Abstract
Context
The relationship of ideal cardiovascular health (CVH) behaviors with preventing early-onset vasomotor symptoms (VMSs) is unknown.
Objective
We investigated the association between CVH metrics and the development of early-onset VMSs in premenopausal women.
Methods
This cohort study included 2541 premenopausal women aged 42 to 52 years without VMSs at baseline. CVH metrics were defined according to the American Heart Association Life Simple 7 metrics. Owing to limited availability of dietary information, CVH metrics were scored from 0 (unhealthy) to 6 (healthy) and classified into 3 groups: poor (0-2), intermediate (3-4), and ideal (5-6) CVH. VMSs, including hot flashes and night sweats, were assessed using the Menopause-Specific Quality of Life questionnaire. Moderate/severe VMSs was defined as a score of 3 or more points (range, 0 to 6; 6 being most bothersome).
Results
During a median follow-up of 4.5 years, 1241 women developed VMSs before menopause. After adjustment for age, parity, education level, and alcohol consumption, the hazard ratio (HR) (95% CI) for developing early-onset VMSs comparing poor CVH group to the ideal group was 1.41 (1.07-1.86). CVH scores were also inversely associated with moderate/severe VMSs in a dose-response manner (P for trend = .004); specifically, multivariable-adjusted HRs comparing intermediate and poor CVH groups to the ideal group were 1.20 (95% CI, 1.02-1.43) and 1.57 (95% CI, 1.08-2.29), respectively.
Conclusion
Unfavorable CVH metrics were significantly associated with an increased risk of early-onset VMSs and its more severe forms among premenopausal women.
Keywords: menopause, vasomotor symptoms, cardiovascular health metrics
Vasomotor symptoms (VMSs), including hot flashes and night sweats, are major menopausal symptoms (1). Approximately 60% to 80% of middle-aged women experience some degree of VMSs before and after menopause (2). VMSs are known to occur near the final menstrual period and last for approximately 1 year after menopause; however, recent research has shown that VMSs can start far earlier than previously reported, even during premenopausal or early menopausal transition stages, and can persist for longer than 10 years after the final menstrual period (3, 4). Severe, frequent, and long-lasting VMSs were significantly related to adverse cardiovascular disease (CVD) profiles, resulting in reduced physical and mental quality of life in postmenopausal women (5-8). Studies of VMSs have focused on perimenopausal or postmenopausal women; however, studies on early-onset VMS occurring in premenopausal women are limited, and its distinctive risk factors and treatment are not well established (9).
CVD is one of the leading causes of death globally and a huge burden on public health (10). To reduce deaths caused by CVDs, the American Heart Association defined ideal cardiovascular health (CVH) metrics in 2010 based on 7 modifiable risk factors and lifestyle behaviors: body mass index (BMI), physical activity, smoking status, blood pressure, diet, fasting glucose, and cholesterol concentrations (11). Although CVH metrics were initially designed as a guideline for CVD prevention, some studies reported that maintaining the ideal CVH metrics was significantly associated with reduced risks of not only cardiovascular events and mortality (12-15) but also subclinical CVD and other non–CVD-related diseases (16-18). There are sex-related differences in patterns of CVD progression and mortality (19); notably, CVD prevalence and incidence among women tend to rapidly increase during menopausal transition because of the withdrawal of endogenous estrogen (20-22). Decreased estrogen levels during menopausal transition could cause endothelial dysfunction, while VMSs are also associated with endothelial dysfunction among middle-aged women and CVD events in later life (23, 24). Given the interrelationship of estrogen deficiency, VMSs, and CVD risk, as well as a lack of preventive measures for VMSs, we hypothesized that adherence to ideal CVH metrics decreases the risk of VMSs. Currently, there are no studies on the benefits of ideal CVH metrics in preventing VMSs. Thus, we investigated the association between ideal CVH metrics and the risk of VMSs in premenopausal women. By investigating the effect of favorable CVH behaviors on preventing early-onset VMSs, adherence to ideal CVH metrics can be promoted as a preventive measure to reduce VMSs and CVD events in women during menopausal transition.
