Abstract
Context
Diabetes is a major risk factor for cardiovascular disease and death but its effect on outcomes in acromegaly is unknown.
Objective
This work aimed to study whether diabetes affects morbidity and mortality in patients with acromegaly.
Methods
A nationwide (Sweden), observational, matched-cohort study was conducted. Patients diagnosed with acromegaly between 1987 and 2020 were identified in the Swedish National Patient Registry and those with concomitant type 2 diabetes in the National Diabetes Registry and Drug Registry. The risk of overall mortality, and cardiovascular mortality and morbidity were estimated using Cox regression.
Results
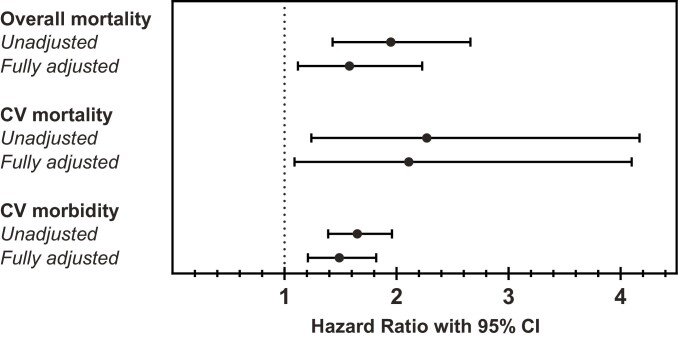
The study included 254 patients with acromegaly and concomitant type 2 diabetes (ACRO-DM group) and 532 without diabetes (ACRO group). Mean (SD) age at baseline was 62.6 (11.4) and 60.0 (12.1) years (P = .004) and the mean (SD) duration of acromegaly was 6.8 (8.1) and 6.0 (6.2) years (P = .098) in the ACRO-DM and ACRO groups, respectively. Overall mean follow-up was 9.2 years. The unadjusted overall mortality rate per 1000 person-years was 35.1 (95% CI, 27.2-44.7) and 20.1 (95% CI, 16.5-24.3) in the respective groups. The hazard ratio (HR) for overall mortality adjusted for multiple confounders was 1.58 (95% CI, 1.12-2.23) in the ACRO-DM group compared with the ACRO group. Cardiovascular mortality (HR 2.11; 95% CI, 1.09-4.10) and morbidity (HR 1.49; 95% CI, 1.21-1.82) were also increased in the ACRO-DM group.
Conclusion
The presence of diabetes in patients with acromegaly was associated with increased overall mortality as well as increased cardiovascular mortality and morbidity.
Keywords: acromegaly, diabetes, mortality, cardiovascular morbidity
Acromegaly is caused by excess secretion of growth hormone (GH), generally from a benign GH-secreting pituitary adenoma, that results in increased levels of insulin-like growth factor 1 (IGF-1) (1). Chronic excess of GH and IGF-1 leads to systemic comorbidities and increased mortality, mainly due to cardiovascular diseases (2-4). Levels of GH/IGF-1, diagnostic delay, age, and hypertension have previously been described as the main determinants of mortality in patients with acromegaly (5, 6). The goals for treatment of acromegaly are therefore to achieve biochemical remission and to treat associated comorbidities (7).
Impaired glucose metabolism is a common complication in acromegaly and the prevalence of diabetes mellitus ranges between 19% and 56% (8, 9). The risk of diabetes in acromegaly is mainly driven by insulin resistance induced by GH that directly attenuates insulin signaling and also stimulates lipolysis, resulting in increased free fatty acids, that consequently increases hepatic gluconeogenesis and reduces glucose uptake in peripheral tissues (9, 10).
The primary treatment of acromegaly is surgical removal of the GH-producing adenoma but approximately half of patients will need additional therapy with medical treatment and/or radiotherapy. First-line medical treatment is a somatostatin analogue that reduces GH secretion and may also reduce tumor remnants; however, it will also reduce insulin secretion and may therefore induce diabetes in susceptible patients (9, 11).
Type 2 diabetes is associated with excess risk of death and cardiovascular diseases, which is related to metabolic control, diabetes duration, and diabetes-related complications such as macrovascular diseases, nephropathy, neuropathy, and retinopathy (12-14). How and to what extent concomitant diabetes influences morbidity and mortality in patients with acromegaly is not known.
The primary aim of this study, therefore, was to investigate overall mortality in acromegalic patients with concomitant type 2 diabetes compared with those without diabetes. Secondary aims were to analyze cardiovascular morbidity and mortality, and the determinants of mortality.
Materials and Methods
Study Design and Data Sources
This was a nationwide, observational, matched-cohort study using health care data from Swedish national health and administrative registries, which contain comprehensive data for each Swedish resident. Patients with acromegaly were identified in the Swedish National Patient Registry (Patient Registry), which gathers health care data on every patient visit or admission within the national hospital system with national coverage since 1987. Study start was set to January 1, 2006 and patients were followed until death, censoring, or end of the study (December 31, 2020).
Via the unique 12-digit personal identification number, data from the Patient Registry were linked to the National Diabetes Registry (NDR), the Prescribed Drug Registry (Drug Registry), the Swedish National Cause of Death Registry (Cause of Death Registry), and the Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) Register. The NDR was started in 1996 and contains detailed clinical data on diabetes including risk factors, treatments, management, and diabetes-related complications for more than 90% of all adult patients with type 2 diabetes in Sweden. The Cause of Death Registry gathers information on date and cause of death for every Swedish resident since 1952. The Drug Registry achieved national coverage on July 1, 2005, and includes information on all prescribed drugs dispensed in Sweden categorized according to the Anatomical Therapeutic Chemical (ATC) classification system. Information on educational level, country of birth, marital status, and annual income was retrieved from the LISA Register, which contains data since 1990 for all Swedish residents aged 16 years or older. The linked data were returned in an anonymized manner by replacing the personal identification numbers with serial numbers. The Swedish registries are held by the National Board of Health and Welfare that ensures high quality for the data (15).
