Abstract
Mapping human brain function is a longstanding goal of neuroscience that promises to inform the development of new treatments for brain disorders. Early maps of human brain function were based on locations of brain damage or brain stimulation that caused a change in function. Over time, this approach was largely replaced by technologies such as functional neuroimaging, which identify brain regions correlated with behaviors or symptoms. Despite their advantages, these technologies reveal correlation, not causation. This creates challenges for interpreting the data generated from these tools and using them to develop treatments for brain disorders. A return to causal mapping of human brain function based on brain lesions and brain stimulation is underway. New approaches can combine these causal sources of information with modern neuroimaging and electrophysiology techniques to gain new insights into the functions of specific brain areas. In this Review, we provide a definition of causality for translational research, propose a continuum along which to assess the relative strength of causal information from human brain mapping studies, and discuss recent advances in causal brain mapping and their relevance for developing treatments.
Introduction
Human brain mapping studies often rely on neurophysiological correlates of symptoms or behavior1–4. This approach has yielded a wealth of knowledge about brain organization5–8, but this knowledge has not routinely translated into therapeutic targets3,9–11. The difference between correlation and causation may be an important reason for this lack of translation12,13. Correlative methods do not distinguish between brain regions that are causing a symptom, compensating for a symptom, or incidentally related to a symptom. As such, interventions based on neurophysiological correlates could improve a symptom, worsen a symptom, or have no effect.
This “causality gap” is a well-recognized problem in human neuroscience10,13,14, and echoes a problem that other disciplines have also faced. For instance, the field of econometrics experienced a “credibility revolution”15 after an influential push to use causal methods in the early 1980s16. By emulating this example, the causality gap in neuroscience may be better framed as a causality opportunity. Wider adoption of causal methods could improve our ability to translate brain mapping findings into successful interventions13,14,17,18. Causal mapping studies using brain lesions and brain stimulation helped lead to mainstream clinical treatments such as deep brain stimulation (DBS) for Parkinson disease19–22 and transcranial magnetic stimulation (TMS) for major depression23,24. These successes may provide a road map for identifying new therapeutic targets based on causal mapping of human brain function.
In this Review, we provide a clinically-relevant definition of causality, summarize different approaches to causal brain mapping, propose a framework for appraising the strength of causality in brain mapping studies, and describe how these studies can be translated into anatomically-targeted treatments for neuropsychiatric disorders.
Brain mapping and causal inference
A brief history of human brain mapping
Early brain maps were based on lesions or stimulation sites that directly caused a functional change, ranging from influential lesion cases such as Phineas Gage25 and H.M.26 to systematic experiments such as Wilder Penfield’s intracranial stimulation studies27–29. However, in the latter part of the 20th century, the advent of functional neuroimaging1,2,4,30 was accompanied by a decline in lesion and stimulation studies (Fig. 1)31. Neuroimaging allowed for noninvasive mapping of human brain function in patients without lesions, including healthy subjects. Neuroimaging correlates often aligned with prior lesion and stimulation results32, and provided additional insights about complex functions such as language and memory33,34.
Fig. 1 |. Evolution of brain mapping literature since 1950.

In the late 20th century, the growth of neuroimaging literature coincided with a decline in brain lesion and brain stimulation literature. The data plotted on the figure were obtained by conducting a Google Ngram search using the terms ‘neuroimaging,’ ‘brain stimulation,’ and ‘brain lesion’31, with the data binned to show between 1950–2019 and the smoothing set to 5.
Nevertheless, this new capability came with a cost, as these correlative tools moved away from the causal inference that was possible with brain lesions and brain stimulation9,14,17,18,30. Some saw this as a minor limitation35, but others criticized functional neuroimaging as “the new phrenology”36. More importantly, despite its popularity, functional neuroimaging was not leading to the development of new clinical treatments14,17,18. This led to essays highlighting the causality gap in human neuroscience14,17 and the need to combine functional neuroimaging with causal techniques17,18.
A brief history of causal inference
The history of causal inference has largely focused on addressing one fundamental problem: to conclude that an intervention caused an outcome, one must determine what would have happened if the intervention had been absent37,38. The counterfactual, or what would happen if an activity had been different, is unobservable by definition. Therefore, definitive causal inference is impossible. Instead, researchers make careful assumptions to estimate the counterfactual by studying the effects of a perturbation, such as a focal disruption of brain activity10,39.
The value of understanding causality was recognized by philosophers as early as Aristotle, who believed that true knowledge is unattainable without asking “why”40,41. Aristotle’s tradition inspired medieval theologians such as the Islamic physician–philosopher ibn Sina and the Catholic priest Thomas Aquinas42. Both ibn Sina and Aquinas reasoned that any causal chain either can be traced infinitely or should end in a primary cause, which Aquinas labeled as “God”42. To explore the concept of free will, ibn Sina also described secondary causes which occur further down the chain set in place by God. As a physician, ibn Sina emphasized the need to modify outcomes by manipulating these secondary causes43.
Philosophers debated this topic for centuries until David Hume codified eight logical “rules” for causal inference in 174844. This early attempt inspired subsequent systems that were concrete enough to apply scientifically. For instance, Robert Koch’s microbiological “postulates” were successfully used to identify the etiology of tuberculosis and cholera45. More recently, Austin Bradford-Hill’s epidemiological “criteria” revealed many important causal relationships, such as the link between smoking and lung cancer46–48.
The Bradford-Hill criteria include temporality (the only mandatory criterion), specificity, effect size, reproducibility, dose-response, physiological plausibility, experimental manipulation, analogy, reversibility (an optional criterion), and coherence at different levels. The link between smoking and lung cancer exemplifies how these criteria can be used to establish a causal relationship: smoking consistently precedes lung cancer (temporality); lung cancer increases specifically after smoking, but not after related exposures such as chewing tobacco and alcohol (specificity); smoking has a large effect on the risk of lung cancer (effect size); the relationship between smoking and lung cancer is consistent across different settings (reproducibility); more smoking leads to greater lung cancer risk (dose-response); smoking causes single-strand DNA breaks, which predispose to neoplasia (physiological plausibility); in animal models, smoking increases cancer risk more than control interventions (experimental manipulation); the effects of smoking are similar to those of other known carcinogens (analogy); some smoking-induced changes are reversible upon cessation of smoking (reversibility); and these criteria are consistently satisfied by in vitro studies, animal studies, human studies, and epidemiological studies (coherence).
Causal inference in biomedical science
The medical sciences did not explicitly focus on causality until ibn Sina’s vision of controlled clinical trials49 was finally realized in the mid-20th century50. Randomized trials changed the face of clinical therapeutics because they established causality by minimizing confounders, as the control group is identical to the intervention group in nearly every way except for the intervention itself. Randomized controlled trials thus became the gold standard for estimating the counterfactual by modeling a situation in which the intervention was absent50.
However, before designing a clinical trial, one must identify components of the causal chain as potential treatment targets51 — otherwise, there is a risk of intervening based on misleading associations52. For instance, tuberculosis rates correlate with malaria rates, body temperature, mycobacterial infection, and travel to endemic countries53. This might lead to the incorrect conclusion that tuberculosis could be treated with antimalarials or antipyretics. In reality, the correlation with malaria is spurious, as both diseases are endemic to lower-income countries, and the correlation with body temperature is due to reverse causation, as tuberculosis causes fever. By contrast, mycobacterial infection is causal, as the counterfactual has been demonstrated with confidence using Koch’s postulates54. Travel to endemic countries is also in the causal chain of events, but only because it increases the risk of mycobacterial infection. Thus, before the first randomized clinical trial evaluated antimycobacterial therapy for tuberculosis, the likely outcome was already well-understood50.
When experimental manipulation in a randomized controlled trial is impractical or unethical, causality can still be assessed observationally using systematic approaches such as the Bradford-Hill criteria55. Importantly, these observational approaches do not necessarily rule out all unmeasured confounders. For instance, it is plausible that the link between smoking and lung cancer is explained by an unobserved genetic factor that independently increases the risk for both. Econometricians often overcome this limitation by identifying natural experiments, in which an intervention is applied in a manner that is practically random. For instance, if lung cancer rates decline consistently in different districts that raise tobacco taxes, this effect is unlikely to be mediated by an unobserved genetic factor. Accordingly, natural experiments can be used to infer causality56,57.
Causal inference in human brain mapping
Human brain mapping researchers often attempt causal inference to achieve one of two objectives. This Review focuses on the objective of symptom localization, which aims to identify causal links between symptoms and neuroanatomy58. When a symptom is successfully localized, it may potentially be treated by modulating the corresponding neuroanatomy. The second objective, mapping the direction of information flow, aims to understand how one brain region can causally influence another. These experiments attempt to estimate the direction of information flow between two or more nodes in the brain using various measures of effective connectivity10. Different methods for symptom localization are summarized in Box 1, while different methods for measuring effective connectivity are summarized in Box 2.
Box 1 |. Neuroimaging techniques for mapping symptoms or behaviors.
Some symptom localization approaches can yield stronger causal inference than others (Fig. 2). Of note, many correlative techniques can provide useful complementary information when combined with causal techniques174.
Task-free neuroimaging
Task-free neuroimaging measures the brain at rest. Blood flow can be measured using positron emission tomography2 or arterial spin labeling175, connectivity can be estimated using resting-state functional MRI (fMRI) and diffusion tensor imaging2, structure can be measured using volumetric MRI57 and electrical activity can be measured using electroencephalography (EEG) or magnetoencephalography176. These measurements can be compared with clinical outcomes to identify neuroanatomical correlates of a behavior. Although task-free neuroimaging does not satisfy any of the criteria for causal inference29,45, it can be combined with causal techniques to better understand the circuit-based mechanisms of a causal relationship55. Task-free neuroimaging can also be used to reliably map brain organization, potentially revealing targets for causal manipulation174.
Task-based neuroimaging
Task-based neuroimaging measures the functional neuroanatomical correlates of a behavioral task. Studies using this approach estimate the counterfactual by comparing an experimental task to carefully designed control tasks. However, task-based imaging studies are rarely used to argue for causality owing to the temporal direction of the experiment — rather than manipulating brain activity and observing the resulting behavior, these studies manipulate the behavior and observe the resulting brain activity3.
Neurofeedback
Neurofeedback is a variant of task-based neuroimaging in which a participant consciously manipulates their own task-based brain activity. As such, this technique modifies brain activity and measures a change in behavior, which allows for stronger causal inference than traditional task-based neuroimaging, which modifies behavior and measures changes in brain activity177,178.
Incidental atrophy
Incidental atrophy patterns can be compared to behavioral outcomes, potentially illustrating specificity (assuming that the atrophy occurs in a near-random pattern) and dose-response (if greater atrophy leads to greater symptom severity). However, temporality is difficult to assess because atrophy progresses slowly. It can be unclear whether the atrophy caused the symptom, the symptom caused the atrophy, or both were caused by an unmeasured variable. For instance, substantia nigra atrophy is associated with anosmia because both phenomena can be caused by Parkinson disease179.
