Abstract
Context
Prolactin is a multifaceted hormone known to regulate lactation. In women with gestational diabetes mellitus (GDM) history, intensive lactation has been associated with lower relative risk of future type 2 diabetes (T2D). However, the role of prolactin in T2D development and maternal metabolism in women with a recent GDM pregnancy has not been ascertained.
Objective
We examined the relationships among prolactin, future T2D risk, and key clinical and metabolic parameters.
Methods
We utilized a prospective GDM research cohort (the SWIFT study) and followed T2D onset by performing 2-hour 75-g research oral glucose tolerance test (OGTT) at study baseline (6-9 weeks postpartum) and again annually for 2 years, and also by retrieving clinical diagnoses of T2D from 2 years through 10 years of follow up from electronic medical records. Targeted metabolomics and lipidomics were applied on fasting plasma samples collected at study baseline from 2-hour 75-g research OGTTs in a nested case-control study (100 future incident T2D cases vs 100 no T2D controls).
Results
Decreasing prolactin quartiles were associated with increased future T2D risk (adjusted odds ratio 2.48; 95% CI, 0.81-7.58; P = 0.05). In women who maintained normoglycemia during the 10-year follow-up period, higher prolactin at baseline was associated with higher insulin sensitivity (P = 0.038) and HDL-cholesterol (P = 0.01), but lower BMI (P = 0.001) and leptin (P = 0.002). Remarkably, among women who developed future T2D, prolactin was not correlated with a favorable metabolic status (all P > 0.05). Metabolomics and lipidomics showed that lower circulating prolactin strongly correlated with a T2D–high risk lipid profile, with elevated circulating neutral lipids and lower concentrations of specific phospholipids/sphingolipids.
Conclusion
In women with recent GDM pregnancy, low circulating prolactin is associated with specific clinical and metabolic parameters and lipid metabolites linked to a high risk of developing T2D.
Keywords: prolactin, gestational diabetes, type 2 diabetes, lactation, maternal metabolism
Lactation is a postpartum activity that has been reported to significantly reduce the risk of multiple cardiometabolic conditions, including cardiovascular diseases, nonalcoholic fatty liver disease, hypertension, metabolic syndrome, and type 2 diabetes (T2D) (1-8). Lactation also has other beneficial effects on both mothers and infants, including reduced infant morbidity and mortality, lower risk of child obesity, and decreased risk of developing ovarian and breast cancer (9, 10). Prolactin, secreted from the pituitary gland, is considered the primary hormone that promotes lactation (11, 12). In addition to its regulation of lactation, prolactin is also a pleiotropic hormone involved in reproduction, metabolism, and osmoregulation (13).
Gestational diabetes mellitus (GDM) is a common metabolic disorder occurring in ~10% or more of all pregnancies (14-16). Women with a GDM history have shown several fold higher risk of developing future T2D compared to non-GDM women (17, 18). The Study of Women, Infant Feeding, and Type 2 Diabetes after GDM Pregnancy (SWIFT), a racially and ethnically diverse prospective research cohort (75% minority), found that increased breastfeeding intensity/duration for 2 months or more was associated with a graded 34% to 57% relative risk reduction in the 2-year incidence of T2D after GDM pregnancy, independent of severity of prenatal glucose intolerance and treatment, pre-pregnancy obesity, socio-demographics, lifestyle behaviors, and perinatal outcomes (5). Furthermore, lactation showed favorable effects on regulating the lipid profiles of women with a GDM history (19-22). Other studies have also demonstrated that intensive breastfeeding after a GDM pregnancy was associated with lower fasting triacylglycerol (TAG) and higher high-density lipoprotein (HDL)-cholesterol (19, 20). Moreover, a longitudinal study by Gunderson et al. showed that higher HDL-cholesterol persisted in women who had lactated for 3 months or longer vs shorter lactation (21). Recently, by applying targeted metabolomic and lipidomic profiling to the SWIFT study, we demonstrated that intensive lactation was associated with significantly decreased fasting glycerolipids (TAGs) and diacylglycerols (DAGs) and increased phospholipids/sphingolipids at early postpartum in women with recent GDM history. Furthermore, women who did not respond metabolically to lactation were more likely to develop T2D (22). These findings suggest that intensive lactation may delay or prevent T2D onset by improving the underlying metabolic status and glucose homeostasis in the early postpartum period.
In recent cross-sectional studies conducted in the general population, higher prolactin levels within the normal range have been associated with improved insulin sensitivity and glucose metabolism, along with lower prevalence of diabetes and metabolic syndrome (normal prolactin range in general population is prolactin < 19-19.40 ng/mL for men and < 19-26.53 ng/mL for women, dependent on different laboratory reference ranges) (23-26). However, findings from prospective observational studies evaluating the association between prolactin and incidence of diabetes in the general population remain conflicting. Some studies have reported that prolactin levels are inversely associated with T2D risk, particularly in women, while others show a positive or null association between prolactin levels and future T2D risk in clinical cohorts consisting of both men and women (27-31). Very recently, a meta-analysis including 6 cross-sectional or longitudinal studies reported that a higher prolactin level (within the normal range) was associated with reduced risk of prevalent but not incident T2D, but these studies included men and did not evaluate lactation history or history of GDM, which may influence T2D risk (32). Despite the important role that prolactin plays in lactation and its potential beneficial effects on T2D risk in the general population, the prospective association between prolactin, circulating metabolic profiles, and incidence of T2D in women with a recent GDM history has not yet been evaluated.
In the present study, a nested case-control study in the SWIFT cohort was conducted aiming to explore early postpartum prolactin, lactation, clinical and metabolic research characteristics, and the maternal circulating metabolome in women with a recent GDM history who were followed for up to 10 years postpartum for the development of T2D.
Methods
SWIFT Cohort Design
The SWIFT study is a prospective, longitudinal research cohort embedded in an integrated healthcare system. The study enrolled a total of 1035 racially and ethnically diverse women with recent GDM pregnancy (via clinical 3-hour 100-g oral glucose tolerance tests (OGTTs) based on Carpenter-Coustan criteria (33)) who delivered a singleton, live-born infant at or after 35 weeks of gestation at Kaiser Permanente Northern California (KPNC) hospitals from September 2008 to December 2011 (34). Women diagnosed with diabetes at study baseline (6-9 weeks postpartum) via 2-hour 75-g research OGTTs (n = 21), dropouts (n = 2), and ineligible women (n = 2) were excluded from the follow-up. A total of 1010 women without diabetes at baseline were followed and tested for future incident T2D onset up to 10 or more years post-baseline. In-person research exams were conducted in a total of 959 participants at baseline and annually for up to 2 years post-baseline (95% participant follow up retention), during which 2-hour 75-g research OGTTs and multiple assessments were performed under research protocols. At each study exam, plasma samples (fasting and 2-hour time point during OGTT) were collected to reclassify the glucose tolerance status; both plasma glucose and insulin assays were performed by the University of Washington, Northwest Lipid Research Laboratories, and samples were stored in the SWIFT Study Biobank. Diagnoses of new-onset T2D since baseline were also obtained electronically from KPNC electronic health records through 10 years post-baseline (2020), and 226 new-onset cases of overt diabetes were identified from women with laboratory testing from research OGTTs or research laboratory tests for diabetes. Plasma and buffy coat samples and aliquots from the research OGTTs were stored in −80 °C freezers in the SWIFT Biobank.
Intensity and duration of breastfeeding/formula feeding for each woman was assessed by trained research staff via telephone calls, mailed feeding diaries, questionnaires during in-person visits and mailed monthly surveys. Based on this information, a lactation intensity/duration ratio (LIR) was calculated as the number of breast milk feeds (on average within 24 hours) divided by the total number of all liquid milk feeds (on average within 24 hours) during the past 7 days to yield a score with a range from 0 to 1 as described previously (35). A score of “1” represents exclusive breastfeeding and a score of “0” represents exclusive formula feeding, with fractional scores representing levels of lactation intensity. We then constructed a summary score by adding the intensity ratios from delivery to 1 month, and > 1 month to 2 months postpartum to obtain a 2-month summary LIR score at study baseline (6-9 weeks postpartum), ranging from 0 to 2.
