Abstract
Background:
Knee osteoarthritis (OA) is a leading cause of pain in older adults. Previous studies indicated clinic-based transcranial direct current stimulation (tDCS) was effective to reduce pain in various populations, but no published studies have reported the efficacy of home-based self-administered tDCS in older adults with knee OA using a randomized clinical study.
Objective:
The purpose of this study was to evaluate the efficacy and feasibility of tDCS on clinical pain intensity in adults with knee OA pain.
Methods:
One hundred twenty participants aged 50e85 years with knee OA pain were randomly assigned to receive fifteen daily sessions of 2 mA tDCS for 20 min (n = 60) or sham tDCS (n = 60) over 3 weeks with remote supervision via telehealth. Clinical pain intensity was measured by the Numeric Rating Scale and Western Ontario and McMaster Universities Osteoarthritis Index. Also, we collected data on the tDCS experience via a questionnaire.
Results:
Participants (68% female) had a mean age of 66 years. Active tDCS significantly reduced pain intensity compared to sham tDCS after completion of the fifteen daily sessions (Cohen’s d = 1.20; p-value < 0.0001). Participants showed high levels of satisfaction with their tDCS experience, and there have been no adverse events.
Conclusion:
We demonstrated that home-based self-administered tDCS was feasible and reduced clinical pain intensity in older adults with knee OA, which can increase its accessibility. Future studies with multi-site randomized controlled trials are needed to validate our findings.
Trial registration:
ClinicalTrials.gov Identifier NCT04016272.
Keywords: Transcranial direct current stimulation, Knee osteoarthritis, Pain, Efficacy, Acceptability, Feasibility
1. Introduction
Arthritis is the leading cause of work disability in the United States (US) with annual costs for medical care and lost earnings of $303.5 billion [1] and the number of people affected is predicted to reach 78 million adults by 2040 [2]. Osteoarthritis (OA) is the most frequent form of arthritis, with knee OA showing the highest incidence [3]. Pain, the main symptom, poses a considerable threat for global functioning [4]. In fact, about 42% of diagnosed individuals report its interference with daily activities [5] and 27% report severe joint pain which gravely compromises quality of life [6]. OA-related pain, intermittent or constant, includes both peripheral and central sensitizations, involving nociceptive, inflammatory and neuropathic pain patterns and increasing the challenge of relieving it [4].
The current standard of care for clinical treatment comprises mainly of the prescription of analgesic medications. This approach has limited efficacy for knee OA pain and can lead to significant adverse effects, especially in older adults [7–9]. Among non-pharmacological interventions, psychological approaches have been traditionally used with inconclusive results on pain relief [10]. Nevertheless, with central sensitization being clearly highlighted in relation to knee OA pain [11,12], non-invasive brain stimulation, more specifically transcranial direct current stimulation (tDCS) [13], is promising as it targets the central nervous system, and has shown a neuromodulatory effect in other chronic pain conditions [14–20]. Briefly, a painless electric current is applied to the scalp and is believed to generate analgesic effects by modulating resting membrane potentials of neurons and pain processing pathways in the targeted cortical area [21]. Recent studies have shown preliminary efficacy in the clinical and home-based settings in patients with fibromyalgia and multiple sclerosis [18,19,22,23].
We previously examined the feasibility and efficacy of tDCS on knee OA pain in the clinical setting with a pilot randomized controlled trial and obtained significant benefits on pain without serious adverse effects [24]. We, then, anticipated that it could be feasible to try this approach in the home setting. Additionally, persons with OA are usually older and have limited mobility and access to transportation while this treatment approach requires daily visits to a clinic. Successfully implementing home-based tDCS would ensure a greater accessibility and sustainability of this treatment approach. Our pilot trial consisted of a two-week home-based tDCS with real-time monitoring (secure videoconferencing) for people suffering from knee OA [25]. The intervention was found acceptable in this setting and significantly reduced pain, setting the stage for a large-scale randomized controlled trial.
Thus, the purpose of this study was to assess the efficacy of a self-administered tDCS treatment in older adults with knee OA on clinical pain intensity. Our hypothesis was that self-administered tDCS would reduce clinical pain intensity compared to sham tDCS. The specific aims were as follows: (1) To determine the efficacy of self-administered tDCS on clinical pain intensity in older adults with symptomatic knee OA, and (2) To investigate the acceptability and feasibility self-administered tDCS in older adults with symptomatic knee OA.
2. Material and methods
Design.
After obtaining ethical approval from the Institutional Review Board (IRB) and registering at www.clinicaltrials.gov (NCT04016272), we conducted a double-blind (participant and experimenter), randomized, sham-controlled, phase II parallel-group study with two groups (sham and active tDCS).
Participants.
In this study, 120 individuals who meet eligibility criteria and give informed consent were randomly assigned to two groups (i.e., 60 participants in each group) using the order of entrance in the study and previous randomization list generated via SAS software (version 9.4) by a statistician with no clinical involvement in this trial based on a covariate adaptive randomization procedure. The randomization balanced the allocation of patients to the study arms with respect to distributions of age, race, and sex.
With this sample size, we expected to be able to detect an effect size of 0.89 with more than 99% power at a significance level of 0.05 after accounting for 10% attrition. The minimum effect size we could detect with this sample size was 0.54 with 80% power at a significance level of 0.05 after accounting for 10% attrition. Thus, our sample size provided sufficient power to detect a clinically meaningful effect.
Similar to our previous work [24], participants who were 50–85 years old were considered eligible if they (1) had symptomatic knee OA based on American College of Rheumatology Clinical criteria (knee radiographs were taken to determine OA severity using Kellgren-Lawrence scores) [26], (2) had had knee OA pain in the past 3 months with an average of at least 30 on a 0–100 numerical rating scale (NRS) for pain, (3) could speak and read English, and (4) had no plan to change medication regimens for pain throughout the trial. According to American College of Rheumatology criteria [26], participants should meet at least 3 of 6 criteria, including age >50 years, stiffness <30 min, crepitus, bony tenderness, bony enlargement, and no palpable warmth. Participants were excluded if they had any concurrent medical conditions that could confound the interpretation of outcome measures, posed a safety risk for any of the assessment or tDCS procedures, or precluded the successful completion of the protocol. Specific exclusion criteria were: (1) prosthetic knee replacement or non-arthroscopic surgery to the affected knee, (2) history of brain surgery, brain tumor, seizure, stroke, or intracranial metal implantation, (3) systemic rheumatic disorders, including rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia, (4) alcohol/substance abuse, (5) current use of sodium channel blockers, calcium channel blockers and NMDS receptor antagonists, (6) diminished cognitive function that would interfere with understanding study procedures (i.e., Mini-Mental Status Exam score ≤23), (7) pregnancy or lactation, (8) hospitalization within the preceding year for psychiatric illness, and (9) no access to a device with internet access that can be used for secure videoconferencing for real-time remote supervision.
