This report provides guidance on the use of brolucizumab for neovascular age-related macular degeneration, given the events of retinal vascular occlusion associated with brolucizumab. Benefit–risk assessment, patient education, and evaluation for inflammation before injection are important components of treatment of neovascular age-related macular degeneration with brolucizumab.
Key words: brolucizumab; intraocular inflammation, retinal vasculitis, retinal vascular occlusion; occlusive retinal vasculitis; adverse events; neovascular age-related macular degeneration
Abstract
Purpose:
Brolucizumab has high efficacy in retinal fluid resolution and provides the possibility for longer dosing intervals in the treatment of neovascular age-related macular degeneration. However, brolucizumab has been associated with events of retinal vasculitis and retinal vascular occlusion typically in the presence of other signs of intraocular inflammation (IOI). The purpose of this report is to provide guidance on the use of brolucizumab for neovascular age-related macular degeneration to a global audience.
Methods:
A literature review was conducted on adverse events related to IOI after administration of brolucizumab in eyes with neovascular age-related macular degeneration.
Results:
Possible risk factors for IOI and retinal vascular occlusion after brolucizumab should be considered before administering brolucizumab. Patients who receive brolucizumab should be educated on the symptoms, signs, and time course of IOI after brolucizumab. Before each injection of brolucizumab, physicians should assess the eye for any signs of inflammation and not treat with brolucizumab if inflammation is detected. Treatment of IOI should be prompt and provided with particular attention to the posterior segment.
Conclusion:
Careful patient selection, patient education, assessment for inflammation, and intensive treatment of possible inflammation are important when using brolucizumab in patients with neovascular age-related macular degeneration.
High intravitreal injection burden and nonadherence are major challenges in the treatment of patients with neovascular age-related macular degeneration (nAMD).1,2 In a recent analysis of real-world data from the Intelligent Research in Sight (IRIS) registry, almost 40% of patients received injections more frequently than every 8 weeks at the 1-year follow-up.3 A global survey of patients and caregivers identified treatment burden as one of the major challenges for nAMD and among the major reasons for nonadherence.4 Data from a systematic review identified nonadherence as a prevalent problem, with up to 57% of patients nonadherent to treatment at 12 months.5 Given that undertreatment represents a risk of long-term vision loss,6 these data suggest there is a need for durable treatments that reduce treatment burden.
Brolucizumab is an anti–vascular endothelial growth factor (VEGF) agent that launched in 2019 for the treatment of nAMD.7 In the phase 3 HAWK and HARRIER studies, visual acuity gains at Week 48 with brolucizumab (q8 or q12 weeks) were noninferior to aflibercept (q8 weeks).8 Rates of retinal fluid presence were lower with brolucizumab than with aflibercept (Figure 1). Furthermore, approximately half of brolucizumab-treated eyes (55.6% and 51.0% in HAWK [6 mg] and HARRIER, respectively) were maintained on a 12-week dosing regimen, suggesting that brolucizumab provides the possibility for greater intervals between injections. Brolucizumab was reported to be generally well tolerated,8 although the incidence of intraocular inflammation (IOI) was higher with brolucizumab 6 mg (HAWK: 5.3%; HARRIER: 2.7%) than with aflibercept (HAWK: 0.3%; HARRIER: 0.8%).9 Based on these studies, brolucizumab has received approval in more than 40 regions worldwide.10,11
Fig. 1.
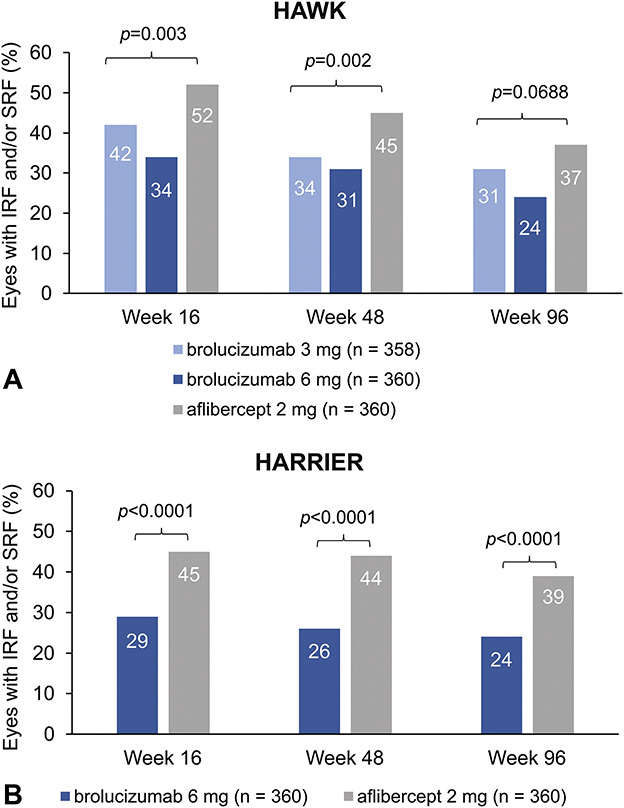
The percentage of eyes with presence of IRF and/or SRF at Week 16, 48, and 96 in the HAWK study (A) and in the HARRIER study (B). One-sided p values are shown for Week 16 and 48. Two-sided P values are shown for Week 96. Adapted from Dugel PU, Singh RP, Koh A, et al. Ophthalmology. 2021;128(1):89–99, with permission from Elsevier.