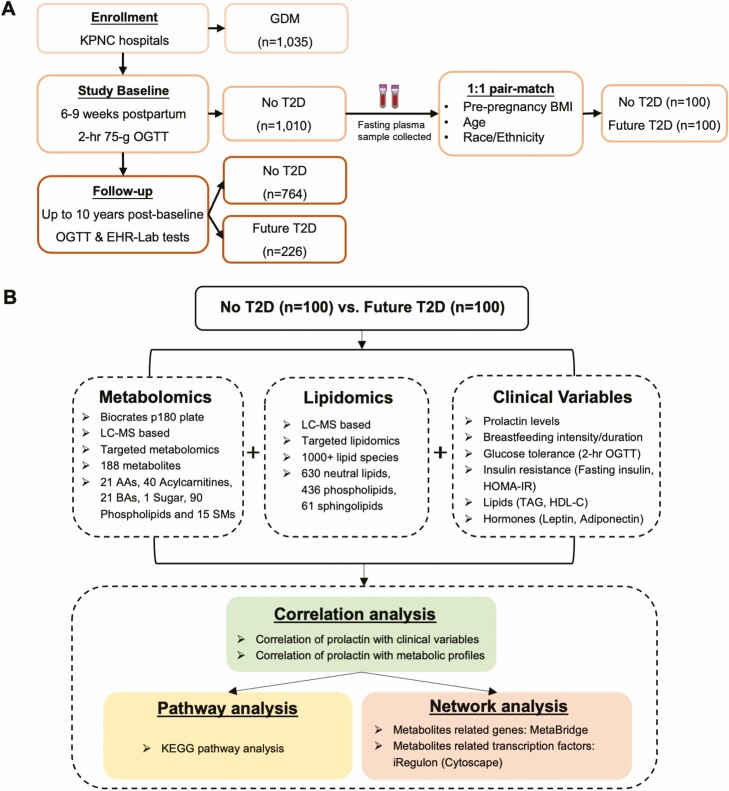
We selected a subset of 200 women (100 future incident T2D cases vs 100 no subsequent T2D controls) from the larger SWIFT cohort using a nested case-control design in which women were matched on age, pre-pregnancy body mass index (BMI), and race/ethnicity as previously described (Tables S1-S2) (36, 37). Fasting plasma samples collected during the 2-hour 75-g research OGTTs performed at study baseline were used to evaluate the metabolic changes at early postpartum by applying targeted metabolomics (Fig. 1).
Figure 1.
Overview of the SWIFT cohort and study design (34). (A) SWIFT cohort enrolled 1035 women with GDM pregnancy that occurred from 2008 to 2011, who attended 3 in-person research exams with research 2-hour 75 g OGTTs starting from 6 to 9 weeks postpartum (study baseline). Of 1010 women with no diabetes at baseline were followed for future T2D onset up to 10 years post-baseline through 2-hour 75-g research OGTTs and electronic health records. A total of 226 participants developed incident T2D during the follow-up period. At baseline, 100 cases (future T2D) and 100 controls (no T2D) were pair-matched and included in the present study. (B) Baseline fasting plasma from the pair-matched 100 cases and 100 controls were measured using targeted metabolomics and lipidomics. Research metabolic variables were also collected for these 200 participants. Bioinformatic analyses, including correlation analysis, pathway analysis, and network analysis, were carried out. Abbreviations: AA, amino acid; BA, biogenic amine; EHR, electronic health record; GDM, gestational diabetes; HDL-C, high-density lipoprotein-cholesterol; KEGG, Kyoto Encyclopedia of Genes and Genomes; KPNC, Kaiser Permanente Northern California; LC-MS, liquid chromatography–mass spectrometry; OGTT, oral glucose tolerance test; SM, sphingomyelin; SWIFT, Study of Women, Infant Feeding, and Type 2 Diabetes after GDM Pregnancy, infant feeding, and type 2 diabetes after GDM pregnancy; T2D, type 2 diabetes; TAG, triglyceride.
Ethics, Consent, and Permissions
The SWIFT study can be located at ClinicalTrials.gov with identifier NCT01967030 (https://clinicaltrials.gov/ct2/show/NCT01967030). The original study and subsequent metabolite assays were approved by the Kaiser Permanente Northern California Institutional Review Board (#CN-04EGund-03-H and #1279812-10), and the additional metabolite assays were approved by Office of Research Ethics at University of Toronto (#38188). All participants enrolled in this study provided written informed consents before conducting any research activities and consented to future studies with their data.
Prolactin Measurement
Prolactin levels were measured using Prolactin (human) ELISA Kit (Cayman Chemical, MI, USA). Briefly, fasting plasma samples, standards, and blank samples (ultrapure water) were added to the plate, followed with 100 µL of anti-prolactin conjugate into each well. After incubation at room temperature for 1 hour, each well was washed 3 times with 300 µL of diluted Wash Buffer. Then 100 µL of TMB Substrate Solution was added into each well, including blank wells. After 15 minutes of incubation at room temperature in the dark, 100 µL of Stop Solution was added to all wells. Finally, the plate was read at 450 nm to obtain prolactin measurements.
Conditional Logistic Regression Analysis
Since the subset of 200 women selected in this study is from the 1:1 pair-matched sample, a matched case-control analysis with prolactin as the predictor variable using conditional logistic regression was performed to account for matching. Multiple covariates, including baseline BMI, baseline fasting plasma glucose and 2-month LIR score (continuous measures), were adjusted in the conditional logistic regression model. The odds ratio (OR) of incident T2D risk for each prolactin quartile relative to the fourth quartile was calculated. Test of trends across increasing prolactin levels were calculated by treating the ordinal categories as continuous variables in the conditional logistic regression models (coded as 1-4 across the 4 quartiles). The conditional logistic regression analysis was performed in open-source software RStudio (Version 1.3.1093) and R (Version 4.0.5).
Targeted Metabolomics and Data Pre-processing
The metabolomics dataset was obtained from our previous studies where the details of performing targeted metabolomics analysis were described (38, 39). Briefly, targeted metabolomics was performed on fasting plasma samples from the 200 participants at study baseline. In this study, the AbsoluteIDQ p180 kit (Biocrates Life Sciences, Innsbruck, Austria) was used to quantify the metabolites and explore the diverse physiological processes. This platform allows the detection of up to 188 metabolites by using mass spectrometry–based techniques, including 21 amino acid (AA), 40 acylcarnitine (AC), 21 biogenic amine (BA), 1 monosaccharide, 90 glycerophospholipids, and 15 sphingomyelin (SM) metabolites. All AAs, BAs, and hexose were analyzed using either deuterated or 13C stable isotope-labeled internal standard and measured by an Agilent 1290 HPLC stack connected to a SCIEX QTRAP 5500 mass spectrometer. An Agilent Eclipse XDB-C18 100*3.0 mm, 3.5 μm column was used. ACs, SMs, and glycerophospholipids were analyzed by flow injection analysis tandem mass spectrometry (FIA-MS/MS) and quantified by internal standard calibration using an Agilent 1290 HPLC stack connected to a SCIEX QTRAP 5500 mass spectrometer. All analyses were performed by the Analytical Facility for Bioactive Molecules (The Hospital for Sick Children, Toronto, ON, Canada) without disclosure of group allocation. For the data pre-processing, metabolites with missing value > 40% were excluded, leaving 141 analytes for the following analysis (39, 40). The remaining missing values were imputed with half of the limit of detection (LOD) value of each metabolite. Log-transformation was performed, and distribution of data was checked. The data pre-processing was performed on the online platform MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/home.xhtml) (41).
Targeted Lipidomic and Data Pre-processing
The lipidomics data was obtained from our recently published paper where the details of lipidomics analysis were described (36). Baseline fasting plasma samples obtained from 200 women were subjected to targeted-lipid profiling performed by Metabolon, Inc. (Morrisville, NC) using gas chromatography–mass spectrometry (GC-MS) and liquid chromatography–mass spectrometry (LC-MS) techniques. Infusion-MS analysis was performed on a Shimadzu LC with nano PEEK tubing and the Sciex Selexlon-5500 QTRAP. The targeted lipidomic profiling allowed the measurement of 1008 lipid species from 15 classes as well as 296 fatty acids. The 1008 lipid species include 26 cholesterol ester (CE), 26 free fatty acid (FFA), 26 monoacylglycerol (MAG), 59 diacylglycerol (DAG) and 493 triacylglycerol (TAG) from the neutral lipid group; 26 lysophosphatidylcholine (LPC), 26 lysophosphatidylethanolamine (LPE), 140 phosphatidylcholine (PC), 216 phosphatidylethanolamine (PE) and 28 phosphatidylinositol (PI) from the phospholipid group; 12 ceramide (CER), 13 dihydroceramide (DCER), 12 hexosylceramide (HCER), 12 lactosylceramide (LCER), and 12 sphingomyelin (SM) species from the sphingolipid group. During the data pre-processing, lipids with > 5% missing values were excluded at baseline, leaving 818 out of 1008 lipid species for the bioinformatic analysis (36, 42). Other data pre-processing was performed as stated above.
