Abstract
Objective
To assess whether, in type 2 diabetes (T2D) patients, lipidomic abnormalities in high-density lipoprotein (HDL) are associated with impaired cholesterol efflux capacity and anti-inflammatory effect, 2 pro-atherogenic abnormalities.
Design and Methods
This is a secondary analysis of the Lira-NAFLD study, including 20 T2D patients at T0 and 25 control subjects. Using liquid chromatography/tandem mass spectrometry, we quantified 110 species of the main HDL phospholipids and sphingolipids. Cholesterol efflux capacity was measured on THP-1 macrophages. The anti-inflammatory effect of HDL was measured as their ability to inhibit the tumor necrosis factor α (TNFα)-induced expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1) on human vascular endothelial cells (HUVECs).
Results
The cholesterol-to-triglyceride ratio was decreased in HDL from T2D patients compared with controls (-46%, P = 0.00008). As expressed relative to apolipoprotein AI, the amounts of phosphatidylcholines, sphingomyelins, and sphingosine-1-phosphate were similar in HDL from T2D patients and controls. Phosphatidylethanolamine-based plasmalogens and ceramides (Cer) were, respectively, 27% (P = 0.038) and 24% (P = 0.053) lower in HDL from T2D patients than in HDL from controls, whereas phosphatidylethanolamines were 41% higher (P = 0.026). Cholesterol efflux capacity of apoB-depleted plasma was similar in T2D patients and controls (36.2 ± 4.3 vs 35.5 ± 2.8%, P = 0.59). The ability of HDL to inhibit the TNFα-induced expression of both VCAM-1 and ICAM-1 at the surface of HUVECs was similar in T2D patients and controls (-70.6 ± 16.5 vs -63.5 ± 18.7%, P = 0.14; and -62.1 ± 13.2 vs -54.7 ± 17.7%, P = 0.16, respectively).
Conclusion
Despite lipidomic abnormalities, the cholesterol efflux and anti-inflammatory capacities of HDL are preserved in T2D patients.
Keywords: HDL, lipidomics, anti-inflammatory effect, cholesterol efflux, type 2 diabetes
The dyslipidemia that is observed in type 2 diabetes (T2D) patients plays a major role in the frequent and early development of atherosclerotic lesions. Both quantitative and qualitative abnormalities in high-density lipoprotein (HDL) are key features of T2D dyslipidemia (1). The concentration of circulating HDL cholesterol is decreased, and HDL particles are triglyceride enriched. In addition, it appears that the sphingophospholipidome of HDL in T2D patients is altered, as reported by Ståhlman et al (2), but data in this field are scarce.
HDL functionality is an important emerging concept. Over the past few years, research has demonstrated that the ability of HDL to promote cholesterol efflux is more strongly inversely associated with incident cardiovascular events than circulating HDL cholesterol level (3, 4). Very recently, in a general population cohort, similar results were reported for the anti-inflammatory capacity of HDL, measured as the ability of HDL to counteract the tumor necrosis factor α (TNFα)-induced expression of vascular cell adhesion molecule 1 (VCAM-1) messenger RNA in human umbilical vascular endothelial cells (HUVECs) (5). The inverse association between the anti-inflammatory capacity of HDL and incident cardiovascular events was independent of both HDL cholesterol concentration and cholesterol efflux capacity (CEC).
The anti-inflammatory effect on endothelial cells implies the binding of HDL to scavenger receptor type BI (SR-BI) and sphingosine-1-phosphate (S1P) receptors, by apolipoprotein (apo) AI and S1P, respectively (6-8). Both in vitro glycoxidation and the increase in triglyceride-to-cholesteryl ester ratio modify apoAI conformation at the surface of HDL, which likely impairs its binding to SR-BI (9, 10). On the other hand, sphingo- and phospholipids play a major role in HDL functions, either by binding to a specific receptor such as S1P, or by modulating the physicochemical properties of HDL (11, 12). Experiments with reconstituted HDL or in vitro enrichment of native HDL with some phospholipid species suggest that changes in the sphingophospholipidome of HDL can influence its anti-inflammatory effect or CEC (13, 14).
The studies that measured HDL CEC in T2D patients reported very contrasted results, varying from decreased to increased, and the reasons for these discrepancies are not entirely elucidated. In particular, most of these studies lack detailed data on HDL composition, even triglyceride and cholesteryl ester content (15-20). As far as HDL anti-inflammatory effect is concerned, studies are scarce, and no data about HDL lipidomics have been reported simultaneously (21-23).
The purpose of this study was thus to measure HDL CEC on macrophages and the ability of HDL to counteract the TNFα-induced expression of adhesion molecules on HUVECs, in T2D patients with well-characterized HDL composition, including the detailed composition of the sphingophospholipidome. We aimed to clarify the extent to which HDL lipid composition abnormalities impair these 2 functions.
Patients and Methods
Subjects
The present work is a secondary analysis of the Lira-NAFLD study (24, 25) (amendment of the clinical trial reg. no. NCT02721888, clinicaltrials.gov). This single-center study was carried out in the University Hospital of Dijon. It was approved by our ethics committee. Twenty T2D patients at T0 of the Lira-NAFLD study and 25 healthy controls were included; written informed consent was obtained for all participants. All the subjects included in the trial between April 2016 (date of the obtention of the amendment) and October 2019 participated, without any additional selection.