Study Population and Study Variables
Adult (age ≥ 18 years) patients diagnosed with acromegaly due to a pituitary adenoma between 1987 and 2020 were identified in the Patient Registry using the codes for acromegaly of the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10), as previously described (3, 16, 17). Patients who were alive at the time of study start (January 1, 2006) were included. To secure high accuracy for our patient selection, only adult patients with both an acromegaly and a pituitary adenoma diagnosis were included (ACRO group). Specifically, the following ICD codes were used: E22.0 (ICD-10) or 253A (ICD-9) for acromegaly in combination with D35.2 or D44.3 (ICD-10) or 237A or 227D (ICD-9) for pituitary adenoma. The date of acromegaly diagnosis was defined as the first specialized health care visit or admission with acromegaly as the main or secondary diagnosis. The diagnosis of pituitary adenoma should have occurred either up to 5 years before or any time after acromegaly diagnosis. Acromegalic patients with a concomitant diagnosis of type 2 diabetes were identified by linking the unique personal identification number to the NDR and the Drug Registry. In particular, patients who had at least one entry in the NDR or an ATC code for diabetes treatment (ie, A10) in the Drug Registry were included in the group of patients with type 2 diabetes (ACRO-DM group). A total of 684 unique patients with acromegaly were identified. Of those, 102 patients developed diabetes after the diagnosis of acromegaly and were therefore at the time point of diabetes diagnosis censured in ACRO group and switched to the ACRO-DM group, resulting in a total of 786 observations.
Patients were followed from January 1, 2006 (study start) to death, censoring, or the end of the study (December 31, 2020). Baseline was defined as the date with both a diagnosis of acromegaly and a diagnosis of type 2 diabetes. Information on acromegaly duration and data on tumor treatment (including pituitary surgery and radiotherapy) were gathered from the Patient Registry. Detailed clinical data (eg, blood pressure, blood lipid levels, body mass index) were not available for the ACRO group, but data on coexisting comorbidities (eg, cardiovascular and renal diseases, sleep apnea, antihypertensive medication) were retrieved using the diagnostic codes for these diseases from the Patient Registry or ATC codes from the Drug Registry. Data on medical treatment for acromegaly and type 2 diabetes were collected from the Drug Registry. The following medical treatments were recorded: somatostatin analogues (SSAs), dopamine agonists (DAs), GH receptor antagonists (GHRAs), lipid-lowering medication, antihypertensive medication, and diabetes treatment (insulin and other diabetes medications).
Outcomes
The risk of all-cause mortality was estimated in acromegalic patients with type 2 diabetes (ACRO-DM group) and those without diabetes (ACRO group). Secondary outcomes included the analysis of cardiovascular mortality and the incidence rate of cardiovascular diseases. Risk factors for mortality and cardiovascular morbidity were analyzed in the ACRO-DM group. In particular, the effect of age, sex, duration of diabetes, glycated hemoglobin A1c (HbA1c) levels, renal function, blood pressure and antihypertensive medication, physical activity, lipid levels and lipid-lowering medication, smoking status, and body mass index on outcomes were analyzed.
Ethics
The study was approved by the regional ethical review board in Gothenburg, Sweden (DNR 604-17) and by the National Board of Health and Welfare, Sweden.
Statistics
Descriptive statistics are presented using means with SD for continuous variables and counts together with percentages for categorical variables. Standardized mean difference (SMD) was used to compare the distribution of baseline variables between the groups and to describe whether a difference (imbalance) between the groups existed. Imbalance is defined as an absolute SMD value greater than 0.2, whereas an SMD less than or equal to 0.2 indicates adequate balance between the groups and less than 0.1 indicates excellent balance.
The mortality rate and incidence rate of cardiovascular diseases were estimated in both groups as the number of deaths or events per 1000 person years, with exact 95% CIs based on the Poisson distribution.
Cumulative mortality was estimated using 1 minus the Kaplan-Meier estimator and compared between the 2 groups using both an unadjusted Cox regression (model 1) and 2 adjusted Cox regressions (models 2 and 3). In model 2, the Cox regression was adjusted for sex and age and, in model 3, the analysis was adjusted using propensity scores in the form of an inverse probability weighted adjusted Cox regression estimating the average treatment effect for everyone. The Cox regression used a robust sandwich estimator for the standard errors to account for person being in both groups. The propensity scores used in the model 3 were estimated using gradient boosting with shrinkage 0.01, interaction depth 3, and number of trees (4990) optimized for SMD between the groups. All variables included in the propensity score model achieved a weighted SMD less than or equal to 0.15, indicating an adequate balance on these variables. Duration of acromegaly, disposable income, education, and age together accounted for 87.5% of the relative importance in the gradient-boosting model used to estimate the propensity scores. Preexisting cardiovascular and chronic kidney diseases were not included in the propensity score model since these diseases may be related to diabetes and thus an effect of diabetes. However, a subanalysis of overall mortality was preformed adding these diseases together with antihypertensive medication and lipid-lowering medication to the propensity score model.
To study the determinants of mortality and cardiovascular morbidity in patients with acromegaly and concomitant diabetes, risk factors for death and cardiovascular diseases were analyzed in this group of patients. The analyses were based on 10 imputed data sets where the imputations were performed using multiple chained equations using the mice package in R. Owing to the small sample size and small number of events, the potential risk factors were evaluated in small Cox regression models containing each variable in addition to sex and age at index. In addition, the effect of glycemic control over time on mortality was analyzed. Specifically, all the available HbA1c values after the index date were collected from the NDR for each patient and a Cox regression model with time-dependent covariates was performed.
