Abstract
Context
Syndromes of severe insulin resistance (SIR) include insulin receptoropathy, in which all signaling downstream of the insulin receptor is lost, and lipodystrophy, in which some signaling pathways are impaired and others preserved. Women with SIR commonly have ovarian hyperandrogenemia; adrenal-derived 11-oxygenated androgens, produced by CYP11B1, have not been studied.
Objective
We aimed to evaluate classic pathway androgens (androstenedione, testosterone) and 11-oxygenated androgens in women with SIR and hyperandrogenemia, and to elucidate the role of insulin receptor signaling for 11-oxygenated androgen production by comparing lipodystrophy and receptoropathy.
Methods
Steroid hormones were quantified using LC-MS/MS in a cross-sectional study of 18 women with hyperandrogenemia and SIR (11 lipodystrophy, 7 receptoropathy) and 23 controls. To assess ovarian vs adrenal origin, steroids were compared in receptoropathy patients with (Ovary+) vs without (Ovary-) ovarian function.
Results
Compared with controls, classic androgens were elevated in both lipodystrophy and receptoropathy, and 11-oxygenated androgens were increased in lipodystrophy (2.9-fold higher 11β-hydroxyandrostenedione (11OHA4), 2.4-fold higher 11-ketoandrostenedione (11KA4), 3.6-fold higher 11-ketotestosterone (11KT); P < 0.01), but not receptoropathy. Product-to-precursor ratios for CYP11B1 conversion of androstenedione to 11OHA4 were similar in lipodystrophy and controls but decreased in receptoropathy (6.5-fold lower than control; P = 0.001). Classic androgens were elevated in Ovary + but not Ovary- patients.
Conclusions
11-Oxygenated androgens are elevated in lipodystrophy but not receptoropathy. In SIR, insulin receptor signaling is necessary for adrenal hyperandrogenemia but not ovarian hyperandrogenemia; excess classic androgens are derived from the ovaries. Insulin receptor signaling increases adrenal 19-carbon steroid production, which may have implications for more common disorders of mild IR.
Keywords: severe insulin resistance, lipodystrophy, insulin receptor, 11-oxygenated androgens, hyperandrogenism, ovaries
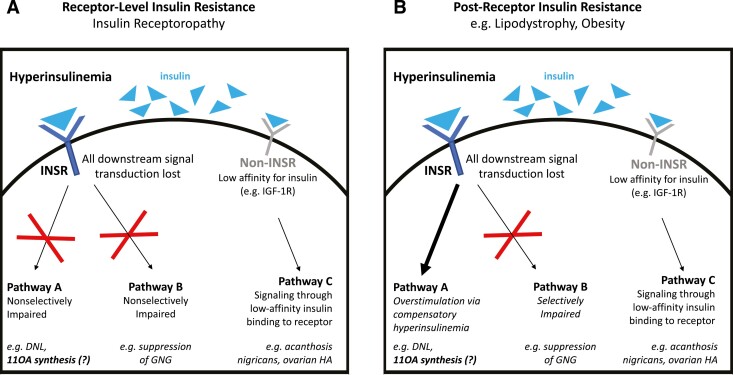
Syndromes of severe insulin resistance (SIR) can be caused by primary disorders of insulin signaling due to dysfunction of the insulin receptor (insulin receptoropathy) or be associated with deficiencies of adipose tissue such as lipodystrophy. In insulin receptoropathies, all insulin signaling pathways are impaired; by contrast, in lipodystrophy, some insulin signaling pathways are impaired while others remain intact (Fig. 1). In female patients, SIR often presents with signs of reproductive dysfunction, including elevated testosterone, subfertility, and a polycystic ovary syndrome (PCOS)-like phenotype. The observed hyperandrogenemia is thought to occur, at least in part, secondary to hyperinsulinemia causing exaggerated ovarian theca cell androgen production, which is analogous to the proposed mechanism of hyperandrogenemia in PCOS (1).
Figure 1.
Subphenotypes of severe insulin resistance (SIR). Defects in insulin signaling can occur at the level of the insulin receptor (receptor-level, e.g., insulin receptoropathy, panel A) or pathways downstream of the insulin receptor (postreceptor, e.g., lipodystrophy, panel B) to cause SIR. Insulin receptoropathy is characterized by nonselective impairment of all signaling pathways downstream of the insulin receptor, whereas in lipodystrophy signaling through some pathways are impaired while other pathways remain intact. In both subphenotypes of SIR, hyperinsulinemia can cause signaling through receptors other than the insulin receptor through excess insulin binding to lower-affinity targets such as the IGF-1 receptor. Abbreviations: SIR, severe insulin resistance; INSR, insulin receptor; DNL, de novo lipogenesis; 11OA, 11-oxygenated androgen; GNG, gluconeogenesis; HA, hyperandrogenism.
A subgroup of patients with PCOS has been shown to have elevations in adrenal-derived androgens in addition to ovarian androgens (2). In healthy women of reproductive age, the ovaries and adrenals produce the “classic” androgen testosterone and its precursor androstenedione. In recent years, there has been increased attention on 11-oxygenated androgens, a class of C19 steroids produced predominantly by the adrenals, which circulate in comparable quantities to steroids of the classic androgen pathway in women (3-5). The initial synthesis of 11-oxygenated androgens occurs exclusively by the adrenal enzyme 11β-hydroxylase (cytochrome P450 11B1, or CYP11B1). CYP11B1 metabolizes androstenedione to 11β-hydroxyandrostenedione (11OHA4) and, to a lesser extent, testosterone to 11β-hydroxytestosterone (11OHT).
Adrenocorticotropic hormone (ACTH) is known to stimulate 11OHA4 production (6), and 11-oxygenated androgens are elevated in congenital adrenal hyperplasia, a disease with characteristic ACTH elevation and adrenal-derived androgen excess (7-9). Other major regulators of 11-oxygenated androgen synthesis have not been identified. In particular, little is known about the regulation of the individual steroidogenic enzymes which govern 11-oxygenated androgen synthesis. Kinyua et al showed that insulin upregulates transcription of CYP11B1, among other adrenal enzymes, both in vitro and in a mouse model of hyperinsulinemia (10). In humans, increased 11-oxygenated androgens have been identified in disorders of mild IR such as PCOS (3, 11) and premature adrenarche (12); however, the molecular mechanisms responsible for these findings are unknown. Serum 11-oxygenated androgens have not been studied in states of profound hyperinsulinemia such as SIR.
