Abstract
Persons with Down syndrome have a form of genetically determined Alzheimer’s disease due to the amyloid precursor protein gene dose effect. Consequently, amyloid plaques and tau neurofibrillary tangles are virtually universal by age 40 years, and the lifetime risk to develop dementia is over 90%. Alzheimeŕs disease is now the main medical problem and leading cause of death in this population. However, diagnosis of dementia remains a clinical challenge due to a lack of awareness and validated diagnostic criteria. Most importantly, there are no treatments to prevent the disease. Unprecedented research activity is rapidly changing the scenario. Biomarkers, including those in plasma, have proven good diagnostic performances. The natural history is strikingly similar to that described in sporadic and autosomal dominant Alzheimeŕs disease suggesting that Down syndrome is an optimal population in which to conduct Alzheimer’s disease prevention trials. Those disease modifying treatments would not only benefit persons with Down syndrome but also the general population.
1. Introduction
Adults with Down syndrome develop the neuropathological hallmarks of Alzheimer’s disease and are at ultra-high risk of developing early onset dementia,1,2 which is now the leading cause of death in this population.3 However, diagnosis of dementia remains a clinical challenge due to a lack of awareness from families, caregivers, and clinicians and validated diagnostic criteria, and despite the excellent diagnostic performance of several biomarkers, including those in plasma.4–6 The most important clinical need is the development of therapies to prevent or delay Alzheimer’s disease. Unfortunately, few trials have been performed so far in this population. This situation is rapidly changing as research groups from around the world are creating new consortia and trial-ready cohorts. Down syndrome presents a unique opportunity in which to perform prevention trials due to the higher prevalence of Down syndrome compared to autosomal dominant Alzheimeŕs disease, and its more homogeneous pathophysiology relative to the sporadic late onset form of Alzheimer’s disease.
In this Review, we summarise data indicating that Alzheimeŕs disease is the main medical problem and cause of death in people with Down syndrome. We discuss how the natural history and clinical presentation of Down syndrome associated Alzheimer’s disease is similar to that of autosomal dominant Alzheimer’s disease. Finally, we explain how people with Down syndrome could possibly be the best population in which to conduct Alzheimer’s disease prevention trials.
2. Epidemiology
Down syndrome is the most frequent cause of intellectual disability of genetic origin, affecting 5.8 million people worldwide.7 Improvements in healthcare and management of co-occurring illnesses have greatly increased life expectancy of people with Down syndrome over the last five decades, and individuals older than 40 years represent a rapidly growing population.8 Consequently, there is an important and urgent need to focus on aging challenges in Down syndrome, with Alzheimer’s disease at the forefront.
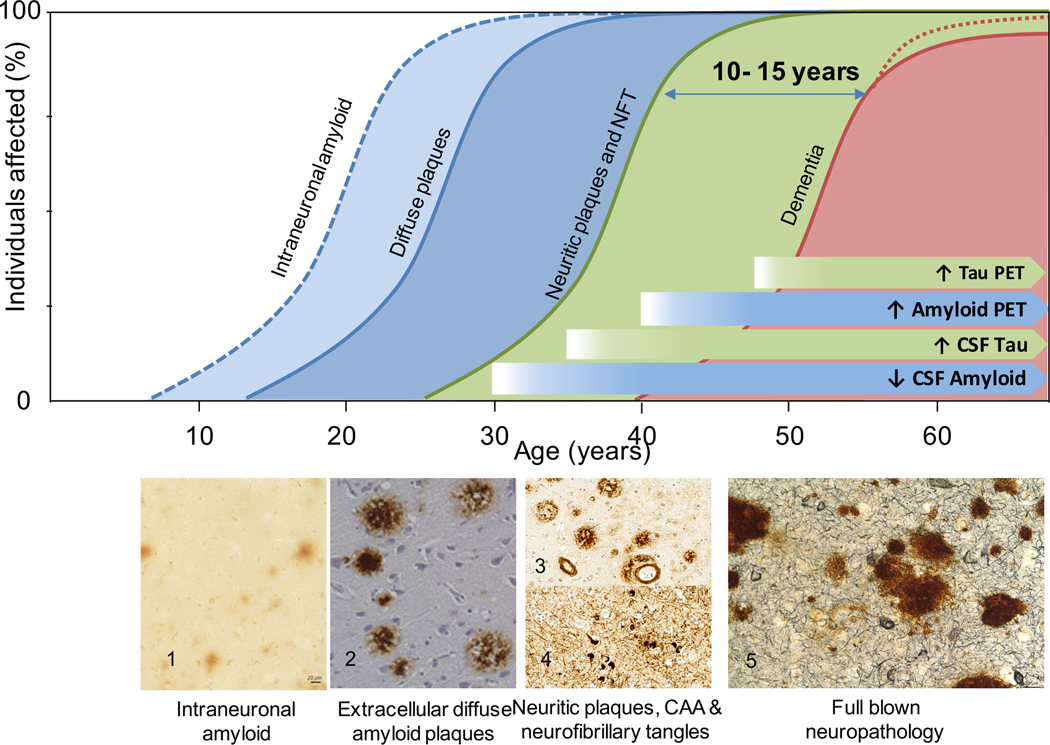
Clinical research and longitudinal studies consistently estimate the lifetime risk of dementia in people with Down syndrome to be over 90%.2,4 Dementia is rare before the age of 40 years, but its incidence and prevalence exponentially increase thereafter, reaching 88–100% in persons with Down syndrome older than 65 years (Figure 1).2
Figure 1: Lifelong accumulation of Alzheimer’s disease neuropathology.
Intraneuronal amyloid accumulation starts in the first decade of life (blue dotted line, 1- frontal cortex). Extracellular diffuse plaques (blue line) start in teenagers and are systematically observed after 30 years of age (2- cingulate gyrus). Amyloid deposition progresses with the accumulation of compact neuritic plaques (3- superior temporal gyrus) in the fourth decade. Tau pathological changes are observed starting in the third decade, with subsequent appearance of neurofibrillary tangles in the fourth decade (NFT) (4- superior temporal gyrus). After 40 years pathological diagnostic criteria for Alzheimer’s disease are fulfilled (green line,3, 4) and pathological changes continue to increase in severity in old age (5- frontal cortex). Amyloid and tau deposition can be detected through in vivo biomarkers. Cerebrospinal fluid changes occur almost 10 years before they are detectable in PET. After age 40, the prevalence of dementia increases exponentially affecting more than 90% of the adults in those older than 60 years of age (red line).
Importantly, Alzheimer’s disease dementia is recorded on 30% 9 of death certificates of people with Down syndrome and is the leading cause of death.9,10 However, this proportion might be an underestimate because dementia is often not recorded as the cause of death.9 In a longitudinal study of adults with Down syndrome, dementia was the proximate cause of death in 70% of cases.3 Future studies should investigate the exact contribution of Alzheimeŕs disease on mortality in Down syndrome.
