Abstract
Purpose
To determine whether 25-hydroxyvitamin D (25-OH D) levels are associated with bone outcomes in a multiracial cohort of young adults.
Methods
This cross-sectional study included 165 participants (83 men, 82 women, 18-30 years of age) who self-identified as Asian, Black, or White. We measured bone microarchitecture and strength of the distal radius and tibia using high-resolution peripheral quantitative computed tomography. We used linear regression to estimate the association between 25-OH D (ng/mL) and bone measurements, adjusting for race, sex, age, weight, height, calcium intake, physical activity, and season.
Results
A total of 43.6% of participants were 25-OH D deficient (<20 ng/mL) with greater prevalence in Asian (38.9%) and Black (43.1%) compared with White (18.0%) participants (P < 0.001). At the distal radius, 25-OH D was positively associated with cortical area, trabecular density, cortical thickness, cortical porosity, and failure load (P < 0.05 for all). At the distal tibia, higher 25-OH D was associated with higher cortical area, trabecular density, trabecular number, failure load, and lower trabecular separation and cortical density (P < 0.05 for all). After multivariable adjustment, those with 25-OH D deficiency had generally worse bone microarchitecture than those with 25-OH D sufficiency. Black individuals had largely more favorable bone outcomes than Asian and White individuals, despite higher prevalence of 25-OH D deficiency.
Conclusions
We found a high prevalence of 25-OH D deficiency in a multiracial cohort of young adults. Lower 25-OH D was associated with worse bone outcomes at the distal radius and tibia at the time of peak bone mass, warranting further attention to vitamin D status in young adults.
Keywords: vitamin D, bone microarchitecture, multiracial cohort
The importance of vitamin D for bone mineralization and homeostasis is well-established. Yet, vitamin D deficiency in adolescents and adults is common with prevalence ranging from 36% to 42% in adult Americans (1-5). Vitamin D deficiency is defined using serum levels of 25-hydroxyvitamin D (25-OH D) below 20 ng/mL (6), and values may vary by season (2) and race/ethnic origin (5). The prevalence of vitamin D deficiency is highest in Black and Asian adults and lowest in White adults (1, 5, 7). Therefore, a recent recommendation statement by US Preventative Services Task Force noted that more studies are needed in the area of vitamin D deficiency in subgroups defined by race and ethnicity (8).
Despite the key roles of vitamin D in bone metabolism, the effects of vitamin D on skeletal health are not well-defined in young adults at the time they attain peak bone mass. Peak bone mass is typically reached by mid-third decade of life for both men and women (9). Previous studies reported that higher 25-OH D concentrations are associated with higher areal bone mineral density (aBMD) measured by dual-energy X-ray absorptiometry (DXA) in adolescents and adults (10-14). Fracture risk is associated with deficits in cortical and trabecular microarchitecture, which cannot be assessed by DXA (15). Furthermore, bone microarchitecture measures by high-resolution peripheral quantitative computed tomography (HR-pQCT) reveal differences in cortical and trabecular bone microstructure by race/ethnic origin (16-19). For example, Black and Asian individuals have more favorable bone microarchitecture and strength than White individuals (17-22). While studies have reported positive associations between 25-OH D and HR-pQCT measures in adolescents (23, 24), little work has focused on the relationship between 25-OH D and bone microarchitecture in young adults at the time of peak bone mass. Further, no studies have addressed these associations by race, though prior work has indicated that the association of vitamin D and bone metabolism may vary by race (25, 26).
Thus, in this study, we aimed to determine the association of serum 25-OH D concentrations and bone microarchitecture and strength in a multiracial cohort of young adults. We also determined whether 25-OH D levels were associated with DXA-derived aBMD and serum markers of bone metabolism. We also assessed differences in bone microstructure by self-identified race and determined whether self-identified race or calcium intake modify associations between 25-OH D and bone microarchitecture.
Methods
Study Design
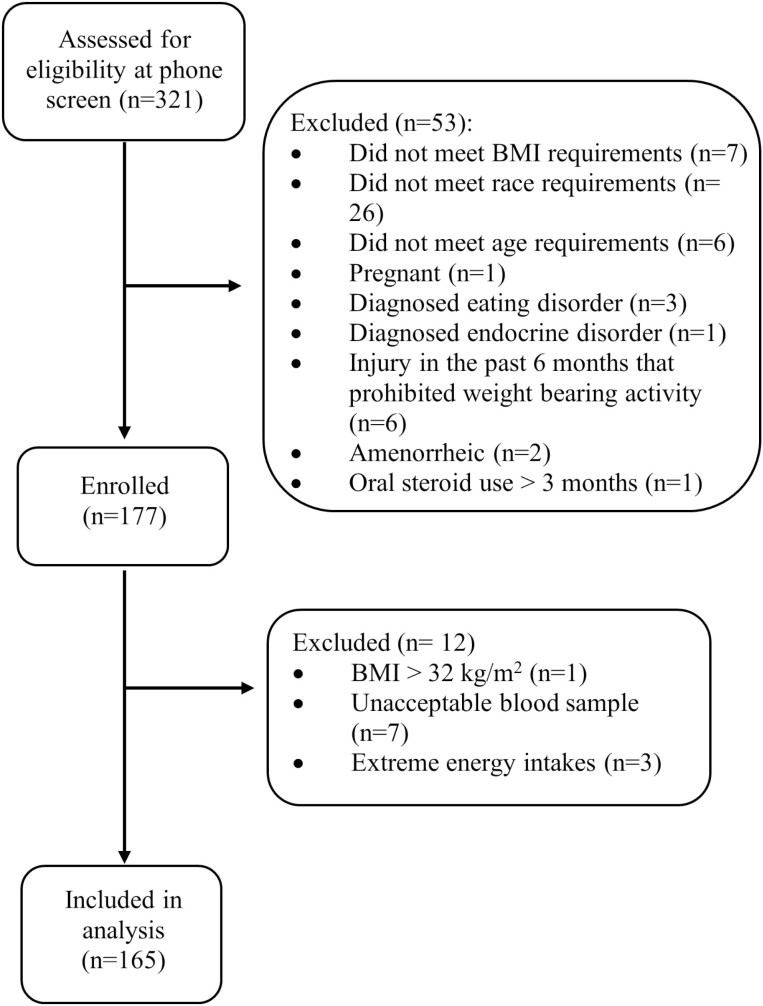
We enrolled men and women, aged 18 to 30 years, from the Boston area from November 2018 to March 2020. In this convenience sample, we recruited subjects from the community via online advertising, flyers, and outreach to local colleges. Inclusion criteria for participation was a body mass index (BMI) between 18 and 30 kg/m2 and a self-identity of Asian, Black/African American, or White/Caucasian. Women were required to be eumenorrheic (>9 menses in the prior 12 months, including 1 menses in the past 60 days). Exclusion criteria included history of bilateral lower leg fractures and prior or current use of medications (eg, bisphosphonates, oral steroids) or medical conditions (eg, diagnosed eating disorder, diabetes, hyperparathyroidism) known to affect bone health. We screened 321 potential participants for this study: 144 screened participants did not participate in the study, including 7 who did not meet the BMI criteria, 26 who did not meet the race criteria, 6 who were outside the age range, 1 who was pregnant, 3 who had a diagnosed eating disorder, 1 with a diagnosed endocrine disorder (postthyroidectomy), 6 who had an injury in the past 6 months that prohibited weight bearing activity, 2 who were amenorrheic, 1 who had prior oral steroid use for greater than 3 months, and 91 who declined to participate in the study (Fig. 1). Following enrollment, 1 participant was excluded for having a BMI > 32 kg/m2 and 7 participants did not have a sufficient blood sample to measure 25-OH D. Four participants were excluded because of extreme energy intakes (≤600 kcal/d or > 4000 kcal for women and > 4200 for men) or from having more than 13 responses missing in the food frequency questionnaire (FFQ). Thus, we used data from 165 participants. Approval for this study was granted by the Institutional Review Board of Partners Health Care and the Human Research Protection Office at the US Army Medical Research and Material Command. Informed written consent was obtained from each subject before participation in the study.