In 2020, the American Society of Retina Specialists circulated safety updates regarding cases of retinal vasculitis (RV) in patients treated with brolucizumab, some of which were reported as occlusive RV with associated vision loss.12 The postmarketing reporting rate for RV and/or retinal vascular occlusion (RO) has been approximately 15.1 per 10,000 injections (as of August 27, 2021).12 To better understand these reports, Novartis commissioned an external Safety Review Committee (SRC) to reassess cases of IOI, endophthalmitis, and retinal artery occlusion among brolucizumab-treated eyes in HAWK and HARRIER.13 Of the 60 eyes evaluated, the SRC reported, 50 eyes (50/1,088; 4.6%) had definite or probable events that were drug-related and in the spectrum of IOI, RV, and/or RO.13 Of these 50 eyes, 36 had concomitant RV (3.3%), of which 23 had concomitant RO (2.1%). The overall rate of at least moderate vision loss (≥15 Early Treatment Diabetic Retinopathy Study letters) by Week 96, however, was comparable between brolucizumab 6 mg and aflibercept in both HAWK (8.1% vs. 7.4%) and HARRIER (7.1% vs. 7.5%).8 Based on the safety findings, a label update was approved by several health authorities.12 In 2021, Novartis issued a statement that physicians should not administer brolucizumab at intervals less than 2 months beyond the first three doses (i.e., dosing intervals should be 8 weeks or more during the maintenance phase), based on the safety findings from the first interpretable 1-year results of the phase three MERLIN study, in which brolucizumab was administered every 4 weeks. In MERLIN, the rates of IOI for brolucizumab and aflibercept were 9.3% versus 4.5%, the rates of RV were 0.8% versus 0.0%, the rates of RO were 2.0% versus 0.0%, and the rates of vision loss ≥15 letters from all causes were 4.8% versus 1.7%.12
Several case reports and case series have described events of RV and/or RO associated with brolucizumab.14–23 A comprehensive table of case study reports was provided by Baumal et al.10 Based on these data, it seemed that a variety of treatments were administered, including topical, intravitreal, sub-Tenon, and systemic corticosteroids. Baumal et al recommended frequent monitoring of patients with IOI for RV and RO and early and intensive treatment for RV and RO. Additional case reports and case series published globally since then are summarized in Table 1. Given that the events can result in vision loss, these cases highlight that early and aggressive therapy is warranted. Guidance on the use of brolucizumab for nAMD has been provided in the literature by several investigators.10,24,25 Since these publications, more insight has emerged from retrospective analysis of real-world evidence from the IRIS Registry and Komodo Healthcare Map and post hoc analyses of the treatment of IOI-related adverse events in HAWK and HARRIER, enabling refinement of recommendations.26,27 In this report, we provide guidance on best practices for the use of brolucizumab for nAMD based on our current understanding of the adverse events and expert opinion.
Table 1.