3. Genetics, pathology, and pathophysiology
Complete trisomy of chromosome 21 is found in 95% of people with Down syndrome; translocations (4%) and mosaicism (1%) occur less frequently. The triplication of amyloid precursor protein gene is both sufficient and necessary to produce early onset dementia in Down syndrome.11 However, other chromosome 21 genes and consequent imbalance in other chromosomes may also affect the age of onset.7,12 For example, the APOE4 haplotype accelerates amyloid deposition and has an earlier symptomatic disease onset by 2 years.1313,14
Alzheimer’s disease neuropathology is almost universal in adults with Down syndrome by the age of 40 years.15 The characteristic lesions, including β-amyloid plaques and neurofibrillary tangles are similar in appearance and distribution to those of the sporadic and autosomal dominant forms (Figure 1 appendix), although some presenilin-1 mutations (especially those mutations in exons 8 and 9) display more frequent cotton-wool plaques.16 The development of the pathology is a decades-long process (Figure 1). The earliest signs of amyloid overexpression are noted as an accumulation within enlarged endosomes and lysosomes, which may start as early as 28 weeks of gestation.17,18 These enlarged endosomes are more numerous normal-sized and clustered in ultrastructural studies (Figure 2 appendix).19 Intracellular Aβ within endosomes can promote mitochondrial dysfunction and oxidative damage.20 Between ages 20 and 30 years β-amyloid systematically deposits as diffuse plaques (although plaques at younger ages have been noted), followed by neuritic plaques in the fourth decade of life.21,22 Neurofibrillary tangles appear after the initial deposition of β-amyloid in a pattern consistent with Braak staging (Figure 1).15 This abnormal tau aggregation plays a critical role in the development of dementia in Down syndrome.23
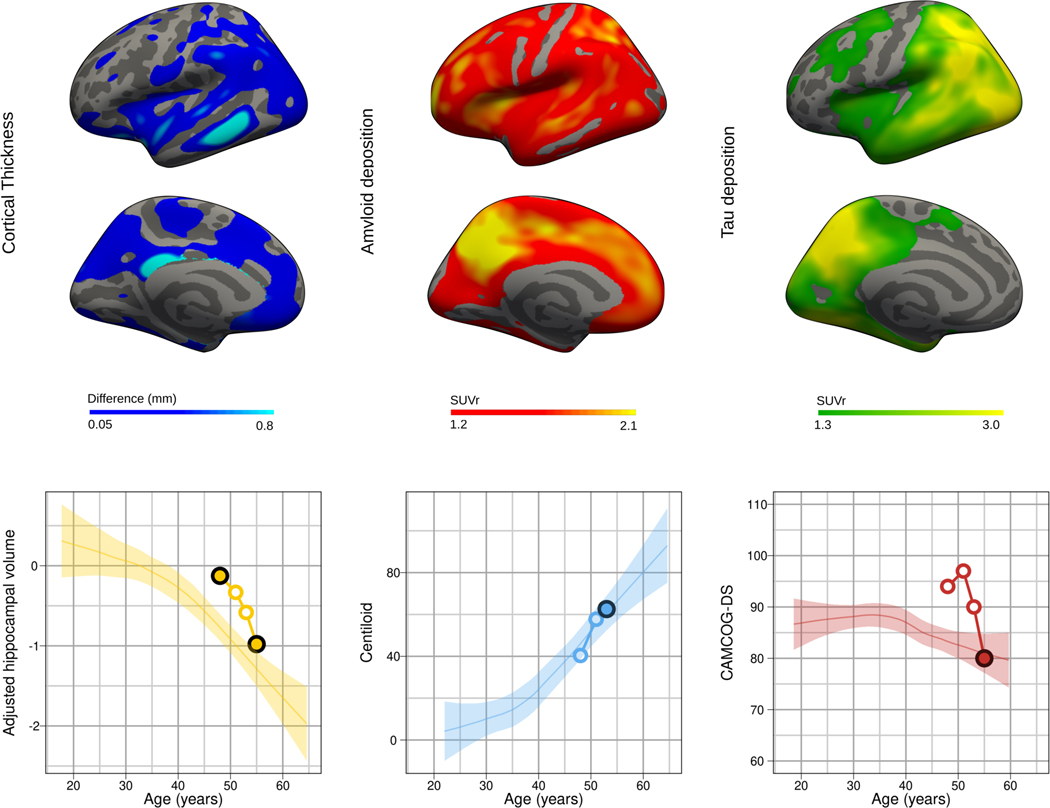
Figure 2. Case study.
The upper row shows the cortical atrophy (difference map between the first and las MRI scan) and both the amyloid and tau PET SUVRs using the Pittsburgh compound B and flortaucipir ligands respectively. The lower row shows the longitudinal intra-individual trajectory of the biomarker changes with respect the described changes with age in Down syndrome as described by Fortea & colleagues.4 The plotted changes with age for the CAMCOG-DS were those described in individuals with mild intellectual disability.44 Coloured dots indicate the different timepoints for assessment and black dots indicate the timepoint in which the displayed neuroimage was performed.
β-amyloid also accumulates in the blood vessels of the brain, as cerebral amyloid angiopathy (Figure 3 appendix). In Down syndrome, the extent of cerebral amyloid angiopathy is higher than in sporadic Alzheimeŕs disease.24 However, it might be lesser than in rare euploid cases with amyloid precursor protein gene duplications,25,26 which more often present with clinical intracerebral haemorrhages, suggesting possible protective factors in Down syndrome brain.26–28
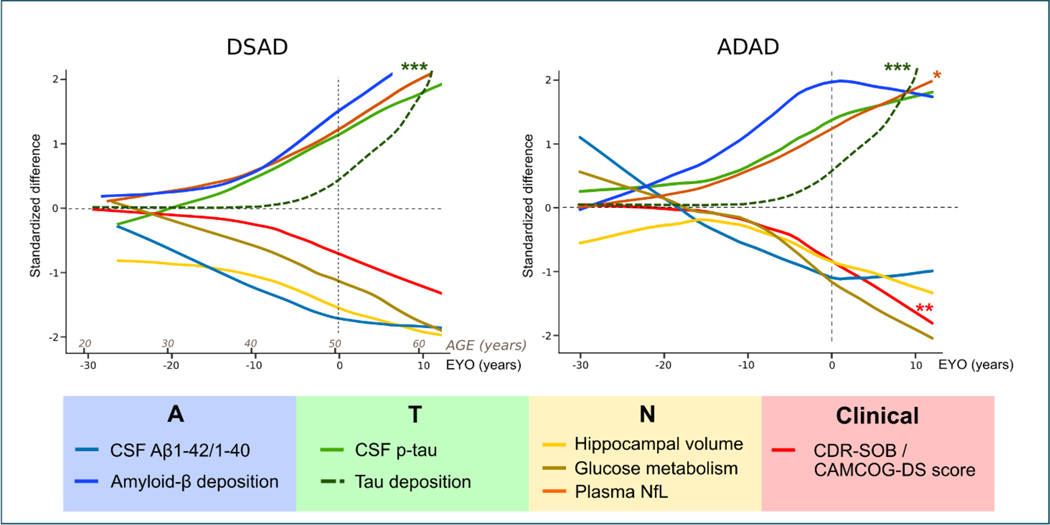
Figure 3: Integrated models of the natural history of Down syndrome-associated Alzheimer’s disease (DSAD) and autosomal dominant Alzheimer’s disease (ADAD).
Comparison of the clinical and biomarker changes (standardised differences) as a function of age in Down syndrome (left) and autosomal dominant Alzheimer’s disease (right).
The DSAD model is based on the study published by Fortea & colleagues4 and includes a hypothetical for tau uptake in PET (***green dotted line) based on the scarce data in Down syndrome.93 The ADAD model is based on that published by Bateman & colleagues.100 We have added the trajectory of plasma neurofilament light based on a large recent study (*orange line),115 and the hypothetical trajectory of Tau deposition measured by Tau-PET (***green dotted line). We have inverted the trajectory of the clinical dementia rating scale sum of boxes (CDR-SOB) to more easily compare the cognitive changes in ADAD and DSAD.
Other pathological substrates have been less studied in Down syndrome. α-synuclein pathology might be found in up to 50% of brains with Down syndrome-associated Alzheimer’s disease.29 In this sense, α-synuclein also appears in very young individuals with autosomal dominant Alzheimer’s disease.30 Phosphorylated TAR-DNA binding protein of 43 kDa (pTDP-43) is significantly reduced in Down syndrome (or autosomal dominant Alzheimer’s disease).31,32 pTDP-43 co-pathology is, thus, relatively rare in younger persons with Alzheimer’s disease irrespective of the genetic aetiology.
