Abstract
Introduction:
Laboratory animal facilities aim to provide excellence in animal care and welfare and support scientific research. Critical to these goals is to ensure a safe work environment for personnel comprising veterinary and animal care, laboratory research, and maintenance staff.
Objective:
Thus, performing occupational risk assessments allows for evaluation of risks from identified hazards associated with a variety of tasks ongoing in laboratory animal facilities.
Methods:
Herein, we present the development of an occupational risk assessment tool purposed to capture the dynamics of work performed in laboratory animal facilities, calculate and prioritize identified risks associated with procedures and processes, and inform and evaluate risk mitigations.
Results:
We also discuss a risk assessment for refining sharps use in nonhuman primate husbandry and care to demonstrate the utility of this tool to improve occupational safety in our animal facility.
Conclusion:
This tool and framework evolve into a holistic occupational risk management system that identifies, evaluates, and mitigates occupational risks; determines risk acceptability; consistently ensures communication and consultation with frontline personnel, stakeholders, senior leadership, and subject matter experts in biosafety, science, and animal care and welfare; and continuously strives to improve and enhance the operations of laboratory animal facilities.
Keywords: biosafety, occupational health, risk assessments, hazards, incidents, animal facilities
Personnel working in laboratory animal facilities, known as vivaria, provide animal care to support research. Animal care and use programs are tasked with the obligation and duty to provide excellence in animal care and welfare and promote the 3 Rs (replacement, reduction, and refinement) of research to safeguard the ethical treatment of animals used to advance scientific knowledge.1 To this end, research animal care and use programs are highly regulated, with oversight at institutional (eg, the Institutional Animal Care and Use Committee, IACUC) and federal (eg, the United States Animal Welfare Act and Animal Welfare Regulations2 and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals3) levels, and are expected to rigorously maintain policies and procedures to ensure compliance. A hallmark of a laboratory animal facility committed to the quality of animal care and welfare is possession and maintenance of external accreditation from AAALAC International.4 In addition to providing animal care and welfare per regulations, policies, and guidelines, such as the Guide for the Care and Use of Laboratory Animals,5 research animal care and use programs must provide a safe working environment to a diverse collective of personnel, such as veterinarians and veterinary technicians, researchers, and animal care, cage wash, facilities, and maintenance staff. A key duty of these programs, and critical to their sustainability and integrity, is implementing means to perform occupational risk assessments to protect staff from biological, chemical, and physical hazards potentially present in the dynamic environment of a vivarium. Performing risk assessments in animal care and use programs is an essential part of safety and occupational health programs,6 with guidance referenced in the Occupational Health and Safety in the Care and Use of Research Animals7 and the Biosafety in Microbiological and Biomedical Laboratories (BMBL).8 Occupational risk assessments of common procedures in animal facilities help determine medical surveillance requirements of personnel, such as respiratory protection or vaccine recommendations; the kind of personal protective equipment (PPE) worn, such as cut- and puncture-resistant gloves; and necessary engineering controls, such as use of animal restraint devices or biological safety cabinets. Herein, we present an occupational risk management framework and tool designed to identify, prioritize, mitigate, and continuously monitor risks in our animal facility, which may serve as a model for other comparative medicine branches (CMBs).
