Abstract
Context
Sleeve gastrectomy (SG) improves metabolic endpoints but is associated with impaired bone outcomes.
Objective
To determine mechanisms contributing to impaired bone health in youth following SG.
Methods
12-month longitudinal observational study in a multidisciplinary tertiary-care hospital, including 64 youth 13-25 years old with moderate-to-severe obesity (51 females); 30 underwent SG and 34 were nonsurgical (NS) controls. SG was undertaken after a combined decision-making process between treatment team and patient. The main outcome measures were fasting blood for enteric peptides, sex steroids, sclerostin, and bone turnover markers (N-terminal propeptide of type 1 procollagen [P1NP] and C-terminal cross-linking telopeptide [CTX]); dual-energy X-ray absorptiometry measures of areal bone mineral density (aBMD) and body composition; high resolution peripheral quantitative computed tomography; measures of volumetric BMD (vBMD); microfinite element analysis of strength estimates (distal radius and tibia).
Results
SG had greater reductions in body mass index (BMI) z-scores, serum estrone, and the free androgen index (FAI) (P ≤ .046), and greater increases in sclerostin, P1NP, and CTX (P ≤ .010) than NS controls. Fasting ghrelin decreased in SG vs NS (P < .0001); fasting peptide YY did not change. Most changes were driven by female SG participants. Among females (the majority of study participants), after controlling for baseline age and race, reductions in total hip aBMD Z-scores were positively associated with changes in BMI, lean mass, estrone, FAI, and ghrelin, and inversely with changes in sclerostin.. Decreases in total vBMD of the radius and tibia were associated positively with decreases in BMI. Increases in CTX were associated with decreases in BMI, lean mass, and ghrelin, and increases in sclerostin.
Conclusion
Bone loss after SG in youth is associated with changes in body composition, sex steroids, sclerostin, and enteric peptides. These are potential targets for future preventative or therapeutic strategies.
Keywords: bariatric surgery, adolescents, bone density, bone microarchitecture, gut peptides, sleeve gastrectomy
With the rising prevalence of obesity, metabolic and bariatric surgery (MBS) is increasingly performed in adolescents and young adults with moderate-to-severe obesity for weight reduction and to ameliorate associated comorbidities (1-3). In adolescents with obesity, the most common MBS is sleeve gastrectomy (SG), which improves many metabolic outcomes (3). However, SG leads to substantial reductions in bone mineral density (BMD) and alterations in bone geometry and microarchitecture in adolescents (4). Adolescence is a critical time for bone accrual toward attainment of peak bone mass, and deficits in bone acquisition following SG during this time may result in deficits in bone health across the lifespan (5). Given the many metabolic benefits of MBS, it is important to determine strategies to prevent the associated bone loss. This requires an understanding of factors that contribute to reduced BMD.
The detrimental effects of MBS on bone are multifactorial, and may include mechanical unloading from weight loss, changes in body composition (such as a reduction in lean mass), calcium and vitamin D deficiency due to decreased absorption postsurgery of these micronutrients, and alterations in hormones that impact bone health, such as the sex steroids and enteric peptides (6-10).
Mechanical unloading following MBS likely increases bone loss from an increase in sclerostin, which inhibits bone formation and increases bone resorption (11, 12). Sclerostin is a product of osteocytes, the mechanosensors of bone (12). Also, weight loss is associated with a reduction in fat and lean mass, and lean mass is a key determinant of BMD at almost every site, weight-bearing and nonweight-bearing, as the pull of muscle on bone is bone anabolic (13, 14). Further, normal calcium and vitamin D status are important for normal bone mineralization and may be altered following MBS (15). In previous studies in adults, estrogen concentrations have been demonstrated to decrease postsurgery from reduced aromatization of androgens, subsequent to loss of subcutaneous fat mass (16, 17). Estrogen inhibits bone resorption through its effects on osteoprotegerin and RANK-L secretion by osteoblasts and may lower sclerostin and preadipocyte factor-1 (pref-1) levels, leading to increased bone formation (18-20). Pref-1 typically inhibits differentiation of mesenchymal stem cells in marrow to osteoblasts (21). SG in adults also alters levels of enteric peptides that can impact bone health (22-24). Serum concentrations of ghrelin (which is bone anabolic) decrease after SG, while concentrations of peptide YY (PYY), an osteoblast inhibitor, have been reported in some studies to increase postsurgery (25, 26).
Of note, data are very limited regarding the impact of SG on sex steroids and enteric peptides in adolescents and young adults. Similarly, the impact of changes in body composition and circulating hormone concentrations on bone has not been assessed in youth undergoing SG. Our objective was to examine the impact of SG in youth on sex steroids, sclerostin, pref-1, and enteric peptides, and to determine whether these alterations impact bone deleteriously. We hypothesized that SG would result in reductions in estradiol and estrone (aromatized from androgens in subcutaneous fat), bioavailable testosterone, and ghrelin, and increases in sclerostin, pref-1, and PYY over 1 year, and that these alterations would be associated with reductions in a marker of bone formation, the N-terminal propeptide of type 1 procollagen (P1NP), and an increase in a marker of bone resorption, C-terminal cross-linking telopeptide (CTX), with reductions in areal and volumetric BMD (vBMD), and alterations in strength estimates (failure load).
Patients and Methods
Participant Selection
We recruited 64 adolescents and young adults, 13-25 years of age, and with moderate-to-severe obesity (body mass index [BMI] ≥ 35 kg/m2 with ≥1 obesity-related comorbidity or a BMI ≥ 40 kg/m2) by advertising in several tertiary-care obesity treatment centers and physician practices between 2015 and 2020. Nonsurgical (NS) controls were also recruited through advertisements in college job boards and institutional recruitment forums. Of these participants, 30 underwent SG, and 34 were NS controls followed with routine management (counseling regarding diet and exercise, sleep habits, and screen time) provided by their primary-care or other providers. Exclusion criteria for both groups included (1) use of oral glucocorticoids or other medications that may affect bone metabolism (other than calcium, vitamin D, or hormonal contraception) within 8 weeks of the baseline visit, (2) use of antipsychotic medications that cause weight gain if treated for less than 6 months or if the dose was not stable for at least 2 months prior to study enrollment, (3) untreated thyroid dysfunction or if the participant was on a stable dose of replacement levothyroxine for fewer than 3 months before study enrollment, and (4) history of smoking more than 10 cigarettes per day or of substance abuse (per DSM-5). We did not exclude females on combined oral hormonal contraception because of the large number of females in this age range who take these medications for management of polycystic ovarian disease or for contraception, but we did exclude participants on depot medroxyprogesterone, given its known and profound effect on bone. Further, we did not exclude females using the progestin-releasing intrauterine device given its limited systemic effects, or those on progestin implants given their limited bone effect (27). The study was approved by our Institutional Review Board and is Health Insurance Portability and Accountability Act compliant. Informed consent was obtained from participants ≥18 years, or parents of participants <18 years. Informed assent was obtained from participants <18 years old. The study is registered in ClinicalTrials.gov (NCT02557438).