The T2D patients were treated with metformin and/or sulfonylurea and/or insulin. Exclusion criteria for T2D patients were hepatic impairment (International Normalized Ratio of ≥ 1.5, or aspartate amino transferase or alanine aminotransferase levels > 3 times the upper limit of the normal reference range) or renal function impairment with glomerular filtration rate < 60 mL/min/1.73 m2. Control subjects were included if they had a body mass index ≤ 28 kg/m2, fasting glycemia < 6.1 mmol/L, triglyceridemia < 1.7 mmol/L, and serum HDL-cholesterol > 1.30 for females and > 1.03 mmol/L for males. Exclusion criteria for controls were liver or kidney diseases and the use of antidiabetic agents. Exclusion criteria for both T2D patients and controls were thyroid disease, alcohol and/or drug abuse, and medications known to affect lipid metabolism (except antidiabetic agents in T2D patients).
Blood was collected in the fasting state in BD Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) with EDTA as the anticoagulant and preservative. The plasma was immediately separated by centrifugation and frozen at -80°C until lipid extraction and analysis.
Outcome Measures
Experiments were performed in the laboratory of INSERM LNC UMR1231 in Dijon, France.
The primary outcome was CEC. The secondary outcomes were the percentage of inhibition of TNFα-induced expression of VCAM-1 and ICAM-1 by HDL, and HDL composition parameters.
Routine Analytical Procedures
Plasma concentrations of glucose, total cholesterol, HDL-cholesterol, triglycerides, apoAI, and total protein were measured on a Dimension Vista analyzer using dedicated reagents (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Free cholesterol was measured on the same analyzer but using reagents from Diasys (Condom, France). Low-density lipoprotein (LDL) cholesterolemia was estimated by the Friedewald equation because triglyceridemia was below 3.88 mmol/L in each subject. Esterified cholesterol in each HDL fraction was calculated as the difference between total and free cholesterol. HbA1c was measured with a G8 HPLC analyzer (Tosoh Bioscience, Tokyo, Japan).
HDL Composition and Lipidomics
HDL isolation
The HDL fraction (density = 1.063-1.210 g/mL) was isolated from 2 mL of plasma by sequential flotation ultracentrifugation at 4°C using a 50.4 rotor in an Optima L80-XP ultracentrifuge (Beckman Coulter, Brea, CA, USA) as previously reported (26). The HDL fraction was extensively dialyzed 3 times against sterilized 0.01% (m/v) EDTA endotoxin-free phosphate-buffered saline for 18 hours at 4°C in the dark, filtered through a 0.22-µm PVDF filter, and immediately used for analysis. From 100 µL of plasma with density adjusted to 1.129 g/mL with KBr (final volume 200 µL), we separated 2 fractions by ultracentrifugation using a TL100.2 rotor in a TL100 ultracentrifuge (Beckman Coulter): the fraction with density < 1.129 g/mL contained HDL2b, 2a, and 3a, and the fraction with density > 1.129 g/mL contained HDL 3b, 3c, and preβ HDL (27). Each fraction was recovered in 100 µL and apoAI was quantified.
Lipid analysis by liquid chromatography coupled with tandem mass spectrometry
Glycerophospholipids and sphingomyelins were extracted from HDL fraction according to the Bligh-Dyer method, whereas S1P and Cer were extracted using the Bielawski method (28, 29) (detailed procedure in Supplemental materials (30)).
The reconstituted extracts were finally injected on a 6460 liquid chromatography-electrospray ionization-tandem mass spectrometry system (Agilent Technologies, Santa Clara, CA, USA), and analyzed according to the methods previously used by our team (31). Lipids were detected using multiple reaction monitoring, and were quantified using a 7-point calibration curve with external standards (34:0 phosphatidylcholine [PC], 19:0 lysophosphatidylcholine [LPC], d18:1/17:0 sphingomyelin [SM], 18:0 lysophosphatidylethanolamine [LPE], 34:0 phosphatidylethanolamine [PE], p18:0/18:1 PE-based plasmalogen, 38:4 phosphatidylinositol [PI], d18:1/20:0 Cer, d18:1/22:0 Cer, d18:1/24:0 Cer, d18:1/24:1 Cer, d18:1 S1P). Linear regressions were applied to calculate lipid concentrations. All internal and external standards were purchased from Avanti Polar Lipids (Alabaster, AL, USA), and solvents were liquid chromatography-mass spectrometry grade quality. For each phospho- or sphingolipid class, we summed the different species belonging to this class.
HDL Ability to Inhibit VCAM-1 and ICAM-1 Expression in HUVECs
We used a protocol adapted from Ashby et al (32). Briefly, HUVECs were isolated from human umbilical cords and cultured in supplemented EBM medium at 37°C 5% CO2. HDL fraction (800 µg/mL apoAI) was added 16 hours before the addition of recombinant TNFα. After 4 hours of incubation at 37°C (5% CO2), cells were trypsinized. Half of the cells were incubated with a mouse anti-VCAM-1 antibody (BD Biosciences, RRID: AB_396003) and the other half with a mouse anti-ICAM-1 antibody (BD Biosciences, RRID: AB_395901). Both antibodies were phycoerythrin-labeled, and fluorescence was quantified by the LSR Fortessa flow cytometer (BD Biosciences). The reference value was obtained by incubating TNFα with phosphate-buffered saline instead of HDL fraction. For each subject, HDL were incubated with 3 different cords and median fluorescence intensities were averaged (detailed procedure in Supplemental materials (30)).
