Abstract
Context
Cushing syndrome (CS) is a rare and serious disease with high mortality. Patients are often diagnosed late in the course of the disease.
Objective
This work investigated whether defined patient populations should be screened outside the at-risk populations defined in current guidelines.
Methods
As part of the prospective German Cushing registry, we studied 377 patients with suspected CS. The chief complaint for CS referral was documented. Using urinary free cortisol, late-night salivary cortisol, and the 1-mg dexamethasone suppression test as well as long-term clinical observation, CS was confirmed in 93 patients and ruled out for the remaining 284.
Results
Patients were referred for 18 key symptoms, of which 5 were more common in patients with CS than in those in whom CS was ruled out: osteoporosis (8% vs 2%; P = .02), adrenal incidentaloma (17% vs 8%, P = 0.01), metabolic syndrome (11% vs 4%; P = .02), myopathy (10% vs 2%; P < .001), and presence of multiple symptoms (16% vs 1%; P < .001). Obesity was more common in patients in whom CS was ruled out (30% vs 4%, P < .001), but recent weight gain was prominent in those with CS. A total of 68 of 93 patients with CS (73%) had typical chief complaints, as did 106 of 284 of patients with ruled-out CS status (37%) according to the Endocrine Society practice guideline 2008.
Conclusion
The 2008 Endocrine Society Practice guideline for screening and diagnosis of CS defined at-risk populations that should undergo testing. These recommendations are still valid in 2022.
Keywords: Cushing disease, hypercortisolism, cortisol, ACTH, diagnostic score, PCOS
Cushing syndrome (CS) is a rare condition (1) that is often diagnosed late in the course of disease, often years after the first onset of symptoms (2). Diagnosis and management of the disease are difficult (3), as CS is typically characterized (and identified) by the presence of multiple symptoms (4). Many symptoms, like hypertension, diabetes, weight gain, or osteoporosis, are very common among the general population, whereas others, like purple striae, are quite specific to CS (5, 6). Obviously, patients can present oligosymptomatically—both initially during primary disease manifestation and especially at recurrence (7, 8). The low incidence of CS and the clinical overlap with pseudo-Cushing states (9), that is, patients with metabolic syndrome or polycystic ovary syndrome (PCOS) (5), can delay a definitive diagnosis and treatment, resulting in increased morbidity and mortality among patients (10-12).
Studies have been performed to identify patients with CS at earlier stages in cohorts with a relevant prevalence of CS (13, 14). In patients with diabetes mellitus, the prevalence of CS is low, ranging between 0% and 3%, depending on patient selection (15-17). The likelihood for hypercortisolism is higher in patients with advanced type 2 diabetes according to a recent meta-analysis (18). The prevalence of CS among patients with hypertension is similarly low (19, 20); however, screening can be recommended in young patients with resistant hypertension or concomitant diabetes (13). Screening approaches in patients with obesity are a matter of debate (13), as results differ greatly between studies (21). However, in general, screening for endogenous hypercortisolism in this group is not recommended (13). Other suggested at-risk populations might be patients with hypogonadotropic hypogonadism (22), young patients with osteoporosis and vertebral or rib fractures (22), patients with central obesity and clinical signs for CS (22), and with adrenal incidentaloma (23, 24). However, the high prevalence of patients with osteoporosis and CS has been questioned (25).
Based on the guideline of the US Endocrine Society in 2008, adult patients with unusual symptoms for their age (eg, osteoporosis, hypertension), with multiple and progressive symptoms, particularly those that are more predictive of CS, and with adrenal incidentaloma compatible with adenoma should be screened. The same applies to children with below average height and above average weight (26). While a few studies proved the value of screening in certain populations (23, 27), a contemporary reassessment is necessary to prove that phenotypic presentation has not shifted because of secular trends in obesity, diabetes, and hypertension epidemiology. We analyzed clinical signs and symptoms that initiated transfer to our tertiary center for screening of CS in recent years to test whether current screening recommendations are still valid (28).