Correlation Analysis at Study Baseline
Spearman correlation analysis was carried out to evaluate the correlation between the prolactin levels, clinical and metabolic variables, and each metabolite/lipid. Spearman correlation analysis was unadjusted. Correlation coefficients (R) and P value were calculated. Clinical and metabolic variables, metabolites, and lipid species that significantly correlated with prolactin levels were identified (α value set at P < 0.05), followed by false discovery rate (FDR) analysis (statistical significance is determined at FDR < 0.2, due to the small dataset) using Benjamini-Hochberg method. The correlation analyses were performed in open-source software RStudio (Version 1.3.1093) and R (Version 4.0.5).
Pathway Analysis and Upstream Transcription Factors Prediction
Using the Kyoto Encyclopedia of Genes and Genomes (KEGG; Kanehisa Laboratories, Kyoto, Japan) pathway database, the significantly prolactin-associated metabolites (P < 0.05) at baseline were subjected to pathway analysis. The KEGG pathway analysis was performed on MetaboAnalyst 4.0. Further, these metabolites were subjected to an online platform MetaBridge (https://www.metabridge.org/) to further identify all of the reactions in which the analytes participate in and all of the potential genes that are involved in these reactions (43). The gene list generated by MetaBridge was subjected to a computational method (iRegulon) to identify the master regulons of these genes using cis-regulatory sequence analysis (44). A network mapping connecting the master regulons and downstream genes was generated using Cytoscape (Cytoscape Consortium, San Diego, CA, USA).
Results
SWIFT Cohort and Study Design
In the SWIFT study (1035 women in total), women classified with overt diabetes based on the 2-hour 75-g research OGTTs at study baseline (6-9 weeks postpartum) (n = 21), dropouts (n = 2), and ineligible women (n = 2) were excluded from the follow-up. Of 1010 women without T2D at study baseline, 959 women also attended in-person research visits at 1 and/or 2 years post-baseline, which included 2-hour research 75-g OGTT to assess the glucose tolerance. We supplemented this research OGTT testing with clinical diagnoses of diabetes from KPNC electronic medical records up to 10 or more years post-baseline through October 2020 (Fig. 1A).
A subset of 200 women (100 future T2D cases and 100 non-T2D matched controls) were selected from the SWIFT cohort for metabolomics and lipidomic assays (Fig. 1B). Those 200 women were pair-matched on age, pre-pregnancy BMI, and race/ethnicity (37). Targeted metabolomic and lipidomic profiling was applied to the fasting plasma samples collected during the 2-hour 75-g research OGTTs performed at study baseline to examine the circulating metabolic profiles.
Prolactin Levels and Risk of Future T2D Within 10 Years of Follow-up
The 200 participants (100 future incident T2D and 100 no T2D) were distributed among 4 prolactin quartiles. The fourth prolactin quartile (>126.24 ng/mL) was used as the reference group. After controlling for the pair-matched design using conditional logistic regression model, the odds ratio (OR) of future T2D was 1.78 (95% CI, 0.83-3.83) for the first quartile (≤34.85 ng/mL), 1.19 (95% CI, 0.54-2.61) for the second quartile (>34.85-60.88 ng/mL), and 0.88 (95% CI, 0.38-2.04) for the third quartile (>60.88-126.24 ng/mL), in a graded manner (Table 1). After adjusting for additional multiple covariates, including baseline BMI, baseline fasting plasma glucose (FPG), and 2-month LIR score, decreasing prolactin quartiles were significantly associated with increased future T2D risk in women with previous GDM (Table 1).
Table 1.
Odds ratio of future T2D among women with recent GDM, by prolactin groups at 6 to 9 weeks postpartum
| Odds ratio | Quartile 1 (≤34.85 ng/mL) | Quartile 2 (>34.85-60.88 ng/mL) | Quartile 3 (>60.88-126.24 ng/mL) | Quartile 4 (>126.24 ng/mL) | P value for trend |
|---|---|---|---|---|---|
| Model 1 | 1.78 (0.83-3.83) | 1.19 (0.54-2.61) | 0.88 (0.38-2.04) | 1.0 (reference) | 0.076 |
| Model 2 | 1.61 (0.74-3.52) | 1.18 (0.53-2.62) | 0.79 (0.33-1.88) | 1.0 (reference) | 0.112 |
| Model 3 | 1.31 (0.53-3.25) | 1.34 (0.51-3.51) | 0.58 (0.20-1.64) | 1.0 (reference) | 0.295 |
| Model 4 | 2.48 (0.81-7.58) | 1.80 (0.64-5.05) | 0.60 (0.21-1.72) | 1.0 (reference) | 0.050 |
Model 1: Adjusted for the pair-match strategy (age, race/ethnicity, and pre-pregnancy BMI).
Model 2: Adjusted for pair-match and postpartum BMI (baseline BMI).
Model 3: Adjusted for pair-match, postpartum BMI, and baseline FPG.
Model 4: Adjusted for pair-match, postpartum BMI, baseline FPG, and 2-month LIR score.
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; LIR, lactation intensity/duration ratio.
Association Between Prolactin Levels and Research Metabolic Characteristics at Baseline
By comparing the research variables in the 4 quartiles of circulating fasting prolactin concentrations, we found that they were directly associated with the 2-month LIR score at 6 to 9 weeks postpartum and total months of lactation duration (P < 0.001) (Table 2). Moreover, at baseline, women with higher prolactin levels had lower pre-pregnancy BMI (P = 0.024) and baseline BMI (P = 0.019), FPG (P = 0.015), fasting insulin (P = 0.023), homeostatic model assessment for insulin resistance (HOMA-IR) (P = 0.018) and leptin (P = 0.014); as well as higher fasting high-density lipoprotein cholesterol (HDL-C, P = 0.008) (Table 2). A Tukey post hoc test revealed that 2-month LIR score was significantly lower in the quartile 1 (P < 0.001) and quartile 2 (P = 0.012) compared with quartile 4, and total lactation duration was also significantly lower in quartile 1 (P < 0.001) and quartile 2 (P = 0.006) compared with quartile 4. Other research metabolic parameters such as pre-pregnancy BMI (P = 0.013), baseline BMI (P = 0.011), FPG (P = 0.009), fasting insulin (P = 0.047), HOMA-IR (P = 0.022), and leptin (P = 0.038) were significantly higher in quartile 1 compared with quartile 4, whereas HDL-C was significantly lower in quartile 1 (P = 0.010) and quartile 3 (P = 0.027) compared to quartile 4.
Table 2.
Clinical and metabolic variables in different quartiles of prolactin levels
| Clinical and metabolic variables | Quartile 1 ≤34.85 (n = 50) |
Quartile 2 >34.85-60.88(n = 50) |
Quartile 3 >60.88-126.24(n = 50) |
Quartile 4 >126.24 (n = 50) |
P value |
|---|---|---|---|---|---|
| Age, mean (SD) | 33.3 (5.5) | 33.5 (4.9) | 34.1 (4.8) | 34.2 (5.5) | 0.800 |
| Pre-pregnancy BMI, kg/m2, mean (SD) | 35.1 (9.5) | 33.3 (7.8) | 33.3 (7.2) | 30.2 (7.1) | 0.024 |
| Race,n (%) | 0.398 | ||||
| Non-Hispanic White | 10 (20%) | 8 (16%) | 9 (18%) | 5 (10%) | |
| Asian | 15 (30%) | 19 (38%) | 19 (38%) | 19 (38%) | |
| Non-Hispanic Black | 9 (18%) | 6 (12%) | 2 (4%) | 3 (6%) | |
| Hispanic | 16 (32%) | 14 (28%) | 20 (40%) | 22 (44%) | |
| Other | 0 (0%) | 3 (6%) | 0 (0%) | 1 (2%) | |
| Prenatal 3-hour 100-g OGTT z-score sum, mean (SD) | 0.17 (2.34) | 0.71 (3.12) | 0.98 (2.64) | 0.28 (2.89) | 0.417 |
| GDM treatment, n (%) | 0.046 | ||||
| Insulin or oral medicine | 27 (54%) | 14 (28%) | 23 (46%) | 18 (36%) | |
| Diet | 23 (46%) | 36 (72%) | 27 (54%) | 32 (64%) | |
| Gestational weight gain, kg, mean (SD) | 8.8 (7.0) | 9.2 (8.9) | 7.7 (6.3) | 10.1 (6.1) | 0.394 |
| Baseline variables (6-9 weeks post-delivery) | |||||
| BMI, kg/m2, mean (SD) | 35.2 (8.6) | 33.5 (7.0) | 32.5 (6.7) | 30.7 (6.4) | 0.019 |
| LIR 2-month intensity score, median(IQR) | 0.71 (0.30-1.70) | 1.73 (0.90-2.00) | 1.96 (1.60-2.00) | 1.92 (1.68-2.00) | <0.001 |
| Total lactation duration, months, median (IQR) | 1.4 (0.7-3.8) | 5.0 (2.3-11.5) | 8.7 (4.3-13.1) | 11.4 (6.2-12.9) | <0.001 |
| FPG, mg/dL, mean (SD) | 100.8 (10.2) | 97.1 (10.9) | 98.7 (10.2) | 94.5 (8.3) | 0.015 |
| 2h-PG, mg/dL, mean (SD) | 117.5 (28.4) | 117.6 (26.8) | 127.3 (36.0) | 115.3 (31.3) | 0.214 |
| Fasting insulin, mU/mL, mean (SD) | 31.8 (17.4) | 29.9 (19.0) | 24.6 (12.2) | 23.6 (12.5) | 0.023 |
| HOMA-IR, median (IQR) | 7.7 (4.5-10.4) | 5.9 (3.9-9.1) | 5.4 (3.7-7.8) | 4.5 (3.5-7.4) | 0.026 |
| Fasting TAG, mg/dL, median (IQR) | 115.5 (72.8-179.0) | 107 (75.8-168.3) | 105.5 (69.0-163.8) | 93.5 (72.5-130.0) | 0.556 |
| Fasting HDL-C, mg/dL, mean (SD) | 47.9 (12.4) | 50.0 (13.7) | 48.8 (11.7) | 56.1 (14.0) | 0.008 |
| Leptin, mean (SD) | 37.0 (16.1) | 35.6 (16.8) | 29.6 (15.9) | 28.5 (14.9) | 0.014 |
| Adiponectin, median (IQR) | 6.4 (5.8-8.2) | 6.5 (4.9-8.8) | 7.1 (5.2-8.4) | 7.3 (5.1-8.6) | 0.929 |
Normal distribution was checked through histogram and Q-Q plot. Data are presented as mean (SD) for continuous variables that are approximately normally distributed. Data are presented as median (IQR) for continuous variables with asymmetrical distributions. Chi-square test was used for categorical variables (n, %), 1-way ANOVA was used for continuous variables (mean, SD), and Kruskal-Wallis test was used for continuous variables (median, IQR). A Tukey post hoc test was performed for relationships that were found to be statistically significant.