Incidental lesions
Incidental lesions can illustrate specificity and dose-response in the same way as incidental atrophy. Furthermore, because lesions occur rapidly, their temporal effects are usually clear. Some lesions are even reversible, as in multiple sclerosis. Thus, lesion studies can provide reasonably strong causal inference3,10,30,58,63. However, because lesions occur in an uncontrolled setting, it is difficult to apply experimental manipulations or estimate the effect of unmeasured pre-lesion factors3,17,29. Lesions can also be challenging to interpret because of diaschisis or downstream effects on distantly connected brain regions147.
Incidental stimulation sites
Incidental stimulation sites exist because therapeutic brain stimulation is often applied imprecisely to approximate targets. For example, TMS is usually targeted using scalp landmarks, leading to incidental variability in the stimulation site on the brain. When the actual stimulation sites are localized retrospectively, near-random variability is revealed in the precise region that is stimulated in any given patient84,95. Thus, the counterfactual can be estimated by comparing the post-treatment state to the pre-treatment state, while specificity can be demonstrated by comparing effective to ineffective stimulation sites. However, this approach still relies on incidental variance (a natural experiment) rather than prospective experimental manipulation, and thus may be subject to unmeasured confounders.
Targeted lesions
Targeted lesions are often created surgically as a therapeutic intervention. In addition to the insights gleaned from incidental lesions, prospectively targeted lesions can be experimentally manipulated, although this is rare given that the procedure is invasive and generally irreversible180,181. This enables strong causal inference, but is currently limited by the inability to assess reversibility.
Targeted stimulation
Targeted stimulation has many of the same advantages as targeted lesions, with the added benefit of reversibility. It also has the same advantages as incidental stimulation sites, with the added benefit of enabling precise experimental manipulation. Thus, targeted stimulation provides data about nearly all of the criteria on the causality continuum10,14,17,18. However, there are often assumptions about the effect of stimulation on neural function that may not be accurate (for example, excitation versus inhibition). Furthermore, to illustrate coherence, targeted stimulation must be complemented with other techniques.
Convergent causal mapping
Convergent causal mapping incorporates multiple causal approaches into a single analysis. For instance, if a symptom is caused by lesioning a specific circuit and the same symptom is relieved by stimulating the same circuit, this cross-modal coherence enables stronger causal inference than either modality alone.
Box 2 |. Mapping causal interactions between brain regions.
Measuring effective connectivity using brain stimulation
Rather than assessing how certain brain regions causally influence specific behaviors, some studies assess how certain brain regions causally influence other brain regions. Causality can be inferred by combining focal stimulation with distant real-time physiological recordings174. For instance, TMS or DBS can be paired with immediate fMRI or EEG. These combinations are high on the causality continuum, as they employ targeted stimulation.
These real-time experiments investigate effective connectivity, which measures the direction of information flow in a brain circuit. If the experiments are rigorously controlled, then alternative and sham stimulation conditions can be used to estimate the counterfactual174. For instance, applying excitatory TMS versus inhibitory TMS to the default mode network (DMN) leads to dissociable effects on DMN connectivity136,143. Similarly, applying excitatory TMS to the frontoparietal control/central executive network (FPCN) can induce immediate changes in connectivity to the default mode network (DMN), while inhibiting the FPCN or stimulating other networks does not induce the same changes137. This capability is consistent across modalities, as intracranial electrical stimulation elicits distinct electrophysiological responses when stimulating the DMN versus the FPCN or the salience network182. This principle of using alternate stimulation targets to estimate the counterfactual also extends to other networks, as TMS to the dorsal attention network versus the DMN can induce network-specific EEG changes138. Collectively, these findings also suggest that TMS may be able to indirectly modify deeper brain regions that cannot be accessed directly.
Effective connectivity can provide mechanistic insights about direct or indirect relationships between sites of interest, and possible directionality and/or timing of signal flow between them. However, it does not offer causal inference about the importance of any of the sites for specific behaviors or functional outcomes183.
Indirectly estimating connectivity
Effective connectivity may be estimated indirectly based on temporal relationships between activity in different brain regions10. These temporal forecasting methods are particularly useful for data with high temporal precision, such as intracranial measurements of local field potentials at the neuronal level184. The most common temporal forecasting approaches are Granger causality and dynamic causal modeling (DCM). Granger causality, which was originally designed for economic time series analysis, quantifies the directional relationship between two variables based on a temporal delay in their activity185. DCM uses a similar principle, but also incorporates multiple regions into a probabilistic graphical model allowing for different hypotheses to be specified by varying its nodes and directed edges. The downstream effects of an incidental fluctuation or behavioral task can then be estimated by selecting the simplest explanatory model186.
These indirect approaches provide weaker causal inference than brain stimulation experiments, as temporal forecasting is not immune to spurious relationships10,187,188. Countless unmeasured confounders could cause a temporal delay189, particularly for techniques with lower temporal resolution such as functional neuroimaging10. Nevertheless, Granger causality and DCM are often misunderstood as tools with strong causal inference, possibly due to misleading nomenclature190,191.
The relationship between brain regions can also be estimated using task-free neuroimaging techniques such as resting-state functional connectivity and diffusion tractography. These approaches are low on the causality continuum, and provide no information about the causal direction of information flow.
For any of these methods to provide causal knowledge, the study must be designed to estimate what would have happened if the intervention had been different40. When direct experimental manipulation is not possible, this may still be accomplished by regressing out potential confounders, fitting progressively larger models that contain the interactions, or estimating how different factors influence the probability of an outcome59. These approaches differ in how they estimate the counterfactual, but can be combined into a single axiomatic definition for causality: an event is causal if, with all else being equal, its presence or absence affects the probability of an outcome10,37,60. Even if the cause is not necessary or sufficient to induce the effect, this probabilistic definition can still reveal treatment targets. For instance, smoking does not always cause lung cancer and lung cancer is not always caused by smoking, but smoking singlehandedly increases the risk of lung cancer. Following this definition, we can reduce the risk of lung cancer with interventions that reduce smoking.
Although the fundamental goal of this approach is to estimate the counterfactual, this is sometimes not enough to clearly demonstrate causality. For instance, task-based fMRI experiments often compare the effects of a task to control conditions that estimate what would have happened if the task were not conducted. However, for the reasons described in Box 1, these experiments are rarely used to argue for causality.
In addition to estimating the counterfactual, causal inference in brain mapping may be assessed by borrowing criteria from Bradford-Hill’s framework61,62 (Fig. 2). Of these criteria, six are particularly useful for assessing the strength of causal inference across different human brain mapping studies: specificity, experimental manipulation, dose-response, coherence/convergence, reversibility, and temporality. Other criteria including effect size, reproducibility, plausibility, and analogy can be studied using any method, and thus are less useful for comparing the strength of causal inference across brain mapping studies.
Fig. 2 |. Appraising causality in human brain mapping studies.
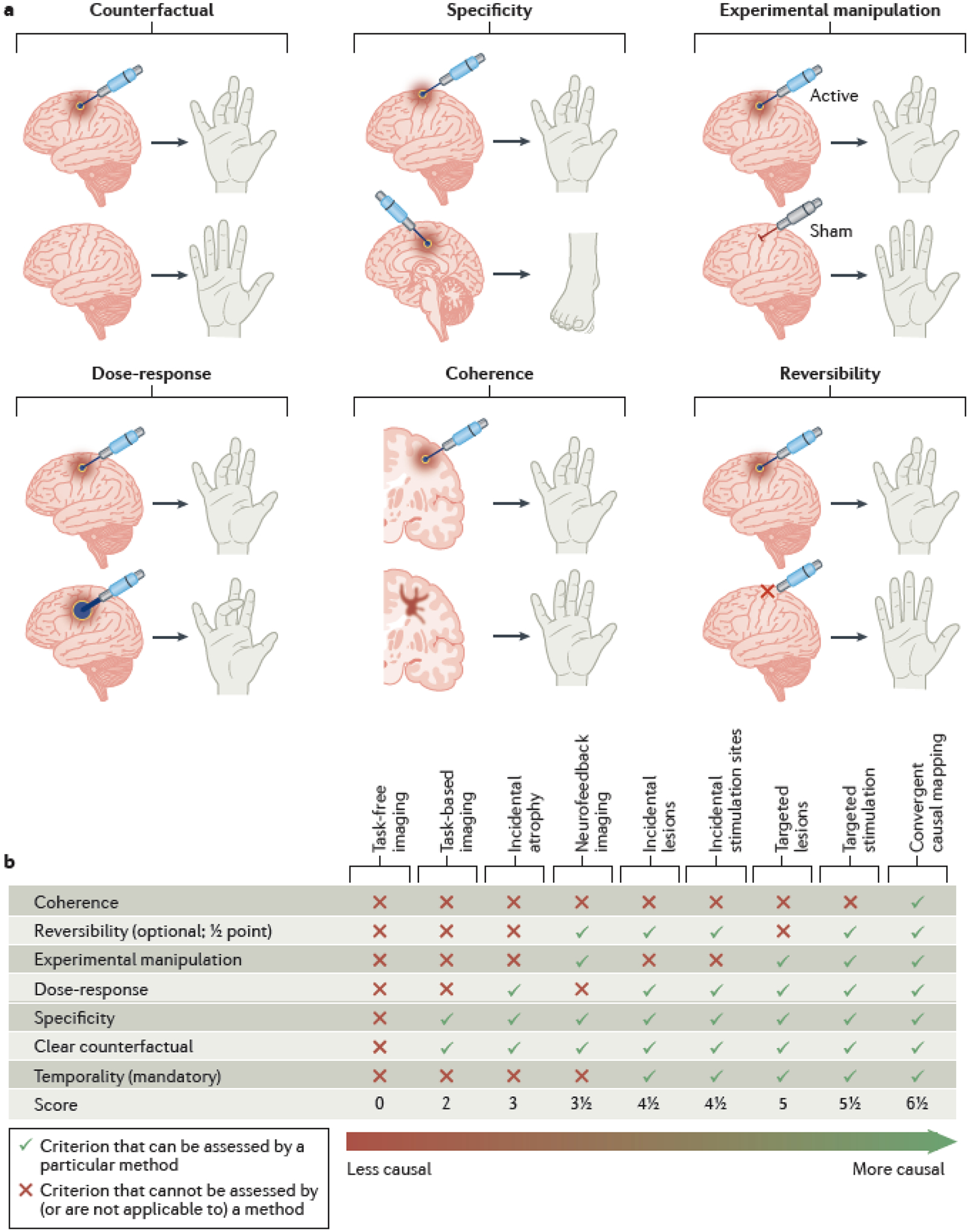
a | Six criteria for appraising causality in human brain mapping studies, adapted from the Bradford-Hill criteria. Intracranial electrical stimulation is used to illustrate each of these criteria. A counterfactual can be estimated by modeling a hypothetical situation in which brain activity was not modulated or was modulated differently. Specificity can be demonstrated when slightly different interventions induce measurably different outcomes. For instance, stimulating different parts of the motor cortex leads to target-specific effects on motor function. An experimental manipulation can be used to selectively modulate activity in a specific brain region compared to a control intervention (in this case a sham). A dose-response relationship is evident when higher-intensity stimulation induces a higher intensity outcome, in this case a more intense muscle contraction. Coherence can be demonstrated if different approaches converge on similar results. For instance, if stimulating a location induces a finger movement, then a lesion at the same location should induce finger weakness. Reversibility can be illustrated by reversal of the behavior when the modification of brain function is discontinued. b | Our proposed causality continuum in human brain mapping. An example of how different brain mapping techniques may be appraised using criteria adapted from the Bradford-Hill criteria, ordered from least causal to most causal along a causality continuum. Targeted brain lesions and brain stimulation satisfy more causality criteria than other brain mapping techniques (described in Box 1 and Box 2).