The primary goal of this study was to evaluate the classic and 11-oxygenated androgen pathways in patients with SIR using liquid chromatography–tandem mass spectrometry (LC-MS/MS), a highly accurate method of steroid profiling. We further aimed to elucidate the role of insulin signaling through the insulin receptor to regulate 11-oxygenated androgens by studying patients with SIR who have intact insulin receptor function with postreceptor insulin resistance (lipodystrophy) vs those with insulin receptoropathy due to pathogenic insulin receptor gene mutations (Type A insulin resistance) or autoantibodies against the insulin receptor (type B insulin resistance).
Methods
Study Subjects
This was an ancillary study of a natural history study of disorders of insulin resistance (NCT00001987). The study was approved by the institutional review board of the National Institutes of Health. Participants or their legal guardians provided written, informed consent; minors provided verbal or written assent as age appropriate. Demographic and clinical data were collected from electronic medical records.
Healthy control subjects were individuals seen in the University of Michigan outpatient clinics for minor health conditions. To-be-discarded serum samples were retrieved from the clinical laboratory and de-identified prior to storage. These samples were obtained with University of Michigan Institutional Review Boards approval (HUM00089758). A waiver of consent was granted for this study.
Patient Eligibility
From the larger cohort of patients who participated in the protocol, patients with a clinical and/or genetically confirmed diagnosis of lipodystrophy or a diagnosis of insulin receptoropathy were assessed for eligibility. Lipodystrophy was diagnosed based on clinical evidence with or without confirmatory molecular genetic testing; insulin receptoropathy was diagnosed by either molecular genetic testing demonstrating pathogenic variants in the insulin receptor gene or by measurement of autoantibodies directed against the insulin receptor (type B insulin resistance). Additional eligibility criteria for inclusion in the primary analysis were: (1) female sex; (2) SIR defined as either fasting insulin > 25 mIU/L OR peak insulin ≥ 250 mIU/L after 75-g oral glucose challenge; (3) hyperandrogenemia defined as total testosterone > 80 ng/dL measured by immunoassay; and (4) availability of serum samples and clinical laboratory test results at the relevant visits. We excluded postmenopausal patients, defined by follicle stimulating hormone ≥ 30 IU/L and absent menstrual periods ≥ 1 year. Patients with recent glucocorticoid use (< 1 month before androgen measurement) were excluded. We assessed patients’ pubertal status based on Tanner staging for breast and pubic hair (Tanner stage II-IV classified as “mid-pubertal,” Tanner stage V for either breast or pubic hair classified as “pubertal”).
We conducted a secondary analysis comparing 11-oxygenated androgens in patients with vs without ovarian function (Ovary + vs Ovary-). All Ovary- patients had suppression of ovarian function due to either oophorectomy or GnRH analog treatment (with or without persistent hyperandrogenism). These analyses included patients from the primary analysis plus 1 additional patient with SIR who had hyperandrogenism that resolved after oophorectomy. For this patient, only a post-oophorectomy sample was available.
The control group included body mass index (BMI)- and age- matched women. Individuals with documented menstrual cycle irregularities, active malignancies, or hypogonadism, and those taking systemic glucocorticoids or sex hormones were excluded from the control cohort. Patients with type 1 diabetes mellitus taking exogenous insulin were not excluded.
Laboratory Testing
For the SIR cohort, blood samples were obtained in the morning after an 8- to 12-hour fast. Measurement of insulin, testosterone, sex hormone–binding globulin (SHBG), hemoglobin A1c, lipids, and serum creatinine was performed using the standard techniques of the NIH Clinical Center Department of Laboratory Medicine. Samples used for measurement of leptin and steroid hormones were frozen at −80 °C until analyzed. For measurement of leptin, serum samples were collected prior to patients’ morning dose of metreleptin and analyzed via radioimmunoassay using a commercial kit (EMD Millipore, catalog number HL-81HK, RRID:AB_2894698 [https://antibodyregistry.org/search.php?q=AB_2894698], assay sensitivity: 0.78 ng/mL, intra- and inter-assay coefficients of variability [CV]: 9.29% and 9.96%, respectively) which quantifies both endogenous leptin and exogenous metreleptin. Steroid hormones were analyzed as described below. For oral glucose tolerance testing (OGTT), patients underwent an overnight fast and subsequently received a 75-g oral glucose challenge; plasma insulin was measured from blood samples drawn at −10, 0, 30, 60, 90, 120, and 180 minutes.
Quantitation of Steroid Hormones by Mass Spectrometry
Fasting morning (6:00 am to 8:00 am) serum samples were collected from patients with SIR. For comparison, morning (7:00 am to 10:00 am) serum samples were obtained from healthy controls, not all of whom were fasting. Serum samples were stored at −80 °C until used for steroid quantitation. The following androgens were measured by LC-MS/MS as previously described (13): androstenedione, testosterone, 11OHA4, 11-ketoandrostenedione (11KA4), 11-ketotestosterone (11KT), 11β-hydroxytestosterone (11OHT), and dehydroepiandrosterone sulfate (DHEAS). Additional adrenal steroids were measured as markers of adrenal steroidogenesis including progesterone, pregnenolone, 11-deoxycorticosterone (11-DOC), corticosterone, 17α-hydroxyprogesterone (17OHP), cortisol, cortisone, pregnenolone sulfate (Preg-S), 17α-hydroxyprogesterone sulfate (17OHP-S), and Androst-5-ene-3β,17β-diol-3-sulfate (5-Adiol-S). All steroid hormones measured by LC-MS/MS are reported in nanomolar concentrations (nmol/L).
Statistical Analyses
Summary statistics are presented as mean ± SD for normally distributed data, and median [25th-75th percentile] for nonnormally distributed data. Nonnormally distributed data were log transformed prior to analyses, except for datasets containing zeros, which were analyzed with nonparametric tests. To assess CYP11B1 enzyme activity, product-to-precursor ratios were calculated based on molar concentrations of each analyte. Area under the curve (AUC) for insulin during OGTTs was calculated using the trapezoidal method. Depending on sample distribution, 2-group comparisons utilized the 2-tailed Mann-Whitney test or unpaired t test with Welch’s correction; 3-group comparisons utilized ordinary analysis of variance (ANOVA) with Tukey’s multiple comparisons test, Welch ANOVA with Dunnett’s T3 multiple comparisons test, or Kruskal-Wallis test with Dunn’s multiple comparisons test. Pearson or Spearman correlation tests were used to assess the relationships between adrenal hormones and insulin levels. Statistical significance was defined as P < 0.05 without correction for multiple comparisons. For all comparisons including control subjects, we performed a sensitivity analysis excluding the 3 control subjects taking exogenous insulin. This was a convenience sample based on available samples and data; thus, no a priori sample size calculations were performed.