Materials and Methods
Patients and Data Acquisition
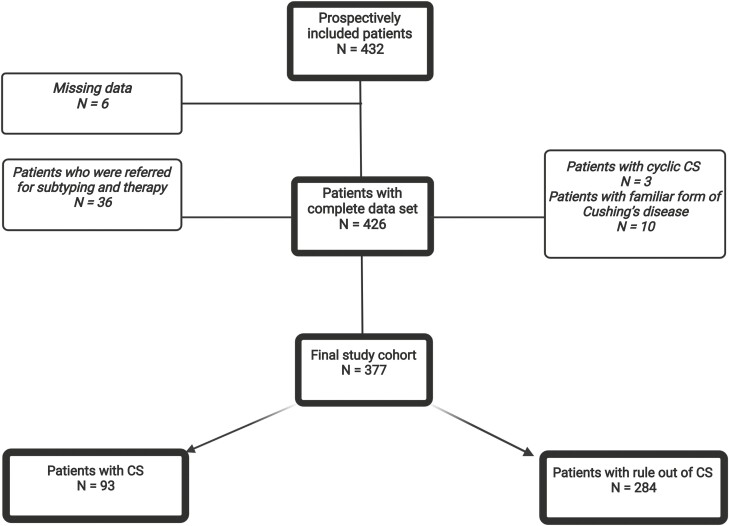
This study is part of the German Cushing Registry. Since 2012, a total of 432 patients have been prospectively evaluated at the Munich tertiary center for CS and formed the basis of this study (Fig. 1). The following patients were excluded: patients who were referred for a second opinion, as biochemical evaluation of CS was already completed (n = 36); patients with cyclic CS (n = 3) or with a familiar form of Cushing disease (n = 10), and patients in whom the presenting problem was not clearly stated (n = 6).
Figure 1.
Patient selection figure created with BioRender.com.
The remaining 377 patients were evaluated according to current guidelines (29), as described previously (30). Clinical signs and symptoms were captured in a standardized fashion. Standard biochemical screening was performed according to guidelines (26, 31), including a sample of 24-hour urine cortisol, late-night salivary cortisol, and 1-mg low-dose dexamethasone suppression test in all patients. We always perform all 3 tests but repeat urinary and salivary sampling a second time only if we think they could be false positive or false negative (eg, false collection). The chief complaint leading to consultation based on the patient’s health records was noted. Final diagnosis was based on signs and symptoms, biochemical testing, surgery outcome, histopathology, and follow-up. Finally, CS was confirmed in 93 patients and ruled out in 284 patients. Subtyping of CS was conducted based on corticotrophin-releasing hormone test, if needed inferior petrosal sinus sampling and imaging, and final diagnosis was confirmed by surgery. In patients with adrenal incidentaloma, catecholamine excess and primary hyperaldosteronism were also excluded.
All patients were categorized according to their chief complaint following the Endocrine Society Practice guideline recommendations into 3 main categories: A) unusual features for age; B) multiple (defined as > 3 typical symptoms for CS) and progressive features; and C) adrenal incidentaloma compatible with adenoma. We also evaluated the negative recommendation against screening in other patient groups (D.).
For statistical analysis, SPSS 26 (IBM) was used. Differences between groups were tested by nonparametric tests. P values less than .05 were considered to be statistically significant. The German Cushing registry was approved by the ethic committee of the LMU Munich. All patients gave written informed consent.
Results
Patient Characteristics
Seventy-six percent of patients were female (in both groups). Patients with CS were older than patients in whom CS was ruled out (median 49 vs 36 years). Blood pressure and glycated hemoglobin A1c were significantly higher in patients with CS (P < .001), while their body mass index (BMI) was similar. As expected, all screening parameters (urinary free cortisol, late-night salivary cortisol, and 1-mg low-dose dexamethasone suppression test) were significantly different between groups (Table 1). In the group of patients with CS, 67% were diagnosed with pituitary CS, 28% with adrenal CS, and 5% with ectopic CS.
Table 1.
Patient characteristics of the study cohorts (shown as median and ranges)
| Cushing syndrome (n = 93) |
Ruled out (n = 284) |
P | |
|---|---|---|---|
| Sex | 76% women | 76% women | – |
| Age, y | 49 (36-58) | 36 (25-52) | < .001 |
| Body mass index | 30 (25-34) | 31 (26-39) | .06 |
| Blood pressure, mm Hg | 141 (130-157)/90 (81-100) | 130 (118-141)/82 (77-90) | < .001 |
| HbA 1c , 4.0%-6.0% | 5.8 (5.4-6.5) | 5.3 (5.1-5.8) | < .001 |
| Serum cortisol 9 am , 1.8-24 µg/dL | 21 (15-29) | 9 (7-13) | < .001 |
| ACTH 9 am , 4-50 pg/mL | ACTH-dependent: 63 (35-92) ACTH-independent: 2.5 (2-5) |
12 (8-19) | < .001 |
| Urinary free cortisol, < 150 µg/24 h | 412 (242-786) | 114 (80-191) | < .001 |
| Late-night salivary cortisol, < 1.5 ng/mL | 7.2 (3.9-11.9) | 1.0 (0.7-1.7) | < .001 |
| 1-mg low-dose dexamethasone suppression test, < 2.0 µg/dL | 13.4 (5.9-22.3) | 1.0 (0.8-1.4) | < .001 |
Abbreviations: ACTH, adrenocorticotropin; HbA1c, glycated hemoglobin A1c.