Materials and Methods
Study Population
In this longitudinal study of middle-aged Korean women, we recruited participants between 2014 and 2018 from the Kangbuk Samsung Health Study, a cohort study of Korean men and women who underwent annual or biannual comprehensive health examinations at the Kangbuk Samsung Hospital Total Healthcare Center clinics in Seoul and Suwon, South Korea. The eligibility criteria for enrollment were as follows: 1) women aged 42 to 52 years; 2) no history of hysterectomy, oophorectomy, or hormone replacement therapy; 3) at least 1 menstrual period in the 3 months before the health screening examinations and no amenorrhea lasting 60 or more days; and 4) no history of a chronic disease that may affect menstrual cycles (malignancy, renal failure, and hypothyroidism or hyperthyroidism). Among 5230 women initially enrolled, 194 who withdrew from the study and 283 who were in their early or late menopausal transition or postmenopausal stages were excluded because we included only premenopausal women in this study. After excluding those who had no information on VMSs or CVH metrics (n = 130) and had a history of coronary disease, heart disease, or stroke (n = 21), the eligible participants at baseline were 4602. For the longitudinal analysis, women who already had VMSs at baseline (n = 1025) did not undergo follow-up examinations (n = 1033), and women with missing information on VMSs (n = 3) during the follow-up period were excluded (Supplementary Fig. 1) (25). Finally, 2541 premenopausal women were included in the study. This study was approved by the institutional review board of Kangbuk Samsung Hospital (No. KBSMC 2022-03-023). All participants provided written informed consent. All the methods in this cohort study were performed in accordance with the relevant guidelines and regulations.
Measurements
Using standardized, structured, and self-administered questionnaires, we obtained data on demographic and clinical characteristics, health-related behaviors, and reproductive factors. Among health-related behaviors, smoking status was categorized as never, former, or current. Physical activity was assessed using the validated Korean version of the International Physical Activity Questionnaire short form (26). Alcohol intake was categorized as less than 10 and greater than or equal to 10 g/day, and education levels were dichotomized as less than university graduates and equal to or greater than university graduates. Parity was defined as the number of pregnancies, including live and stillbirths. Diet was measured using a 103-item self-administered food frequency questionnaire validated for use in Korea and designed to assess dietary habits during the previous year (27). The total energy and nutrient intake were calculated using the food composition tables developed by the Korean Nutrition Society (28).
The participants wore a lightweight gown with no shoes. Trained experts measured height, weight, and body composition. BMI was calculated as the individual’s body weight divided by the height squared. Blood pressure (BP) was measured 3 times using automatic BP equipment (53000-E2, Welch Allyn) after a 5-minute rest. We used the average BP based on the second and third BP measurements. Blood samples were collected from the antecubital vein after at least 10 hours of fasting. The serum total cholesterol and triglyceride concentrations were determined using an enzymatic colorimetric assay. High-density lipoprotein and low-density lipoprotein cholesterol levels were directly measured using a homogenous enzymatic colorimetric assay. serum fasting glucose levels were measured using the hexokinase method on Modular DPP systems (Roche Diagnostics) until 2015, and the Cobas 8000 c702 (Roche Diagnostics) thereafter. Glycated hemoglobin A1c levels were determined using a turbidimetric inhibition immunoassay on the Cobas Integra 800 (Roche Diagnostics) until January 2018 and the Cobas 8000 c513 (Roche Diagnostics) thereafter (RRID:AB_2909460 and AB_2909459).
CVH metrics were defined according to the American Heart Association Life Simple 7 metrics (11). Ideal CVH metrics were defined as follows: 1) smoking: never or former smoker; 2) BMI less than 23; 3) physical activity: 150 min/week or more of moderate-intensity physical activity, 75 min/week or more of vigorous-intensity physical activity, or 150 min/week or more of moderate- or vigorous-intensity physical activity; 4) total cholesterol less than 200 mg/dL; 5) BP less than 120/80 mm Hg; 7) fasting glucose less than 100 mg/dL; and 6) diet: 4 or 5 healthy dietary components as defined next (11). The ideal dietary metric was determined based on the intake of the following 5 healthy dietary components: fruits and vegetables (≥ 450 g/day), fish (≥ 198 g/week), fiber-rich whole grains (≥ 85 g/day), sodium (< 1500 mg/day), and sugar-sweetened beverages (≤ 1 L/week). To calculate the ideal CVH score, each ideal CVH metric was considered as 1 point, and the number of ideal CVH metrics was added for each participant (range, 0-7 points). However, diet information was available in only a fraction of the participants (n = 1430, 56.3%); thus, the CVH score without diet metrics was used, ranging from 0 to 6 points, and was divided into 3 groups of poor (0-2), intermediate (3-4), and ideal (5-6) CVH (reference).
The VMSs included hot flashes and night sweats. To determine the presence and degree of VMSs, the validated Korean version of the Menopause-Specific Quality of Life questionnaire was used at baseline (29, 30). Participants indicated whether they had experienced VMSs during the past month and described how bothersome the symptoms were on a 7-point Likert scale, from “not bothered at all” (0) to “extremely bothersome” (6) (29, 31). For statistical analysis, the raw scores of VMS intensity were recoded to an 8-point grading system, including zero: the answer “No” was recoded as zero, and “Yes, but not bothered at all” was converted to one. The degree of increase in VMS severity, ranging from 1 to 6, was rescored from 2 to 7. If the participant responded “No” to hot flashes or night sweats, we considered that the participant did not have VMSs. Women who answered “Yes” and experienced hot flashes or night sweats were considered to have VMSs. Furthermore, we considered women with one or two recoded points as having mild VMSs and those with 3 or more recoded points as having moderate/severe VMSs. Early-onset VMS was defined as the occurrence of VMSs before menopause.