Participants were recruited in Southeast Texas. The study was advertised around local institutions (e.g., UTHealth) and communities by using study flyers. Moreover, our recruitment strategy involved screening and recruiting potential participants from the UT Physicians Orthopedics Clinic.
tDCS Intervention.
Participants received a brief training to explain the procedure and how to use the device. Participants were trained at the baseline visit until they feel comfortable using the tDCS device. Participants were shown how to apply and use the tDCS device, then asked to demonstrate this to the investigator, and given feedback until they can comfortably apply and use the tDCS device. After the research staff confirms that the participant has understood all details of the stimulation, the tDCS device and device kit organized by day for ease of use were given to the participant with written instructions with pictures in the form of a manual. For pain treatment, tDCS is typically delivered with the anode electrode placed over the primary motor cortex (M1) and with the cathode electrode placed over the supraorbital region (SO) [17,21]. A panel of experts from the European Chapter of the International Federation of Clinical Neurophysiology recommends 20-min M1-SO stimulation using 2 mA electrical current intensity for possible efficacy among populations with chronic pain [13]. tDCS with a constant current intensity of 2 mA was applied for 20 min per session daily for 3 weeks via the Soterix 1 × 1 tDCS mini-CT Stimulator device (Soterix Medical Inc., NY; 6.5 inches long, 3 inches wide, 0.7 inches thick) with headgear and 5 × 7 cm saline-soaked surface sponge electrodes. Electrical current was ramped up and ramped down over 30 s at the beginning and end of the stimulation period, respectively. The sponge electrodes snap into the custom headgear, which was secured to the participant’s head for simple and fail-safe electrode preparation. This single-position headgear with clearly labeled sponge markers eliminated room for user error and helped conserve the placement of the montage. Participants could only administer a stimulation session via the Soterix 1 × 1 tDCS mini-CT Stimulator device after being provided a single-use unlock code for each session by the research staff once proper contact quality was achieved (only the on/off button was adjustable by the study participants; they were not able to adjust the device settings) during real-time remote supervision. This device has built-in double-blinding protocols requiring the entry of a five-digit code to initiate stimulation. Each subject-specific code, used to activate the programmed stimulation sequence, is one-time use only and can only be activated only once. The experimenter and participant were blind to group allocation. After the participant entered the unlock code, the screen on the device showed a timer that counted down the minutes until the end of the session. At 20 min, the device turned off automatically, and study staff instructed the participant to remove the headset and discard the sponges and to safely store all materials for the next session. For sham stimulation, the electrodes were placed in the same positions as for active stimulation, but the stimulator only delivered 2 mA current for 30 s. This sham stimulation method has been shown to be reliable and indistinguishable from active treatment [14,27].
Clinical Pain Intensity.
Following the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations for clinical trials involving chronic pain [28], clinical pain intensity changes assessed through a numeric rating scale (NRS) was our primary outcome measure for data analysis purposes with a primary endpoint being at 3 weeks (at the end of the 15 treatment sessions) and a secondary endpoint at 3 months. Clinical pain intensity was measured by asking participants to rate their average knee pain over the past 24 h via NRS from 0 (no pain) to 100 (worst pain imaginable). The NRS is a reliable and well-validated measure with good ability to detect pain change in adults with knee OA [29]. We also collected Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [30], which ranges from 0 to 96, with higher scores indicating worse OA symptoms. The WOMAC has been reported to have a Cronbach’s alpha coefficient of 0.86–0.89 among patients with knee and hip OA.
Acceptability and Feasibility.
We collected data on participants’ tDCS experience via a questionnaire, adapted from Gillick et al. [31] and Cha et al. [32], at the conclusion of tDCS treatment on a 0 (strongly disagree) to 10 (strongly agree) scale: 1) It was easy to prepare the device and accessories; 2) The device was unnecessarily complex; 3) The device was easy to use; 4) I felt the video conferences with a technical person were helpful; 5) I would imagine that most people would learn to use this device quickly; 6) The device was cumbersome to use; 7) I felt confident using the device; 8) I needed to learn a lot of things before I could get going with this device; 9) The effectiveness of the treatment increased over the course of treatment; 10) Overall, I felt that transcranial electrical stimulation treatment benefited me. Participants were also encouraged to elaborate their answers in free form.
We evaluated the presence and severity of possible side effects of treatment at the end of each session on a 0 (not at all) to 10 (highest degree) scale. By using open-ended questions, the participants were asked whether they experienced any side effects, and they were then asked specifically about tingling, itching sensation, burning sensation, pain at the stimulation site, fatigue, nervousness, headache, difficulty concentrating, mood change, and changes in vision or visual perception. If any side effects were reported, the degree of relatedness to the intervention was assessed on a 5-point scale. This approach has been used in our previous study and frequently in other studies [24,33,34].
Data analysis.
All statistical analyses were conducted using R Statistical Software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria). The raw data was examined for missing values and outliers. Unless otherwise stated, statistical significance was established based on p < 0.05. The normality distribution assumption was checked via Shapiro’s test. The 3-week NRS and WOMAC score changes from baseline were compared between the active and the sham tDCS groups, using Wilcoxon rank-sum test. A multivariate linear model was further employed for two-group comparison of 3-week clinical pain intensity changes with controlling for gender, age, and race. Bonferroni correction was employed for Type I error control when needed. A multivariate linear model was also employed to regress the 3-week NRS changes against multiple selected potential confounding factors, and stepwise model selection technique was then used to identify the parsimonious model. To verify whether the effects of tDCS was sustained or not at 3 months, the NRS changes from baseline to 3 months were compared with those from baseline to 3 weeks using paired Wilcoxon rank-sum test. Profile analysis based on a linear mixed-effects model was also performed to compare the trends of NRS and WOMAC score changes over time between the two groups. In addition, a generalized estimating equation (GEE) model was used to investigate the relationships between the longitudinal observations of NRS changes (from baseline to 1 week, 2 weeks, 3 weeks, 1 month, 2 months, and 3 months) and the same set of potential confounding factors mentioned before. Finally, sex-stratified analyses were performed to understand the outcome differences due to gender.