Chief Complaint for Consultation and Screening in Patients With Cushing Syndrome and Ruled-out CS
We identified 18 different chief complaints for which patients were transferred to the tertiary center (Table 2). The 3 most common reasons were obesity/weight gain (n = 89, 24% of patients), adrenal or pituitary incidentaloma (n = 40, 11%), and hypertension (n = 40, 11%). The frequencies of the chief complaints are listed in Table 2. Half the patients screened for CS belonged to groups that are not counted as the priority groups recommended by the 2008 guidelines (see Tables 2 and 3). CS was diagnosed in 12% of those patients, compared to 39% among those who fall into the screening groups A to C as recommended by the 2008 guidelines.
Table 2.
Reasons for consultation/screenings in the study cohorts
| CS (N = 93) |
CS ruled out (N = 284) |
|
|---|---|---|
| Reason for consultation/chief complaint in accordance to guideline recommendation | ||
| Unusual features for age (group A) | ||
| Osteoporosis/osteopenia | 8% (N = 7) | 2% (N = 5) |
| Hypertension | 5% (N = 5) | 12% (N = 35) |
| Multiple and progressive features (group B) | ||
| Multiple symptomsa | 16% (N = 15) | 1% (N = 3) |
| Metabolic syndrome | 11% (N = 10) | 4% (N = 12) |
| Myopathy | 10% (N = 9) | 2% (N = 5) |
| “PCOS” symptoms (acne, hirsutism, menstrual changes) | 6% (N = 6) | 8% (N = 22) |
| Adrenal incidentaloma (group C) | ||
| Incidentaloma | 17% (N = 16) | 8% (N = 24) |
| ∑ in recommended group | N = 68 (73%) | N = 106 (37%) |
| Reason for consultation/chief complaint in other groups (group D) | ||
| Obesity/weight gain | 4% (N = 4) | 30% (N = 85) |
| Fatigue/tiredness | 3% (N = 3) | 5% (N = 15) |
| Visual Cushing diagnosis (by external physician)b | 3% (N = 3) | 5% (N = 15) |
| Edema | 3% (N = 3) | 4% (N = 12) |
| Lab resultsc | 2% (N = 2) | 4% (N = 12) |
| Suspicious clinical signsd | 2% (N = 2) | 1% (N = 4) |
| Psychiatric disorders | 1% (N = 1) | 3% (N = 8) |
| “Visual diagnosis” (by patient or family)d | 1% (N = 1) | 2% (N = 7) |
| Sweating | 1% (N = 1) | 2% (N = 5) |
| Other | 5% (N = 5) | 5% (N = 15) |
| ∑ in this group | N = 25 (27%) | N = 178 (63%) |
Abbreviations: BDI, Beck Depression Inventory; CS, Cushing syndrome; PCOS, polycystic ovary syndrome.
a Multiple symptoms: more than 3 symptoms that can be typical for CS (eg, hypertension AND diabetes AND sleeping disorders).
b Visual diagnosis (by physician): any physician suspected CS just by the clinical appearance of the patient (most often during consultation for an unrelated clinical problem).
c Lab results: Serum cortisol was elevated in a measurement (measurement without initial suspicion of CS).
dVisual diagnosis (by patient): Patient looked up their own appearance on the internet and suspected CS or patient knows someone with CS and suspects they might suffer from it as well.
d Clinical signs: moon face (twice), striae (3 times), signs of aging.
Table 3.
Summary of screening recommendations
| Screening group | No. of patients in total | No. of patients diagnosed with CS |
|---|---|---|
| Group A: patients with unusual features for age | 52 | 12 (23%) |
| Group B: patients with multiple and progressive features, particularly those that are more predictive of CS | 82 | 40 (49%) |
| Group C: patients with adrenal incidentaloma compatible with adenoma | 40 | 16 (40%) |
| Summary groups A-C | 174 | 68 (39%) |
| Group D: recommendation against widespread testing for CS in any other patient group | 203 | 25 (12%) |
Abbreviation: CS, Cushing syndrome.