Methods
Following a screening visit to confirm eligibility, study visits were performed prior to SG (baseline) and 12 months after surgery. SG participants followed the standard postoperative nutrition guidelines provided by their clinical team. NS controls similarly had study visits at baseline and again after 12 months of routine management. These participants were followed by their pediatricians/internists over the study duration with recommendations around diet, exercise, sleep habits, and screen time, and the frequency of follow-up was based on provider recommendations. Twenty-nine participants in each group returned for the 12-month follow-up visit. One participant in the SG group and 5 participants in the NS group did not complete the 12-month study visit (unresponsive on contact). Subjects self-reported race and ethnicity. At each visit, a thorough history was obtained, and a physical examination performed. Height was measured with a wall-mounted stadiometer as the mean of 3 measurements, and weight was measured to the nearest 0.1 kg with an electronic scale. Participants underwent dual-energy X-ray absorptiometry (DXA) scans at the spine, hip, and whole body less head, and high-resolution peripheral quantitative computed tomography (HRpQCT) scans at the distal radius and tibia. Moderate-to-vigorous physical activity was determined by recall using the Paffenbarger questionnaire (28). Fasting blood samples were obtained for bone turnover markers (P1NP and CTX), estradiol, estrone, sclerostin, pref-1, ghrelin, PYY, calcium, phosphorus, 25-hydroxy vitamin D (25OHD), and parathyroid hormone (PTH). Samples for bone turnover markers, estrogens, sclerostin, pref-1, ghrelin, and PYY were stored at –80°C. Depending on serum levels of 25OHD, NS participants were advised to take vitamin D supplementation through their primary-care providers as follows: for 25OHD levels between 20 and 30 ng/mL, 4000 IU of vitamin D supplementation per day; for 25OHD levels between 12 and 20 ng/mL, 50 000 IU per week for 2 months; for 25OHD levels below 12 ng/mL, 50 000 IU per week for 3 months. Surgical participants had a strict regimen of supplements and were asked to follow the guidelines of their clinical team. All participants were provided 1200 mg of calcium and 800 IU of vitamin D daily for the duration of the study. Not all 58 enrolled participants who completed both the baseline and 12-month visits had all study end points available at 12 months; however, all 58 participants had at least 1 or more study end points available at the 12-month timepoint. Reasons for missing data included motion artifact for HRpQCT scans, which rendered a few scans unusable for determining bone endpoints, and difficult blood draws in a few subjects, which resulted in insufficient volume of sample available for all biochemical testing.
Biochemical Analysis
Serum P1NP concentrations were measured by radioimmunoassay (Orion Diagnostics, Espoo, Finland; intra-assay coefficient of variation (CV) 3.5-5.3%, interassay CV 3.6-5.4%, sensitivity 0.7 μg/L) and serum CTX by an enzyme-linked immunosorbent assay (ELISA) (Immunodiagnostic Systems, Fountain Hills, AZ; intra-assay CV 5.2-6.8%, interassay CV 5.6-7.4%, sensitivity 0.02 ng/mL). Serum estradiol and estrone concentrations were measured by liquid chromatography tandem mass spectrometry (intra-assay CV <5%, interassay CV 12%, sensitivity 1 pg/mL), total testosterone by liquid chromatography tandem mass spectrometry (males: intra-assay CV <2%, interassay CV 7% RSD, sensitivity 1 ng/dL; females: intra-assay CV <5%, interassay CV 5%, sensitivity 1 ng/dL), and sex hormone binding globulin (SHBG) by a chemiluminescent immunoassay (Beckman Coulter, Fullerton, CA, intra-assay CV 4.5-4.8%, interassay CV 5.2-5.5%, sensitivity 0.3 nmol/L). ELISA was used to measure serum concentrations of sclerostin (R&D systems, intra-assay CV 2%, interassay CV 9.5%, sensitivity 1.74 pg/mL), pref-1 (R&D systems, DuoSet assay), ghrelin (EMD Millipore Corporation, MO; intra-assay CV 1.32%, interassay CV sensitivity 50 pg/mL), and PYY (Millipore Corporation, Billerica, MA, intra-assay CV 17-18%, interassay CV 12-18%, sensitivity 10 pg/mL). Calcium and phosphorus were measured using standard assays by a reference laboratory (Quest Diagnostics), while glucose concentrations were measured by the core laboratory of our institution. Serum 25OHD was measured by an immunochemiluminometric assay (Quest Diagnostics) and PTH by a chemiluminescent immunoassay (Beckman Coulter,Fullerton, CA; intra-assay CV 1.6-2.6%, interassay CV 2.8-5.8%, sensitivity 1 pg/mL). The free androgen index (FAI) was used as a measure of physiologically active testosterone and calculated as a ratio of fasting total testosterone (in nmol/L) divided by fasting SHBG (in nmol/L), multiplied by 100 (29).
Areal Bone Mineral Density and Body Composition Assessment
DXA (Hologic 4500 A, Apex software version 13.3; Hologic Inc, Waltham, MA) was used to assess areal BMD (aBMD) of the lumbar spine, total hip, femoral neck, and whole body less head, as well as measures of body composition (fat and lean mass). Coefficients of variation for aBMD, fat mass, and lean mass for our institution are 0.8% to 1.1%, 2.1%, and 1.0%, respectively. The Hologic pediatric database was used to calculate aBMD Z-scores for participants <20 years of age and the adult database for participants ≥20 years of age.
Volumetric Bone Mineral Density and Failure Load Assessment
HRpQCT (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) was used to measure vBMD, while failure load was estimated using microfinite element analysis via mathematical modeling of the effects of simulated mechanical load on bone (30). The scans were obtained at the nondominant limb unless there was a history of fracture, in which case the nonfractured side was assessed. As all subjects were at or near final adult height, we used a fixed region of interest defined by a distal edge 22.5 and 9.5 mm from the tibia and radius endplates, respectively. Same-day reproducibility for repeated measurements at our center is 0.2% to 1.4% for vBMD. The scans were matched at baseline and 12 months, with an average match percentage of 89% (range 69-100%) at the radius and 92.6% (range 72-100%) at the tibia. Two baseline scans were of poor quality and not included.
Statistical Analysis
JMP software version 16.0 was used for analysis. All data were initially assessed for normality using the Shapiro Wilk test and reported as mean ± standard error of the mean (for normally distributed data) or median and interquartile range (for data not normally distributed). For between group comparisons, the Student t-test or the Wilcoxon rank sum test was used depending on data distribution. For within-group comparisons (baseline vs 12-month data), the paired t-test or Wilcoxon signed rank test was used depending on data distribution. The study was powered at 96% at an alpha level of 0.0167 to detect meaningful difference in study endpoints with 25 completers in each group. The lower alpha value was selected because the protocol included a third group not included in the current analysis (participants undergoing gastric bypass surgery) to control for multiple comparisons. The gastric bypass cohort is still being recruited. Multivariate regression analysis was used to determine differences between groups after adjusting for baseline age, sex, and race. Spearman correlation analysis was performed for female participants to determine associations of 12-month change in bone parameters with 12-month changes in body composition and hormones; multivariate regression analysis was used to control for age and race. Only females were included in correlation analysis because they constituted the majority of the participants, and because of the marked differences in gonadal steroids in males vs females. We did not evaluate these associations in males given the small sample size. P < .05 was used to denote statistical significance.