Design of an Occupational Risk Assessment Tool for Laboratory Animal Facilities
The Occupational Risk Management Process
At its core, a risk assessment is asking the following questions: What can go wrong? How likely will it happen? What are the consequences if it does happen?9 Risk assessments should be done before a standard operating procedure (SOP) is performed, especially a new procedure, and in response to an incident to identify root causes and implement corrective actions. In addition, risk assessments should be done at least annually to review work procedures and recommended mitigations and to continuously monitor for risks and improve work environments in animal facilities. While the terms risks and hazards are used interchangeably, a hazard is something that has the potential to cause harm, such as a hypodermic needle. A risk is a function of the likelihood and consequence of an event occurring due to a hazard, such as a needle stick injury. Previous works detailing the history of, critical need for, and technical guidance of laboratory risk assessment and management equipped us with the foundational knowledge necessary to develop our occupational risk assessment tool specifically for research animal care and use programs.10 -12 In addition, the European Committee for Standardization (CEN) laboratory biorisk management standard was immensely helpful in developing our framework.13
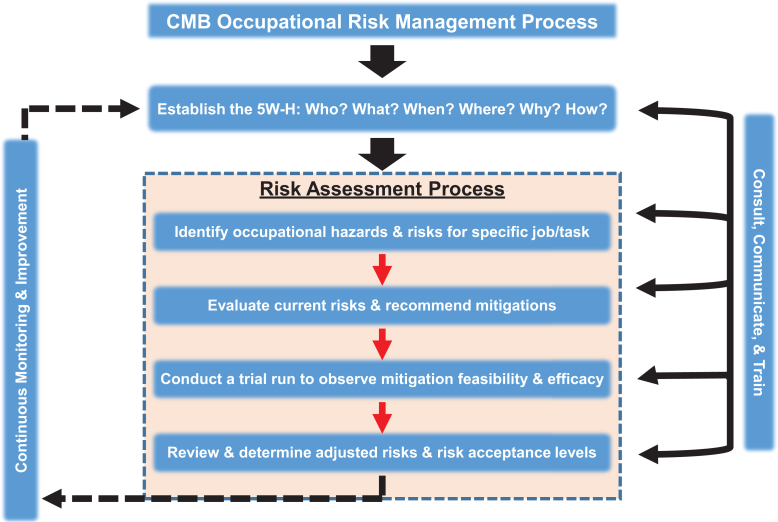
Performing an occupational risk assessment is part of a larger, systematic management process intended to identify, evaluate, and mitigate risks. A key first step in our occupational risk management process is establishing the who, what, when, where, why, and how (the 5W-H) of the procedure and its accompanying risk assessment. Examples of 5W-H questions may include the following: Who is performing the procedure? What is the procedure? When is the procedure performed? Where is the procedure being performed (eg, the animal biosafety level)? Why is the risk assessment being performed (eg, in response to an incident)? How will the risk assessment identify risks and mitigate them? This process involves consulting with frontline staff performing the procedures and subject matter experts such as veterinary, scientific, facilities and maintenance, and safety and occupational health personnel. Also, engaging these diverse teams and consistently communicating with personnel and stakeholders, such as researchers and the IACUC, is pivotal. Another component involves training personnel when recommended mitigations drive changes in SOPs to make tasks safer. This occupational risk management process continuously monitors for risks to improve the safety landscape while achieving quality and excellence in animal care and research support. This process integrates occupational safety into the daily operations of an animal facility as it involves frontline staff throughout the process, creates buy-in, and empowers personnel to be a participating voice in enhancing the safety of their work environment. We have developed a CMB occupational risk assessment form (Supplementary Form 1) to capture the essence of our occupational risk management process (Figure 1), which we adapted from the International Organization for Standardization (ISO) 31000 standard on risk management.14