When such criteria are applied rigorously, causal inference from observational data can approach the strength of causal inference from randomized controlled trials57. However, there is no established system for estimating the strength of causal inference from human brain mapping data. Building on Hume’s framework and the many systematic approaches that it inspired, we suggest a ‘causality continuum’ for human brain mapping (Fig. 2b). While it is impossible to conclusively determine the causal role of an intervention, this continuum may be applied to score the relative strength of causality for different brain mapping techniques.
Conceptual causal mapping frameworks
Among different human brain mapping approaches, brain lesions and brain stimulation are the furthest along the causality continuum, assuming that a study using one of these approaches is rigorously designed to estimate the counterfactual and to demonstrate specificity10,18. However, the number of possible lesions or stimulation sites is essentially infinite, as is the number of possible behavioral outcomes, making it impractical to prospectively disentangle the behavioral effects of every single brain region from all other brain regions30. This practical challenge can be overcome by identifying natural experiments with incidental or near-random variance in the location of the lesion or the stimulation site. Using large datasets, it is possible to test for anatomical specificity by comparing hundreds of incidental lesions or stimulation sites across the brain63. This can yield a large-scale map of brain regions associated with certain lesion-induced symptoms64,65 or stimulation-induced responses66. This is commonly achieved by comparing the locations of lesion or stimulation locations that modify a symptom, by comparing the connectivity of these lesions or stimulation sites, or by modifying symptoms in real-time with direct stimulation and recording.
Mapping near-random brain lesions
Most early lesion and stimulation studies were based on case reports or small case series in which a lesion or a stimulation was sufficient to cause a certain outcome. These studies provided useful knowledge about the neuroanatomy of clearly measurable functions, such as sensation, movement, and vision. However, some higher-order functions are difficult to study using individual cases, as stimulation of higher association areas does not always induce a measurable response67. This limitation can be mitigated using a probabilistic approach to causality, in which it is determined how a lesion or a stimulation modifies the probability of the outcome63. The probabilistic approach still lends itself to identifying therapeutic targets, as a treatment that reduces the probability of a symptom can still be clinically useful37.
In a probabilistic study, large datasets are used to compare the effects of multiple lesions or stimulation sites. This was first illustrated with voxel lesion symptom mapping (VLSM), which compares symptom-causing lesions with other lesions63. For example, in Vietnam Veterans with penetrating brain injury, lesions to the amygdala and anterior temporal lobe reduced the risk of posttraumatic stress disorder68, while lesions to the posterior temporal lobe increased the risk of anxiety69. The probabilistic approach used in VLSM has also been expanded to brain stimulation studies — for instance, in patients receiving prefrontal TMS for major depression, relatively anterior and lateral stimulation sites are more effective than relatively posterior and medial sites70–72. However, the statistical power of VLSM is limited because most lesions and stimulation sites do not overlap with one another (Fig. 3a)58, so large sample sizes are required to detect statistical associations at any given brain location. VLSM also does not directly account for the role of diaschisis and inter-connected brain networks30,58, although network-level relationships may become apparent with sufficient spatial coverage and sample size.
Fig. 3 |. Heteogeneous lesions causing the same symptom can complicate causal inference.

a | If lesions causing the same symptom (but not other lesions) intersect a common brain region (area of overlap for lesions 1–3; outlined in red), one can infer a causal role of this region in symptom generation. If other lesions causing the same symptom fail to intersect this brain region (lesions 4–6), this causal inference becomes weaker. b | If lesion locations causing the same symptom (but not any other lesions) are connected to a common hub region (lesions 1–6), then the connectivity with this hub region defines a brain network (orange) that encompasses all lesion locations causing the symptom. One can then infer a causal role of this brain network in symptom generation. Note that the hub region is not necessarily causally linked to the symptom, but rather defines a network that is causally linked to the symptom.
Localizing symptoms to brain circuits
When similar symptoms are induced by non-overlapping lesions in different patients, it can be challenging to localize the symptom to specific neuroanatomy (Fig. 3a). These individual differences may be explained by damage to a common network with different components that have been affected across individuals67,73–84 — in other words, lesions in different locations caused the same symptom because they intersected the same brain circuit58.
To test for localization to brain circuits, lesion and stimulation data can be combined with circuit maps derived from brain imaging or electrophysiology data (Box 3). This approach has been used widely in studies that employ lesion network mapping (LNM) and related techniques, which use correlative neuroimaging to explain heterogeneous causal findings58. Rather than simply comparing lesion locations, LNM first identifies the circuit connected to each lesion and then compares the circuits (Fig. 3b), as lesions in different brain regions may cause a similar symptom if they damage the same brain circuit58. In this manner, correlative neuroimaging can reveal a mechanistic connection between seemingly discordant causal results.
Box 3 |. Circuit mapping approaches that may be combined with causal techniques.
Several imaging techniques can provide reliable information about the organization of brain circuits. These approaches can be combined with causal information from lesions or brain stimulation sites to map effects to brain circuits. Each brain mapping technique has different strengths and limitations:
Structural connectivity
Diffusion MRI can measure the locations of white matter tracts, enabling us to study the effects of stimulating different parts of the same tract86,90,97,110. This provides high spatial resolution, but is unable to map polysynaptic relationships or crossing white matter fibers.
Functional connectivity
This approach estimates connectivity by measuring correlations between spontaneous fluctuations of distant brain regions, usually measured with fMRI. Unlike diffusion MRI, this technique can estimate the effects of a stimulation site on whole-brain polysynaptic networks75,76. However, it is limited by the assumption that temporal correlation implies a connection between different brain regions.
Normative connectivity
Rather than measuring structural or functional connectivity in individuals, it is possible to use a large connectome database to estimate the whole-brain connectivity of a particular brain region. This technique provides greater reliability at the expense of mapping inter-individual variability in brain architecture58.
Surface electrophysiology
Electroencephalography (EEG) or magnetoencephalography (MEG) can be used to measure the immediate electrical effects of stimulating distant brain regions, providing high temporal precision14. These techniques enable the study of the rapid propagation of the stimulation to the rest of the brain, but are limited by poor spatial resolution.
Intracranial electrophysiology
Owing to greater anatomical precision than surface EEG, intracranial EEG (iEEG) enables precise mapping of specific behaviors. However, these tools are invasive and are usually only practical in specialized clinical centres where patients are evaluated for surgical treatment of refractory epilepsy66.
Other approaches
In theory, it may be possible to define a priori circuits using any neuroimaging approach, including task fMRI, effective connectivity, regional cerebral blood flow, or volumetric imaging. These approaches have not yet been widely used to map the circuits connected to specific lesions or stimulation sites, so methodological validation is still needed.
Because LNM maps the whole-brain connectivity of each lesion, every patient has data for every voxel in the brain, providing LNM with more statistical power than VLSM. LNM also quantifies the degree to which a lesion or stimulation site overlaps with different circuits, thus uncovering dose-response relationships85. Many recent studies have illustrated the potential of using normative brain connectivity to detect a causal link between lesions or stimulation sites and different clinical outcomes, even when VLSM shows no significant overlap. For instance, brain lesions are more likely to cause amnesia if they are connected to the subiculum85. DBS sites are more likely to relieve motor symptoms of Parkinson’s disease if they are connected to the supplementary motor area86. TMS sites are more likely to relieve depression if they are functionally anti-correlated to the subgenual cingulate72. Similar approaches have now been used to map the causal neuroanatomy of movement disorders86–91, mood disorders71,90,92–95, anxiety-related disorders96–98, psychotic disorders99–101, disorders of consciousness102–104, and various other neuropsychiatric phenomena105–109.
Of note, most LNM studies define the circuit using a normative connectome database, which acts as a ‘wiring diagram’ that depicts the expected connectivity profile of any lesion location. This wiring diagram is based on resting-state functional connectivity or diffusion tractography data from a large cohort of participants without brain lesions or stimulation. Ongoing work is underway to compare wiring diagrams generated by different methods, including structural versus functional connectivity110,111 and normative versus individualized connectivity75,76, each of which may independently add value75,76,111.
There are also some disadvantages to studying lesions and stimulation sites at the circuit level. These approaches can increase the risk of erroneous causal reasoning by adding an extra layer of complexity. If a brain lesion causes a particular symptom, it is reasonable to infer that the lesion location is causally involved in the symptom. If different lesion locations causing the same symptom are connected to one common hub, it is tempting to infer a causal relationship between the symptom and the hub. However, the hub simply defines the connectivity network that encompasses the lesion locations that cause a symptom. In other words, the causal inference is for the network, not the hub that defines the network. The hub may represent an important treatment target, but it is one step removed from the causal inference applied to the lesion locations themselves. One would need to independently study the effect of lesions or stimulation to the hub region to infer a causal link between the hub and the symptoms.
Real-time causal mapping
Another approach to causal inference addresses the temporality, experimental manipulation, and reversibility criteria by using real-time stimulation experiments in which different brain regions are focally stimulated to induce transient effects.
The most precise tool for real-time mapping of human brain function is intracranial electrical stimulation (iES) in conscious individuals66, although recent studies suggest that thermal stimulation may achieve similar effects112,113. These studies are usually conducted in patients who are implanted with multifocal electrode arrays for seizure monitoring before surgery for epilepsy. While these electrodes are implanted, investigators can use them to deliver focal stimulation and measure changes in a participant’s subjective experiential state or experimental task performance66,114. This method also enables simultaneous intracranial EEG (iEEG) recording during and after stimulation, yielding measures of effective connectivity. While iES does not provide whole-brain coverage in the same manner as LNM and other circuit-level analyses, one can still estimate the counterfactual by comparing clinical outcomes from thousands of possible stimulation sites across multiple patients67.
Although iES dates back to Penfield’s work in the mid-20th century, a recent renaissance in iES research has been accelerated by the development of neuroimaging and circuit-based techniques66. High resolution imaging methods can localize the stimulated brain areas with millimeter precision to calculate the extent of the brain targeted by a given electrical dose115.