Results
Baseline Characteristics and Changes Over 12 Months in Anthropometric Measures and Body Composition
Participants in the SG and NS groups did not differ for age, sex, race, or height (Table 1). Weight and BMI were higher in SG vs NS at baseline. As expected, weight, BMI, and BMI z-scores decreased significantly in the SG vs NS group, with a significant within-group decrease in weight, BMI, and BMI z-scores following SG. Groups did not differ for physical activity at baseline or 12 months. At baseline, fat mass was higher in the SG vs NS group, while lean mass did not differ. Lean mass and fat mass decreased in the SG vs NS group; within-group changes were significant in the SG group for both measures. At baseline, 3 NS participants and 1 SG participant had HbA1c concentrations ranging between 6.2% and 8.8%, and at 12 months all participants had HbA1C concentrations of less than 6%.
Table 1.
Baseline characteristics and changes over a year in anthropometric measures and body composition for sleeve gastrectomy and nonsurgical groups
| Baseline measures | P value | Change over 12 months | P value | P value adjusted for baseline age, sex, and race | |||
|---|---|---|---|---|---|---|---|
|
Surgical
(n = 30) |
Nonsurgical
(n = 34) |
Surgical
(n = 29) |
Nonsurgical
(n = 29) |
||||
| Clinical characteristics | |||||||
| Age (years) | 18.2 ± 0.4 (n = 30) |
17.7 ± .5 (n = 34) |
.550 | — | — | — | — |
| Sex (F/M) | 24/6 (n = 30) |
27/7 (n = 34) |
1.000 | — | — | — | — |
| Race (non-Black/black) | 7/23 (n = 30) |
6/28 (n = 34) |
.687 | — | — | — | — |
| Height (cm) | 169.1 (160.8, 174.2) (n = 30) |
165.2 (161.2, 171.3) (n = 34) |
.637 | .4 (–0.3, 1.2)* (n = 29) |
.4 (–0.1, 1.4)** (n = 29) |
.674 | .219 |
| Weight (kg) | 127.3 (111.3, 144.7) (n = 30) |
117.9 (99.5, 127.2) (n = 34) |
.007 † | –32.6 (–45.3, –26.7)**** (n = 29) |
2.6 (–2.3, 5.5) (n = 29) |
<.0001 | <.0001 |
| BMI (kg/m2) | 44.8 (41.7, 50.0) (n = 30) |
41.6 (37.9, 45.8) (n = 34) |
.003 † | –12.1 (–15.4, –9.1)**** (n = 29) |
0.6 (–1.2, 1.7) (n = 29) |
<.0001 | <.0001 |
| BMI z-score | 2.48 (2.34, 2.73) (n = 30) |
2.41 (2.21, 2.61) (n = 34) |
.113† | –0.70 (–0.99, –0.46)**** (n = 29) |
–0.03 (–0.10, –0.003)* (n = 29) |
<.0001 | <.0001 |
| Moderate to vigorous activity (hours/week) | 25.3 (9.3, 46.4) (n = 28) |
24.8 (13.4, 31.3) (n = 34) |
.826 | 9.0 (–4.5, 23.3) (n = 25) |
3.3 (–14.0, 22.5) (n = 29) |
.410 | .334 |
| Body composition (by DXA) | |||||||
| Total lean mass (kg) | 62.4 (56.1, 70.2) (n = 30) |
59.6 (51.2, 67.7) (n = 34) |
.168† | –10 (–1.3, –5.9)**** (n = 29) |
1.5 (–0.1, 3)** (n = 29) |
<.0001 | <.0001 |
| Total fat mass (kg) | 64.6 ± 2.4 (n = 30) |
55.2 ± 1.8 (n = 34) |
.002 † | –25.4 ± 2.2**** (n = 29) |
0.1 ± 1.0 (n = 29) |
<.0001 | <.0001 |
Data are presented as mean ± standard error of the mean or median (interquartile range); significant P values are in bold.
Between group differences in baseline measures and changes over 12 months were compared using the Student t-test for parametric data and the 2-sample Wilcoxon rank sum test for nonparametric data.
Abbreviations: BMI, body mass index; DXA, dual-energy X-ray absorptiometry.
† P < .05 after adjusting for baseline age, sex and race (Black vs non-Black) for baseline measures.
Within-group changes over 12-months were assessed using paired t-test (for parametric data) and using Wilcoxon signed rank test (for non-parametric data):
*P < .05,
** P < .01;
*** P < .001, and
**** P < .0001. indicate a significant within-group change over 12 months
Changes Over 12 Months in Bone Parameters
DXA and HRpQCT measures
Data for a subset of participants have been reported previously and are summarized here (4). Baseline bone parameters did not differ between SG vs NS groups. Significant within-group reductions were noted in aBMD at the lumbar spine, total hip, and femoral neck in the SG group, which significantly differed from changes in the NS group; differences between groups persisted after controlling for age, sex, and race (Table 2).
Table 2.
One-year change in DXA variables, HRpQCT bone parameters and bone turnover markers
| Baseline measures | P value | Change over 12 months | P value | P value adjusted for baseline age, sex, and race | |||
|---|---|---|---|---|---|---|---|
|
Surgical
(n = 30) |
Nonsurgical
(n = 34) |
Surgical
(n = 29) |
Non–Surgical
(n = 29) |
||||
| DXA variables | |||||||
| LS BMD Z-scores | 1.10 (0.50, 1.53) (n = 30) |
1.20 (0.55, 1.65) (n = 33) |
1.000 | –0.30 (–0.55, 0)**** (n = 29) |
0 (–0.25, 0.25) (n = 29) |
.015 | .027 |
| Total hip BMD Z-scores | 1.50 (0.75, 2.1) (n = 29) |
1.65 (0.78, 2.13) (n = 34) |