Statistical Analysis
The characteristics of the study population were presented across the CVH metric categories using descriptive statistics. Incident early-onset of VMSs that occurred before women reached menopause was the primary outcome of the present study. Person-years of follow-up were calculated from the baseline visit to the time of VMSs occurrence, the time of menopause, or the last time the questionnaire survey was completed, whichever came first. Even though VMSs occurrence was known to have occurred between 2 visits (visit with the first report of VMSs and the previous visit), the precise time at which it developed was unknown. Thus, a parametric proportional hazards model was used to account for interval censoring (stpm command in Stata) (32) and estimate hazard ratios (HRs) with 95% CIs for incident early-onset VMSs according to groups of poor, intermediate, and ideal CVH metrics. Furthermore, we conducted a sensitivity analysis using moderate/severe VMSs as a secondary outcome. Potential confounders included age at baseline, parity, education level, and alcohol intake. For the linear trend test, the number of each category was included as a continuous variable in the model. All statistical analyses were performed using Stata version 17.0 (Stata Corp LP) and R version 4.1.0 (R Foundation for Statistical Computing). Statistical significance was defined as a 2-sided P value of less than .05.
Results
Table 1 presents the demographic and clinical characteristics of the 2541 study participants without VMSs at baseline according to the CVH metric categories. The mean age was 44.6 ± 2.3 years. The mean BMI, systolic BP, and diastolic BP were 22.3 ± 2.9, 103.0 ± 11.0, and 66.2 ± 8.6 mm Hg, respectively. The proportions of poor, intermediate, and ideal CVH groups were 3.8%, 39.9%, and 56.3%, respectively. Women who had higher ideal CVH metric score were on average younger; had lower BMI, BPs, total cholesterol, and fasting glucose levels; and were more likely to have higher education levels.
Table 1.
Demographic and clinical characteristics of the study population
| Characteristics | Overall (n = 2541) | CVH metrics score | ||
|---|---|---|---|---|
| Poor (0-2) | Intermediate (3-4) | Ideal (5-6) | ||
| (n = 96) | (n = 1015) | (n = 1430) | ||
| Age, y | 44.6 ± 2.33 | 45.29 ± 2.59 | 44.83 ± 2.37 | 44.44 ± 2.27 |
| BMI | 22.28 ± 2.94 | 26.08 ± 3.04 | 23.62 ± 3.1 | 21.08 ± 2.05 |
| Age at menarche, y | 13.89 ± 1.38 | 13.59 ± 1.4 | 13.85 ± 1.39 | 13.94 ± 1.37 |
| Parity, % | 2262 (89.0) | 83 (86.5) | 905 (89.2) | 1274 (89.1) |
| Higher education, % | 2058 (81.0) | 67 (69.8) | 810 (79.8) | 1181 (82.6) |
| High alcohol intakea, % | 270 (10.6) | 17 (17.7) | 137 (13.5) | 116 (8.1) |
| Current smokers, % | 33 (1.3) | 7 (7.3) | 21 (2.1) | 5 (0.4) |
| High physical activity, % | 1240 (48.8) | 19 (19.8) | 287 (28.3) | 934 (65.3) |
| Diabetes, % | 44 (1.7) | 7 (7.3) | 30 (3.0) | 7 (0.5) |
| Hypertension, % | 95 (3.8) | 23 (24.0) | 52 (5.1) | 20 (1.4) |
| SBP, mm Hg | 103.02 ± 11.03 | 121.98 ± 14.33 | 105.75 ± 11.49 | 99.81 ± 8.32 |
| DBP, mm Hg | 66.19 ± 8.62 | 78.95 ± 10.09 | 68.02 ± 8.94 | 64.03 ± 7.11 |
| Fasting glucose, mg/dL | 92.64 ± 11.24 | 110.35 ± 20.99 | 94.85 ± 13.22 | 89.88 ± 6.26 |
| Total cholesterol, mg/dL | 191.24 ± 30.38 | 216.92 ± 29.97 | 202.95 ± 30.38 | 181.2 ± 26.15 |
| LDL-C mg/dL | 117.94 ± 28.42 | 144.75 ± 31.62 | 128.94 ± 28.56 | 108.34 ± 23.75 |
| HDL-C, mg/dL | 67.35 ± 15.91 | 60.41 ± 17.07 | 66.16 ± 16.28 | 68.66 ± 15.39 |
| Triglycerides, mg/dL | 73 (56-97) | 113 (85-151] | 83 (64-111] | 65 (52-86) |
| hs-CRP, mg/L | 0.03 (0.02-0.06) | 0.08 (0.04-0.15) | 0.04 (0.02-0.07) | 0.03 (0.02-0.05) |
Data are presented as means ± SD, medians (interquartile range), or numbers (percentages).