Group A consisted of patients who had unusual features for their age. These were, for example, osteoporosis and osteopenia, which were chief complaints in 12 patients, of whom 7 (58%) received a final diagnosis of CS. Hypertension, mostly of new onset, was found in the consultation recommendation notes of 40 patients, and CS was confirmed in 5 (13%). Group B patients had multiple and progressive features, particularly those that are more predictive of CS. Eighteen patients presented with multiple symptoms (defined as > 3 typical symptoms for CS), and CS was diagnosed in 15 (83%). Details regarding these symptoms are presented in Table 4. Myopathy was the chief complaint in 14 patients, and CS was confirmed in 9 (64%). Metabolic syndrome was also common (22 patients), with 10 (45%) having CS. Twenty-eight women presented with hyperandrogenic symptoms, and 21% had confirmed CS. Finally, group C patients had adrenal or pituitary incidentaloma compatible with adenoma. There were 40 patients in this group, of whom 16 (40%) received a final diagnosis of CS.
Table 4.
Symptoms in patients with chief complaint “multiple symptoms”
| Symptom/Sign | No. of patients (N = 18) |
|---|---|
| Recent weight gain | 17 |
| Arterial hypertension | 8 |
| Myopathy | 7 |
| Menstrual irregularities (in females) and amenorrhea | 7 |
| Fatigue | 5 |
| Diabetes (new onset or worsening) | 4 |
| Sleeping disorders | 3 |
| Sterility (in females) | 3 |
| Depression | 3 |
| Hematoma | 3 |
| Osteoporosis | 3 |
| Edema | 3 |
| Hair loss | 2 |
| Moon face | 2 |
| Hirsutism (in females) | 2 |
| Sweating | 2 |
| Gastrointestinal symptoms | 1 |
| Palpitations | 1 |
| Incidentaloma | 1 |
| Low serum potassium | 1 |
| Loss of libido | 1 |
| Poor wound healing | 1 |
| Purple striae | 1 |
| Acne | 1 |
| Facial fullness | 1 |
| Abscess | 1 |
Group D consisted of 203 patients who did not fall in one of the aforementioned categories. Of those, 25 (27%) received a final diagnosis of CS. Details are depicted in Table 2.
Likelihood of Cushing Syndrome
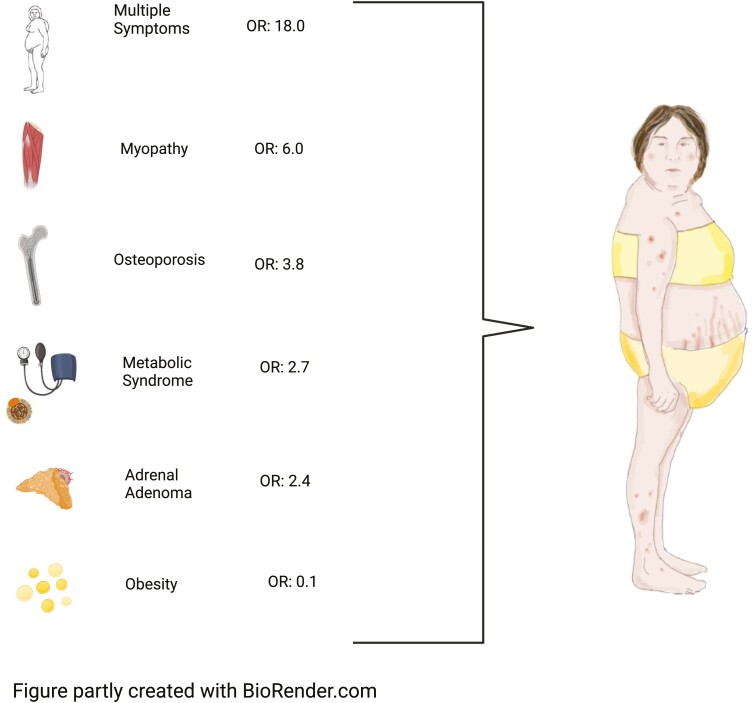
Of the chief complaints, 6 were significantly different between patients with confirmed CS and those where CS was ruled out (Table 5). Five were significantly more frequent in CS: osteoporosis (8% vs 2%; P = .02), incidentaloma (17% vs 8%; P = .01), metabolic syndrome (11% vs 4%; P = .02), myopathy (10% vs. 2%; P < .001), and multiple symptoms (16% vs 1%; P < .001). Obesity or weight gain were more common in those in whom CS was ruled out (30% of patients vs 4% in CS; P < .001). Multiple symptoms were the most important aspect increasing probability for CS (odds ratio [OR] 18.0 [5.1-63.8]), while weight gain/obesity decreased probability for CS (OR 0.11 [0.04-0.30]) (Fig. 2).
Table 5.