Abbreviations: 2h-PG, 2-hour post-load plasma glucose; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; LIR, lactation intensity/duration ratio; TAG, triglyceride.
Previous studies suggested the normal prolactin level at early postpartum was less than 100 ng/mL (45-48). Therefore, we stratified the subset of women (n = 200) into 2 subgroups: women with normal prolactin levels (<100 ng/mL, n = 131) and women with abnormal prolactin levels (≥100 ng/mL, n = 69). We then compared clinical and key metabolic variables across different quartiles of prolactin within each subgroup. We found the prolactin levels were associated with lactation intensity (P < 0.001) and duration (P < 0.001), as well as FPG (P = 0.013) and 2-hour-PG (P = 0.027) in the normal prolactin subgroup (Table S3) (37). However, these associations were not found in the abnormal prolactin subgroup (Table S4) (37).
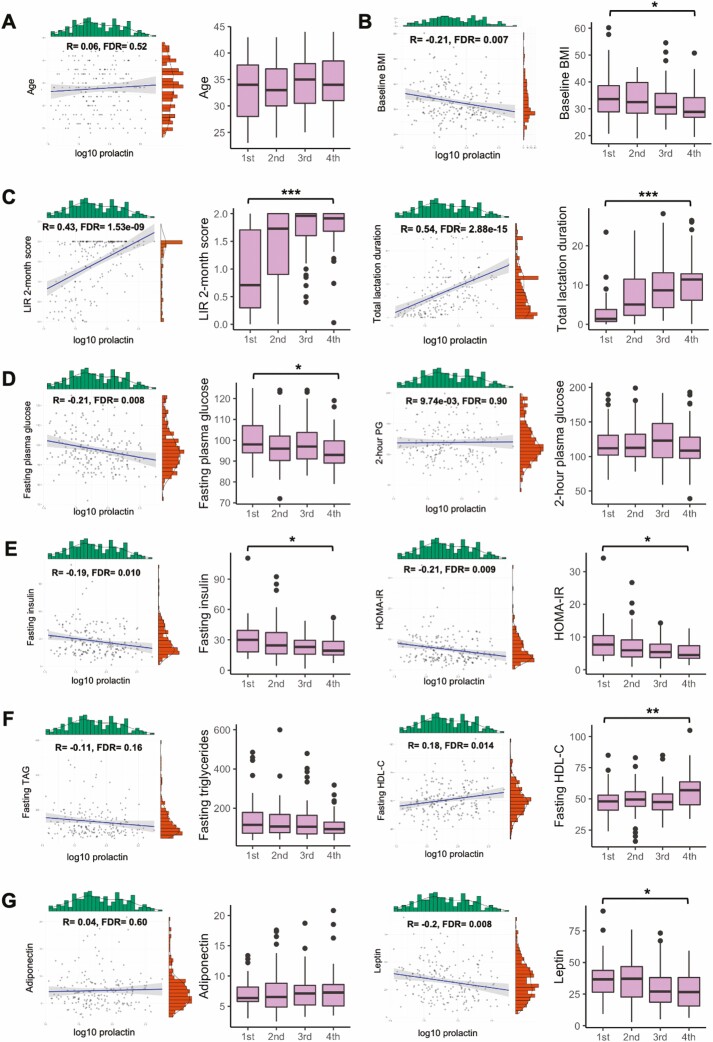
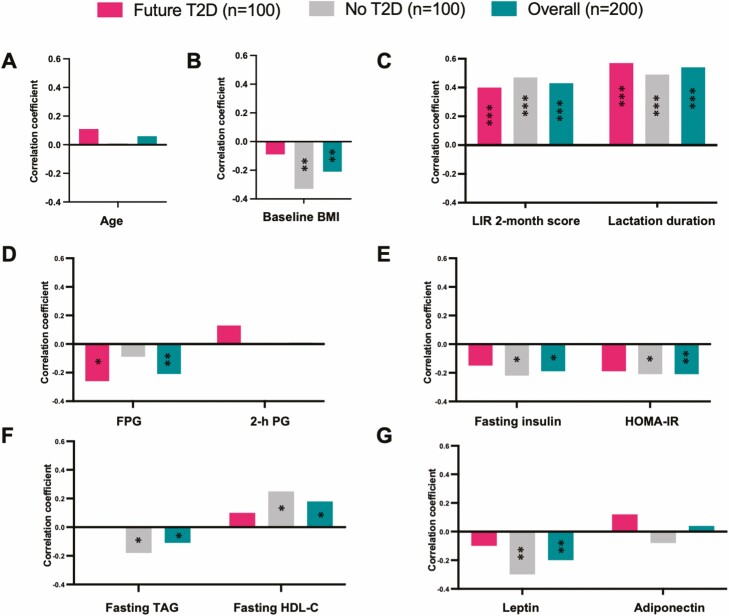
In addition, correlations between prolactin concentrations and clinical and metabolic parameters were evaluated at early postpartum (6-9 weeks postpartum). Prolactin was significantly positively correlated with lactation intensity at baseline (R = 0.43, FDR = 1.53e-09), total months of lactation duration (R = 0.54, FDR = 2.88e-15) and fasting HDL-C at baseline (R = 0.18, FDR = 0.014), but negatively correlated with other baseline variables: fasting TAG (R = -0.11, FDR = 0.16), baseline BMI (R = −0.21, FDR = 0.007), fasting insulin levels (R = −0.19, FDR = 0.010), FPG (R = −0.21, FDR = 0.008), HOMA-IR (R = −0.21, FDR = 0.009), and leptin (R = −0.20, FDR = 0.008) (Fig. 2). We further stratified the 200 women into 2 subgroups based on future T2D status: future incident T2D (n = 100) and no T2D (n = 100). In both subgroups, prolactin levels were significantly and similarly positively correlated with lactation intensity at 6 to 9 weeks postpartum (no T2D: R = 0.47, FDR = 5.45e-06; future T2D: R = 0.40, FDR = 2.63e-04) and total months of lactation duration (no T2D: R = 0.49, FDR = 2.66e-06; future T2D: R = 0.57, FDR = 8.72e-09) (Fig. 3). In the no T2D subgroup, were prolactin levels correlated with baseline BMI (R = −0.33, FDR = 0.004), fasting insulin (R = −0.22, FDR = 0.058), HOMA-IR (R = −0.21, FDR = 0.065), fasting TAG (R = −0.18, FDR = 0.10), fasting HDL-C (R = 0.25, FDR = 0.024) and leptin (R = −0.30, FDR = 0.006) (Fig. 3). Interestingly, prolactin levels were not correlated with these clinical and metabolic variables in the future T2D subgroup (Fig. 3). These data suggested that compared with women who do not develop T2D within the follow-up period, prolactin during the early postpartum period does not correlate with the clinical and metabolic parameters related to increased T2D risk in women who later developed T2D.