Results
Patient Characteristics
We identified 22 female patients with a diagnosis of lipodystrophy or insulin receptoropathy with hyperandrogenemia; we excluded n = 2 patients who were postmenopausal, and n = 2 patients who did not meet criteria for SIR. Ultimately, 18 female patients with SIR and hyperandrogenemia met criteria for inclusion in the primary analysis (Table 1). Of these, 11 (61%) had a diagnosis of lipodystrophy (7 generalized, 4 partial) and 7 (39%) had a diagnosis of insulin receptoropathy (2 Rabson-Mendenhall syndrome, 1 heterozygous insulin receptor (INSR) variant [type A], 4 type B). Of the 15 pubertal patients, 2 (13%) were taking androgen receptor antagonists. Patients’ median age and mean BMI were 19 (interquartile range: 17-30) years and 23.4 ± 4.5 kg/m2, respectively. Mean fasting insulin was 67.1 (47.9-185.7) mIU/L, with no difference between patients who did and those who did not take injected insulin. Fasting insulin was similar between SIR subgroups. However, AUC for insulin during the OGTT was greater in the insulin receptoropathy group vs lipodystrophy, suggesting that subjects with insulin receptoropathy experience a greater degree of hyperinsulinemia than subjects with lipodystrophy in the postprandial state. SHBG was lower in the lipodystrophy subgroup vs insulin receptoropathy (18 vs 53 nM; P = 0.019), consistent with a failure of insulin to suppress hepatic SHBG synthesis in insulin receptoropathy (1); however, both total and free testosterone were comparable between groups. Creatinine was within normal limits for all patients. Measurements of fasting insulin, AUC insulin, SHBG, % hemoglobin A1c, and creatinine were not available for n = 1 patient with lipodystrophy.
Table 1.
Characteristics of female patients with severe insulin resistance
| All SIR (N = 18) | Lipodystrophy (N = 11) | Insulin receptoropathy (N = 7) | Control (N = 23) | |
|---|---|---|---|---|
| Age (years) | 19 [17-30] | 20 [17-33] | 17 [17-26] | 19 [17-31] |
| BMI (kg/m2) | 23.4 ± 4.5 | 23.5 ± 5.1 | 23.3 ± 3.8 | 21.7 ± 4.6 |
| Diagnosis | Generalized lipodystrophy (2 acquireda, 3 AGPAT2, 1 BSCL2, 1 unknown) | INSR gene mutation (2 Rabson- Mendenhall [homozygous], 1 heterozygous) | N/A | |
| Familial partial lipodystrophy (1 LMNA, 2 PPARG, 1 unknownb) | Type B, autoantibody to insulin receptor(N = 4) | |||
| Reproductive status | Mid-pubertal, 3 (17%) | Pubertal, 11 (100%) | Mid-pubertal, 3 (43%) | ND |
| Pubertal, 15 (83%) | Pubertal, 4 (57%) | |||
| Race | Black, 15 (83%) | Black, 3 (27%) | Black, 7 (100%) | Black, 4 (17%) |
| White, 3 (17%) | White, 6 (55%) | White, 19 (83%) | ||
| Multiracial, 1 (6%) | Multiracial, 1 (9%) | |||
| Unknown, 1 (6%) | Unknown, 1 (9%) | |||
| Fasting insulin (mIU/L) | 67.1 [47.9-185.7] | 65.1 [50.8-125.9] | 135.0 [40.3-189.4] | ND |
| Insulin AUC on OGTT (mIU*min/L) | 41,518 [24,111-95,823] | 28,566 [18,164-46,180] | 69,220 [42,658-163,264] | ND |
| Hemoglobin A1c (%) | 7.3 ± 1.4 | 7.5 ± 1.3 | 7.1 ± 1.7 | ND |
| SHBG (nmol/L) | 18.0 (14.5-53.5) | 16.5 (10.3-27.8) | 53.0 (18.0-104.0) | ND |
| Total testosterone (ng/dL)* | 192.0 (92.0-358.0) | 134.5 (95.4-231.5) | 314.0 (89.5-777.0) | 29.0 (19.0-39.0) |
| Free testosterone (ng/dL)† | 3.9 (2.6-7.0) | 3.8 (2.7-5.6) | 5.3 (2.4-7.4) | ND |
| Serum leptin (ng/dL) | 9.0 [2.9-14.3] | 3.7 [1.4-14.4] | 12.9 [5.3-14.2] | ND |
| Exogenous insulin use | 9 (50%) | 8 (73%) | 1 (14%) | 3 (13%) |
| Metreleptin use | 9 (50%) | 9 (82) | 0 (0%) | 0 |
| ACEi/ARB use | 6 (33%) | 5 (45%) | 1(14%) | 3 (13%) |
| Antiandrogen use | 2 (11%) | 1 (14%); Cyproterone | 1 (14%); Spironolactone | 0 |
Data presented as mean ± SD for normally distributed data, or median [25th-75th percentile] for nonnormally distributed data, or N (%) for categorical data.
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AUC, area under curve; ND, no data; OGTT, oral glucose tolerance test; SIR, severe insulin resistance;
a Due to juvenile dermatomyositis;
b Negative for LMNA and PPARG.
*Measured by immunoassay.
†Calculated based off measured Total Testosterone, SHBG, and Albumin.
A total of 23 healthy controls were included. The median age (19 [17-31] years) and BMI (21.7 ± 4.6 kg/m2) of the controls were similar to the SIR cohort. The majority of control subjects were White (83%, vs 17% Black). Three control subjects were taking exogenous insulin therapy for management of type 1 diabetes.
Classic and 11-Oxygenated Androgens Are Increased in SIR
In our cohort of patients with SIR (lipodystrophy plus insulin receptoropathy), adrenal steroid hormones including androgens and androgen precursors were consistently higher compared with healthy controls (Table 2). Androstenedione and testosterone were 3.7-fold and 5.7-fold higher in SIR vs controls (P < 0.0001), respectively. 11-oxygenated androgens were similarly elevated, with 11OHA4, 11KA4, and 11KT being 2.3-fold, 1.8-fold, and 3.1-fold higher in SIR vs controls (P ≤ 0.02). In contrast, only DHEAS and Preg-S were decreased in SIR compared with controls (2.6-fold and 1.8-fold lower, respectively). Several other steroids were increased in SIR including progesterone, 17α-hydroxyprogesterone, deoxycorticosterone, and 11-deoxycortisol (11dF). Insulin did not correlate significantly with any steroid hormones measured (P > 0.05 for all rs) for the entire SIR cohort and within the subphenotypes of lipodystrophy and insulin receptoropathy.
Table 2.