Figure 1.
Enrollment flow diagram.
Vitamin D Assessment
Fasting blood draws were collected in the morning for each subject. Serum was isolated and stored at -80 °C until analysis. 25-OH D levels (ng/mL) were assessed via immunoassay from blood samples (Quest Diagnostics, Marlborough, MA). Vitamin D sufficiency was defined as ≥ 20 ng/mL and deficiency as <20 ng/mL as recommended by the Institute of Medicine (6).
Bone Measurements
The primary outcome for this study was HR-pQCT-derived bone microarchitecture and strength at the distal radius and tibia. Secondary outcomes included DXA-derived aBMD and bone turnover markers (BTMs).
Bone microarchitecture and strength
We measured bone microarchitecture and strength by micro-finite element analysis (μFEA) at the distal radius and tibia via HR-pQCT (XtremeCT II, Scanco Medical AG, Basserdorf, Switzerland; isotropic voxel size of 61 μm). The scanned region of interest was determined relative to the individual’s bone length to overcome potential confounding of differences in height and/or bone length. The scan regions started at 4% of radial length and the 7% of tibial length (distal) and extended proximally for 168 slices. The nondominant limb was scanned unless there was a prior fracture in that limb, in which case the contralateral limb was scanned. Quality control was maintained with daily scanning of the manufacturer’s phantom. Each scan was immediately reviewed for motion artifact and was repeated up to 2 times if there was significant motion artifact. We scored movement artifact on a 5-point scale, from 1 = no movement to 5 = severe movement artifact (27). Scans with movement artifact >3 were excluded (2 scans at the distal radius and 1 scan at the distal tibia).
We measured total, cortical, and trabecular cross-sectional areas, cortical and trabecular thickness, cortical porosity (%), trabecular separation (mm), and trabecular number (mm). We also used 3-dimensional HR-pQCT images to perform linear μFEA to estimate failure load (Newtons [kN]) under axial compression. In this method, each voxel in the HR-pQCT image is converted to a linear isotropic hexahedral element, assuming a Young’s modulus of 10 GPa, and Poisson’s ratio of 0.3 for all elements. The finite element model is then subjected to axial compression, with a compressive strain of 1% applied along the vertical axis with the top and bottom surfaces fully constrained (28). HR-pQCT scan acquisition, analysis, and reporting followed published guidelines (29).
Short-term reproducibility for HR-pQCT measurements at the distal radius in our laboratory ranged from 1.7% to 2.5% for density measurements, from 1.8% to 12.7% for cortical microarchitecture parameters, from 0.8% to 3.0% for trabecular microarchitecture parameters, and was 4.4% for μFEA estimated failure load. Short-term reproducibility for HR-pQCT measurements at the distal tibia in our laboratory ranged from 0.5% to 0.9% for density measurements, from 1.2% to 9.6% for cortical microarchitecture parameters, from 0.8% to 2.9% for trabecular microarchitecture parameters, and was 2.8% for μFEA estimated failure load.
Areal Bone Mineral Density
We used DXA to assess the posteroanterior spine, femoral neck, and total hip aBMD (g/cm2) (DXA, QDR Discovery, Hologic, Inc., Bedford, MA). Quality control was maintained through daily measurements of a Hologic CXA anthropomorphic spine phantom and visual review of every scan image by an investigator experienced in bone densitometry. The in vivo scanning precision at our institution is 0.005, 0.006, and 0.009 g/cm2 for posteroanterior spine, total hip, and femoral neck, respectively.
Serum BTMs
We measured C-terminal telopeptides of type I collagen (CTX-1), a marker of bone resorption, intact amino-terminal propeptide of type I procollagen (intact P1NP), a marker of bone formation, and intact serum parathyroid hormone (iPTH) and sclerostin. Specifically, CTX-1 was analyzed using chemiluminescence technology (IDS-iSYS CTX-I CrossLaps assay). The reportable range of the assay was 0.033 to 6.000 ng/mL. Five levels of controls were used to assess the quality of each CTX-1 run and coefficients of variation were <10% for each run. Intact P1NP was analyzed using chemiluminescence technology (IDS-iSYS Intact PINP). The reportable range of the assay was 2 to 230 ng/mL. iPTH was analyzed using immunoassay (IDS-iSYS Intact PTH assay). The reportable range of the assay was 5 to 5000 pg/mL. Serum controls were assayed using 3 lots of reagents in duplicate twice per day for 20 days on 3 instruments. Ten levels of controls were used to assess the quality of each serum PTH run, and coefficients of variation were <10% for each run. Sclerostin was analyzed by an enzyme-linked immunosorbent assay (Quantikine Human SOS Immunoassay). The reportable range of the assay was 67.0 to 300 pg/mL. Three levels of controls were used to assess the quality of each sclerostin run and coefficients of variation were <11% for each run. All assays were performed at the Immunoassay Core Facility at Maine Medical Center Research Institute (Scarborough, ME).