All these processes can initiate and exacerbate neuroinflammation (Figure 4 appendix), noted as higher levels of expression various cytokines and chemokines,33,34 and as microglial activation and dysfunction.34,35 In part, a consequence of neuroinflammation, is significant oxidative damage in the brains of persons with Down syndrome.36,37 Plasma inflammatory proteins may be important as a biomarker for Alzheimer’s disease in Down syndrome. Two recent studies using a proteomic profile approach found that some of the markers that best discriminate mild cognitive impairment in adults with Down syndrome and distinguished Alzheimer’s disease from cognitive stable people with Down syndrome were markers of inflammation.38,39
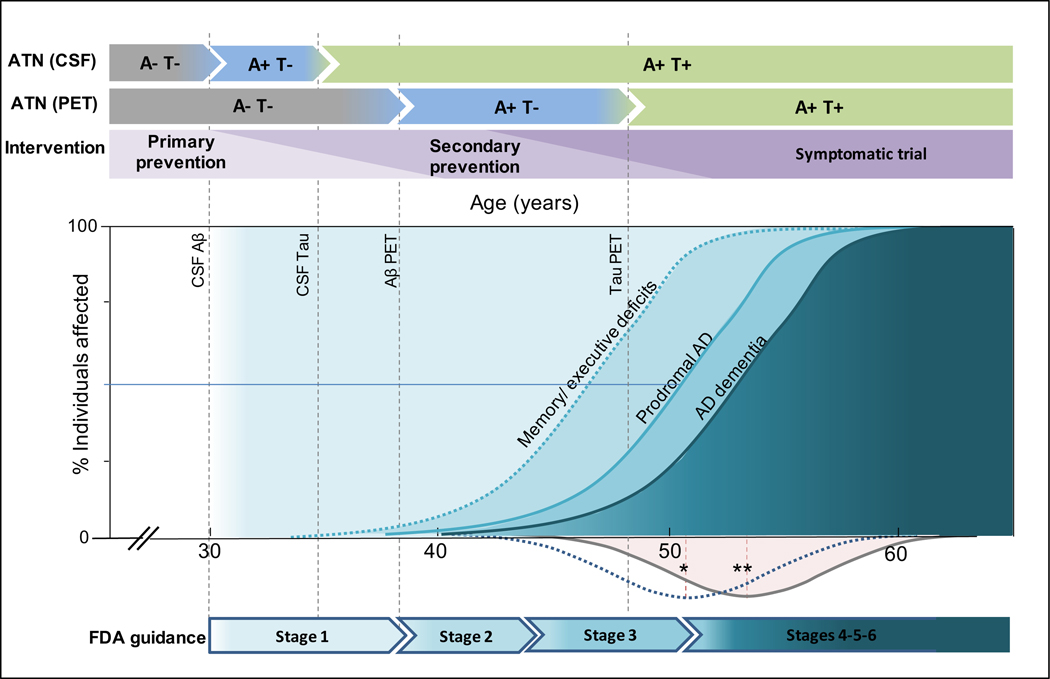
Figure 4. Framework for clinical trials in Down syndrome.
The two upper arrows for the AT(N) reflect the different timings for the earliest changes in CSF and PET, which in turn would affect the definition of primary or secondary prevention trials (third arrow). The model includes the different clinical outcomes reflecting the clinical progression of Alzheimer disease in Down syndrome. Subtle memory/executive deficits can appear from 35 years of age, prodromal Alzheimer’s disease appears with a mean age of presentation of 50·8 years (*) while dementia presents with a mean age of onset of 53·8 (**) years of age. The Gaussians below the X-axis reflect de density of prodromal and AD dementia diagnosis in Fortea et al paper.4 The vertical dotted lines reflect the earliest biomarker changes for the amyloid and tau biomarkers. The lower arrow summarizes the age spans in which different types of intervention trials could be performed in adults with Down syndrome, following the new Food and Drug Administration guidanceº.112
In addition, immune dysregulation is inherent to Down syndrome, which is associated with defects in T-cell maturation, B-cell function, and pro-oxidative state. Four (out of the six) interferon receptors are coded in chromosome 21, leading to a chronic inflammatory state.40 Finally, several comorbidities associated with Down syndrome, such as periodontitis, 41 might produce a low-grade peripheral chronic inflammation and may influence Alzheimeŕs disease pathophysiology. However, there is still limited data. Further investigation is warranted, as they might offer new avenues for intervention.
4. Clinical presentation and diagnosis
4.1. Diagnosis
There is a long lag (up to 20 years) between the development of pathology and the onset of the prodromal stage of Alzheimer’s disease in Down syndrome (Figure 1).4,15 The diagnosis of prodromal Alzheimer’s disease requires a change in cognition from previous level of functioning. Dementia is diagnosed when cognitive decline affects activities of daily living. However, in persons with Down syndrome, the variable degree of premorbid intellectual disability makes these definitions difficult, as well as the validation of population norms.
Figure 2 presents a typical case study and neuroimaging changes across 7 years in a 48-year-old man with Down syndrome. The initial presentation was depressive symptoms but no behavioural changes. Pittsburgh Compound-B PET showed amyloid deposition in the striatum and precuneus and no major atrophy. A follow-up visit 3 years later (age 51 years) showed amyloid accumulation on imaging. Two years later (age 53 years), a diagnosis of prodromal Alzheimer’s disease was made; signs and symptoms included lack of motivation, slower thinking, poorer memory, stubbornness, repetitive language, word-finding problems, and an inability to keep up with conversation. Brain atrophy was evident. At a clinical visit 2 years later (aged 55 years), a diagnosis of Alzheimer’s disease dementia was made. Both brain atrophy and cognitive decline (as measured by CAMCOG-DS) further progressed. At this visit, [18-F]-AV-1451 PET was done, which showed widespread cortical tau uptake with a typical AD regional distribution.
In the general population, due to dementia mimics and lack of awareness, dementia can frequently be misdiagnosed and underdiagnosed.42 These problems are exacerbated in Down syndrome due to an even larger lack of awareness from families, caregivers and clinicians. Consultations for cognitive decline are often only done when activities of daily living are substantially affected or when behavioural problems emerge. Given these challenges, population-based health plans in Europe to screen for Alzheimeŕs disease have shown a substantial increase in detection.43
The diagnosis of symptomatic Alzheimeŕs disease in Down syndrome is a challenge. Early symptoms can be mistaken as part of lifelong intellectual disability44 or obscured by coexisting medical comorbidities that might impact cognition, such as obstructive sleep apnoea, hypothyroidism and depression.45–47 In contrast, due to the younger age of onset of dementia, differential diagnosis rarely includes other neurodegenerative dementias. There are no validated or widely accepted clinical diagnostic criteria. In most specialized clinics and research settings, a physician and neuropsychologist make the diagnosis of prodromal or Alzheimer’s disease dementia independently or use a case consensus process.4,48,49
4.2. Assessment and neuropsychology
Most neuropsychological test batteries used in the general population are of limited use in adults with Down syndrome, as most individuals score at floor levels. Adapted tests are required, such as the Down Syndrome Mental Status Examination, the Test for Severe Impairment, the Cambridge Cognitive Examination for Older Adults with Down’s Syndrome, the Arizona Cognitive Test Battery or the modified Cued Recall Test.7,15,50–54 Given differences in premorbid intellectual levels, studies have emphasized tracking within-person change over time4,55 and focused on adjusted clinical cut-offs for screening for Alzheimeŕs disease.44,50,56 Assessment of decline should be patient-specific, taking into consideration the personal best level of achievement. A caveat is that adults with profound or severe intellectual disability have often been excluded from research, highlighting the critical need for valid and reliable measures of cognitive functioning and dementia symptoms in low functioning adults.50
Research advancements over the past 5–10 years provide new insights into the clinical presentation. Much of the early research suggested an initial frontal-type clinical presentation.57 However, the clinical picture has evolved over the past 5–10 years as a consequence of the creation of large Down syndrome research international consortia,50,58 which have assembled larger sample sizes and more advanced analytic methods (e.g., meta-analyses and machine-learning approaches) to determine the sequence of cognitive and behavioural changes. Moreover, identification of imaging and fluid biomarkers in the past 5 years has created new opportunities to investigate cognitive indicators of the transition from preclinical to prodromal Alzheimeŕs disease in Down syndrome.
This research now recognizes a similar clinical presentation to that of sporadic and autosomal dominant Alzheimeŕs disease. Declines in episodic memory are consistently reported early in the disease course,44,55,59–62 particularly in individuals who are amyloid positive at presentation or in those who become amyloid positive in the follow-up.55 Declines in attention as well as in executive functions are also found early. These initial declines are closely followed by declines in visuospatial ability, verbal fluency, motor coordination and planning.55,59–62 Direct tests of cognition capture decline earlier than informant reports.50,59 Also, while mild cognitive impairment in the general population is considered to have minimal impact on functional skills, decline in everyday living skills is seen in people with early prodromal Alzheimer’s disease and Down syndrome,50,63 perhaps because everyday living skills are more cognitively demanding in this population.
4.3. Late onset myoclonic epilepsy in Down syndrome
Epilepsy is one of the most frequent neurological comorbidities in Down syndrome with prevalence estimates of up to 13%.64 Epilepsy prevalence has a bimodal distribution with onset in early childhood and after the 5th decade of life, the later presentation being in close relation to symptomatic Alzheimer’s disease. Up to 75% of adults with Down syndrome and dementia develop epilepsy,65 a higher proportion than in sporadic (1.5%- 12.7%) and autosomal dominant Alzheimer’s disease (2.8%−41.7%).66 In fact, the occurrence of a first episode of an untriggered seizure after the age 40–45 years is highly suggestive of symptomatic Alzheimer’s disease.