1.000 | –0.75 (–1.25, –0.40)**** (n = 28) |
–0.10 (–0.20, 0.10) (n = 29) |
<.0001 | <.0001 |
| FN BMD Z-scores | 1.40 (0.85, 1.90) (n = 29) |
1.60 (0.80, 2.35) (n = 34) |
.798 | –0.70 (–1.28, –0.30)**** (n = 28) |
0 (–0.40, 0.25) (n = 29) |
<.0001 | <.0001 |
| Whole body BMD Z-scores | -0.15 (-0.90, 0.43) (n = 30) |
-0.10 (-0.73, 0.80) (n = 34) |
.576 | 0 (–0.35, 0.50) (n = 29) |
0 (–0.40, 0.20) (n = 29) |
.906 | .878 |
| HRpQCT variables | |||||||
| Radius | |||||||
| Total vBMD (mgHA/cm3) | 345.3 ± 11.8 (n = 26) |
349.7 ± 11.3 (n = 31) |
.792 | –5.7 ± 3.0 (n = 20) |
2.7 ± 3.14 (n = 22) |
.076 | .083 |
| Cortical vBMD (mgHA/cm3) | 836.5 (796.8, 850.5) (n = 26) |
836.0 (809.3, 879.8) (n = 31) |
.537 | 15.6 (6.40, 28.7)*** (n = 20) |
6.20 (–6.50, 15.9)* (n = 22) |
.031 | .325 |
| Trabecular vBMD (mgHA/cm3) | 201.0 (171.9, 223.9) (n = 26) |
204.8 (169.2, 221.0) (n = 31) |
.904 | –7.00 (–17.58, –0.80)** (n = 20) |
0.70 (–2.20, 2.95) (n = 22) |
.002 | .001 |
| Failure load (N) | 4597 (4060, 5029) (n = 26) |
4510 (3952, 5423) (n = 31) |
.846 | –94.5 (–203.5, 105.3) (n = 20) |
3.5 (–185.0, 148.3) (n = 22) |
.458 | .575 |
| Tibia | |||||||
| Total vBMD (mgHA/cm3) | 342.7 (293.0, 373.9) (n = 23) |
356.6 (330.0, 381.8) (n = 34) |
.207 | 1.90 (–14.00, 5.70) (n = 21) |
4.10 (2.70, 7.45)*** (n = 25) |
.050 | .010 |
| Cortical vBMD (mgHA/cm3) | 869.2 (844.8, 881.6) (n = 23) |
867.7 (850.3, 898.0) (n = 34) |
.510 | 12.0 (3.4, 24.7)*** (n = 21) |
6.4 (4.1, 12.6)** (n = 25) |
.363 | .598 |
| Trabecular vBMD (mgHA/cm3) | 207.5 (197.0, 236.3) (n = 23) |
218.0 (197.5, 240.8) (n = 34) |
.542 | 2.3 (–15.6, 5.0) (n = 21) |
2.4 (–0.8, 3.7) (n = 25) |
.612 | .038 |
| Failure load (N) | 12912 (11798, 13782) (n = 23) |
12696 (11361, 14367) (n = 34) |
.770 | 135.0 (–544.5, 656.5) (n = 21) |
142.0 (–7.8, 452.8)* (n = 24) |
.420 | .220 |
| Bone markers | |||||||
| P1NP (ug/L) | 78.1 (64.2, 105.9) (n = 29) |
76.7 (61.2, 99.5) (n = 33) |
.905 | 9.8 (–9.9, 29.9) (n = 27) |
–14.1 (–27.9, 0.3)* (n = 28) |
.007 | .058 |
| CTX (ng/mL) | 0.49 (0.37, 0.70) (n = 28) |
0.42 (0.27, 0.79) (n = 34) |
.713 | 0.27 (0.10, 0.37)** (n = 27) |
–0.09 (–0.16, 0.02)* (n = 29) |
.0001 | .0001 |
Data are presented as mean ± standard error of the mean or median (interquartile range); significant P values are in bold.
Between-group differences in baseline measures and changes over 12 months were compared using the Student t-test for parametric data and the 2-sample Wilcoxon rank sum test for nonparametric data.
For between group comparisons at baseline: †P < .05 after adjusting for baseline age, sex and race (Black vs non-Black). Within-group changes over 12-months were assessed using paired t-test (for parametric data) and using Wilcoxon signed rank test (for non-parametric data):
*P < 0.05,
** P < 0.01;
*** P < 0.001, and
**** P < .0001 indicate a significant within-group change over 12 months.
Abbreviations: LS, lumbar spine; FN, femoral neck; vBMD, volumetric bone density; P1NP, N-terminal propeptide of type 1 procollagen; CTX, C-terminal cross-linking telopeptide.
Radius
Trabecular vBMD decreased while cortical vBMD increased in the SG group. An increase in cortical vBMD was observed in the NS group as well. SG and NS groups differed significantly for 12-month change in cortical and trabecular vBMD; the change in trabecular vBMD remained significant after adjusting for baseline age, sex, and race.
Tibia
The SG group did not demonstrate the 12-month increase in total vBMD and failure load observed in the NS group; both groups had an increase in cortical vBMD over 12 months. SG vs NS groups differed significantly for 12-month change in total and trabecular vBMD after adjusting for baseline age, sex, and race, with greater increases noted in the NS group.
Bone turnover markers
The SG group demonstrated a significant within-group increase in CTX, while the NS group had a within-group decrease in both P1NP and CTX over 12 months. The groups differed for changes in P1NP and CTX over 12 months. Changes in CTX remained significant after adjusting for baseline age, sex, and race. After stratification by sex, females had a significant reduction in P1NP in the SG vs NS groups 9.9 (–8.0, 31.0) vs –10.9 (–24.3, 2.8) µg/L, P = .027), while male participants tended to have an increase in CTX 0.20 (–0.21, 0.58) vs –0.16 (–0.45, 0.03) ng/mL, P = .083) (Table 2).
Changes Over 12 Months in Biochemical Parameters
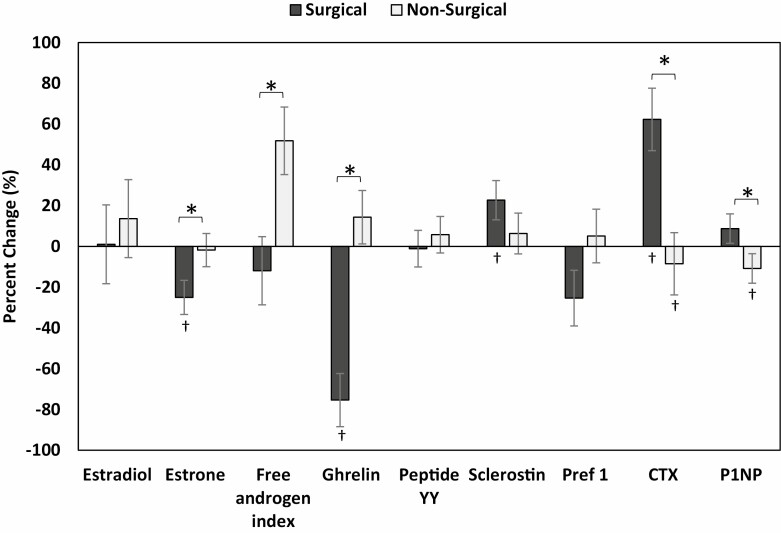
Biochemical parameters did not differ between SG and NS groups at baseline. Absolute changes in these parameters over 12. months are shown in Table 3, while percent changes are shown in Fig. 1.
Table 3.