Abbreviations: BMI, body mass index; CVH, cardiovascular health; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
a High alcohol intake greater than or equal to 10 g/day.
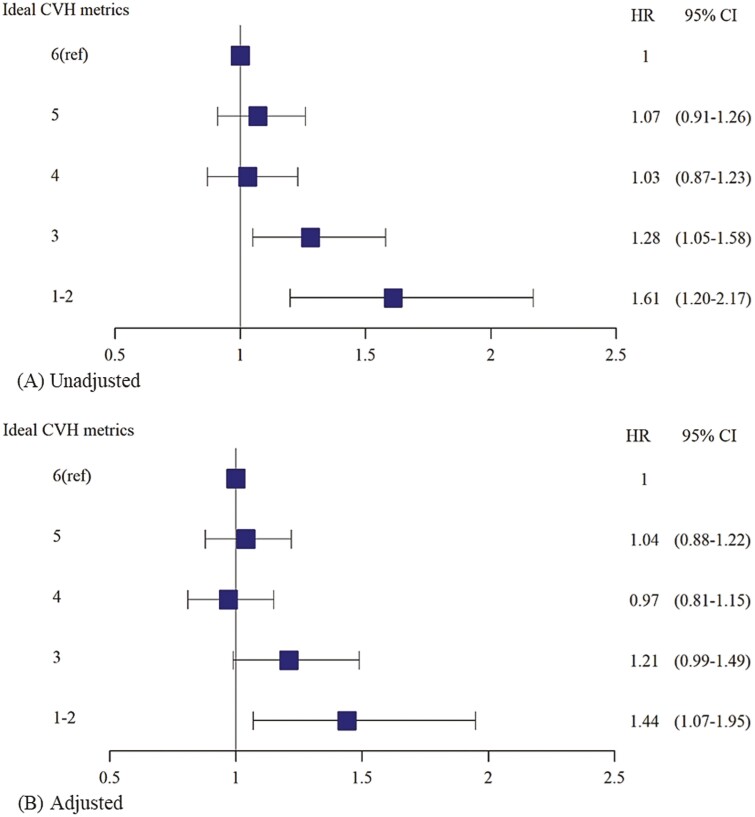
Table 2 shows the longitudinal association of CVH metrics with the incidence of early-onset VMSs among premenopausal women without prevalent VMSs at baseline. During 11 201.7 person-years of the follow-up period, 1241 women developed incident early-onset VMSs (incidence rate, 11.1 per 100 person-years). The median follow-up duration was 4.5 years (interquartile range, 3.4-5.6 years). Compared to women with the ideal CVH group, those with intermediate and poor CVH had unadjusted HRs for early-onset VMSs, which were 1.06 (95% CI, 0.94-1.19) and 1.54 (95% CI, 1.17-2.03), respectively (P for trend = .021). After adjusting for age, parity, education levels, and alcohol intake, the HRs (95% CIs) for developing early-onset VMSs were 1.01 (0.90-1.14) and 1.41 (1.07-1.86) in participants with intermediate and poor CVH, respectively, compared with the ideal group (P for trend = .166). In the sensitivity analysis using ideal CVH metrics of 6 points as a reference, the results were similar (Fig. 1). In sensitivity analysis using each VMS component as an outcome, women with poor CVH had significant adjusted HRs (95% CIs) of 1.65 (1.21-2.24) for developing hot flashes symptoms and 1.35 (1.02-1.80) for incident night sweats symptoms, compared to those with ideal CVH metrics (Supplementary Table 1) (25).
Table 2.
Longitudinal association between cardiovascular health metrics and early-onset vasomotor symptoms in premenopausal women
| PY | Early-onset VMS | Incidence rate | Unadjusted | Age-adjusted | Multivariable-adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|
| (cases per 100 PY) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| CVH metrics | |||||||||
| Ideal (5-6) | 6352.4 | 682 | 10.7 | Ref | Ref | Ref | |||
| Intermediate (3-4) | 4452.3 | 502 | 11.3 | 1.06 | (0.94-1.19) | 1.02 | (0.91-1.15) | 1.01 | (0.90-1.14) |
| Poor (0-2) | 396.96 | 57 | 14.4 | 1.54 | (1.17-2.03) | 1.41 | (1.07-1.86) | 1.41 | (1.07-1.86) |
| P for trend | .021 | .135 | .166 |
Multivariable-adjusted for age, parity, education levels, and amount of alcohol intake.
Abbreviations: CVH, cardiovascular health; HR, hazard ratio; PY, person-year; Ref, reference; VMS, vasomotor symptom.
Figure 1.
Hazard ratios (95% CI) for developing early-onset vasomotor symptoms among premenopausal women in A, unadjusted, and B, adjusted models.