The most frequent epileptic seizures in this context are generalized tonic-clonic or myoclonic seizures, which usually coexist and have a morning predominance.67 Sleep abnormalities might contribute to worse seizure control. The interictal electroencephalograph is variable but might yield a low diagnostic performance. The characteristic epileptiform activity consists in polyspike waves and slow background activity.68 Due to these specific clinical characteristics the term, “Late-Onset Myoclonic Epilepsy in Down syndrome” has been coined. Given the high risk of recurrence, chronic treatment with an antiepileptic (oftentimes levetiracetam) should be recommended. These epileptic seizures are probably underdiagnosed and inadequately treated.69
5. Biomarkers and Alzheimeŕs disease natural history
5.1. Biofluid biomarkers
Cerebrospinal fluid biomarkers are incorporated into the diagnostic criteria for sporadic Alzheimeŕs disease, and commonly used in clinical trials.57,70 There are fewer studies in Down syndrome, but all have consistently shown a similar biochemical signature of symptomatic Alzheimer’s disease, with a 50% reduction in the β-amyloid 42/40 ratio and a two-fold increase in total tau and 181-phosphorylated tau concentrations in symptomatic patients.4,5,71,72 These cerebrospinal fluid biomarkers have excellent diagnostic performance.4–6 Some studies suggest synaptic biomarkers such as neurogranin6 or neuronal pentraxin-2, a protein implicated in inhibitory circuit function,73 or nerve growth factor pathway biomarkers may also be useful.74
Blood-based biomarkers have obvious advantages. The development of ultrasensitive technology, such as Single Molecule Array, has enabled the development of new blood-based biomarkers.75 Plasma neurofilament light chain concentrations, a biomarker of neurodegeneration, also have excellent diagnostic and prognostic performance.4,76,77 Although neurofilament light chain concentrations are not specific to Alzheimer’s disease, but rather a biomarker of axonal damage, they are highly indicative of symptomatic Alzheimer’s disease in Down syndrome, as other neurodegenerative disorders are exceedingly rare.77
Novel plasma phosphorylated-tau assays have been recently developed and tested in Down syndrome. A recent study has shown that 181phospho-tau concentrations start to increase in the mid-30s, and are highly accurate for the diagnosis of symptomatic Alzheimer’s disease.78 Plasma concentrations correlate with cerebrospinal 181phospho-tau concentrations, as well as with neurodegeneration biomarkers such as neurofilament light chain levels, cortical thinning and brain hypometabolism in Alzheimer’s disease-related brain regions.78
Adults with Down syndrome have higher plasma β-amyloid 1–42 and 1–40 concentrations compared to euploid controls, but these biomarkers have not yet proven to be useful for diagnosing symptomatic Alzheimer’s disease.79,80 Of note, there are no reports in Down syndrome with the novel mass spectrometry techniques that accurately detect brain amyloidosis in sporadic Alzheimer’s disease.81
Plasma neurofilament light chain and phospho-tau concentrations will most likely change clinical practice and clinical trials in Down syndrome. Admittedly, there are still challenges, shared with the overall Alzheimeŕs disease field, to standardize and develop universal cut-offs for clinical practice. However, impressive advances are being made as a result of enormous efforts and funding. In short, plasma biomarkers can now stage the whole AT(N) framework82 and have the potential to become useful, easy, and cost-effective screening tools to detect symptomatic Alzheimeŕs disease in Down syndrome.
5.2. Neuroimaging biomarkers
Several studies in the last five years have been performed using multimodal MRI and / or PET with amyloid and tau tracers in adults with Down syndrome.83
MRI structural features reflect both neurodevelopmental and Alzheimer’s disease related changes in Down syndrome. Neurodevelopmental brain anatomy abnormalities include smaller whole brain volumes and brachycephalia with smaller frontal lobes, hippocampi, cerebellum and brain stem.84 The atrophy pattern associated with Alzheimer’s disease involves posterior dominant cortical thinning with atrophy of hippocampus, thalamus, and striatum in a similar pattern to what is seen in sporadic Alzheimer’s disease.83,85
Amyloid-PET radioligands indicate the presence of neuritic plaques in accordance with the Thal staging system described in sporadic Alzheimeŕs disease86 in the parieto-temporal, precuneus, posterior cingulate, and frontal regions. Some adults with Down syndrome might present with initial increased uptake in the striatum, a finding that is described in some cases of autosomal dominant Alzheimer’s disease.87 The significance of this early striatal uptake remains uncertain. It might reflect less efficient clearance mechanisms in the striatum in the context of amyloid overproduction, but this remains speculative.
Studies using 18fluorodeoxyglucose (18FDG)-PET, a measure of regional brain metabolism, also show the same regional pattern of hypometabolism in Down syndrome described in sporadic Alzheimeŕs disease involving the parietal, precuneus and posterior cingulate.88,89 Of note, some reports show regional hypermetabolism in young individuals with Down syndrome as a consequence of potential compensatory activity and/or less efficient glycolysis.90–92
There are a very small number of studies using tau-PET tracers in Down syndrome, but the available data also shows the expected pattern in accordance with the tau Braak staging system.93,94 As in sporadic Alzheimeŕs disease, tau uptake only occurs in amyloid positive individuals, and correlates with brain atrophy, hypometabolism as well as cognitive decline.94,95
The more frequent cerebral amyloid angiopathy found in pathological studies has a correlate in imaging studies (Figure 3 appendix). Findings on the modified Boston criteria for CAA (i.e. lobar intracerebral haemorrhage, lobar microbleeds, cortical superficial siderosis) are more frequent in Down syndrome than in sporadic Alzheimer’s disease, but similar to autosomal dominant cases.28 Other cerebrovascular disease biomarkers, such as white matter hyperintensities, enlarged perivascular spaces and infarcts, are also frequently found in adults with Down syndrome despite the lower prevalence of traditional vascular risks factors (namely hypertension) compared to the general population.96 This recent study found a monotonic increase in these markers along the Alzheimeŕs disease continuum suggesting cerebrovascular disease is a core feature of the disease (and not simply a comorbidity). Similar findings are reported in autosomal dominant Alzheimeŕs disease.97
More advanced MRI techniques have also been studied in adults with Down syndrome. Using diffusion weighted sequences; the integrity of white matter tracts has been mapped. A pattern of white matter abnormalities is proposed, in which the late myelinating commissural and limbic fibres are affected by disease before the early myelinating ones.98 Of note, developmental abnormalities in myelination are likely to overlap with those due to Alzheimeŕs disease pathology. Functional MRI also shows pre-symptomatic changes in Down syndrome in the default mode network,99 the large scale network primarily affected in Alzheimer’s disease.
5.3. Natural history
Over the past decade, significant progress has been made in understanding the natural history of Alzheimer’s disease in Down syndrome. A predictable sequence of events in biomarker changes begins more than two decades before the onset of dementia, in a strikingly similar order and timing to that described in autosomal dominant Alzheimer’s disease.4,100 Figure 3 compares the sequence of clinical and biomarkers changes in Down syndrome and autosomal dominant Alzheimer’s disease.