Results
Patient Characteristics
The study included 254 patients with acromegaly and concomitant type 2 diabetes and 532 patients without diabetes. Baseline characteristics of the 2 groups are shown in Table 1. Mean (SD) age at baseline was 62.6 (11.4) years in the ACRO-DM group and 60.0 (12.1) years in the ACRO group (P = .004). Mean (SD) duration of acromegaly was 6.8 (8.1) and 6.0 (6.2) years in the respective groups (P = .098). Mean (SD) duration of diabetes was 2.4 (3.8) years. Mean follow-up was 9.2 years. Mean income and level of education were lower in the ACRO-DM group, while there was no difference in marital status, and frequency of hypopituitarism and diabetes insipidus between the groups (see Table 1). The frequency of preexisting cardiovascular diseases including heart failure, and atrial fibrillation as well as chronic kidney diseases, ocular complications, all neoplasms, and use of antihypertensive and lipid-lowering medication, was higher in the ACRO-DM group compared with the ACRO group (see Table 1). The variables included in the propensity scores and the SMD achieved after adjustment are shown in Table 2. Mean (SD) HbA1c was 55.0 (14.5) mmol/mol [7.1% (3.5%)] and body mass index 30.7 (5.4) in the ACRO-DM group (data not available for the ACRO group).
Table 1.
Baseline characteristics of acromegaly patients with and without type 2 diabetes
| Characteristics | ACRO (n = 532) | ACRO-DM (n = 254) | SMD |
|---|---|---|---|
| Age, y | 60.0 (12.1) | 62.6 (11.4) | 0.22 |
| Women | 244 (45.9) | 130 (51.2) | 0.11 |
| Acromegaly duration, y | 6.0 (6.2) | 6.8 (8.1) | 0.12 |
| Mean incomea | 2136.6 (1904.5) | 1851.7 (1200.8) | 0.18 |
| Born in Sweden | 439 (82.7) | 194 (76.4) | 0.16 |
| Marital status | 0.12 | ||
| Married | 307 (57.8) | 140 (55.1) | – |
| Divorced | 93 (17.5) | 52 (20.5) | – |
| Single | 92 (17.3) | 39 (15.4) | – |
| Widowed | 39 (7.3) | 23 (9.1) | – |
| Educational level | 0.22 | ||
| Low | 136 (25.8) | 87 (34.9) | – |
| Intermediate | 230 (43.6) | 104 (41.8) | – |
| High | 161 (30.6) | 58 (23.3) | – |
| Diabetes treatment | |||
| Oral medications | 0 | 102 (40.2) | – |
| Insulin | 0 | 45 (17.7) | – |
| Antihypertensive medication | 241 (45.3) | 186 (73.2) | 0.59 |
| Lipid-lowering medication | 90 (16.9) | 98 (38.6) | 0.50 |
| Hypopituitarism | 121 (22.7) | 45 (17.7) | 0.13 |
| Diabetes insipidus | 7 (1.3) | 4 (1.6) | 0.02 |
| Comorbidities | |||
| Cardiovascular diseases | 196 (36.8) | 154 (60.6) | 0.49 |
| Heart failure | 20 (3.8) | 20 (7.9) | 0.18 |
| Atrial fibrillation | 25 (4.7) | 22 (8.7) | 0.16 |
| Chronic kidney diseases | 2 (0.4) | 12 (4.7) | 0.28 |
| Ocular complications | 0 | 10 (3.9) | 0.29 |
| Sleep apnea | 46 (8.6) | 32 (12.6) | 0.13 |
| Carpal tunnel syndrome | 12 (2.3) | 9 (3.5) | 0.08 |
| Neoplasms | 52 (9.8) | 40 (15.7) | 0.18 |
| Duration of diabetes, y | – | 2.37 (3.8) | – |
| Systolic blood pressure, mm Hg | – | 135.7 (16.4) | – |
| Diastolic blood pressure, mm Hg | – | 80.8 (10.6) | – |
| HbA1c, mmol/mol | – | 55.0 (14.5) | – |
| Body mass index | – | 30.7 (5.4) | – |
| Creatinine, µmol/L | – | 77.1 (37.8) | – |
Data are shown as n (%) or mean (SD).
Abbreviations: ACRO, acromegaly alone; ACRO-DM, acromegaly and type 2 diabetes; HbA1c, glycated hemoglobin A1c; SEK, Swedish krona; SMD, standardized mean difference.
a Income in 100 SEK per year. 100 SEK is equivalent to £85.40 (approximately US $11.45).
Table 2.
Variables included in the propensity score model and standardized mean difference achieved after adjustment
| Variables | SMD after adjustmenta |
|---|---|
| Age | 0.11 |
| Sex | 0.07 |
| Acromegaly duration | 0.05 |
| Mean income | 0.15 |
| Marital status | 0.04 |
| Educational level | 0.08 |
| Hypopituitarism | 0.03 |
| Sleep apnea | 0.05 |
| Visual field defects | 0.03 |
| Preindex treatment for acromegaly | |
| Pituitary surgery | 0.03 |
| Radiation therapy | 0.002 |
| Somatostatin antagonists | 0.02 |
| Dopamine agonists | 0.08 |
| Growth hormone receptor antagonists | 0.06 |
Abbreviations: SMD, standardized mean difference.
a All variables achieved a weighted SMD ≤ 0.15, indicating an adequate balance for these variables between the acromegaly and type 2 diabetes and acromegaly alone groups.
The frequencies of pituitary surgery (68% in the ACRO vs 71% in the ACRO-DM group, P = .62) and use of radiotherapy (16% in the ACRO vs 16% in the ACRO-DM group, P ≥ .999) were similar among the 2 groups during the entire study period (Table 3). A total of 466 (59%) patients received medical therapy in the entire study cohort. The use of SSAs (30% in the ACRO vs 37% in the ACRO-DM group, P = .055) and GHRAs (6% in the ACRO vs 10% in the ACRO-DM group, P = .063) showed a trend for a higher frequency in the ACRO-DM group (see Table 3).
Table 3.