Serum concentrations in nmol/L of adrenal steroids differ in patients with severe insulin resistance (SIR) vs healthy controls
| Analyte(nmol/L) | SIR (all) N = 18 |
Lipodystrophy N = 11 |
Insulin receptoropathy N = 7 |
Control N = 23 |
P value (SIR vs control) |
P value (LD vs R vs Control) |
|---|---|---|---|---|---|---|
| A4 | 9.2 (5.3-14.4) | 8.8 (5.0-10.9) | 13.0 (5.3-21.1) | 2.4 (1.6-3.2) | <0.0001 | <0.0001a,b |
| T | 5.6 (2.9-14.0) | 4.1 (2.8-6.1) | 13.6 (3.6-42.0) | 1.0 (0.7-1.2) | <0.0001 | <0.0001a,b |
| 11OHA4 | 6.0 (3.5-8.5) | 8.2 (4.3-13) | 3.6 (1.0-5.2) | 2.9 (2.3-4.6) | 0.02 | 0.0007a,c |
| 11OHT | 0.4 (0.2-0.8) | 0.55 (0.20-0.90) | 0.3 (0.2-0.5) | 0.3 (0.2-0.4) | 0.13 | 0.052 |
| 11KA4 | 1.0 (0.5-1.5) | 1.4 (1.0-1.7) | 0.7 (0.3-1.0) | 0.6 (0.4-0.8) | 0.015 | 0.0035a |
| 11KT | 2.0 (0.9-2.8) | 2.7 (1.7-2.9) | 1.0 (0.8-1.7) | 0.7 (0.6-1.2) | 0.0008 | <0.0001a,c |
| Progesterone | 0.5 (0.3-0.8) | 0.4 (0.3-0.9) | 0.5 (0.4-0.8) | 0.3 (0.2-4.3) | 0.024 | 0.061 |
| 17-OHP | 4.1 (2.0-6.1) | 4.3 (1.9-7.1) | 3.9 (2.4-5.8) | 1.2 (0.6-1.8) | 0.0008 | 0.004a,b |
| DOC | 0.7 (0.6-1.3) | 0.68 (0.61-0.80) | 0.9 (0.6-1.9) | 0.14 (0.09-0.19) | <0.0001 | <0.0001a,b |
| Corticosterone | 5.4 (2.5-7.6) | 5.5 (2.6-11.5) | 5.6 (2.2-5.8) | 5.2 (3.8-9.1) | 0.29 | 0.34 |
| 11dF | 0.7 (0.6-1.5) | 1.07 (0.61-2.18) | 0.7 (0.6-0.7) | 0.49 (0.35-0.75) | 0.0031 | 0.0021a |
| Cortisol | 265.6 (167.5-356.2) | 271.9 (168.1-370.3) | 231.9 (105.7-291.7) | 279.4 (201.1-378.9) | 0.44 | 0.21 |
| Cortisone | 73.8 (55.3-91.7) | 76.6 ± 33.1 | 69.3 ± 31.1 | 74.5 (22.7) | 0.77 | 0.59 |
| DHEAS | 1529 (943-2842) | 2184 (943-4638) | 1311 (934-2046) | 3419 (2531-4768) | 0.0043 | 0.0066b |
| Preg-S | 59.4 (30.9-86.3) | 79.6 (34.4-100.1) | 57.8 (22.7-70.7) | 104.2 (28.0-199.5) | 0.0025 | 0.0079a,b |
| 17OH Preg-S | 9.9 (7.6-13.4) | 10.3 (9.2-16.9) | 7.9 (4.1-10.3) | 12.9 (9.4-16.8) | 0.19 | 0.16 |
| 5-Adiol-S | 121.8 (91.9-197.7) | 164.4 (87.4-303.1) | 98.8 (93.4-121.4) | 181.6 (131.3-240.5) | 0.16 | 0.014b |
Data presented as mean ± SD for normally distributed data, or median [25th-75th percentile] for nonnormally distributed data.
Abbreviations and conversion factors for steroid hormones (multiply by value to convert from nmol/L to ng/dL): 11OHA4, 11β-hydroxyandrostenedione (30.24); 11OHT, 11β-hydroxytestosterone (30.44); 11dF, 11-deoxycortisol (34.65); 11KA4, 11-ketoandrostenedione (30.04); 11KT, 11- ketotestosterone (30.24); 17-OHP, 17α-hydroxyprogesterone (33.05); 17OHP-S, 17α-hydroxyprogesterone sulfate (41.25); 5-Adiol-S, androst-5-ene-3β,17β-diol-3-sulfate (37.05); A4, androstenedione (28.6); corticosterone (34.65); cortisol (36.25); cortisone (36.05); DHEAS, dehydroepiandrosterone sulfate (36.85); DOC, 11-deoxycorticosterone (33.05); LD, lipodystrophy; Preg-S, pregnenolone sulfate (39.65); SIR, severe insulin resistance; T, testosterone (28.8).
a P < 0.05 for LD vs control;
b P < 0.05 for insulin receptoropathy vs control;
c P < 0.05 for LD vs insulin receptoropathy.
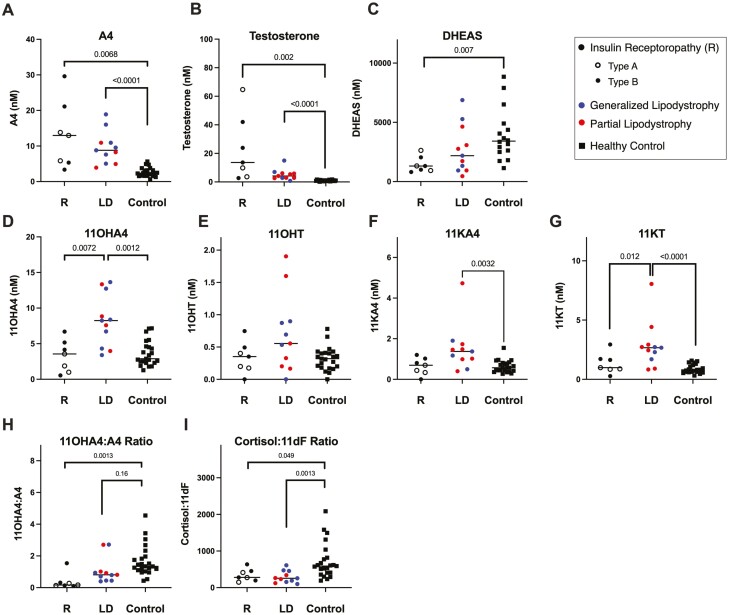
11-Oxygenated Androgens Are Increased in Lipodystrophy but not Insulin Receptoropathy
Androgens were evaluated in 2 SIR subphenotypes, lipodystrophy and insulin receptoropathy (Table 2, Fig. 2). Testosterone and the classic pathway androgen precursors, androstenedione and 17-hydroxyprogesterone, were similarly increased in both lipodystrophy and insulin receptoropathy relative to controls. However, DHEAS and 5-Adiol-S were decreased relative to controls in the insulin receptoropathy group only (Fig. 2C). The 11-oxygenated-androgens 11OHA4, 11KA4, and 11KT were elevated in lipodystrophy but not in insulin receptoropathy (Figs 2D, 2F, 2G). 11OHT was comparable among controls and both SIR subphenotypes (Fig. 2F). Relative to controls, patients with lipodystrophy had 2.9-fold higher 11OHA4 (P = 0.0012), 2.4-fold higher 11KA4 (P = 0.0032), and 3.6-fold higher 11KT (P < 0.0001). Furthermore, 2 11-oxygenated-androgens, 11OHA4 and 11KT, were higher in the lipodystrophy vs insulin receptoropathy subgroups by 2.3-fold and 2.7-fold, respectively. A sensitivity analysis excluding the 3 control subjects taking exogenous insulin showed no difference in these results.