Measurement of Covariates
We captured medical history, dietary intake, and physical activity history via questionnaires. Race was self-reported as either Asian, Black, or White and participants were required to have had at least 3 grandparents that identified as that same self-reported race. Physical activity history was assessed through the bone-specific physical activity questionnaire, which evaluates past and current activity patterns (30). Dietary intake was assessed via a semiquantitative Block’s FFQ (31). The FFQ was completed based on the subject’s food intake over the previous year and reviewed by study staff. Questionnaires with >13 food items left blank or extreme energy intakes were considered invalid and excluded. Total calcium was calculated as sum of intake from diet and supplements. Height without shoes (inches) was measured to the nearest quarter inch with a stadiometer. Weight, in light clothing (pounds), was measured with a calibrated scale. We calculated BMI as mass (kg) divided by height squared (m2). Recognizing the influence of season on vitamin D, the month of study enrollment was recorded and used to determine season. Season was defined as: October-December = fall, January-March = winter, April-June = spring, and July-September = summer.
Statistical Analysis
Variables are reported as mean ± SD or as the proportion of individuals in the total sample as well as by categories of 25-OH D (sufficient [≥ 20 ng/mL] or deficient [<20 ng/mL]). We tested differences in demographic variables between the sufficient and deficient groups using t-tests for continuous variables and χ 2 tests for categorical variables. We conducted separate analyses for each bone outcome (from HR-pQCT, DXA-derived aBMD, and BTMs). 25-OH D was modelled as a continuous variable (ng/mL) and by category (sufficient vs deficient). We used multiple linear regression to estimate the association between concentration of 25-OH D (ng/mL) and bone measures at the distal radius and tibia. The HR-pQCT data were standardized by subtracting each data point from the mean and dividing by the SD. The BTMs iPTH and P1NP were log transformed. We used beta coefficients to estimate difference in bone measures per 1 SD higher 25-OH D. We also computed the adjusted mean of each bone measure for categories of 25-OH D. The models for HR-pQCT and DXA-derived aBMD were adjusted for race, sex, age, weight, height, total calcium intake, physical activity, and season of visit. The models for BTMs were adjusted for race, sex, age, weight, height, and season of visit. Multiple linear regression was used to assess the main effect of race and any interactions of 25-OH D with race. Interactions and the main effect of race were considered significant if P for interaction was <0.05. If P was <0.05 for the main effect of race, pairwise comparisons were used to further understand the directionality and differences in bone by race. Multiple linear regression was also used to assess the interactions of 25-OH D and calcium intake (mg/d). Interactions were considered significant if P for interaction was <0.05. Analyses were stratified for significant interactions. Comparisons with a P value <0.05 were reported as statistically significant. We used Stata/IC version 16.0 (StataCorp LP, College Station, TX) for all statistical analyses.
Results
Subject Characteristics
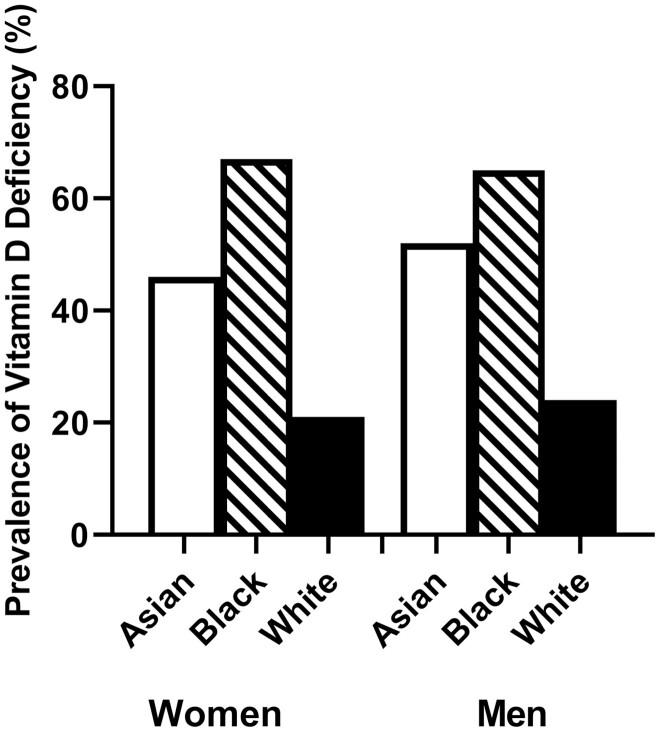
The final study sample included 83 men and 82 women (Table 1), including 58 who identified as Asian (n = 27 women, 31 men), 48 who identified as Black (n = 25 women, 23 men), and 59 who identified as White (n = 30 women, 29 men). Subjects had a mean age of 23.7 ± 3.1 years and mean BMI of 24.1 ± 3.1 kg/m2 (Table 1). Mean serum concentration of 25-OH D was 22.8 ± 10 ng/mL, and 43.6% of participants met criteria for vitamin D deficiency. More Asian (48%) and Black (65%) individuals were vitamin D deficient compared with White (22%) individuals (Table 1, Fig. 2). Participants with sufficient levels of vitamin D were largely enrolled in the summer and fall and had higher total calcium intake (from diet and supplements) than those who were deficient in vitamin D. Those with sufficient vitamin D levels tended to have higher physical activity levels than those with vitamin D deficiency, though this did not reach statistical significance (Table 1).
Table 1.
Demographic characteristics of study subjects
| 25-OH D (ng/mL) | ||||
|---|---|---|---|---|
| Total | Sufficient (≥20 ng/mL) |
Deficient (<20 ng/mL) |
||
| n = 165 | n = 93 | n = 72 | P | |
| Sex | 0.576 | |||
| Man | 83 (50.3%) | 45 (48.4%) | 38 (52.8%) | |
| Woman | 82 (49.7%) | 48 (51.6%) | 34 (47.2%) | |
| Race | <0.001 | |||
| Asian | 58 (35.2%) | 30 (32.3%) | 28 (38.9%) | |
| Black | 48 (29.1%) | 17 (18.3%) | 31 (43.1%) | |
| White | 59 (35.8%) | 46 (49.4%) | 13 (18.0%) | |
| Age, y | 23.7 (3.1) | 23.9 (2.9) | 23.3 (3.3) | 0.223 |
| Height, inches | 67.4 (4.0) | 67.5 (4.1) | 67.3 (4.1) | 0.748 |
| Weight, lb | 156.3 (27.6) | 155.8 (25.2) | 156.9 (30.5) | 0.812 |
| BMI, kg/m2 | 24.1 (3.1) | 24.0 (2.6) | 24.3 (3.7) | 0.561 |
| Total BPAQ | 29.0 (20.3) | 31.3 (22.3) | 26.0 (17.0) | 0.093 |
| 25-OH D, ng/mL | 22.8 (10.0) | 29.6 (7.6) | 14.0 (3.7) | < 0.001 |
| Season of enrollment | 0.062 | |||
| Fall | 19 (11.5%) | 13 (14.0%) | 6 (8.3%) | |
| Winter | 67 (40.6%) | 32 (34.4%) | 35 (48.6%) | |
| Spring | 51 (30.9%) | 27 (29.0%) | 24 (33.3%) | |
| Summer | 28 (17.0%) | 21 (22.6%) | 7 (9.7%) | |
| Total calcium intake, mg/d | 746.8 (355.9) | 823.2 (408.6) | 648.2 (242.2) | 0.002 |
Values are mean (SD) or n (%). Bold indicates P < 0.05 for differences between vitamin D-sufficient and vitamin D-deficient groups.
