a casual lunch in a physician office and sharing information about a new product. Education payments are also small, with a median value of $9, and can include payment for educational classes or events, or materials like textbooks and medical journal articles (CMS 2019). The targeted physician has very limited financial stake in the ongoing relationship, but may nevertheless respond to the informative or persuasive nature of the interaction or due to psychological motivations such as gift-exchange.
For completeness, we also include a third, smaller category of payments for travel and lodging. Our sample reports 18,000 travel transactions, accounting for only 5 percent of total detailing expenditures. Transfers in this category are of intermediate value, averaging $260 per transaction. Consistent with their low frequency, we generally do not have sufficient statistical power to estimate the relationship between travel payments and prescription volume. We control for travel payments in all regressions.
Payments are spread over both time and space. Figure 3 shows, for each physician specialty and payment type, the cumulative share of physicians who received payments and the average cumulative number of payments associated with each drug. Although there are different time trends in payment rates by drug, payments keep accumulating over the entire period of our study, with largely similar promotional strategies. Appendix Figure A2 shows the distribution of payments to hospital referral regions (HRR) with different population sizes. The relative ranking of payment penetration by regional population is also quite stable.
Figure 3:
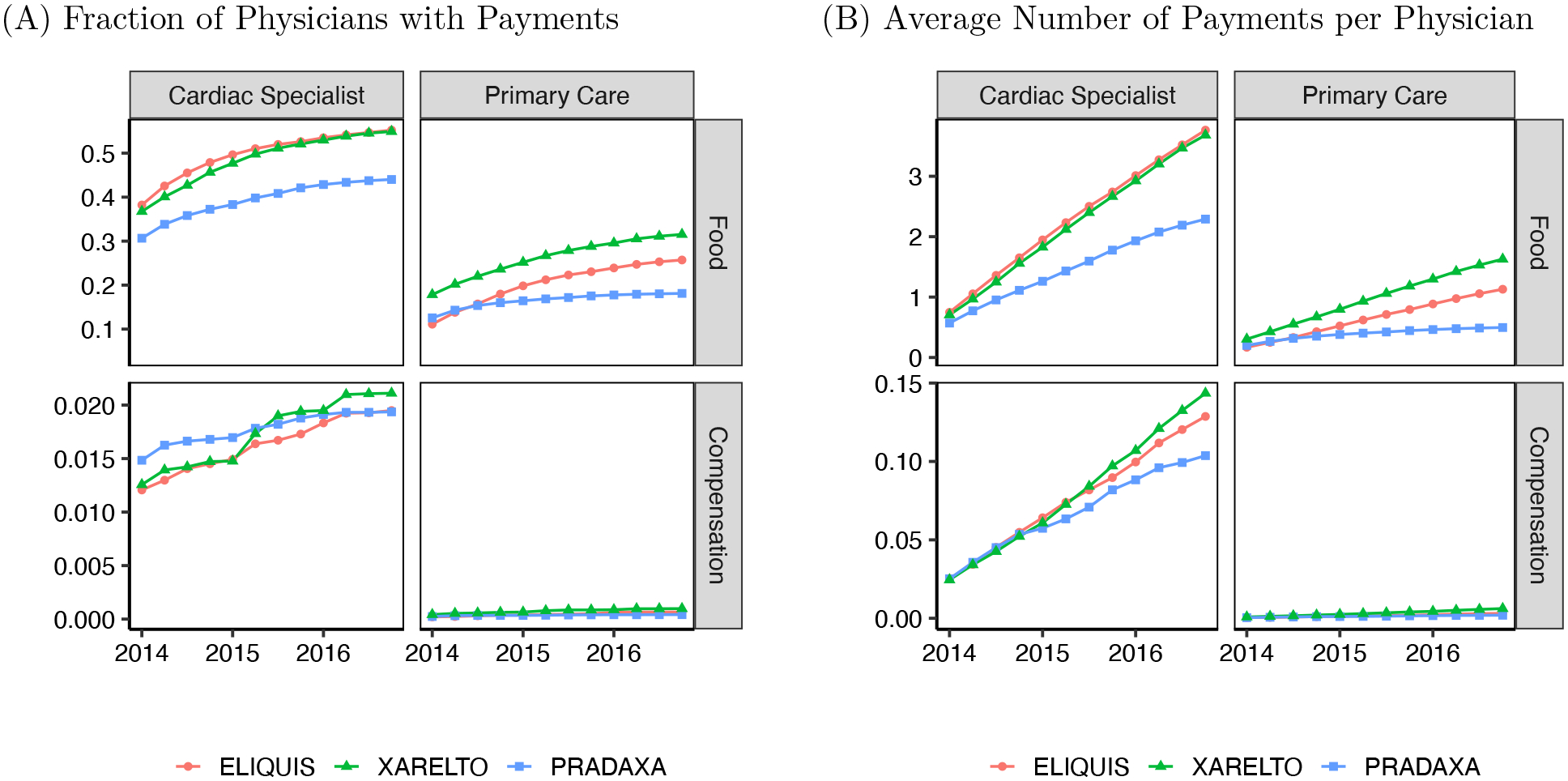
Cumulative Payments over Time, by Type of Payment and Medical Specialty
Notes: Figures show cumulative information on the volume of payments associated with each of the three NOACs in our sample. Panel A shows the fraction of physicians who received at least one payment. Panel B shows information on the average number of payments per physician, including zero payments. Note that facet vertical axes have different scales. In each panel, column of facets shows data for a different medical specialty: cardiac specialties (left) and primary care (right). Each row of facets shows payments of a different type: Food category includes education, food, and beverage transfers; Compensation includes compensation for services and consulting fees. Section 1 describes the specialty and payment category definitions.
Physician characteristics
We use the Physician Compare database to identify the physician’s primary specialty, experience (measured as years since medical school graduation), and group practice affiliations.
1.2. Patterns of Payments, Prescriptions, and Peer Connections
Physicians who share patients with many peer physicians are more likely to receive compensation payments. Figure 4 sorts physicians by decile of number of peers (i.e., network degree) within each HRR and specialty type; it then plots how the average number of pharmaceutical payments per physician varies across the distribution of peer group size. While physicians with relatively few peers are less likely to receive food from pharmaceutical companies, there is little difference in the rate of food transfer among the top four deciles of the distribution. By contrast, highly connected physicians in the top deciles of the peer count distribution are more likely to be targeted with compensation payments, a pattern we see for both cardiac specialists and primary care physicians. These patterns suggest that large payments may be strategically targeted to highly connected doctors, who are better positioned to amplify the payment’s impact. A caveat to interpreting this relationship is that physicians with more peer connections may also see more patients in their own practice.
Figure 4:
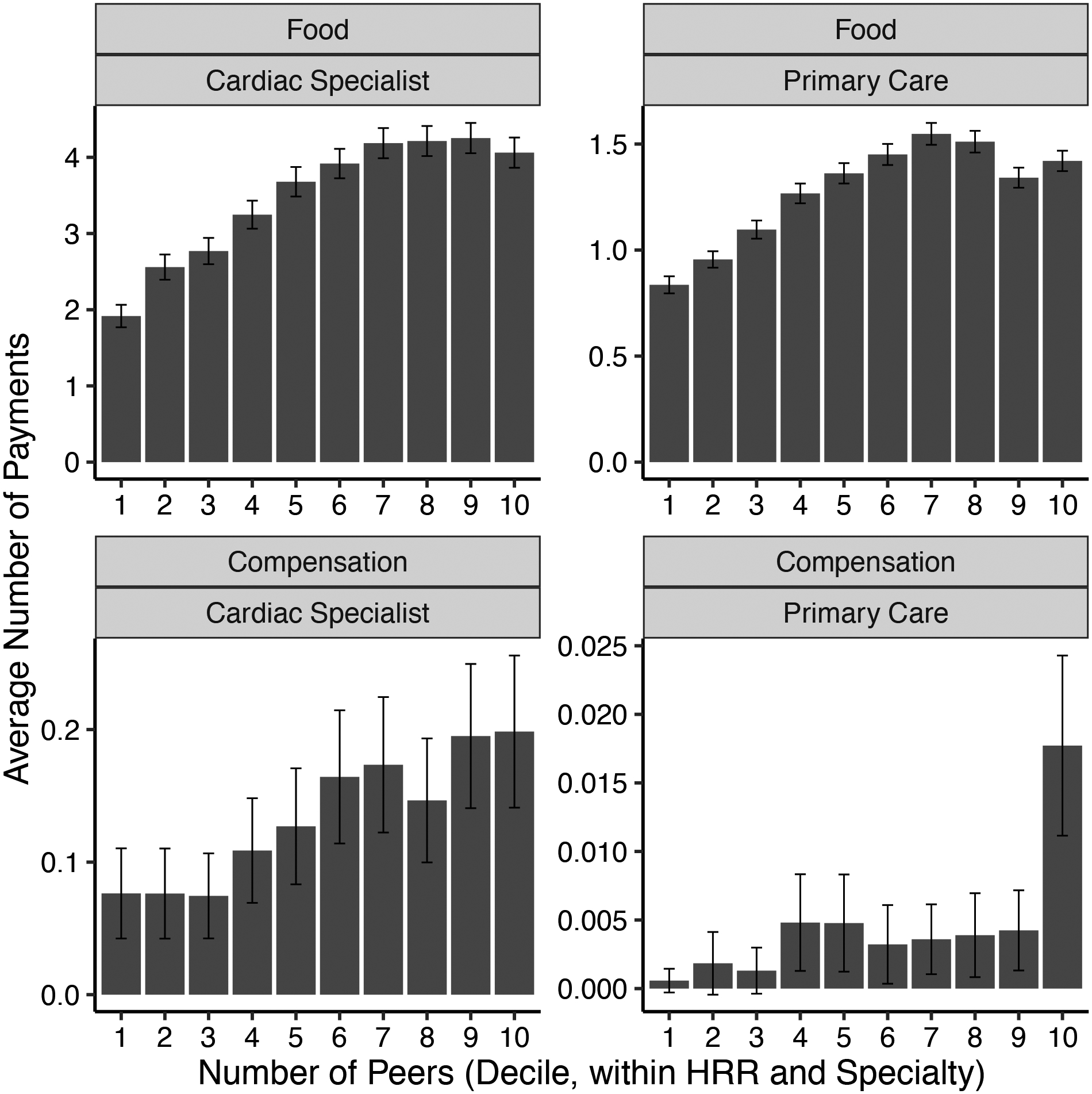
Average Number of Payments by Recipient Number of Peers
Notes: For each specialty and type of payment, figure shows the average number of payments made to each physician (y-axis), by deciles of the recipient’s number of peers (x-axis). Deciles are calculated separately for each HRR and specialty. Error bars show 95 percent confidence interval for the mean. Note that facet vertical axes have different scales. Each row of facets shows payments of a different type: Food category includes education, food, and beverage transfers; Compensation includes compensation for services and consulting fees. Each column shows data for a different medical specialty: cardiac specialties (left) and primary care (right). For specialty definitions see Section 1.