Summary of Recent Global Case Studies and Case Series of IOI, Retinal Vasculitis, and Retinal Vascular Occlusive Events after Intravitreal Brolucizumab Treatment in Patients With nAMD
| Study | N | Presenting Symptoms | Examination Findings | Treatment | Resolution |
| Angerer, 202035 | 1 eye | Swollen eyelids and foreign body sensation initially; eye pain and vision loss | Vision was 1/30 on hand chart (from 0.05), internal pressure of 33 mm Hg, moderate Descemet folds, AC cells, fundus view was reduced, and choroidal detachment | Ofloxacin eye drops TID initially; intravenous aciclovir 500 mg TID, prednisolone acetate eye drops 5 times daily; switch to 900-mg oral valganciclovir BID (because of initial suspicion of herpes zoster–associated uveitis); subsequently, parabulbar administration of fortecortin and systemic prednisolone 100 mg daily | Pale optic disc, “ghosting” of the central arteries, obliterations of retinal vessels in the periphery, intraretinal bleeding. Vision no longer increased |
| Iyer 202019 | 1 eye | Pain, ocular aches, floaters, and decreased vision | VA decreased from 20/70–20/200, AC cells (0.5+), vitreous debris, arterial plaques, vascular sheathing, boxcarring, and retinal whitening; subsequently, VA decreased to count fingers at 3 feet, fine keratic precipitates, AC cells (2+), posterior debris and haze, and worse retinal whitening | Topical 1% prednisolone acetate; subsequently, 0.05% difluprednate drops every 2 hours and oral high-dose methylprednisolone pulse therapy, pars plana vitrectomy with intravitreal triamcinolone injection | Vision improved to 20/200 |
| Antaki 202120 | 1 eye | Pain, vision loss | VA reduced to light perception (from 20/150), moderate to severe vitritis, arterial sheathing, diffuse arterial and venous narrowing, perivenular hemorrhages, filling defects, and peripheral nonperfusion | — | — |
| Enriquez 202136 | 14 eyes of 13 patients | Symptoms of 1 patient described: floaters and blurred vision | IOI with anterior segment and/or vitreous cells | Topical and/or oral corticosteroids, sub-Tenon triamcinolone acetonide, vitrectomy, and/or intraoperative triamcinolone; or no treatment | 11 eyes switched to a different anti-VEGF |
| Hikichi, 202121 | 3 eyes of 3 patients | Ocular redness, pain, and decreased vision | BCVA decreased to 0.3 (from 1.2), ciliary hyperemia, AC (1+) cells, fine keratic precipitates without fibrin material, anterior vitreous cells (2+), slight vitreous haze; subsequently, hypopyon and vitreous opacities, BCVA decreased to HM | Topical 0.01% betamethasone sodium phosphate QID; subsequently, sub-Tenon 20 mg triamcinolone acetonide | Anterior chamber inflammation resolved; subsequently, hypopyon resolved, vitreous opacities decreased with improved BCVA to 0.5 |
| Ocular pain, redness, and blurred vision | BCVA decreased to 0.06 (from 0.3), conjunctival hyperemia, AC cells (1+), fine keratic precipitates, vitreous cells, vitreous opacities, intraretinal hemorrhage, vitreous haze, and sheathed inferior and inferotemporal retinal veins | Topical 0.01% betamethasone sodium phosphate QID and sub-Tenon 20-mg triamcinolone acetonide | Anterior chamber inflammation resolved, vitreous opacities decreased, BCVA recovered to 0.3, resolution of retinal hemorrhage, and reduction of the sheathed retinal vessels | ||
| Floaters and gradual progression to blurry vision without redness or pain | BCVA decreased to 0.6 (from 0.7), no ciliary hyperemia, rare AC cells, fine keratic precipitates, some vitreous opacities, sheathed retinal arteries and veins, and IRF and SRF recurrence | Topical 0.01% betamethasone sodium phosphate QID and sub-Tenon 20-mg triamcinolone acetonide injection | Vitreous opacities resolved, BCVA recovered to 0.7, and sheathed retinal vessels seemed to improve | ||
| Kataoka, 202122 | 3 eyes of 3 patients | Blurry vision described as “white out” | VA reduced to 0.1 (from 0.6), AC cells (1+), fine keratic precipitates, vitreous cells (1+), no hypopyon, aqueous flare, vitreous haze, sheathing of peripheral retinal arteries and veins, blot retinal hemorrhage, leakage from veins at arcades and the peripheral retina, leakage from optic nerve head, and appearance of subretinal hyperreflective material | Oral prednisolone 30 mg per day and sub-Tenon triamcinolone acetonide injection (20 mg/0.5 mL) and 0.1% betamethasone eye drops QID | Vascular sheathing disappeared, no evidence of inflammation on FA, subretinal hyperreflective material decreased, VA recovered to 0.8, and aqueous flare decreased to levels comparable with fellow eye |