Figure 1.
The comparative medicine branch (CMB) occupational risk management process adapted from ISO 31000. Critical to this process is establishing the 5W-H to identify job-specific hazards and risks, evaluate current risks, recommend mitigations, assess mitigation feasibility and efficacy, and determine adjusted risks and risk acceptance. Consistently consulting and communicating with staff and continuously monitoring for risks are pivotal to this process.
Current Risk Identification and Calculation
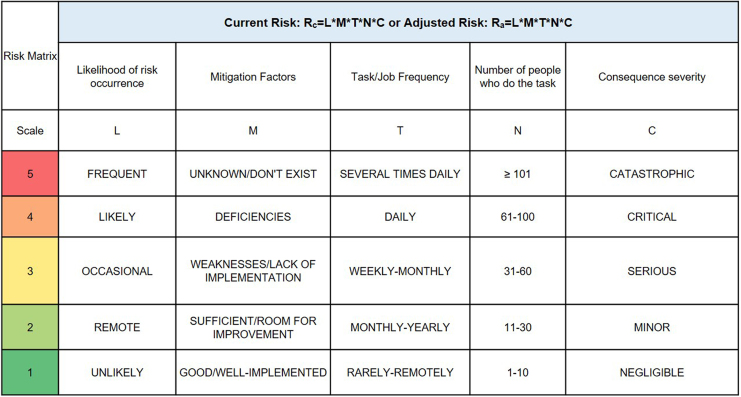
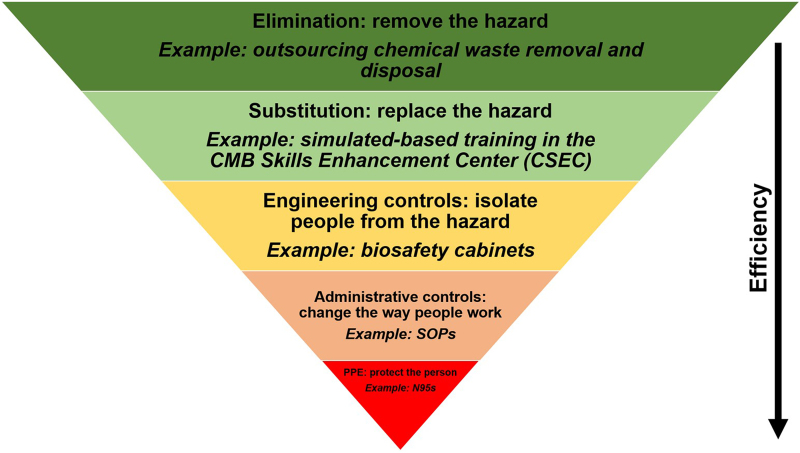
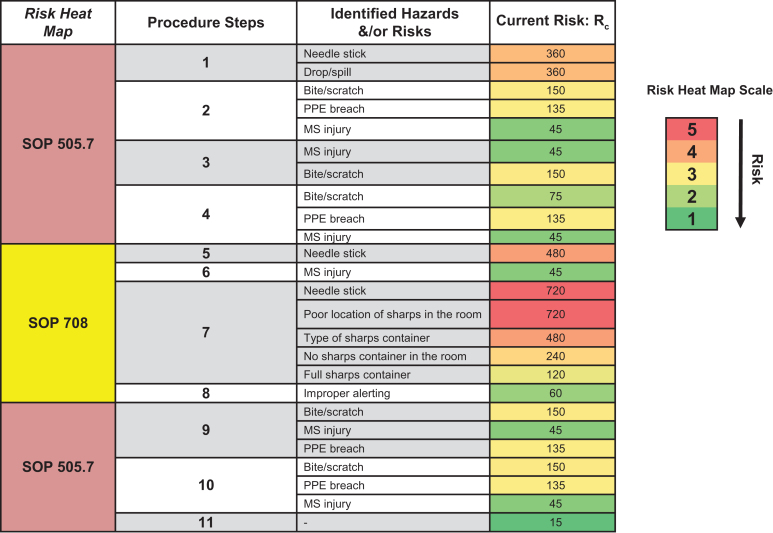
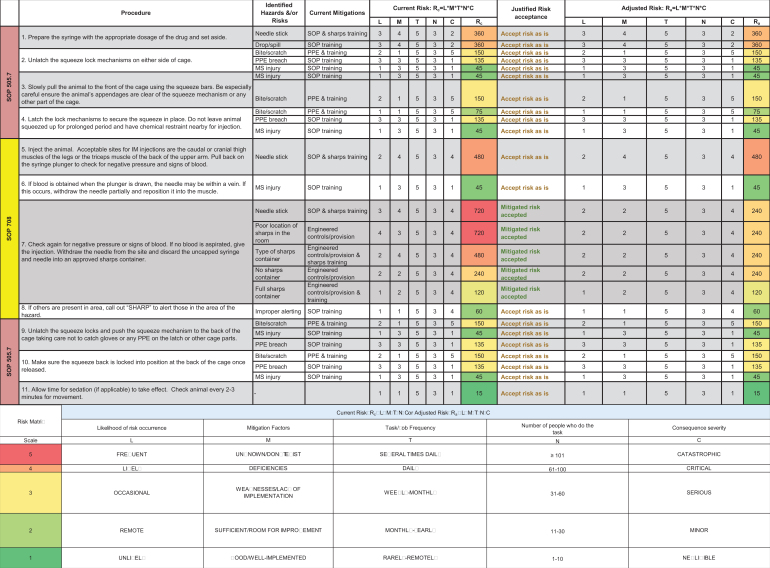
To calculate the current risks associated with a job task in an animal facility, communicating and working with frontline personnel, relevant subject matter experts, and stakeholders is necessary to provide consensus on what are the highest priority risks to recommend feasible mitigations. To quantitatively determine risk, one can multiply likelihood (L) and consequence severity (C) of a hazardous event. However, we expanded this equation, in a similar process outlined in Carvalho and Melol,15 to account for other variables that affect occupational risks and to capture the dynamic workspace and operations of animal facilities. These variables, in addition to likelihood (L) and consequence severity (C), are mitigation efficacy (M), task/job frequency (T), and the number of people doing the task (N). We developed this multiplicative matrix (Figure 2) to calculate the current risk (Rc) associated with job tasks outlined in SOPs. To note, adjusted risk (Ra) is calculated using the same matrix after implementing mitigations and determining their efficacy. A value from 1 to 5 is assigned to each variable pertaining to identified occupational risks from a procedure (Figure 2). The calculated Rc values and the colorimetric scale (green = lower-valued risks; red = higher-valued risks) aid in identifying risk “hot spots” and generating a Rc heat map to prioritize risks in need of mitigation. To demonstrate the use of this matrix and developed risk assessment tool, we discuss a risk assessment performed on nonhuman primate (NHP) husbandry and care later in this report. Included in the developed risk management program is a hierarchical scheme of the following types of mitigations (from most to least effective; Figure 3): elimination to remove the hazard, substitution to replace the hazard, engineering controls to isolate personnel from hazards, administrative controls to change the way people work, and PPE to directly protect personnel. Thus, this process of discussing identified risks, efficacy of current mitigations, and calculating Rc allows for a gap analysis that prioritizes risks and recommends mitigations to enhance occupational safety.