Figure 2.
Correlation analysis of prolactin and research metabolic variables. Correlation between log10 prolactin levels and research metabolic variables; and the distribution of indicated variable within 4 quartiles of prolactin levels (first quartile: ≤34.85 ng/mL, second quartile: >34.85-60.88 ng/mL; third quartile: >60.88-126.24 ng/mL; fourth quartile: >126.24 ng/mL). (A) Age. (B) Baseline BMI. (C) Lactation intensity/duration (LIR 2-month score and total lactation duration). (D) Glucose tolerance variables (Research FPG and 2-hour PG). (E) Insulin resistance variables (fasting insulin and HOMA-IR). (F) Lipids (TAG and HDL-C). (G) Hormones (adiponectin and leptin). *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: 2-h PG, 2-hour plasma glucose; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LIR, lactation intensity/duration ratio; TAG, triglyceride.
Figure 3.
Correlation of prolactin levels and research metabolic variables in the subgroups. Spearman correlation analysis of prolactin levels and research metabolic variables in the following 3 subgroups: future T2D (n = 100), no T2D (n = 100), and overall (n = 200). (A) Age. (B) Baseline BMI. (C) Lactation intensity/duration (LIR 2-month score and total lactation duration). (D) Glucose tolerance variables (FPG and 2-hour PG). (E) Insulin resistance variables (fasting insulin and HOMA-IR). (F) Lipids (TAG and HDL-C). (G) Hormones (adiponectin and leptin). Significance of correlation analysis: *FDR < 0.2, **FDR < 0.01, ***FDR < 0.001. Abbreviations: 2-h PG, 2-hour plasma glucose; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LIR, lactation intensity/duration ratio; TAG, triglyceride.
Prolactin-Associated Metabolites, Pathways, and Master Regulons at Study Baseline
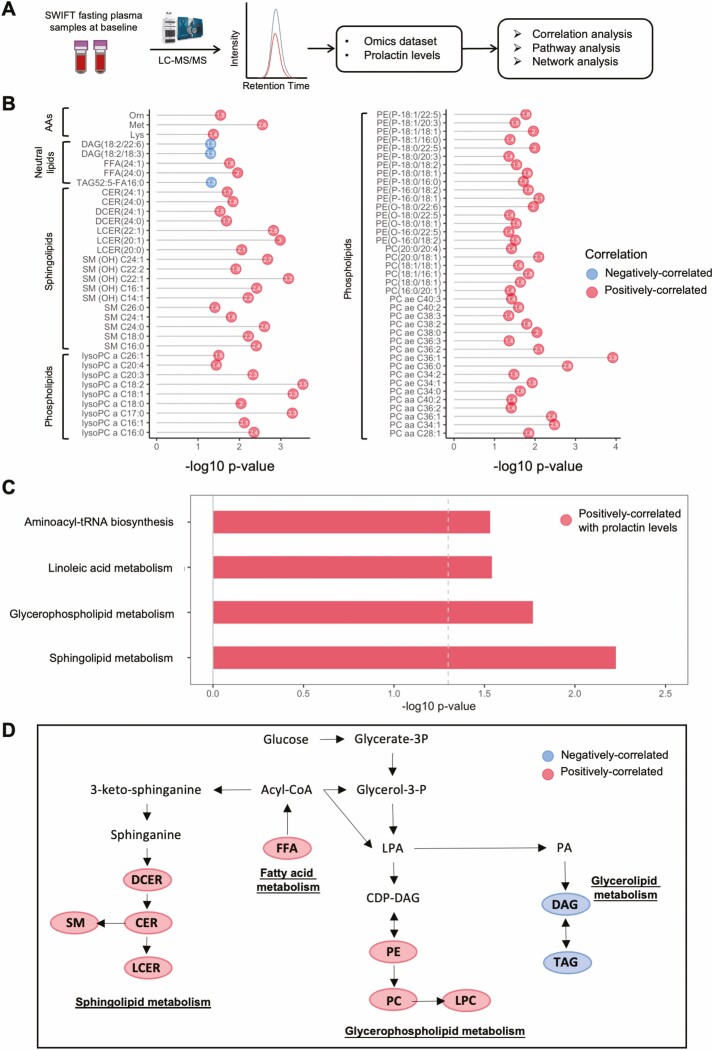
Correlation analyses were carried out to identify prolactin-associated metabolites and lipid species (Fig. 4A). A total of 73 metabolites (3 amino acids, 5 neutral lipids, 17 sphingolipids, and 48 phospholipids) were significantly positively correlated with prolactin levels at baseline (Fig. 4B). To identify metabolic pathways associated with prolactin levels at baseline, these 73 metabolites were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. We observed 4 prolactin-correlated metabolic pathways, including sphingolipid metabolism (P = 0.006), glycerophospholipid metabolism (P = 0.017), aminoacyl-tRNA biosynthesis (P = 0.029), and linoleic acid metabolism (P = 0.029) (Fig. 4C). These prolactin-associated pathways (fatty acid, neutral lipid, sphingolipid, and glycerophospholipid metabolism) are closely linked and share common substrates including phosphatidate and fatty acyl-CoA (Fig. 4D), suggesting that prolactin is associated with a pathway shift away from neutral lipids sources toward sphingolipids and phospholipids in the early postpartum period.
Figure 4.
Identification of prolactin-associated metabolites/lipids and metabolic pathways. (A) Workflow of the identification of prolactin-associated metabolites/lipids and metabolic pathways. (B) Bubble plot showing the significantly prolactin-correlated metabolites and lipid species (FDR < 0.20). Red points indicate positively correlated with prolactin levels and blue points denote negatively correlated with prolactin levels. (C) Significantly prolactin-correlated metabolic pathways (P < 0.05) at baseline analyzed by KEGG pathway analysis. Red indicates positively correlated with prolactin levels. (D) Integrated metabolic pathway of lipid biosynthesis (fatty acids, neutral lipids, glycerophospholipids, and sphingolipids metabolism). Red indicates positive-correlated, and blue denotes negative-correlated. Abbreviations: AA, amino acid; KEGG, Kyoto Encyclopedia of Genes and Genomes; FFA, free fatty acid; CER, ceramide; DAG, diacylglycerol; DCER, dihydroceramide; LCER, lactosylceramide; LC-MS, liquid chromatography–mass spectrometry; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; TAG, triacylglycerol.
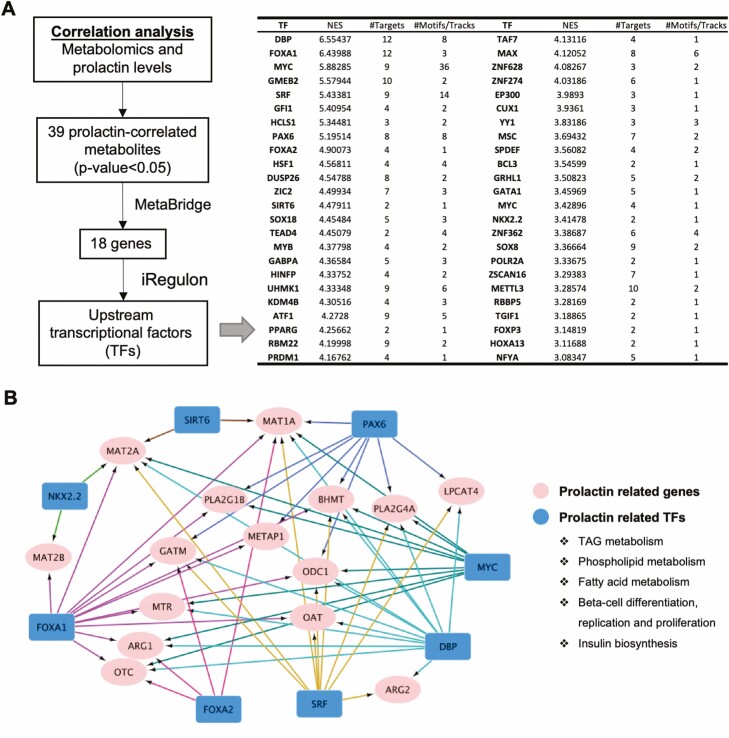
To gain a deeper understanding of the biological changes linked to prolactin, we used MetaBridge to cross-link genes with the prolactin-correlated metabolites (FDR < 0.2, Table S5 (37)) using the metabolomics dataset (43). iRegulon was then applied to detect master regulons from the set of genes and establish a regulatory network (44). The 39 prolactin-correlated metabolites identified were linked to 18 genes using MetaBridge (Fig. 5A). By using iRegulon analysis, these 18 genes were further matched to 48 upstream transcription factors (i.e., FOXA1, FOXA2, MYC, PAX6, SIRT6, DBP, etc.), the majority of which are implicated in lipid metabolism (fatty acids, triglycerides, and phospholipids), pancreatic beta-cell differentiation/proliferation, and insulin biosynthesis (Fig. 5A-5B).