Abbreviations: 25-OH D, 25-hydroxy vitamin D; BMI, body mass index; BPAQ, Bone Specific Physical Activity Questionnaire.
Figure 2.
Prevalence of vitamin D deficiency (25-OH D < 20 ng/mL) by race and sex.
Relationship Between Serum Vitamin D and Bone Microarchitecture
At both the distal radius and the distal tibia, bone morphology and microarchitecture were generally more favorable with higher serum levels of 25-OH D (Table 2). At the distal radius, after multivariable adjustment, higher 25-OH D levels were significantly associated with higher cortical area, trabecular density, cortical thickness, cortical porosity, and failure load (Table 2). At the distal tibia, after multivariable adjustment, higher 25-OH D levels were significantly associated with higher total area, cortical area, trabecular density, trabecular number and failure load, and with lower trabecular separation and cortical density (Table 2).
Table 2.
Bone density and microarchitecture by vitamin D status (mean [SD]) and the association between serum 25-OH D and standardized HR-pQCT measurements, expressed as beta coefficient and standard error (SE)
| Sufficient (≥20 ng/mL) | Deficient (<20 ng/mL) | P a | β coefficient (SE)a |
P a | |
|---|---|---|---|---|---|
| Distal radius | n = 92 | n = 71 | n = 163 | ||
| Size/morphology | |||||
| Tt.Ar, mm2 | 309.8 (61.5) | 301.2 (65.1) | 0.091 | 0.010 (0.006) | 0.095 |
| Ct.Ar, mm2 | 66.1 (13.7) | 63.8 (15.7) | 0.012 | 0.022 (0.006) | 0.001 |
| Ct.Ar/Tt.Ar, % | 21.8 (4.8) | 21.5 (4.3) | 0.393 | 0.013 (0.009) | 0.159 |
| Tb.Ar, mm2 | 243.8 (56.7) | 237.3 (57.3) | 0.301 | 0.005 (0.007) | 0.423 |
| Density | |||||
| Tt.BMD, mgHA/cm3 | 330 (57) | 323 (52) | 0.145 | 0.015 (0.009) | 0.082 |
| Ct.BMD, mgHA/cm3 | 896 (42) | 894 (50) | 0.647 | -0.005 (0.008) | 0.510 |
| Tb.BMD, mgHA/cm3 | 177 (39) | 170 (41) | 0.040 | 0.016 (0.008) | 0.040 |
| Microarchitecture | |||||
| Ct.Th, mm | 1.06 (0.20) | 1.04 (0.21) | 0.152 | 0.019 (0.008) | 0.021 |
| Ct.Po, % | 0.44 (0.25) | 0.43 (0.37) | 0.164 | 0.025 (0.008) | 0.003 |
| Tb.Th, mm | 0.234 (0.021) | 0.231 (0.020) | 0.018 | 0.015 (0.008) | 0.053 |
| Tb.Sp, mm | 0.609 (0.089) | 0.631 (0.117) | 0.094 | -0.012 (0.008) | 0.164 |
| Tb.N, 1/mm | 1.53 (0.19) | 1.48 (0.22) | 0.190 | 0.012 (0.007) | 0.176 |
| µFEA | |||||
| FL, kN | 4.13 (1.54) | 3.97 (1.64) | 0.033 | 0.023 (0.007) | 0.002 |
| Distal tibia | n = 92 | n = 72 | n = 164 | ||
| Size/morphology | |||||
| Tt.Ar, mm2 | 730.1 (144.5) | 698.3 (129.5) | 0.011 | 0.012 (0.007) | 0.068 |
| Ct.Ar, mm2 | 142.7 (29.8) | 143.7 (33.5) | 0.405 | 0.012 (0.006) | 0.033 |
| Ct.Ar/Tt.Ar, % | 20.0 (4.4) | 20.8 (3.9) | 0.445 | 0.002 (0.008) | 0.853 |
| Tb.Ar, mm2 | 587.5 (136.6) | 554.5 (113.5) | 0.021 | 0.010 (0.007) | 0.170 |
| Density | |||||
| Tt.BMD, mgHA/cm3 | 347 (56) | 346 (54) | 0.400 | 0.013 (0.008) | 0.108 |
| Ct.BMD, mgHA/cm3 | 942 (39) | 954 (40) | 0.009 | -0.023 (0.008) | 0.007 |
| Tb.BMD, mgHA/cm3 | 200 (41) | 191 (47) | 0.016 | 0.025 (0.008) | 0.002 |
| Microarchitecture | |||||
| Ct.Th, mm | 1.61 (0.33) | 1.66 (0.35) | 0.818 | 0.008 (0.007) | 0.291 |
| Ct.Po, % | 1.42 (0.75) | 1.44 (1.03) | 0.785 | 0.010 (0.009) | 0.246 |
| Tb.Th, mm | 0.271 (0.034) | 0.269 (0.034) | 0.342 | 0.012 (0.008) | 0.124 |
| Tb.Sp, mm | 0.687 (0.112) | 0.741 (0.160) | 0.002 | -0.026 (0.008) | 0.002 |
| Tb.N, 1/mm | 1.39 (0.21) | 1.31 (0.24) | 0.004 | 0.025 (0.008) | 0.002 |
| µFEA | |||||
| FL (kN) | 12.7 (3.2) | 12.6 (3.4) | 0.046 | 0.016 (0.006) | 0.005 |
Bold = P < 0.05.
Abbreviations: 25-OH D, 25-hydroxyvitamin D; µFEA, micro-finite element analysis; Ct.Ar, cortical area; Ct.BMD, cortical density; Ct.Po, cortical porosity; Ct.Th, cortical thickness; FL (failure load); HR-pQCT, high-resolution peripheral quantitative polymerase chain reaction; Tb.Ar, trabecular area; Tb.BMD, trabecular density; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Tt.Ar, total area; Tt.BMD, total density.
a Adjusted for race, sex, age, height, weight, total calcium, physical activity, and season.