Table 2 displays summary statistics, stratifying the sample by physicians’ eventual payment exposure status. This table is restricted to our analysis sample for consistency with the subsequent regression results. Specifically, we impose two sample requirements to ensure the physician is actively treating Medicare enrollees. First, the doctor must have at least one peer provider as defined by the CMS Referral Patterns data. Second, the physician must prescribe at least one anticoagulant claim in our sample (for any of the anticoagulant drugs, including warfarin) over the three-year study period. These two restrictions together drop 17 percent of the physicians listed in Physician Compare from our sample. We further require that physicians who receive their first observed payment during our sample period (January 1, 2014 through December 31, 2016) have two quarters of pre-payment data and two quarters of post-payment data. We impose this restriction for own compensation, own travel, and own food payments, as well as peer compensation payments. This restriction ensures that we have a balanced panel for at least four quarters around the first payment event.
Table 2:
Summary Statistics by Payment Status
| Own Payments | Peer Payments | |||||
|---|---|---|---|---|---|---|
| None | Food or Travel | Compensation | None, Food, or Travel | Compensation | All Physicians | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| A. Prescription volume | ||||||
| Prescribed patients (per qtr) | 0.320 | 1.115 | 5.938 | 0.452 | 1.124 | 0.548 |
| Newly prescribed patients (per qtr) | 0.025 | 0.081 | 0.388 | 0.033 | 0.085 | 0.041 |
| Fraction of Anticoagulant Prescriptions (%) | 13.7 | 20.2 | 35.7 | 15.2 | 19.7 | 15.8 |
| B. Physician characteristics | ||||||
| Percent cardiologists | 8.0 | 21.9 | 81.3 | 9.3 | 27.8 | 12.0 |
| Experience (Years) | 21.0 | 23.8 | 25.1 | 21.6 | 22.7 | 21.8 |
| C. Physician network characteristics | ||||||
| N of shared-patient peers | 16.6 | 28.1 | 62.0 | 15.4 | 46.0 | 19.7 |
| N of strong shared-patient peers | 8.5 | 15.4 | 35.1 | 7.9 | 25.5 | 10.3 |
| N of group-practice peers | 89.1 | 47.2 | 77.3 | 79.6 | 66.7 | 77.7 |
| N of excluded 2nd-degree peers | 181.9 | 264.6 | 436.3 | 173.5 | 392.4 | 204.8 |
| D. Promotional Payments | ||||||
| Total own pharma payments ($) | 0 | 148 | 38,261 | 103 | 344 | 137.9 |
| N of quarters with food payment | 0 | 4.112 | 8.378 | 0.979 | 2.018 | 1.127 |
| N of quarters with compensation | 0 | 0 | 5.082 | 0.001 | 0.034 | 0.013 |
| N of peer-quarters with compensation | 0.763 | 1.672 | 3.216 | 0 | 7.102 | 0.895 |
| Percent of observations | 72.8 | 26.9 | 0.3 | 85.7 | 14.3 | 100 |
| N of doctors | 135,428 | 70,550 | 972 | 154,539 | 41,423 | 166,420 |
| N of doctor-drug-quarter observations | 3,982,128 | 1,470,264 | 14,028 | 4,686,072 | 780,348 | 5,466,420 |
| N of observations for fraction outcome* | 2,513,742 | 1,197,940 | 13,038 | 3,130,133 | 594,587 | 3,724,720 |
Notes: Table shows summary statistics for the main sample of 5,466,420 physician-drug-quarter observations; this is a panel of 166,420 physicians over 12 quarters and for three NOAC drugs. Columns 1–3 show statistics for subsets of physicians who directly received different types of pharmaceutical payments: no payments, payments for food or travel, or payments for compensation. Columns 4–5 show statistics for the subset of physicians whose peers received compensation payments, and the complement set of those whose peers did not receive such payments. Column 6 shows statistics for the entire sample. In Panel A, prescribed patients is the number of unique beneficiaries filling prescriptions for the drug in the quarter. Newly prescribed patients are prescribed patients without any anticoagulant prescription in the preceding year. Fraction of Anticoagulant Prescriptions is the share of target drug prescriptions out of all filled anticoagulant prescriptions written by the physician in the quarter. This statistic is based on the subset of 3,724,720 physician-quarters with at least one anticoagulant prescriptions. Definitions of payment types, physician medical specialties, and shared-patient peers are discussed in Section 1.
Table 2 reports that 73 percent of doctors in our sample receive no payments directly. 27 percent of doctors receive food or travel payments for each drug, and these doctors average $148 in payments for the target drug over the 12 quarters of our sample. The 0.3 percent of physicians who receive compensation payments for each drug draw much larger transfers from pharmaceutical companies, averaging $38,261 per doctor cumulatively over 12 quarters. Cardiac specialists make up only 22 percent of food or travel recipients, but account for 81 percent of compensation recipients.
Appendix Figure A3 shows the distribution of the number of quarters with payments over the study period for paid physicians. Though the modal payee received just one food or compensation payment, most payees receive multiple payments. Physicians receiving food payments for a particular drug are paid in 4 out of 12 quarters on average, while those receiving compensation payments average 5 quarters with payments. A limitation of our data is that we do not observe payments before the third quarter of 2013. If some physicians were paid only prior to the third quarter of 2013, we will treat them as if they were never paid in our analysis, which would bias our results on payment effects toward zero. As Figure A3 shows, this is not likely to be a large group, since most paid doctors receive repeated payments.
Although fewer than one percent of doctors receive compensation payments, many more doctors are indirectly exposed to compensated peers since paid doctors are highly connected. 14 percent of doctors in our sample are linked to a compensation-paid physician for a given drug. Appendix Table A1 reports the correlation between each type of payment exposure within a quarter. Consistent with the partial overlap in shared-patient and group-practice peer affiliations discussed above, the correlation between the number of compensation payments to peers of either type is 0.237. The correlation between own food receipt and peer compensation exposures is only 0.091, suggesting that these events often occur independently, affecting different physicians at different points in time.
Table 2 also illustrates that physicians directly and indirectly targeted with payments use the promoted drug more intensely. Doctors whose peers receive compensation payments prescribe the NOAC to 1.12 patients per quarter, on average, compared to 0.45 patients per quarter for doctors whose peers do not receive compensation payments. We explore this relationship in our regression analysis.
Note that the prescription volumes reported here cover only a modest fraction of doctors’ overall patient panel. We observe prescriptions for a 40 percent sample of Medicare Part D enrollees and only 72 percent of Medicare beneficiaries were enrolled in Part D as of 2015 (Hoadley et al. 2015), suggesting our sample covers roughly a quarter of all Medicare beneficiaries. Further, only two-thirds percent of NOAC prescriptions are for patients 65 years or older (Agency for Healthcare Research and Quality 2014). Assuming that the impact of pharmaceutical payments on prescribing patterns for non-Part D enrollees is similar, we can roughly scale our quarterly patient counts up to the full population by multiplying our estimates by a factor of 5.4. For simplicity, we report unscaled results.
2. Identification and Estimation
Our analysis focuses on estimating pharmaceutical payments’ spillover effects on the peers of targeted physicians. We apply the partial population identification approach, described in Moffitt (2001): identification relies on the fact that some doctors are directly treated (i.e. they themselves receive a large payment) while their peers remain untreated. Exploiting this variation, we compare prescriptions by peers of paid and unpaid physicians, before and after payments are made. This variation isolates changes in peer characteristics over time (i.e. payment exposure) that we argue are plausibly unrelated to changes in the focal doctor’s prescription outcome except through possible peer effects.
By focusing on peer spillovers from discrete payment events, we avoid some of the econometric concerns commonly associated with peer effect studies that directly correlate peer outcomes with own outcomes, including reflection bias, weak instrument bias, and exclusion bias (Manski 1993; Angrist 2014; Caeyers and Fafchamps 2016). We lay out our regression model below, and then discuss identification in more detail.
2.1. Regression models of payment impact
We model prescription decisions as a function of payments, including payments both directly made to the physician and payments to the index doctor’s peer. We begin with a graphical event study around the first payment exposure of each type and then move to a specification that accounts for the accumulating impact of each transfer.
Let Yitd denote the prescription volume of drug d, by doctor i, in quarter t. With slight abuse of notation, let Gi denote the group of direct peers of i in the network G; we model the network as undirected and unweighted. Because peer relationships are intransitive, j ∈ Gi does not imply Gj = Gi, i.e. peer groups vary even among connected peers. We focus only on the effect of payments on direct first-degree peers of recipients. If payments also influence higher-degree peers, our regression coefficients would be biased toward zero.9
Event study.
Our first approach is to graphically analyze prescription patterns before and after the first payment event. To flexibly capture the different effects of various payment types, every specification accounts separately for own and peer exposure to each payment type: food, travel, and compensation. We estimate the model:
| (1) |
where r(idt) indexes event time in quarters relative to the physician’s first payment (of each type) for the index drug. Our main model pools all drugs together, estimating the average effects of payments associated with each drug on prescriptions of that drug, Yitd. The terms αid and βdts are doctor-drug and drug-quarter-specialty fixed effects, respectively. Xidt includes a vector of differential time trends. The vector Zid defines indicator variables for whether doctor i ever receives each of the three types of payments for drug d; it is multiplied by δ(i,d,t), which are the parameters describing how prescription volume changes relative to the quarter of the doctor’s first payment of each type. The vector ZGi,d defines indicator variables for whether doctor i has a peer who ever receives each of the three payment types for drug d; ηr(i,d,t) are the parameters describing how prescription volume changes relative to the quarter of the doctor’s first peer payment exposure.