Treatment for acromegaly during the entire study period
| Acromegaly treatment | ACRO (n = 532) | ACRO-DM (n = 254) | SMD |
|---|---|---|---|
| Pituitary surgery | 364 (68.4) | 179 (70.5) | 0.05 |
| Radiation therapy | 85 (16.0) | 40 (15.7) | 0.01 |
| Somatostatin analogues | 159 (29.9) | 94 (37.0) | 0.15 |
| Dopamine agonists | 100 (18.8) | 54 (21.3) | 0.06 |
| Growth hormone receptor antagonists | 33 (6.2) | 26 (10.2) | 0.15 |
Data are shown as n (%).
Abbreviations: ACRO, acromegaly alone; ACRO-DM, acromegaly and type 2 diabetes; SMD, standardized mean difference.
Overall Mortality
The unadjusted mortality rate from any causes per 1000 person-years was 35.1 (95% CI, 27.2-44.7) for the ACRO-DM group and 20.1 (95% CI, 16.5-24.3) for the ACRO group. The corresponding rates for mortality from cardiovascular causes were 9.6 (95% CI, 5.7-15.1) and 4.5 (95% CI, 2.9-6.7). The incidence rate for cardiovascular disease per 1000 person-years was 281.6 (95% CI, 243.2-324.4) in the ACRO-DM group and 135.6 (95% CI, 121.9-150.5) in the ACRO group.
The ACRO-DM group had an excess overall mortality. Specifically, the hazard ratio (HR) adjusted for age and sex for overall mortality among patients with diabetes compared with those without diabetes was 1.81 (95% CI, 1.32-2.49) (Table 4 and Fig. 1). The corresponding HR after adjustment using propensity score was 1.58 (95% CI, 1.12-2.23), showing that despite adjustment for multiple factors, patients with acromegaly and concomitant diabetes still have excess mortality (see Table 4). A subanalysis of overall mortality adding preexisting cardiovascular and chronic kidney diseases, antihypertensive medication, and lipid-lowering medication to the propensity score model led to a similar result with an HR for overall mortality of 1.51 (95% CI, 1.04-2.19).
Table 4.
Hazard ratios for overall mortality and cardiovascular mortality and morbidity among patients with acromegaly and type 2 diabetes vs patients with acromegaly without diabetes
| Outcome | HR (95% CI) | P |
|---|---|---|
| Death from any cause | ||
| Unadjusted HR | 1.95 (1.43-2.66) | < .001 |
| Age- and sex-adjusted HR | 1.81 (1.32-2.49) | < .001 |
| Propensity score–adjusted HR | 1.58 (1.12-2.23) | .0096 |
| Death from cardiovascular causes | ||
| Unadjusted HR | 2.27 (1.24-4.17) | .008 |
| Age- and sex-adjusted HR | 2.17 (1.18-3.98) | .0124 |
| Propensity score–adjusted HR | 2.11 (1.09-4.10) | .0276 |
| Cardiovascular diseases | ||
| Unadjusted HR | 1.65 (1.39-1.96) | < .001 |
| Age- and sex-adjusted HR | 1.51 (1.27-1.80) | < .001 |
| Propensity score–adjusted HR | 1.49 (1.21-1.82) | < .001 |
Abbreviation: HR, hazard ratio.
Figure 1.
Hazard ratios for overall mortality, and cardiovascular (CV) mortality and morbidity. Unadjusted and fully adjusted using propensity score hazard ratios in patients with acromegaly and type 2 diabetes compared with patients with acromegaly without diabetes.
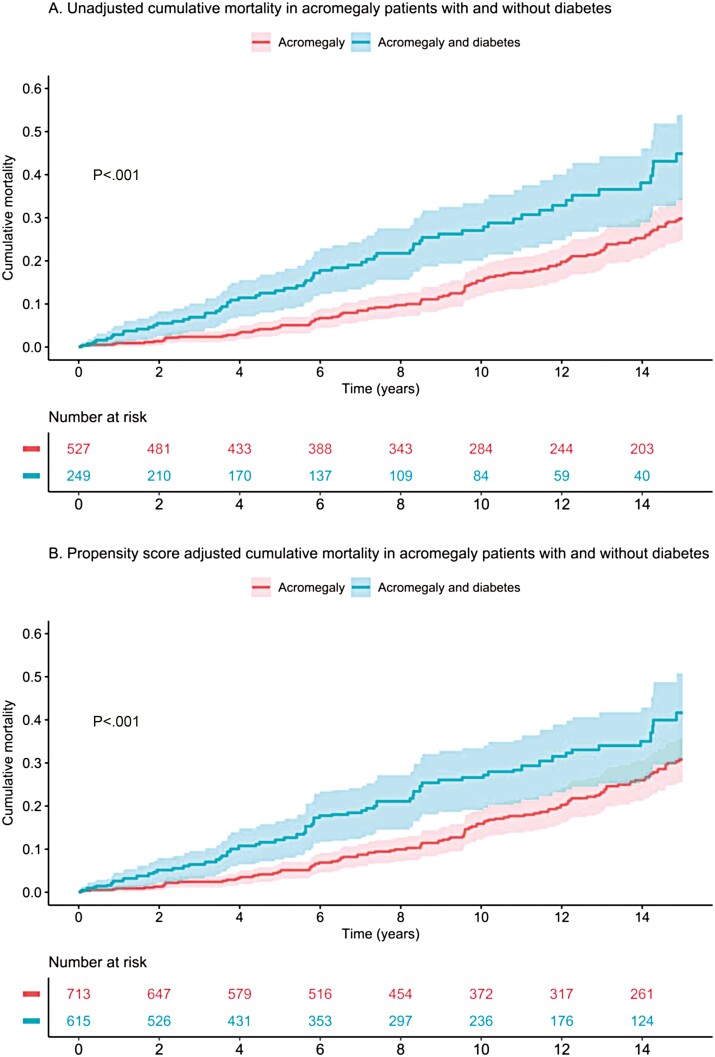