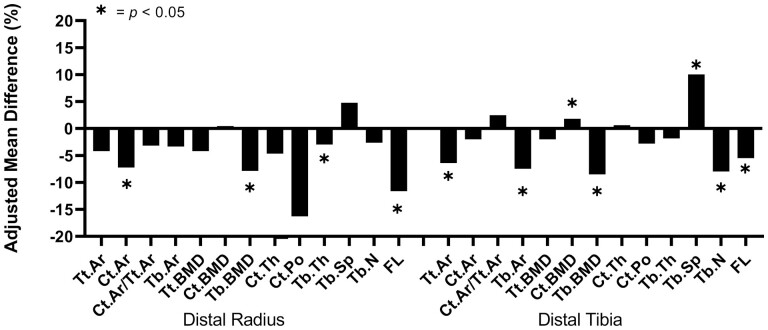
We observed similar patterns when comparing bone density and microarchitecture in individuals with vitamin D sufficiency (≥ 20 ng/mL) vs deficiency (<20 ng/mL)(Table 2, Fig. 3). At the distal radius, participants who were deficient in vitamin D had significantly lower adjusted means of failure load (-11.6%), cortical area (-7.2%), trabecular density (-7.9%), and trabecular thickness (-3.0%) compared with those who had adequate vitamin D levels (Fig. 3a). At the distal tibia, participants who were deficient in vitamin D had significantly lower adjusted means of total area (-6.4%), failure load (-5.5%), trabecular area (-7.5%), trabecular density (-8.5%), and trabecular number (-8.0%), as well as higher trabecular separation (10.0%) and cortical density (1.8%) compared with those who had sufficient levels of vitamin D (Fig. 3b).
Figure 3.
Percent differences between 25-OH D sufficient and deficient subjects in adjusted means of HR-pQCT-derived bone parameters at the distal radius and tibia. Values are adjusted for race, sex, age, height, weight, total calcium, physical activity, and season.
Areal Bone Mineral Density
Consistent with our observations at the peripheral skeleton, higher levels of 25-OH D were associated with higher aBMD of the femoral neck (β ± standard error [SE] = 0.004 ± 0.001, P = 0.001), total hip (β ± SE = 0.005 ± 0.001, P ≤ 0.001), and lumbar spine aBMD (β±SE = 0.003 ± 0.001, P = 0.006) after multivariable adjustment. Participants with vitamin D deficiency had significantly lower adjusted mean values of femoral neck (-7.0%), total hip (-7.1%), and lumbar spine (-5.4%) aBMD compared with those who were sufficient in vitamin D (Table 3).
Table 3.
Comparison of DXA aBMD in subjects who were 25-OH D deficient vs sufficient, expressed as multivariable adjusted mean values (± SE) in each group
| Vitamin D Sufficient (≥ 20 ng/mL) | Vitamin D Deficient (< 20 ng/mL) | ||
|---|---|---|---|
| n = 93 | n = 72 | P a | |
| Femoral neck aBMD, g/cm2 | 0.963 (0.016) | 0.896 (0.019) | 0.008 |
| Total hip aBMD, g/cm2 | 1.058 (0.014) | 0.984 (0.017) | 0.001 |
| Lumbar spine aBMD, g/cm2 | 1.084 (0.013) | 1.025 (0.016) | 0.006 |
Bold = P < 0.05.
Abbreviations: 25-OH D, 25-hydroxyvitamin D; aBMD, areal bone mineral density; DXA, dual-energy X-ray absorptiometry; SE, standard error.
a Adjusted for race, sex, age, height, weight, total calcium, physical activity, and season.
25-OH D and Bone Turnover Markers
25-OH D was significantly inversely associated with iPTH after multivariable adjustment (β ± SE = -0.015 ± 0.003 pg/mL, P ≤ 0.001). Vitamin D levels were not associated with CTX1, P1NP, or sclerostin after adjusting for covariates. iPTH was lower in individuals sufficient in vitamin D compared to those deficient in vitamin D (adjusted mean: 33.36 ± 1.48 vs 43.50 ± 1.79 pg/mL, P ≤ 0.001). CTX1, P1NP, and sclerostin levels were similar in vitamin D-deficient and vitamin D-sufficient groups.
Main Effect of Race, Interaction With Race, and Interaction With Total Calcium Intake
Race did not modify the associations between serum 25-OH D and HR-pQCT outcomes (P value for interaction = 0.06-0.96), DXA-derived aBMD (P value for interaction = 0.31-0.73), or BTMs (P value for interaction = 0.48-0.95). Further, no significant interactions were observed between 25-OH D and total calcium intake in the analyses of HR-pQCT (P value for interaction = 0.15-0.96) or DXA-derived aBMD (0.80-0.98).
HR-pQCT variables and DXA-derived aBMD varied by self-identified race (Table 4). In general, Black individuals had more favorable bone density and morphology compared with Asian and White individuals. For example, Black subjects had greater total and trabecular density, cortical and trabecular thickness, and failure load at the tibia compared with Asian and White individuals. Also, for a few variables, White individuals had better HR-pQCT outcomes than Asian participants.
Table 4.