Cerebrospinal β-amyloid levels decrease after there are widespread diffuse plaques in the brain, beginning in the early 30s, at least 20 years before prodromal Alzheimeŕs disease diagnosis. Of note, there might be a period of pseudo-normality in these levels, as changes might start at younger ages. Thus, higher cerebrospinal fluid β-amyloid 42 concentrations are found in children with Down syndrome than in age-matched euploid controls.101 Amyloid tracers in positron emission tomography detect plaques a decade later, most likely due to preferential binding of fibrillar deposits. These changes are in agreement with those reported in Dominantly Inherited Alzheimer’s disease Network or in the Colombian kindred.100,102
Tau biomarkers show a similar dissociation, cerebrospinal fluid and plasma tau are elevated at age 35 years, but increased tau positron emission tomography uptake occurs more than a decade later in amyloid positive individuals.4,78 A similar gap and time-lag is described in autosomal dominant Alzheimer’s disease.103 Of note, recent work with plasma exosomes of neuronal origin shows elevated amyloid and tau levels in children and adolescents with Down syndrome opening a new window to study Alzheimeŕs disease pathophysiology.104
Different neurodegeneration biomarkers abnormalities begin at different ages. Plasma neurofilament light levels start to increase in the early 30s, but is further increased in the 40s and symptomatic stages. Brain metabolism shows a linear decrease, with significant differences in the late 30s, nearly 13 years before prodromal Alzheimer’s disease diagnosis. Hippocampal volumes reflect Down syndrome-associated neurodevelopmental differences, but also show Alzheimeŕs disease-related atrophy in the early 40s. A decline in cognitive scores is observed shortly thereafter. The median age of prodromal Alzheimer’s disease diagnosis is around 51 years, and that of Alzheimer’s disease dementia between 53 and 55 years.4,105 Death typically occurs between 57 and 60 years of age.81,105,106
In short, clinical and biomarker changes in Down syndrome associated Alzheimer’s disease are similar in their direction, magnitude, and temporality to those in sporadic and autosomal dominant forms. Furthermore, the patterns of cerebral amyloid and tau uptake, atrophy, and hypometabolism show that Alzheimer’s disease in persons with Down syndrome targets the same cortical regions affected in the other forms.4
6. Existing treatments and clinical trials
Currently, there are no approved drugs for the treatment of Alzheimer’s disease dementia in persons with Down syndrome. A Cochrane review in 2015 concluded that there is insufficient evidence to determine whether drugs approved for the treatment of sporadic Alzheimer’s dementia (cholinesterase inhibitors and the glutamate receptor antagonist memantine) are effective in treating cognitive decline in persons with Down syndrome.107 Only small clinical trials using drugs approved for treatment of Alzheimeŕs disease in persons with Down syndrome have been performed (e.g. four clinical trials evaluated the efficacy of donepezil in a total of 192 participants and a relatively larger trial in 173 tested memantine).107,108 However, given the clear evidence of cholinergic deficits in adults with Down’s syndrome 109 and the anecdotal evidence that cholinesterase inhibitors might increase survival,105 a common clinical practice is to use cholinesterase inhibitors, but not memantine.108 As with many psychotropic medications, there may be increased adverse events in adults with Down syndrome with cholinesterase inhibitors, which are, nonetheless generally well tolerated.107
There is a clear ethical imperative to perform clinical trials to reduce disease burden in persons with Down syndrome. Anti-amyloid therapies are obvious candidates and should be tested in adults with Down syndrome with a close monitoring of vasculogenic oedema and haemorrhages given the more prominent cerebral amyloid angiopathy. However, Alzheimeŕs disease has a complex pathophysiology that most likely will require a multidimensional approach including, but not limited to anti-tau and anti-inflammatory therapies. However, despite the clinical need (and advantages), only a handful of such trials have been conducted in adults with Down syndrome in the last 5 years.110 We highlight several clinical trials in Table 1. In this sense, the ongoing trials and infrastructure built for autosomal dominant Alzheimeŕs disease111 should inspire progress in Down syndrome.
Table 1:
Clinical trials of pharmacological interventions for people with Down síndrome
| Study type | Setting | Eligibility criteria | Investigational agent | Dosage | Primary endpoint | Result | |
|---|---|---|---|---|---|---|---|
| NCT01594346 | 36-month randomised, double-blind, placebo-controlled phase 3 trial | Recruitment occurred at 21 sites with research experience in adults with Down syndrome in five countries (Australia, Canada, Ireland, UK, and USA) | Adults with Down syndrome aged 50 years or older (n=337) | Alpha-tocopherol (vitamin E) versus placebo | 1000 international units twice per day orally | Rate of change on the Brief Praxis Test | One or more non-serious adverse event: 96% treatment group vs 93% placebo group (p=0·90). Serious adverse events (ie, medical conditions requiring hospitalisation or substantial care): 31·5% treatment group vs 32·0% placebo group (p=1·00). No between-group difference in Brief Praxis Test (generalised estimating equation: b=0·159; CI 20·732–1·049; p=0·729). |
| NCT01791725 (DS201) | 4-week randomised, double-blind, placebo-controlled phase 2 trial | Three centres in the USA | Adults with Down syndrome without dementia (n=26) | ELND005 (scyllo-Inositol; cyclohexane-1,2,3,4,5,6- hexol) versus placebo | 250 mg once or twice per day orally | Safety and tolerability | Treatment-emergent adverse events: five (41·7%) participants in the 250 mg twice daily group, two (40·0%) in the 250 mg once daily group, and none in the placebo group. All events were mild in intensity. |
| NCT02738450 (3 Star) | 12-month, placebo-controlled, phase 1b multicentre study plus 12-month follow-up | Four centres in the USA | Adults with Down syndrome aged 25–45 years (n=16) | ACI-24 (liposomal vaccine against aggregated β-amyloid peptides) versus placebo | Seven subcutaneous injections of 300 or 1000 μg | Safety and tolerability, antibody titre | Not yet available; a phase 2a trial is planned (NCT04373616 |
Down syndrome is arguably the best population in which to conduct Alzheimeŕs disease prevention trials: First, similar to autosomal dominant Alzheimer’s disease, adults with Down syndrome have a high risk for developing symptomatic Alzheimer’s disease, and a predictable sequence of pathogenic events. Second, Alzheimeŕs disease in Down syndrome is more homogeneous than sporadic Alzheimer’s disease, in which many co-pathologies frequently coexist. However, due to the triplication of other genes in chromosome 21, there might be differences in some processes (such as neuroinflammation) that might impact the generalizability of some of the findings.
Figure 4 summarizes the age-spans in which primary and secondary prevention trials as well as trials in symptomatic individuals could be performed in adults with Down syndrome, as well as following the new Food and Drug Administration guidance.112 The relatively high numbers of people with Down syndrome ensure recruitment for any stage of the disease. Admittedly, there are additional challenges regarding informed consent and feasibility. Nonetheless, the aforementioned studies support that a substantial proportion of adults with Down syndrome are capable and willing perform such trials.
7. Future directions
Randomized clinical trials are the most urgent unmet clinical need for people with Down syndrome. Such trials will require reliable, valid, and sensitive clinical measures for inclusion and monitoring disease progression. A collective effort is underway by the research community, regulators and pharmacological industry to agree on the outcome measures for the different stages of the disease. We are now on the cusp of being able to launch clinical trials utilizing data from a number of longitudinal biomarker studies in this population.4,48,113
Another major unmet clinical need is the development of validated and widely accepted diagnostic criteria for the preclinical, prodromal, and dementia stages of Down syndrome associated Alzheimeŕs disease, similar to those developed for sporadic Alzheimeŕs disease by the National Institute of Aging-Alzheimer’s Association (NIA-AA criteria),114 and incorporating standard Alzheimer’s disease biomarker data.138
There are few studies assessing the relationship of inflammatory and Alzheimeŕs disease biomarkers, and a relative paucity of data longitudinal tau-PET, plasma or cerebrospinal fluid data. Also, more studies assessing the relationships (and interactions) between biomarkers and cognitive decline are needed. Biomarker studies will also elucidate if the AT(N) biomarker classification system can be applied to adults with Down syndrome.113 Finally, future studies should assess the effects of potential risk and protective factors. These should include cognitive reserve and resilience, genetic studies (both genome wide association studies and the influence of mosaicism, which might temper the phenotype), co-occurring illnesses, as well as socio-demographic and environmental factors.
There are reasons for optimism. Research in Down syndrome-associated Alzheimeŕs disease is benefiting from unprecedented research activity and funding (Table 2). These initiatives will undoubtedly accelerate the discovery of disease modifying treatments that would not only benefit persons with Down syndrome but also the general population.