Figure 2.
The comparative medicine branch occupational risk matrix. This tool calculates current risk or adjusted risk by multiplying (scale: 1-5) the following factors: likelihood of risk occurrence (L), mitigation factors (M), task/job frequency (T), number of people who do the task (N), and consequence severity (C). Numbers calculated help inform the risk landscape and assist in prioritizing, identifying, and mitigating risk hot spots associated with a specific job/task.
Figure 3.
Relevant risk mitigations in laboratory animal facilities. In descending order of efficiency, with examples provided, there are 5 key means to mitigate risk: elimination, substitution, engineering controls, administrative controls, and personal protective equipment (PPE). CMB, comparative medicine branch; SOP, standard operating procedure.
Assessing Mitigation Efficacy, Calculating Adjusted Risk, and Determining Risk Acceptance
To determine the feasibility of recommended mitigations, it is necessary to “test drive” these mitigations. This involves working with frontline staff to receive their input and feedback to refine work processes. As part of this phase of the occupational risk management process, the adjusted risk (Ra) landscape is determined using the aforementioned matrix to generate a corresponding heat map. Ra should be lower than Rc. Given animal facilities have a dual obligation to keep personnel safe and ensure the well-being of housed animals, it is essential that recommended mitigations maintain occupational safety and animal welfare.
Based on the matrix’s design, risk will never be zero as there will always be hazards present in an animal facility. A critical discussion point that arises, as current risks, recommended and assessed mitigations, and adjusted risks are collectively determined, is risk acceptability. Criteria used to determine if a risk is acceptable include severity of consequences, access to resources to sustain proper mitigations, and goals and aims of the department and institution. As an example, an institution may choose to outsource removing chemical waste for proper disposal to eliminate associated occupational risks. Thus, the institution decides that having frontline staff handle chemical waste disposal is not an acceptable risk and selects elimination as a mitigation strategy (Figure 3). In the context of an animal facility, it is important to consider risk acceptance or elimination against the backdrop of both occupational safety and health and animal welfare and care.
Occupational Risk Management in Practice: Sharps Refinement and Use in Macaque Husbandry and Care
We have applied this occupational risk assessment tool to the daily operations of our facility, and to demonstrate the use of this tool, we discuss below a risk assessment performed to refine hypodermic needle (referred to as sharps in the text) use in macaque husbandry and care. While other kinds of sharps, such as scalpels, suture needles, and a plethora of surgical instruments, are used in macaque husbandry and care programs, the focus of this risk assessment was on hypodermic needles. Our facility provides daily care for old world NHPs (OW NHPs) such as rhesus, pig-tailed, and long-tailed macaques on IACUC-approved protocols.
B virus is a herpesvirus naturally present in macaques and is a high-consequence zoonotic disease associated with these animals. Macaques usually present with no symptoms and are thus asymptomatic, but they can still shed the virus. Bites, scratches, and mucous membrane exposures to macaque bodily fluids, such as blood and urine, can lead to fatal encephalitis in humans if proper treatment is not sought immediately. The death of Ms. Elizabeth Griffin, a technician working with macaques, from a mucous membrane exposure to the B virus drove the fields of lab animal science and biosafety to revamp occupational safety and health guidelines regarding work with macaques.16 -19
Our animal care, veterinary, and research personnel often administer intramuscular (IM) injections in macaques for veterinary care (eg, sedation for physical exams) or for research-related procedures (eg, experimental infections with biological agents). Across the macaque colony, IM injections may be administered by personnel multiple times per day. The impetus to make this specific procedure safer was the potentially high consequence of a needle stick (ie, occupational exposure to B virus) when working with macaques.