Bone density, morphology, and microarchitecture by self-identified race (sex-adjusted mean [SD])
| Asian | Black | White | P a | |
|---|---|---|---|---|
| Distal radius | n = 60 | n = 56 | n = 59 | |
| Size/morphology | ||||
| Tt.Ar (mm2) | 285.9 (6.5) | 312.6 (6.7) | 317.8 (6.5) | 0.001 b , c |
| Ct.Ar (mm2) | 62.7 (1.5) | 69.1 (1.5) | 63.9 (1.5) | 0.007 b , d |
| Ct.Ar/Tt.Ar (%) | 22.2 (0.6) | 22.5 (0.6) | 20.7 (0.6) | 0.088 |
| Tb.Ar (mm2) | 223.2 (6.3) | 243.6 (6.6) | 253.9 (6.4) | 0.003 b , c |
| Density | ||||
| Tt.BMD (mgHA/cm3) | 326 (7) | 342 (7) | 319 (7) | 0.067 |
| Ct.BMD (mgHA/cm3) | 903 (5) | 884 (5) | 896 (5) | 0.049 b |
| Tb.BMD (mgHA/cm3) | 165 (5) | 188 (5) | 171 (5) | 0.001 b , d |
| Microarchitecture | ||||
| Ct.Th (mm) | 1.05 (0.02) | 1.11 (0.03) | 1.01 (0.02) | 0.018 d |
| Ct.Po (%) | 0.39 (0.04) | 0.48 (0.04) | 0.44 (0.04) | 0.300 |
| Tb.Th (mm) | 0.232 (0.002) | 0.240 (0.002) | 0.228 (0.002) | 0.001 b , d |
| Tb.Sp (mm) | 0.651 (0.012) | 0.589 (0.013) | 0.608 (0.012) | 0.002 b , c |
| Tb.N (1/mm) | 1.45 (0.03) | 1.55 (0.03) | 1.53 (0.03) | 0.011 b , c |
| µFEA | ||||
| FL (kN) | 3.7 (0.2) | 4.8 (0.2) | 3.7 (0.2) | <0.001 b , d |
| Distal tibia | n = 60 | n = 57 | n = 59 | |
| Size/morphology | ||||
| Tt.Ar (mm2) | 676.2 (15.6) | 721.7 (16.0) | 739.0 (15.7) | 0.015 b , c |
| Ct.Ar (mm2) | 133.1 (2.7) | 159.0 (2.8) | 140.3 (2.8) | <0.001 b , d |
| Ct.Ar/Tt.Ar (%) | 20.1 (0.5) | 22.4 (0.5) | 19.3 (0.5) | <0.001 b , d |
| Tb.Ar (mm2) | 543.1 (15.3) | 562.8 (15.7) | 598.7 (15.4) | 0.037 c |
| Density | ||||
| Tt.BMD (mgHA/cm3) | 339 (7) | 374 (7) | 335 (7) | <0.001 b , d |
| Ct.BMD (mgHA/cm3) | 944 (5) | 957 (5) | 944 (5) | 0.108 |
| Tb.BMD (mgHA/cm3) | 190 (5) | 208 (5) | 192 (5) | 0.033 b , d |
| Microarchitecture | ||||
| Ct.Th (mm) | 1.55 (0.04) | 1.83 (0.04) | 1.56 (0.04) | <0.001 b , d |
| Ct.Po (%) | 1.49 (0.11) | 1.36 (0.11) | 1.39 (0.11) | 0.653 |
| Tb.Th (mm) | 0.269 (0.004) | 0.282 (0.004) | 0.262 (0.004) | 0.001 b , d |
| Tb.Sp (mm) | 0.741 (0.017) | 0.718 (0.018) | 0.673 (0.017) | 0.022 c |
| Tb.N (1/mm) | 1.30 (0.03) | 1.34 (0.03) | 1.42 (0.03) | 0.011 c |
| µFEA | ||||
| FL (kN) | 11.6 (0.3) | 14.1 (0.3) | 12.3 (0.3) | <0.001 b , d |
Bold = P < 0.05.
Abbreviations: 25-OH D, 25-hydroxyvitamin D; µFEA, micro-finite element analysis; Ct.Ar, cortical area; Ct.BMD, cortical density; Ct.Po, cortical porosity; Ct.Th, cortical thickness; FL (failure load); HR-pQCT, high-resolution peripheral quantitative polymerase chain reaction; Tb.Ar, trabecular area; Tb.BMD, trabecular density; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Tt.Ar, total area; Tt.BMD, total density.
a Adjusted for sex.
b P < 0.05, Asian vs Black individuals.
c P < 0.05, Asian vs White individuals.
d P < 0.05, Black vs White individuals.
Discussion
The purpose of this cross-sectional study was to delineate the associations between serum 25-OH D levels and bone density, microarchitecture, and strength in a multiracial cohort of young adults. We found a high prevalence of vitamin D deficiency, and that, generally, lower 25-OH D was associated with worse bone microarchitecture and strength, lower DXA-derived aBMD, and higher levels of iPTH across all races. Neither race nor total calcium intake significantly modified the association of 25-OH D and bone outcomes of HR-pQCT and DXA, though bone density and microarchitecture varied by race.
Consistent with prior studies (1-5, 32, 33), we observed a high prevalence (43.6%) of vitamin D deficiency in this cohort of young adults. Notably, we found that vitamin D deficiency varied by race, with Asian and Black individuals having a 2- to 3-fold higher prevalence of vitamin D deficiency than White individuals. This observation is in line with prior studies reporting that vitamin D deficiency varies by race and tends to be highest in adults identifying as Asian or Black (1-5, 32-36). In particular, a study using data from the National Health and Nutrition Examination Survey from 2005 to 2006 reported that 41.6% of adults were 25-OH D deficient, with the highest and lowest prevalence of vitamin D deficiency seen in Black (82.1%) and White (30%) individuals, respectively (1).
We found that lower 25-OH D was generally associated with worse bone density and microstructure outcomes from HR-pQCT at the appendicular skeleton. At the distal radius, lower 25-OH D was associated with lower cortical area, cortical thickness, trabecular density, and failure load. At the distal tibia, lower 25-OH D was associated with lower total area, cortical area, trabecular density, trabecular number, failure load, and higher trabecular separation and cortical density. Our findings in young adults are consistent with prior studies that have investigated the association of 25-OH D and HR-pQCT measures in other cohorts of adolescents and older adults (23, 24, 37-39). In particular, in a cross-sectional study of adolescent boys and girls in Hong Kong, lower 25-OH D was significantly associated with worse cortical and trabecular bone measures at the distal radius (23). Further, a prospective study in Australia reported that lower 25-OH D levels in adolescence were associated with worse bone microarchitecture at both the distal radius and distal tibia in early adulthood (24). In a cross-sectional study of mostly vitamin D-sufficient adult women and men in Canada, lower 25-OH D was significantly associated with lower trabecular BMD and trabecular thickness at the distal tibia (37). Another cross-sectional study of older women with at least 1 prevalent low-impact fracture reported that lower 25-OH D was associated with lower cortical BMD at the distal radius (38). Last, a large cross-sectional study in men aged 20 to 65 years found that lower 25-OH D levels were significantly associated with worse trabecular bone measures at the distal tibia (39).
Our finding of lower 25-OH D being associated with lower DXA-derived aBMD measures at the axial skeleton is also consistent with several previous studies (10-13). For instance, in individuals at the age of peak bone mass, 25-OH D was reported to be positively correlated with DXA-derived aBMD (10, 11). Serum 25-OH D levels are also positively associated with aBMD at the axial skeleton in children and adolescents (12), whereas a 25-OH D insufficiency was associated with low spine aBMD in premenopausal women (13). In postmenopausal women 25-OH D less than 25 ng/mL was associated with lower aBMD t scores (14).