Odds ratios for different symptoms
| Reasons for consultation | CS ruled out (N = 284) |
CS (N = 93) |
P | Odds ratio | CI |
|---|---|---|---|---|---|
| Obesity/weight gain | 30% | 4% | < .001 | 0.11 | 0.04-0.30 |
| Incidentaloma | 8% | 17% | .01 | 2.4 | 1.2-4.7 |
| Metabolic syndrome | 4% | 11% | .02 | 2.7 | 1.1-6.5 |
| Osteoporosis | 2% | 8% | .02 | 3.8 | 1.1-12.9 |
| Myopathy | 2% | 10% | < .001 | 6.0 | 2.0-18.3 |
| Multiple symptomsa | 1% | 16% | < .001 | 18.0 | 5.1-63.8 |
| Visual diagnosis (by external physician)b | 5% | 3% | .3 | ||
| Lab resultsc | 4% | 2% | .4 | ||
| Hypertension | 12% | 5% | .07 | ||
| Visual diagnosis (by patient)d | 2% | 1% | .2 | ||
| “PCOS” symptoms (acne, hirsutism, menstrual changes) | 8% | 6% | .7 | ||
| Fatigue/tiredness | 5% | 3% | .3 | ||
| Edema | 4% | 3% | .7 | ||
| Psychiatric disorders | 3% | 1% | .2 | ||
| Sweating | 2% | 1% | .4 | ||
| Clinical signsd | 1% | 2% | .6 | ||
| Other | 5% | 5% | ≥ .999 |
Abbreviations: BDI, Beck Depression Inventory; CS, Cushing syndrome; PCOS, polycystic ovary syndrome.
a Multiple symptoms: more than 3 symptoms that can be typical for CS (eg, hypertension AND diabetes AND sleeping disorders).
b Visual diagnosis (by physician): any physician suspected CS just by the clinical appearance of the patient (most often during a consultation for an unrelated clinical problem).
c Lab results: Serum cortisol was elevated in a measurement (measurement without initial suspicion of CS).
d Visual diagnosis (by patient): Patient looked up their appearance on the internet and suspected CS or patient knows someone with CS and suspects they might suffer from it as well.
d Clinical signs: moon face (twice), striae (3 times), signs of aging.
Figure 2.
Reasonable screening for Cushing syndrome (CS). OR, odds ratio.
The Obesity Phenotype
Obesity and weight gain were the most frequent chief complaint to screen for CS (n = 89, thereof 85 CS ruled out, 4 CS) but also the presenting problem that made diagnosis of CS most unlikely. Obesity or weight gain was in fact the “only” chief complaint in these patients; they did not have another major clinical problem.
In 70 of 89 patients obesity was a lifelong problem (often since childhood). Seventeen of 89 patients reported a very recent weight gain (in the last year). In 2 patients, onset was unclear/unknown.
In patients in whom CS was ruled out, the majority suffered from lifelong obesity (69/83, 83%). Among the 4 patients with confirmed CS, acute weight gain within 1 year was present in the 3 patients with pituitary CS, but not in the 1 patient with adrenal CS. The BMI of patients that presented with weight gain/obesity was higher in the ruled-out group than in patients with CS (38 [29–43] vs 33 [26–36]; P = not significant).
Discussion
The main finding of this study is that the 2008 Endocrine Society guideline recommendations are valid and identified roughly 73% of cases presenting with unusual features for age, multiple and progressive features, and incidentaloma in our prospective series covering 10 years between 2012 and 2021. On the other hand, other chief complaints than those mentioned earlier were present in 27% of patients with CS, which should be considered in clinical practice.
Implications for Clinical Practice
In our experience, half the patients submitted for screening of CS in our specialized center (see Table 1) did not belong to one of the recommended groups for screening. As CS is a severe disease when left untreated or diagnosed late—leading to an increased mortality and morbidity (10, 32, 33)—sensitivity of screening should be conceptually high to avoid false-negative results. Although the frequency of CS was low in some categories of the chief complaints, our data do not argue against screening in those instances.
Psychiatric disorders were a very uncommon reason for patient referrals for screening. This is interesting, as depression or/and other psychiatric disorders are very common in patients with CS, affecting up to 80% of patients (5, 34, 35). The prevalence of depression (36) is high but CS seems to be seldom suspected in this group. Future studies are needed to analyze the prevalence of CS in patients with psychiatric disorders. PCOS-like symptoms were a common reason for consultation. In a retrospective study, Brzana et al (37) showed a high prevalence of former treatment for suspected PCOS in patients with confirmed CS. It seems to be very reasonable to screen women with these symptoms or this diagnosis for CS as well because the prevalence of CS seems to be high in this group but so far, there have not been any prospective studies to evaluate the prevalence of CS among patients with PCOS.