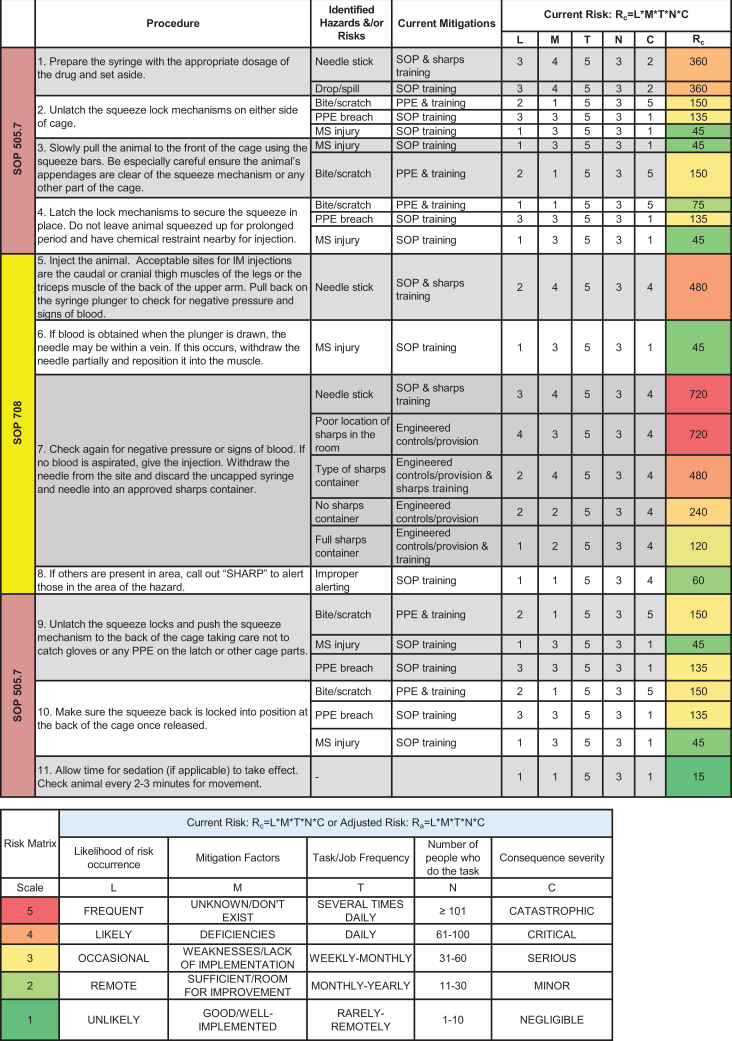
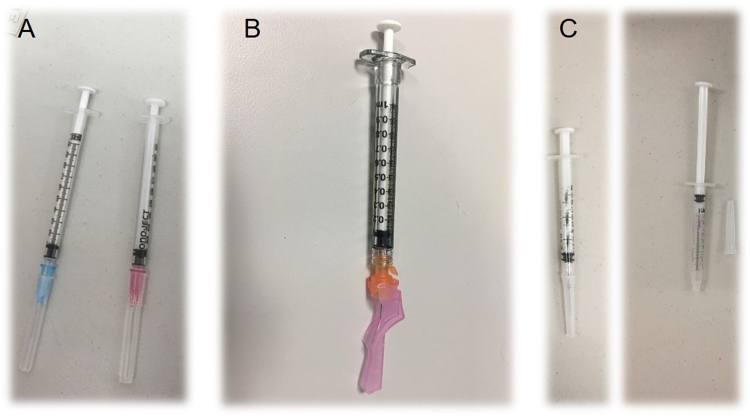
The animal facility’s safety representative coordinated and streamlined this risk assessment process with a team of animal care technicians, veterinarians, and project managers. The team discussed the relevant SOPs that outlined all procedural steps, including drawing up medication with a hypodermic needle and syringe, using a squeeze cage as an engineering control to physically restrain the macaque against the front of the cage to give the injection, and disposing the used sharp in an approved container, which is kept in close proximity to the user. The team also discussed risks, likelihood of occurrence, current mitigations, task/job frequencies, and consequence severity. Figure 4 outlines the procedure covered in 3 SOPs, identified hazards and risks, current mitigations, task/job frequencies, and Rc calculations. The Rc heat map (Figure 5) provided a clear representation of risk hot spots, the highest of which were potential needle sticks after giving the injection and the type of sharps container used. The team primarily focused on investigating the use of safety engineered sharps (such as retractable needle-syringe systems) and comparing them to the friction-top syringe needles being used in the facility (Figure 6A). Specifically, technicians on the team assessed 2 types of safety engineered sharps: (1) a safety shield, Luer-lock hypodermic needle with a cover that, when activated, locks the needle inside to provide a safety shield (BD Luer-Lok syringe with detachable needle; Figure 6B) and (2) a retractable hypodermic needle in which the needle retracts barely into the syringe when activated (Dynarex Safety Syringe with fixed needle; Figure 6C).