Cholesterol Efflux Capacity
The capacity of apoB-depleted plasma samples to support cholesterol efflux was analyzed using the human monocyte cell line THP-1 (Sigma-Aldrich, Saint-Louis, MO, USA). THP-1 macrophages in which ABCA1 or ABCG1 were stably silenced (THP-1 ΔABCA1 and THP-1 Δ ABCG1) were kindly given by Wilfried Le Goff (INSERM-UMR_S1166, Sorbonne Université, Paris, France) (33, 34). Cells were cultured in 96-well plates, charged with 10 nM Bodipy-labeled cholesterol in ethyl acetate and with 10 µM TO901317 as LXR agonist to enhance the expression of ABC transporters. Cells were incubated with apoB-depleted plasma (5% v/v) for 4 hours (37°C, 5% CO2) and fluorescence was read at 485/535 nm using a SPARK microplate reader platform (Tecan, Grödig, Austria), both in supernatant and in cells after lysis. Each fluorescence value was corrected by autofluorescence obtained in wells containing apoB-depleted plasma but without Bodipy cholesterol. Percent efflux was calculated by the following formula: [fluorescence in supernatant ÷ (fluorescence in supernatant + fluorescence in lysat)] × 100. All assays were performed in triplicate. ABCA1-dependent CEC was calculated as the difference between THP-1 CEC and THP-1 ΔABCA1 CEC. ABCG1-dependent CEC was calculated as the difference between THP-1 CEC and THP-1 ΔABCG1 CEC (detailed procedure in Supplemental materials (30)).
Statistics
The sample size necessary to demonstrate a 4-point decrease in CEC (SD = 4) in T2D patients compared with controls, with a power equal to 0.9, and a risk alpha equal to 0.05, was 18 (Biosta TGV, software epiR, package R version 0.9-96).
Data are reported as mean ± SD. Statistical calculations were performed using the StatView software package (version 5.0). The results between controls and T2D patients were compared using the nonparametric Mann-Whitney U test. A 2-tailed probability level of 0.05 was accepted as statistically significant. The level of significance of the P value was adjusted as needed for multiple comparisons using the Bonferroni method.
Results
The clinical and biochemical characteristics of the recruited T2D patients and control subjects are shown in Table 1. Glycemic control was poor in the T2D patients (HbA1c = 10.03 ± 2.32%). Serum triglycerides were 2.6-fold higher in T2D patients compared with controls (P < 0.00001), and LDL and HDL cholesterol were, respectively, 20% (P = 0.035) and 40% (P < 0.00001) lower. ApoAI concentration in the plasma fraction with density > 1.129 g/mL was similar between T2D patients and controls (0.62 ± 0.08 vs 0.59 ± 0.11 g/L, P = 0.35), whereas the apoAI concentration in the fraction with density < 1.129 g/mL was decreased by 29% in T2D patients compared with controls (0.78 ± 0.18 vs 1.10 ± 0.19, P < 0.001)
Table 1.
Clinical and biological characteristics of the study subjects
| T2D patients (n = 20) |
Controls (n = 25) |
P value | |
|---|---|---|---|
| Age (y) | 55.9 ± 13.4 | 48.5 ± 9.0 | 0.051 |
| Sex ratio (M/F) | 11/9 | 12/13 | |
| BMI (kg/m2) | 36.8 ± 8.1 | 22.4 ± 2.3 | < 0.00001 |
| Fasting blood glucose (mmol/L) | 9.39 ± 2.77 | 4.77 ± 0.47 | < 0.00001 |
| HbA1c (%) | 10.03 ± 2.32 | NA | |
| Metformin + sulfonylurea users | 35% | NA | |
| Metformin + insulin users | 20% | NA | |
| Sulfonylurea + insulin users | 20% | NA | |
| Metformin + sulfonylurea + insulin users | 15% | NA | |
| eGFR CKD-EPI (mL/min/1.73 m2) | 100 ± 17 | 101 ± 17 | 0.86 |
| Serum triglycerides (mmol/L) | 2.38 ± 1.01 | 0.92 ± 0.38 | <0.00001 |
| Serum total cholesterol (mmol/L) | 4.61 ± 1.07 | 5.39 ± 1.03 | 0.035 |
| Serum HDL cholesterol (mmol/L) | 1.06 ± 0.35 | 1.77 ± 0.42 | < 0.00001 |
| Serum LDL cholesterol (mmol/L) | 2.56 ± 0.82 | 3.19 ± 0.89 | 0.035 |
| Serum apoAI in fraction with d > 1.129 g/mL (g/L) | 0.62 ± 0.08 | 0.59 ± 0.11 | 0.35 |
| Serum apoAI in fraction with d < 1.129 g/mL (g/L) | 0.78 ± 0.18 | 1.10 ± 0.19 | < 0.001 |
Data are presented as mean ± SD.
Abbreviations: apoAI, apolipoprotein AI; BMI, body mass index; d, density; eGFR CKD-EPI, estimated glomerular filtration rate Chronic Kidney Disease-Epidemiology Collaboration; F, female; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; T2D, type 2 diabetes.
HDL Composition
The relative lipid and protein composition of HDL is detailed in Table 2. The triglyceride and cholesteryl ester contents were respectively 68% higher (P = 0.012, limit of significance after Bonferroni adjustment) and 23% lower (P = 0.00008) in T2D patients than in control subjects (when expressed in mass units). Thus, the cholesteryl ester/triglyceride ratio was 46% lower in HDL from T2D patients (P = 0.00008). These HDL were also less rich in free cholesterol (-29%, P = 0.00008). The proportions of total proteins tended to be higher in T2D patients than in controls (+5.5%, at the limit of significance) but the proportion of apoAI was similar in the two groups.
Table 2.