3. Results
Participants.
A total of 129 participants were assessed for eligibility, and of those, 6 participants declined to participate because they were not interested in this study. Therefore, 123 participants were randomly assigned to receive either active tDCS or sham tDCS. Of those, one participant for the active group and 2 participants for the sham group withdrew from the study because the patients could not attend the remaining sessions for personal reasons. All 120 participants who continued in the study completed all sessions and assessments. Fig. 1 depicts the flow of participants throughout the study. The mean age was 66 years (SD = 8.41) with 68.3% of females (n = 82). The average duration of OA was 70 months, approximately 6 years. The baseline demographic and clinical characteristics of participants are presented in Table 1.
Fig. 1.
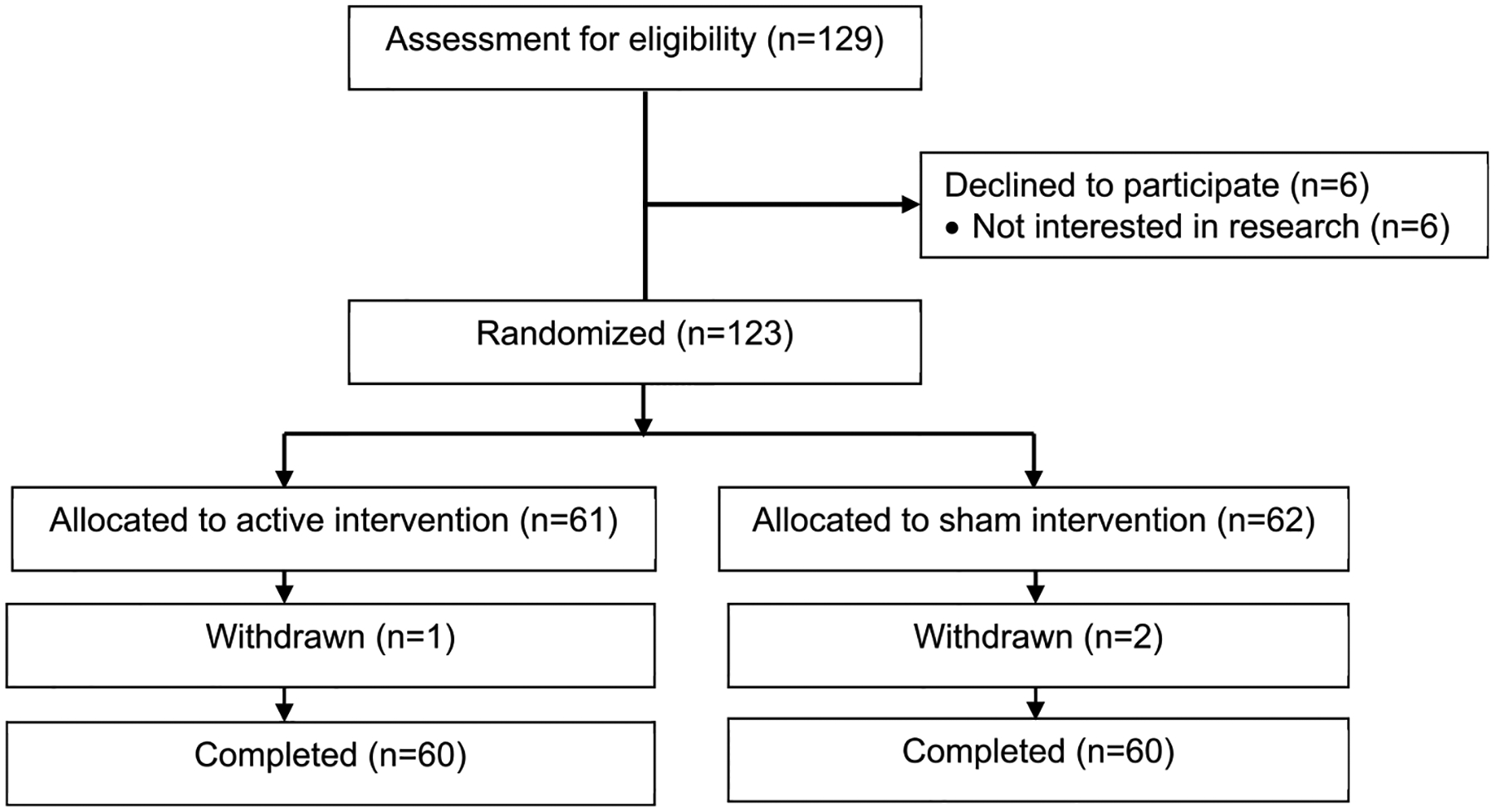
Study flow diagram.
Table 1.
Baseline demographic and clinical characteristics of the participants.