| Floaters | AC cells (1+), fine keratic precipitates, vitreous cells (1+), aqueous flare, vitreous haze, sheathing on peripheral retinal arteries and veins, blot retinal hemorrhage, filling defects, areas of nonperfusion in the peripheral retina, and segmental leakage in peripheral veins; subsequently, VA worsened from 0.5 to 0.3 | Oral prednisolone 30 mg per day, sub-Tenon triamcinolone acetonide injection (20 mg/0.5 mL), and 0.1% betamethasone eye drops QID | Vascular sheathing disappeared, VA recovered to 0.5, aqueous flare decreased to levels comparable with fellow eye, and no evidence of inflammation on FA | ||
| Blurry vision, floaters, and redness | VA reduced to 0.6 (from 1.5), slight conjunctival injection, AC cell (1+), fine keratic precipitates, vitreous cell (2+), thick vitreous opacities, blot hemorrhage in the peripheral retina, filling defects in the peripheral retinal arteries and veins, an area of nonperfusion in the peripheral retina, and diffuse leakage from retinal vessels and optic nerve head; subsequently VA worsened to 0.3 | Oral prednisolone 30 mg per day, sub-Tenon triamcinolone acetonide injection (20 mg/0.5 mL), and 0.1% betamethasone eye drops QID | Decreased leakage, VA recovered to 1.5, improvement in vitreous haze, and disappearance of retinal hemorrhage | ||
| Kaupke, 202137 | 1 eye | VA loss that had persisted for 2 days, redness, and feeling of pressure | AC cells and conjunctival injection; subsequently, VA reduced to HM from 0.1, retinal hemorrhages, and increased AC irritation | Dexamethasone and gentamicin eye drops QID; subsequently, prednisolone 40 mg increased to 100 mg for 3 days | 5 months after first presentation, the eye was completely blind and showed retinal atrophy |
| Kessler, 202130 | 2 eyes of 1 patient | “Thundercloud” appearance OS after first brolucizumab injection; vision loss OU after second brolucizumab injection | BCVA was 0.1 OD and 0.05 OS, granulomatous precipitates, pronounced AC flare, and numerous vitreous cells. Vision OS was blurred, and ischemia of the upper hemisphere of the retina was visible. Vessels showed leakage in OU | Vitrectomies with subsequent intravitreal dexamethasone implants OU; intravenous methylprednisolone 1 g for 3 days, 500 mg for 2 days, and 100 mg for the final 6 days; local prednisolone acetate hourly and ofloxacin QID; oral cortisone therapy | No renewal of inflammation and subsequently VA stabilized OU at 0.32 |
| Leclaire, 202138 | 1 eye | Pain and visual deterioration | BCVA decreased to 1/15 m (from 0.4), AC cells, deposits on the endothelium, vitreous opacities, perivascular leakage, nonperfused areas, and vascular occlusions | Intravenous prednisolone 80 mg daily | AC and vitreous cleared, BCVA improved to 0.125, and perivascular ischemia was visible over the vascular arches |
| Maruko, 202123 | 12 eyes of 12 patients | — | IOI consisting of AC cells and/or vitreous cells (12 eyes), with IOI + RV in 4 eyes and IOI + RV + RO in 2 eyes. RO was located outside the vascular arcades | IOI was treated with 0.1% betamethasone eye drops; RV was treated with sub-Tenon injection of triamcinolone acetonide 20 mg | VA decreased from 74 ETDRS letters to 35, then improved to 65 in 1 eye with RO. VA decreased from 59 letters to 50, then recovered to 65 in the other eye with RO |
| Narayanan, 202139 | 1 eye | Loss of vision, mild pain, redness | VA reduced to HM (from 20/250), minimal conjunctiva congestion, no lid edema, and 1-mm hypopyon, intense vitritis. Microbiological testing and polymerase chain reaction were negative | Topical steroids administered hourly, vitrectomy biopsy with intravitreal ceftazidime and vancomycin, topical moxifloxacin 6 times daily, prednisolone acetate hourly, and homatropine eye drops TID | Inflammation resolved significantly, and fundus details were visible. No evidence of vasculitis. VA improved to 20/600 |
| Riedel, 202131 | 2 eyes of 1 patient | VA reduced to 0.2 OD (from 0.5) and HM OS (from 0.63) | Vitreous cells, papilledema, thin partly white arteries, cotton-wool spots, bleeding along vessels, highly reflective band in the outer plexiform layer, delayed retinal filling, prolonged arteriovenous passage time, and vascular occlusion | Oral prednisolone 50 mg, oral esomeprazole 40 mg, dexamethasone drops QID, and dorzolamide hydrochloride drops BID. OS also received intravitreal dexamethasone through implant | VA improved to 0.5 OD and 1/35 of a meter OS |
AC, anterior chamber; BCVA, best-corrected visual acuity; BID, 2 times per day; ETDRS, Early Treatment Diabetic Retinopathy Study; FA, fluorescein angiography; HM, hand motion; IOI, intraocular inflammation; IRF, intraretinal fluid; OD, right eye; OS, left eye; OU, both eyes; QID, 4 times per day; RO, retinal vascular occlusion; RV, retinal vasculitis; SRF, subretinal fluid; TID, 3 times per day; VA, visual acuity.