Figure 4.
Identification of hazards and risks from nonhuman primate (NHP) intramuscular (IM) injections. The risk assessment team identified hazards and risks for each step of the NHP IM injection procedure and collectively worked together to calculate current risk (Rc) and generate the Rc heat map to prioritize and mitigate risk hot spots. MS, musculoskeletal; PPE, personal protective equipment.
Figure 5.
Rc heat map analysis. The Rc heat map analysis summarized identified hazards and risks calculated using the comparative medicine branch occupational risk matrix. The heat map analysis revealed that getting a needle stick after an intramuscular injection and the type of sharps container used were risk hot spots warranting further mitigation. The team primarily focused on refining sharps use in macaque husbandry and care given the severity of occupational exposure to the B virus. MS, musculoskeletal; PPE, personal protective equipment; SOP, standard operating procedure.
Figure 6.
Comparison of sharps to refine nonhuman primate intramuscular injections. Prior to this risk assessment, friction top syringes with needles were used (A). To enhance safety and reduce the risk of needle sticks, the risk assessment team considered safety shield, Luer-lock syringes with needles (B) and retractable syringes with needles (C).
Using the relevant SOPs for NHP IM injections, the team designed and executed a trial run in the facility’s mock laboratory, which is free of biological agents and animals and solely used for training purposes. As part of the mock trial, the team used an NHP cage with a squeeze mechanism and an intramuscular simulator model, which was strapped to the cage and brought forward and back using the squeeze mechanism (Figure 7). Animal care technicians on the team assessed the different sharps using the intramuscular simulator model strapped to the NHP cage. They found that the safety shield, Luer-lock syringe needles did not fit completely through the spacing of the cage bars due to the shield (Figure 8), which is pushed forward by the user over the needle after injection until it locks into place and then is discarded in a sharps container. To note, the retractable syringe needles easily fit through the spacing of the cage bars (Figure 9), and the retractable mechanism was activated after giving the injection by pushing the plunger until fully seated, with no space between the needle holder base and plunger, and retracting the plunger and needle holder base together, resulting in the needle being contained within the barrel of the syringe. This mechanism protects the user from exposed needles.
Figure 7.
Mock trial setup consisting of the intramuscular (IM) simulated model and a nonhuman primate squeeze cage. The IM simulated model was strapped to the cage and squeezed to the front of the bottom and top units of the cage.
Figure 8.
Mock trial of safety shield sharps. Using an intramuscular (IM) simulator model, personnel assessed the efficacy of the safety shield, Luer-lock sharps. Personnel found that the shields prevented them from effectively administering an IM injection.
Figure 9.
Mock trial of retractable sharps. Using an intramuscular simulator model, personnel assessed the efficacy of retractable sharps. Briefly, personnel injected the model and activated the retraction mechanism via a push-then-pull motion to bring the needle inside the barrel of the syringe. These sharps eliminated occupational exposure to needles.