| Sham tDCS (n = 60) | Active tDCS (n = 60) | P-value | |
|---|---|---|---|
| Age, years, M (SD) | 66.60 (8.43) | 65.32 (8.41) | 0.54 |
| Gender, n (%) | 0.70 | ||
| Male | 18 (30.0%) | 20 (33.3%) | |
| Female | 42 (70.0%) | 40 (66.7%) | |
| Race, n (%) | 0.23 | ||
| American Indian or Alaska Native | 0 (0.0%) | 2 (3.3%) | |
| Asian | 2 (3.3%) | 6 (10.0%) | |
| African American | 22 (36.7%) | 19 (31.7%) | |
| White | 32 (53.3%) | 26 (43.3%) | |
| Hispanic or Latino | 4 (6.7%) | 7 (11.7%) | |
| BMI, kg/m2, M (SD) | 32.52 (8.30) | 32.70 (8.73) | 0.90 |
| OA duration, months, M(SD) | 69.25 (82.87) | 71.35 (75.86) | 0.89 |
| Marital Status, n (%) | 0.70 | ||
| Married | 29 (48.3%) | 33 (55.0%) | |
| Widowed | 9 (15.0%) | 4 (6.7%) | |
| Divorced | 10 (16.7%) | 10 (16.7%) | |
| Separated | 2 (3.3%) | 1 (1.7%) | |
| Never married | 7 (11.7%) | 7 (11.7%) | |
| Living with partner | 3 (5.0%) | 5 (8.3%) | |
| NRS, M(SD) | 50.63 (21.77) | 55.05 (21.96) | 0.27 |
| WOMAC, M(SD) | 43.88 (15.58) | 42.00 (17.05) | 0.53 |
| K/L radiographic score, n (%) | 0.37 | ||
| Grade 1 | 7 (11.7%) | 9 (15.0%) | |
| Grade 2 | 20 (33.3%) | 20 (33.3%) | |
| Grade 3 | 29 (48.3%) | 22 (36.7%) | |
| Grade 4 | 4 (6.7%) | 9 (15.0%) |
Note. M = Mean, SD = Standard Deviation, BMI = Body Mass Index; NRS = Numeric Rating Scale of Pain, ranging 0 (no pain) to 100 (worst pain imaginable); WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index, ranging from 0 to 96, with higher scores indicating worse osteoarthritis symptoms; K/L = Kellgren/Lawrence (grade 1: doubtful joint space narrowing and possible osteophytic lipping; grade 2: definite osteophytes and possible joint space narrowing, grade 3: multiple osteophytes, definite joint space narrowing, sclerosis, possible bony deformity, grade 4: large osteophytes, marked joint space narrowing, severe sclerosis and definite bony deformity).
Clinical Pain Intensity.
The pain intensity (NRS) outcome was found skewed while close to Gaussian. Using Wilcoxon rank-sum test, the NRS changes from baseline to 3 weeks were found significantly different between the active and the sham tDCS groups (Cohen’s d = 1.20; p-value < 0.0001); however, WOMAC changes from baseline to 3 weeks was found not significantly different between the two groups (Cohen’s d = 0.23; p-value = 0.10). Table 2 shows the NRS and WOMAC changes at 3 weeks and 3 months from baseline for both the sham and the active groups. The average decrease in NRS at 3 weeks from baseline was 24.07 ± 21.55 for the active group while it was only 1.08± 16.46 for the sham group. The average decrease in NRS at 3 months from baseline was 14.27± 24.94 for the active group while only 0.43± 25.42 for the sham group. The WOMAC score decreased by 10.95± 14.20 at 3 weeks from baseline for the active group and 8.08± 10.56 for the sham group; at 3 months from baseline, the WOMAC score decreased by 8.92± 16.35 for the active group and 8.67± 14.64 for the sham group. Finally, responders were defined as those subjects who had at least a 30% reduction in NRS scores from baseline to 3 weeks; for the active tDCS group, 36 responders and 24 non-responders were found; and for the sham tDCS group, 14 responders and 46 non-responders were found.
Table 2.
Comparison of pain intensity changes between two groups at 3 weeks and 3 months from baseline.
| Variable | Sham group (n = 60) | Active group (n = 60) | Effect size (d) | Wilcoxon-Statistic | P value |
|---|---|---|---|---|---|
| NRS Change (3-week) | −1.08 ± 16.46 | −24.07 ± 21.55 | 1.20 | 2879.5 | <0.0001 |
| NRS Change (3-month) | −0.43 ± 25.42 | −14.27 ± 24.94 | 0.55 | 2392.5 | <0.01 |
| WOMAC Change (3-week) | −8.08 ± 10.56 | −10.95 ± 14.20 | 0.23 | 2110 | 0.10 |
| WOMAC Change (3-month) | −8.67 ± 14.64 | −8.92 ± 16.35 | 0.02 | 1912.5 | 0.56 |
Note. Mean ± standard deviation is presented in the first two columns. NRS, Numeric Rating Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Regarding the influence of sociodemographic factors on pain intensity changes at 3 weeks, the multivariate regression analysis in relation to gender, age, and race showed that: 1) the pain intensity changes were not significantly different between male and female; 2) age was not significantly associated with pain intensity changes; 3) only the pain intensity changes of American Indian or Alaska Native was significantly different from those of American Caucasians (p-value = 0.04). However, a further examination of our data showed that the number of American Indian or Alaska Native was very small in this study (n = 2, preventing us from making any reliable conclusion). After including other potential confounding factors in the multivariate linear model, we performed stepwise variable selection and found that treatment group (p-value < 0.0001), baseline score for pain, stiffness, and function as measured by the WOMAC (p-value < 0.001), and baseline pain intensity score as measured by the NRS (p-value < 0.0001) were significant with respect to 3-week NRS changes.
In addition, to examine the sustained effects of tDCS, we compared the 3-week pain intensity changes with the 3-month pain intensity changes from baseline using paired Wilcoxon test for the treatment group. The corresponding p-value was less than 0.01 and thus there existed a significant difference between the 3-week and the 3-month effects (3-week vs baseline Cohen’s d = 1.12; 3-month vs baseline Cohen’s d = 0.57). While the mean decrease in NRS scores at 3 months was found less than that at 3 weeks (3-week vs 3-month Cohen’s d = 0.42), the NRS scores at 3 months were also found significantly less than those at the baseline (p-value < 0.001). Therefore, we could conclude that even if the tDCS effect diminishes over time, the improvement conferred by tDCS can be sustained at three months.
Tables 3 and 4 show the profile analysis results based on linear mixed-effects model that was performed to compare the temporal trends in NRS and WOMAC changes from baseline to 3 weeks between the active and the sham groups (see also Figs. 2 and 3), with statistical significance. For the active tDCS group, a decreasing trend of NRS over time was found significant while no significance was detected in the NRS score trend for the sham tDCS group (Table 3; the overall p-value corresponding to the interaction between the time factor and the treatment group factor is less than 0.01). However, the profile analysis results suggested that WOMAC trends over 3 weeks were not significantly different between the active and the sham tDCS groups (Table 4; the overall p-value corresponding to the interaction between the time factor and the treatment factor is greater than 0.05).
Table 3.