To estimate specifications that allow for pre-trends, we first estimate a model that excludes the pre-treatment quarter parameters from the δ(i,d,t) and ηr(i,d,t) terms; instead, the model includes a differential linear pre-trend for each type of own and peer payment (food, travel, and compensation), as well as the full vector of indicator variables for post-treatment quarters.10 As a second step, we residualize the outcome variable by the estimated pre-trend and then estimate a version of equation (1) with a full array of pre- and post- treatment quarter parameters. This final specification allows us to directly remove the linear pre-trend from the post-period and graphically assess the presence of nonlinear trends in the pre-treatment period. We report results both with and without implementing this detrending procedure.
The pre-period is uncontaminated by early payments because the graph simply focuses on quarters before and after the first observed payment of each type. All doctors identifying the pre- and post-payment effects were required to have no observed earlier payments over at least four sampled quarters before that first payment. For doctors who received payments in the second half of 2013, over which period we only have payment but not prescription data, we included separate time trends to account for the possibility that early payments targeted different recipients than later ones. Because our payment data set begins in Quarter 3 of 2013, presumably after some payments have been made, our estimates of payment effects may be biased toward zero since we cannot identify the first payment over the drug’s complete history. The effects of early payments will be absorbed in the fixed effects and differential time trends of paid physicians.
Main specification.
The event study graphs illustrate a trend break in prescription volume after the first payment. A key reason for this apparent trend break is that most doctors in our sample receive repeated payments in the post-period. Thus, for the primary regression specification reported in our tables, we use the running sum of paid quarters as the key independent variable to capture the individual impact of each payment. We estimate:
| (2) |
where Pitd denotes a vector of variables that count the number of quarters up to time t with payments of each type (food, travel, compensation) made to physician i for drug d. PGi,d,t similarly counts the number of payments of each type made to doctor d’s peers (Gi, d, t) up to time t.11 The control variables in equation (2) parallel those in equation (1), including the same set of fixed effects. We continue to include differential time trends by own and peer payment type (food, travel, and compensation) for doctors who receive payments in 2013, before the beginning of our Part D sample. In addition, this specification includes additively separable trends by own and peer payment type for any doctor who is paid for the first time during our sample period, which allows for differential pre-trends for doctors paid during our sample.
The key parameters of interest are the δ vector, which captures the effect of each additional quarter with own pharmaceutical payments of each type, and the η vector, which captures the effect of the number of peer-quarter pairs that received each type of payment to date.
2.2. Discussion of identification
The main identification concern is the endogeneity of peer payments: peers of paid physicians may have had high prescription rates even in the absence of their peer’s payment. Such a correlation could arise because of homophily (payments may target enthusiastic adopters with likeminded peers) or common shocks (payments may target locations with positive demand shocks).
To address concerns about endogeneity, we use new payments to the focal doctor’s peers to generate changes over time in exposure to high-prescribing peers with greater enthusiasm for the new drugs. Through the inclusion of doctor-drug fixed effects, the framework accounts for the possibility that payments are associated with unobserved time-invariant physician characteristics. These control variables allow us to differentiate correlated tastes for technology among peers from the impact of payment exposure. For example, if pharmaceutical transfers target doctors who were already high-volume prescribers or who already had high prescribing peers, this would not bias our findings.
Threats to the identification could still arise with this approach if payments coincide with changes in prescription volume for the target drug, which would have occurred even in the absence of payment. One benefit of focusing on the peers of targeted doctors is that these peers have not been directly selected by the pharmaceutical company, making it more plausible that they would otherwise experience parallel trends to other doctors of the same specialty and eventual payment status. We assess parallel trends through event study graphs described below.
We also consider bias that may arise from common local shocks to patient preferences or physician information, such as direct-to-consumer advertising, changes in local disease burden or information disseminated at local conferences. We verify that our results are robust to the inclusion of fixed effects for the interaction of hospital referral region, drug, and quarter, which capture differential regional time patterns in drug adoption. In addition, we perform a robustness check using a matched sample, whereby every paid physician and his or her peers is matched to an unpaid physician in the same hospital referral region, specialty, and with a similar number of peers. We describe these and other robustness tests at length in Section 3.3.
3. Results
3.1. Baseline estimates of payment influence
Figure 1 shows the relationship between payment exposure and prescription volume, before and after the first payment exposure of every type, obtained from estimating equation (1). Panel (A), shows estimates that do not account for differential pre-trends by the doctor’s eventual payment status. These graphs illustrate that paid doctors are indeed on a trend of increasing use even prior to their first payment; this pattern holds for doctors who receive compensation and food payments, as well as for doctors exposed to compensated peers. Panel (B) displays the same results using a more flexible specification that allows for differential pre-trends, as described in Section 2.1. These plots that account for differential pre-trends show a trend break, with accelerating growth in prescription volume after the first payment.
Prescription volume deviates further from the trend as more quarters elapse following the first payment. This pattern is especially salient following the first food and peer compensation payments. Recall that many doctors are exposed to repeated payments of the same type; the growth in post-period prescribing may reflect the accumulating impact of subsequent payments. For this reason, in our main specification, which we discuss next, we avoid simple pre/post comparisons and instead model prescription volume as a function of cumulative payment exposure. We use the main specification to guide our interpretation of the effect sizes.
Table 3 reports regression estimates, using equation (2), of the impact of individual payments on different measures of prescription volume. The key independent variables in these regressions count the number of quarters to date in which the doctor received a payment of each type, or the number of exposures to date to peer payments of each type. Column 1 reports that each quarter with a compensation payment increases the paid doctor’s prescription fills by 0.37 patients per quarter. Smaller transfers have smaller estimated effects: a food payment increases a doctor’s own prescribing by 0.06 additional beneficiaries per quarter. Each exposure to a peer’s compensation payment leads to a 0.02 increase in quarterly prescribed beneficiaries.
Table 3:
The Influence of Payments on Target Drug Prescription Volumes
| Dependent Variable: | |||
|---|---|---|---|
| Number of Prescribed Patients | Newly Prescribed Patients | Fraction of Anticoagulant Prescriptions | |
| (1) | (2) | (3) | |
| Payment count, by type: | |||
| Own Compensation | 0.3684 (0.1156) | 0.0286 (0.0159) | 0.0093 (0.0058) |
| Own Food | 0.0584 (0.0037) | 0.0048 (0.0006) | 0.0039 (0.0007) |
| Peer Compensation | 0.0197 (0.0061) | 0.0020 (0.0009) | 0.0019 (0.0009) |
| Peer Food | −0.0006 (0.0014) | 0.0002 (0.0002) | 0.0004 (0.0004) |
| Mean of dependent variable | 0.5486 | 0.0409 | 0.1588 |
| N (Doctor × Drug × Quarter) | 5,466,420 | 5,466,420 | 3,724,720 |
Notes: Estimates of equation (2); each column reports key coefficient estimates from a separate regression. The dependent variables capture different prescription volume measures. The independent variables capture the counts of different types of payments made to the prescribing physicians (“Own”) or to others with whom the prescribing physicians shared patients (“Peers”). Food includes payments for food and beverages, and educational items. Compensation includes payments for consulting, speaking, and other services. See Section 1 for detailed definitions. Physician-drug, specialty-drug-quarter fixed effects, controls for all other types of payments, and payment-type-specific linear time trends included in all specifications. Standard errors are clustered within doctor.
To contextualize these magnitudes, we compare the estimated increases in prescription volume to the 2014–2016 average prescription volume for each group of recipients. Compensation payments increase prescriptions by the paid physician by 6 percent from a mean of 5.94 prescribed beneficiaries per quarter. Food transfers increase prescriptions by the paid physician by 5 percent from a mean of 1.11 prescribed beneficiaries per quarter. Exposure to a compensated peer increases prescribing by 1.8 percent from the average 1.12 prescribed beneficiaries per quarter.
These findings are consistent with each type of payment promoting prescriptions in a different way. Recipients of compensation payments have deep ties to pharmaceutical firms; they typically receive repeated payments, climbing into the tens of thousands of dollars over multiple years. Thus, they may have strong incentives to prescribe the promoted NOAC in order to maintain a relationship with the pharmaceutical company and signal their belief in the product’s clinical value. Viewed in this light, it is perhaps unsurprising that physicians’ prescription volume responds to compensation payments. By contrast, food payments reflect a brief engagement with a salesperson in exchange for a low value transfer (the median payment is worth less then $15). Hence the pecuniary incentives to have a “quid pro quo” trade with a sales representative in exchange for this small transfer are modest at best. The professional reputation of recipient physicians is not entwined with the drug’s commercial success or clinical use. Rather, the influence of these payments suggests the informative or persuasive nature of the interactions.
The influence of exposure to compensated peers is particularly notable. The highly skewed nature of the industry payment distribution, with most spending going to large compensation payments for a very small set of physicians, suggests that peer effects may be an explicit part of the marketing strategy. This strategy is consistent with anecdotal evidence from industry insiders (see Elliot 2016; Thomas et al. 2014). A back-of-the-envelope calculation further suggests that spillover effects are an essential part of the return on these large payments.12 In the next section, we discuss additional evidence related to the possible nature of this influence.
Prescription renewals may contribute to the peer effects we uncovered: e.g., when a primary care physician renews a prescription initiated by a compensated cardiologist. In addition, as the primary care physician becomes more familiar with the new drug, she may choose to initiate new prescriptions with the drug. To quantify the latter effect, we exclude prescription renewals by restricting our prescription volume outcome to only include patients without any prior prescription for anticoagulants in the previous year. As reported in Table 3, column 2, between 8 and 10 percent of the effect of payments on total prescription volume is driven by prescriptions for patients with no prior anticoagulant use. This result extends to doctors with exposure to compensated peers, suggesting the peer spillover effects of payments also spur prescriptions of the target drug to new patients.