Some recent studies provide examples of how iES-induced effects can be used to validate anatomical boundaries defined by other techniques such as functional neuroimaging or even animal models. For example, visual perception of faces is modified by stimulation of a patch of the posterior fusiform face area defined by task-based fMRI80. A will to persevere can be induced by stimulation of a hub in the anterior cingulate cortex defined by resting-state fMRI79. An altered sense of self can be induced by stimulation of part of the posteromedial cortex implicated in self-referential thought from task-based fMRI116. Furthermore, the probability of observing any behavioral response to stimulation aligns with brain network boundaries defined by resting state fMRI67. Finally, a dissociative state can be induced by stimulating the posterior cingulate cortex, a target identified based on an optogenetic mouse model of dissociation117. These studies illustrate how iES can add value to results from correlative neuroimaging experiments or studies in animal models, potentially facilitating their translation into clinical treatments.
Clinical causal brain mapping
Optimizing brain stimulation targets
Causal research is uniquely valuable in medicine because of its potential to identify targets for clinical intervention. If a particular factor is causal, it affects the probability of an outcome with all else being equal. Therefore, modifying that factor should modify the probability of that outcome. In clinical neuroscience, this principle can be tested using existing data in patients who received therapeutic TMS and DBS. If a particular brain region is causally implicated in a symptom, then stimulating that region should modify the probability or severity of that symptom. For instance, TMS can be used to localize the language centres in individual patients by studying the effects of targeted inhibition on speech function118,119. This approach can be used by neurosurgeons to avoid damaging language function during surgery120.
Furthermore, circuit mapping studies have used connectivity to gain insight into the most effective TMS and DBS targets. In major depression, the most effective TMS sites are functionally anti-correlated to the subgenual cingulate cortex71,72,94, while the most effective DBS sites overlap with tracts that connect the subgenual cingulate to the orbitofrontal cortex and the limbic system121,122. In Parkinson disease, the most effective DBS sites for motor symptoms are structurally connected to the supplementary motor area and functionally anti-correlated to the primary motor cortex86. In obsessive-compulsive disorder, the most effective DBS sites overlap with a tract that connects the subthalamic nucleus to the prefrontal cortex96,97 and are functionally connected to the anterior cingulate, insula, and precuneus123. In each case, stimulation sites might be refined to better target the therapeutic connections or to potentially avoid connections associated with DBS side effects90. Whether this improves clinical outcomes remains to be validated prospectively.
Beyond optimizing treatment targets for any given disorder, causal circuit mapping approaches have been used to disentangle which symptom clusters respond to different stimulation sites. In two cohorts of patients with major depression, TMS sites connected to a particular circuit were more likely to improve “dysphoric” symptoms such as sadness and suicidality, while TMS sites connected to a different circuit were more likely to improve “anxiosomatic” symptoms such as insomnia and sexual dysfunction98. Disentangling symptom clusters in this fashion lays the foundation for personalized target selection124 and highlights the need for deeper phenotyping, which may reveal more distinct treatment targets for distinct symptoms.
Identifying brain stimulation targets
This framework may be extended to identify new TMS and DBS targets based on lesions that modify different symptoms. If damage to a particular circuit can cause a symptom, it is reasonable to hypothesize that stimulation of the same circuit may relieve that symptom. In fact, a similar approach was used to identify some of the neuromodulation targets currently in clinical use. Early studies on incidental stroke lesions led to identification of the dorsolateral prefrontal cortex as an effective TMS target for depression24,125,126, while early findings on therapeutic lesioning led to identification of the ventral intermediate nucleus, subthalamic nucleus, and globus pallidus interna as effective DBS targets for Parkinson disease19,20,22,127.
Broadly, these successes suggest that brain lesions can inform treatment targeting with brain stimulation. The causal circuit mapping approach also illustrates coherence across multiple methods, a rarely met criterion on the causality continuum. For example, while LNM can help us detect a causal link between lesions and outcomes based on overlap with a network, the causal agent is still the lesion location, not the network hub (Fig. 3). To determine if the network hubs are causally implicated in the behavior, targeted stimulation could then be used, thereby meeting the coherence criterion. This postulate is illustrated by two recent examples of coherence between LNM and iES results. First, lesions connected to the dorsal cingulate can disrupt volitional will105, while iES to the dorsal cingulate can induce a will to persevere (Fig. 4a)79. Second, lesions connected to the right posterior fusiform gyrus can disrupt facial recognition107, while iES of the right posterior, but not the left or anterior, fusiform gyrus can distort face perception80,128,129 (Fig. 4b). These findings illustrate how stimulation and lesions can provide complementary information about the function of a network hub.
Fig. 4 |. Coherence between LNM and iES studies.
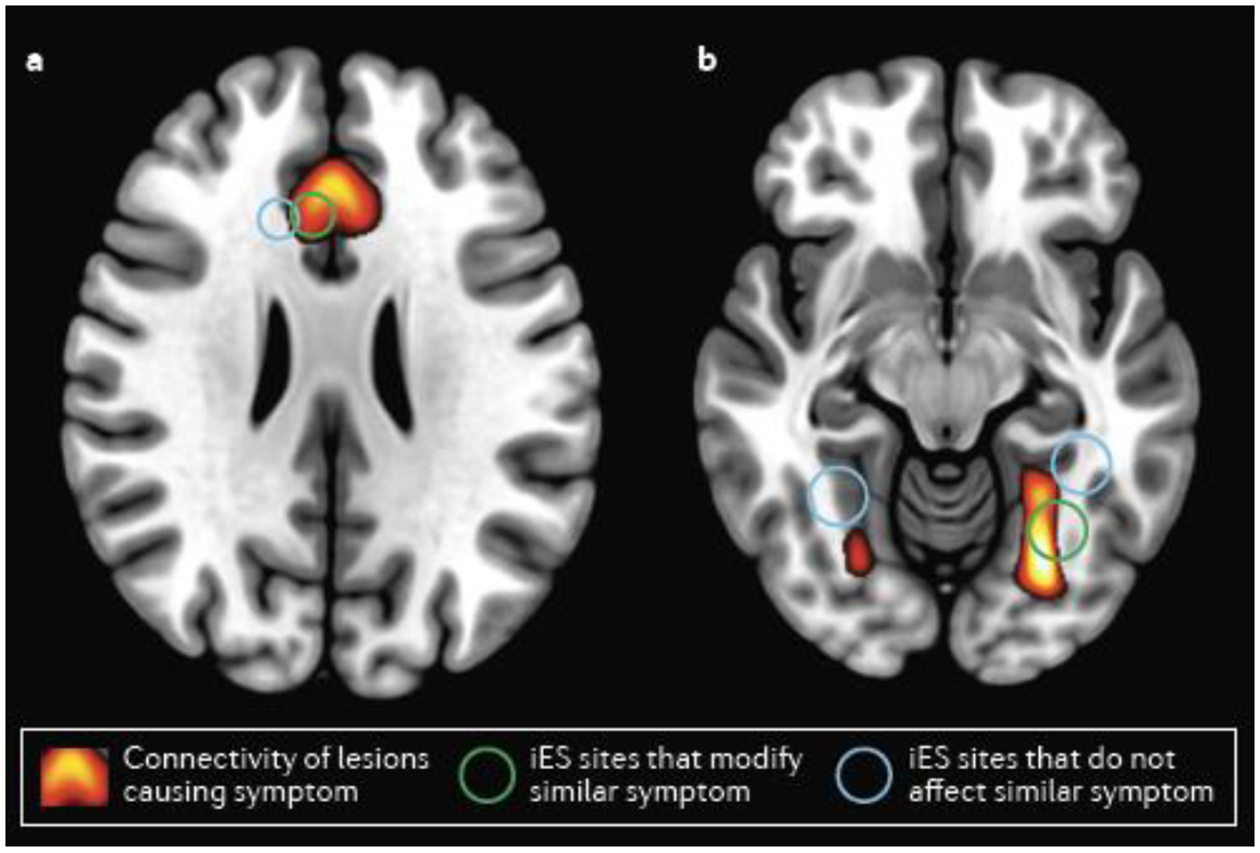
a | Lesion network mapping (LNM) revealed that lesions causing decreased will to perform actions are connected to the anterior cingulate (warm colors). Intracranial electrical stimulation (iES) sites overlapping with this network (green circle) can induce a will to persevere, unlike other nearby iES sites (white). b | LNM revealed that lesions causing disordered face perception are connected to the right posterior fusiform gyrus (FG, warm colors). iES to the right posterior FG (green circle), but not the right anterior FG or the left FG (white circles), causes distorted face perception.
A similar postulate suggests that different stimulation modalities can also provide complementary causal information. If a circuit is causally implicated in a symptom, then the symptom should be affected by different types of stimulation to that circuit. A review of published TMS and DBS targets explored this topic across 14 different disorders, finding that published TMS targets for various disorders were connected to the same brain regions as published DBS targets for the same disorders109. The authors thus hypothesized that better DBS targets may be identified based on the connectivity of effective TMS targets, and vice versa. If TMS and DBS sites converge on the same circuit, the strength of the causal inference linking this circuit to the symptom increases.
A recent study systematically tested this hypothesis by examining depression severity in 461 patients with brain lesions (five datasets), 151 patients receiving therapeutic TMS (four datasets), and 101 patients receiving therapeutic DBS (five datasets)95. Across all fourteen datasets, this analysis revealed a common circuit that was connected to the lesions that caused depression, the TMS sites that improved depression, and the DBS sites that worsened or improved depression (Fig. 5). Connectivity to this circuit also predicted the clinical efficacy of TMS and DBS sites in a leave-one-dataset-out cross-validation. Similar concordance was also observed for motor symptoms of Parkinson disease — lesions causing parkinsonism were connected to the same circuit as DBS sites that relieve parkinsonism, and both were connected to the primary motor cortex, the most common TMS target for parkinsonism95. Thus, the connectivity of brain lesions causing a symptom may reveal effective stimulation targets for the same symptom95. This provides a clear model for how convergent results can strengthen causal inference — if a circuit is causally linked to a symptom, then different manipulations of the circuit should modify the probability or severity of that symptom. Subsequent studies have expanded this principle to other symptoms and disorders, finding that lesion locations can predict which DBS targets are most likely to modify tics in Tourette disorder130 and cognitive symptoms in Parkinson disease131.
Fig. 5 |. Coherence between TMS, DBS, and brain lesions.