Profile analysis results for Numeric Rating Scale.
| Variable | Estimate | Standard Error | 95% Confidence Intervals | t Value | P value | |
|---|---|---|---|---|---|---|
| Intercept | 50.63 | 3.02 | 44.74 | 56.53 | 16.76 | <0.01 |
| Week 1 | −5.87 | 2.51 | −10.76 | −0.98 | −2.34 | 0.02 |
| Week 2 | −7.43 | 2.51 | −12.32 | −2.54 | −2.96 | <0.01 |
| Week 3 | −1.08 | 2.51 | −5.97 | 3.81 | −0.43 | 0.67 |
| active tDCS | 4.42 | 4.27 | −3.92 | 12.76 | 1.03 | 0.30 |
| Week 1: active tDCS | −3.25 | 3.55 | −10.17 | 3.67 | −0.92 | 0.36 |
| Week 2: active tDCS | −9.63 | 3.55 | −16.55 | −2.72 | −2.71 | <0.01 |
| Week 3: active tDCS | −22.98 | 3.55 | −29.90 | −16.07 | −6.48 | <0.0001 |
Note. The baseline is used as the reference level for the time factor, and the sham tDCS group is used as the reference level for the intervention factor. “Week 1: active tDCS” denotes the interaction between the time factor and the treatment.
Table 4.
Profile analysis results for Western Ontario and McMaster Universities Osteoarthritis Index.
| Variable | Estimate | Standard Error | 95% Confidence Intervals | t Value | P value | |
|---|---|---|---|---|---|---|
| Intercept | 43.88 | 2.27 | 39.44 | 48.33 | 19.31 | <0.0001 |
| Week 1 | −4.85 | 1.44 | −7.65 | −2.05 | −3.38 | <0.001 |
| Week 2 | −7.70 | 1.44 | −10.50 | −4.90 | −5.37 | <0.0001 |
| Week 3 | −8.08 | 1.44 | −10.88 | −5.29 | −5.63 | <0.0001 |
| active tDCS | −1.88 | 3.21 | −8.17 | 4.40 | −0.59 | 0.56 |
| Week 1: active tDCS | 0.12 | 2.03 | −3.84 | 4.07 | 0.06 | 0.95 |
| Week 2: active tDCS | −3.88 | 2.03 | −7.84 | 0.07 | −1.91 | 0.06 |
| Week 3: active tDCS | −2.87 | 2.02 | −6.82 | 1.09 | −1.41 | 0.16 |
Note. The baseline is used as the reference level for the time factor, and the sham tDCS group is used as the reference level for the intervention factor. “Week 1: active tDCS” denotes the interaction between the time factor and the treatment.
Fig. 2.
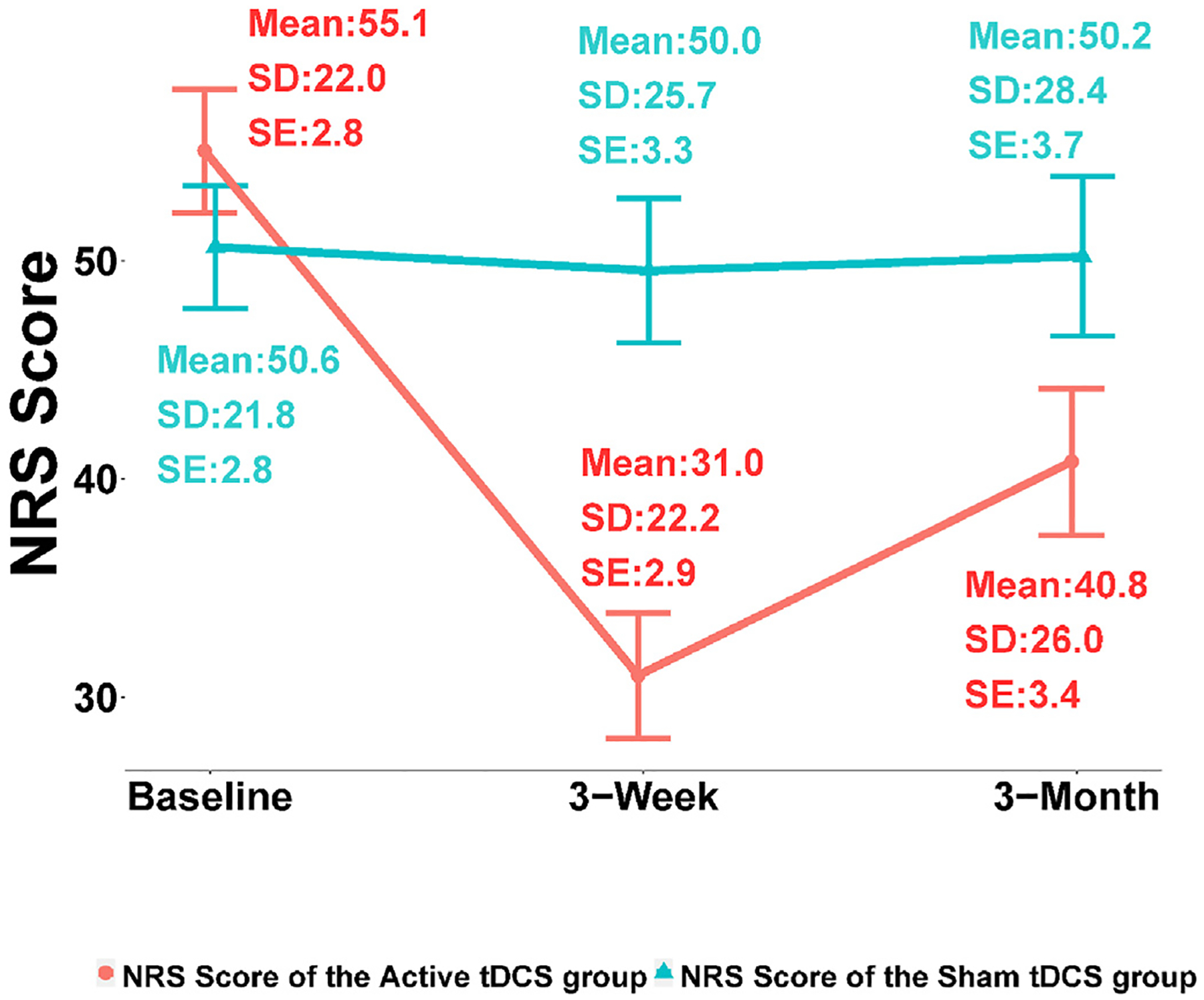
Numeric rating score changes. SD: standard deviation, SE: standard error.
Fig. 3.