To assess whether the estimated increases in prescription volume were driven by an increase in the total volume of anticoagulant prescriptions or, alternatively, by doctors shifting patients toward the target drug, we consider a third outcome measure: the fraction of anticoagulant prescriptions that were for the target drug (defined for the 68 percent of doctor-drug-quarters with nonzero anticoagulant prescriptions). Results are reported in Table 3, column 3. Own food payments and exposure to compensated peers are associated with a significant increase in market share of the target drug. We investigate the competitive business stealing and market expanding effects of these payments further in Section 4.
3.2. Potential channels for peer influence
Exposure to compensated peers may induce prescription changes in a few possible ways. Recall that compensation payments mostly target specialists. When a primary care physician observes patients return from a specialist consultation with a prescription for a NOAC, the primary care physician may infer that the specialist sees that drug as clinically superior to other options and update her beliefs about the drug’s quality. Alternatively, the compensated physician may directly “proselytize” about the drug to his peers. While we cannot directly distinguish these mechanisms, we perform several analyses to illuminate the possible channels.
We find evidence supporting the channel of indirect learning by observing peer prescription choices. To briefly foreshadow, peer effects are larger when the two physicians share more patients in common, suggesting they have more opportunities for passive learning. Although compensated physicians may have more opportunities to promote the drug through direct proselytizing to physicians in their own group practice, we find that peer effects are similar for shared-patient peers inside and outside the physician’s group practice. Finally, we exploit the network structure and find that compensation payments to peers-of-peers (who do not have a direct connection to the index doctor) increase the index doctor’s prescribing. The rest of this section reports these findings in detail.
Relationship Type and Strength
In Table 4, we consider additional measures of the strength and type of peer relationship. First, we separate baseline patient-sharing peer relationships according to whether the two doctors have an above- or below-median count of shared patients. (The median relationship in our sample involves 21 shared Medicare Fee for Service patients.) Results reported in column 2. Compensation payments have a larger effect on peers with stronger ties: a compensation payment to a strongly-tied shared-patient peer increases the index doctor’s prescription volume by 0.028 patients per quarter. The effect on more weakly tied shared-patient peers is significantly smaller, although still positive, at 0.008 additional prescribed patients per quarter.
Table 4:
The Influence of Payments on Prescription Volume, Different Peer Definitions
|
Dependent Variable: Number of Prescribed Patients |
|||
|---|---|---|---|
| (1) | (2) | (3) | |
| Compensation Payment Count, by Recipient Type: | |||
| Own | 0.3684 (0.1156) | 0.3684 (0.1155) | 0.3679 (0.1156) |
| Shared-Patient Peer | 0.0197 (0.0061) | 0.0284 (0.0077) | 0.0221 (0.0061) |
| Below-Median Shared-Patient Peer | −0.0200 (0.0093) | ||
| Group Practice and Shared-Patient Peer | −0.0091 (0.0145) | ||
| Group Practice and not Shared-Patient Peer | 0.0134 (0.0045) | ||
| N (Doctor×Drug×Quarter) | 5,466,420 | 5,466,420 | 5,466,420 |
Notes: Estimates of equation (2). The dependent variable is the number of prescribed patients. Independent variables capture the counts of exposure to compensation payments, for different types of recipients. “Own” denotes payments to the prescribing physician. “Shared-Patient Peer” denotes a payment recipient with whom the prescriber shared at least 11 Medicare patients. “Below-Median Shared-Patient Peer” denotes a payment recipient with whom the prescriber shared less than 21 patients (the median number for our sample), a proxy for a weaker relationship. “Group Practice and Shared Patients Peer” coefficients report the difference in payment impact for those with both group practice and shared-patient ties, relative to those with only shared-patient ties. Finally, the “Group Practice Peer and not Shared-Patient Peer” coefficient reports the impact of payments to peers who share a group practice affiliation but do not meet the definition of a shared-patient peer. See Section 1 for exact definitions. Physician-drug, specialty-drug-quarter fixed effects, controls for all other types of payments, and payment-type-specific linear time trends included in all specifications. Standard errors are clustered within doctor.
Table 4 also explores peer relationships based on group practice affiliation. We distinguish three types of peer relationships: doctors who share patients, doctors who share both patients and a group practice affiliation, and doctors who only share a group practice affiliation. The results suggest that doctors who share patients with a compensation-paid peer will increase their prescribing volume by 0.020 per quarter in our sample. This effect is not significantly different for peers who not only share patients but also a group practice affiliation with a compensation-paid peer.13 Doctors who only share a group practice affiliation (but do not have shared patients) with the compensation-paid peer increase their prescription volume of the target drug by 0.013 patients per quarter.
This evidence suggests that patient sharing may be an important channel through which compensated physicians influence their peers. Peer effects are larger when the two physicians have more common patients, and patient sharing relationships predict influence independently of shared group practice affiliations. These patterns also suggest that social ties among patients are unlikely to explain our results.14
Peer Spillovers in Prescription
To further test for indirect peer spillovers in prescription behavior, we adapt the approach of De Giorgi et al. (2010) and conduct an instrumental variables exercise, exploiting the fact that patient-sharing peer networks are intransitive. In our sample, only a third of connected triplets are fully connected. This feature allows us to use cross-sectional variation in payments to “excluded peers”, i.e. peers of peers who are not also the index doctor’s direct peers, as instrumental variables for the average prescribing volume of the doctor’s direct peers. The IV regressions control for payments to direct peers and payments to the index doctor. Because this framework exploits payments to “excluded peers,” it suggests that our estimated peer effects are not exclusively driven by proselytizing activity by compensated physicians. This method is described in more detailed in Appendix A.1. Results are reported in Appendix Table A2.
Using this approach, we find supportive evidence of peer spillovers in prescribing. As expected, the first stage shows that compensation payments to the index doctor’s excluded peers predict higher average prescribing of the target drug among the doctor’s direct peers. The second stage results show that an average (instrumented) increase of one prescribed patient per quarter among a physician’s direct peers is estimated to increase the index doctor’s prescribing by 0.27 additional patients. These results suggest that observing colleagues’ prescribing patterns may be an important mechanism behind the documented peer effects, and that peers of compensated doctors may play a role in amplifying the rippling influence of compensation payments through the network.
3.3. Robustness
In this section, we report the results of a series of robustness checks, showing that the estimated effects of physician payments are robust to varying the sample, set of control variables, or functional form of the outcome.
Effects by drug, physician specialty, and physician experience
Appendix Table A3 reports results of separately estimating equation (2) for different subsamples. Peer payments increase prescription volume for both primary care providers and cardiac specialists, in a similar proportion to mean prescribing rates for both groups. These patterns suggest that our main findings do not reflect efforts by physicians to attract pharmaceutical funding for themselves, once they learn that a peer has received compensation payments. Only 0.1% of primary care physicians receive payments compared to 4% of cardiologists, yet they both respond as strongly to peer compensation exposure. We also find similar proportional increases for both low and high experience physicians. Finally, both own payments and exposure to compensated peers increase prescription volume for each of the three NOACs under study. The peer effects of compensation payments are not statistically distinguishable across drugs.
Additional controls and matched sample estimates
Table 5 reports results of a set of robustness checks. First, Column 2 tests robustness to adding HRR-drug-quarter fixed effects; these rich controls capture local trends in NOAC diffusion, accounting for correlated local shocks in the promotion of specific products, such as direct-to-consumer advertising, local promotional events, or medical conferences. Results remain nearly identical to the baseline specification (reproduced for reference in column 1). In column 3 of Table 5, we add a vector of control variables for payments made by the same manufacturer of the index NOAC for interactions that promote other products. Accounting for these other relationships physicians may have with the pharmaceutical firms promoting NOACs does not substantively change our estimated effects.
Table 5:
The Influence of Payments on Target Drug Prescription Volume: Robustness Checks
|
Dependent Variable:
Number of Prescribed Patients |
|||||
|---|---|---|---|---|---|
| Specification: | |||||
| Baseline | HRR-drug-quarter Fixed Effects | Controls for Other Payments | Matched Sample, Flexible Trends | Matched Sample, Without Trends | |
| (1) | (2) | (3) | (4) | (5) | |
| Payment Count, by Type | |||||
| Own Compensation | 0.3684 (0.1156) | 0.3664 (0.1104) | 0.3679 (0.1146) | 0.4184 (0.1474) | 0.4948 (0.1224) |
| Own Food | 0.0584 (0.0037) | 0.0557 (0.0036) | 0.0561 (0.0037) | 0.0668 (0.0064) | 0.0676 (0.0047) |
| Peer Compensation | 0.0197 (0.0061) | 0.0192 (0.0060) | 0.0245 (0.0060) | 0.0249 (0.0077) | 0.0289 (0.0055) |
| Peer Food | −0.0006 (0.0014) | −0.0012 (0.0013) | −0.0014 (0.0013) | −0.0030 (0.0027) | −0.0071 (0.0022) |
| Mean dependent variable | 0.5486 | 0.5488 | 0.5486 | 0.7750 | 0.7750 |
| N (Doctor × Drug × Quarter) | 5,466,420 | 5,463,264 | 5,466,420 | 1,913,556 | 1,913,556 |
Notes: Table compares estimates obtained from different variants of equation (2). Column 1 repeats our baseline estimates of the effects of payments of different types on the quarterly number of prescribed beneficiaries, already shown in Table 3. Column 2 shows an extended specification that includes HRR-drug-quarter fixed effects, in addition to all terms of the baseline specification (HRR is missing for a small number of observations, hence the slightly smaller sample size). Column 3 shows an extended specification that includes separate controls for payments made by the pharmaceutical company that promotes the target drug in association with any other drugs except for the target drug. Columns 4 and 5 show alternative estimates of the effects of payments on prescriptions, obtained using a sample that includes recipients of compensation payments and matched non-recipients. Matching was first performed exactly on specialty and drug then coarsely on group practice network degree, shared-patient network degree, and years of experience. Details of the matching procedure are described in Appendix Section A.2 and Appendix Table A8. Physician-drug, specialty-drug-quarter fixed effects, controls for all other types of payments, and payment-type-specific linear time trends included in all specifications. Standard errors are clustered within doctor. Column 5 excludes differential time trends for doctors paid during our Medicare Part D sample period.