Obesity
Obesity, which was the most common reason for patient referrals to our center, was associated with a very low pretest probability of diagnosing CS. Furthermore, as known by a study by Baid et al (38) , biochemical screening tests can be falsely abnormal in patients with obesity, another argument against widespread screening in this group. Similar results were reported by Catargi et al (39) in 200 obese patients with type 2 diabetes mellitus. A quarter of them showed abnormal results in the 1-mg low-dose dexamethasone suppression test, and CS was finally confirmed in only 4 patients. Controversially, Javorsky and colleagues (40) identified in a small but multicentric study 12 patients in whom CS was diagnosed after performance of bariatric surgery. However, half these patients suffered additionally from hypertension and/or diabetes mellitus (40). Based on these data and other case reports, there are screening recommendations for CS in patients seeking bariatric surgery (41-43). In contrast, in a recent clinical score for CS, a BMI above 30 was a negative predictor for CS (44). In a study by Abraham et al (45), quality of life was assessed by the 36-Item Short Form Health Survey in obese patients and patients with CS, showing significant differences between the groups; while obese patients had a better mean physical component summary score, the mean mental component summary score was lower. This might be additionally helpful to help decide which patients with obesity should be screened for CS (45). Our data endorse the recommendation against screening in patients with long-lasting obesity as a singular problem or main complaint. In our cohort, patients with obesity had few other features typical for CS. However, obesity should not be discounted when other features consistent with CS are present. All in all, patients with obesity should be carefully clinically evaluated to decide whether to screen them for CS.
Other Recommendations and Economical Perspectives
Referring to additional expert reviews, it should be noted that there are authors who plead for a more expansive screening approach, for example, in patients with diabetes, stating that the prevalence of CS is underrated in this condition (46). Our data suggest that the prevalence might indeed be higher in those patient groups. Tabarin and Perez (14), however, argue against systematic screening approaches because of their limited benefit. Viewed from another angle, approaches in the diagnosis of CS should be within a reasonable economical frame. As known from a US study, patients with CS have significantly higher health care costs, whereas costs decrease substantially after successful surgery (47). A Canadian cost-of-illness analysis showed similar results (48). To minimize health care–related costs, it would be beneficial to diagnose patients as early as possible. However, to date, it is unclear how to offer extended screening approaches that are reasonably economical.
Limitations and Strengths
This study has limitations; the most important is its monocentric design. In some health care systems the referral for consultations might be more selective because of reimbursement policies, which can affect screening outcomes. Further prospective studies could be beneficial to validate our findings in other health care settings. Our data might be valid for the health care system in Germany, but could differ considerably in other countries and health care systems. In addition, we cannot comment on racial disparities. However, based on the available literature, we do not expect any (49). The strength of the study depends on the high number of patients and its prospective design.
Conclusion
Besides several screening approaches, 2 clinical scores have been developed in recent years to identify patients who should be screened for CS (44, 50). To date, validation studies for these scores are missing. To that point, their value in everyday clinical practice remains uncertain. Analyzing presenting problems in patients with suspected CS revealed that 5 reasons for screening increase the likelihood of having CS (myopathy, metabolic syndrome, osteoporosis, adenoma, and multiple CS-specific symptoms), while obesity as chief complaint is the single factor to significantly decrease probability of CS. Although clinical practice differs from official recommendations, our study underlines the validity of the recommendations of the 2008 Endocrine Society Practice guideline: Patients falling into 1 of the 3 at-risk groups of patients having a reasonable to high likelihood for CS justify screening.
Acknowledgment
This work is part of the German Cushing’s Registry CUSTODES.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- BMI
body mass index
- CS
Cushing syndrome
- PCOS
polycystic ovary syndrome
Contributor Information
Leah T Braun, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Frederick Vogel, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Stephanie Zopp, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Thomas Marchant Seiter, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
German Rubinstein, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Christina M Berr, Department of Endocrinology, I. Medical Clinic, University Hospital, University of Augsburg, 86156 Augsburg, Germany.
Heike Künzel, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Felix Beuschlein, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany; Klinik für Endokrinologie, Diabetologie und Klinische Ernährung, Universitätsspital Zürich (USZ) und Universität Zürich (UZH), 8091 Zurich, Switzerland.
Martin Reincke, Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität München, 80336 Munich, Germany.
Financial Support
This work was supported by the Else Kröner-Fresenius Stiftung (grant Nos. 2012_A103 and 2015_A228 to M.R.); the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, Projektnummer: 314061271-TRR 205 to M.R. and F.B.); is supported by the Clinician Scientist Program RISE (Rare Important Syndromes in Endocrinology, to L.T.B.), supported by the Else-Kröner-Fresenius Stiftung and Eva Luise und Horst Köhler Stiftung; the DFG (No. 413635475 to F.V.); and the Munich Clinician Scientist Program (MCSP) of the LMU München.