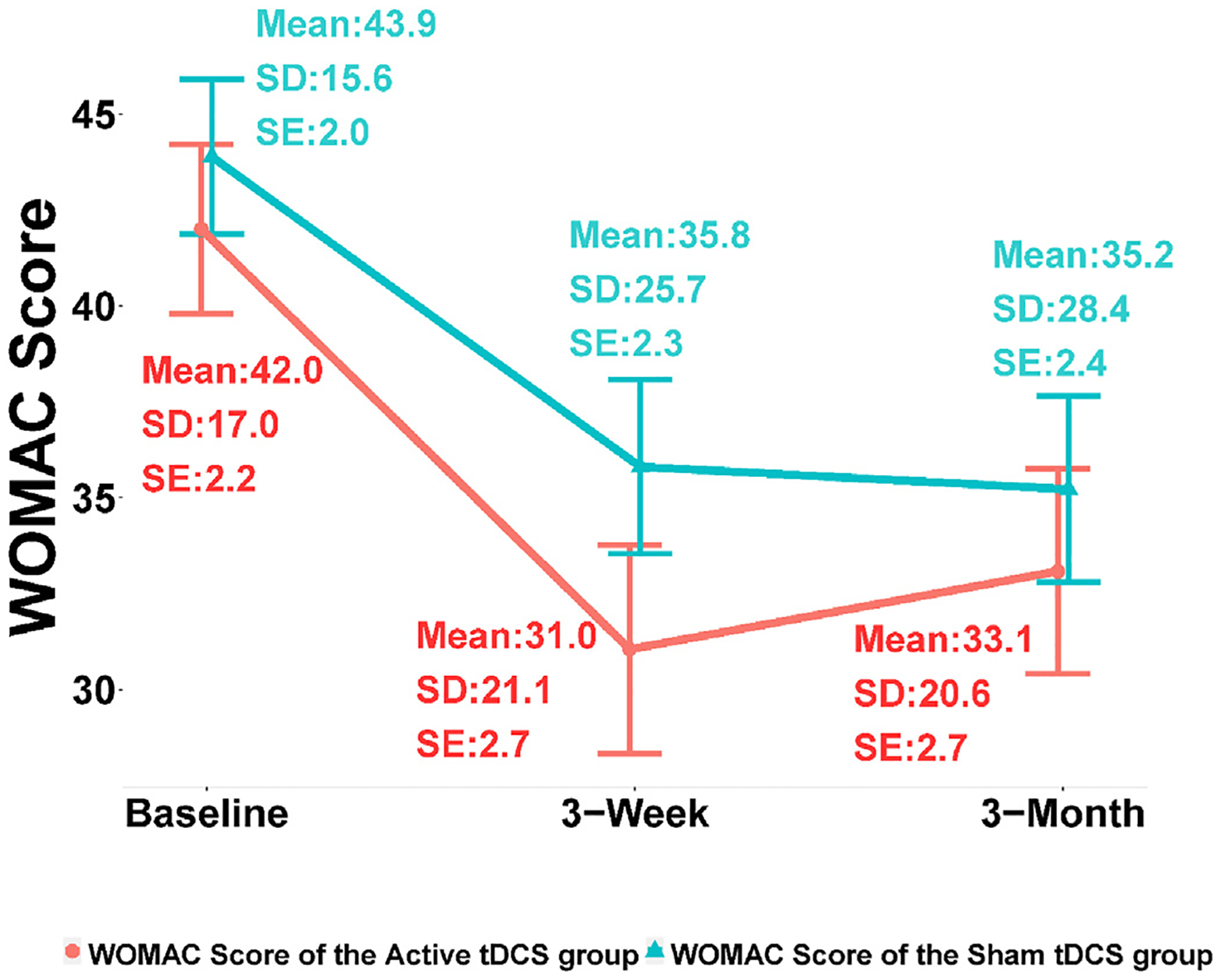
WOMAC score changes. SD: standard deviation, SE: standard error.
Finally, a GEE model was employed to regress the NRS changes over all the six time points from baseline (week 1, week 2, week 3, month 1, month 2, and month 3) against selected covariates. The treatment group (p-value < 0.0001), age (p-value < 0.01), gender (p-value < 0.0001), African American with respect to American Caucasian (p-value < 0.001), BMI (p-value < 0.01), and baseline of NRS (p-value < 0.0001) were found statistically significant. American Indians or Alaska Natives were not taken into consideration due to the very small sample size. Based on sex-stratified analysis, we did not find statistically significant differences between male and female in both groups. However, when analyzing the data of males and females separately, baseline BMI and baseline WOMAC score were found significant for females while age and OA months were found significant for males.
Acceptability and Feasibility.
Home-based tDCS was well received and participants showed high levels of satisfaction with their experience as shown in Table 5. Overall, they found that the device was easy to use and felt confident using it. They also appreciated to receive guidance remotely via videoconference. All participants tolerated tDCS well without experiencing any serious adverse effects.
Table 5.
Descriptive results for the tDCS experience questionnaire (N = 120).
| Items of the tDCS experience questionnaire | Mean (SD) |
|---|---|
| 1. It was easy to prepare the device and accessories (0–10) | 9.41 (1.50) |
| 2. The device was unnecessarily complex (0–10) | 0.89 (2.53) |
| 3. The device was easy to use (0–10) | 9.59 (1.36) |
| 4. I felt the video conferences with a technical person were helpful (0–10) | 9.41 (1.68) |
| 5. I would imagine that most people would learn to use this device quickly (0–10) | 9.32 (1.48) |
| 6. The device was cumbersome to use (0–10) | 1.27 (2.64) |
| 7. I felt confident using the device (0–10) | 9.70 (1.17) |
| 8. I needed to learn a lot of things before I could get going with this device (0–10) | 1.05 (2.50) |
| 9. The effectiveness of the treatment increased over the course of treatment (0–10) | 6.58 (3.40) |
| 10. Overall, I felt that transcranial electrical stimulation treatment benefited me (0–10) | 6.72 (3.35) |
Note. SD = Standard Deviation. 0 = strongly disagree, 10 = strongly agree.