Considerations for Treating With Brolucizumab
Identification of patients for treatment with brolucizumab
The decision to use brolucizumab in a patient with nAMD is based on a benefit–risk assessment. Brolucizumab has been shown to have a benefit in reducing retinal fluid and to allow for 12-week dosing intervals in some patients; however, the risk of RO and vision loss after brolucizumab must be considered.8,13,28,29 It is notable that despite RV and RO events after brolucizumab, the rates of moderate vision loss were similar between brolucizumab- and aflibercept-treated patients,28 highlighting that inadequate control of exudation may also be an important cause of vision loss, and this must be considered during treatment decision-making. Risk of IOI-related adverse events after brolucizumab has been estimated from the incidence of these events in clinical trials and real-world databases.13,27 According to the post hoc analysis of HAWK and HARRIER by the SRC, the rate of RO after brolucizumab was 2.1% (23/1,088), and the rate of any form of IOI and losing 15 or more letters after brolucizumab was 0.7% (8/1,088).13 According to retrospective analyses of the IRIS Registry and Komodo Healthcare Map, the incidences of RV and/or RO after administration of brolucizumab were 0.6% with up to 6 months of follow-up.27 No significant difference was found between treatment-naive eyes and eyes that were switched from another anti-VEGF in rates of IOI-related adverse events after brolucizumab.23,27 In addition to the general incidence of these events, risk factors for IOI and RO should be considered to help indicate possible increased risk of RO for certain patients. The post hoc analysis of HAWK and HARRIER found that female sex and Japanese ethnicity were possible risk factors for IOI after brolucizumab.23 Retrospective analyses of the IRIS Registry and Komodo Healthcare Map replicated the finding of female sex as a potential risk factor for IOI and/or RO (IRIS: odds ratio [OR] = 2.23) and for RV and/or RO (IRIS: OR = 2.89) after brolucizumab.27 Risk of RV and/or RO was estimated to be 0.65% for women versus 0.22% for men (IRIS).27 In addition, history of IOI and/or RO within the 12 months before the initiation of brolucizumab was found to be a potential risk factor for IOI and/or RO (IRIS: OR = 4.69) and for RV and/or RO (IRIS: OR = 12.53) after brolucizumab.27 Risk of RV and/or RO among those with previous IOI and/or RO compared with those without previous IOI and/or RO was 3.97% versus 0.32% (IRIS).27 These findings suggest history of IOI or RO is a relatively strong risk factor for RV and/or RO. Physicians should inquire about a patient's history of IOI and RO for the benefit–risk assessment. The consequences of vision loss after brolucizumab for a particular eye should also be considered. For instance, patients with poor vision in the fellow eye may have a different perspective than those with good vision in the fellow eye. The risk of bilateral vision loss after brolucizumab should also be acknowledged when conducting a benefit–risk assessment for bilateral injections.30,31 Importantly, the patient's preferences should be considered when deciding whether to use brolucizumab.
Patient Counseling and Education
Before initiation of treatment with brolucizumab, the risk of RO and vision loss after brolucizumab should be explained to the patient along with an explanation that the RO typically occurs in the presence of other signs of IOI.7,15 Patients should be educated on the signs and symptoms associated with IOI after brolucizumab and the importance of immediately returning to the clinic if the patient experiences such signs or symptoms. Signs and symptoms reported in patients who developed RV and RO after brolucizumab have included reduced or blurred vision, floaters, pain or discomfort, redness, and scotoma.14,15,21,22 Signs and symptoms reported in recent cases of IOI after brolucizumab are shown in Table 1. The likely time course of symptom onset from the last brolucizumab injection is also important for patient education. In a post hoc analysis of HAWK and HARRIER, the median time to the onset of the IOI-related adverse event was 25.5 days (range = 1–91) in patients who received brolucizumab.13 Similarly, a postmarketing study of 26 eyes with RV after administration of brolucizumab found that the mean time to presentation of the adverse event was 26 days (range = 3–63).15 Based on these findings, IOI symptoms after brolucizumab may develop anytime between injections.