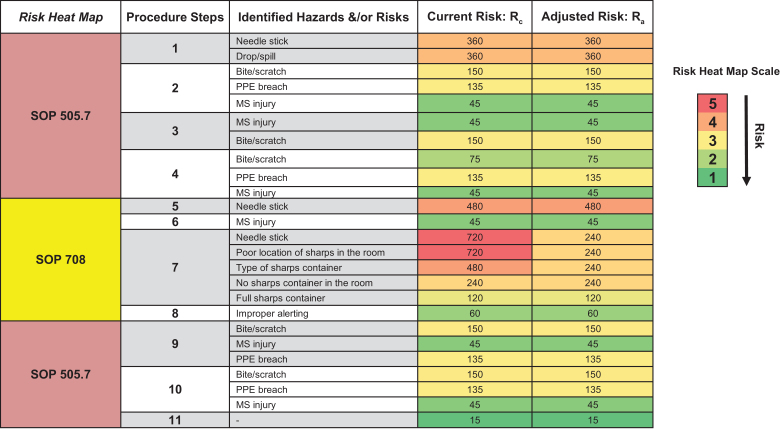
Based on this mock trial, the team decided to move forward with the retractable syringe needles and did a pilot study with 2 long-tailed macaques on an IACUC-approved training protocol. These animals were housed in an NHP cage with a squeeze mechanism, one animal in the top unit and one in the bottom unit. The animal care technicians used these retractable syringe needles to administer IM injections. The team discussed all findings, determined risk acceptance, and calculated Ra (Figure 10). The use of retractable syringe needles drove down the risk of needle sticks after giving IM injections, as indicated in the Rc and Ra heat maps (Figure 11). The team communicated this risk assessment to CMB senior leadership; stakeholders, such as principal investigators and research staff; and veterinary care teams. The team recommended the use of these sharps when specifically performing NHP IM injections (other injection modalities were not assessed in this risk assessment) and updated relevant SOPs. CMB personnel were trained on how to use these safety sharps, and the team continuously monitored for risks and any animal welfare concerns with these safety sharps being used en masse across the NHP colony.
Figure 10.
Reevaluation of hazards and risks from nonhuman primate (NHP) intramuscular (IM) injections. The risk assessment team discussed the mock trial and pilot study findings and collectively worked together to calculate adjusted risk (Ra), generate the Ra heat map, and determine risk acceptance. MS, musculoskeletal; PPE, personal protective equipment; SOP, standard operating procedure.
Figure 11.
Ra heat map analysis. The Ra heat map analysis summarized adjusted risks calculated using the comparative medicine branch (CMB) occupational risk matrix. The heat map analysis revealed that the use of retractable sharps decreased the likelihood of getting a needle stick after giving an intramuscular (IM) injection. MS, musculoskeletal; PPE, personal protective equipment; SOP, standard operating procedure.
About 3 months following the use of these safety sharps in the facility, there were no needle stick incidents associated with giving NHP IM injections. However, there was a concern that these safety sharps may compromise animal care as the further push of the plunger needed to activate the retractable mechanism, with the needle still in the animal, may lead to the user accidently hitting a vein or nerve. Withdrawing the needle from the animal and then activating the retractable mechanism still presented the risk of a needle stick to staff. Given this concern, the team convened to find retractable sharps that allowed for preremoval needle retraction to prevent occupational exposure while minimizing any discomfort and harm to NHPs. The team identified safety syringe needles that retract using a spring-loaded mechanism in which the user activates retraction after injection, but before withdrawing the needle from the animal, by pressing the top of the plunger without further advancing the needle. The needle then retracts into the syringe barrel and then is discarded into a sharps container. The animal care technicians assessed these safety sharps when performing IM injections in rhesus macaques on an IACUC-approved training protocol (data not shown), and the risk assessment team recommended the use of these retractable sharps when performing NHP IM injections. The team communicated these findings to CMB personnel and senior leadership and stakeholders, revised SOPs, and trained CMB personnel. In addition, the team maintained that contingency plans/SOPs instructing staff on how to respond to occupational needle stick exposures remain in place. Currently, the team continues monitoring for risks and animal welfare concerns. Thus far, there are still no needle stick injuries associated with NHP IM injections or present concerns regarding animal welfare. This risk assessment demonstrated occupational risk management in practice and highlighted the importance of involving frontline personnel in mitigating risks in animal facilities and consistent communication.
Conclusions and Discussion
Laboratory animal facilities present a dynamic occupational risk landscape. The report herein presented the development of an occupational risk assessment tool intended to identify and prioritize risk hot spots, generate practical mitigations, and continuously monitor for risks. In addition, we highlighted its utility in decreasing needle stick injuries when personnel administer IM injections in NHPs. Involving frontline personnel in the risk assessment process institutes a positive culture of safety and empowers staff to participate in efforts to improve occupational safety. In summary, this tool and management process illustrates a systematic means of identifying, evaluating, and mitigating occupational risks to augment the safety operations of laboratory animal facilities.