Figure 5.
Network analysis of master transcription factors and genes involved in the prolactin-associated metabolism at early postpartum. (A) Flow chart of the identification of co-expressed genes and master transcription factors (TFs) from prolactin-correlated metabolites using MetaBridge and iRegulon. (B) iRegulon analysis depicting the regulatory network between the master regulons associated with prolactin and their downstream target genes. Abbreviations: NES, normalized enrichment score; TF, transcription factor.
Identification of Prolactin Cutoff Value That Is Associated With New Onset of T2D
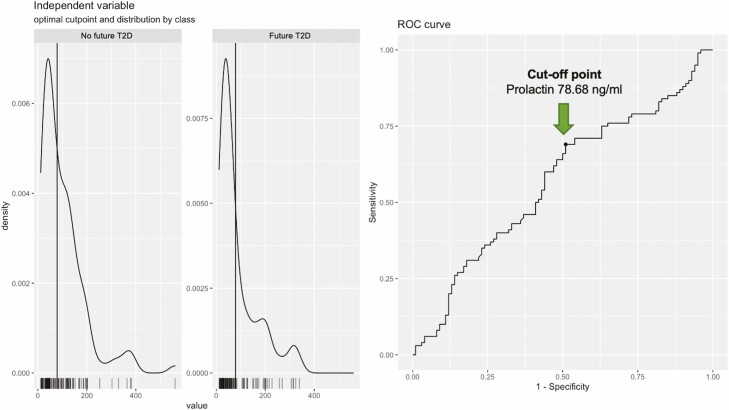
By using package “cutpointr” in R, we also identified a cutoff point of prolactin levels (78.68 ng/mL) at which a clinical outcome (newly onset of T2D in this case) was found (Fig. 6), which may help to identify women with high risk of developing future T2D after recent GDM pregnancy.
Figure 6.
Identification of cutoff point of prolactin level. A cutoff point of prolactin levels (78.68 ng/mL) was identified by using package “cutpointr” in R, at which level the glucose tolerance outcomes (i.e., new onset of T2D) were found in the current study. The left showing the distribution of predictor values per class, the right showing the receiver operating characteristic (ROC) curve.
Discussion
In the present study, we selected a subset of 200 women from a large well-characterized prospective GDM research cohort (SWIFT study) who underwent follow-up laboratory testing for new onset of T2D for 10 or more years post-baseline. We showed a higher circulating prolactin level (quartile) was associated with higher lactation intensity and duration, and a graded lower incidence rate of future T2D during follow-up. Furthermore, prolactin was found to be significantly positively correlated with fasting plasma HDL-C, but negatively correlated with pre-pregnancy BMI, baseline BMI, FPG, fasting plasma insulin, HOMA-IR, and fasting plasma leptin. However, this association among clinical and metabolic parameters and prolactin was only observed in women who did not develop future T2D. Moreover, prolactin was negatively correlated with neutral lipid levels, but positively with phospholipid and sphingolipid metabolism. This supports the hypothesis that higher prolactin, related to breastfeeding, is associated with a more favorable lipid profile and lower relative risk of future T2D. Prolactin levels and their influence on maternal lipid metabolism may be one possible mechanism underlying the benefits of lactation.
Prolactin is a hormone whose primary role is the regulation of lactation. Indeed, we found that the circulating prolactin levels were significantly positively correlated with lactation intensity and duration. Prolactin secreted by lactotrophs was reported to initiate responses that are crucial for the feeding of infants, including mammary epithelial cell proliferation and differentiation (49, 50). In addition to regulating lactation, prolactin is also involved in other functions, including metabolic homeostasis. In preclinical studies, it has been reported that prolactin plays a pivotal role in glucose metabolism through effects on pancreatic β-cell mass and insulin production (51, 52). Prolactin also has a role in peripheral insulin sensitivity (53) and orexigenic actions in the central nervous system, promoting positive energy balance (54-57). In addition, prolactin might also affect energy homeostasis through modulation of lipid metabolism (58). We showed here that prolactin levels correlated with a series research and metabolic parameters such as pre-pregnancy BMI (negatively), baseline BMI (negatively), FPG (negatively), fasting insulin (negatively), HOMA-IR (negatively), HDL-C (positively), and leptin (negatively), suggesting prolactin was closely linked to maternal metabolism in the early postpartum period. When we stratified the 200 women into 2 subgroups based on the T2D status up to 10 years post-baseline, we found that the beneficial correlation between prolactin and these clinical and metabolic parameters at baseline were observed only in women who did not develop T2D, not in those who later developed T2D, ostensibly related to reduced prolactin (37). These findings suggest that lower prolactin levels are associated with increased T2D risk, independent of obesity and socio-demographics, possibly driven by a T2D-related nonbeneficial early postpartum metabolic profile. This is consistent with our previous findings; that women with recent GDM pregnancy who displayed a less favorable metabolic profile within the overall intensive lactation group at early postpartum are more likely to develop future T2D (22).
By using targeted/quantitative metabolomics, we found prolactin was negatively correlated with neutral lipids, but positively correlated with phospholipid and sphingolipid metabolism at 6 to 9 weeks postpartum. This is consistent with previous reports suggesting that prolactin might affect energy homeostasis through modulation of lipid metabolism (58, 59). Our previous nested case-control study in SWIFT women showed that future T2D risk was positively associated with neutral lipids but negatively associated with phospholipids and sphingolipids (36, 39). Interestingly, the prolactin-correlated lipid profile that we observed in the present study indicates that a favorable shift from neutral lipid to phospholipid and sphingolipid might be a mechanism of protective benefits of prolactin on future T2D risk. Further KEGG pathway analysis suggested prolactin is linked to fatty acid, sphingolipid, glycerophospholipid, and amino-tRNA pathways to support the nutritional substrate demands of lactogenesis. To identify the master transcriptional regulators linked to prolactin-correlated metabolites, we cross-referenced the 39 prolactin-correlated metabolites to genes and upstream transcription factors using MetaBridge and iRegulon. We found that the prolactin-correlated metabolites were regulated by a clusters of transcription factors including FOXA1, FOXA2, MYC, SIRT6, PPARG, and YY1, involved in the metabolism of lipids including fatty acid, triglyceride, and phospholipid species (60-65). In addition, DBP, FOXA1, FOXA2, MYC, SRF, PAX6, and NKX2.2, are associated with pancreatic beta-cell differentiation, replication, proliferation, and insulin biosynthesis (66-71). Our findings supported that higher prolactin during the postpartum period not only regulates lactation but also possibly maternal metabolism and beta-cell function to meet the new demands of a feeding infant.
Women with a history of GDM have a 7-fold higher risk of developing T2D in later life compared with women without GDM history (17, 18). Our studies are among the first prospective longitudinal studies to evaluate metabolic risk prior to lactation (i.e., prenatal OGTT glucose tests) and show protective associations, including up to 50% reduction in the relative risk of developing T2D in women within 2 years after GDM pregnancy (4, 5). Other studies that began to follow-up on women during mid- to late-life many years beyond childbearing have shown much weaker or null associations and did not assess GDM history (72-75). Very recently, our group showed that intensive lactation significantly restrained maternal lipogenesis and profoundly changed the early postpartum lipid profile in women with recent GDM, which could be a biochemical mechanism underlying the metabolic benefits of lactation (22). Here we show that the lowest quartile group for prolactin levels had the highest T2D risk. Since the primary role of prolactin is the regulation of lactation, part of the correlation of prolactin with reduced T2D risk may be due to higher lactation intensity, which we accounted for in model 4 (Table 1). After controlling for the lactation intensity, the risk of future T2D increased significantly for the lowest prolactin category, distinguishing the lactation-associated impact on prolactin and subsequent T2D. In the general population, higher prolactin was also reported to be associated with lower T2D risk (23, 76). Taken together, this information provides a better understanding of prolactin in human health and disease. However, more studies are required to gain a better understanding of the physiological role of prolactin, perhaps in part by employing preclinical models.