Disclosures
The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1. Steffensen C, Bak AM, Rubeck KZ, Jørgensen JOL. Epidemiology of Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):1-5. [DOI] [PubMed] [Google Scholar]
- 2. Rubinstein G, Osswald A, Hoster E, et al. Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J Clin Endocrinol Metab. 2020;105(3):e12–e22. [DOI] [PubMed] [Google Scholar]
- 3. Newell-Price J, Grossman A. Diagnosis and management of Cushing’s syndrome. Lancet. 1999;353(9170):2087-2088. [DOI] [PubMed] [Google Scholar]
- 4. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602. [DOI] [PubMed] [Google Scholar]
- 5. Braun LT, Riester A, Oßwald-Kopp A, et al. Toward a diagnostic score in Cushing’s syndrome. Front Endocrinol (Lausanne). 2019;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33-M38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605-1617. [DOI] [PubMed] [Google Scholar]
- 8. Braun LT, Zopp S, Vogel F, et al. Signs, symptoms and biochemistry in recurrent Cushing disease: a prospective pilot study. Endocrine. 2021;73(3):762-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scaroni C, Albiger NM, Palmieri S, et al. Altogether to Beat Cushing’s Syndrome (ABC) Study Group. Approach to patients with pseudo-Cushing’s states. Endocr Connect. 2020;9(1):R1-R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Javanmard P, Duan D, Geer EB. Mortality in patients with endogenous Cushing’s syndrome. Endocrinol Metab Clin North Am. 2018;47(2):313-333. [DOI] [PubMed] [Google Scholar]
- 11. Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167(3):311-326. [DOI] [PubMed] [Google Scholar]
- 12. Ross EJ, Linch DC. Cushing’s syndrome—killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet. 1982;2(8299):646-649. [DOI] [PubMed] [Google Scholar]
- 13. Shimon I. Screening for Cushing’s syndrome: is it worthwhile? Pituitary. 2015;18(2):201-205. [DOI] [PubMed] [Google Scholar]
- 14. Tabarin A, Perez P. Pros and cons of screening for occult Cushing syndrome. Nat Rev Endocrinol. 2011;7(8):445-455. [DOI] [PubMed] [Google Scholar]
- 15. Reimondo G, Pia A, Allasino B, et al. Screening of Cushing’s syndrome in adult patients with newly diagnosed diabetes mellitus. Clin Endocrinol (Oxf). 2007;67(2):225-229. [DOI] [PubMed] [Google Scholar]
- 16. Terzolo M, Reimondo G, Chiodini I, et al. Screening of Cushing’s syndrome in outpatients with type 2 diabetes: results of a prospective multicentric study in Italy. J Clin Endocrinol Metab. 2012;97(10):3467-3475. [DOI] [PubMed] [Google Scholar]
- 17. Mullan K, Black N, Thiraviaraj A, et al. Is there value in routine screening for Cushing’s syndrome in patients with diabetes? J Clin Endocrinol Metab. 2010;95(5):2262-2265. [DOI] [PubMed] [Google Scholar]
- 18. Aresta C, Soranna D, Giovanelli L, et al. When to suspect hidden hypercortisolism in type 2 diabetes: a meta-analysis. Endocr Pract. 2021;27(12):1216-1224. [DOI] [PubMed] [Google Scholar]
- 19. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193-202. [DOI] [PubMed] [Google Scholar]
- 20. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12(5):609-615. [DOI] [PubMed] [Google Scholar]
- 21. Tiryakioglu O, Ugurlu S, Yalin S, et al. Screening for Cushing’s syndrome in obese patients. Clinics. 2010;65(1):9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Findling JW, Raff H. Screening and diagnosis of Cushing’s syndrome. Endocrinol Metab Clin North Am. 2005;34(2):385-402, ix-x. [DOI] [PubMed] [Google Scholar]
- 23. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 24. Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieman LK. Screening for reversible osteoporosis: is cortisol a culprit? Ann Intern Med. 2007;147(8):582-584. [DOI] [PubMed] [Google Scholar]
- 26. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med. 2007;147(8):541-548. [DOI] [PubMed] [Google Scholar]
- 28. Nieman LK. Diagnosis of Cushing’s syndrome in the modern era. Endocrinol Metab Clin North Am. 2018;47(2):259-273. [DOI] [PubMed] [Google Scholar]