Acknowledgments
We thank our colleagues in the Comparative Medicine Branch for administrative and technical support and assistance and the LLS Fellowship Program.
Authors’ Note
The views, findings, and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Research Ethics Statement
This study was ethically reviewed and approved.
Statement of Human and Animal Rights
The study was conducted according to the institution’s animal care and use committee.
Statement of Informed Consent
No written or oral informed consent statements were required.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Centers for Disease Control and Prevention’s National Center for Emerging and Zoonotic Infectious Diseases and the Laboratory Leadership Service (LLS) Fellowship (awarded to A.M.C.) Program.
Prior Presentation
This work was presented at the 2017 National American Association for Laboratory Animal Science (AALAS) Meeting, October 2017, Austin, TX.
Supplemental Material
Supplemental material for this article is available online.
References
- 1. Russell W, Burch R. The Principles of Humane Experimental Technique. London, UK: Methuen & Co Ltd; 1959. [Google Scholar]
- 2. Animal Welfare Act and Regulations “Blue Book.” 2017. https://www.aphis.usda.gov/animal_welfare/downloads/AC_BlueBook_AWA_FINAL_2017_508comp.pdf. Accessed April 23, 2018.
- 3. Public Health Service Policy on Humane Care and Use of Laboratory Animals. 2015. https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf. Accessed April 23, 2018.
- 4. AAALAC International. https://www.aaalac.org/. Accessed April 23, 2018.
- 5. National Academies Press. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 6. Van Sluyters R. A guide to risk assessment in animal care and use programs: the metaphor of the 3-legged stool. ILAR J. 2008;49(4):372–378. [DOI] [PubMed] [Google Scholar]
- 7. National Academies Press. Occupational Health and Safety in the Care and Use of Research Animals. Washington, DC: National Academies Press; 1997. [Google Scholar]
- 8. U.S. Department of Health and Human Services Public Health Service Centers for Disease Control and Prevention National Institutes of Health. HHS Publication No. (CDC) 21-1112. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. 2009.
- 9. Stanley K, John GB. On the quantitative definition of risk. Risk Anal. 1981;1(1):11–27. [Google Scholar]
- 10. CRC Press. Laboratory Biorisk Management: Biosafety and Biosecurity. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 11. Caskey S, Gaudioso J, Salerno R, et al. Sandia: Biosafety Risk Assessment Report. Albuquerque, NM: Sandia National Laboratories; 2010. [Google Scholar]
- 12. Astuto-Gribble LM, Caskey SA. Laboratory Biosafety and Biosecurity Risk Assessment Technical Guidance Document. Albuquerque, NM: Sandia National Laboratories; 2014. [Google Scholar]
- 13. European Committee for Standardization. CEN Workshop Agreement: CWA 15793. In: Laboratory Biorisk Management. Brussels, Belgium: European Committee for Standardization (CEN; ); 2011. [Google Scholar]
- 14. International Organization for Standardization. ISO 31000: 2018-Risk Management. In: Risk Management-Guidelines. International Organization for Standardization (ISO); 2018. [Google Scholar]
- 15. Carvalho F, Melo RB. Stability and reproducibility of semi-quantitative risk assessment methods within the occupational health and safety scope. Work. 2015;51(3):591–600. [DOI] [PubMed] [Google Scholar]
- 16. Fatal cercopithecine herpesvirus 1 (B virus) infection following a mucocutaneous exposure and interim recommendations for worker protection. MMWR Morb Mortal Wkly Rep. 1998;47(49):1073–1076, 1083. [PubMed] [Google Scholar]
- 17. Cohen J, Davenport D, Stewart J, Deitchman S, Hilliard J, Chapman L. Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1). Clin Infect Dis. 2002;35(10):1191–1203. [DOI] [PubMed] [Google Scholar]
- 18. Cercopithecine herpesvirus 1 (B virus) infection resulting from ocular exposure. Appl Occup Environ Hyg. 2001;16(1):32–34. [DOI] [PubMed] [Google Scholar]
- 19. Elizabeth R. Griffin Research Foundation. http://www.ergriffinresearch.org/. Accessed April 23, 2018.