Table 3 shows the sensitivity analyses using moderate/severe VMSs as end points. Compared with a group of ideal CVH, women with intermediate and poor CVH had significant unadjusted HRs (95% CIs) of 1.28 (1.08-1.51) and 1.79 (1.24-2.60) for developing moderate/severe VMSs, respectively (P for trend < .001). After adjustment for confounders, the multivariable-adjusted HRs (95% CIs) for incident moderate/severe VMSs were 1.20 (1.02-1.43) and 1.57 (1.08-2.29) in premenopausal women with the groups of intermediate and poor CVH, respectively, compared with the reference group. There were significant trends in ideal CVH metric scores with moderate/severe and early-onset VMSs (P for trend = .004). The Kaplan-Meier curves for early-onset and moderate/severe VMSs by CVH metrics groups are presented in Supplementary Fig. 2 (25). Women with poor CVH metrics at baseline were likely to have early-onset and moderate/severe VMSs sooner over time than those with ideal CVH metrics. In the sensitivity analysis without restriction prior to menopause during follow-up, while including women who reached menopause during follow-up, the results were similar (Supplementary Table 2) (25).
Table 3.
Longitudinal association between cardiovascular health metrics and early-onset of moderate/severe vasomotor symptoms in premenopausal women
| PY | Early-onset VMS | Incidence rate | Unadjusted | Age-adjusted | Multivariable-adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|
| (cases per 100 PY) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| CVH metrics | |||||||||
| Ideal (5-6) | 6660.18 | 288 | 4.3 | Ref | Ref | Ref | |||
| Intermediate (3-4) | 4657.29 | 254 | 5.5 | 1.28 | (1.08-1.51) | 1.22 | (1.03-1.44) | 1.20 | (1.02-1.43) |
| Poor (0-2) | 425.05 | 31 | 7.3 | 1.79 | (1.24-2.60) | 1.60 | (1.10-2.32) | 1.57 | (1.08-2.29) |
| P for trend | < .001 | .003 | .004 |
Multivariable-adjusted for age, parity, education levels, and amount of alcohol intake.
Abbreviations: CVH, cardiovascular health; HR, hazard ratio; PY, person-year; Ref, reference; VMS, vasomotor symptom.
In addition, we investigated the association between each component of the CVH metrics and incident early-onset VMSs (Table 4). After multivariable adjustment for potential confounders, nonsmoking status, normal BMI, and normal BP were significantly and independently associated with lower risks of early-onset VMSs. In contrast, normal fasting glucose concentrations tended to be associated with a lower risk of VMS, but this association was not statistically significant. However, moderate or high physical activity was positively associated with the risk of early-onset VMSs.
Table 4.
Longitudinal association between each component of ideal cardiovascular health metrics and early-onset vasomotor symptoms in premenopausal women
| CVH metrics | PY | Early-onset VMS | Incidence rate | Age-adjusted | Multivariable-adjusted |
|---|---|---|---|---|---|
| (cases per 100 PY) | HR (95% CI) | HR (95% CI) | |||
| Current smoking | |||||
| Yes | 137.62 | 19 | 13.8 | Ref | Ref |
| No | 11 064.04 | 1222 | 11.0 | 0.60 (0.38-0.95) | 0.89 (0.37-0.94) |
| Physical activity | |||||
| Low | 5799.54 | 608 | 10.5 | Ref | Ref |
| Moderate/high | 5402.12 | 633 | 11.7 |
1.15 (1.03-1.28) |
1.15 (1.03-1.29) |
| Body mass index | |||||
| ≥ 23 | 3696.87 | 472 | 12.8 | Ref | Ref |
| < 23 | 7504.79 | 769 | 10.3 |
0.79 (0.71-0.89) |
0.78 (0.65-0.93) |
| 90th vs 10th percentile |
0.75 (0.58-0.98) |
0.76 (0.89-0.98) |
|||
| Total cholesterol, mg/dL | |||||
| ≥ 200 | 4089.51 | 461 | 11.3 | Ref | Ref |
| < 200 | 7112.15 | 780 | 11.0 |
1.00 (0.89-1.12) |
0.98 (0.88-1.11) |
| 90th vs 10th percentile |
0.90 (0.70-1.16) |
0.90 (0.70-1.15) |
|||
| Blood pressure, mm Hg | |||||
| ≥ 120/80 | 1037.76 | 136 | 13.1 | Ref | Ref |
| < 120/80 | 10163.9 | 1105 | 10.9 |
0.77 (0.64-0.92) |
0.78 (0.65-0.93) |
| 90th vs 10th percentile |
0.72 (0.56-0.92) |
0.73 (0.70-1.15) |
|||
| Fasting glucose, mg/dL | |||||
| ≥ 100 | 1469.09 | 187 | 12.7 | Ref | Ref |
| < 100 | 9732.57 | 1054 | 10.8 |
0.84 (0.72-0.99) |
0.86 (0.73-1.01) |
| 90th vs 10th percentile |
0.79 (0.62-1.01) |
0.80 (0.63-1.02) |
Multivariable-adjusted for age, parity, education levels, and amount of alcohol intake.