In the present study, we reported that the prolactin levels in women with recent GDM pregnancy were negatively associated with risk of future T2D. We further showed that prolactin was correlated with favorable research metabolic parameters in the subset of women who did not develop T2D during follow-up, but not in women who later developed T2D during the same follow-up period. Prolactin was found to be negatively correlated with neutral lipids, but positively correlated with phospholipid and sphingolipid metabolism, indicating that a favorable shift from neutral lipid to phospholipid and sphingolipid may be a possible mechanism of protective benefits of prolactin on future T2D risk. These data suggest that elevated levels of prolactin are linked to reduced T2D risk by regulating maternal lipid metabolism. Overall, our study provides a perspective on prolactin and maternal metabolism. The strength of our study is the usage of the prospective cohort SWIFT, a racially and ethnically diverse women with recent GDM pregnancy, as well as the use of high-throughput metabolomics/lipidomics to profile the metabolic changes correlated with prolactin levels at early postpartum. These strengths distinguish our study from previous reports, providing a unique insight into the relationship of prolactin and diabetes risk in women with recent GDM. Some limitations of our study include the relatively small number of subject samples assayed, the fact that only one postpartum time point was examined and that only one cohort was studied. Future lipidomics and metabolomics performed on a larger sample size and in an independent cohort would further illuminate the association between prolactin levels, T2D risk, and maternal metabolism. Despite these limitations, this study provides a perspective on the correlation of prolactin and maternal metabolism, further advancing our understanding of prolactin and its relationship with diabetes risk in women.
Glossary
Abbreviations
- BMI
body mass index
- DAG
diacylglycerol
- FDR
false discovery rate
- FPG
fasting plasma glucose
- GDM
gestational diabetes mellitus
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment for insulin resistance
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KPNC
Kaiser Permanente Northern California
- LIR
lactation intensity/duration ratio
- OGTT
oral glucose tolerance test
- SWIFT
Study of Women, Infant Feeding, and Type 2 Diabetes after GDM Pregnancy
- T2D
type 2 diabetes
- TAG
triacylglycerol
Contributor Information
Ziyi Zhang, Department of Physiology, Faculty of Medicine, University of Toronto, Ontario M5S 1A8, Canada; Department of Endocrinology, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, Zhejiang 310016, China.
Anthony L Piro, Department of Physiology, Faculty of Medicine, University of Toronto, Ontario M5S 1A8, Canada.
Amina Allalou, Department of Physiology, Faculty of Medicine, University of Toronto, Ontario M5S 1A8, Canada.
Stacey E Alexeeff, Division of Research, Kaiser Permanente Northern California, Oakland, CA 94612, USA.
Feihan F Dai, Department of Physiology, Faculty of Medicine, University of Toronto, Ontario M5S 1A8, Canada.
Erica P Gunderson, Division of Research, Kaiser Permanente Northern California, Oakland, CA 94612, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California 91101, USA.
Michael B Wheeler, Department of Physiology, Faculty of Medicine, University of Toronto, Ontario M5S 1A8, Canada; Metabolism Research Group, Division of Advanced Diagnostics, Toronto General Hospital Research Institute, Toronto, Ontario M5G 2C4, Canada.
Funding
This study is supported by research grants from Canadian Institutes of Health Research (CIHR): FRN 143219 (M.B.W.), https://www.cihr-irsc.gc.ca. The National Institute of Child Health and Human Development (NICHD): R01HD050625 (E.P.G.), https://www.nichd.nih.gov/. The National Institute of Digestive, Diabetes and Kidney Disease (NIDDK): R01DK118409 (E.P.G.), https://www.niddk.nih.gov/. National Natural Science Foundation of China: 82100702 (Z.Z.), https://www.nsfc.gov.cn. China Scholarship Council fellowships (Z.Z.), https://www.csc.edu.cn/. Banting and Best Diabetes Centre (Z.Z., A.L.P.) https://bbdc.org/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Z.Z.: Conceptualization, data analysis, investigation, visualization, methodology, writing—original draft preparation, writing—review and editing. A.L.P.: Conceptualization, writing—original draft preparation, writing—review and editing. A.A.: Conceptualization, prolactin dataset generation, writing—review and editing. S.E.A.: Methodology, writing—review and editing. F.F.D.: Conceptualization, supervision, investigation, writing—original draft preparation and editing. E.P.G.: SWIFT cohort recruitment and enrollment, study design, protocol and data collection, conceptualization, methodology, biospecimens, funding, writing—review and editing. M.B.W.: Conceptualization, supervision, resources, funding acquisition, methodology, investigation, writing—original draft preparation, writing—review and editing. All authors read and approved the final manuscript.
Conflict of Interest
The authors of this manuscript have the following competing interests: M.B.W. has declared a research grant from Janssen Pharmaceuticals Company (shared with E.P.G.). E.P.G. has declared the following competing interest: R01HL145808-01 (E.P.G., PI). R01DK118409-01 (E.P.G., PI). R01HD095128-01 (E.P.G., Co-I). R21HL145419-01 (E.P.G., Co-I). R01DK106201-01 (E.P.G., PI). R01HD022965 (E.P.G., Consultant). American Institute for Research (AIR) (E.P.G., PI). Janssen Pharmaceuticals Company, funding ended 2018 (E.P.G., PI) (shared with M.B.W.).
Data Availability
Requests to access the analytic datasets from qualified researchers trained in human subjects' confidentiality protocols may be sent to the corresponding authors.
Clinical Trial Information
ClinicalTrials.gov Identifier: NCT01967030.
References
- 1. Ajmera VH, Terrault NA, VanWagner LB, et al. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. J Hepatol. 2019;70(1):126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gunderson EP, Jacobs DR Jr., Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes. 2010;59(2):495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunderson EP, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Intern Med. 2018;178(3): 328-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunderson EP, Hurston SR, Ning X, et al. ; Study of Women, Infant Feeding and Type 2 Diabetes After GDM Pregnancy Investigators. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;163(12):889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appiah D, Lewis CE, Jacobs DR, et al. The association of lactation duration with visceral and pericardial fat volumes in parous women: the CARDIA study. J Clin Endocrinol Metab. 2021;106(6):1821-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkegaard H, Bliddal M, Stovring H, et al. Breastfeeding and later maternal risk of hypertension and cardiovascular disease - the role of overall and abdominal obesity. Prev Med. 2018;114:140-148. [DOI] [PubMed] [Google Scholar]
- 8. Parikh NI, Gonzalez JM, Anderson CAM, et al. ; American Heart Association Council on E, Prevention, Council on Arteriosclerosis T. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902-e916. [DOI] [PubMed] [Google Scholar]
- 9. Dewey KG, Gungor D, Donovan SM, et al. Breastfeeding and risk of overweight in childhood and beyond: a systematic review with emphasis on sibling-pair and intervention studies. Am J Clin Nutr. 2021;114(5):1774-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandyousefi S, Davis JN, Gunderson EP. Association of infant diet with subsequent obesity at 2-5 years among children exposed to gestational diabetes: the SWIFT study. Diabetologia. 2021;64(5):1121-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernard V, Young J, Binart N. Prolactin - a pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15(6):356-365. [DOI] [PubMed] [Google Scholar]
- 12. McNeilly AS. Lactation and the physiology of prolactin secretion. Postgrad Med J. 1975;51(594):231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Vicchi F, De Winne C, Brie B, Sorianello E, Ladyman SR, Becu-Villalobos D. Metabolic functions of prolactin: Physiological and pathological aspects. J Neuroendocrinol. 2020;32(11):e12888. [DOI] [PubMed] [Google Scholar]
- 14. The global challenge of diabetes. Lancet. 2008;371(9626):1723. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34(2):173-99, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. [DOI] [PubMed] [Google Scholar]
- 18. Puhkala J, Raitanen J, Kolu P, Tuominen P, Husu P, Luoto R. Metabolic syndrome in Finnish women 7 years after a gestational diabetes prevention trial. BMJ Open. 2017;7(3):e014565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR Jr. The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82(3):451-455. [PubMed] [Google Scholar]
- 20. Gunderson EP, Kim C, Quesenberry CP Jr., et al. Lactation intensity and fasting plasma lipids, lipoproteins, non-esterified free fatty acids, leptin and adiponectin in postpartum women with recent gestational diabetes mellitus: the SWIFT cohort. Metabolism. 2014;63(7):941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunderson EP, Lewis CE, Wei GS, Whitmer RA, Quesenberry CP, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109(3):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z, Lai M, Piro AL, et al. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: the SWIFT study. BMC Med. 2021;19(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang T, Lu J, Xu Y, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care. 2013;36(7):1974-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chahar C, Chahar K, Ankit BS, Gadhwal A, Agrawal RP. Association of serum prolactin level with impaired glucose regulation and diabetes. J Assoc Physicians India. 2017;65(3):34-39. [PubMed] [Google Scholar]