Follow-up Visits
For patients who receive brolucizumab, it has been considered that additional follow-up visits beyond those required for monitoring and treatment of nAMD may help increase the likelihood of early detection of IOI and RO; however, additional visits work against the purpose of using brolucizumab to reduce treatment burden. As an alternative, thorough patient education, in addition to examining for IOI and RO at visits for nAMD, may be adequate if the patient knows to return to the clinic immediately on experiencing symptoms of IOI. Physicians may consider having a clinical staff member call the patient to conduct a screening for symptoms of IOI as a supplement to patient education. Prophylaxis for IOI has also been suggested as a possible way to avoid the need for additional follow-up visits; however, there is no evidence to support the effectiveness of prophylaxis for IOI or RO after brolucizumab. Furthermore, because most patients will never develop IOI or RO, this would involve administering prophylaxis to many patients who will never develop these adverse events.
Preinjection Assessment and Evaluation of Intraocular Inflammation
Before each injection of brolucizumab, it is important to assess the patient for signs of inflammation using a dilated slit-lamp examination, dilated fundoscopy, and optical coherence tomography because brolucizumab is contraindicated in the presence of active IOI.7,32 Although the majority of IOI cases after brolucizumab (74%) were found to occur in the first 6 months of treatment in HAWK and HARRIER, 14% occurred between 6 and 12 months, and an additional 12% of cases occurred after 12 months.13 Therefore, physicians should remain vigilant for signs of inflammation even after the first 6 months. Additional data are required to verify whether most IOI cases occur in the first 6 months. Full characterization of the location and severity of the inflammation, including the use of wide-field imaging and angiography, should be conducted to guide treatment decisions.10,24
Signs of IOI have been found in the anterior and/or the posterior segments in postmarketing studies of RV after brolucizumab. In a study of 26 eyes of 25 patients with RV after brolucizumab, IOI at presentation was found to be only anterior for 31% of eyes, only posterior for 27%, and both anterior and posterior for 35%. In 8% of eyes, IOI manifested only as RV.15 In another study of 15 eyes from 12 patients with RV, vitreous cells or opacities were observed in all eyes, and anterior chamber cells were observed in most eyes (73%).14 Anterior findings also included fine keratic precipitates, Descemet folds, flare, and corneal edema.14 Various features of RV and RO have been reported after brolucizumab, including vascular sheathing, boxcarring, narrowing, and occlusion, as well as filling defects, staining, choroidal hypofluorescence, and optic nerve leakage.14,15,21,22 IOI after brolucizumab is considered more severe if there is RV and even more severe if there is RO.14 Occlusions may affect the macula and/or the peripheral retina.14,15
Conditions to consider in the differential diagnosis of IOI after brolucizumab include infectious endophthalmitis and systemic diseases that may trigger RV.10,18 One important differentiating characteristic between infectious endophthalmitis and IOI after brolucizumab is that infectious endophthalmitis presents on average 3 to 4 days from the last intravitreal injection, whereas IOI after brolucizumab has been found to present on average approximately 26 days from the last intravitreal injection.13,15,33 However, the timing of presentation may not always distinguish between these conditions, and initial signs may be similar making the distinction challenging.10,24
Treatment of Intraocular Inflammation and Retinal Vascular Occlusion After Brolucizumab
The following treatment recommendations for IOI or RO after brolucizumab are the opinions of the authors. Treatment of IOI or RO after brolucizumab should be prompt and intensive.10 If any inflammation or RO is detected, brolucizumab should be discontinued and treatment for nAMD should be resumed with a different anti-VEGF agent when medically appropriate. This is a conservative approach, given that IOI does not always progress to RO.13 For inflammation confined to the anterior chamber, frequent administration of potent topical corticosteroids may be adequate to control the inflammation; however, there is the risk that inflammation will develop in the posterior segment of the eye,18 in which case topical corticosteroids would not suffice. Therefore, careful monitoring of the posterior segment for the development of inflammation is necessary, and administration of intravitreal, sub-Tenon, or systemic corticosteroids may be considered to address the potential development of posterior segment inflammation.21,22,26 If posterior segment inflammation is suspected or detected, intravitreal, sub-Tenon, or systemic corticosteroids should be administered. In case studies of RV or RO after brolucizumab, visual acuity returned to that observed before treatment with brolucizumab and sheathing of retinal vessels improved after injection of corticosteroids into the sub-Tenon capsule with or without oral corticosteroids.21,22 Other treatment methods would need to be considered for patients in whom corticosteroids are not tolerated or are contraindicated. Post hoc analyses of HAWK and HARRIER found that IOI-related events were typically treated with topical corticosteroids, and intraocular or systemic corticosteroids were rarely administered.26 Therefore, it is currently unknown what the approximate rates of RO and vision loss from brolucizumab would be with more intensive treatment of IOI. A summary of the authors' recommendations, based on expert opinion, is provided in Figure 2.