Abbreviations: CVH, cardiovascular health; HR, hazard ratio; PY, person-year; Ref, reference; VMS, vasomotor symptom.
Discussion
In the present cohort study of premenopausal women, women with unfavorable CVH behaviors, indicated by lower scores on ideal CVH metrics, were likely to have a higher risk of developing early-onset VMSs than those with high levels of ideal CVH metrics. The inverse relationships of ideal CVH metrics with moderate/severe and early-onset VMSs were slightly stronger than the results without consideration of severity. Of the CVH metric components, nonsmoking status, normal BMI status, and normal BP were significantly associated with decreased risks of developing early-onset VMSs; a similar tendency was also observed for ideal fasting glucose levels but did not reach statistical significance. In our study, the association between CVH metrics and incident VMS was significant but modest regarding the effect size. However, given the high prevalence of VMS, reportedly up to 70% (2), the implication of CVH on VMS risk can be important at the population level and CVH is also easily applicable in clinical practice settings, potentially an acceptable preventive measure for VMS among middle-aged women even in the premenopausal stage. Furthermore, given that hormone therapy is the main treatment modality for VMSs with no other approved therapies, our findings suggest a possible role of adherence to ideal CVH metrics as a preventive measure to reduce VMSs in premenopausal women.
Despite increasing evidence supporting a relationship between early-onset VMSs and CVD risk factors, CVD events, and related mortality (22, 33-36), no study has investigated the effect of ideal CVH metrics on preventing early-onset VMSs among premenopausal women. A cross-sectional study conducted on 5857 postmenopausal women without CVD found that women with VMSs were more likely to have adverse CVD risk factors, including abnormal lipid levels, high BP, and high BMI (37). In another previous study, pooled individual-level data from 23 365 middle-aged women in 6 prospective studies investigated the relationships between the frequency and severity of VMSs and the risk of incident CVD. Severe VMSs, rather than frequent VMSs, were significantly associated with an increased risk of CVD (5). Regarding the timing of incident VMSs, early- or late-onset VMSs were more likely to have a higher risk of CVD. A community-based prospective study using data from the Framingham Heart Study investigated whether unfavorable CVH metrics affect the progression of coronary artery calcification, which is a major risk factor for CVD among middle-aged women with low baseline CVD risk (38). This study found that, as the number of ideal CVH metrics components decreased, the risk of coronary artery calcification progression increased.
Furthermore, we found that each favorable CVH component, including nonsmoking status, normal BMI and BPs, and ideal fasting glucose levels, had a protective role in reducing the risks of early-onset VMSs, which is in line with previous reports (39-42). According to an individual-level pooled study of 8 observational studies among middle-aged women, current smokers with obesity (BMI ≥ 25) had significantly higher risks of frequent/severe VMSs than never smokers with normal weight (39). Cross-sectional results, using data from the US Study of Women’s Health Across the Nation, revealed that higher BP was significantly associated with more frequent VMSs at baseline compared to normal BP among middle-aged women (41). Another population-based study conducted on 4895 women aged 45 to 50 years reported that diabetes was significantly related to early severe VMSs (42). On the other hand, unexpectedly, in our study, women with ideal physical activity were likely to have increased risks of early-onset VMSs compared to those with low physical activity. However, the reason for this finding remains unclear. Previous studies on the association between physical activity and VMSs have reported mixed results (43, 44). Physical activity may increase body core temperature and thus stimulate more VMSs, while a protective role of physical activity has also been proposed given its beneficial effects, including neuroendocrine (eg, endorphin, serotonin), body composition, thermoregulation, and psychological effects (45-48). In a randomized controlled trial using the intervention of physical activity in 121 middle-aged women during a 2-week period, the acute exercise bout decreased hot flashes based on subjective (self-report) and objective measures using 24-hour Biolog sternal skin conductance recordings (48). In that study, daily physical activity did not affect VMSs at the between-person level; however, at the within-person level, performing moderate- to high-intensity exercise was associated with increased reporting of VMSs, especially among women with low fitness levels (48). On the other hand, a cross-sectional study, conducted on 1113 women with information on menopause-related symptoms, reported that self-reported physical activity levels were significantly and inversely correlated with VMSs (44). Thus, the association between physical activity and VMS can differ depending on the fitness level of women. Further studies with detailed information on the type of physical activity and fitness level can help us better understand the association between physical activity and VMS.