Relative composition of HDL in the study subjects
| T2D patients (n = 20) |
Controls (n = 25) |
P value | |
|---|---|---|---|
| Free cholesterol (% total weight) | 3.46 ± 0.75 | 4.88 ± 1.21 | 0.00008 |
| Esterified cholesterol (% total weight) | 14.2 ± 3.0 | 18.4 ± 2.8 | 0.00008 |
| Triglycerides (% total weight) | 5.20 ± 2.35 | 3.09 ± 0.88 | 0.012 |
| Phospholipids + sphingolipids (% total weight) | 29.3 ± 2.7 | 28.3 ± 3.8 | 0.41 |
| Proteins (% total weight) ApoAI (% total weight) |
47.8 ± 3.2 35.5 ± 3.6 |
45.3 ± 2.9 36.3 ± 3.3 |
0.011 0.53 |
| Esterified cholesterol/ triglycerides | 3.46 ± 1.54 | 6.37 ± 1.39 | 0.00008 |
| Fructosamines (µmol/g proteins) | 9.23 ± 1.94 | 6.99 ± 1.89 | 0.00064 |
Data are presented as mean ± SD. For triglycerides, free and esterified cholesterol, phospho- or sphingolipids and total proteins, differences were statistically significant when P < 0.01, according to Bonferroni adjustment.
Abbreviations: apoAI, apolipoprotein AI; BMI, body mass index.
Phospholipid and Sphingolipid Classes
The proportions of the different phospholipid and sphingolipid classes relative to HDL apoAI are presented in Table 3. PC, SM, and S1P proportions were very similar between T2D patients and controls. The SM/PC ratio was also comparable between the 2 groups (0.108 ± 0.037 vs 0.121 ± 0.031, P = 0.30).
Table 3.
Relative composition of phospholipid and sphingolipid classes in HDL fractions
| T2D patients (n = 20) |
Controls (n = 25) |
P value | |
|---|---|---|---|
| Glycerophospholipids (nmol/mg) | |||
| PC/apoAI | 913 ± 149 | 877 ± 206 | 0.307 |
| LPC/apoAI | 31.2 ± 11.8 | 24.6 ± 7.2 | 0.280 |
| PE/apoAI | 5.00 ± 2.17 | 3.55 ± 1.77 | 0.026 |
| LPE/apoAI | 1.21 ± 0.057 | 0.96 ± 0.44 | 0.077 |
| LPC + LPE/apoAI | 32.4 ± 12.2 | 25.6 ± 7.5 | 0.060 |
| PI/apoAI | 36.6 ± 12.8 | 29.9 ± 12.2 | 0.073 |
| PE-based plasmalogens/apoAI | 1.26 ± 0.63 | 1.73 ± 0.88 | 0.038 |
| Sphingolipids (nmol/mg) | |||
| SM/apoAI | 96 ± 30 | 103 ± 33 | 0.483 |
| Cer/apoAI | 0.387 ± 0.136 | 0.512 ± 0.206 | 0.053 |
| S1P/apoAI | 0.068 ± 0.020 | 0.071 ± 0.012 | 0.689 |
Data are presented as mean ± SD. The value for each phospho- or sphingolipid class is the sum of the different species belonging to this class. Statistical analysis has been performed on the sums, representing a unique value for each class.
Abbreviations: apoAI, apolipoprotein AI; Cer, ceramide; PC, phosphatidylcholine; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; S1P, sphingosine-1-phosphate; SM, sphingomyelin.
In T2D patients, HDL was 41% richer in PE than in controls (P = 0.026) and 27% (P = 0.0601) richer in LPE + LPC. PE-based plasmalogens were 27% lower in T2D patients (P = 0.038). The HDL Cer/apoAI ratio tended to be lower in T2D patients compared with controls (-24%, P = 0.053) and PI/apoAI tended to be higher (+23%, P = 0.073).
HDL Functionality
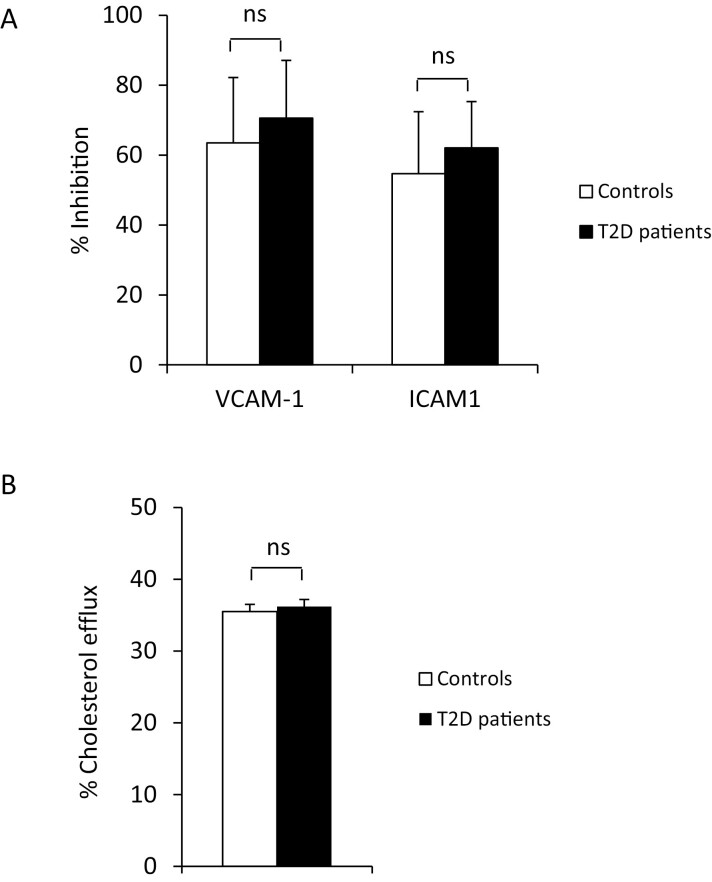
The ability of HDL from T2D patients to inhibit the TNFα-induced expression of both VCAM-1 and ICAM-1 at the surface of HUVEC was similar as that of HDL from control subjects (-70.6 ± 16.5 vs -63.5 ± 18.7%, P = 0.14 and -62.1 ± 13.2 vs -54.7 ± 17.7%, P = 0.16, respectively) (Fig. 1A).