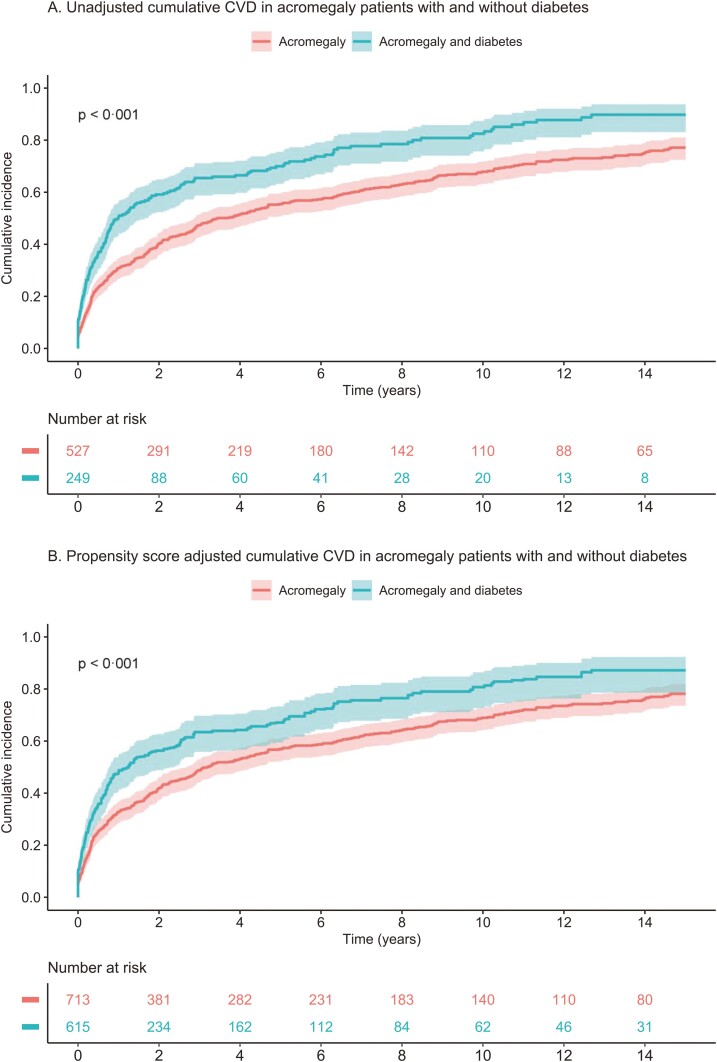
When cardiovascular mortality was analyzed, the HR was 2.17 (95% CI, 1.18-3.98) after adjustment for age and sex, and 2.11 (95% CI, 1.09-4.1) after adjustment using propensity score. In addition, the ACRO-DM group had an increased risk of cardiovascular diseases compared with the ACRO group (see Table 4 and Fig. 1) with an HR of 1.51 (95% CI, 1.27-1.80) adjusted for age and sex, and 1.49 (95% CI, 1.21-1.82) after propensity score adjustment. Kaplan-Meier analysis of all-cause mortality and cardiovascular morbidity in acromegaly patients with and without type 2 diabetes in an unadjusted model and after propensity score adjustment are shown in Figs. 2 and 3, respectively.
Figure 2.
Kaplan-Meier plots for all-cause mortality. A, Unadjusted, and B, fully propensity score–adjusted plots in patients with acromegaly and type 2 diabetes compared with patients with acromegaly without diabetes.
Figure 3.
Kaplan-Meier plots for cardiovascular morbidity. A, Unadjusted, and B, fully propensity score–adjusted plots in patients with acromegaly and type 2 diabetes compared with patients with acromegaly without diabetes. CVD, cardiovascular disease.
Risk factors for overall mortality were analyzed in patients with acromegaly and concomitant diabetes. Age was significantly associated with an increased risk of death (HR 1.10; 95% CI, 1.07-1.13), whereas sex (HR women 0.79; 95% CI, 0.48-1.29), body mass index (HR 0.99; 95% CI, 0.92-1.06), smoking status (HR 1.00; 95% CI, 0.39-2.56), systolic blood pressure (HR 1.00; 95% CI, 0.98-1.02), diastolic blood pressure (HR 1.01; 95% CI, 0.98-1.04), and lipid levels (HR 0.87; 95% CI, 0.48-1.57) were not. Finally, the duration of diabetes (HR 1.07; 95% CI, 1.00-1.16) and glycemic control at baseline (HbA1c) (HR 1.02; 95% CI, 0.99-1.04) showed minor effect on overall mortality. When the effect of glycemic control over time (i.e., all the Hba1C values available in the NDR) on mortality was analyzed, a similar result was found, with a HR for overall mortality of 1.02 (95% CI, 1.00-1.04).
The analysis of predictive factors for cardiovascular morbidity showed that age (HR 1.03; 95% CI, 1.02-1.04), diabetes duration (HR 1.09; 95% CI, 1.05-1.13), diastolic blood pressure (HR 1.02; 95% CI, 1.00-1.04), body mass index (HR 1.05; 95% CI, 1.01-1.09), and treatment with lipid-lowering medication (HR 1.60; 95% CI, 1.08-2.36) or antihypertensive medication (HR 2.1; 95% CI, 1.21-3.64) were associated with an increased risk of cardiovascular morbidity.
Discussion
In this nationwide Swedish study, patients with acromegaly and concomitant type 2 diabetes had excess mortality, which was 60% higher than in patients without diabetes despite adjustment for multiple confounding factors. In addition, mortality due to cardiovascular causes was increased 2-fold and the risk of cardiovascular diseases was 50% higher in acromegalic patients with diabetes than in those without.