- 25. Ruiz-Herrera X, de Los Rios EA, Diaz JM, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. 2017;158(1):56-68. [DOI] [PubMed] [Google Scholar]
- 26. Wagner R, Heni M, Linder K, et al. Age-dependent association of serum prolactin with glycaemia and insulin sensitivity in humans. Acta Diabetol. 2014;51(1):71-78. [DOI] [PubMed] [Google Scholar]
- 27. Li J, Rice MS, Huang T, et al. Circulating prolactin concentrations and risk of type 2 diabetes in US women. Diabetologia. 2018;61(12):2549-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balbach L, Wallaschofski H, Volzke H, Nauck M, Dorr M, Haring R. Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC Endocr Disord. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang T, Xu Y, Xu M, et al. Circulating prolactin and risk of type 2 diabetes: a prospective study. Am J Epidemiol. 2016;184(4):295-301. [DOI] [PubMed] [Google Scholar]
- 30. Retnakaran R, Ye C, Kramer CK, et al. Maternal serum prolactin and prediction of postpartum beta-cell function and risk of prediabetes/diabetes. Diabetes Care. 2016;39(7):1250-1258. [DOI] [PubMed] [Google Scholar]
- 31. Therkelsen KE, Abraham TM, Pedley A, et al. Association between prolactin and incidence of cardiovascular risk factors in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faria de Castro L, Alves Dos Santos A, Augusto Casulari L, Ansaneli Naves L, Amorim Amato A. Association between variations of physiological prolactin serum levels and the risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2020;166:108247. [DOI] [PubMed] [Google Scholar]
- 33. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768-773. [DOI] [PubMed] [Google Scholar]
- 34. Gunderson EP, Matias SL, Hurston SR, et al. Study of Women, Infant Feeding, and Type 2 diabetes mellitus after GDM pregnancy (SWIFT), a prospective cohort study: methodology and design. BMC Public Health. 2011;11:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piper S, Parks PL. Use of an intensity ratio to describe breastfeeding exclusivity in a national sample. J Hum Lact. 2001;17(3):227-232. [DOI] [PubMed] [Google Scholar]
- 36. Lai M, Al Rijjal D, Rost HL, Dai FF, Gunderson EP, Wheeler MB. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. Elife. 2020;9:e59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Z, Piro AL, Allalou A, Alexeeff SE, Dai FF, Gunderson EP, Wheeler MB. Prolactin and maternal metabolism in women with a recent GDM pregnancy and links to future T2D: the SWIFT study. 10.7910/DVN/ADEL9O, Harvard Dataverse, V1. Deposited May 7, 2022. Published June 24, 2022. [DOI] [PMC free article] [PubMed]
- 38. Allalou A, Nalla A, Prentice KJ, et al. A predictive metabolic signature for the transition from gestational diabetes mellitus to type 2 diabetes. Diabetes. 2016;65(9):2529-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai M, Liu Y, Ronnett GV, et al. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: a metabolic profiling study. PLoS Med. 2020;17(5):e1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St John-Williams L, Blach C, Toledo JB, et al. Targeted metabolomics and medication classification data from participants in the ADNI1 cohort. Sci Data. 2017;4:170140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486-W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pang Y, Kartsonaki C, Lv J, et al. Adiposity, metabolomic biomarkers, and risk of nonalcoholic fatty liver disease: a case-cohort study. Am J Clin Nutr. 2022;115(3):799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hinshaw SJ, Lee AHY, Gill EE, Hancock REW. MetaBridge: enabling network-based integrative analysis via direct protein interactors of metabolites. Bioinformatics. 2018;34(18):3225-3227. [DOI] [PubMed] [Google Scholar]
- 44. Janky R, Verfaillie A, Imrichova H, et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput Biol. 2014;10(7):e1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skouby SO, Kuhl C, Hornnes PJ, Andersen AN. Prolactin and glucose tolerance in normal and gestational diabetic pregnancy. Obstet Gynecol. 1986;67(1):17-20. [PubMed] [Google Scholar]
- 46. Walker M. Breastfeeding management for the clinician: using the evidence. Jones & Bartlett Learning, 2017. Burlington, Massachusetts, USA. [Google Scholar]
- 47. Wambach K, Spencer B. Breastfeeding and human lactation. Jones & Bartlett Learning, 2021. Burlington, Massachusetts, USA. [Google Scholar]
- 48. Tay CC, Glasier AF, McNeilly AS. Twenty-four hour patterns of prolactin secretion during lactation and the relationship to suckling and the resumption of fertility in breast-feeding women. Hum Reprod. 1996;11(5):950-955. [DOI] [PubMed] [Google Scholar]
- 49. Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299(5603):117-120. [DOI] [PubMed] [Google Scholar]
- 50. Bridges RS. Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol. 2015;36:178-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143(4):1378-1385. [DOI] [PubMed] [Google Scholar]
- 52. Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocana A. Growth factors and beta cell replication. Int J Biochem Cell Biol. 2006;38(5-6):931-950. [DOI] [PubMed] [Google Scholar]
- 53. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006;17(3):110-116. [DOI] [PubMed] [Google Scholar]
- 54. Sauve D, Woodside B. Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res. 2000;868(2):306-314. [DOI] [PubMed] [Google Scholar]
- 55. Arumugam R, Fleenor D, Freemark M. Effects of lactogen resistance and GH deficiency on mouse metabolism: pancreatic hormones, adipocytokines, and expression of adiponectin and insulin receptors. Endocrine. 2007;32(2):182-191. [DOI] [PubMed] [Google Scholar]
- 56. Perez Millan MI, Luque GM, Ramirez MC, et al. Selective disruption of dopamine D2 receptors in pituitary lactotropes increases body weight and adiposity in female mice. Endocrinology. 2014;155(3):829-839. [DOI] [PubMed] [Google Scholar]
- 57. Luque GM, Lopez-Vicchi F, Ornstein AM, et al. Chronic hyperprolactinemia evoked by disruption of lactotrope dopamine D2 receptors impacts on liver and adipocyte genes related to glucose and insulin balance. Am J Physiol Endocrinol Metab. 2016;311(6):E974-E988. [DOI] [PubMed] [Google Scholar]
- 58. Ling C, Hellgren G, Gebre-Medhin M, et al. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology. 2000;141(10):3564-3572. [DOI] [PubMed] [Google Scholar]
- 59. Ramos-Roman MA, Syed-Abdul MM, Adams-Huet B, Casey BM, Parks EJ. Lactation versus formula feeding: insulin, glucose, and fatty acid metabolism during the postpartum period. Diabetes. 2020;69(8):1624-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432(7020):1027-1032. [DOI] [PubMed] [Google Scholar]
- 61. Edmunds LR, Sharma L, Kang A, et al. c-Myc programs fatty acid metabolism and dictates acetyl-CoA abundance and fate. J Biol Chem. 2014;289(36):25382-25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pan G, Diamanti K, Cavalli M, Lara Gutierrez A, Komorowski J, Wadelius C. Multifaceted regulation of hepatic lipid metabolism by YY1. Life Sci Alliance. 2021;4(7):e202000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moya M, Benet M, Guzman C, et al. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS One. 2012;7(1):e30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khan D, Ara T, Ravi V, et al. SIRT6 transcriptionally regulates fatty acid transport by suppressing PPARgamma. Cell Rep. 2021;35(9):109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith SA. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem Soc Trans. 2002;30(Pt 6):1086-1090. [DOI] [PubMed] [Google Scholar]
- 66. Nakabayashi H, Ohta Y, Yamamoto M, et al. Clock-controlled output gene Dbp is a regulator of Arnt/Hif-1beta gene expression in pancreatic islet beta-cells. Biochem Biophys Res Commun. 2013;434(2):370-375. [DOI] [PubMed] [Google Scholar]
- 67. Gosmain Y, Katz LS, Masson MH, Cheyssac C, Poisson C, Philippe J. Pax6 is crucial for beta-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol Endocrinol. 2012;26(4):696-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Doyle MJ, Sussel L. Nkx2.2 regulates beta-cell function in the mature islet. Diabetes. 2007;56(8):1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gao N, Le Lay J, Qin W, et al. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol Endocrinol. 2010;24(8):1594-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bastidas-Ponce A, Roscioni SS, Burtscher I, et al. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic beta-cells. Mol Metab. 2017;6(6):524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosselot C, Kumar A, Lakshmipathi J, et al. Myc is required for adaptive beta-cell replication in young mice but is not sufficient in one-year-old mice fed with a high-fat diet. Diabetes. 2019;68(10):1934-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rameez RM, Sadana D, Kaur S, et al. Association of maternal lactation with diabetes and hypertension: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):e1913401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601-2610. [DOI] [PubMed] [Google Scholar]
- 74. Villegas R, Gao YT, Yang G, et al. Duration of breast-feeding and the incidence of type 2 diabetes mellitus in the Shanghai Women’s Health Study. Diabetologia. 2008;51(2):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jager S, Jacobs S, Kroger J, et al. Breast-feeding and maternal risk of type 2 diabetes: a prospective study and meta-analysis. Diabetologia. 2014;57(7):1355-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6(1). doi:10.3390/diseases6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests to access the analytic datasets from qualified researchers trained in human subjects' confidentiality protocols may be sent to the corresponding authors.