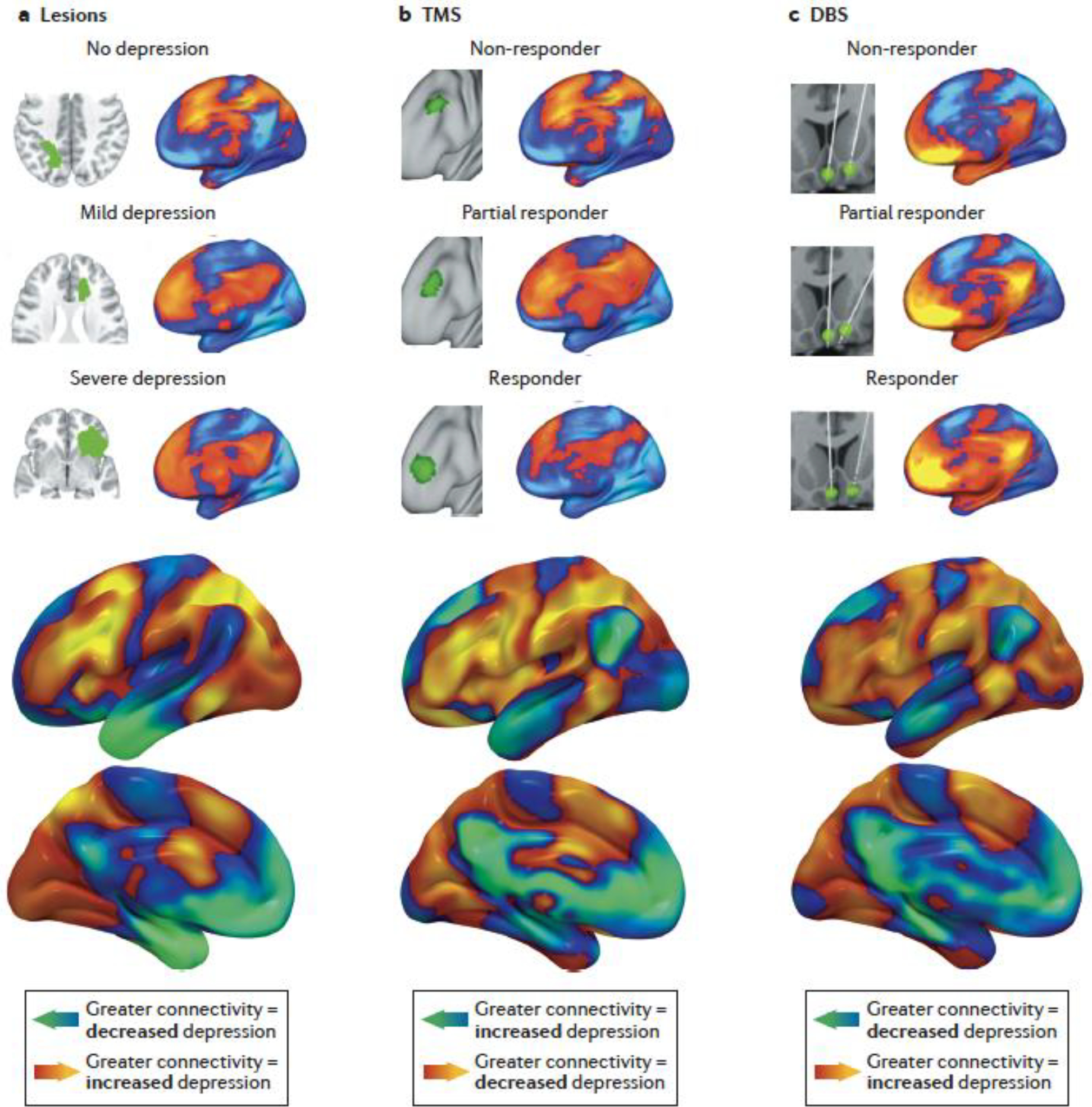
A common brain circuit was connected to brain lesions that cause depression, TMS sites that relieve depression, and DBS sites that modify depression. a | For 461 incidental brain lesions (top left), whole-brain connectivity was estimated using a normative connectome database (top right). This connectivity map was compared with depression severity, yielding a map of the whole-brain circuit connected to lesions that increase depression severity (bottom). b | The same procedure was conducted for 151 incidentally-variable TMS sites, yielding a map of the whole-brain circuit connected to TMS sites that improve depression. The color scale was inverted for this map because TMS sites that improve depression were expected to be anti-correlated to lesion locations associated with lower depression severity109. c | The same procedure was also conducted for 101 DBS sites, yielding a map of the whole-brain circuit connected to DBS sites that modify depression. These three maps were significantly more similar than expected by chance. Adapted with permission from REF. 95.
Roadmap for future research
Integrating correlative and causal maps
Beyond LNM, which directly combines correlative and causal techniques into a single analysis, there are several ways to test for coherence (or lack thereof) between correlative and causal techniques.
This principle was nicely illustrated in two recent studies, in which task-based iEEG (causality score = 2) was used to identify a medial-lateral dissociation between simple tone perception and complex speech perception. These correlative results were then used to guide a causal manipulation (iES, causality score = 5.5), revealing that medial sites induced hallucinations of simple sounds, while lateral sites interrupted complex word perception132,133. Thus, by combining advanced analysis of correlative data with causal stimulation, these studies discovered a novel medial-lateral gradient in the auditory cortex.
Causal techniques can also be used to compare different correlative brain mapping results. For instance, a recent study used task-based fMRI (causality score = 2) to compare different approaches for parcellating the brain based on task-free neuroimaging (causality score = 0)134. Another recent study used incidental lesions (causality score = 5.5) to compare different approaches for defining ‘cortical hubs’ based on resting-state fMRI (causality score = 0)135. In both cases, the more causal technique showed that some of the correlative approaches were more functionally relevant than the others. Importantly, the strength of causal inference in such studies is defined by the more causal technique, not the less causal technique. In the future, techniques further along the causality continuum should be used to test results from less causal methods whenever feasible.
By contrast, correlative imaging can also be used as a biomarker when comparing the effects of different causal techniques, especially techniques that do not induce a measurable clinical effect. For instance, functional neuroimaging can be used to estimate the effect of TMS to different targets in the prefrontal cortex, even when these targets do not induce clear behavioral changes136–138. In addition, by using correlative imaging to measure the physiological effects of an intervention before the clinical effects become apparent, it may be possible to derive useful therapeutic biomarkers139.
Finally, multifocal stimulation protocols may be designed to target different parts of a circuit defined by correlative techniques. The effect of stimulating different nodes of the same circuit could differ, and stimulating one site may modify excitability of a secondary site132. Various studies have attempted multifocal TMS and some have suggested that it may be safe to combine TMS with DBS applied to the same circuit, but more work is needed to identify optimal multifocal stimulation approaches140–143.
Best practices for causal brain mapping
Different human brain mapping techniques can yield insights at different levels of causal inference. It is important to recognize which level of causal inference applies to a given study and to form appropriate conclusions as a result, especially when the goal is uncovering a therapeutic target. Here, we provide some guidelines that may be helpful in this regard.
First, purely correlative studies should avoid making claims of causality and be cautious in suggesting therapeutic relevance. While correlative studies can plausibly identify biomarkers for diagnosis and monitoring139, there is a fundamental gap between correlative results and identifying treatment targets — while some correlations may reflect a causal relationship, others could be spurious or reverse-causal.
Second, the strength of causal inference should be explicitly assessed in studies that make any causal conclusions, including limitations. Before proposing a treatment target, brain mapping results should be explicitly compared with results from studies using approaches further along the causality continuum. For instance, atrophy-derived brain maps are more likely to be causal if they agree with lesion-derived maps of the same behavior144. If there is concordance across different causal mapping approaches, and consistency as one moves higher up on the causality continuum, this increases the probability that a correlative finding provides a useful mechanistic insight towards a treatment target.
Third, lesion and stimulation studies should explicitly consider the degree of concordance between modalities. This may become increasingly common as more lesion and stimulation datasets become available. We provided several examples of concordance between lesion and brain stimulation data (Fig. 4 and 5)95,130,131 which increases confidence in a causal relationship.
Fourth, to identify treatment targets, it is useful to focus not only on the strength of causality but also on its clinical relevance. For instance, if stimulating a specific region or circuit modifies the probability of specific symptoms, this is clinically meaningful and may represent a therapeutic target. By contrast, if stimulating a specific region or circuit induces an electrophysiological or hemodynamic response in a distant region, this information may be less clinically relevant. This knowledge may be useful for mechanistic and methodological purposes, but it does not imply that the distant region should be used as a treatment target.
Fifth, there is a critical difference between the strength of causality and the overall quality of a study. Our proposed causality continuum applies to studies that are well-designed and well-executed with rigorous methods and appropriate controls. If a study uses unjustified methods or lacks an appropriate control group, then the strength of causal inference cannot be reliably assessed, regardless of which methods were used. Thus, the causality continuum is only one of many dimensions that should be assessed when appraising a human brain mapping study.
Last, perhaps most importantly, prospective randomized clinical trials are still needed to translate causal circuit mapping results into practical treatments. Most existing clinical trials compare the effect of treatment with a sham treatment or placebo. This approach is valuable to determine the effect of the treatment modality, but it does not clarify the effect of the targeted circuit. To address this, prospective trials could isolate the effect of the targeted circuit by comparing the clinical effects of stimulating different brain circuits. If different targets can modulate different symptoms, these trials would be a powerful tool for testing circuit-based hypotheses and translating these findings into treatments.
Limitations and open questions
The mechanisms by which lesions or stimulations change an outcome remain unclear. It is relatively straightforward to understand why brain damage from a structural lesion will cause a loss of function, but it is unclear how some lesions can lead to positive outcomes145,146. Lesions are generally presumed to be inhibitory, but homeostatic compensation and diaschisis may lead to excitatory effects elsewhere the brain147,148. The effects of focal brain damage may extend beyond the lesion itself and these effects may differ for gray matter versus white matter lesions148,149. A better understanding of the neural mechanisms of diaschisis may shed light on these open questions.
The mechanisms of transcranial and intracranial stimulation can add complexity to the interpretation of causal brain mapping studies, as the roles of different stimulation parameters remain unclear. What are the effects of different stimulation frequencies and intensities150,151? How are different cortical layers affected152? How are outcomes related to cognitive states or tasks153? These and other factors may influence which neurons are affected by a stimulation154,155. Subtle variations in the stimulated field can lead to measurable differences in behavioral outcomes156, which may explain why some of Penfield’s experiments have failed to replicate157.
Results have been particularly heterogenous in the TMS literature. This is partly related to dosing – over the last two decades, TMS studies have used progressively higher doses and observed progressively larger effects158. However, individual differences and mechanisms remain poorly-understood. For example, high-frequency TMS is conventionally thought to increase cortical excitability, but up to 30% of individuals may show no change in or even a decrease in excitability159. Similarly, TMS has been hypothesized to function by modulating NMDA receptor-mediated plasticity, which can be modified by various drugs160, but these drugs can have minimal effect on TMS clinical outcomes161. As such, even a rigorously-designed TMS study may produce limited effect sizes, potentially underestimating the true causal relationship between the targeted brain region and behavioral effect.