Fig. 2.
Recommendations for assessment, patient education, and management for IOI after brolucizumab based on the authors' opinions. Anterior uveitis, intermediate uveitis, and posterior uveitis are defined according to the SUN working group.34
Conclusions
This report provides guidance on the use of brolucizumab for nAMD based on the opinion of the authors. The decision to use brolucizumab depends on a benefit–risk assessment. Patients who receive brolucizumab should be educated on the signs and symptoms of IOI after brolucizumab, the possible time course of IOI, and the need to immediately return to the clinic if any signs or symptoms arise. Based on recommendations from Novartis, brolucizumab should not be administered more frequently than every 2 months after the initial three loading doses. Before each injection of brolucizumab, physicians should assess the anterior and posterior segments of the eye for signs of inflammation. If inflammation is detected, brolucizumab should be discontinued, and a full examination should be conducted to characterize the location and severity of the inflammation for the purpose of guiding treatment decisions. Treatment of IOI after brolucizumab should be prompt and intensive. Physicians should be aggressive in treating possible inflammation in the posterior segment of the eye.
Acknowledgments
Medical writing support was provided by Ann Todd (IMPRINT Science, New York, NY) and was funded by Novartis Pharma AG. This manuscript was developed in accordance with Good Publication Practice (GPP3) guidelines. The authors had full control of the content and made the final decision on all aspects of this publication.
Footnotes
Medical writing support was funded by Novartis Pharma AG (Basel, Switzerland). F.G. Holz reports research grants and personal fees from Acucela, Allergan, Apellis Pharmaceuticals, Bayer, Bioeq/Formycon, Chengdu Kanghong Pharmaceutical Group, Geuder, Heidelberg Engineering, IVERIC bio, Novartis, Roche/Genentech, and Zeiss and personal fees from Boehringer Ingelheim, Graybug Vision, Lin BioScience, Pixium Vision, Stealth BioTherapeutics, Aerie, and Oxurion. T. Iida received grants from NIDEK (Japan) and Senju Pharmaceutical (Japan); grants and personal fees from Alcon Pharma (Japan) and Santen Pharmaceutical (Japan); and personal fees from Bayer Yakuhin (Japan). T. Iida also received research support from Canon (Japan), Kowa Pharmaceuticals (Japan), and Topcon (Japan) outside the submitted work. I. Maruko received grants from Japan Society for the Promotion of Science KAKENHI (Grant Number JP20K09781); grants and personal fees from Alcon Pharma; and personal fees from Bayer Yakuhin, Santen Pharmaceutical, Alcon Japan, Topcon, Senju Pharmaceutical, and NIDEK outside the submitted work. S.R. Sadda reports being a consultant for Allergan/AbbVie, Amgen, Apellis Pharmaceuticals, CenterVue, 4D Molecular Therapies, Heidelberg Engineering, IVERIC Bio, Nanoscope Therapeutics, Novartis, Optos, Oxurion, Regeneron Pharmaceuticals, and Roche/Genentech and research instruments from CenterVue, Carl Zeiss Meditec, Heidelberg Engineering, Nidek, Optos, and Topcon.
None of the authors has any financial/conflicting interests to disclose.
Contributor Information
Tomohiro Iida, Email: iida@twmu.ac.jp.
Ichiro Maruko, Email: imaruko@twmu.ac.jp.
SriniVas R. Sadda, Email: vassadda@gmail.com.
References
- 1.Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol 2015;38:620–627. [DOI] [PubMed] [Google Scholar]
- 2.Ehlken C, Helms M, Böhringer D, et al. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol 2017;12:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacCumber MW. Real-world injection intervals in wet AMD. Retina Today May/June 2020 Special Report.
- 4.Varano M, Eter N, Winyard S, et al. Current barriers to treatment for wet age-related macular degeneration (wAMD): findings from the wAMD patient and caregiver survey. Clin Ophthalmol 2015;9:2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology 2021;128:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer DS, Heier JS, Brown DM, et al. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009;116:1731–1739. [DOI] [PubMed] [Google Scholar]
- 7.Beovu. Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2022. [Google Scholar]
- 8.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020;127:72–84. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen QD, Das A, Do DV, et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 2020;127:963–976. [DOI] [PubMed] [Google Scholar]
- 10.Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and/or vascular occlusion after brolucizumab treatment. Ophthalmol Retina 2021;5:519–527. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-related retinal vasculitis: emerging disconnect between clinical trials and real world. Eye (Lond) 2021;35:1292–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beovu® (brolucizumab): global use and safety information for healthcare professionals. Available at: https://www.brolucizumab.info. Accessed June 1, 2021.