Figure 1.
HDL anti-inflammatory effect and ability to induce cholesterol efflux. (A) Anti-inflammatory effect was measured on HUVEC incubated with HDL (800 µg/mL apoAI) for 16 hours before stimulating VCAM-1 and ICAM-1 expression by 100 UI/mL recombinant TNFα. HDL-induced inhibition was calculated by comparison with TNFα-induced VCAM-1 and ICAM-1 expression without HDL. (B) Cholesterol efflux was measured on THP-1 macrophages loaded with Bodipy-labeled cholesterol and incubated with apolipoprotein B-depleted plasma (5% v/v) for 4 hours. Percent of efflux was calculated with the following formula: [fluorescence in supernatant ÷ (fluorescence in supernatant + fluorescence in lysat)] × 100. NS, not significant.
CEC of apoB-depleted plasma was similar in T2D patients and controls (36.2 ± 4.3 vs 35.5 ± 2.8%, P = 0.59) (Fig. 1B). The ABCA1-dependent pathway was responsible for 30.5 ± 7.1% of total CEC in T2D patients, vs 29.1 ± 6.1% in controls (P = 0.30). The ABCG1-dependent pathway represented 3.4 ± 3.4 and 4.3 ± 3.6% of total CEC in T2D patients and controls, respectively (P = 0.41), and the non-ABCA1/ABCG1-dependent pathway represented 61.1 ± 9.3 and 61.0 ± 10.4% (P = 0.86).
Discussion
Here, we report simultaneously the CEC and anti-inflammatory effect of HDL and a detailed HDL lipidomic analysis in poorly controlled T2D patients compared with lean normolipidemic controls. Despite some lipidomic abnormalities, we found that neither CEC nor the anti-inflammatory effect of HDL were impaired.
Inflammation was studied by quantifying the expression of adhesion molecules at the surface of vascular endothelial cells. These cells play a crucial role in the first steps of the development of atherosclerotic lesions by recruiting inflammatory cells in the subendothelial space. A few previous publications reported that the anti-inflammatory effect of HDL is impaired in T2D patients (21-23). However, these studies were performed either in very few subjects by pooling samples, or with a concentration of HDL far lower than what was used in our work (ie, 800 µg/mL), or by measuring the HDL inflammatory index, which cannot be compared with the expression of adhesion molecules at the surface of endothelial cells. These important methodological differences can easily explain the discrepancies between studies.
The suppression of the TNFα-induced expression of VCAM-1 and ICAM-1 by HDL depends on NF-kB inhibition, which results from different mechanisms, including the activation of endothelial NO synthesis via the PI3kinase/Akt pathway, 3β-hydroxysteroid-δ24 reductase, and heme oxygenase. SR-BI and S1P receptors are of major importance for the activation of all these enzymes (6-8, 35, 36). The cholesteryl ester-to-triglyceride ratio of HDL in our T2D patients was greatly decreased, which is characteristic of this disease (1, 2). Such a modification has been demonstrated to impair some HDL functions. The replacement of cholesteryl esters by triglyceride molecules in HDL reduces the exposure of apoAI to the aqueous phase and impairs HDL antioxidant activity. It could also modify apoAI affinity for SR-BI. However, in the present study, the decrease in the cholesteryl ester-to-triglyceride ratio had no deleterious effect on the ability of HDL to counteract TNFα-induced expression of adhesion molecules. At first sight, this appears to contrast with the experiment having showed that the triglyceride enrichment induced by intralipid infusion impairs the ability of HDL to reduce VCAM-1 and ICAM-1 expression (37). But such impairment is observed mainly when the HDL triglyceride content is very high, which was not the case in most of our T2D patients. It must be noted that triglyceride enrichment does not always impair HDL functionality. Indeed, replacing cholesteryl esters with triglycerides in the core of spherical reconstituted HDL had no effect on their ability to inhibit VCAM-1 expression (38). On the other hand, we previously demonstrated that in vitro triglyceride enrichment did not change HDL-induced NO production by HUVEC, a process for which HDL binding to SR-BI and S1P receptor is also crucial (22). Similarly, it did not change the amount of total neutral lipids taken up by SR-BI, suggesting that the affinity to SR-BI is unaltered (39).
Very few data have been published in T2D regarding HDL composition in phospholipids and sphingolipids. S1P amount, which is of major importance for HDL anti-inflammatory effect, was not decreased in T2D patients compared with control subjects, similarly to what has been reported (40). This contributes to maintaining a normal HDL anti-inflammatory effect. The decrease in HDL total Cer was also observed by Ståhlman et al. in hypertriglyceridemic T2D patients (2), and by our group in subjects with metabolic syndrome and hypertriglyceridemia but normal glycemia (26). Cer activates NF-kB, which is a positive transcriptional factor of VCAM-1 and ICAM-1 (41). Thus, a decrease in HDL Cer is probably not deleterious in terms of inflammation. The decrease in plasmalogens in HDL is also currently observed in T2D patients but this phospholipid subclass is, to our knowledge, mainly implied in the antioxidant effect. SM has been reported to be more effective than PC to confer an anti-inflammatory profile to an apoAI mimetic peptide (13). The normal HDL anti-inflammatory effect observed in T2D patients is hence consistent with the normal HDL SM/PC ratio observed in our study.