Columns 4 and 5 of Table 5 report the results from a matched sample, where we matched each recipient of a compensation payment at any point during 2014–2016 with physicians who did not receive any such payments, matching on specialty, hospital referral region, experience and number of peers. Details of the matching procedure can be found in Appendix Section A.2. Columns 4 and 5 replicate the baseline specification on the matched sample, with and without differential time trends for paid physicians. The estimated effect of peer compensation exposure in the matched sample is 0.025 or 0.029, respectively, slightly larger than the baseline estimate of 0.020 patients per quarter.
Random effects and sample restrictions
In Appendix Table A4, we report results varying the specification and the estimating sample. Column 1 reproduces our baseline estimates for reference (from Table 3), which include doctor-drug fixed effects. Column 2 substitutes the fixed effects with random effects at the doctor-drug level, while controlling for indicator variables for whether the doctor was ever exposed to each payment type for the index drug. In column 3, we estimate our baseline fixed effects model on a restricted sample of doctors, excluding doctors who received a payment of any type for the target drug or who were exposed to a compensation-paid peer prior to Quarter 3 of 2014. In both the random effect and the restricted sample specifications, estimated peer compensation effects are similar at 0.021 or 0.020, compared with the baseline of 0.020 patients per quarter.
Extensive- versus intensive-margin effects of different payment types
Appendix Figure A5 reports the results of estimating equation (2), replacing the dependent variable with an indicator for whether the physician prescribed the drug to at least Q patients, for different values of Q. Results show that each payment type affects different parts of the prescription volume distribution. As expected, compensation payments appear to increase prescribing along the intensive margin, not the extensive margin. Directly receiving a compensation payment increases the probability recipients prescribe the promoted drug to at least 3 to 10 in-sample patients, but not the probability that they prescribe to at least 1 or 2 patients. By contrast, food payments increase prescriptions on the extensive margin. Exposure to a compensated peer has its largest effects on the margin of prescribing to at least 2–4 patients per quarter.
In Appendix Table A5, we test whether the first observed payment (during our sample period) has a differential impact relative to subsequent payments. Point estimates suggest that a doctor’s first payment of each type has a slightly smaller estimated effect than subsequent payments.15
4. Payment Effects on Rival Drugs
In Section 3, we found that promotional payments increase prescriptions of the targeted drug. This increase may come at the expense of rival drugs or benefit them, depending on whether payments lead doctors to substitute the advertised drug for its rivals or induce increased prescription of rival drugs in the same class. We now consider specifications that examine possible market expansion and business stealing by estimating the cross-drug effects of pharmaceutical payments. Results from this analysis are reported in Table 6.
Table 6:
The Effects of Payments on Prescription of Target versus Rival Drugs
|
Dependent Variable:
Number of Patients Prescribed: |
||
|---|---|---|
| Warfarin | Target NOAC | |
| (1) | (2) | |
| Payments promoting: | ||
| Payment count, by type: | Any NOAC | Target NOAC |
| Own Compensation | 0.0159 (0.0536) | 0.3772 (0.1160) |
| Own Food | 0.0104 (0.0030) | 0.0577 (0.0037) |
| Peer Compensation | 0.0050 (0.0041) | 0.0194 (0.0061) |
| Peer Food | 0.0054 (0.0015) | −0.0006 (0.0013) |
| Rival NOACs | ||
| Own Compensation | −0.1163 (0.0347) | |
| Own Food | 0.0047 (0.0015) | |
| Peer Compensation | −0.0012 (0.0022) | |
| Peer Food | −0.0018 (0.0005) | |
| Mean dependent variable | 1.4864 | 0.5486 |
| N (Doctor × Drug × Quarter) | 1,796,544 | 5,466,420 |
Notes: Estimates using equation (2) with the dependent variables being the quarterly number of prescribed patients within different segments of the anticoagulant market: warfarin, (the off-patent and unpromoted incumbent) in column 1, and the target NOAC in column 2. The independent variables capture the counts of different types of payments made to the prescribing physicians (“Own”) or to others with whom the prescribing physicians shared patients (“Peers”). See Section 1 for definitions of Food and Compensation. Different panels show payments associated with any NOAC, the target NOAC, and other rival NOACs, as labeled (see Section 4 for details). Physician-drug, specialty-drug-quarter fixed effects, controls for all other types of payments, and payment-type-specific linear time trends included in all specifications. Standard errors are clustered within doctor.
We first examine whether NOAC promotion steals business from the non-advertised incumbent warfarin; results are reported in Table 6 column 1. We find that NOAC promotion has negligible effects on warfarin prescribing. Warfarin may remain the preferred option for many patients for a variety of reasons: it is prescribed for a broader range of indications than those covered by the initial NOAC labels, has a large base of existing patients who may stick with it, and is much less expensive than the newer options.
Next, we examine the cross-drug effects of payments among the three heavily promoted NOAC drugs. These three NOACs are particularly close substitutes for each other; they have a similar biologic mechanism, similar administration requirements, and being on-patent they are all much more expensive than warfarin. To study business stealing within this market segment, we estimate a version of equation (2) with measures of exposure to payments for both the index and competitor NOAC drugs. If these marketing efforts have business stealing effects across NOAC drugs, then we would expect payments for other NOACs would reduce prescriptions for the index drug.
The results of this analysis are reported in column 2 of Table 6. The estimated own-drug effects of payments are nearly identical to our baseline estimates. Turning to cross-drug effects of payments, we find that receiving compensation payments associated with rival NOACs reduces prescriptions for the index NOAC. This suggests that compensation payments partly increase prescriptions by stealing business from competitors. This (negative) cross-drug effect of compensation payments is a about a third as large as the (positive) own-drug effect. Adding up the positive own-drug effect and the negative cross-drug effect (summed over two competing NOACs), the estimates imply that compensation payments still spur a net increase in total NOAC prescribing by the paid physician. Food payments are estimated to have positive spillovers on prescriptions of rival NOACs, also expanding total NOAC prescribing. We find no cross-drug effects for indirect exposure to compensated peers; the point estimate is small and not statistically distinguishable from zero, despite having a small standard error.
Taken together, these findings imply that all types of payment exposure increase total anticoagulant prescribing. We find the market expansion plausible, given two important contextual observations. First, Abaluck et al. (2020) find that only about half of patients who meet atrial fibrillation treatment guidelines are actually treated with anticoagulants. Second, warfarin (the incumbent therapy), unlike NOACs, requires regular blood tests and dosing adjustments to maintain optimal therapeutic control. Thus, it is plausible that some providers and patients would prefer to initiate treatment with a NOAC, even if they would not have previously found the patient suitable for treatment with warfarin. Both observations suggest potential room for market growth.
It is helpful to compare these results with previous research on direct-to-consumer advertising, which has focused specifically on these sorts of competitive effects. Shapiro (2018b) reports that direct-to-consumer advertising of antidepressants had market expanding effects with positive spillovers to rival products, while Sinkinson and Starc (2019) find evidence of business stealing effects among branded statin competitors but positive spillovers to non-advertised rivals. In our context of physician advertising, we find that food payments have small positive spillovers on other anticoagulants, whereas compensation payments induce business stealing from branded rivals. This is consistent with the lighter touch of a meal interaction leading the physician to expand their use of oral anticoagulants in general, with this expansion tipped towards the promoted drug. The more intense relationships built through compensation payments induce compensation recipients to substitute towards the promoted drug instead of competitors.
5. Payment Effects on Guideline Adherence
A highly contested question is how pharmaceutical detailing payments impact patient welfare. Given the evidence described above that payment exposure spurs anticoagulant market growth, welfare effects will depend on the benefits of the drug for the marginal prescribed patients. On the one hand, payments may lead physicians to over-prescribe high cost drugs. On the other hand, pharmaceutical companies argue that detailing improves welfare by educating physicians about new drugs and providing up-to-date information to support better practice.16
To shed light on this question, we analyzed whether pharmaceutical payments increase adherence to evidence-based clinical guidelines on anticoagulant prescriptions. Because guidelines are not available to cover all indications for anticoagulant drugs, we narrow our focus to atrial fibrillation, which is a common reason for prescribing anticoagulants. There are two popular risk scores to assess the risks and benefits of anticoagulation for patients with atrial fibrillation: the HAS-BLED and CHADS2 scores (Pisters et al. 2010; Lip et al. 2011; Gage et al. 2001, 2004). The HAS-BLED score estimates risk of bleeding for patients on anticoagulant drugs, which is the major safety concern that should be weighed against the stroke reduction benefits of the drug. The CHADS2 score estimates the gains from anticoagulation.17 Note that current guidelines provide little guidance on selecting among the various anticoagulant drugs; rather, they focus on determining whether the patient is appropriate for anticoagulation drugs at all (Manningm et al. 2019).
If doctors were to increase their adherence to the HAS-BLED guidelines, we would expect fewer prescription fills for patients at high risk of bleeding. To test whether this is the case, we construct the HAS-BLED risk score for each atrial fibrillation patient; details are described in Appendix Section A.3. Because we do not observe all the factors that underlie this guideline, some high-risk patients will be mis-classified as “low-risk”. But “high-risk” had sufficiently many risk-factors observed in our data and therefore are less likely to be misclassified.
We first evaluate payment effects on total anticoagulant prescriptions for high and low HAS-BLED score patients. Total anticoagulant prescriptions cover any anticoagulant (including all NOACs or warfarin) to capture the market-wide guideline adherence effects. In these specifications, we estimate a version of equation (2) that includes the same set of fixed effects, except for drug fixed effects (since drugs are pooled). Results are in Columns 1–3 of Table 7. Point estimates suggest that own food, own compensation, and peer compensation payments each increase prescription volume for both low and high-risk patients. Exposure to peer compensation is estimated to increase total anticoagulant prescribing among high bleeding risk patients by 0.6 percent, and the 95 percent confidence interval is bounded below by a 0.4 percent decrease in prescribing to high bleeding risk patients. Own food payments significantly increase the number of high-risk patients prescribed anticoagulants by 0.047 and increase the number of low-risk patients prescribed by 0.034; these both amount to a 1.7 percent increase from the subgroups’ mean prescription volume.