4. Discussion
To our knowledge, this is the first randomized controlled trial evaluating the efficacy of home-based, self-administered tDCS for patients with OA pain. We found that home-based tDCS with real-time remote supervision was associated with significant improvement in clinical pain intensity up to three months after a three-week treatment. The treatment was acceptable and well tolerated by older adults with knee OA and did not cause any significant adverse effects. Overall, patients were highly satisfied with their experience. These phase II results corroborate our previous preliminary findings on the feasible and acceptable application of a two-week treatment with tDCS in the home setting for older adults with OA and extend these by providing evidence of efficacy on a longer term, i.e., 3 months [25].
Our findings are also coherent with previous studies on home-based tDCS in individuals with other chronic conditions. Brietzke et al. [22] found considerable reductions in pain in individuals with fibromyalgia (n = 20). Nevertheless, while we did not find any significant improvement in physical function as measured by the WOMAC, Caumo et al. [23] recorded decrease in disability (n = 48) in women with fibromyalgia. Additionally, after using a double-blind controlled design, we can confirm that we observed similar results to those obtained in the clinical setting with ideal implementation conditions [24], which provides additional data on the brain regions (i.e., M1-SO) to target for the treatment of knee OA. Although tDCS underlying mechanisms need to be further studied, consistent with previous studies, our results suggest that tDCS could improve endogenous central pain inhibition in older adults with knee OA by mitigating the impact of central sensitization and modulating brain activity in processing pain. This rationale is supported, for instance, by the fact that a relationship was found between improvements in clinical pain intensity and improvements in experimental pain sensitivity [35]. Additionally, another study targeting solely patients with knee OA exhibiting a dysfunctional descending pain inhibitory system observed tDCS parallel benefits on both experimental pain modulation and clinical pain severity [36].
Home-based implementation of tDCS is very meaningful for a population with reduced mobility. Daily commutes to a clinic inhibit the benefits of this promising approach and reduce its accessibility [37]. Moreover, although used as an adjunct, it has been shown in previous studies that tDCS induced decreased opioid use [22]. Reducing the reliance on pharmacological treatment is a considerable advantage, especially in an older population with several comorbidities and other pharmacologic therapies potentially harmful in this vulnerable subgroup. Beside the secondary effects of medications, polypharmacy has been associated with a decreased level of physical activity in patients with knee OA [38]. Hence, in addition to addressing nociception and related suffering, by reducing pain intensity as well as pain-related cognitions and medication intake, home-based tDCS has the potential to promote emotional and physical functioning and prevent disability.
This study has a few limitations. Self-administered tDCS may have some variability, and it may not confer the same accuracy and consistency as clinic-based, professionally administered stimulation sessions. To minimize this possibility, we followed the standards and guidelines for remotely supervised tDCS [39] to maximize reproducibility and participant comfort. Research staff were properly trained for participant interactions, and participants were trained at the baseline visit until they feel comfortable using the tDCS device that is specifically designed for remote use. Each participant was remotely supervised by trained research staff at each stimulation session to ensure the correct technique and to monitor any adverse events. The proposed study translated tDCS into the home environment and is very much like a real-world setting. Additionally, we did not see any changes on WOMAC scores. This finding might be partially explained by the tDCS dosage we used in the current study. Although we followed current tDCS guidelines [13], they are not specific to OA pain and several parameters need to be examined [20]. For instance, a longer duration of treatment, longer sessions or higher electrical intensity might be needed to see changes on measures such as WOMAC, as suggested by other studies with different populations obtaining significant results on proxy measures of pain [22,23]. Lastly, we did not measure any biomarkers or assess neuronal mechanisms in this study. However, this trial was focused on efficacy. These variables will be important for future mechanistic studies on tDCS targeting OA pain [22,23,40].
Our findings provide avenues for future research. Our results are promising regarding the long-term efficacy of this approach with benefits sustained 3 months after the last treatment. Future trials need to explore optimal dosage for knee OA pain relief on two aspects: the magnitude of the effect and the duration and maintenance of benefits. This will allow the appreciation of the tangible [41]. For instance, a dose-response has been observed in another study with patients with fibromyalgia, which was reflected by a large effect size after an extended period treatment (60 sessions over 12 weeks) [22]. Regarding the maintenance of benefits, in the context of a chronic condition, we need to determine if an extended period of treatment also prolongs the duration of benefits and when the treatment should be repeated. Additionally, to reduce opioid consumption, but also to address pain refractory to such treatment, it would be relevant to assess the effects of combining tDCS with another non-pharmacological approach. Based on the biopsychosocial model of chronic pain, psychological and biological factors are interrelated in the central processing of pain [42,43]. Thus, adding a psychological approach, such as a mindfulness-based intervention, could potentiate the central effects of tDCS on chronic pain [44,45].
5. Conclusions
We showed that self-administered, remotely supervised tDCS at home including 5 daily 20-min sessions for 3 weeks is feasible, acceptable, and significantly improves pain intensity in older adults with knee OA, which sets the stage for future studies involving larger samples and effectiveness assessment.
Funding
This study was supported by the NIH/NINR Grant R15NR018050. The authors declare no conflict of interest.
Abbreviations:
- tDCS
transcranial direct current stimulation
- OA
osteoarthritis
- M1
primary motor cortex
- SO
supraorbital region
- NRS
Numeric Rating Scale
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Footnotes
CRediT authorship contribution statement
Geraldine Martorella: Writing – original draft, preparation. Kenneth Mathis: Conceptualization, Methodology, Funding acquisition. Hongyu Miao: Conceptualization, Methodology, Writing – original draft, Formal analysis, Funding acquisition. Duo Wang: Visualization, Investigation, Software. Lindsey Park: Validation, Investigation, Resources, Data curation. Hyochol Ahn: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].CDC. Arthritis 2021. Available from: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/arthritis.htm.