We measured CEC using apoB-depleted plasma and macrophages, similarly to large epidemiological studies that highlighted the inverse relationship between CEC and cardiovascular risk (3, 4, 42). In particular, THP-1 was chosen in some of them (3, 42). Several studies have investigated CEC in T2D, with conclusions varying from decreased to increased CEC (15-20). CEC has been evaluated using different protocols in terms of cell type (macrophages, fibroblasts, hepatocytes, transfected cells), the stimulation or not of cholesterol transporters, the tracer (radioactive or fluorescent), the acceptor and its quantity (HDL, total plasma, apoB-depleted plasma), or incubation time. All these differences often make the comparison between studies difficult and are likely to explain the divergent results. Our conclusion is similar to that in the study by Annema et al (15). that was also performed on THP-1 macrophages with a 5-hour incubation time (vs 4 hours in the present study) with apoB-depleted plasma, and with sample sizes higher than 130 in both controls and T2D patients. Dullaart et al., working with plasma and fibroblasts, also reported normal CEC in T2D (16). Our study differs from these 2 previous studies in that glycemia was much higher in T2D patients, and our work extends the conclusion that CEC is not impaired in T2D, to poorly controlled T2D patients. The use of HDL isolated by ultracentrifugation instead of apoB-depleted plasma may also be a source of discrepancy because of the elimination of some preβ-HDL, which play an important role in ABCA1-dependent cholesterol efflux (20). The selection criteria for T2D patients and controls are also likely to explain divergent results. Indeed, Shiu et al. reported a lower CEC in T2D patients compared with controls but the groups were matched on HDL-cholesterol, body mass index, and triglycerides, which is not representative of usual T2D patients compared with healthy subjects (19), contrary to our study and others that reported normal or even higher CEC (15-17).
We observed that ABCA1-dependent cholesterol efflux represents about 30% of CEC on THP-1 macrophages stimulated by an LXR ligand, which is concordant with previously reported results (33). Cholesterol efflux occurs via ABCA1 after apoAI binding. The acceptors of cholesterol for the ABCA1-dependent pathway are pre-β-HDL, HDL3b, and 3c (33). The acceptors of ABCG1-dependent cholesterol efflux are larger HDL, but this pathway is minor in THP-1 (34). All HDL subclasses contribute to cholesterol efflux by the non-ABCA1/ABCG1-dependent pathways (mainly passive diffusion). We separated serum into 2 fractions with a cutoff at a density equal to 1.129 g/mL, and quantified apoAI in each of them. ApoAI concentrations were similar in T2D patients and controls in the fraction with a density > 1.129 g/mL which contains pre-β-HDL, HDL3b, and 3c (27). This is concordant with a similar ABCA-1-dependent CEC. Non-ABCA1/ABCG1-dependent cholesterol efflux depends on all HDL subclasses (33). It was not decreased in our T2D patients, whereas apoAI in the less dense HDL fraction was decreased. This observation may be surprising at first glance, but it can be explained by previous findings. Indeed, ABCA1-dependent cholesterol efflux is the most important determinant of total efflux from macrophages, whereas passive diffusion has a weaker impact (43). In addition, serum total apoAI concentration does not appear to be the main determinant of serum apoB-depleted plasma CEC on macrophages (15, 16, 44), it is rather the concentration of small HDL including preβ, and consequently the fraction of apoAI in these HDL.
As far as the sphingophospholipidome is concerned, the nature of sphingo- or phospholipid molecules influences CEC, but this has been shown either with reconstituted HDL with a unique sphingo- or phospholipid class or with enrichments higher than those observed in current diseases. This is the case for LPC because in vitro HDL enrichment was found to diminish CEC, but with an amount of LPC much higher than that reported in our study and usually observed in T2D patients (26). Among the other abnormalities of the sphingophospholipidome we observed in our T2D patients, to our knowledge, none has been demonstrated to inhibit CEC. Our study suggests that a moderate decrease in plasmalogens or increase in PE and PI do not alter HDL CEC in T2D patients. The content of HDL in total phospholipids (45-47) and in SM (13, 48) was associated with CEC, but these settings were both normal in our T2D cohort.
Some limitations need to be considered. Our groups of controls and T2D patients were small. However, such sample sizes allowed to demonstrate a 4-point decrease in CEC in T2D patients compared with controls, with a power equal to 0.9. We observed no decreasing trend. So, there is little chance that our conclusions are biased by type 2 error. Our sample sizes also made it possible to evidence a 15-point decrease in the anti-inflammatory effect. Again, no decreasing trend was observed. Second, our T2D patients were 7 years older than controls. If age had any influence on HDL functionality, it would rather be a negative one. Moreover, we calculated correlation coefficients for age and our quantitative variables in controls on the one hand, and in T2D patients on the other hand, and none was statistically significant. Thus, an influence of age on our results for both HDL functionality and composition is unlikely. Finally, each of our experiments was performed on 1 cell type for CEC and for the anti-inflammatory effect, and our results should be extrapolated to other cell types with caution. However, it must be noted that the cell types used in this study are the same as those used to demonstrate a link between HDL functionality and cardiovascular risk (3, 5).
In conclusion, in addition to the well-known decreased cholesteryl ester-to-triglyceride ratio, HDL from T2D patients with poor glycemic control compared with HDL from lean normolipidemic control subjects, show abnormalities of the sphingophospholipidome. These abnormalities consist mainly in a decrease in Cer, plasmalogens, and an increase in PE and LPE + LPC. These modifications are not associated with impairments in the ability of HDL to counteract the TNFα-induced expression of adhesion molecules on HUVEC. ApoB-depleted plasma CEC was also unaltered. Thus, some HDL functions appear to be preserved in T2D patients and an impairment of these functions cannot be considered as a major cause of the increased cardiovascular risk in T2D.