Diabetes mellitus is a common complication of acromegaly, occurring in more than 20% of patients at diagnosis, with a prevalence that progressively increases with longer disease duration (9). Understanding the effect of diabetes on long-term outcomes in patients with acromegaly is important for the improvement of treatment strategies and management of the disease. Patients with acromegaly have a 30% excess overall mortality, with cardiovascular disorders being the main cause of death (3, 18). GH and IGF-1 levels are strong determinants of mortality (6) and biochemical remission is therefore likely to improve outcome, with some studies showing that, in well-controlled patients, the mortality rate can be reduced to that expected in the general population (6).
Chronic GH/IGF-1 excess plays an important role in the development of acromegaly-associated complications such as hypertension and type 2 diabetes that, in turn, may affect mortality (5, 19). The role of hypertension in acromegaly has been extensively studied (5) and shown to be independently associated with cardiac abnormalities such as left ventricular hypertrophy, and diastolic and systolic dysfunction (20, 21). Moreover, in a cohort of 2090 patients with acromegaly (5), hypertension was associated with a 3-fold higher mortality compared with those without hypertension. Whether type 2 diabetes mellitus affects mortality in acromegaly in a similar manner has been sparsely studied.
Diabetes is a strong and well-known independent risk factor for cardiovascular morbidity and mortality (22, 23). A large nationwide study, including more than 400 000 patients with type 2 diabetes, showed that the risk of death from cardiovascular causes was 33% higher among those with diabetes than in the background population (13). Glycemic control, diabetes duration, and diabetes-related complications such as macrovascular diseases, nephropathy, neuropathy, and retinopathy had an adverse effect on outcomes (12-14). Our study is, however, the first study to evaluate the effect of type 2 diabetes on mortality and morbidity in a nationwide cohort of patients with acromegaly.
In our study cohort, patients with acromegaly and diabetes were older (62.6 vs 60.0 years) and had higher prevalence of cardiovascular diseases (61% vs 37%), atrial fibrillation (9 vs 5%), and heart failure (8% vs 4%), antihypertensive medication (73% vs 45%), compared with those without diabetes (see Table 1). This is in line with data from the French Acromegaly Registry, which showed that patients with acromegaly and concomitant diabetes were older, had a longer duration of acromegaly, a higher body mass index, and a higher frequency of hypertension compared with acromegalic patients without diabetes (24). In another study including 130 patients with acromegaly (20), patients with impaired glucose metabolism had an increased prevalence of diastolic and systolic dysfunction and those with both diabetes and hypertension had more severe cardiac abnormalities with the highest prevalence of left ventricular hypertrophy, diastolic filling abnormalities, and impaired systolic function at rest. These studies together with our findings clearly indicate increased cardiovascular disease among acromegalic patients with diabetes.
In our study, the risk of death from any cause was 2-fold higher in patients with acromegaly and concomitant diabetes than in those without diabetes in an unadjusted model, whereas in a fully adjusted model using propensity score, the excess overall mortality remained 60% higher. Furthermore, in the fully adjusted model, mortality from cardiovascular causes was 2-fold higher and the risk of cardiovascular comorbidities 50% higher in patients with diabetes than in those without diabetes. There have been some indications in previous studies that concomitant diabetes affects outcome in acromegaly. In a retrospective analysis of 208 patients with acromegaly with 72 deaths, the deceased had an increased prevalence of diabetes compared with surviving patients (59% vs 15%) (6). Another survey, including 1512 acromegalic patients, showed that diabetes was a predictor of mortality in a univariate model (odds ratio 1.09; 95% CI, 1.02-3.51; P = .04), although the statistical significance disappeared in multivariable analysis (25).
The different treatment strategies offered to patients with acromegaly may have various effects on glucose metabolism. It has been reported that biochemical control obtained with surgery normalizes glucose metabolism in 23% to 58% of patients with preexisting diabetes (9). The effect of medical treatment is, however, more diverse. Conventional SSAs (eg, octreotide and lanreotide) bind to somatostatin receptors (SSTR) 2 and 5, with a higher affinity to SSTR2. These receptors play a crucial role in glucose regulation, and treatment with SSAs appears to suppress insulin and glucagon secretion and may lead to a detrimental effect on glucose homeostasis (9). In a recent meta-analysis including 1297 acromegalic patients, first-generation SSAs reduced insulin levels and increased HbA1c without affecting fasting plasma glucose (11). A second-generation SSA (pasireotide) has been introduced in 2014. Pasireotide binds to 4 of the 5 SSTRs (SSTR1, SSTR2, SSTR3, and SSTR5) with the highest affinity for SSTR5 (ie, 40-100 times higher than conventional SSAs), and suppresses insulin and incretin secretion, leading to impaired glucose metabolism in a significant proportion of patients (26, 27). On the other hand, GHRAs (eg, pegvisomant) ameliorate glucose metabolism by improving insulin sensitivity (28). Finally, DAs seem to reduce HbA1c and fasting plasma glucose levels as shown in patients with type 2 diabetes (29), although data in patients with acromegaly are lacking. In our study cohort, 32% of patients received SSAs, 20% DAs, and 8% GHRAs. Subgroup analyses of morbidity and mortality in these different treatment groups were not performed because of the small sample size. The use of SSAs and GHRAs was somewhat more frequent in the ACRO-DM group (see Table 3) but all the treatments were included in the propensity score. It will be of interest to see whether new medical treatment options for acromegaly can be developed with improved efficacy and a reduced negative effect on glucose metabolism (30).
Our propensity score–adjusted data do show that diabetes has detrimental effects on long-term outcome in patients with acromegaly. Age seems to be the main determinant of mortality, but we cannot exclude that the duration of diabetes and glycemic control play a role on outcome in acromegaly as they do in patients with diabetes alone (13).