Table 7:
The Effect of Payments, by Risk of Severe Drug Side Effects
| Dependent variable: | ||||||
|---|---|---|---|---|---|---|
| Patients Prescribed Any Anticoagulant | Patients Prescribed the Targeted Anticoagulant | |||||
| All Patients | Low Bleeding Risk | High Bleeding Risk | All Patients | Low Bleeding Risk | High Bleeding Risk | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Payment Count, by Type | ||||||
| Own Compensation | 0.3590 (0.2306) | 0.1037 (0.1273) | 0.2553 (0.1582) | 0.5149 (0.1683) | 0.2054 (0.0964) | 0.3095 (0.1126) |
| Own Food | 0.0809 (0.0115) | 0.0335 (0.0060) | 0.0474 (0.0084) | 0.0654 (0.0074) | 0.0246 (0.0039) | 0.0408 (0.0054) |
| Peer Compensation | 0.0288 (0.0175) | 0.0120 (0.0086) | 0.0168 (0.0136) | 0.0198 (0.0113) | 0.0151 (0.0061) | 0.0047 (0.0080) |
| Peer Food | −0.0014 (0.0062) | 0.0028 (0.0025) | −0.0042 (0.0054) | −0.0013 (0.0034) | −0.0018 (0.0015) | 0.0005 (0.0028) |
| Mean Dep. Var. | 4.7635 | 1.9595 | 2.8049 | 1.0153 | 0.4489 | 0.5664 |
| N (Doctor × Drug × Quarter) | 1,554,036 | 1,554,036 | 1,554,036 | 3,688,884 | 3,688,884 | 3,688,884 |
Notes: Table shows estimates of the impact of pharmaceutical payments on anticoagulant prescriptions, for the sample of patients diagnosed with atrial fibrillation and who received at least one anticoagulant prescription during the study period. The different columns show results separately by major bleeding risk—an adverse risk of NOAC use—based on the HAS-BLED risk score. The sample is partitioned by bleeding risk based on our calculation of the HAS-BLED score. See Section 5 for details. Columns 1–3 show estimates of the impact of payments (pooled across all three NOAC drugs) on the total number of anticoagulants prescribed per quarter (pooled across all anticoagulants, including NOACs and warfarin). Columns 4–6 show similar estimates, but where both payments and the prescription volume outcomes are measured separately for each NOAC in our sample. Physician-drug, specialty-drug-quarter fixed effects, controls for all other types of payments, and payment-type-specific linear time trends included in all specifications. Standard errors are clustered within doctor.
In columns 4–6 of Table 7, we disaggregate the data by drug to test whether promotional efforts increase guideline-concordant prescribing for the target drug, which we would expect if any physician education that occurred with the detailing was drug-specific. Again, we find no significant evidence that doctors are decreasing their prescribing to high-risk patients. In Appendix Section A.3 we perform another analysis of guideline concordance that incorporates compliance with the CHADS2 guideline to assess expected clinical benefits, the results of which are reported in Appendix Table A6. We find no evidence that pharmaceutical payments increased adherence to the CHADS2 guideline either.
Our results suggest that payments increase average prescription volume for high-risk and low-value patients. We argue that such findings are hard to reconcile with the idea that payments strictly improve physician’s information or guideline adherence; at least in some cases, payments may induce low-value prescribing.
6. Assessing the Aggregate Impact of Payments
So far, we have focused on estimating the average effect of a single detailing payment on prescription volumes of the targeted doctor and her peers. A natural related question is: what is the aggregate impact of pharmaceutical payments on diffusion of the marketed products? To explore this topic, we use our estimated model as a quantification framework to perform several counterfactual analyses.
Our counterfactual analysis combines the estimated unit-effects of each payment type with information on the number and timing of payments, and the network position and geographic location of paid physicians. We use unit-effect estimates from the random effects version of equation (2) (reported in column 2 of Table A4), which allows us to relax our sampling restrictions discussed in Section 1.2 and extend the counterfactual analysis to cover the entire sample.18
Using this framework, we compare actual prescription volumes with counterfactual prescriptions under two alternative payment influence schemes: (1) payments only affecting paid doctors themselves (zeroing out any peer-payment impact), and (2) no payments. Figure 5 summarizes the results of this analysis, describing the total impact of pharmaceutical payments made over the three years of our study on prescription volume. Panel (A) shows the estimated average payment impact on the direct recipient and on the recipient’s peers. Even though compensation payments are much rarer than food payments (see Panel (B)), compensation payments make a substantial contribution to the aggregate impact of payments on prescription volume, primarily through their peer effects (see Panel (C)). Spending on new oral anticoagulants in the United States reached an estimated annual volume of $7.6 billion by Quarter 4 of 2016. Our estimates suggest that absent payments, the market would have only been $6.2 billion. About $387 million in prescription spending is attributable to indirect effects of payments on recipients’ peers, amounting to a quarter of the total payment impact. This may be a conservative estimate of peer effects’ total contribution, because it accounts for only first degree patient-sharing relationships, overlooking other forms of peer relationships.
Figure 5:
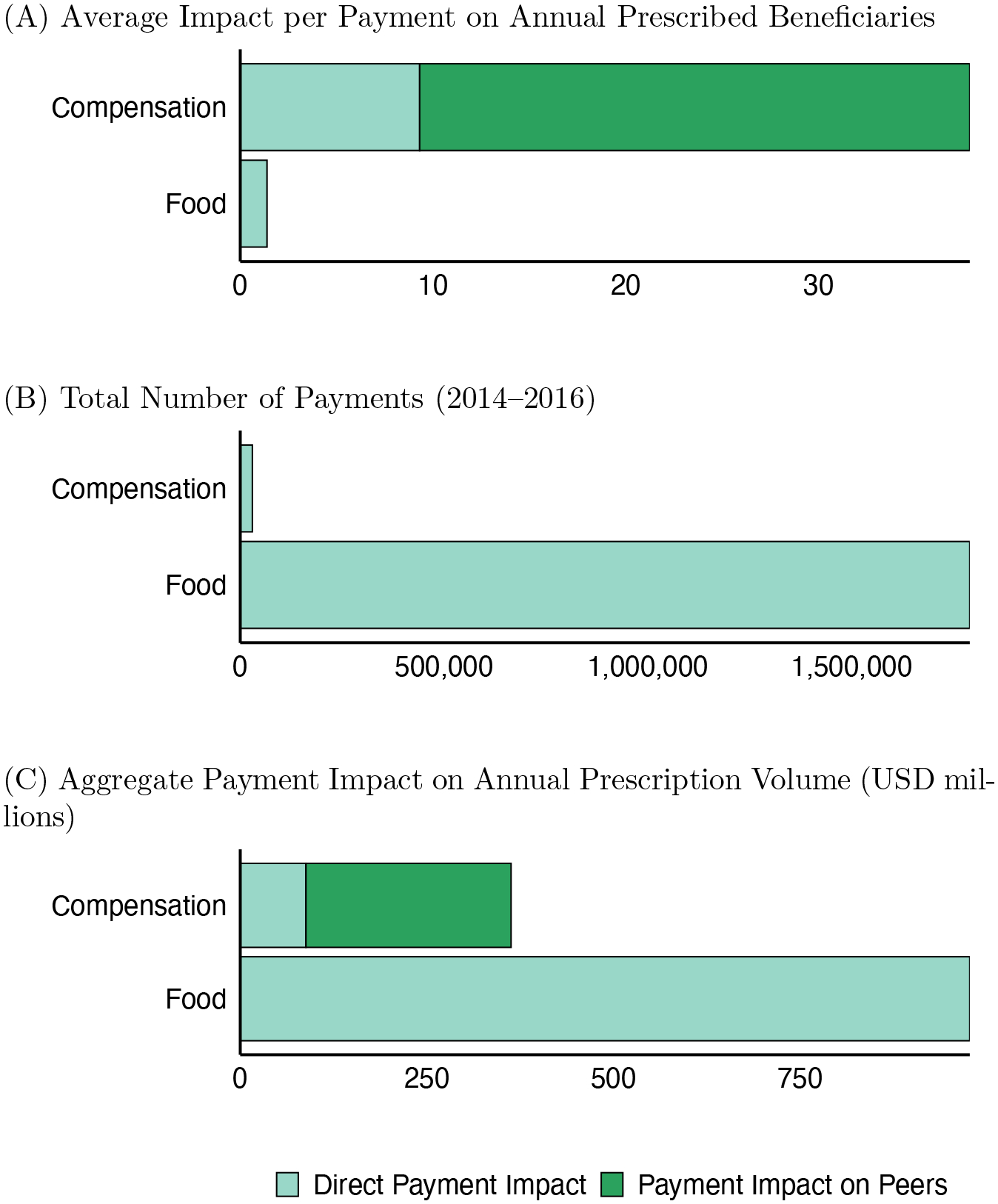
Pharmaceutical Payment Impact on Prescription Volumes
Notes: Figure shows estimates of direct and peer effects of pharmaceutical payments on prescription volume, by type of payment. Panel (A) shows the estimated effect of a single payment of each type on the annual number of unique beneficiaries prescribed the target drug by the payment recipient (light shade) and by all of the recipient’s peers (dark shade). The impact of peer food payments (− 0.0022, s.e. = 0.0281) is shown as zero. Panel (B) shows the number of pharmaceutical payments and in-kind transfers associated with NOAC drugs in 2014–2016. Panel (C) shows the estimated overall contribution of payments to annual NOAC prescription spending by direct recipients (light shade) and their peers (dark shade) in the United States. To obtain estimates of total spending in US dollars, we multiply the estimated counterfactual number of beneficiaries per quarter with the quarterly average cost of prescriptions of each drug. These average costs are fairly stable over our sample period, as seen in Appendix Figure A4. Panel (A) is based on a 40 percent sample of Medicare Part D beneficiaries; dollar estimates in Panel (C) are annualized and rescaled by a factor of 5.4 to extrapolate to all US prescriptions. This scaling factor is discussed in Section 1. Data sources are described in Section 6. Underlying data are reported in Appendix Table A7.
We also explore how pharmaceutical payments contribute to geographic variation in the diffusion of NOACs. Donohue et al. (2012) reports that variation in the propensity to prescribe brand-name (rather than generic) drugs is a major contributor to total variation in Medicare Part D spending. Differences in pharmaceutical detailing intensity across regions is one potential explanation for these differences. Panel (A) of Appendix Figure A6 shows that regions with higher initial adoption are exposed to more subsequent detailing payments; Panel (B) shows that as a result, regions with high initial adoption have greater predicted growth in NOAC prescriptions. This evidence suggests that payments increase, rather than decrease, spatial disparity in the diffusion of NOACs.