Acknowledgments
The authors are indebted to Véronique Grivet and Cécile Breuiller (Department of Endocrinology and Metabolic Diseases, CHU Dijon Bourgogne, Dijon, France) for help during the recruitment of patients, to the midwife of the Maternity Unit of Dijon University hospital for the collection of umbilical cords, to Marion Xolin and Laurence Loiodice for their valuable technical assistance, and to Suzanne Rankin for proofreading.
Contributor Information
Damien Denimal, INSERM LN C UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Department of Biochemistry, CHU Dijon Bourgogne, 21070 Dijon, France.
Sara Benanaya, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France.
Serge Monier, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Flow Cytometry Platform, Fédération de Recherche Santé STIC/DIMACELL, Université Bourgogne-Franche Comté, 21000 Dijon, France.
Isabelle Simoneau, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Department of Endocrinology and Metabolic Diseases, CHU Dijon Bourgogne, 21070 Dijon, France.
Jean-Paul Pais de Barros, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Lipidomic Analytical Platform, Université Bourgogne-Franche Comté, 21000 Dijon, France.
Wilfried Le Goff, Institute of Cardiometabolism and Nutrition, INSERM-UMR_S1166, Sorbonne Université, 75013 Paris, France.
Benjamin Bouillet, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Department of Endocrinology and Metabolic Diseases, CHU Dijon Bourgogne, 21070 Dijon, France.
Bruno Vergès, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Department of Endocrinology and Metabolic Diseases, CHU Dijon Bourgogne, 21070 Dijon, France.
Laurence Duvillard, INSERM LNC UMR1231, Université Bourgogne-Franche Comté, 21000 Dijon, France; Department of Biochemistry, CHU Dijon Bourgogne, 21070 Dijon, France.
Financial Support
The authors acknowledge grants from Agence Nationale de la Recherche under the program “Investissements d’Avenir” with reference ANR-11-LABX-0021 (LipSTIC Labex). They also acknowledge financial support from the University of Burgundy-Franche-Comte, the National Institute of Health and Medical Research (INSERM), the Region Burgundy-Franche Comte, and the Fonds Européens de Développement Régional.
Author Contributions
L.D. wrote the manuscript. L.D. and D.D. designed the study. I.S., B.V., and B.B. recruited patients and controls. W.L.G. provided modified cells. B.V., D.D., and W.L.G. reviewed the manuscript. D.D., S.B., J.P.P.d.B., and S.M. performed the biochemical analyses and in vitro studies and the liquid chromatography-mass spectrometry measures. L.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
No potential conflicts of interest relevant to this article were reported.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ståhlman M, Fagerberg B, Adiels M, et al. . Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: impact on small HDL particles. Biochim Biophys Acta. 2013;1831(11):1609–1617. [DOI] [PubMed] [Google Scholar]
- 3. Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. HDL (high-density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. 2019;39(9):1874–1883. [DOI] [PubMed] [Google Scholar]
- 4. Rohatgi A, Khera A, Berry JD, et al. . HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia C, Anderson JLC, Gruppen EG, et al. . High-density lipoprotein anti-inflammatory capacity and incident cardiovascular events. Circulation. 2021;143(20):1935–1945. [DOI] [PubMed] [Google Scholar]
- 6. Kimura T, Tomura H, Mogi C, et al. . Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281(49):37457–37467. [DOI] [PubMed] [Google Scholar]
- 7. Kimura T, Tomura H, Mogi C, et al. . Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal. 2006;18(6):841–850. [DOI] [PubMed] [Google Scholar]
- 8. McGrath KC, Li XH, Puranik R, et al. . Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29(6):877–882. [DOI] [PubMed] [Google Scholar]
- 9. Nobecourt E, Davies MJ, Brown BE, et al. . The impact of glycation on apolipoprotein A-I structure and its ability to activate lecithin: cholesterol acyltransferase. Diabetologia. 2007;50(3):643–653. [DOI] [PubMed] [Google Scholar]
- 10. Curtiss LK, Bonnet DJ, Rye KA. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: a surface plasmon resonance study. Biochemistry. 2000;39(19):5712–5721. [DOI] [PubMed] [Google Scholar]
- 11. Darabi M, Guillas-Baudouin I, Le Goff W, Chapman MJ, Kontush A. Therapeutic applications of reconstituted HDL: when structure meets function. Pharmacol Ther. 2016;157:28–42. [DOI] [PubMed] [Google Scholar]
- 12. Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54(11):2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwendeman A, Sviridov DO, Yuan W, et al. . The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56(9):1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rached F, Lhomme M, Camont L, et al. . Defective functionality of small, dense HDL3 subpopulations in ST segment elevation myocardial infarction: relevance of enrichment in lysophosphatidylcholine, phosphatidic acid and serum amyloid A. Biochim Biophys Acta. 2015;1851(9):1254–1261. [DOI] [PubMed] [Google Scholar]
- 15. Annema W, Dikkers A, de Boer JF, et al. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: the CODAM study. Sci Rep. 2016;6:27367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dullaart RPF, Pagano S, Perton FG, Vuilleumier N. Antibodies against the C-terminus of ApoA-1 are inversely associated with cholesterol efflux capacity and HDL Metabolism in subjects with and without type 2 diabetes mellitus. Int J Mol Sci. 2019;20(3):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Low H, Hoang A, Forbes J, et al. . Advanced glycation end-products (AGEs) and functionality of reverse cholesterol transport in patients with type 2 diabetes and in mouse models. Diabetologia. 2012;55(9):2513–2521. [DOI] [PubMed] [Google Scholar]