Finally, when attempting to map causal information to brain circuits, it’s important to note that brain circuits can be defined in different ways5,162–165. Human brain circuits can be defined using thousands of individual voxels63,100,166, hundreds of distinct parcels5,162,164,165, or a handful of functionally independent networks6,163, and can be defined at the group or individual level167–170. This heterogeneity leads to high researcher degrees of freedom and may contribute to poor reproducibility of neuroimaging results171. This fragmentation was illustrated by a recent study in which 70 different research teams were asked to test the same hypotheses in the same neuroimaging dataset, and no two teams chose the same analytical procedure172. While many causal mapping studies have been robust to subtle methodological variations93,98, others have yielded different results when using different approaches173. Methodological standardization may improve reproducibility (one of Bradford-Hill’s criteria), and thus help move the entire field closer to causal inference.
Conclusions
Owing to decades of research in clinical and systems neuroscience, the field has amassed a wealth of knowledge about the organization of different brain regions. The recent re-emergence of lesion and stimulation-based studies has also led to a rapid growth in our ability to infer the causal role of these different regions, potentially enabling more informed clinical trials of targeted neuromodulation. To achieve this, causality should be interpreted as ‘stronger’ or ‘weaker’ along a continuous spectrum, and purely correlative findings should be explicitly acknowledged as such. By using tools and approaches that are further along the causality continuum, we can come closer to translating brain maps into clinical treatment targets for neuropsychiatric disease. Following the example set by the field of econometrics15, this may help human brain mapping achieve its own credibility revolution.
Acknowledgments
The authors thank the National Institute of Mental Health, the Brain & Behavior Research Foundation, Brigham & Women’s Hospital, and Stanford University BioX for funding the present work.
Competing interests
SHS serves as a consultant for Kaizen Brain Center, Acacia Mental Health, and Magnus Medical. SHS owns stock in Brainsway Inc (publicly traded) and Magnus Medical (not publicly traded). SHS and MDF have jointly received investigator-initiated research support from Neuronetics Inc. None of these organizations were involved in the present work. SHS and MDF each own independent intellectual property on the use of brain network mapping to target neuromodulation. The present work did not utilize any of this intellectual property. The authors report no other conflicts of interest related to the present work.
References
- 1.Fox MD & Raichle ME Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews neuroscience 8, 700–711 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME A brief history of human brain mapping. Trends in neurosciences 32, 118–126 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Genon S, Reid A, Langner R, Amunts K & Eickhoff SB How to Characterize the Function of a Brain Region. Trends in Cognitive Sciences 22, 350–364, doi: 10.1016/j.tics.2018.01.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gusnard DA & Raichle ME Searching for a baseline: functional imaging and the resting human brain. Nature reviews neuroscience 2, 685–694 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Glasser MF et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178, doi: 10.1038/nature18933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power JD et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullmore E & Sporns O The economy of brain network organization. Nature Reviews Neuroscience 13, 336–349 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK What we can do and what we cannot do with fMRI. Nature 453, 869–878, doi: 10.1038/nature06976 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Jonas E & Kording KP Could a Neuroscientist Understand a Microprocessor? PLoS computational biology 13, e1005268, doi: 10.1371/journal.pcbi.1005268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid AT et al. Advancing functional connectivity research from association to causation. Nature Neuroscience 22, 1751–1760, doi: 10.1038/s41593-019-0510-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laumann TO & Snyder AZ Brain activity is not only for thinking. Current Opinion in Behavioral Sciences 40, 130–136, doi: 10.1016/j.cobeha.2021.04.002 (2021). [DOI] [Google Scholar]
- 12.Etkin A A Reckoning and Research Agenda for Neuroimaging in Psychiatry. The American journal of psychiatry 176, 507–511, doi: 10.1176/appi.ajp.2019.19050521 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Weichwald S & Peters J Causality in Cognitive Neuroscience: Concepts, Challenges, and Distributional Robustness. Journal of Cognitive Neuroscience 33, 226–247, doi: 10.1162/jocn_a_01623 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Etkin A Addressing the Causality Gap in Human Psychiatric Neuroscience. JAMA Psychiatry 75, 3–4, doi: 10.1001/jamapsychiatry.2017.3610 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Angrist JD & Pischke J-S The credibility revolution in empirical economics: How better research design is taking the con out of econometrics. Journal of economic perspectives 24, 3–30 (2010). [Google Scholar]
- 16.Leamer EE Let’s Take the Con Out of Econometrics. The American Economic Review 73, 31–43 (1983). [Google Scholar]
- 17.Etkin A Mapping Causal Circuitry in Human Depression. Biol Psychiatry 86, 732–733, doi: 10.1016/j.biopsych.2019.09.009 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Bergmann TO & Hartwigsen G Inferring Causality from Noninvasive Brain Stimulation in Cognitive Neuroscience. Journal of Cognitive Neuroscience 33, 195–225, doi: 10.1162/jocn_a_01591 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Pycroft L, Stein J & Aziz T Deep brain stimulation: An overview of history, methods, and future developments. Brain and Neuroscience Advances 2, 2398212818816017, doi: 10.1177/2398212818816017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano AM et al. Effect of GPi pallidotomy on motor function in Parkinson’s disease. The Lancet 346, 1383–1387, doi: 10.1016/S0140-6736(95)92404-3 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Groiss SJ, Wojtecki L, Sudmeyer M & Schnitzler A Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2, 20–28, doi: 10.1177/1756285609339382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benabid AL et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. The Lancet 337, 403–406 (1991). [DOI] [PubMed] [Google Scholar]
- 23.George MS et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 48, 962–970, doi: 10.1016/s0006-3223(00)01048-9 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Rubio B, Pallardó F & Catalá MD Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet (London, England) 348, 233–237, doi: 10.1016/s0140-6736(96)01219-6 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Harlow JM Passage of an iron rod through the head. The Boston Medical and Surgical Journal (1828–1851) 39, 0_1 (1848). [PMC free article] [PubMed] [Google Scholar]
- 26.Scoville WB & Milner B Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry 20, 11 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penfield W & Jasper H Epilepsy and the functional anatomy of the human brain. (1954).
- 28.Penfield W & Perot P The brain’s record of auditory and visual experience: a final summary and discussion. Brain 86, 595–696 (1963). [DOI] [PubMed] [Google Scholar]
- 29.Penfield W & Boldrey E Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937). [Google Scholar]
- 30.Rorden C & Karnath H-O Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience 5, 812–819, doi: 10.1038/nrn1521 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Michel J-B et al. Quantitative analysis of culture using millions of digitized books. Science 331, 176–182, doi: 10.1126/science.1199644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaillard R et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 50, 191–204 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Petersen SE, Fox PT, Posner MI, Mintun M & Raichle ME Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 331, 585–589, doi: 10.1038/331585a0 (1988). [DOI] [PubMed] [Google Scholar]
- 34.Dolan RJ & Fletcher P Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388, 582–585 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Zalesky A, Fornito A & Bullmore E On the use of correlation as a measure of network connectivity. NeuroImage 60, 2096–2106, doi: 10.1016/j.neuroimage.2012.02.001 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Uttal WR The new phrenology: The limits of localizing cognitive processes in the brain. (The MIT press, 2001). [Google Scholar]
- 37.Pearl J Causality. (Cambridge University Press, 2009). [Google Scholar]
- 38.Angrist JD & Pischke J-S Mostly harmless econometrics: An empiricist’s companion. (Princeton university press, 2008). [Google Scholar]
- 39.Holland PW Statistics and causal inference. Journal of the American statistical Association 81, 945–960 (1986). [Google Scholar]
- 40.Pearl J & Mackenzie D The book of why: the new science of cause and effect. (Basic books, 2018). [Google Scholar]
- 41.Gotthelf A Aristotle’s conception of final causality. The Review of Metaphysics, 226–254 (1976). [Google Scholar]
- 42.Black DL Mental Existence in Thomas Aquinas and Avicenna. Mediaeval Studies 61, 45–79 (1999). [Google Scholar]
- 43.Taylor R Primary and Secondary Causality. (The Routledge Companion to Islamic Philosophy, 2012). [Google Scholar]
- 44.Smith NK The philosophy of David Hume: a critical study of its origins and central doctrines. (1941).
- 45.Koch R in 10th International Medical Congress.