These counterfactual exercises captures only partial equilibrium responses. A complete industry payment ban may induce changes to drug pricing, direct to consumer advertising, or even market entry decisions. Theory developed by Inderst and Ottaviani (2012) highlights that disclosure of industry payments, as mandated with the Open Payments data, may directly affect payment amounts and consumer response to physician recommendations; the amount, distribution, and impact of payments may be different in the absence of these disclosure requirements. Modeling these effects is beyond the scope of the current analysis, but highlights important directions for future work.
7. Conclusion
Pharmaceutical companies pay physicians large sums of money, in the form of payments for services or in-kind transfers. In 2015, 48 percent of US doctors received an industry payment (Tringale et al. 2017). Using rich administrative data on prescriptions, payments, and physician networks, and exploiting variation in both the timing and targeting of payments, this study has shown that pharmaceutical payments lead to a significant and persistent increase in the prescription of new anticoagulant drugs. We find no evidence that payments improved adherence to clinical guidelines.
Furthermore, the majority of industry spending on provider payments goes to large payments that compensate a small set of specialized and highly connected physicians. These compensation payments have substantial spillover effects—they lead not only to increased prescriptions by recipients, but also by the recipients’ peers. The cumulative effect of peer spillovers from each large payment is several times greater than the direct effect on the paid physician. Overall, spillover effects of pharmaceutical payments account for about a quarter of their estimated impact on prescription volumes.
Our results suggest that learning from peers is an important channel through which pharmaceutical payments impact clinical practice. This finding corroborates accounts of marketing strategies that leverage influential individuals for wider impact. More broadly, these results suggest that peer influence may be an important channel for adoption of new technologies in medicine.
This project leaves several open questions. Our research takes as given the observed promotional strategies. Future work could investigate whether alternative payment schemes yield different effects. Additionally, it would be interesting to explore whether similar peer effects arise when practice patterns change for reasons other than pharmaceutical payments. Our findings suggest that policy interventions to increase prescribing of recommended drugs may achieve a broader reach by targeting highly connected physicians, but rigorous evaluation should test whether peer spillovers extend to other settings.
Supplementary Material
Acknowledgments
For helpful comments and suggestions, we thank Liran Einav, Ben Golub, Alan Griffith, Erzo F.P. Luttmer, Angelo Mele, Kyle Myers, David Molitor, Seth Richards-Shubik, Jon Skinner, Doug Staiger, and seminar participants at Tel Aviv University, Dartmouth College, Stanford University, Imperial College London, ASHEcon, the ASSA Annual Meeting in Atlanta, the Fifth Annual Network Science and Economics Conference at Indiana University, the Barcelona GSE Summer Forum, and the NBER Summer Institute. The authors gratefully acknowledge research support by NIH grants PO1 AG19783. Dan Zeltzer acknowledges support from The Foerder Institute for Economic Research at Tel Aviv University and the Pinhas Sapir Center for Development. We thank Stephanie Tomlin and Weiping Zhou for help obtaining and managing the data. The content is solely the responsibility of the authors.
Footnotes
Over 2014–2016, industry payments of greater than $1,000, which amount to the top 3 percent of all payments by value, accounted for $5.1 billion in promotional payments, or nearly 80 percent of the total transfers ($6.5 billion). The top one percent, with payment value greater than $2,800, account for half of the total value transferred.
Our findings echo an early case study of hybrid corn diffusion by Ryan and Gross (1943), which found that sales representatives were an important source of original information about the technology, and then peer networks played a key role in subsequent diffusion. They are also consistent with earlier evidence from marketing research showing that drug adoption choices are correlated with the adoption of self-reported peers (Nair et al. 2010; Iyengar et al. 2011) or geographically proximate colleagues (Manchanda et al. 2008), and that both may be mediated by detailing phone calls and office visits.
The FDA first approved Pradaxa on October 19, 2010, Xarelto on July 1, 2011, and Eliquis on December 28, 2012. This slight variation in the introduction of drugs means that we have a chance to observe slightly different stages in the life cycle of product introduction. A fourth NOAC, Savaysa, was introduced in 2015, but this happens at the very end of our sample, and accordingly accounts for less than 0.1% of the anticoagulant prescriptions in our data. Therefore, we exclude it from our primary analysis. For completeness, we include Savaysa as part of the total class of oral anticoagulants for analyses in Section 5.
Global Anticoagulants Market Expected to Reach $43 Billion By 2025. Allied Market Research Report. https://www.alliedmarketresearch.com/press-release/anticoagulant-drugs-market.html. Accessed November 2020.
Anticoagulants - prices and information, https://www.goodrx.com/anticoagulants. Accessed November 2020.
We define primary care physicians as those whose primary specialty recorded in the Physician Compare database is one of: Family Practice, Internal Medicine, General Practice, or Geriatric Medicine. Cardiologists are defined as physicians whose primary specialty is one of: Cardiology, Interventional Cardiology, or Cardiac Surgery.
Barnett et al. (2011) find that 82 percent of physician pairs sharing at least nine patients report an advice or referral relationship, compared with only 19 percent of physician pairs with one shared patient.
We use Physician Compare for name and address information. As both Physician Compare and Open Payments are maintained by CMS, more than 97 percent of our matches are exact matches on last name, first name, and state. The remaining matches include slight misspellings; we match these remaining records by blocking on state and first letter of last name and using fuzzy string matching with the Jaro-Winkler distance.
A third of the physicians in our sample are indirectly connected to a compensated physician, through a common peer; four out of five physicians are connected to a compensated physician through a path of length three or less. Assuming effects decay as they ripple through the network, further indirect effects are likely small.
We pursue this two-step procedure for the graphical analysis to surmount the underidentification problem that would otherwise arise when trying to include both differential time trends and dummy variables for time relative to first payment (Borusyak and Jaravel 2017).
Both the Pidt and PGi,d,t variables are set to zero for doctors who are never (own or peer) paid and for doctors who receive their first (own or peer) payment of this type in the two quarters before our Part D sample begins.
Assume an average revenue of $800 per prescribed beneficiary per quarter (see Appendix Figure A4) and consider that spending on food payments is approximately $30 per transaction. Food payments are estimated to yield 0.06 new prescriptions each quarter in our 40 percent Medicare sample, which extrapolates to an additional quarterly revenue of $260 when both Medicare and non-Medicare patients are considered. Compensation payments of $3,000 per quarter would appear less profitable when we only consider the effect on the paid physician: a compensation payment generates $1,600 of additional revenue from prescriptions by the paid physician. Including additional prescriptions from the 62 (on average) peers of a compensated physician suggests that peer spillovers alone generate an additional $5,300 per compensation payment. We discuss the aggregate impact of payments further in Section 6.
The estimate increase for this group is 0.013 additional beneficiaries, which is the sum of the “Shared patient” and the “Group practice and shared patient” coefficients reported in Table 4, column 2.
If information diffusion through patients’ social networks drove our results, we would have expected stronger peer effects among physicians affiliated with the same practice, who may be more likely to serve socially tied patients. But we find similar influence when physicians only share patients but practice in different locations.
While we focus on drugs that were introduced shortly before our sample period, payments from before the third quarter of 2013 are censored. The finding that later payments do not have a smaller effect on prescriptions than early payments suggests the potential bias due to this censoring is likely small.
Note that guideline adherence is only a proxy for decision quality. A more comprehensive welfare analysis, e.g., using chart data, could account for physician decision quality more directly and evaluate whether newly prescribed patients benefit from treatment.
For a quick reference guide to clinical scoring for atrial fibrillation, see MDCalc https://www.mdcalc.com/has-bled-score-major-bleeding-risk and https://www.mdcalc.com/chads2-score-atrial-fibrillation-stroke-risk. Accessed November 2020.
Using random effects allows us to include doctors who were paid at the very beginning or very end of the study period and so did not meet the requirements for panel balance, for which we do not have fixed effect estimates. This decision has only a slight effect on the counterfactual estimates. Using fixed effects with the restricted sample of doctors would lead us to conclude that payments increased total prescription volume by 21% instead of 23%. Similarly, incorporating cross-drug effects of promotional payments would not change the main conclusions of this analysis. The effect of compensation payments on the paid physician would be smaller, but the peer effects would remain nearly unchanged.
Contributor Information
Leila Agha, Department of Economics, Dartmouth College, and NBER..
Dan Zeltzer, School of Economics, Tel Aviv University..
References
- Abaluck Jason, Agha Leila, Chan David C Jr, Singer Daniel, and Zhu Diana, “Fixing Misallocation with Guidelines: Awareness vs. Adherence,” NBER Working Paper No. 27467, 2020. [Google Scholar]
- Agency for Healthcare Research and Quality, “Medical Expenditure Panel Survery,” 2014. data retrieved from https://meps.ahrq.gov.
- Agha Leila and Molitor David, “The Local Influence of Pioneer Investigators on Technology Adoption: Evidence from New Cancer Drugs,” The Review of Economics and Statistics, 2018, 100 (1), 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrist Joshua D, “The perils of peer effects,” Labour Economics, 2014, 30, 98–108. [Google Scholar]
- Banerjee Abhijit, Chandrasekhar Arun G., Duflo Esther, and Jackson Matthew O, “The diffusion of microfinance,” Science, 2013, 341 (6144), 1236498. [DOI] [PubMed] [Google Scholar]
- Barnett Michael L., Landon Bruce E., O’Malley A. James, Keating Nancy L., and Christakis Nicholas A., “Mapping physician networks with self-reported and administrative data,” Health Services Research, 2011, 46 (5), 1592–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borusyak Kirill and Jaravel Xavier, “Revisiting event study designs,” Available at SSRN 2826228, 2017. [Google Scholar]
- Bramoullé Yann, Djebbari Habiba, and Fortin Bernard, “Identification of peer effects through social networks,” Journal of Econometrics, 2009, 150 (1), 41–55. [Google Scholar]
- Caeyers Bet and Fafchamps Marcel, “Exclusion Bias in the Estimation of Peer Effects,” NBER Working Paper No. 22565, 2016. [Google Scholar]
- Campbell John J., Understanding Pharma: The Professional’s Guide to how Pharmaceutical and Biotech Companies Really Work, Pharmaceutical Institute, 2008. [Google Scholar]
- Carey Colleen, Lieber Ethan M.J., and Miller Sarah, “Drug firms payments and physicians prescribing behavior in Medicare Part D,” Journal of Public Economics, 2021, 197, 104402. [Google Scholar]
- Center for Disease Control, “Blood Clots: A Serious but Preventable Medical Condition,” 2019. https://www.cdc.gov/ncbddd/dvt/documents/blood-clots-fact-sheet.pdf, Accessed November 2020.