- 18. Yassine HN, Belopolskaya A, Schall C, Stump CS, Lau SS, Reaven PD. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism. 2014;63(5):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shiu SW, Wong Y, Tan KC. Pre-β1 HDL in type 2 diabetes mellitus. Atherosclerosis. 2017;263:24–28. [DOI] [PubMed] [Google Scholar]
- 20. Apro J, Tietge UJ, Dikkers A, Parini P, Angelin B, Rudling M. Impaired cholesterol efflux capacity of high-density lipoprotein isolated from interstitial fluid in type 2 diabetes mellitus-brief report. Arterioscler Thromb Vasc Biol. 2016;36(5):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgantini C, Natali A, Boldrini B, et al. . Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60(10):2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ebtehaj S, Gruppen EG, Parvizi M, Tietge UJF, Dullaart RPF. The anti-inflammatory function of HDL is impaired in type 2 diabetes: role of hyperglycemia, paraoxonase-1 and low grade inflammation. Cardiovasc Diabetol. 2017;16(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sang H, Yao S, Zhang L, et al. . Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J Clin Endocrinol Metab. 2015;100(3):870–879. [DOI] [PubMed] [Google Scholar]
- 24. Petit JM, Cercueil JP, Loffroy R, et al. . Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the lira-NAFLD study. J Clin Endocrinol Metab. 2017;102(2):407–415. [DOI] [PubMed] [Google Scholar]
- 25. Vergès B, Hassid J, Rouland A, et al. . Liraglutide reduces plasma PCSK9 in patients with type 2 diabetes not treated with statins. Diabetes Metab. 2022;48(2):101284. [DOI] [PubMed] [Google Scholar]
- 26. Denimal D, Monier S, Brindisi MC, et al. . Impairment of the ability of HDL from patients with metabolic syndrome but without diabetes mellitus to activate eNOS: correction by S1P enrichment. Arterioscler Thromb Vasc Biol. 2017;37(5):804–811. [DOI] [PubMed] [Google Scholar]
- 27. Martin SS, Jones SR, Toth PP. High-density lipoprotein subfractions: current views and clinical practice applications. Trends Endocrinol Metab. 2014;25(7):329–336. [DOI] [PubMed] [Google Scholar]
- 28. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 29. Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39(2):82–91. [DOI] [PubMed] [Google Scholar]
- 30. Duvillard L. Supplemental materials for Normal HDL cholesterol efflux and anti-inflammatory capacities in type 2 diabetes despite lipidomic abnormalities. Figshare repository. Deposited 27 May 2022. 10.6084/m9.figshare.19898593 [DOI] [PMC free article] [PubMed]
- 31. Denimal D, Nguyen A, Pais de Barros JP, et al. . Major changes in the sphingophospholipidome of HDL in non-diabetic patients with metabolic syndrome. Atherosclerosis. 2016;246:106–114. [DOI] [PubMed] [Google Scholar]
- 32. Ashby DT, Rye K-A, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(9):1450–1455. [DOI] [PubMed] [Google Scholar]
- 33. Du XM, Kim MJ, Hou L, et al. . HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133–1142. [DOI] [PubMed] [Google Scholar]
- 34. Larrede S, Quinn CM, Jessup W, et al. . Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol. 2009;29(11):1930–1936. [DOI] [PubMed] [Google Scholar]
- 35. Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3β-hydroxysteroid-Δ24 reductase expression and inducing heme oxygenase-1. Circ Res. 2013;112(2):278–288. [DOI] [PubMed] [Google Scholar]
- 36. Keul P, Polzin A, Kaiser K, et al. . Potent anti-inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: a new aspect of HDL dysfunction and its therapy. FASEB J. 2019;33(1):1482–1495. [DOI] [PubMed] [Google Scholar]
- 37. Patel S, Puranik R, Nakhla S, et al. . Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis. 2009;204(2):424-428. [DOI] [PubMed] [Google Scholar]
- 38. Baker PW, Rye K-A, Gamble JR, Vadas MA, Barter PJ. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res. 1999;40(2):345–353. [PubMed] [Google Scholar]
- 39. Greene DJ, Skeggs JW, Morton RE. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI). J Biol Chem. 2001;276(7):4804–4811. [DOI] [PubMed] [Google Scholar]
- 40. Tong X, Peng H, Liu D, et al. . High-density lipoprotein of patients with type 2 diabetes mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells: relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc Diabetol. 2013;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boon J, Hoy AJ, Stark R, et al. . Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62(2):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yubero-Serrano EM, Alcalá-Diaz JF, Gutierrez-Mariscal FM, et al. . Association between cholesterol efflux capacity and peripheral artery disease in coronary heart disease patients with and without type 2 diabetes: from the CORDIOPREV study. Cardiovasc Diabetol. 2021;20(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30(4):796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Ghazali A, Deodhar S, Saldanha S, et al. . Molecular patterns of extreme and persistent cholesterol efflux capacity. Arterioscler Thromb Vasc Biol. 2021;41(10):2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwala AP, Rodrigues A, Risman M, et al. . HDL phospholipid content and cholesterol efflux capacity are reduced in patients with very high HDL-C and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35(6):1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fournier N, Paul J-L, Atger V, et al. . HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler Thromb Vasc Biol. 1997;17(11):2685–2691. [DOI] [PubMed] [Google Scholar]
- 47. Yancey PG, Kawashiri M, Moore R, et al. . In vivo modulation of HDL phospholipid has opposing effects on SR-BI- and ABCA1-mediated cholesterol efflux. J Lipid Res. 2004;45(2):337–346. [DOI] [PubMed] [Google Scholar]
- 48. Subbaiah PV, Gesquiere LR, Wang K. Regulation of the selective uptake of cholesteryl esters from high density lipoproteins by sphingomyelin. J Lipid Res. 2005;46(12):2699–2705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.