One could argue that the higher proportion of cardiovascular and chronic kidney diseases at baseline among ACRO-DM patients could affect our results (see Table 1). However, it is important to note that these comorbidities may be associated with diabetes itself and adjusting for these factors could result in an underestimation of the effect of diabetes on outcome. On the other hand, it is not possible to exclude that these complications are associated with chronic overexposure to GH/IGF-1. Therefore, a subanalysis of overall mortality was performed adding these variables to the propensity score model and similar findings were observed. This subanalysis therefore strengthens the conclusion that diabetes has a significant effect on mortality in patients with acromegaly.
The strengths of the present study are the large unselected nationwide population and the long follow-up period. Another major strength is the high reliability of the data that were retrieved from official national registers that cover the entire Swedish population. In addition, all patients diagnosed with acromegaly due to a pituitary adenoma were included nationwide regardless of the center and of the burden of disease, limiting the effects of selection bias.
There are some study limitations to be considered: first, the retrospective nature of this study and the lack of detailed clinical and biochemical data for patients with acromegaly (eg, tumor size, GH and IGF-1 levels, and posttreatment biochemical status) that were not available in the registers. Specifically, the lack of biochemical data is the main weakness since GH and IGF-1 are associated with an increased risk of diabetes and excess mortality and the increased use of medical treatment for acromegaly in the ACRO-DM group may suggest that this group had increased disease activity.
In addition, there are some clinical variables of interest affecting cardiovascular risk that would have added value to our analysis such as body mass index, physical activity, smoking status, lipids, etc, which were not available in the ACRO group.
In conclusion, this study is the first analysis of the effect of diabetes on long-term outcomes in patients with acromegaly. We have shown that the coexistence of diabetes is associated with excess overall and cardiovascular mortality and increased risk of cardiovascular disease in comparison with patients with acromegaly without diabetes. Our findings highlight the importance of optimizing management of acromegaly to prevent the development of diabetes.
Acknowledgments
The authors dedicate this paper to our late colleague and friend Ann-Marie Svensson. The authors are grateful to Associate Professor Katarina Eeg-Olofsson and the statistician Erik Bülow for their contribution to this manuscript. The authors acknowledge Peter Todd (Tajut Ltd, Kaiapoi, New Zealand) for third-party writing assistance in drafting of this manuscript, for which he received financial compensation from ALF funding.
Glossary
Abbreviations
- ACRO
acromegaly alone
- ACRO-DM
acromegaly and type 2 diabetes
- ATC
Anatomical Therapeutic Chemical
- DA
dopamine agonist
- GH
growth hormone
- GHRA
growth hormone receptor antagonists
- HbA1c
glycated hemoglobin A1c
- HR
hazard ratio
- ICD
International Classification of Diseases
- IGF-1
insulin-like growth factor 1
- LISA
Longitudinal Integrated Database for Health Insurance and Labour Market Studies
- NDR
National Diabetes Registry
- SMD
standardized mean difference
- SSA
somatostatin analogue
- SSTR
somatostatin receptor
Contributor Information
Daniela Esposito, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, 41345 Gothenburg, Sweden; Department of Endocrinology, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Daniel S Olsson, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, 41345 Gothenburg, Sweden; Department of Endocrinology, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Stefan Franzén, Health Metrics Group, Sahlgrenska Academy, University of Gothenburg, 41345 Gothenburg, Sweden.
Mervete Miftaraj, National Diabetes Register, Centre of Registers, 41345 Gothenburg, Sweden.
Jonatan Nåtman, National Diabetes Register, Centre of Registers, 41345 Gothenburg, Sweden.
Soffia Gudbjörnsdottir, National Diabetes Register, Centre of Registers, 41345 Gothenburg, Sweden; Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, 41345 Gothenburg, Sweden.
Gudmundur Johannsson, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, 41345 Gothenburg, Sweden; Department of Endocrinology, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Financial Support
This work was supported by the Swedish government under the ALF agreement (ALFGBG-873321) and an unrestricted research grant from Pfizer AB. The funding source did not have any involvement in the study design or in any other phase of the project (collection, analysis, and interpretation of data; writing of the report; decision to submit the paper for publication).
Author Contributions
D.E., D.S.O., G.J., and S.F. contributed to the study concept and design. S.F. and J.N. performed the statistical analyses. D.E. had the primary responsibility for writing the paper. All authors contributed to data interpretation, and reviewed and revised subsequent versions of the manuscript. D.E., D.S.O., S.F., J.N., and G.J. vouch for the integrity of the analyses.
Disclosures
D.E. has received lecture fees from Ipsen. D.S.O. has been a consultant for Novo Nordisk, Sandoz, Ipsen, and Pfizer AB; has received unrestricted grants from Sandoz and Pfizer AB; and is an employee at AstraZeneca as of August 30, 2021. S.F. is an employee at AstraZeneca as of October 4, 2021. S.G. has received personal fees (lectures fees and research grants) from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi, all outside this work. G.J. has served as a consultant for Novo Nordisk, Shire, and Astra Zeneca; and has received lecture fees from Eli Lilly, Ipsen, Novartis, Novo Nordisk, Merck Serono, Otsuka, and Pfizer AB. All other authors have nothing to declare.
Data Availability
Raw data from this study are stored by the NDR and may be accessed on request to the NDR.