Twelve-month change in sex steroids, sclerostin, pref-1, and enteric peptides following sleeve gastrectomy vs routine management
| Baseline measures | P value | Change over 12 months | P value | P value adjusted for baseline age, sex, and race | |||
|---|---|---|---|---|---|---|---|
|
Surgical
(n = 30) |
Nonsurgical
(n = 34) |
Surgical
(n = 27) |
Nonsurgical
(n = 29) |
||||
| Estradiol (pg/mL) | 45.50 (31.55, 54.70) (n = 29) |
48.10 (3.85, 76.80) (n = 33) |
.521 | –7.70 (–31.70, 27) (n = 27) |
–8.6 (–34.50, 16.18) (n = 28) |
.371 | .329 |
| Estrone (pg/mL) | 50.2 (39.3, 84.0) (n = 29) |
46.3 (38.4, 76.7) (n = 33) |
.667 | –17.3 (–39.6, 3.3)*** (n = 27) |
–2.9 (–12.8, 12.5) (n = 27) |
.019 | .091 |
| Total testosterone (ng/dL) | 35.2 (30.7, 60.1) (n = 28) |
42.3 (27.4, 71.2) (n = 33) |
.587 | –5.3 (–15.2, 10.5) (n = 27) |
1.9 (–5.8, 16.3) (n = 29) |
.297 | .022 |
| SHBG (nmol/L) | 23.09 (16.37, 40.46) (n = 29) |
24.77 (14.48, 33.90) (n = 33) |
.778 | 15.28 (6.12, 40.32)**** (n = 27) |
–0.45 (–7.65, 6.08) (n = 28) |
.0003 | .0004 |
| Free androgen index (ng/mL) | 6.40 (2.60, 10.17) (n = 28) |
6.30 (3.58, 22.60) (n = 33) |
.659 | –1.27 (–5.15, 0.33) (n = 26) |
0.65 (–2.53, 4.51) (n = 28) |
.046 | .738 |
| Sclerostin (pg/mL) | 130.2 (106.3, 161.9) (n = 28) |
148.0 (104.7, 212.7) (n = 34) |
.511 | 14.9 (–14.6, 63.0)* (n = 27) |
–17.4 (–58.3, 49.8) (n = 28) |
.010 | .155 |
| Pref 1 (pg/mL) | 328.2 (128.7, 531.5) (n = 25) |
243.3 (85.7, 346.8) (n = 31) |
.401 | –47.9 (–153.3, 12.3)* (n = 23) |
–9.5 (–52.4, 21.0) (n = 27) |
.150 | .181 |
| Ghrelin (pg/mL) | 206.1 (97.3, 415.8) (n = 29) |
183.6 (129.8, 314.0) (n = 32) |
.549 | –179.1 (–291.4, –80.9)**** (n = 24) |
23.9 (–34.3, 137.1) (n = 27) |
<.0001 | <.0001 |
| PYY (pg/mL) | 80.0 (65.0, 111.0) (n = 29) |
91.0 (61.5, 106.0) (n = 33) |
.927 | –5.0 (–25.0, 7.0) (n = 27) |
–8.0 (–17.8, 16.0) (n = 29) |
.692 | .383 |
| 25OHD (ng/mL) | 21.9 (17.1, 33.9) (n = 30) |
21.9 (17.2, 3.1) (n = 33) |
.563 | 0.9 (–3, 9) (n = 27) |
–0.5 (–6.8, 1.8) (n = 29) |
.127 | .039 |
| PTH (pg/mL) | 37.1 (31.3, 45.4) (n = 29) |
37.7 (29.2, 52.6) (n = 33) |
.730 | 0.8 (–14.1, 3.4) (n = 27) |
0.1 (–8.3, 13.5) (n = 28) |
.471 | .239 |
Data are presented as median (interquartile range) since data were non-parametric); significant P values are bolded.
Between group differences in baseline measures and changes over 12 months were compared using the 2-sample Wilcoxon rank sum test.
For between group comparisons at baseline: †P < .05 after adjusting for age, sex, and race.
Within-group changes over 12-months were assessed using Wilcoxon signed rank test:
*P < 0.05,
** P < 0.01;
*** P < 0.001 and
**** P < .0001. indicate a significant within-group change over 12 months
Abbreviations: SHBG, sex hormone binding globulin; PYY, peptide YY; 25OHD, 25-hydroxycholecalciferol; PTH, parathyroid hormone.
Figure 1.
Percent change in biochemical parameters in the nonsurgical and surgical groups (after controlling baseline age, sex and race) over 12 months, *P < .05 for between group comparison, †P < .05 for within-group change.
Sex steroids
The SG group demonstrated a significant within-group decrease in estrone and increase in SHBG over 12 months and differed from NS for changes in estrone, SHBG, and the FAI. After controlling for age, sex, and race, 12-month change in estrone trended lower, while change in SHBG was higher in the SG vs NS groups; SG had greater reductions in testosterone over 12 months. After stratification by sex, most differences in the SG vs NS groups were only seen in females, who had a significant decrease in estrone (–21.8 [–41.0, –4.8] vs –3.2 [–27.4, 16.1] pg/mL, P = .038), total testosterone (–9.3 [–16.2, 2.7] vs –0.7 [–6.5, 7.6] ng/dL, P = .022), and FAI (–1.51 [–5.2, –0.6] vs 0.18 [–2.8, 2.7], P = .019), and a significant increase in SHBG (22.6 [6.3, 42.6] vs 0 [–7.6, 7.7] nmol/L, P = .001) levels in the SG vs NS groups. At baseline, 5 participants in the SG group and 12 participants in the NS group were on contraceptive medications, and at 12 months 7 participants in the SG group and 14 participants in the NS group were on such medications. Participants who were on hormonal contraceptives at baseline continued on the same medication at 12 months. Further, 2 participants in each group started on oral contraceptives over the 12-month study duration. On excluding participants in both groups who were on hormonal contraceptive medications, our results did not change substantially. Similarly, on only excluding the 2 participants in each group who started oral contraceptives after the baseline visit, our results did not change substantially.
Male participants demonstrated a marked increase in total testosterone (235.0 [187.5, 352.0] vs 35.5 [–11.3, 84.5] ng/dL, P = .014) and SHBG (8.6 [2.0, 12.3] vs –3.7 [–9.4, 1.9] nmol/L, P = .023) levels, with no net change in FAI (26.9 [–4.3, 62.7] vs 11.9 [–1.0, 32.8], P = .523) in the SG vs NS groups.
Reductions in estrone were positively associated with reductions in BMI, lean mass, and fat mass, while reductions in the FAI were also positively associated with reductions in lean mass (data not shown).
Sclerostin and pref-1
Over 12 months, sclerostin increased while pref-1 decreased in the SG group. The 12-month change in sclerostin differed significantly between groups in univariate analysis but not after adjusting for age, sex, and race. Differences between groups for sclerostin and pref-1 were no longer evident after sex stratification. Increases in sclerostin were inversely associated with reductions in BMI, and lean and fat mass (data not shown). Associations with changes in Pref-1 were not evaluated as circulating concentrations did not change following SG.
Enteric peptides
Significant within- and between-group decreases in ghrelin were noted in the SG vs NS groups that persisted after controlling for age, sex, and race. Groups did not differ for changes in PYY over 12 months. After stratification by sex, the SG group demonstrated a significant reduction in ghrelin in both females (–181.1 [–290.8, –13.7] vs 53.9 [–46.7, 154.6] pg/mL, P = .0001) and males (–89.0 [–310.6, 61.1] vs 5.56 [–41.6, 41.7] pg/mL, P = .023). Reductions in ghrelin were positively associated with reductions in BMI, lean mass, and fat mass (data not shown). Associations with changes in PYY were not evaluated as circulating concentrations did not change following SG.
25OHD and PTH
The SG group had greater increases in 25OHD than the NS group after adjusting for age, sex, and race. There was no within- or between-group change in PTH levels over 12 months. After stratification by sex, females (but not males) demonstrated an increase in 25OHD levels (2 [–2.5, 11.7] vs 0 [–6.5, 1.3] ng/mL, P = .047) in the SG vs NS groups. Associations with changes in PTH were not evaluated as circulating concentrations did not change following SG.
Associations Between Changes in Bone Parameters and Changes in Body Composition and Biochemical Parameters
Table 4 shows associations of changes in bone parameters with changes in body composition, sex steroids, sclerostin, and enteric peptides over 12 months in all female participants. Overall, the magnitude of the associations was similar or slightly lower within the SG group. Male participants were not included in this association analysis because of known differences in sex hormones between the sexes, and the smaller number of males in the study.
Table 4.