- 46.Bradford Hill A The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine 58, 295–300, doi: 10.1177/003591576505800503 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedak KM, Bernal A, Capshaw ZA & Gross S Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol 12, 14, doi: 10.1186/s12982-015-0037-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araújo L, Dalgalarrondo P & Banzato C On the notion of causality in medicine: Addressing Austin Bradford Hill and John L. Mackie. Archives of Clinical Psychiatry 41, 56–61, doi: 10.1590/0101-60830000000010 (2014). [DOI] [Google Scholar]
- 49.Sajadi MM, Mansouri D & Sajadi MR Ibn Sina and the clinical trial. Annals of internal medicine 150, 640–643, doi: 10.7326/0003-4819-150-9-200905050-00011 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Bradford Hill A Memories of the British streptomycin trial in tuberculosis: the first randomized clinical trial. Controlled Clinical Trials 11, 77–79 (1990). [DOI] [PubMed] [Google Scholar]
- 51.Gillies D Causality, Probability, and Medicine. (Taylor & Francis, 2018). [Google Scholar]
- 52.Kaplan RM & Irvin VL Likelihood of Null Effects of Large NHLBI Clinical Trials Has Increased over Time. PLOS ONE 10, e0132382, doi: 10.1371/journal.pone.0132382 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baluku JB et al. Prevalence of Malaria and TB Coinfection at a National Tuberculosis Treatment Centre in Uganda. Journal of Tropical Medicine 2019, 3741294, doi: 10.1155/2019/3741294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch R The etiology of tuberculosis. Mittheilungen aus dem Kaiserlichen Gesundheitsamte 2, 1–88 (1884). [Google Scholar]
- 55.Hernán MA & Robins JM Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiology 183, 758–764, doi: 10.1093/aje/kwv254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinescu IE, Lawlor PN & Kording KP Quasi-experimental causality in neuroscience and behavioural research. Nature human behaviour 2, 891–898, doi: 10.1038/s41562-018-0466-5 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Cook TD, Shadish WR & Wong VC Three conditions under which experiments and observational studies produce comparable causal estimates: New findings from within-study comparisons. Journal of Policy Analysis and Management 27, 724–750, doi: 10.1002/pam.20375 (2008). [DOI] [Google Scholar]
- 58.Fox MD Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med 379, 2237–2245, doi: 10.1056/NEJMra1706158 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Williamson J Establishing Causal Claims in Medicine. International Studies in the Philosophy of Science 32, 33–61, doi: 10.1080/02698595.2019.1630927 (2019). [DOI] [Google Scholar]
- 60.Russo F & Williamson J Interpreting causality in the health sciences. International studies in the philosophy of science 21, 157–170 (2007). [Google Scholar]
- 61.Evans AS Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med 49, 175–195 (1976). [PMC free article] [PubMed] [Google Scholar]
- 62.Cassidy JM, Mark JI & Cramer SC Functional connectivity drives stroke recovery: shifting the paradigm from correlation to causation. Brain, doi: 10.1093/brain/awab469 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates E et al. Voxel-based lesion–symptom mapping. Nature Neuroscience 6, 448–450, doi: 10.1038/nn1050 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Wu O et al. Role of Acute Lesion Topography in Initial Ischemic Stroke Severity and Long-Term Functional Outcomes. Stroke 46, 2438–2444, doi:doi: 10.1161/STROKEAHA.115.009643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbetta M et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron 85, 927–941, doi: 10.1016/j.neuron.2015.02.027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parvizi J & Kastner S Promises and limitations of human intracranial electroencephalography. Nature Neuroscience 21, 474–483, doi: 10.1038/s41593-018-0108-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox KCR et al. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nature human behaviour 4, 1039–1052, doi: 10.1038/s41562-020-0910-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koenigs M et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci 11, 232–237, doi: 10.1038/nn2032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knutson KM et al. Injured brain regions associated with anxiety in Vietnam veterans. Neuropsychologia 51, 686–694, doi: 10.1016/j.neuropsychologia.2013.01.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herbsman T et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry 66, 509–515, doi: 10.1016/j.biopsych.2009.04.034 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Fox MD, Buckner RL, White MP, Greicius MD & Pascual-Leone A Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 72, 595–603, doi:10.1016/j.biopsych.2012.04.028 10.1016/j.biopsych.2012.04.028. Epub 2012 Jun 1. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weigand A et al. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry 84, 28–37, doi: 10.1016/j.biopsych.2017.10.028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon EM et al. Individual-specific features of brain systems identified with resting state functional correlations. Neuroimage 146, 918–939, doi: 10.1016/j.neuroimage.2016.08.032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon EM, Laumann TO, Adeyemo B & Petersen SE Individual Variability of the System-Level Organization of the Human Brain. Cereb Cortex 27, 386–399, doi: 10.1093/cercor/bhv239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqi SH, Weigand A, Pascual-Leone A & Fox MD Identification of personalized TMS targets based on subgenual cingulate connectivity: an independent replication. Biol Psychiatry (2021. (In Press)). [DOI] [PubMed] [Google Scholar]
- 76.Cash RFH, Cocchi L, Lv J, Fitzgerald PB & Zalesky A Functional Magnetic Resonance Imaging–Guided Personalization of Transcranial Magnetic Stimulation Treatment for Depression. JAMA Psychiatry, doi: 10.1001/jamapsychiatry.2020.3794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howell B et al. Quantifying the axonal pathways directly stimulated in therapeutic subcallosal cingulate deep brain stimulation. Human brain mapping 40, 889–903, doi: 10.1002/hbm.24419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddiqi SH et al. Repetitive Transcranial Magnetic Stimulation with Resting-State Network Targeting for Treatment-Resistant Depression in Traumatic Brain Injury: A Randomized, Controlled, Double-Blinded Pilot Study. J Neurotrauma 36, 1361–1374, doi: 10.1089/neu.2018.5889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parvizi J, Rangarajan V, Shirer WR, Desai N & Greicius Michael D. The Will to Persevere Induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron 80, 1359–1367, doi: 10.1016/j.neuron.2013.10.057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schrouff J et al. Fast temporal dynamics and causal relevance of face processing in the human temporal cortex. Nature Communications 11, 656, doi: 10.1038/s41467-020-14432-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yih J, Beam DE, Fox KCR & Parvizi J Intensity of affective experience is modulated by magnitude of intracranial electrical stimulation in human orbitofrontal, cingulate and insular cortices. Social cognitive and affective neuroscience 14, 339–351, doi: 10.1093/scan/nsz015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Downar J et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 76, 176–185, doi: 10.1016/j.biopsych.2013.10.026 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Drysdale AT et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med, doi:10.1038/nm.4246 10.1038/nm.4246. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cash RFH et al. Using Brain Imaging to Improve Spatial Targeting of Transcranial Magnetic Stimulation for Depression. Biol Psychiatry, doi: 10.1016/j.biopsych.2020.05.033 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Ferguson MA et al. A human memory circuit derived from brain lesions causing amnesia. Nature Communications 10, 3497, doi: 10.1038/s41467-019-11353-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horn A et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol 82, 67–78, doi: 10.1002/ana.24974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joutsa J, Horn A, Hsu J & Fox MD Localizing parkinsonism based on focal brain lesions. Brain 141, 2445–2456, doi: 10.1093/brain/awy161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corp DT et al. Network localization of cervical dystonia based on causal brain lesions. Brain 142, 1660–1674, doi: 10.1093/brain/awz112 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joutsa J et al. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol 84, 153–157, doi: 10.1002/ana.25285 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Irmen F et al. Left prefrontal impact links subthalamic stimulation with depressive symptoms. Ann Neurol, doi: 10.1002/ana.25734 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Laganiere S, Boes AD & Fox MD Network localization of hemichorea-hemiballismus. Neurology 86, 2187–2195, doi: 10.1212/wnl.0000000000002741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padmanabhan JL et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry, doi: 10.1016/j.biopsych.2019.07.023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cotovio G et al. Mapping mania symptoms based on focal brain damage. The Journal of Clinical Investigation 130, 5209–5222, doi: 10.1172/JCI136096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cash RFH et al. Subgenual Functional Connectivity Predicts Antidepressant Treatment Response to Transcranial Magnetic Stimulation: Independent Validation and Evaluation of Personalization. Biol Psychiatry 86, e5–e7, doi: 10.1016/j.biopsych.2018.12.002 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Siddiqi SH et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nature human behaviour (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baldermann JC et al. Connectivity Profile Predictive of Effective Deep Brain Stimulation in Obsessive-Compulsive Disorder. Biol Psychiatry 85, 735–743, doi: 10.1016/j.biopsych.2018.12.019 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Li N et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications 11, 3364, doi: 10.1038/s41467-020-16734-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siddiqi SH et al. Distinct Symptom-Specific Treatment Targets for Circuit-Based Neuromodulation. The American journal of psychiatry 177, 435–446, doi: 10.1176/appi.ajp.2019.19090915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim NY et al. Lesions causing hallucinations localize to one common brain network. Molecular Psychiatry 26, 1299–1309, doi: 10.1038/s41380-019-0565-3 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Boes AD et al. Network localization of neurological symptoms from focal brain lesions. Brain : a journal of neurology 138, 3061–3075, doi: 10.1093/brain/awv228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Darby RR, Laganiere S, Pascual-Leone A, Prasad S & Fox MD Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain : a journal of neurology 140, 497–507, doi: 10.1093/brain/aww288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Snider SB et al. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Human brain mapping 41, 1520–1531, doi: 10.1002/hbm.24892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fischer DB et al. A human brain network derived from coma-causing brainstem lesions. Neurology 87, 2427–2434, doi: 10.1212/wnl.0000000000003404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joutsa J, Shih LC & Fox MD Mapping holmes tremor circuit using the human brain connectome. Ann Neurol 86, 812–820, doi: 10.1002/ana.25618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Darby RR, Joutsa J, Burke MJ & Fox MD Lesion network localization of free will. Proc Natl Acad Sci U S A 115, 10792–10797, doi: 10.1073/pnas.1814117115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Darby RR, Horn A, Cushman F & Fox MD Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A 115, 601–606, doi: 10.1073/pnas.1706587115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen AL et al. Looking beyond the face area: lesion network mapping of prosopagnosia. Brain 142, 3975–3990, doi: 10.1093/brain/awz332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohen AL et al. Tuber Locations Associated with Infantile Spasms Map to a Common Brain Network. Ann Neurol 89, 726–739, doi: 10.1002/ana.26015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fox MD et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A 111, E4367–4375, doi: 10.1073/pnas.1405003111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horn A & Fox MD Opportunities of connectomic neuromodulation. NeuroImage 221, 117180, doi: 10.1016/j.neuroimage.2020.117180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowren M et al. Post-Stroke Cognitive and Motor Outcomes Predicted from Lesion Location and Lesion Network Mapping. Brain (2022. (In Press)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Long Michael A. et al. Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron 89, 1187–1193, doi: 10.1016/j.neuron.2016.01.032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ibayashi K et al. Focal Cortical Surface Cooling is a Novel and Safe Method for Intraoperative Functional Brain Mapping. World Neurosurgery 147, e118–e129, doi: 10.1016/j.wneu.2020.11.164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Borchers S, Himmelbach M, Logothetis N & Karnath HO Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nature reviews. Neuroscience 13, 63–70, doi: 10.1038/nrn3140 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Winawer J & Parvizi J Linking Electrical Stimulation of Human Primary Visual Cortex, Size of Affected Cortical Area, Neuronal Responses, and Subjective Experience. Neuron 92, 1213–1219, doi: 10.1016/j.neuron.2016.11.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parvizi J et al. Altered sense of self during seizures in the posteromedial cortex. Proceedings of the National Academy of Sciences 118, e2100522118, doi: 10.1073/pnas.2100522118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vesuna S et al. Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94, doi: 10.1038/s41586-020-2731-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knecht S et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nature neuroscience 5, 695–699 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Pascual‐Leone A, Gates JR & Dhuna A Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology 41, 697–702 (1991). [DOI] [PubMed] [Google Scholar]