- 29. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braun LT, Fazel J, Zopp S, et al. The effect of biochemical remission on bone metabolism in Cushing’s syndrome: a 2-year follow-up study. J Bone Miner Res. 2020;35(9):1711-1717. [DOI] [PubMed] [Google Scholar]
- 31. Reimondo G, Pia A, Bovio S, et al. Laboratory differentiation of Cushing’s syndrome. Clin Chim Acta. 2008;388(1-2):5-14. [DOI] [PubMed] [Google Scholar]
- 32. Valassi E, Tabarin A, Brue T, et al. High mortality within 90 days of diagnosis in patients with Cushing’s syndrome: results from the ERCUSYN registry. Eur J Endocrinol. 2019;181(5):461-472. [DOI] [PubMed] [Google Scholar]
- 33. Vogel F, Braun LT, Rubinstein G, et al. Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J Clin Endocrinol Metab. 2020;105(12):e4490-e4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol (Oxf). 1996;45(6):715-720. [DOI] [PubMed] [Google Scholar]
- 35. Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ayuso-Mateos JL, Vázquez-Barquero JL, Dowrick C, et al. ODIN Group. Depressive disorders in Europe: prevalence figures from the ODIN study. Br J Psychiatry. 2001;179(4):308-316. [DOI] [PubMed] [Google Scholar]
- 37. Brzana J, Yedinak CG, Hameed N, Plesiu A, McCartney S, Fleseriu M. Polycystic ovarian syndrome and Cushing’s syndrome: a persistent diagnostic quandary. Eur J Obstet Gynecol Reprod Biol. 2014;175:145-148. [DOI] [PubMed] [Google Scholar]
- 38. Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab. 2009;94(10):3857-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Catargi B, Rigalleau V, Poussin A, et al. Occult Cushing’s syndrome in type-2 diabetes. J Clin Endocrinol Metab. 2003;88(12):5808-5813. [DOI] [PubMed] [Google Scholar]
- 40. Javorsky BR, Carroll TB, Tritos NA, et al. Discovery of Cushing’s syndrome after bariatric surgery: multicenter series of 16 patients. Obes Surg. 2015;25(12):2306-2313. [DOI] [PubMed] [Google Scholar]
- 41. Fleseriu M, Ludlam WH, Teh SH, Yedinak CG, Deveney C, Sheppard BC. Cushing’s syndrome might be underappreciated in patients seeking bariatric surgery: a plea for screening. Surg Obes Relat Dis. 2009;5(1):116-119. [DOI] [PubMed] [Google Scholar]
- 42. Stiles LES. Cushing syndrome and bariatric surgery: why, when, and how to evaluate preoperatively. Surg Obes Relat Dis. 2009;5(1):119-121. [DOI] [PubMed] [Google Scholar]
- 43. Savastano S, Pivonello R, Colao A. Bariatric surgery for obesity and hidden Cushing syndrome. Surg Obes Relat Dis. 2009;5(1):121-122. [DOI] [PubMed] [Google Scholar]
- 44. Parasiliti-Caprino M, Bioletto F, Frigerio T, et al. A new clinical model to estimate the pre-test probability of Cushing’s syndrome: the Cushing score. Front Endocrinol (Lausanne). 2021; 12:747549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abraham SB, Abel BS, Rubino D, Nansel T, Ramsey S, Nieman LK. A direct comparison of quality of life in obese and Cushing’s syndrome patients. Eur J Endocrinol. 2013;168(5):787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guaraldi F, Salvatori R. Cushing syndrome: maybe not so uncommon of an endocrine disease. J Am Board Fam Med. 2012;25(2):199-208. [DOI] [PubMed] [Google Scholar]
- 47. Swearingen B, Wu N, Chen SY, Pulgar S, Biller BMK. Health care resource use and costs among patients with Cushing disease. Endocr Pract. 2011;17(5):681-690. [DOI] [PubMed] [Google Scholar]
- 48. Van Uum S, Hurry M, Petrella R, Koch C, Dranitsaris G, Lacroix A. Management of patients with Cushing’s disease: a Canadian cost of illness analysis. J Popul Ther Clin Pharmacol. 2014;21(3):e508-e517. [PubMed] [Google Scholar]
- 49. Ioachimescu AG, Goswami N, Handa T, Pappy A, Veledar E, Oyesiku NM. Racial disparities in acromegaly and Cushing’s disease: a referral center study in 241 patients. J Endocr Soc. 2022;6(1):bvab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. León-Justel A, Madrazo-Atutxa A, Alvarez-Rios AI, et al. Spanish CRISALIDA Study Group. A probabilistic model for Cushing’s syndrome screening in at-risk populations: a prospective multicenter study. J Clin Endocrinol Metab. 2016;101(10):3747-3754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.