Table 2:
Observational studies of cognition and biomarkers in people with Down syndrome
| Sites | Objectives | Variables | |
|---|---|---|---|
| Down Alzheimer Barcelona Neuroimaging Initiative cohort | Population-based cohort of Catalonia (Spain) centralised in a university tertiary hospital | To study the natural history of Alzheimer’s disease in Down syndrome through biomarkers; to recruit a trial-ready cohort | Longitudinal clinical and cognitive data; biofluid biomarkers; genetics; neuroimaging biomarkers; sleep studies; EEG; brain banking |
| The LonDowns Consortium | Four university, clinical, and research centres in the UK | To explore the cognitive, genetic and cellular factors underlying individual differences in susceptibility to Alzheimer’s disease; to study individual differences in cognitive abilities and brain activity | Longitudinal clinical and cognitive data; biofluid biomarkers; genetics; neuroimaging biomarkers |
| Horizon 21 consortium | Ten university, clinical, and research centres from eight European countries (France, Germany, Ireland, Netherlands, Norway, Spain, Sweden, and UK) | To identify factors that influence Alzheimer’s disease development in people with Down syndrome; to develop clinical trials to prevent or slow the disease in this population | Longitudinal clinical and cognitive data; biofluid biomarkers; genetics; neuroimaging biomarkers; sleep studies |
| Alzheimer’s Biomarkers Consortium— Down Syndrome | Nine academic centres in the USA and UK | To identify biomarkers of cognitive impairment and dementia; to identify crucial factors that link cerebral amyloid deposition to neurodegeneration and dementia; to understand the relationships between biomarkers; to provide rapid public access to all data | Longitudinal clinical and cognitive data; biofluid biomarkers; neuroimaging biomarkers; genetics |
| TRC-DS | 14 academic, clinical, and research sites in three countries (USA, UK, and Spain) | To analyse the relationships between cognitive measures and biomarkers of Alzheimer’s disease; to identify endpoints for Alzheimer’s disease clinical trials in people with Down syndrome that best reflect disease progression | Longitudinal cognitive and clinical assessment; biofluid biomarkers; genetics; neuroimaging biomarkers |
| Lumind IDSC LIFE-DSR | Ten academic centres in the USA | To better understand the cognitive and behavioural changes along with the health issues found in adults with Down syndrome as they progress towards Alzheimer’s disease | Longitudinal health examination; blood biomarkers, cognitive measures, MRI, PET scan, CSF biomarkers, plasma biomarkers |
Supplementary Material
Acknowledgments
The authors would like to acknowledge Jordi Pegueroles and Mateus Aranha, from the Memory Unit of Sant Pau Hospital (Barcelona, Spain); Dr. Iban Aldecoa from the NTB-Biobanc-IDIBAPS-Hospital Clínic (Barcelona, Spain); Alessandra C Martini at the Department of Pathology and Laboratory Medicine University of California, Irvine (USA); Marie-Claude Potier and Alexandra Botté from Paris Brain Institute (ICM), Sorbonne Université, Hôpital de la Pitié-Salpêtrière, Paris (France) for helping in acquiring, providing, and assembling images for figures.
JF and MCI gratefully acknowledge Fundació Catalana Síndrome de Down for their global support and the members of the Alzheimer Down Unit and the Memory Unit from the Hospital de la Santa Creu I Sant Pau for their daily work and dedication.
All authors want to thank all the persons with Down syndrome who participate in research projects and their families. Their generosity is enabling and will enable important advances in the field of Alzheimer’s disease.
Declaration of interests / Conflict of interest statement
JF reports grants from the Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III (PI14/01126 and PI17/01019) and the CIBERNED program (Program 1, Alzheimer Disease and SIGNAL study, www.signalstudy.es), partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una manera de hacer Europa. This work was also supported by the National Institutes of Health (NIA grants 1R01AG056850 - 01A1; R21AG056974 and R01AG061566), Fundació La Marató de TV3 (20141210), grants from Fundació Víctor Grífols i Lucas, and by the Generalitat de Catalunya (SLT006/17/00119). JF is a consultant to Novartis and Merck, has received conference fees from Esteve, NovoNordisk, Biogen, has been part of AC Immune and Lundbeck advisory boards, and owns a patent (EP18382175.0).
SHZ is supported by CPFT (Cambridgeshire & Peterborough NHS Foundation Trust) and is part of The Alzheimer’s Biomarkers Consortium—Down Syndrome (ABC-DS), funded by the National Institute on Aging and the Eunice Kennedy Shriver National Institute for Child Health and Human Development (U01 AG051406 and U01 AG051412).
SH reports grants from NICHD core grant (NICHD U54 HD090256) to support time working on papers; and grants from NIH (R01AG031110, U01AG051406).
MSR is a consultant to AC Immune and Alzheon. He has received research support from the NIH, Avid, Baxter, Eisai, Elan, Genentech, Janssen, Lilly, Merck, and Roche.
EH reports funding to support acquisition of tissue supported by funding to EH from NIH/NIA U19AG068054, NIH/NICHD R01HD064993, NIH/NIA P30AG066519 and Brightfocus grant #BFF17-008. EH has been a consultant for AC Immune.
MCI reports grants from the Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III (PI18/00335) and the CIBERNED program (Program 1, Alzheimer Disease and SIGNAL study, www.signalstudy.es), partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una manera de hacer Europa; Alzheimer’s Association and Global Brain Health Institute (GBHI_ALZ-18-543740), Jérôme Lejeune Foundation (Project #1913, Cycle 2019B), and Societat Catalana de Neurologia (Premi Beca Fundació SCN 2020).
The manuscript describes independent research, and the views expressed of the authors and not necessarily those of the funders. The sponsors did not take part in the design and conduct of the review; or the decision to submit the article for publication.
Footnotes
Search strategy and selection criteria
We searched PubMed for research studies published since database inception until May 2021. All relevant articles relating to Alzheimer disease (AD) in individuals with Down syndrome (DS) were identified for consideration. Search terms include: (“Down syndrome” OR “Down’s syndrome” OR Downs) AND (Alzheimer OR Alzheimer’s OR dementia). Additional search terms for specific sections were considered. There were no language restrictions. The final reference list was generated on the basis of relevance to the topics covered in this Review.
References
- 1.Mann DMA. The pathological association between down syndrome and Alzheimer disease. Mech Ageing Dev 1988; 43: 99–136. [DOI] [PubMed] [Google Scholar]
- 2.McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res 2017; 61: 843–52. [DOI] [PubMed] [Google Scholar]
- 3.Hithersay R, Startin CM, Hamburg S, et al. Association of Dementia with Mortality among Adults with Down Syndrome Older Than 35 Years. JAMA Neurol 2019; 76: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortea J, Vilaplana E, Carmona-Iragui M, et al. Clinical and biomarker changes of Alzheimer’s Disease in adults with Down Syndrome: a cross-sectional study. Lancet 2020; 395: 1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortea J, Carmona-Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol 2018; 17: 860–9. [DOI] [PubMed] [Google Scholar]
- 6.Henson RL, Doran E, Christian BT, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in a cohort of adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down’s syndrome. Lancet Neurol 2016; 15: 622–36. [DOI] [PubMed] [Google Scholar]
- 8.de Graaf G, Buckley F, Dever J, Skotko BG. Estimation of live birth and population prevalence of Down syndrome in nine U.S. states. Am J Med Genet Part A 2017; 173: 2710–9. [DOI] [PubMed] [Google Scholar]
- 9.Englund A, Jonsson B, Zander CS, Gustafsson J, Annerén G. Changes in mortality and causes of death in the Swedish Down syndrome population. Am J Med Genet Part A 2013; 161: 642–9. [DOI] [PubMed] [Google Scholar]
- 10.Landes SD, Stevens JD, Turk MA. Cause of death in adults with Down syndrome in the United States. Disabil Health J 2020; 13: 100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doran E, Keator D, Head E, et al. Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer’s Disease: The Role of APP. J Alzheimer’s Dis 2017; 56: 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiseman FK, Pulford LJ, Barkus C, et al. Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain 2018; 141: 2457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejanin A, Iulita M, Vilaplana E, et al. Effect of APOEɛ4 on clinical and multimodal biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Jama Neurol 2021; Online ahead of print. DOI: 10.1001/jamaneurol.2021.1893. [DOI] [Google Scholar]
- 14.Prasher VP, Sajith SG, Rees SD, et al. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer’s disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry 2008; 23: 1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol 2019; 15: 135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol 2009; 118: 37–52. [DOI] [PubMed] [Google Scholar]
- 17.Gyure K, Durham R, Stewart W, Smialek J, Troncoso J. Intraneuronal Aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med 2001; 125: 489–92. [DOI] [PubMed] [Google Scholar]