Figure 2.
11-Oxyandrogens are elevated in patients with lipodystrophy (LD) but not insulin receptoropathy (R) relative to healthy controls. A-G) Serum concentrations of steroid hormones differ between patients with lipodystrophy, insulin receptoropathy, and controls. H-I) Product-to-precursor ratios for 2 steroid hormone conversion reactions catalyzed by CYP11B1 in the adrenal gland, I) in the 11-oxygenated androgen pathway and G) in the classic pathway. Patients with insulin receptoropathy have decreased ratios of 11OHA4:A4 relative to controls and patients with lipodystrophy, while the ratio of cortisol:11dF is decreased in both SIR subphenotypes. P values shown for 1-way ANOVA comparison. Abbreviations and conversion factors (multiply by value to convert from nmol/L to ng/dL): 11OHA4, 11β-hydroxyandrostenedione (30.24); 11OHT, 11β-hydroxytestosterone (30.44); 11KA4, 11-ketoandrostenedione (30.04); 11KT, 11-ketotestosterone (30.24); A4, androstenedione (28.6); DHEAS, dehydroepiandrosterone sulfate (36.85); LD, lipodystrophy; R, insulin receptoropathy; T, testosterone (28.8).
CYP11B1 Product-Precursor Ratios Differ by SIR Subgroup
To assess CYP11B1 enzyme activity, we calculated direct product-to-precursor ratios for adrenal CYP11B1-catalyzed reactions (Fig. 2). Ratios for 11OHA4:androstenedione (Fig. 2I) were numerically but not statistically lower for patients with lipodystrophy (0.8 [0.4-1.0]) compared with controls (1.3 [1.0-1.9]) (P = 0.16), but the ratios were significantly lower in the insulin receptoropathy subgroup (0.2 [0.1-1.2]) relative to both lipodystrophy (P = 0.024) and controls (P = 0.005) (Fig. 3A). In contrast, the cortisol:11dF ratio was decreased in both the lipodystrophy (256.2 [155.1-455.8]) and insulin receptoropathy (278.6 [195.6-453.0]) subgroups relative to controls (563.9 ([390.2-782.3]) (Fig. 2I). Similarly, in a sensitivity analysis excluding the 3 control subjects taking exogenous insulin, these findings were unchanged.
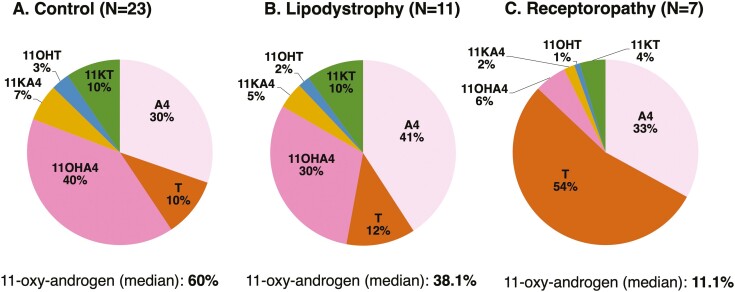
Figure 3.
Distinct relative proportions of the classic and 11-oxygenated androgen pathways. Relative percentages for individual steroids, total classic pathway androgens (sum of androstenedione and testosterone), and total 11-oxygenated androgens (sum of 11OHA4, 11OHT, 11KA4, and 11KT) were significantly different among (A) healthy controls, (B) patients with lipodystrophy, and (C) patients with insulin receptoropathy (P < 0.05 for 1-way ANOVA). Abbreviations: 11dF, 11-deoxycortisol; 11OHA4, 11β-hydroxyandrostenedione; 11OHT, 11β-hydroxytestosterone; 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; A4, androstenedione; LD, lipodystrophy; T, testosterone.
Proportions of Circulating Steroids Differ by SIR Subgroup
The proportion of 11-oxygenated androgens differed (P < 0.0001) between the controls and patients with lipodystrophy and insulin receptoropathy (Fig. 3). In control subjects, 11-oxygenated androgens comprised the majority (60%) of androgens, with 11OHA4 predominating (40%). Controls had comparable proportions of 11KT and testosterone (both 10%) in the circulation. In lipodystrophy, fewer than half (38%) of circulating androgens were 11-oxygenated androgens; androstenedione was the major circulating androgen (41%). Relative proportions of 11KT (10%) and testosterone (12%) were similar in lipodystrophy and controls. Conversely, 11-oxygenated androgens comprised a small proportion of circulating androgens (11.1%) in patients with insulin receptoropathy, and the classic androgen pathway dominated (testosterone 54% and androstenedione 33%).