- 13.Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology 2021;128:1050–1059. [DOI] [PubMed] [Google Scholar]
- 14.Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology 2020;127:1345–1359. [DOI] [PubMed] [Google Scholar]
- 15.Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis 2020;4:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep 2020;18:100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondapalli SSA. Retinal vasculitis after administration of brolucizumab resulting in severe loss of visual acuity. JAMA Ophthalmol 2020;138:1103–1104. [DOI] [PubMed] [Google Scholar]
- 18.Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep 2020;18:100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer PG, Peden MC, Suñer IJ, et al. Brolucizumab-related retinal vasculitis with exacerbation following ranibizumab retreatment: a clinicopathologic case study. Am J Ophthalmol Case Rep 2020;20:100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antaki F, Vadboncoeur J. Retinal vasculitis after intravitreal injection of brolucizumab. Can J Ophthalmol 2022;57:e40. [DOI] [PubMed] [Google Scholar]
- 21.Hikichi T. Three Japanese cases of intraocular inflammation after intravitreal brolucizumab injections in one clinic. Jpn J Ophthalmol 2021;65:208–214. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka K, Horiguchi E, Kawano K, et al. Three cases of brolucizumab-associated retinal vasculitis treated with systemic and local steroid therapy. Jpn J Ophthalmol 2021;65:199–207. [DOI] [PubMed] [Google Scholar]
- 23.Maruko I, Okada AA, Iida T, et al. Brolucizumab-related intraocular inflammation in Japanese patients with age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol 2021;259:2857–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holz FG, Heinz C, Wolf A, et al. [Intraocular inflammation with brolucizumab use: patient management-diagnosis-therapy]. Ophthalmologe 2021;118:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-foreseeable workflow in the current scenario. Eye (Lond) 2021;35:1548–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, Albini TA, Seres A, et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina 2022;6:97–108. [DOI] [PubMed] [Google Scholar]
- 27.Khanani AM, Zarbin MA, Barakat MR, et al. Safety outcomes of brolucizumab in neovascular age-related macular degeneration: results from the IRIS registry and Komodo Healthcare Map. JAMA Ophthalmol 2022;140:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2021;128:89–99. [DOI] [PubMed] [Google Scholar]
- 29.Bulirsch LM, Saßmannshausen M, Nadal J, et al. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol:2021. Published online April 12, 2021. [DOI] [PMC free article] [PubMed]
- 30.Kessler LJ, Mayer CS, Son HS, et al. Bilateral vasculitis following intravitreal brolucizumab injection(in German). Ophthalmologe 2022;119:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel AM, Lackerbauer C, Lohmann CP, Ulbig M. [Bilateral occlusive vasculitis after intravitreal injection of brolucizumab in neovascular age-related macular degeneration]. Ophthalmologe 2022;119:75–78. [DOI] [PubMed] [Google Scholar]
- 32.Summary Beovu of Product Characteristics. Dublin, Ireland: Novartis Europharm Limited; 2022. [Google Scholar]
- 33.Rayess N, Rahimy E, Storey P, et al. Postinjection endophthalmitis rates and characteristics following intravitreal bevacizumab, ranibizumab, and aflibercept. Am J Ophthalmol 2016;165:88–93. [DOI] [PubMed] [Google Scholar]
- 34.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 2005;140:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angerer MPM, Neuburger M, Hille K, Horn PC. [Vaso-occlusive retinitis following intravitreal injection of brolucizumab]. Ophthalmologe 2021;118:1048–1050. [DOI] [PubMed] [Google Scholar]
- 36.Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol 2021;139:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaupke N, Stübiger N, Dulz S, et al. [Acute unilateral loss of vision after intravitreal injection of a VEGF inhibitor]. Ophthalmologe 2021;118:1276–1279. [DOI] [PubMed] [Google Scholar]
- 38.Leclaire MD, Lauermann J, Alten F, Eter N. [Intraocular inflammation with occlusive retinal vasculitis following intravitreal injection of brolucizumab]. Ophthalmologe 2022;119:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan R, Tyagi M, Gupta SR, et al. Immediate onset of sterile endophthalmitis with hypopyon after intravitreal brolucizumab in a case of polypoidal choroidal vasculopathy. Indian J Ophthalmol 2021;69:469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]