We also observed a few associations that were unexpected, namely that higher 25-OH D was significantly associated with higher cortical porosity (at the distal radius) and with lower cortical density (at the distal tibia). The reason for these observations is unclear, though it is plausible these were due to chance because we conducted a large number of tests for association. Of note, at both the distal radius and the tibia, higher 25-OH D was significantly associated with increased failure load, indicating increased bone strength overall. Further, although higher 25-OH D was associated with increased cortical porosity as a continuous variable, there were no differences in cortical porosity of the distal radius or tibia when comparing vitamin D-deficient and vitamin D-sufficient groups. In these young adults, the absolute value of cortical porosity at the distal radius was low (0.43% in those with sufficient 25-OH D and 0.36% in those with deficient 25-OH D), suggesting that, in young adults, cortical porosity does not contribute substantially to overall bone strength as assessed by µFEA. It is unclear why higher 25-OH D was associated with lower cortical density at the tibia. It is possible that our statistical model did not fully adjust for possible confounding because of race and/or season, as they were both associated with cortical BMD.
Contrary to our hypothesis, self-identified race did not modify the association between 25-OH D and bone health. This is inconsistent with some, but not all previous studies of racially and ethnically diverse cohorts (7, 26). Gutiérrez and colleagues studied 4206 National Health and Nutrition Examination Survey participants, reporting that aBMD significantly decreased as 25-OH D decreased in White and Mexican-American participants, but not in Black participants (26). Another study investigated the relationship between 25-OH D and DXA-derived aBMD in 1114 Black, White, and Hispanic men aged 20 to 79 years (7), finding that 25-OH D was associated with DXA-derived aBMD in White men, but not among Black or Hispanic men (7). Given that these race interactions were observed in large cohort studies, it is likely that our study has insufficient power to detect differences in the association between vitamin D and bone health by race. However, a study using iliac crest bone biopsies showed that although 25-OH D was lower in Black than White women, there were no notable differences in bone mineralization at the tissue level (40). Furthermore, our observation of generally more favorable bone outcomes in Black vs Asian and White subjects, despite higher prevalence of vitamin D deficiency in Black subjects suggests that bioavailability of 25-OH D may vary and/or that other factors, not accounted for in our analyses, are influencing bone density and microarchitecture in these young adults. We previously had similar findings in a cohort of Black and White men and women, where Black individuals had more favorable bone microarchitecture and strength at the distal tibia than White individuals (20). It is clear that potential modification of the association between 25-OH D and bone outcomes by race is complex and remains incompletely understood.
This study has several important strengths. First, we enrolled a multiracial cohort of both women and men, at the age of peak bone mass, allowing us to test whether self-identified race modified the association between vitamin D and bone outcomes. A unique aspect of our study was that we assessed the association of 25-OH D with HR-pQCT measures in a multiracial cohort of young adults. In addition, we performed the HR-pQCT measurements at sites relative to individual bone length, thereby mitigating any confounding effects of subject-specific variations in limb length or height. All analyses were adjusted for key covariates, including race, sex, height, weight, physical activity, and season of enrollment.
Our study also has several limitations. The cross-sectional study design limited our ability to identify a causal relationship between 25-OH D and bone mass, microarchitecture, and strength. Our sample size precluded stratification by race and sex. Also, our recruitment of volunteers from the community may not include individuals who are representative of the general US population or those living in different locations. We also used the same threshold for vitamin D deficiency across all races, which may not be appropriate (41). Further, we did not assess bioavailable vitamin D, which may be important in multiracial cohorts (42, 43), though Nielsen et al reported that free 25-OH D, from either direct measurements or calculated from polyclonal vitamin D binding protein assays, reflected total 25-OH D regardless of race (44). We relied on self-reported race, rather than using genetic ancestry markers as some studies have done (34). However, the self-identification of our participants’ race can be interpreted in the context of race as a social construct. Further, the covariates of physical activity and calcium intake were self-reported measures, which can introduce random misclassification bias. Last, residual confounding may be present because it is likely that people with higher 25-OH D engage in other healthy behaviors including increased physical activity, vitamin D supplementation, or healthy eating habits. Despite these limitations, these findings highlight the important role of vitamin D on bone health in young adults at the time of peak bone mass.
Conclusion
In summary, we found a high prevalence of vitamin D deficiency in young adults. Further, lower vitamin D is associated with worse bone microarchitecture and strength at both the distal radius and tibia at the time of peak bone mass. Whether skeletal deficits at the time of peak bone mass lead to increased fracture risk later in life remains uncertain, but these findings warrant further attention to vitamin D status in young adults. Future studies are needed to test whether vitamin D supplementation would enhance attainment and consolidation of bone density, microstructure and strength in young adults with vitamin D deficiency.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the US Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official US Army, Department of Defense endorsement of approval of the products or services of these organizations. This paper has been approved for public release with unlimited distribution. This research was supported in part by appointments to the Department of Defense Research Participation Program at the US Army Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education. We acknowledge support from the US Department of Defense, Defense Health Program, and Joint Program Committee (W811XWH-15-C-0024 and W81XWH-16-1-0652) and the National Institutes of Health shared instrumentation grant (S10 RR023405).
Glossary
Abbreviations
- 25-OH D
25-hydroxyvitamin D
- µFEA
micro-finite element analysis
- aBMD
areal bone mineral density
- BMI
body mass index
- BTM
bone turnover marker
- CTX-1
C-terminal telopeptides of type I collagen
- DXA
dual-energy X-ray absorptiometry
- FFQ
food frequency questionnaire
- HR-pQCT
high-resolution peripheral quantitative computed tomography
- iPTH
intact serum parathyroid hormone
- P1NP
amino-terminal propeptide of type I procollagen
- SE
standard error
Contributor Information
Margaret Garrahan, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA.
Sarah Gehman, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA.
Sara E Rudolph, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA.
Adam S Tenforde, Harvard Medical School, Boston, MA 02215, USA; Spaulding Rehabilitation Hospital, Cambridge, MA 02138, USA.
Kathryn E Ackerman, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA; Harvard Medical School, Boston, MA 02215, USA; Boston Children’s Hospital, Boston, MA 02215, USA.
Kristin L Popp, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA; Harvard Medical School, Boston, MA 02215, USA; United States Army Research Institute of Environmental Medicine, Natick, MA 01760, USA.
Mary L Bouxsein, Endocrine Unit, Massachusetts General Hospital, Boston, MA 02114, USA; Harvard Medical School, Boston, MA 02215, USA; Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA.
Shivani Sahni, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02131, USA.
Funding
US Army Medical Research Acquisition Activity (W811XWH-15-C-0024 and W81XWH-16-1-0652).