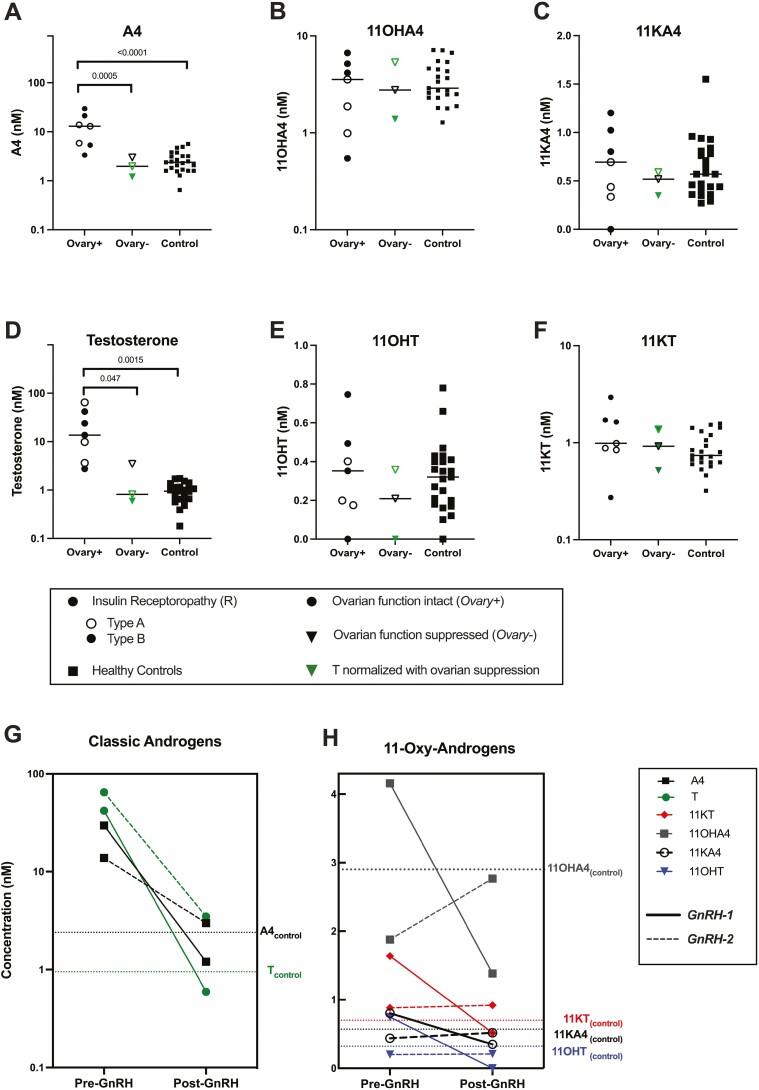
Ovarian Function Does Not Affect 11-Oxygenated Androgen Concentration in Patients With Insulin Receptoropathy
To evaluate the relative contributions of the adrenals and ovaries to circulating 11-oxygenated androgens, we compared androgens in pubertal patients with (Ovary+) vs without (Ovary-) functioning ovaries (Fig. 4). Within the insulin receptoropathy cohort, 7 patients were Ovary + and 3 patients were Ovary-. All patients with lipodystrophy were Ovary+, so we could not compare ovarian function subgroups in this cohort. Compared to both Ovary- and controls, Ovary + had higher androstenedione (PANOVA < 0.0001) and testosterone (PANOVA = 0.01) (Fig. 4A-4B). However, 11-oxygenated androgens were comparable among all 3 groups (Fig. 4C-4F).
Figure 4.
Elevations of classical androgens, but not adrenally derived 11-oxygenated androgens, depend on ovarian function in SIR patients with insulin receptoropathy. A-F) Serum concentrations of classic pathway vs 11-oxygenated androgens in subjects with insulin receptoropathy and healthy controls. Ovary + includes 7 patients with intact ovarian function (circles); Ovary- includes 3 patients with suppressed ovarian function (triangles). Green icons represent Ovary- patients with testosterone <80 ng/dL measured by LC-MS/MS. All Ovary- patients had testosterone <80 ng/dL measured by immunoassay. P values shown for 1-way ANOVA. G-H) Biochemical response to ovarian suppression with GnRH analog therapy in 2 hyperandrogenemic patients with insulin receptoropathy. Ovarian suppression led to G) normalization of classical androgen levels, testosterone (black closed circle) and androstenedione (A4, purple square), and H) inconsistent effects on 11-oxygenated androgen concentrations (11OHA4, gray square; 11KA4, black open circle; 11OHT, blue triangle; 11KT, red diamond). Median concentrations of steroid hormones in control subjects (N = 23) are represented by dotted lines. Abbreviations and conversion factors (multiply by value to convert from nmol/L to ng/dL): 11OHA4, 11β-hydroxyandrostenedione (30.24); 11OHT, 11β-hydroxytestosterone (30.44); 11KA4, 11-ketoandrostenedione (30.04); 11KT, 11-ketotestosterone (30.24); A4, androstenedione (28.6); T, testosterone (28.8).
Two patients (GnRH-1 and GnRH-2) were treated with GnRH analogs as part of clinical care, with the aim of suppressing ovarian function to alleviate hyperandrogenism. GnRH-1 was a 33-year-old woman with type B insulin resistance, and GnRH-2 was a 16-year-old girl with Rabson-Mendenhall syndrome caused by homozygous Ile119Met INSR variant. GnRH-2 (but not GnRH-1) also received add-back estrogen and progesterone therapy. Steroid hormone levels were quantified before and after GnRH analog therapy, allowing for intra-individual comparisons of androgens in the Ovary + and Ovary- states (Fig. 4). Prior to GnRH analog, both patients had substantially elevated androstenedione and testosterone (Fig. 4G), but concentrations of 11-oxygenated androgen levels were comparable to those observed in control subjects (Fig. 4H). In both patients, GnRH analog led to a striking decrease in androstenedione and testosterone (mean change: −87% and −97%). In GnRH-1, all 11-oxygenated androgen metabolites decreased less than androstenedione and testosterone with treatment, while in GnRH-2 all 11-oxygenated androgen were modestly increased. In GnRH-1, fasting insulin decreased by 42% after treatment (247.0 to 143.7 mIU/L), while fasting insulin for GnRH-2 significantly worsened to exceed the upper limit of the assay (247.5 to > 1000 mIU/L).
Discussion
In the present study, we demonstrate that among female patients with SIR and hyperandrogenemia, adrenal-derived 11-oxygenated androgens are elevated in patients with lipodystrophy but not those with insulin receptoropathy. Despite high androstenedione in both subphenotypes of SIR, we found that the adrenal-derived, CYP11B1-produced 11-oxygenated androgens were elevated only in lipodystrophy patients, suggesting that the adrenal androgen excess observed in states of hyperinsulinism is driven by excess insulin signaling through its receptor.
In humans, patients with SIR provide a natural model to study the pathophysiology of hyperandrogenism in hyperinsulinemic states. Both subphenotypes of SIR, insulin receptoropathy and lipodystrophy, exhibit profound hyperinsulinemia as a compensatory response to diminished action of insulin to stimulate cellular glucose uptake. Insulin receptoropathies are characterized by a loss of signaling in all intracellular signal transduction pathways downstream of the insulin receptor (receptor-level IR). By contrast, in lipodystrophy, as in obesity, some insulin receptor signaling pathways are impaired while other pathways can be overstimulated by hyperinsulinemia (postreceptor IR), a concept known as “selective insulin resistance” (14). Divergent clinical findings can sometimes be found according to these IR subphenotypes, such as dyslipidemia (Fig. 1) (15, 16). In contrast, concordant phenotypes can be ascribed to decreased insulin receptor signaling in both groups (e.g., inability of insulin to suppress hepatic gluconeogenesis), or alternatively to insulin receptor-independent processes (e.g., acanthosis nigricans caused by excess insulin stimulation of keratinocyte IGF-1 receptors (17)). We observed adrenal hyperandrogenemia in lipodystrophy (postreceptor IR) but not insulin receptoropathy (receptor-level IR), suggesting that increased insulin receptor signaling is needed for this effect.