Associations of changes in bone turnover markers and bone parameters with changes in body composition measures, sex steroids, and enteric peptides over 12-months in cohort with female participants only
| 12-month change | BMI | Lean Mass | Estrone | FAI | Sclerostin | Ghrelin |
|---|---|---|---|---|---|---|
| ρ | ρ | ρ | ρ | ρ | ρ | |
| Bone turnover markers | ||||||
| P1NP | –0.455 * , a | –0.251 | –0.042 | 0.060 | 0.546 * | –0.306 |
| CTX | –0.559 * , a | –0.571 * , a | –0.173 | –0.112 | 0.401 * , a | –0.423 * , a |
| DXA | ||||||
| LS BMD Z-scores | 0.212 | 0.202 | 0.170 | 0.342 * | –0.089 | 0.198 |
| Total hip BMD Z-scores | 0.699 * , a | 0.735 * , a | 0.387 * , a | 0.425 * , a | –0.165a | 0.358 * |
| FN BMD Z-scores | 0.463 * , a | 0.502 * , a | 0.257a | 0.089 | –0.052 | 0.352 * , a |
| Whole body BMD Z-scores | –0.273 | –0.063 | 0.147 | 0.163 | –0.005 | 0.024 |
| HRpQCT | ||||||
| Radius | ||||||
| Total vBMD | 0.404 * , a | 0.277 | 0.263 | –0.000 | 0.043 | 0.070 |
| Cortical vBMD | –0.105 | –0.252 | 0.098 | 0.058 | 0.076 | –0.287 |
| Trab vBMD | 0.617 * , a | 0.424 * , a | 0.128 | –0.013 | –0.100 | 0.341 a |
| Failure load | 0.156 | 0.040 | 0.111 | 0.220 | –0.050 | 0.222 |
| Tibia | ||||||
| Total vBMD | 0.342 * , a | 0.285 | 0.221 | 0.085a | –0.217 | 0.280 |
| Cortical vBMD | –0.113 | –0.142 | –0.095 | –0.350 * | –0.380 * | 0.068 |
| Trabecular vBMD | –0.034 | –0.086 | 0.215 | 0.053a | –0.057 | 0.012 |
| Failure load | 0.109 | 0.091 | 0.257 | –0.110 | –0.138 | –0.012 |
Spearman correlations (ρ) are reported.
Abbreviations: BMI, body mass index; BMD, bone mineral density; FAI, free androgen Index; P1NP, N-terminal propeptide of type 1 procollagen; CTX, C-terminal cross-linking telopeptide; LS, lumbar spine; FN, femoral neck; vBMD, volumetric bone mineral density.
*P < 0.05 (also in bold);
a P < .05 after controlling for baseline age and race.
Association of changes in bone parameters with changes in body composition
Inverse associations were noted of changes in BMI with changes in CTX and P1NP, and of changes in lean mass with changes in CTX over 12 months. Greater decreases in BMI and lean mass predicted greater decreases in total hip and femoral neck BMD Z-scores, and radial trabecular vBMD. Similarly, decreases in BMI were associated with decreases in radial and tibial total vBMD. All associations remained significant after controlling for age and race.
Associations of changes in bone parameters with changes in sex steroids
Decreases in estrone were associated with decreases in total hip and femoral neck BMD Z-scores after controlling for age and race. No significant associations were noted of changes in estradiol with changes in bone parameters (data not shown). Decreases in FAI were associated with decreases in lumbar spine and total hip BMD Z-scores and with increases in tibial cortical vBMD. After controlling for age and race, decreases in FAI were associated with decreases in femoral neck BMD Z-scores and tibial total vBMD.
Associations of changes in parameters with changes in sclerostin
Increases in sclerostin were associated with increases in P1NP and CTX, and with decreases in tibial cortical vBMD over 12 months. After controlling for age and race, associations remained significant for changes in CTX over 12 months.
Associations of changes in bone parameters with changes in enteric peptides
Decreases in ghrelin were associated with decreases in total hip and femoral neck BMD Z-scores, and with increases in CTX over 12 months. After controlling for age and race, decreases in ghrelin continued to be associated with decreases in femoral neck BMD Z-scores and also with decreases in radial trabecular vBMD, and with increases in CTX.
Associations of changes in bone parameters with changes in 25OHD and PTH
Changes in 25OHD were associated positively with changes in tibial cortical vBMD (r = 0.513, P = .002), while increases in PTH were associated with decreases in tibial cortical vBMD (r = – 0.372, P = .026) over 12 months. After controlling for age and race, these associations continued to be significant.
Discussion
Adolescents and young adults with moderate-to-severe obesity who underwent SG demonstrated the expected reductions in BMI, fat mass, and lean mass, and changes in DXA and HRpQCT endpoints compared with NS controls. Further, our data demonstrate reductions in the sex steroids (estrone and FAI), increases in sclerostin, and reductions in ghrelin over 12 months in the SG vs NS groups. The SG group also had a significant increase in CTX, a marker of bone resorption, compared with the NS group. We observed associations between changes in body composition, sex steroids, sclerostin, and ghrelin with changes in bone parameters over the 12-month study period. To our knowledge, this is the first study reporting changes in sex steroids, sclerostin, and enteric peptides following SG, and associations of changes in these parameters with changes in bone endpoints over 12 months.
Changes in Body Composition and Bone Parameters
Data from this larger cohort replicate DXA and HRpQCT findings previously reported in a subset of our patients following SG (4). Positive associations of changes in BMI with changes in bone parameters are consistent with the effect of mechanical unloading from weight loss and associated changes in body composition on bone outcomes (3, 4, 7, 31). We observed an increase in P1NP and CTX levels after SG associated with reductions in BMI, fat mass, and lean mass, indicating that increased bone turnover likely contributes to the decrease in bone density after surgery; these findings are similar to those reported in a study in adults following SG (32).
Changes in Sex Steroids, Sclerostin, and Pref-1
While estradiol is primarily synthesized by the aromatization of testosterone to estradiol in the gonads and to a lesser extent in other tissues, aromatization of the adrenal androgens to estrone in adipose tissue is a significant source of estrogen in people with obesity (33, 34). In a meta-analysis of adults undergoing a variety of MBS procedures, circulating estradiol decreased after MBS. However, in the current study, we observed reductions only in estrone but not estradiol after SG (16). The association of decreases in estrone with decrease fat mass is consistent with loss of adipose tissue causing a reduction in aromatization of androgens to estrone (16, 35). Further, Escobar-Morreale et al (16) reported an increase in total testosterone in males and a decrease in females with an increase in SHBG in both sexes (16). Our study showed no significant change in total testosterone levels for the group as a whole; however, female participants demonstrated a decrease and male participants demonstrated an increase in these levels. Overall, our participants did demonstrate an increase in SHBG with an associated decrease in the FAI after SG. However, after controlling for age, sex, and race, there was a significant reduction in total testosterone after surgery, while the reduction in FAI was no longer evident. The positive association between FAI and lean mass has been previously established (36).