- –, “Stroke Statistics,” 2020. https://www.cdc.gov/stroke/facts.htm, Accessed November 2020.
- Chan David C., “Influence and Information in Team Decisions: Evidence from Medical Residency,” Working Paper, 2018. [Google Scholar]
- CMS, “Physician Shared Patient Patterns,” 2013. data retrieved from http://downloads.cms.gov/foia/physician-shared-patient-patterns-2013-days30.zip.
- –, “40% Research Identifiable Medicare Part D Database,” 2013–2016. data dictionaries are available at https://www.resdac.org/cms-data/files/pde.
- –, “Open Payments Databases,” 2013–2016. https://openpaymentsdata.cms.gov/.
- –, “Natures of Payment,” 2019. https://www.cms.gov/OpenPayments/About/Natures-of-Payment, Accessed November 2020.
- Coleman James, Katz Elihu, and Menzel Herbert, “The Diffusion of an Innovation Among Physicians,” Sociometry, 1957, 20 (4), 253–270. [Google Scholar]
- David Guy, Markowitz Sara, and Richards-Shubik Seth, “The effects of pharmaceutical marketing and promotion on adverse drug events and regulation,” American Economic Journal: Economic Policy, 2010, 2 (4), 1–25. [Google Scholar]
- DeJong Colette, Aguilar Thomas, Tseng Chien-Wen, Lin Grace A., Boscardin W. John, and Dudley R. Adams, “Pharmaceutical industry–sponsored meals and physician prescribing patterns for Medicare beneficiaries,” JAMA Internal Medicine, 2016, 176 (8), 1114–1122. [DOI] [PubMed] [Google Scholar]
- Donohue Julie M., Guclu Hasan, Gellad Walid F., Chang Chung-Chou H., Huskamp Haiden A., Choudhry Niteesh K., Zhang Ruoxin, Lo-Ciganic Wei-Hsuan, Junker Stefanie P., Anderson Timothy, and Richars-Shubik Seth, “Influence of peer networks on physician adoption of new drugs,” PLOS One, 2018, 13 (10), e0204826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- –, Morden Nancy E., Gellad Walid F., Bynum Julie P., Zhou Weiping, Hanlon Joseph T., and Skinner Jonathan, “Sources of Regional Variation in Medicare Part D Drug Spending,” New England Journal of Medicine, 2012, 366 (6), 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot Carl, “The Drug Pushers,” The Atlantic, April 2016. [Google Scholar]
- Gage Brian F., Waterman Amy D., Shannon William, Boechler Michael, Rich Michael W., and Radford Martha J., “Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation,” JAMA, 2001, 285 (22), 2864–2870. [DOI] [PubMed] [Google Scholar]
- –, van Walraven Carl, Pearce Lesly, Hart Robert G., Koudstaal Peter J., Boode BSP, and Petersen Palle, “Selecting patients with atrial fibrillation for anticoagulation: Stroke risk stratification in patients taking aspirin,” Circulation, 2004, 110 (16), 2287–2292. [DOI] [PubMed] [Google Scholar]
- Galeotti Andrea, Golub Benjamin, and Goyal Sanjeev, “Targeting interventions in networks,” arXiv preprint arXiv:1710.06026, 2017. [Google Scholar]
- Giorgi Giacomo De, Pellizzari Michele, and Redaelli Silvia, “Identification of social interactions through partially overlapping peer groups,” American Economic Journal: Applied Economics, 2010, 2 (2), 241–275. [Google Scholar]
- Goldenberg Jacob, Han Sangman, Lehmann Donald R, and Hong Jae Weon, “The role of hubs in the adoption process,” Journal of Marketing, 2009, 73 (2), 1–13. [Google Scholar]
- Golub Ben and Sadler Evan, “Learning in social networks,” in Bramoullé Yann, Galeotti Andrea, and Rogers Brian, eds., The Oxford Handbook of the Economics of Networks, Oxford University Press, 2016. [Google Scholar]
- Grennan Matthew, Myers Kyle, Swanson Ashley, and Chatterji Aaron, “Physician-Industry Interactions: Persuasion and Welfare,” NBER Working Paper No. 24864, 2018. [Google Scholar]
- Hoadley John F, Cubanski Juliette, and Neuman Patricia, “Medicare’s Part D drug benefit at 10 years: firmly established but still evolving,” Health Affairs, 2015, 34 (10), 1682–1687. [DOI] [PubMed] [Google Scholar]
- Inderst Roman and Ottaviani Marco, “Competition through commissions and kick-backs,” American Economic Review, 2012, 102 (2), 780–809. [Google Scholar]
- Iyengar Raghuram, Van den Bulte Christophe, and Valente Thomas W., “Opinion leadership and social contagion in new product diffusion,” Marketing Science, 2011, 30 (2), 195–212. [Google Scholar]
- Larkin Ian, Ang Desmond, Steinhart Jonathan, Chao Matthew, Patterson Mark, Sah Sunita, Wu Tina, Schoenbaum Michael, Hutchins David, Brennan Troyen, and Loewenstein George, “Association between academic medical center pharmaceutical detailing policies and physician prescribing,” JAMA, 2017, 317 (17), 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip Gregory Y.H., Frison Lars, Halperin Jonathan L., and Lane Deirdre A., “Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score,” Journal of the American College of Cardiology, 2011, 57 (2), 173–180. [DOI] [PubMed] [Google Scholar]
- Manchanda Puneet, Xie Ying, and Youn Nara, “The role of targeted communication and contagion in product adoption,” Marketing Science, 2008, 27 (6), 961–976. [Google Scholar]
- Manningm Warren J, Singer Daniel E, and Lip Gregory YH, “Nonvalvular atrial fibrillation: Anticoagulant therapy to prevent thromboembolism,” 2019. UpToDate®, https://www.uptodate.com/contents/nonvalvular-atrial-fibrillation-anticoagulant-therapy-to-prevent-thromboembolism, Accessed November 2020.
- Manski Charles F., “Identification of endogenous social effects: The reflection problem,” The Review of Economic Studies, 1993, 60 (3), 531–542. [Google Scholar]
- Mekaj Ymer H., Mekaj Agon Y., Duci Shkelzen B., and Miftari Ermira I, “New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events,” Therapeutics and Clinical Risk Management, 2015, 11, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt Robert A, “Policy interventions, low-level equilibria, and social interactions,” Social Dynamics, 2001, 4 (45–82), 6–17. [Google Scholar]
- Nair Harikesh S, Manchanda Puneet, and Bhatia Tulikaa, “Asymmetric social interactions in physician prescription behavior: The role of opinion leaders,” Journal of Marketing Research, 2010, 47 (5), 883–895. [Google Scholar]
- Navathe Amol and David Guy, “The formation of peer reputation among physicians and its effect on technology adoption,” Journal of Human Capital, 2009, 3 (4), 289–322. [Google Scholar]
- Ornstein Charles, Weber Tracy, and Jones Ryann Grochowski, “We Found Over 700 Doctors Who Were Paid More Than a Million Dollars by Drug and Medical Device Companies,” ProPublica, October 17 2019. [Google Scholar]
- Oster Emily and Thornton Rebecca, “Determinants of technology adoption: Peer effects in menstrual cup take-up,” Journal of the European Economic Association, 2012, 10 (6), 1263–1293. [Google Scholar]
- Pisters Ron, Lane Deirdre A., Nieuwlaat Robby, De Vos Cees B., Crijns Harry J.G.M., and Lip Gregory Y.H., “A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey,” Chest, 2010, 138 (5), 1093–1100. [DOI] [PubMed] [Google Scholar]
- Ryan Bryce and Gross Neal C, “The diffusion of hybrid seed corn in two Iowa communities.,” Rural Sociology, 1943, 8 (1), 15. [Google Scholar]
- Sacarny Adam, Olenski Andrew R., and Barnett Michael L., “Association of Quetiapine Overuse Letters With Prescribing by Physician Peers of Targeted Recipients: A Secondary Analysis of a Randomized Clinical Trial,” JAMA Psychiatry, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Lisa M. and Woloshin Steven, “Medical Marketing in the United States, 1997–2016,” JAMA, 01 2019, 321 (1), 80–96. [DOI] [PubMed] [Google Scholar]
- Shapiro Bradley T., “Informational shocks, off-label prescribing, and the effects of physician detailing,” Management Science, 2018, 64 (12), 5925–5945. [Google Scholar]
- –, “Positive spillovers and free riding in advertising of prescription pharmaceuticals: The case of antidepressants,” Journal of Political Economy, 2018, 126 (1), 381–437. [Google Scholar]
- Silver David, “Haste or Waste? Peer Pressure and Productivity in the Emergency Department,” The Review of Economic Studies, August 2020. eprint available at https://academic.oup.com/restud/advance-article-pdf/doi/10.1093/restud/rdaa054/33895062/rdaa054.pdf. [Google Scholar]
- Sinkinson Michael and Starc Amanda, “Ask your doctor? Direct-to-consumer advertising of pharmaceuticals,” The Review of Economic Studies, 2019, 86 (2), 836–881. [Google Scholar]
- Thomas Katie, Armendariz Agustin, and Cohen Sarah, “Detailing Financial Links of Doctors and Drug Makers,” The New York Times, September 30, 2014. [Google Scholar]
- Tringale Kathryn R, Deborah Marshall, Mackey Tim K, Michael Connor, Murphy James D, and Hattangadi-Gluth Jona A, “Types and distribution of payments from industry to physicians in 2015,” JAMA, 2017, 317 (17), 1774–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltzer Dan, “Gender homophily in referral networks: Consequences for the medicare physician earnings gap,” American Economic Journal: Applied Economics, 2020, 12 (2), 169–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