Our study had several limitations. CVH metric components, including smoking status, physical activity, alcohol intake, and VMSs, were assessed using self-reported questionnaires, which may lead to misclassification. Of the CVH metrics, dietary components were not included because of their availability in only a fraction of participants and no assessment for whole-grain intake, which is equivalent to brown rice in Korea, but is not included in the food frequency questionnaire used in our study. Additionally, there remains the possibility of residual confounding due to unmeasured confounders. Finally, since our study cohort comprised relatively healthy middle-aged Korean women who had low proportion of poor CVH group members (3.8%), our results may not be generalizable to other populations of different ethnicity and higher prevalence of comorbidities. Nonetheless, this is the first study to suggest that achieving ideal CVH metrics might prevent early-onset VMSs based on longitudinal cohort data. We also evaluated the bothersome degree of VMSs using questionnaires and presented a significant association of favorable CVH behavior with low risks of incident moderate/severe VMSs. Furthermore, our study had a prospective design, a large sample size of a well-characterized population of premenopausal women, and the use of carefully standardized clinical, lifestyle, and laboratory measures, which allowed us to account for the consideration of multiple potential confounders.
Conclusion
In this cohort study of Korean premenopausal women, unfavorable CVH behaviors were significantly associated with an increased risk of early-onset VMSs. This association is more pronounced in the development of moderate/severe VMSs. Further research is needed to establish whether promoting ideal CVH metrics, a feasible and effective measure, could help prevent VMSs and CVD events in middle-aged women of diverse ethnicities.
Glossary
Abbreviations
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- CVH
cardiovascular health
- HR
hazard ratio
- VMS
vasomotor symptom
Contributor Information
Hye Rin Choi, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Institute of Medical Research, School of Medicine, Sungkyunkwan University, Suwon, 16419, Republic of Korea.
Yoosoo Chang, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 03181, Republic of Korea; Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul 06355, Republic of Korea.
Yejin Kim, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea.
Yoosun Cho, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea.
Jeonggyu Kang, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea.
Min-Jung Kwon, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Department of Laboratory Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea.
Ria Kwon, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Institute of Medical Research, School of Medicine, Sungkyunkwan University, Suwon, 16419, Republic of Korea.
Ga-Young Lim, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Institute of Medical Research, School of Medicine, Sungkyunkwan University, Suwon, 16419, Republic of Korea.
Kye-Hyun Kim, Department of Obstetrics and Gynecology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 03181, Republic of Korea.
Hoon Kim, Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul 03080, Republic of Korea.
Yun Soo Hong, Departments of Epidemiology and Medicine, and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA.
Jihwan Park, Departments of Epidemiology and Medicine, and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA.
Di Zhao, Departments of Epidemiology and Medicine, and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA.
Juhee Cho, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul 06355, Republic of Korea; Departments of Epidemiology and Medicine, and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA.
Eliseo Guallar, Departments of Epidemiology and Medicine, and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA.
Hyun-Young Park, Department of Precision Medicine, National Institute of Health, Korea Disease Control and Prevention Agency, Cheongju 28159, Republic of Korea.
Seungho Ryu, Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 04514, Republic of Korea; Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 03181, Republic of Korea; Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul 06355, Republic of Korea.
Financial Support
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (Nos. 2014ER630200, 2017ER630200, 2020ER710200, 2020ER710201, and 2020ER710202) and by the SKKU Excellence in Research Award Research Fund, Sungkyunkwan University, 2020.
Author Contributions
Y. Chang and S. Ryu planned, designed, and directed this study, including quality assurance and control. H.R. Choi planned the study concept, analyzed the data, designed the analytic strategy, and drafted the manuscript. Y. Kim, R. Kwon, and G. Lim collected the data and conducted a literature review. J. Kang, M. J. Kwon, Y. Cho, K. H. Kim, H. Kim, J. Cho, Y. Hong, J. Park, Zhao Di, H. Y. Park, and E. Guallar interpreted the results and conducted critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation. Am J Public Health. 2006;96(7):1226-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sayan S, Pekin T, Yıldızhan B. Relationship between vasomotor symptoms and metabolic syndrome in postmenopausal women. J Int Med Res. 2018;46(10):4157-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avis NE, Crawford SL, Greendale G, et al. ; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu D, Chung HF, Dobson AJ, et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: a pooled analysis of six prospective studies. Am J Obstet Gynecol. 2020;223(6):898.e1-898.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muka T, Oliver-Williams C, Colpani V, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gast GCM, Grobbee DE, Pop VJM, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51(6):1492-1498. [DOI] [PubMed] [Google Scholar]