Disclosures
S.S. has institutional grants from Dairy Management Inc., has reviewed grants for American Egg Board’s Egg Nutrition Center, and is a scientific advisor to Institute for the Advancement of Food and Nutrition Sciences.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48-54. [DOI] [PubMed] [Google Scholar]
- 2. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531. [DOI] [PubMed] [Google Scholar]
- 4. Looker AC, Schleicher RL. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011;( 59):8. [PubMed] [Google Scholar]
- 5. Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SAM.. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract. 2012;18(6):914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Preventive Services Task Force. Screening for vitamin D deficiency in adults: US preventive services task force recommendation statement. JAMA. 2021;325(14):1436-1442. [DOI] [PubMed] [Google Scholar]
- 9. Teegarden D, Proulx WR, Martin BR, et al. Peak bone mass in young women. J Bone Miner Res. 1995;10(5):711-715. [DOI] [PubMed] [Google Scholar]
- 10. Sadat-Ali M, Al Elq AH, Al-Turki HA, Al-Mulhim FA, Al-Ali AK. Influence of vitamin D levels on bone mineral density and osteoporosis. Ann Saudi Med. 2011;31(6):602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Välimäki VV, Alfthan H, Lehmuskallio E, et al. Vitamin D status as a determinant of peak bone mass in young finnish men. J Clin Endocrinol Metab. 2004;89(1):76-80. [DOI] [PubMed] [Google Scholar]
- 12. Pekkinen M, Viljakainen H, Saarnio E, Lamberg-Allardt C, Mäkitie O. Vitamin D is a major determinant of bone mineral density at school age. PLoS One. 2012;7(7):e40090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adami S, Bertoldo F, Braga V, et al. 25-hydroxy vitamin D levels in healthy premenopausal women: association with bone turnover markers and bone mineral density. Bone. 2009;45(3):423-426. [DOI] [PubMed] [Google Scholar]
- 14. Napoli N, Strollo R, Sprini D, Maddaloni E, Rini GB, Carmina E. Serum 25-OH vitamin D in relation to bone mineral density and bone turnover. Int J Endocrinol. 2014;2014:e487463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samelson EJ, Broe KE, Xu H, et al. Cortical and trabecular bone microarchitecture predicts incident fracture independently of DXA bone mineral density and FRAX in older women and men: the Bone Microarchitecture International Consortium (BoMIC). Lancet Diabetes Endocrinol. 2019;7(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Bergh JP, Szulc P, Cheung AM, Bouxsein M, Engelke K, Chapurlat R. The clinical application of high-resolution peripheral computed tomography (HR-pQCT) in adults: state of the art and future directions. Osteoporos Int. 2021;32(8):1465-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu XS, Walker MD, McMahon DJ, et al. Better skeletal microstructure confers greater mechanical advantages in Chinese-American women versus white women. J Bone Miner Res. 2011;26(8):1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker MD, Liu XS, Stein E, et al. Differences in bone microarchitecture between postmenopausal Chinese-American and White women. J Bone Miner Res. 2011;26(7):1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boutroy S, Walker MD, Liu XS, et al. Lower cortical porosity and higher tissue mineral density in Chinese American versus White women. J Bone Miner Res. 2014;29(3):551-561. [DOI] [PubMed] [Google Scholar]
- 20. Popp KL, Hughes JM, Martinez-Betancourt A, et al. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone. 2017;103:200-208. [DOI] [PubMed] [Google Scholar]
- 21. Putman MS, Yu EW, Lee H, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013;28(10):2177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Putman MS, Yu EW, Lin D, Darakananda K, Finkelstein JS, Bouxsein ML. Differences in trabecular microstructure between black and white women assessed by individual trabecular segmentation analysis of HR-pQCT images. J Bone Miner Res. 2017;32(5):1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung TF, Cheuk KY, Yu FWP, et al. Prevalence of vitamin D insufficiency among adolescents and its correlation with bone parameters using high-resolution peripheral quantitative computed tomography. Osteoporos Int. 2016;27(8):2477-2488. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Wu F, Winzenberg T, Jones G. The association of vitamin D in youth and early adulthood with bone mineral density and microarchitecture in early adulthood. Calcif Tissue Int. 2019;104(6):605-612. [DOI] [PubMed] [Google Scholar]
- 25. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50(1):111-118. [DOI] [PubMed] [Google Scholar]
- 28. Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842-848. [DOI] [PubMed] [Google Scholar]
- 29. Whittier DE, Boyd SK, Burghardt AJ, et al. Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos Int. 2020;31(9):1607-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weeks BK, Beck BR. The BPAQ: a bone-specific physical activity assessment instrument. Osteoporos Int. 2008;19(11):1567-1577. [DOI] [PubMed] [Google Scholar]
- 31. Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139(12):1190-1196. [DOI] [PubMed] [Google Scholar]
- 32. Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86(1):150-158. [DOI] [PubMed] [Google Scholar]
- 33. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771-777. [DOI] [PubMed] [Google Scholar]
- 34. Hsu S, Hoofnagle AN, Gupta DK, et al. Race, ancestry, and vitamin D metabolism: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2020;105(12):e4337-e4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S-564S. [DOI] [PubMed] [Google Scholar]
- 37. Boyd SK, Burt LA, Sevick LK, Hanley DA. The relationship between serum 25(OH)D and bone density and microarchitecture as measured by HR-pQCT. Osteoporos Int. 2015;26(9): 2375-2380. [DOI] [PubMed] [Google Scholar]
- 38. Paranhos-Neto FP, Neto LV, Madeira M, et al. Vitamin D deficiency is associated with cortical bone loss and fractures in the elderly. Eur J Endocrinol. 2019;181(5):509-517. [DOI] [PubMed] [Google Scholar]
- 39. Chaitou A, Boutroy S, Vilayphiou N, et al. Association of bone microarchitecture with parathyroid hormone concentration and calcium intake in men: the STRAMBO study. Eur J Endocrinol. 2011;165:151-159. [DOI] [PubMed] [Google Scholar]
- 40. Qiu S, Rao SD. Effect of serum 25-hydroxyvitamin D concentrations on skeletal mineralization in black and white women. J Bone Miner Metab. 2021;39(5):843-850. [DOI] [PubMed] [Google Scholar]
- 41. Willett AM. Vitamin D status and its relationship with parathyroid hormone and bone mineral status in older adolescents. Proc Nutr Soc. 2005;64(2):193-203. [DOI] [PubMed] [Google Scholar]
- 42. Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D–binding protein modifies the vitamin D–bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielson CM, Jones KS, Chun RF, et al. Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101(5):2226-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.