Estrogen impacts bone metabolism through multiple mechanisms, with primarily antiresorptive and to a lesser extent bone anabolic effects (likely mediated via reductions in sclerostin and pref-1) (18-20, 37), but data are lacking regarding the impact of decreases in estrogen on bone endpoints after SG. For the first time, we demonstrate associations between decreases in estrone after SG and decreases in BMD Z-scores at the hip and femoral neck in female participants after adjusting for baseline age and race. Similarly, the positive effect of testosterone on bone accrual during adolescence has been well established in the literature (38). Consistent with this, we observed positive associations between decreases in FAI and decreases in BMD Z-scores at the lumbar spine and hip. However, in contrast to established knowledge, we observed inverse associations of changes in in FAI with changes in tibial total and trabecular vBMD. The mechanism underlying this unexpected relationship merits further investigation,
Sclerostin, a hormone secreted by osteocytes, which reduces osteoblastic activity and bone formation while increasing osteoclastic bone resorption (39), is believed to be a mediator of the effects of estrogen on bone (18). Sclerostin is also impacted by mechanical loading, with an increase in sclerostin expected following the unloading of bone with weight loss (12). Consistent with this, and similar to a study in adults following SG (32), we observed an increase in sclerostin following surgery associated with the decrease in BMI, and lean and fat mass. The increase in sclerostin was associated with reductions in BMD Z-scores at hip and femoral neck, and tibial cortical vBMD on correlational analysis, although this was no longer significant after controlling for age and race.
Pref-1 is a transcription factor that inhibits osteoblast differentiation in bone marrow, and studies have reported a reduction in pref-1 levels following estrogen administration, suggesting that bone anabolic effects of estrogen may partially be mediated via reductions in pref-1 (20). In contrast to the expected increase in pref-1 given reductions in estrone following surgery, we observed a decrease in pref-1 levels in the SG group, though this did not differ significantly from changes observed in the NS group. This adds to the very sparse literature regarding the impact of SG on pref-1 levels, and merits further study.
Changes in 25OHD and PTH
Decreased absorption of calcium and vitamin D after bariatric surgery has previously been reported to be associated with poor bone outcomes (40). We noted greater increases in 25OHD levels in SG vs NS after controlling for covariates, likely a consequence of robust vitamin D supplementation after SG, with no changes in PTH over time. The changes in 25OHD were positively associated with changes in tibial cortical vBMD while changes in PTH were negatively associated, consistent with known effects of vitamin D and PTH on bone endpoints (41).
Changes in Enteric Peptides
While several studies in adults have reported on the trajectory of enteric peptides after SG (25, 26), data in youth are limited. A meta-analysis of 28 studies in adults reported that ghrelin decreased and PYY increased after SG (25). Because ghrelin levels change across puberty (42), data from adult studies cannot be extrapolated to adolescents. Consistent with studies in adults (25), we observed a decrease in fasting ghrelin levels following SG associated with decreases in BMI, lean mass, and fat mass. This is in contrast to previous cross-sectional studies in adolescents (both overweight and underweight) that report an inverse association of ghrelin with body fat (41, 43) and not consistent with the general understanding that ghrelin (an appetite-stimulating hormone) tracks inversely with body weight. This discrepancy is likely because of the resection of the primary site of ghrelin secretion, the gastric fundus, in SG. Ghrelin is bone anabolic, and previous studies in adults have demonstrated positive associations between changes in ghrelin and reductions in lumbar spine BMD after SG (41). Similarly, we observed positive associations of changes in ghrelin with changes in femoral neck BMD Z-scores and radial trabecular vBMD over 12 months.
Like ghrelin, PYY levels change across puberty, and therefore studies in adults cannot be extrapolated to adolescents (43). In contrast to studies in adults undergoing SG that have reported increases in PYY following surgery (25), and cross-sectional studies in youth with obesity (44) and anorexia nervosa (45) reporting a negative relationship of PYY levels with BMI and fat mass, we found no change in fasting PYY concentrations over 12 months in the SG group despite significant reductions in BMI. Similarly, a 1-year longitudinal study in adults demonstrated no change in fasting PYY after SG (26). Arakawa et al postulated that this lack of change in PYY may be a consequence of decreased gastric emptying and decreased hormone secretion following SG (26), possibly because of sleeve dilatation following SG (46). While PYY inhibits osteoblastic activity and bone formation (47), we found no associations between changes in PYY and changes in bone endpoints. Previous studies in adolescents have not assessed changes in PYY following SG.
Our study has limitations, including a relatively small sample size and short duration of follow-up of study participants following SG. Additionally, our study was observational and we demonstrate associations that do not prove causation. Future studies with a larger number of participants, including males, followed over a longer duration will be important to confirm our findings and determine whether trajectories of sex steroids, enteric peptides, and other hormones change over time. Conversely, our study has many strengths. Data for adolescents and young adults following SG are sparse, and ours is 1 of the few studies reporting changes in sex steroids, sclerostin, pref-1, and enteric peptides in relation to bone outcomes following SG. Another strength of the study is our NS control group, not available to some other studies in adolescents with obesity (2, 48, 49).
In conclusion, our study suggests that bone loss after SG in youth with moderate-to-severe obesity is a multifactorial process. Changes in body composition, sex steroids (particularly estrone and FAI), sclerostin, and ghrelin are associated with bone changes after SG. Future studies are necessary that follow youth for longer durations postsurgery to determine long-term trajectories of these biochemical parameters and their associations with bone endpoints, and how best to utilize this information to devise preventative and therapeutic strategies.
Glossary
Abbreviations
- 25OHD
25-hydroxy vitamin D
- aBMD
areal bone mineral density
- BMI
body mass index
- CT
computed tomography
- CTX
C-terminal cross-linking telopeptide
- DXA
dual-energy X-ray absorptiometry
- ELISA
enzyme-linked immunosorbent assay
- FAI
free androgen index
- HRpQCT
high-resolution peripheral quantitative computed tomography
- MBS
metabolic and bariatric surgery
- NS
nonsurgical
- P1NP
N-terminal propeptide of type 1 procollagen
- pref-1
preadipocyte factor-1
- PTH
parathyroid hormone
- PYY
peptide YY
- SEM
standard error of the mean
- SG
sleeve gastrectomy
- SHBG
sex hormone binding globulin; vBMD, volumetric bone mineral density
Contributor Information
Supritha Nimmala, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Snimarjot Kaur, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Vibha Singhal, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; MGH Weight Center, Boston, MA 02114, USA.
Deborah M Mitchell, Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Endocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Fatima Cody Stanford, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; MGH Weight Center, Boston, MA 02114, USA.
Mary L Bouxsein, Center for Advanced Orthopaedic Studies, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Meghan Lauze, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Carolyn Huynh, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Clarissa C Pedreira, Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Hang Lee, MGH Biostatistics Center and Harvard Medical School, Boston, MA 02114, USA; Department of Medicine and Harvard Medical School, Boston, MA 02114, USA.
Miriam A Bredella, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Madhusmita Misra, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Funding
This work was supported by National Institutes of Health grants R01DK103946 (M.M., M.A.B.), K23DK110419-01(VS), P30DK040561 (V.S., F.C.S.), K24DK109940 (M.A.B.), K24HD071843 (M.M.), L30DK118710 (F.C.S.), P30DK057521 (V.S.), 1UL1TR002541-01, 1UL1TR001102, 1S10RR023405-01.
Conflicts of Interest
Deborah M. Mitchell: consultant for Amolyt; Madhusmita Misra: consultant for Sanofi and AbbVie and has served on the scientific advisory board for AbbVie and Ipsen. All other authors declare no conflicts of interest.