- 18.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid β deposition in sporadic alzheimer’s disease and down syndrome: Differential effects of APOE genotype and presenilin mutations. Am J Pathol 2000; 157: 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botté A, Lainé J, Xicota L, et al. Ultrastructural and dynamic studies of the endosomal compartment in down syndrome. Acta Neuropathol Commun 2020; 8: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busciglio J, Pelsman A, Wong C, et al. Altered metabolism of the amyloid β precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron 2002; 33: 677–88. [DOI] [PubMed] [Google Scholar]
- 21.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski TM, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis 1996; 3: 16–32. [DOI] [PubMed] [Google Scholar]
- 22.Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: A regional quantitative analysis. Exp Neurol 1998; 150: 296–304. [DOI] [PubMed] [Google Scholar]
- 23.Perez SE, Miguel JC, He B, et al. Frontal cortex and striatal cellular and molecular pathobiology in individuals with Down syndrome with and without dementia. Acta Neuropathol 2019; 137: 413–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Head E, Phelan MJ, Doran E, et al. Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta Neuropathol Commun 2017; 5: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann DMA, Davidson YS, Robinson AC, et al. Patterns and severity of vascular amyloid in Alzheimer’s disease associated with duplications and missense mutations in APP gene, Down syndrome and sporadic Alzheimer’s disease. Acta Neuropathol 2018; 136: 569–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buss L, Fisher E, Hardy J, et al. Intracerebral haemorrhage in Down syndrome: Protected or predisposed? [version 1; referees: 2 approved]. F1000Research 2016; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmona-Iragui M, Videla L, Lleó A, Fortea J. Down syndrome, Alzheimer disease, and cerebral amyloid angiopathy: The complex triangle of brain amyloidosis. Dev Neurobiol 2019; 79. DOI: 10.1002/dneu.22709. [DOI] [PubMed] [Google Scholar]
- 28.Carmona-Iragui M, Balasa M, Benejam B, et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer’s disease. Alzheimer’s Dement 2017; 13: 1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippa CF, Schmidt ML, Lee VMY, Trojanowski JQ. Antibodies to α-synuclein detect lewy bodies in many down’s syndrome brains with alzheimer’s disease. Ann Neurol 1999; 45: 353–7. [DOI] [PubMed] [Google Scholar]
- 30.Alafuzoff I, Kovacs GG. Comorbidities. Handb Clin Neurol 2018; 145: 573–7. [DOI] [PubMed] [Google Scholar]
- 31.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s Syndrome: Association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 2011; 122: 703–13. [DOI] [PubMed] [Google Scholar]
- 32.Lippa CF, Rosso AL, Stutzbach LD, Neumann M, Lee VMY, Trojanowski JQ. Transactive response DNA-binding protein 43 burden in familial Alzheimer disease and Down syndrome. Arch Neurol 2009; 66: 1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcock DM, Hurban J, Helman AM, et al. Down syndrome individuals with Alzheimer’s disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer’s disease. Neurobiol Aging 2015; 36: 2468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores-Aguilar L, Iulita MF, Kovecses O, et al. Evolution of neuroinflammation across the lifespan of individuals with down syndrome. Brain 2020; 143: 3653–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martini AC, Helman AM, McCarty KL, et al. Distribution of microglial phenotypes as a function of age and Alzheimer’s disease neuropathology in the brains of people with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cenini G, Dowling ALS, Beckett TL, et al. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta - Mol Basis Dis 2012; 1822: 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strydom A, Dickinson MJ, Shende S, Pratico D, Walker Z. Oxidative stress and cognitive ability in adults with Down syndrome. Neuro-Psychopharmacology Biol Psychiatry 2009; 33: 76–80. [DOI] [PubMed] [Google Scholar]
- 38.Petersen ME, Zhang F, Schupf N, et al. Proteomic profiles for Alzheimer’s disease and mild cognitive impairment among adults with Down syndrome spanning serum and plasma: An Alzheimer’s Biomarker Consortium–Down Syndrome (ABC–DS) study. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen M, Zhang F, Krinsky-McHale SJ, et al. Proteomic profiles of prevalent mild cognitive impairment and Alzheimer’s disease among adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan KD, Evans D, Pandey A, et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep 2017; 7: 14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamer A, Fortea J, Videla S, et al. Periodontal disease’s contribution to Alzheimer’s disease progression in Down syndrome. Alzheimer’s Dement (Amsterdam, Netherlands) 2016; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol 2002; 55: 929–37. [DOI] [PubMed] [Google Scholar]
- 43.Blesa R, Trias C, Fortea J, Videla S. Alzheimer’s disease in adults with Down syndrome: a challenge. T21RS Sci Soc Bull 2015. [Google Scholar]
- 44.Benejam B, Videla L, Vilaplana E, et al. Diagnosis of prodromal and Alzheimer’s disease dementia in adults with Down syndrome using neuropsychological tests. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12. DOI: 10.1002/dad2.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cody KA, Piro-Gambetti B, Zammit MD, et al. Association of sleep with cognition and beta amyloid accumulation in adults with Down syndrome. Neurobiol Aging 2020; 93: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dekker AD, Sacco S, Carfi A, et al. The Behavioral and Psychological Symptoms of Dementia in Down Syndrome (BPSD-DS) Scale: Comprehensive Assessment of Psychopathology in Down Syndrome. J Alzheimer’s Dis 2018; 63: 797–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giménez S, Videla L, Romero S, et al. Prevalence of Sleep Disorders in Adults With Down Syndrome: A Comparative Study of Self-Reported, Actigraphic, and Polysomnographic Findings. J Clin Sleep Med 2018; 14: 1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Handen BL, Lott IT, Christian BT, et al. The Alzheimer’s Biomarker Consortium-Down Syndrome: Rationale and methodology. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12. DOI: 10.1002/dad2.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Startin CM, Hamburg S, Hithersay R, et al. The LonDownS adult cognitive assessment to study cognitive abilities and decline in Down syndrome. Wellcome Open Res 2016; 1. DOI: 10.12688/wellcomeopenres.9961.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krinsky-McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer’s dementia in adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball S, Holland T, Huppert F, Treppner P, Dodd K. The CAMDEX-DS: the Cambridge Examination for Mental Disorders of Older People with Down syndrome and other intellectual disabilities. 2006. [Google Scholar]
- 52.Lautarescu BA, Holland AJ, Zaman SH. The Early Presentation of Dementia in People with Down Syndrome: a Systematic Review of Longitudinal Studies. Neuropsychol Rev 2017; 27: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinai A, Hassiotis A, Rantell K, Strydom A. Assessing specific cognitive deficits associated with dementia in older adults with down syndrome: Use and validity of the Arizona Cognitive Test Battery (ACTB). PLoS One 2016; 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edgin JO, Anand P, Rosser T, et al. The Arizona cognitive test battery for down syndrome: Test-retest reliability & practice effects. Am J Intellect Dev Disabil 2017; 122: 215–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartley SL, Handen BL, Devenny D, et al. Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer’s disease in Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beresford-Webb JA, Mak E, Grigorova M, Daffern SJ, Holland AJ, Zaman SH. Establishing diagnostic thresholds for Alzheimer’s disease in adults with Down syndrome: the Cambridge Examination for Mental Disorders of Older People with Down’s Syndrome and Others with Intellectual Disabilities (CAMDEX-DS). BJPsych Open 2021; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. [DOI] [PubMed] [Google Scholar]
- 58.Esbensen AJ, Hooper SR, Fidler D, et al. Outcome measures for clinical trials in down syndrome. Am J Intellect Dev Disabil 2017; 122: 247–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Firth NC, Startin CM, Hithersay R, et al. Aging related cognitive changes associated with Alzheimer’s disease in Down syndrome. Ann Clin Transl Neurol 2018; 5: 741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartley SL, Handen BL, Devenny D, et al. Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome. Neurobiol Aging 2017; 58: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hithersay R, Baksh RA, Startin CM, et al. Optimal age and outcome measures for Alzheimer’s disease prevention trials in people with Down syndrome. Alzheimer’s Dement 2020; : 1–10. [DOI] [PubMed] [Google Scholar]
- 62.Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer’s disease in Down syndrome. Alzheimer’s Dement 2019; 15: 245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pulsifer MB, Evans CL, Hom C, et al. Language skills as a predictor of cognitive decline in adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meeus M, Kenis S, Wojciechowski M, Ceulemans B. Epilepsy in children with Down syndrome: not so benign as generally accepted. Acta Neurol Belg 2015; 115: 569–73. [DOI] [PubMed] [Google Scholar]