References
- 1. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558-2573. doi: 10.1056/NEJMra062453 [DOI] [PubMed] [Google Scholar]
- 2. Lesén E, Granfeldt D, Houchard A, et al. Comorbidities, treatment patterns and cost-of-illness of acromegaly in Sweden: a register-linkage population-based study. Eur J Endocrinol. 2017;176(2):203-212. doi: 10.1530/EJE-16-0623 [DOI] [PubMed] [Google Scholar]
- 3. Esposito D, Ragnarsson O, Granfeldt D, Marlow T, Johannsson G, Olsson DS. Decreasing mortality and changes in treatment patterns in patients with acromegaly from a nationwide study. Eur J Endocrinol. 2018;178(5):459-469. doi: 10.1530/EJE-18-0015 [DOI] [PubMed] [Google Scholar]
- 4. Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Español de Acromegalia, REA). Eur J Endocrinol. 2004;151(4):439-446. doi: 10.1530/eje.0.1510439 [DOI] [PubMed] [Google Scholar]
- 5. Vila G, Luger A, van der Lely AJ, et al. Hypertension in acromegaly in relationship to biochemical control and mortality: global ACROSTUDY outcomes. Front Endocrinol (Lausanne). 2020;11:577173. doi: 10.3389/fendo.2020.577173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89(2):667-674. doi: 10.1210/jc.2003-031199 [DOI] [PubMed] [Google Scholar]
- 7. Melmed S, Bronstein MD, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-561. doi: 10.1038/s41574-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102-152. doi: 10.1210/er.2002-0022 [DOI] [PubMed] [Google Scholar]
- 9. Pivonello R, Auriemma RS, Grasso LFS, et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20(1):46-62. doi: 10.1007/s11102-017-0797-7 [DOI] [PubMed] [Google Scholar]
- 10. Hannon AM, Thompson CJ, Sherlock M. Diabetes in patients with acromegaly. Curr Diab Rep. 2017;17(2):8. doi: 10.1007/s11892-017-0838-7 [DOI] [PubMed] [Google Scholar]
- 11. Cozzolino A, Feola T, Simonelli I, et al. Somatostatin analogs and glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab. 2018;103(6):2089-2099. doi: 10.1210/jc.2017-02566 [DOI] [PubMed] [Google Scholar]
- 12. Salehidoost R, Mansouri A, Amini M, Aminorroaya Yamini S, Aminorroaya A. Diabetes and all-cause mortality, a 18-year follow-up study. Sci Rep. 2020;10(1):3183. doi: 10.1038/s41598-020-60142-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720-1732. doi: 10.1056/NEJMoa1504347 [DOI] [PubMed] [Google Scholar]
- 14. Cusick M, Meleth AD, Agrón E, et al. ; Early Treatment Diabetic Retinopathy Study Research Group. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: Early Treatment Diabetic Retinopathy Study report no. 27. Diabetes Care. 2005;28(3):617-625. doi: 10.2337/diacare.28.3.617 [DOI] [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Prolonged diagnostic delay in acromegaly is associated with increased morbidity and mortality. Eur J Endocrinol. 2020;182(6):523-531. doi: 10.1530/EJE-20-0019 [DOI] [PubMed] [Google Scholar]
- 17. Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Incidence of benign and malignant tumors in patients with acromegaly is increased: a nationwide population-based study. J Clin Endocrinol Metab. 2021;106(12):3487-3496. doi: 10.1210/clinem/dgab560 [DOI] [PubMed] [Google Scholar]
- 18. Bolfi F, Neves AF, Boguszewski CL, Nunes-Nogueira VS. Mortality in acromegaly decreased in the last decade: a systematic review and meta-analysis. Eur J Endocrinol. 2018;179(1):59-71. doi: 10.1530/EJE-18-0255 [DOI] [PubMed] [Google Scholar]
- 19. Gadelha MR, Kasuki L, Lim, DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2019;40(1):268-332. doi: 10.1210/er.2018-00115 [DOI] [PubMed] [Google Scholar]
- 20. Colao A, Baldelli R, Marzullo P, et al. Systemic hypertension and impaired glucose tolerance are independently correlated to the severity of the acromegalic cardiomyopathy. J Clin Endocrinol Metab. 2000;85(1):193-199. doi: 10.1210/jcem.85.1.6318 [DOI] [PubMed] [Google Scholar]
- 21. López-Velasco R, Escobar-Morreale HF, Vega B, et al. Cardiac involvement in acromegaly: specific myocardiopathy or consequence of systemic hypertension? J Clin Endocrinol Metab. 1997;82(4):1047-1053. doi: 10.1210/jcem.82.4.3876 [DOI] [PubMed] [Google Scholar]
- 22. Seshasai SRK, Kaptoge S, Thompson A, et al. ; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829-841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lind M, Garcia-Rodriguez LA, Booth GL, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia. 2013;56(12):2601-2608. doi: 10.1007/s00125-013-3063-1 [DOI] [PubMed] [Google Scholar]
- 24. Fieffe S, Morange I, Petrossians P, et al. ; French Acromegaly Registry. Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. Eur J Endocrinol. 2011;164(6):877-884. doi: 10.1530/EJE-10-1050 [DOI] [PubMed] [Google Scholar]
- 25. Arosio M, Reimondo G, Malchiodi E, et al. ; Italian Study Group of Acromegaly. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol. 2012;167(2):189-198. doi: 10.1530/EJE-12-0084 [DOI] [PubMed] [Google Scholar]
- 26. Gadelha MR, Gu F, Bronstein MD, et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr Connect. 2020;9(12):1178-1190. doi: 10.1530/EC-20-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gadelha MR, Bronstein MD, Brue T, et al. ; Pasireotide C2402 Study Group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875-884. doi: 10.1016/S2213-8587(14)70169-X [DOI] [PubMed] [Google Scholar]
- 28. Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ. Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J Clin Endocrinol Metab. 2009;94(7):2459-2463. doi: 10.1210/jc.2008-2086 [DOI] [PubMed] [Google Scholar]
- 29. Andersen IB, Andreassen M, Krogh J. The effect of dopamine agonists on metabolic variables in adults with type 2 diabetes: a systematic review with meta analysis and trial sequential analysis of randomized clinical trials. Diabetes Obes Metab. 2021;23(1):58-67. doi: 10.1111/dom.14183 [DOI] [PubMed] [Google Scholar]
- 30. Gadelha MR, Wildemberg LE, Kasuki L. The future of somatostatin receptor ligands in acromegaly. J Clin Endocrinol Metab. 2022;107(2):297-308. doi: 10.1210/clinem/dgab726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data from this study are stored by the NDR and may be accessed on request to the NDR.