Data Availability
Dataset generated during and analyzed during the current study are not publicly available as the study is ongoing, but are available from the corresponding author on reasonable request.
Clinical Trials Registration
NCT02557438. Study registered in September 2015.
References
- 1. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242-9. [DOI] [PubMed] [Google Scholar]
- 2. Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg. 2013;48(12):2401-7. [DOI] [PubMed] [Google Scholar]
- 3. Singhal V, Youssef S, Misra M. Use of sleeve gastrectomy in adolescents and young adults with severe obesity. Curr Opin Pediatr. 2020;32(4):547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Misra M, Singhal V, Carmine B, et al. . Bone outcomes following sleeve gastrectomy in adolescents and young adults with obesity versus non-surgical controls. Bone. 2020;134:115290. doi: 10.1016/j.bone.2020.115290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon CM, Zemel BS, Wren TAL, et al. . The determinants of peak bone mass. J Pediatr. 2017;180:261-269. doi: 10.1016/j.bone.2020.115290 [DOI] [PubMed] [Google Scholar]
- 6. Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2(2):165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061-5. [DOI] [PubMed] [Google Scholar]
- 9. Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes (Lond). 2012;36(11):1373-9. [DOI] [PubMed] [Google Scholar]
- 10. Misra M, Bredella MA. Bone metabolism in adolescents undergoing bariatric surgery. J Clin Endocrinol Metab. 2021;106(2):326-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219(1):1-9. [DOI] [PubMed] [Google Scholar]
- 12. Spatz JM, Ellman R, Cloutier AM, et al. . Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28(4):865-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ackerman KE, Nazem T, Chapko D, et al. . Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96(10):3123-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ackerman KE, Putman M, Guereca G, et al. . Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012;51(4):680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laird E, Ward M, McSorley E, Strain JJ, Wallace J. Vitamin D and bone health; potential mechanisms. Nutrients. 2010;2(7):693-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Carretero JIB. Prevalence of “obesity-associated gonadal dysfunction” in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(4):390-408. [DOI] [PubMed] [Google Scholar]
- 17. Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70(2):473-479. [DOI] [PubMed] [Google Scholar]
- 18. Fujita K, Roforth MM, Demaray S, et al. . Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women. J Clin Endocrinol Metab. 2014;99(1):e81-e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95(4):1991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faje AT, Fazeli PK, Katzman D, et al. . Inhibition of Pref-1 (preadipocyte factor 1) by oestradiol in adolescent girls with anorexia nervosa is associated with improvement in lumbar bone mineral density. Clin Endocrinol (Oxf). 2013;79(3):326-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hei SS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23(11):1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim TY, Shoback DM, Black DM, et al. . Increases in PYY and uncoupling of bone turnover are associated with loss of bone mass after gastric bypass surgery. Bone. 2020;131:115115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Shen X, Wan C, et al. . Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: differential signalling via Akt and ERK. Cell Biochem Funct. 2012;30(4):297-302. [DOI] [PubMed] [Google Scholar]
- 24. Fukushima N, Hanada R, Teranishi H, et al. . Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20(5):790-8. [DOI] [PubMed] [Google Scholar]
- 25. McCarty TR, Jirapinyo P, Thompson CC. Effect of sleeve gastrectomy on ghrelin, GLP-1, PYY, and GIP gut hormones: a systematic review and meta-analysis. Ann Surg. 2020;272(1):72-80. [DOI] [PubMed] [Google Scholar]
- 26. Arakawa R, Febres G, Cheng B, Krikhely A, Bessler M, Korner J. Prospective study of gut hormone and metabolic changes after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. PLoS One. 2020;15(7):e0236133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30(12):2391-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simpson K, Parker B, Capizzi J, et al. . Validity and reliability question 8 of the Paffenbarger Physical Activity Questionnaire among healthy adults. J Phys Act Health. 2015;12(1):116-23. [DOI] [PubMed] [Google Scholar]
- 29. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-72. [DOI] [PubMed] [Google Scholar]
- 30. Boutroy S, van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392-399. [DOI] [PubMed] [Google Scholar]
- 31. Jaruvongvanich V, Vantanasiri K, Upala S, Ungprasert P. Changes in bone mineral density and bone metabolism after sleeve gastrectomy: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(8):1252-1260. [DOI] [PubMed] [Google Scholar]
- 32. Muschitz C, Kocijan R, Marterer C, et al. . Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891-901. [DOI] [PubMed] [Google Scholar]
- 33. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116-24. [DOI] [PubMed] [Google Scholar]
- 34. Corona G, Rastrelli G, Monami M, et al. . Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168(6):829-43. [DOI] [PubMed] [Google Scholar]
- 35. Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol. 2019;10:626. doi: 10.3389/fendo.2019.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mouser JG, Loprinzi PD, Loenneke JP. The association between physiologic testosterone levels, lean mass, and fat mass in a nationally representative sample of men in the United States. Steroids. 2016;115:62-66. doi: 10.1016/j.steroids.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 37. Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106(10):1203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rey RA, Grinspon RP. Androgen treatment in adolescent males with hypogonadism. Am J Men’s Health. 2020;14(3):155798832092244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29-37. doi: 10.1016/j.bone.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corbeels K, Verlinden L, Lannoo M, et al. . Thin bones: vitamin D and calcium handling after bariatric surgery. Bone Rep. 2018;8:57-63. doi: 10.1016/j.bonr.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carrasco F, Basfi-Fer K, Rojas P, et al. . Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014;24(6):877-84. [DOI] [PubMed] [Google Scholar]
- 42. Ferrero S, Inui A, Argente J. Active ghrelin levels during fetal life and childhood [3] (multiple letters). J Pediatr. 2004;145(6):862-863. [DOI] [PubMed] [Google Scholar]
- 43. Lloyd B, Ravi P, Mendes N, Klibanski A, Misra M. Peptide YY levels across pubertal stages and associations with growth hormone. J Clin Endocrinol Metab. 2010;95(6):2957-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandes SP, Alessi J, Santos ZEA, de Mello ED. Association between eating behavior, anthropometric and biochemical measurements, and peptide YY (PYY) hormone levels in obese adolescents in outpatient care. J Pediatr Endocrinol Metab. 2020;33(7):873-877. [DOI] [PubMed] [Google Scholar]
- 45. Misra M, Miller KK, Tsai P, et al. . Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91(3):1027-33. [DOI] [PubMed] [Google Scholar]
- 46. Disse E, Pasquer A, Pelascini E, et al. . Dilatation of sleeve gastrectomy: myth or reality? Obes Surg. 2017;27(1):30-37. [DOI] [PubMed] [Google Scholar]
- 47. Schiellerup SP, Skov-Jeppesen K, Windeløv JA, et al. . Gut hormones and their effect on bone metabolism. Potential drug therapies in future osteoporosis treatment. Front Endocrinol. 2019;10:75. doi: 10.3389/fendo.2019.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alqahtani AR, Elahmedi MO, Qahtani A al. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):842-850. [DOI] [PubMed] [Google Scholar]
- 49. Inge TH, Courcoulas AP, Jenkins TM, et al. . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset generated during and analyzed during the current study are not publicly available as the study is ongoing, but are available from the corresponding author on reasonable request.