- [2].Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable Activity limitation among US adults, 2015–2040. Arthritis Rheumatol 2016;68(7):1582e7. 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].O’Neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep 2018;16(5):611–6. 10.1007/s11914-018-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology 2018;57(suppl_4):iv43–50. 10.1093/rheumatology/kex419. [DOI] [PubMed] [Google Scholar]
- [5].Barbour KE, Helmick CG, Boring M, Zhang X, Lu H, Holt JB. Prevalence of doctor-diagnosed arthritis at state and county levels - United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65(19):489–94. 10.15585/mmwr.mm6519a2. [DOI] [PubMed] [Google Scholar]
- [6].Barbour KE, Boring M, Helmick CG, Murphy LB, Qin J. Prevalence of severe joint pain among adults with doctor-diagnosed arthritis - United States, 2002–2014. MMWR Morb Mortal Wkly Rep 2016;65(39):1052–6. 10.15585/mmwr.mm6539a2. [DOI] [PubMed] [Google Scholar]
- [7].Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 2018;320(24):2564–79. 10.1001/jama.2018.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Interagency pain research coordinating committee-national pain strategy overview. Bethesda, MD: National Institutes of Health; 2016. Available from: https://www.iprcc.nih.gov/National-Pain-Strategy/Overview. [Google Scholar]
- [9].HEAL initiative-pain management effectiveness research network. Bethesda, MD: National Institutes of Health; 2019. [Google Scholar]
- [10].Zhang L, Fu T, Zhang Q, Yin R, Zhu L, He Y, et al. Effects of psychological interventions for patients with osteoarthritis: a systematic review and meta-analysis. Psychol Health Med 2018;23(1):1–17. 10.1080/13548506.2017.1282160. [DOI] [PubMed] [Google Scholar]
- [11].Bartholomew C, Lack S, Neal B. Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression. Scand J Pain 2019;20(1):11e27. 10.1515/sjpain-2019-0079. [DOI] [PubMed] [Google Scholar]
- [12].Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23(7):1043–56. 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- [13].Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128(1):56–92. 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- [14].Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 2006;54(12):3988–98. 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- [15].Simis M, Reidler JS, Duarte Macea D, Moreno Duarte I, Wang X, Lenkinski R, et al. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Pract 2015;15(5):423–32. 10.1111/papr.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain 2010;11(5):436–42. 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- [17].Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manag 2010;39(5):890–903. 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- [18].Kang JH, Choi SE, Park DJ, Xu H, Lee JK, Lee SS. Effects of add-on transcranial direct current stimulation on pain in Korean patients with fibromyalgia. Sci Rep 2020;10(1):12114. 10.1038/s41598-020-69131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Young J, Zoghi M, Khan F, Galea MP. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: randomized controlled trial. Pain Med 2020;21(12):3451–7. 10.1093/pm/pnaa128. [DOI] [PubMed] [Google Scholar]
- [20].Pinto CB, Teixeira Costa B, Duarte D, Fregni F. Transcranial direct current stimulation as a therapeutic tool for chronic pain. J ECT 2018;34(3):e36–50. 10.1097/YCT.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008;1(3):206–23. 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- [22].Brietzke AP, Zortea M, Carvalho F, Sanches PRS, Silva DPJ, Torres I, et al. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain 2020;21(1–2):212–24. 10.1016/j.jpain.2019.06.013. [DOI] [PubMed] [Google Scholar]
- [23].Caumo W, Alves RL, Vicuna P, Alves C, Ramalho L, Sanches PRS, et al. Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: a randomized, double-blind sham-controlled study. J Pain 2021. 10.1016/j.jpain.2021.11.002. [DOI] [PubMed] [Google Scholar]
- [24].Ahn H, Woods AJ, Kunik ME, Bhattacharjee A, Chen Z, Choi E, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: an experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul 2017;10(5):902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahn H, Sorkpor S, Miao H, Zhong C, Jorge R, Park L, et al. Home-based self-administered transcranial direct current stimulation in older adults with knee osteoarthritis pain: an open-label study. J Clin Neurosci 2019;66:61–5. 10.1016/j.jocn.2019.05.023. [DOI] [PubMed] [Google Scholar]
- [26].Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29(8):1039–49. [DOI] [PubMed] [Google Scholar]
- [27].Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006;117(4):845e50. 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [28].Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238–44. 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- [29].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63(Suppl 11):S240–52. 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- [30].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12): 1833–40. [PubMed] [Google Scholar]
- [31].Gillick BT, Feyma T, Menk J, Usset M, Vaith A, Wood TJ, et al. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys Ther 2015;95(3):337–49. 10.2522/ptj.20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cha YH, Urbano D, Pariseau N. Randomized single blind sham controlled trial of adjunctive home-based tDCS after rTMS for mal de debarquement syndrome: safety, efficacy, and participant satisfaction assessment. Brain Stimul 2016;9(4):537e44. 10.1016/j.brs.2016.03.016. [DOI] [PubMed] [Google Scholar]
- [33].Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016;9(5):641e61. 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul 2012;5(2):155–62. 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ahn H, Suchting R, Woods AJ, Miao H, Green C, Cho RY, et al. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: randomized sham-controlled pilot clinical study. J Pain Res 2018;11:2071–82. 10.2147/JPR.S173080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tavares DRB, Okazaki JEF, Santana MVA, Pinto A, Tutiya KK, Gazoni FM, et al. Motor cortex transcranial direct current stimulation effects on knee osteoarthritis pain in elderly subjects with dysfunctional descending pain inhibitory system: a randomized controlled trial. Brain Stimul 2021;14(3): 477–87. 10.1016/j.brs.2021.02.018. [DOI] [PubMed] [Google Scholar]
- [37].Riggs A, Patel V, Paneri B, Portenoy RK, Bikson M, Knotkova H. At-home transcranial direct current stimulation (tDCS) with telehealth support for symptom control in chronically-ill patients with multiple symptoms. Front Behav Neurosci 2018;12:93. 10.3389/fnbeh.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thanoo N, Gilbert AL, Trainor S, Semanik PA, Song J, Lee J, et al. The relationship between polypharmacy and physical activity in those with or at risk of knee osteoarthritis. J Am Geriatr Soc 2020;68(9):2015–20. 10.1111/jgs.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci 2015;9:26. 10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zortea M, Ramalho L, Alves RL, Alves C, Braulio G, Torres I, et al. Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front Neurosci 2019;13: 1218. 10.3389/fnins.2019.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021;24(4):256–313. 10.1093/ijnp/pyaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gatchel RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. Am Psychol 2004;59(8):795–805. 10.1037/0003-066X.59.8.795. [DOI] [PubMed] [Google Scholar]
- [43].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133(4):581–624. 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- [44].Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KM. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cognit Behav Ther 2016;45(1):5–31. 10.1080/16506073.2015.1098724. [DOI] [PubMed] [Google Scholar]
- [45].Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med 2017;51(2):199–213. 10.1007/s12160-016-9844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