- 8. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119(4):753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e 360. [DOI] [PubMed] [Google Scholar]
- 11. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586-613. [DOI] [PubMed] [Google Scholar]
- 12. Lachman S, Peters RJ, Lentjes MA, et al. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2016;23(9):986-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol. 2017;40(12):1339-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol. 2016;214:279-283. [DOI] [PubMed] [Google Scholar]
- 15. Kim JY, Ko YJ, Rhee CW, et al. Cardiovascular health metrics and all-cause and cardiovascular disease mortality among middle-aged men in Korea: the Seoul Male Cohort Study. J Prev Med Public Health. 2013;46(6):319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang Y, Ngandu T, Laatikainen T, et al. Cardiovascular health metrics from mid-to late-life and risk of dementia: a population-based cohort study in Finland. PLoS Med. 2020;17(12):e1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Yao Y, Wang Y, et al. Ideal cardiovascular health metrics and the risk of non-alcoholic fatty liver disease: a cross-sectional study in northern China. Liver Int. 2019;39(5):950-955. [DOI] [PubMed] [Google Scholar]
- 18. Shpilsky D, Bambs C, Kip K, et al. Association between ideal cardiovascular health and markers of subclinical cardiovascular disease. Clin Cardiol. 2018;41(12):1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart. 2016;102(11):825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agrinier N, Cournot M, Dallongeville J, et al. Menopause and modifiable coronary heart disease risk factors: a population based study. Maturitas. 2010;65(3):237-243. [DOI] [PubMed] [Google Scholar]
- 21. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99(9):1165-1172. [DOI] [PubMed] [Google Scholar]
- 22. Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767-776. [DOI] [PubMed] [Google Scholar]
- 23. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801-1811. [DOI] [PubMed] [Google Scholar]
- 24. Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306(5):H628-H640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi HR, Chang Y, Kim Y, et al. Supplementary data for “Ideal cardiovascular health metrics and risk of incident early-onset vasomotor symptoms among premenopausal women.” figshare. 2022. Accessed on April 22, 2022. 10.6084/m9.figshare.19634355.v2. [DOI]
- 26. Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. [DOI] [PubMed] [Google Scholar]
- 27. Ahn Y, Kwon E, Shim JE, et al. Validation and reproducibility of food frequency questionnaire for Korean Genome Epidemiologic Study. Eur J Clin Nutr. 2007;61(12):1435-1441. [DOI] [PubMed] [Google Scholar]
- 28. Korean Nutrition Society. Food Value: Nutrient Composition Table for Foods. Korean Nutrition Society; 2006. [Google Scholar]
- 29. Park JH, Bae SH, Jung YM. Validity and reliability of the Korean version of the Menopause-Specific Quality of Life [article in Korean]. J Korean Acad Nurs. 2020;50(3):487-500. [DOI] [PubMed] [Google Scholar]
- 30. Sydora BC, Fast H, Campbell S, Yuksel N, Lewis JE, Ross S. Use of the Menopause-Specific Quality of Life (MENQOL) questionnaire in research and clinical practice: a comprehensive scoping review. Menopause. 2016;23(9):1038-1051. [DOI] [PubMed] [Google Scholar]
- 31. Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24(3):161-175. [DOI] [PubMed] [Google Scholar]
- 32. Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175-2197. [DOI] [PubMed] [Google Scholar]
- 33. Simon T. Why is cardiovascular health important in menopausal women? Climacteric. 2006;9(Suppl 1):13-18. [DOI] [PubMed] [Google Scholar]
- 34. Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. 2018;21(2):96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thurston RC, Aslanidou Vlachos HE, Derby CA, et al. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc. 2021;10(3):e017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gast GCM, Samsioe GN, Grobbee DE, Nilsson PM, van der Schouw YT. Vasomotor symptoms, estradiol levels and cardiovascular risk profile in women. Maturitas. 2010;66(3):285-290. [DOI] [PubMed] [Google Scholar]
- 38. Hwang SJ, Onuma O, Massaro JM, et al. Maintenance of ideal cardiovascular health and coronary artery calcium progression in low-risk men and women in the Framingham Heart Study. Circ Cardiovasc Imaging. 2018;11(1):e006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson DJ, Chung HF, Seib CA, et al. Obesity, smoking, and risk of vasomotor menopausal symptoms: a pooled analysis of eight cohort studies. Am J Obstet Gynecol. 2020;222(5):478.e1-478.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159(12):1189-1199. [DOI] [PubMed] [Google Scholar]
- 41. Jackson EA, El Khoudary SR, Crawford SL, et al. Hot flash frequency and blood pressure: data from the Study of Women’s Health Across the Nation. J Womens Health (Larchmt). 2016;25(12):1204-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012;97(10):3487-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2011(5):CD006108. [DOI] [PubMed] [Google Scholar]
- 44. El Hajj A, Wardy N, Haidar S, et al. Menopausal symptoms, physical activity level and quality of life of women living in the Mediterranean region. PLoS One. 2020;15(3):e0230515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851-1861. [DOI] [PubMed] [Google Scholar]
- 46. Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137(1):1-13. [DOI] [PubMed] [Google Scholar]
- 47. Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc. 1997;29(1):58-62. [DOI] [PubMed] [Google Scholar]
- 48. Elavsky S, Gonzales JU, Proctor DN, Williams N, Henderson VW. Effects of physical activity on vasomotor symptoms: examination using objective and subjective measures. Menopause. 2012;19(10):1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.