- 65.Gholipour T, Mitchell S, Sarkis RA, Chemali Z. The clinical and neurobehavioral course of Down syndrome and dementia with or without new-onset epilepsy. Epilepsy Behav 2017; 68: 11–6. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y, Lavrencic L, Radford K, et al. Systematic review of coexistent epileptic seizures and Alzheimer’s disease: Incidence and prevalence. J Am Geriatr Soc 2021; Epub ahead. DOI: 10.1111/jgs.17101. [DOI] [PubMed] [Google Scholar]
- 67.Santoro JD, Pagarkar D, Chu DT, et al. Neurologic complications of Down syndrome: a systematic review. J Neurol 2020. DOI: 10.1007/s00415-020-10179-w. [DOI] [PubMed] [Google Scholar]
- 68.Lefter S, Costello DJ, McNamara B, Sweeney B. Clinical and EEG features of seizures in adults with down syndrome. J Clin Neurophysiol 2011; 28: 469–73. [DOI] [PubMed] [Google Scholar]
- 69.Altuna M, Giménez S, Fortea J. Epilepsy in Down Syndrome : A Highly Prevalent Comorbidity. J Clin Med 2021; 10: 2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lleó A, Cavedo E, Parnetti L, et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat Rev Neurol 2015; 11: 41–55. [DOI] [PubMed] [Google Scholar]
- 71.Dekker AD, Fortea J, Blesa R, De Deyn PP. Cerebrospinal fluid biomarkers for Alzheimer’s disease in Down syndrome. Alzheimer’s Dement (Amsterdam, Netherlands) 2017; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fagan A, Henson R, Li Y, et al. Comparison of CSF biomarkers in Down syndrome and autosomal dominant Alzheimer disease: a cross-sectional study. Lancet Neurol 2021; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belbin O, Xiao MF, Xu D, et al. Cerebrospinal fluid profile of NPTX2 supports role of Alzheimer’s disease-related inhibitory circuit dysfunction in adults with down syndrome. Mol Neurodegener 2020; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pentz R, Iulita MF, Ducatenzeiler A, et al. Nerve growth factor (NGF) pathway biomarkers in Down syndrome prior to and after the onset of clinical Alzheimer’s disease: A paired CSF and plasma study. Alzheimer’s Dement 2020; : 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer ‘ s disease. 2019; 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petersen ME, Rafii MS, Zhang F, et al. Plasma Total-Tau and Neurofilament Light Chain as Diagnostic Biomarkers of Alzheimer’s Disease Dementia and Mild Cognitive Impairment in Adults with Down Syndrome. J Alzheimers Dis 2021; 79: 671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carmona-Iragui M, Alcolea D, Al. E. Prognostic performance and longitudinal changes in plasma Neurofilament light levels in adults with Down syndrome: a multicentre longitudinal study. Lancet Neurol 2021; Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lleó A, Al E. Phosphorylated tau 181 in plasma as a biomarker for Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Nat Commun 2021; Accepted. [Google Scholar]
- 79.Schupf N, Patel B, Pang D, et al. Elevated plasma beta-amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol 2007; 64: 1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol 2016; 15: 673–84. [DOI] [PubMed] [Google Scholar]
- 81.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet (London, England) 2021; 6736. DOI: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alcolea D, Delaby C, Muñoz L, et al. Use of plasma biomarkers for AT(N) classification of neruodegenerative dementias. J Neurol Neurosurg Psychiatry 2021; Epub ahead. [DOI] [PubMed] [Google Scholar]
- 83.Neale N, Padilla C, Fonseca LM, Holland T, Zaman S. Neuroimaging and other modalities to assess Alzheimer’s disease in Down syndrome. NeuroImage Clin. 2018; 17: 263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol 2010; 9: 623–33. [DOI] [PubMed] [Google Scholar]
- 85.Annus T, Wilson LR, Acosta-Cabronero J, et al. The Down syndrome brain in the presence and absence of fibrillar β-amyloidosis. Neurobiol Aging 2017; 53: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen AD, McDade E, Christian B, et al. Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer’s disease from late-onset amyloid deposition. Alzheimer’s Dement 2018; 14: 743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villemagne VL, Ataka S, Mizuno T, et al. High striatal amyloid β-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol 2009; 66: 1537–44. [DOI] [PubMed] [Google Scholar]
- 88.Lao PJ, Brickman AM. Multimodal neuroimaging study of cerebrovascular disease, amyloid deposition, and neurodegeneration in Alzheimer’s disease progression. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2018; 10: 638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zammit MD, Laymon CM, Betthauser TJ, et al. Amyloid accumulation in Down syndrome measured with amyloid load. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matthews DC, Lukic AS, Andrews RD, et al. Dissociation of Down syndrome and Alzheimer’s disease effects with imaging. Alzheimer’s Dement Transl Res Clin Interv 2016; 2: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haier RJ, Alkire MT, White NS, et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology 2003; 61: 1673–9. [DOI] [PubMed] [Google Scholar]
- 92.Lengyel Z, Balogh E, Emri M, et al. Pattern of Increased Cerebral FDG Uptake in Down Syndrome Patients. Pediatr Neurol 2016; : 270–5. [DOI] [PubMed] [Google Scholar]
- 93.Tudorascu DL, Laymon CM, Zammit M, et al. Relationship of amyloid beta and neurofibrillary tau deposition in Neurodegeneration in Aging Down Syndrome (NiAD) study at baseline. Alzheimer’s Dement Transl Res Clin Interv 2020; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rafii MS, Lukic AS, Andrews RD, et al. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimer’s Dis 2017; 60: 439–50. [DOI] [PubMed] [Google Scholar]
- 95.Tudorascu DL, Anderson SJ, Minhas DS, et al. Comparison of longitudinal Aβ in nondemented elderly and Down syndrome. Neurobiol Aging 2019; 73: 171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lao PJ, Gutierrez J, Keator D, et al. Alzheimer-Related Cerebrovascular Disease in Down Syndrome. Ann Neurol 2020; 88: 1165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016; 79: 929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosas HD, Hsu E, Mercaldo ND, et al. Alzheimer-related altered white matter microstructural integrity in Down syndrome: A model for sporadic AD? Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson M, Muir K, Reddy D, Webster R, Kapoor C, Miller E. Prognostic accuracy of fetal MRI in predicting postnatal neurodevelopmental outcome. Am J Neuroradiol 2020; 41: 2146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bateman RJ, Xiong C, Benzinger T, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012; 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Englund H, Annerén G, Gustafsson J, et al. Increase in beta-amyloid levels in cerebrospinal fluid of children with Down syndrome. Dement Geriatr Cogn Disord 2007; 24: 369–74. [DOI] [PubMed] [Google Scholar]
- 102.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: A cross-sectional study. JAMA Neurol 2015; 72: 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDade E, Bateman RJ. Tau positron emission tomography in autosomal dominant Alzheimer disease small windows, big picture. JAMA Neurol 2018; 75: 536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamlett ED, Goetzl EJ, Ledreux A, et al. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimer’s Dement 2017; 13: 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sinai A, Mokrysz C, Bernal J, et al. Predictors of Age of Diagnosis and Survival of Alzheimer’s Disease in Down Syndrome. J Alzheimer’s Dis 2017; 61: 717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: A retrospective cohort study. Lancet Neurol 2011; 10: 213–20. [DOI] [PubMed] [Google Scholar]
- 107.Livingstone N, Hanratty J, Macdonald G, Mcshane R. Pharmacological interventions for cognitive decline in people with Down syndrome. Cochrane Database Syst Rev 2015; 2015: Art. No.: CD011546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanney M, Prasher V, Williams N, et al. Memantine for dementia in adults older than 40 years with Down’s syndrome (MEADOWS): a randomised, double-blind, placebo-controlled trial. Lancet; 379: 528–36. [DOI] [PubMed] [Google Scholar]
- 109.Godridge H, Reynolds GP, Czudek C, Calcutt NA, Benton M. Alzheimer-like neurotransmitter deficits in adult Down’s syndrome brain tissue. J Neurol Neurosurg Psychiatry 1987; 50: 775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strydom A, Coppus A, Blesa R, et al. Alzheimer’s disease in Down syndrome: An overlooked population for prevention trials. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018; 4: 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moulder KL, Snider BJ, Mills SL, et al. Dominantly inherited alzheimer network: Facilitating research and clinical trials. Alzheimer’s Res Ther 2013; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.U.S. Department of Health and Human Services, (FDA) F and DA, (CDER) C for DE and R, (CBER) C for BE and R. Early Alzheimer’s Disease: Developing Drugs For Treatment, Guidelines for Industry. 2018 https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf (2018).
- 113.Rafii MS, Ances BM, Schupf N, et al. The AT(N) framework for Alzheimer’s disease in adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol 2020; 19: 513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.