- 120.Lefaucheur JP & Picht T The value of preoperative functional cortical mapping using navigated TMS. Neurophysiologie clinique = Clinical neurophysiology 46, 125–133, doi: 10.1016/j.neucli.2016.05.001 (2016). [DOI] [PubMed] [Google Scholar]
- 121.Riva-Posse P et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 76, 963–969, doi: 10.1016/j.biopsych.2014.03.029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riva-Posse P et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Molecular psychiatry 23, 843–849, doi: 10.1038/mp.2017.59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li N et al. A Unified Functional Network Target for Deep Brain Stimulation in Obsessive-Compulsive Disorder. Biol Psychiatry, doi: 10.1016/j.biopsych.2021.04.006 (2021). [DOI] [PubMed] [Google Scholar]
- 124.Nestor Sean M., M.D., Ph.D., & Blumberger Daniel M., M.D., M.Sc. Mapping Symptom Clusters to Circuits: Toward Personalizing TMS Targets to Improve Treatment Outcomes in Depression. American Journal of Psychiatry 177, 373–375, doi: 10.1176/appi.ajp.2020.20030271 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Robinson RG, Kubos KL, Starr LB, Rao K & Price TR Mood disorders in stroke patients. Importance of location of lesion. Brain 107 (Pt 1), 81–93, doi: 10.1093/brain/107.1.81 (1984). [DOI] [PubMed] [Google Scholar]
- 126.Robinson RG & Szetela B Mood change following left hemispheric brain injury. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 9, 447–453 (1981). [DOI] [PubMed] [Google Scholar]
- 127.Bergman H, Wichmann T & DeLong MR Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438, doi: 10.1126/science.2402638 (1990). [DOI] [PubMed] [Google Scholar]
- 128.Parvizi J et al. Electrical Stimulation of Human Fusiform Face-Selective Regions Distorts Face Perception. The Journal of Neuroscience 32, 14915–14920, doi: 10.1523/jneurosci.2609-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rangarajan V et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 12828–12836, doi: 10.1523/jneurosci.0527-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ganos C et al. A neural network for tics: insights from causal brain lesions and deep brain stimulation. Brain, doi: 10.1093/brain/awac009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reich MM et al. A brain network for deep brain stimulation induced cognitive decline in Parkinson’s disease. Brain, doi: 10.1093/brain/awac012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hamilton LS, Oganian Y, Hall J & Chang EF Parallel and distributed encoding of speech across human auditory cortex. Cell 184, 4626–4639.e4613, doi: 10.1016/j.cell.2021.07.019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hamilton LS, Edwards E & Chang EF A spatial map of onset and sustained responses to speech in the human superior temporal gyrus. Current Biology 28, 1860–1871. e1864 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Zhi D, King M & Diedrichsen J Evaluating brain parcellations using the distance controlled boundary coefficient. bioRxiv, 2021.2005.2011.443151, doi: 10.1101/2021.05.11.443151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Warren DE et al. Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences 111, 14247–14252, doi: 10.1073/pnas.1322173111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eldaief MC, Halko MA, Buckner RL & Pascual-Leone A Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences 108, 21229–21234, doi: 10.1073/pnas.1113103109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen AC et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences 110, 19944–19949, doi: 10.1073/pnas.1311772110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ozdemir RA et al. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proceedings of the National Academy of Sciences 117, 8115–8125, doi: 10.1073/pnas.1911240117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gallen CL & D’Esposito M Brain Modularity: A Biomarker of Intervention-related Plasticity. Trends Cogn Sci 23, 293–304, doi: 10.1016/j.tics.2019.01.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu D et al. Multifocal transcranial stimulation in chronic ischemic stroke: A phase 1/2a randomized trial. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 29, 104816, doi: 10.1016/j.jstrokecerebrovasdis.2020.104816 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Brys M et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology 87, 1907–1915, doi: 10.1212/WNL.0000000000003279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Magsood H, Syeda F, Holloway K, Carmona IC & Hadimani RL Safety Study of Combination Treatment: Deep Brain Stimulation and Transcranial Magnetic Stimulation. Frontiers in Human Neuroscience 14, doi: 10.3389/fnhum.2020.00123 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Deng Z, Lisanby SH & Peterchev AV in 2010. Annual International Conference of the IEEE Engineering in Medicine and Biology. 6821–6824. [DOI] [PubMed] [Google Scholar]
- 144.Tetreault AM et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain 143, 1249–1260, doi: 10.1093/brain/awaa058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joutsa J et al. Identifying therapeutic targets from spontaneous beneficial brain lesions. Annals of Neurology 84, 153–157, doi: 10.1002/ana.25285 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Kapur N Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain 119 (Pt 5), 1775–1790, doi: 10.1093/brain/119.5.1775 (1996). [DOI] [PubMed] [Google Scholar]
- 147.Carrera E & Tononi G Diaschisis: past, present, future. Brain 137, 2408–2422, doi: 10.1093/brain/awu101 (2014). [DOI] [PubMed] [Google Scholar]
- 148.Sperber C Rethinking causality and data complexity in brain lesion-behaviour inference and its implications for lesion-behaviour modelling. Cortex; a journal devoted to the study of the nervous system and behavior 126, 49–62, doi: 10.1016/j.cortex.2020.01.004 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Griffis JC, Metcalf NV, Corbetta M & Shulman GL Structural Disconnections Explain Brain Network Dysfunction after Stroke. Cell reports 28, 2527–2540.e2529, doi: 10.1016/j.celrep.2019.07.100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Klomjai W, Katz R & Lackmy-Vallee A Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of physical and rehabilitation medicine 58, 208–213, doi:10.1016/j.rehab.2015.05.005 10.1016/j.rehab.2015.05.005. Epub 2015 Aug 28. (2015). [DOI] [PubMed] [Google Scholar]
- 151.Su D et al. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson’s disease: a meta-analysis of controlled trials. Scientific Reports 8, 14456, doi: 10.1038/s41598-018-32161-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Radman T, Ramos RL, Brumberg JC & Bikson M Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2, 215–228.e213, doi: 10.1016/j.brs.2009.03.007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Feredoes E, Heinen K, Weiskopf N, Ruff C & Driver J Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci U S A 108, 17510–17515, doi: 10.1073/pnas.1106439108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duffley G, Anderson DN, Vorwerk J, Dorval AD & Butson CR Evaluation of methodologies for computing the deep brain stimulation volume of tissue activated. Journal of neural engineering 16, 066024, doi: 10.1088/1741-2552/ab3c95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thielscher A, Antunes A & Saturnino GB Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference 2015, 222–225, doi: 10.1109/embc.2015.7318340 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Raij T et al. Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biol Psychiatry 84, 129–137, doi: 10.1016/j.biopsych.2017.10.022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Curot J et al. Awake Craniotomy and Memory Induction Through Electrical Stimulation: Why Are Penfield’s Findings Not Replicated in the Modern Era? Neurosurgery 87, E130–e137, doi: 10.1093/neuros/nyz553 (2020). [DOI] [PubMed] [Google Scholar]
- 158.Cole EJ et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. The American journal of psychiatry 177, 716–726, doi: 10.1176/appi.ajp.2019.19070720 (2020). [DOI] [PubMed] [Google Scholar]
- 159.Maeda F, Keenan JP, Tormos JM, Topka H & Pascual-Leone A Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Experimental brain research 133, 425–430, doi: 10.1007/s002210000432 (2000). [DOI] [PubMed] [Google Scholar]
- 160.Brown JC et al. NMDA receptor partial agonist, d-cycloserine, enhances 10 Hz rTMS-induced motor plasticity, suggesting long-term potentiation (LTP) as underlying mechanism. Brain Stimul 13, 530–532, doi: 10.1016/j.brs.2020.01.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Minzenberg MJ & Leuchter AF The effect of psychotropic drugs on cortical excitability and plasticity measured with transcranial magnetic stimulation: Implications for psychiatric treatment. Journal of Affective Disorders 253, 126–140, doi: 10.1016/j.jad.2019.04.067 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Schaefer A et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex 28, 3095–3114, doi: 10.1093/cercor/bhx179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeo BT et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165, doi:10.1152/jn.00338.2011 10.1152/jn.00338.2011. Epub 2011 Jun 8. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gordon EM et al. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cerebral cortex 26, 288–303, doi: 10.1093/cercor/bhu239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Destrieux C, Fischl B, Dale A & Halgren E Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC & Wager TD Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665, doi:10.1038/nmeth.1635 https://www.nature.com/articles/nmeth.1635#supplementary-information (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Laumann TO et al. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87, 657–670, doi: 10.1016/j.neuron.2015.06.037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gordon EM et al. Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807 e797, doi: 10.1016/j.neuron.2017.07.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang D et al. Parcellating cortical functional networks in individuals. Nat Neurosci 18, 1853–1860, doi: 10.1038/nn.4164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hacker CD et al. Resting state network estimation in individual subjects. Neuroimage 82, 616–633, doi: 10.1016/j.neuroimage.2013.05.108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cohen AL & Fox MD Reply: The influence of sample size and arbitrary statistical thresholds in lesion-network mapping. Brain 143, e41–e41, doi: 10.1093/brain/awaa095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Botvinik-Nezer R et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88, doi: 10.1038/s41586-020-2314-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Salvalaggio A et al. Reply: Lesion network mapping predicts post-stroke behavioural deficits and improves localization. Brain 144, e36–e36, doi: 10.1093/brain/awab004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bergmann TO et al. Concurrent TMS-fMRI for causal network perturbation and proof of target engagement. NeuroImage 237, 118093, doi: 10.1016/j.neuroimage.2021.118093 (2021). [DOI] [PubMed] [Google Scholar]
- 175.Golay X, Hendrikse J & Lim TC Perfusion imaging using arterial spin labeling. Topics in Magnetic Resonance Imaging 15, 10–27 (2004). [DOI] [PubMed] [Google Scholar]
- 176.Fischl B FreeSurfer. Neuroimage 62, 774–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.MacInnes JJ, Dickerson KC, Chen NK & Adcock RA Cognitive Neurostimulation: Learning to Volitionally Sustain Ventral Tegmental Area Activation. Neuron 89, 1331–1342, doi: 10.1016/j.neuron.2016.02.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bauer CCC et al. Real-time fMRI neurofeedback reduces auditory hallucinations and modulates resting state connectivity of involved brain regions: Part 2: Default mode network -preliminary evidence. Psychiatry Res 284, 112770, doi: 10.1016/j.psychres.2020.112770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tarakad A & Jankovic J Anosmia and Ageusia in Parkinson’s Disease. International review of neurobiology 133, 541–556, doi: 10.1016/bs.irn.2017.05.028 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Williams ZM, Bush G, Rauch SL, Cosgrove GR & Eskandar EN Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neuroscience 7, 1370–1375, doi: 10.1038/nn1354 (2004). [DOI] [PubMed] [Google Scholar]
- 181.Sheth SA et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221, doi: 10.1038/nature11239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shine JM et al. Distinct patterns of temporal and directional connectivity among intrinsic networks in the human brain. The Journal of Neuroscience, 1574–1517, doi: 10.1523/jneurosci.1574-17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rafiei F, Safrin M, Wokke ME, Lau H & Rahnev D Transcranial magnetic stimulation alters multivoxel patterns in the absence of overall activity changes. Human Brain Mapping n/a, doi: 10.1002/hbm.25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buzsáki G, Anastassiou CA & Koch C The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience 13, 407–420, doi: 10.1038/nrn3241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Granger CWJ & Newbold P Forecasting economic time series. (Academic Press, 1977). [Google Scholar]
- 186.Friston KJ, Harrison L & Penny W Dynamic causal modelling. Neuroimage 19, 1273–1302, doi: 10.1016/s1053-8119(03)00202-7 (2003). [DOI] [PubMed] [Google Scholar]
- 187.Barnett L, Barrett AB & Seth AK Misunderstandings regarding the application of Granger causality in neuroscience. Proc Natl Acad Sci U S A 115, E6676–e6677, doi: 10.1073/pnas.1714497115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Friston K Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS biology 7, e33, doi: 10.1371/journal.pbio.1000033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Webb JT, Ferguson MA, Nielsen JA & Anderson JS BOLD Granger Causality Reflects Vascular Anatomy. PLOS ONE 8, e84279, doi: 10.1371/journal.pone.0084279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Granger CW Time series analysis, cointegration, and applications. American Economic Review 94, 421–425 (2004). [Google Scholar]
- 191.Mehler D & Kording K The lure of causal statements: Rampant mis-inference of causality in estimated connectivity. (2018).