Current models of hyperandrogenemia in SIR have implicated the ovaries as the major source of excess androgen. Insulin directly stimulates ovarian theca cell proliferation and synthesis of androstenedione and testosterone (18, 19), analogous to proposed mechanisms of PCOS (20). Insulin-lowering therapies (i.e., insulin sensitizers) in SIR are associated with androgen reduction (21-23), supporting the idea that excess insulin promotes a hyperandrogenic phenotype that is reversible through improving insulin resistance. Furthermore, women with SIR experience normalization of hyperandrogenism after oophorectomy or ovarian suppression with GnRH analog (1), supporting a primary ovarian source for androgens in SIR. Consistent with these models, we found high androstenedione and testosterone only in patients with intact ovarian function.
The proposed mechanism of insulin’s pro-steroidogenic actions on the ovaries is controversial. Studies of insulin receptor-knockout mice have failed to detect insulin-induced increases of ovarian androgens (24), leading some to conclude that insulin binding to its receptor is necessary to stimulate ovarian androgen production (25). However, a recent large study of patients with SIR showed elevated ovarian androgens in both insulin receptoropathy (receptor-level IR) and lipodystrophy (postreceptor IR), suggesting that insulin can cause hyperandrogenemia independent of insulin receptor signaling. It is possible that only extremely high concentrations of insulin activate signaling through lower-affinity targets; the IGF-1 receptor has been proposed as one potential pathway. Our findings also support a role for an insulin receptor-independent ovarian hyperandrogenemia in states of extreme hyperinsulinemia. By contrast, the elevations in 11-oxygenated androgens in lipodystrophy but not insulin receptoropathy suggest that adrenal hyperandrogenemia is dependent on excess insulin signaling through its receptor. Furthermore, this observation suggests that little if any ovarian-derived androstenedione and testosterone circulate to the adrenals and are converted to 11-oxygenated metabolites. Consistent with this model, other studies have found that circulating gonadal-derived androgens do not meaningfully act as substrates for adrenal 11-oxygenated androgen metabolism. For instance, Davis et al. showed that administering exogenous testosterone to postmenopausal women does not alter 11-oxygenated androgen concentrations (26). Therefore, in lipodystrophy (postreceptor IR), adrenal hyperandrogenemia is likely not an indirect consequence of insulin’s effects to increase ovarian androgen synthesis; instead, our data support the notion that excess insulin receptor signaling directly increases adrenal 19-carbon steroid synthesis. It is plausible that the adrenal androgen excess observed in conditions of mild insulin resistance, such as in PCOS, can be mechanistically explained by increased insulin receptor signaling in the adrenals, as has been observed in children of mothers with PCOS (27).
We hypothesized that excess insulin signaling through the insulin receptor in lipodystrophy upregulates adrenal steroidogenic enzymes such as CYP11B1 to cause 11-oxygenated androgen excess. We observed in patients with insulin receptoropathy that product-to-precursor ratios of CYP11B1-catalyzed reactions were decreased relative to controls, despite high levels of the precursors, 11dF and androstenedione. In contrast, patients with lipodystrophy had ratios of 11OHA4:androstenedione that were not significantly lower than controls and higher than insulin receptoropathy, despite a striking 4-fold increase of androstenedione. These findings suggest preserved CYP11B1 expression or activity in lipodystrophy (postreceptor IR), but not insulin receptoropathy (receptor-level IR), which may occur secondary to differences in transcriptional regulation or altered development of the zona reticularis (28). Furthermore, the high 11-deoxysteroid production in lipodystrophy suggests that other steroidogenic enzymes upstream of CYP11B1 are upregulated by insulin signaling through its receptor. Experimental models in mice and in adrenocortical cell cultures have shown that hyperinsulinemia signaling through the insulin receptor upregulates numerous adrenal steroidogenic enzymes, including not only CYP11B1 but also HSD3B2, independent of ACTH (10, 29). We found higher 11-oxygenated androgens, yet significantly lower Preg-S and a nonsignificant trend toward lower DHEAS, 5-Adiol-S, and 17OH-Preg-S in lipodystrophy patients (Table 2), consistent with increased activity of adrenal HSD3B2 and possibly other enzymes early in the androgen biosynthesis pathways. Our data provides the first evidence in humans supporting a direct role for insulin receptor signaling in regulating adrenal androgen synthesis.
Strengths of this study include evaluation of a novel class of androgens in the context of chronic and severe hyperinsulinemia without confounding effects of obesity, the use of LC-MS/MS for steroid quantitation, and inclusion of age, sex, and BMI-matched controls. Our study has several limitations. As an ancillary study of a larger natural history study, our analysis was intrinsically limited based on availability of historical patient data via chart review. Subjects were not matched by ethnicity across cohorts. There is some evidence to suggest that androgen profiles in PCOS differ based on ethnicity (3, 4, 11, 30), and in theory a similar phenomenon could contribute to the effects observed in the present study. Additionally, plasma ACTH, adrenal volume, results of dynamic adrenal testing, and menstrual phase were not available, limiting our ability to evaluate the hypothalamic-pituitary-adrenal axis (31, 32). However, we did not observe universal elevations of all adrenal hormones, suggesting that elevated 11-oxygenated androgens are not occurring in the context of a global increase in adrenal steroidogenesis or other adrenal pathology. Moreover, CYP11B1 product-precursor relationships only provide a crude approximation of enzymatic activity, as serum hormone concentrations may not represent the precursor pool for adrenal enzymes. Finally, lipodystrophy and insulin receptoropathy are exceedingly rare disorders. Furthermore, within the insulin receptoropathy group there was genotypic and phenotypic heterogeneity, including both homozygous and heterozygous mutations of the insulin receptor. Although this heterogeneity might have variable effects on loss of insulin receptor signaling, none of the patients in this group showed elevated 11-oxygenated androgens. Consequently, our study of 18 patients was underpowered to detect correlations between insulin and analytes of interest. However, we were able to observe significant and consistent differences in androgen levels of these subgroups despite a small sample size, which is an advantage of studying rare disease groups. These findings highlight major differences in physiology in patients with and without insulin receptor signaling.
In summary, this is the first study to characterize 11-oxygenated androgens in female patients with SIR and hyperandrogenemia. Here we observe that 11-oxygenated androgens are elevated in SIR associated with postreceptor signaling impairment (lipodystrophy), but not in SIR due to primary disorders of the insulin receptor (insulin receptoropathy). Modulation of adrenal steroidogenesis by insulin receptor signaling potentially has clinical implications in women with SIR and other disorders of androgen excess. Insulin-receptor dependent insulin signaling may represent an important mechanism for the regulation of 11-oxygenated androgen synthesis not only in SIR but also in common disorders of mild IR such as PCOS. The ovaries are primarily responsible for elevated classic pathway androgens but do not contribute to excess 11-oxygenated androgens in SIR.
Glossary
Abbreviations
- 11OHA4
11β-hydroxyandrostenedione
- 11dF
11-deoxycortisol
- 11KA4
11-ketoandrostenedione
- 11KT
11-ketotestosterone
- 11OHT
11β-hydroxytestosterone
- 5-Adiol-S
androst-5-ene-3β,17β-diol-3-sulfate
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- AUC
area under the curve
- BMI
body mass index
- CYP11B1
11β-hydroxylase member 1
- DHEAS
dehydroepiandrosterone sulfate
- INSR
insulin receptor
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- OGTT
oral glucose tolerance test
- PCOS
polycystic ovary syndrome
- Preg-S
pregnenolone sulfate
- SHBG
sex hormone–binding globulin
- SIR
severe insulin resistance
Contributor Information
Dalia Walzer, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Adina F Turcu, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Smita Jha, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Brent S Abel, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Richard J Auchus, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Department of Pharmacology, University of Michigan, Ann Arbor, MI 48109, USA.
Deborah P Merke, The National Institutes of Health Clinical Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD 20892, USA.
Rebecca J Brown, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
This work was supported in part by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (R.J.B., B.S.A., and S.J.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (D.P.M.). A.F.T. was supported by grant K08DK109116 from the National Institute of Diabetes and Digestive and Kidney Diseases. D.W. was supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the American Association for Dental Research and the Colgate-Palmolive Company.
Disclosure Statement
D.P.M. received unrelated research funds from Diurnal Limited through the National Institutes of Health Cooperative Research and Development Agreement. Other authors state that they have no conflicts of interest.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
ClinicalTrials.gov registration no. NCT00001987.
References
- 1. Huang-Doran I, Kinzer AB, Jimenez-Linan M, et al. Ovarian hyperandrogenism and response to gonadotropin-releasing hormone analogues in primary severe insulin resistance. J Clin Endocrinol Metab. 2021;106(8):2367-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmina E, Lobo RA. Prevalence and metabolic characteristics of adrenal androgen excess in hyperandrogenic women with different phenotypes. J Endocrinol Invest. 2007;30(2):111-116. [DOI] [PubMed] [Google Scholar]
- 3. O’Reilly MW, Kempegowda P, Jenkinson C, et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tosi F, Villani M, Garofalo S, et al. Clinical value of serum levels of 11-oxygenated metabolites of testosterone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;107(5):e2047-e2055. [DOI] [PubMed] [Google Scholar]
- 5. Carmina E, Stanczyk FZ, Chang L, Miles RA, Lobo RA. The ratio of androstenedione:11 beta-hydroxyandrostenedione is an important marker of adrenal androgen excess in women. Fertil Steril. 1992;58(1):148-152. [DOI] [PubMed] [Google Scholar]
- 6. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248-1261. [DOI] [PubMed] [Google Scholar]
- 8. Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2018;178:221-228. [DOI] [PubMed] [Google Scholar]
- 9. Turcu AF, Mallappa A, Elman MS, et al. 11-oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinyua AW, Doan KV, Yang DJ, et al. Insulin Regulates adrenal steroidogenesis by stabilizing SF-1 activity. Sci Rep. 2018;8(1):5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida T, Matsuzaki T, Miyado M, et al. 11-oxygenated C19 steroids as circulating androgens in women with polycystic ovary syndrome. Endocr J. 2018;65(10):979-990. [DOI] [PubMed] [Google Scholar]
- 12. Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-Oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95-96. [DOI] [PubMed] [Google Scholar]
- 15. Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119(2):315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baykal AP, Parks EJ, Shamburek R, et al. Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI Insight. 2020;5(14):e137180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance. Am J Clin Dermatol. 2004;5(3):199-203. [DOI] [PubMed] [Google Scholar]
- 18. Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83(6):2001-2005. [DOI] [PubMed] [Google Scholar]
- 19. Tosi F, Negri C, Perrone F, et al. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):1712-1719. [DOI] [PubMed] [Google Scholar]
- 20. Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;122(1-3):42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255-263. [DOI] [PubMed] [Google Scholar]
- 22. Lungu AO, Zadeh ES, Goodling A, Cochran E, Gorden P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2012;97(2):563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klubo-Gwiezdzinska J, Lange M, Cochran E, Semple RK, Gewert C, Brown RJ, Gorden P. Combined Immunosuppressive therapy induces remission in patients with severe type B insulin resistance: a prospective cohort study. Diabetes Care. 2018;41(11):2353-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu S, Divall S, Nwaopara A, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63(4):1270-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis SR, Turcu AF, Robinson PJ, Bell RJ. Exogenous testosterone does not influence 11-oxygenated C19 steroid concentrations in healthy postmenopausal women. J Endocr Soc. 2019;3(3):670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torchen LC, Sisk R, Legro RS, Turcu AF, Auchus RJ, Dunaif A. 11-Oxygenated C19 steroids do not distinguish the hyperandrogenic phenotype of PCOS daughters from girls with obesity. J Clin Endocrinol Metab. 2020;105(11):e3903-e3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pignatti E, Altinkilic EM, Bräutigam K, Grössl M, Perren A, Zavolan M, Flück CE. Cholesterol deprivation drives DHEA biosynthesis in human adrenals. Endocrinology. 2022;163(7): bqac076. [DOI] [PubMed] [Google Scholar]
- 29. Kristiansen SB, Endoh A, Casson PR, Buster JE, Hornsby PJ. Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult human adrenocortical cells. Steroids. 1997;62(2):258-265. [DOI] [PubMed] [Google Scholar]
- 30. Huang Z, Yong E-L. Ethnic differences: is there an Asian phenotype for polycystic ovarian syndrome? Best Pract Res Clin Obstet Gynaecol. 2016;37:46-55. [DOI] [PubMed] [Google Scholar]
- 31. Reincke M, Faßnacht M, Väth S, Mora P, Allolio B. Adrenal incidentalomas: a manifestation of the metabolic syndrome? Endocr Res. 1996;22(4):757-761. [DOI] [PubMed] [Google Scholar]
- 32. Muscogiuri G, Sorice GP, Prioletta A, et al. The size of adrenal incidentalomas correlates with insulin resistance. Is there a cause-effect relationship? Clin Endocrinol (Oxf). 2011;74(3):300-305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.