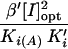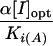Abstract
Various ethyl and benzyl spermine analogues, including the anticancer agent N1,N12-bis(ethyl)spermine, were studied for their ability to affect the growth of cultured Escherichia coli cells, to inhibit [3H]putrescine and [3H]spermine uptake into cells, and to modulate the peptidyltransferase activity (EC 2. 3. 2. 12). Relative to other cell lines, growth of E. coli was uniquely insensitive to these analogues. Nevertheless, these analogues conferred similar modulation of in vitro protein synthesis and inhibition of [3H]putrescine and [3H]spermine uptake, as is seen in other cell types. Thus, both ethyl and benzyl analogues of spermine not only promote the formation and stabilization of the initiator ribosomal ternary complex, but they also have a sparing effect on the Mg2+ requirements. Also, in a complete cell-free protein-synthesizing system, these analogues at low concentrations stimulated peptide bond formation, whereas at higher concentrations, they inhibited the reaction. The ranking order for stimulation of peptide-bond formation by the analogues was N4,N9-dibenzylspermine > N4,N9-bis(ethyl)spermine ≅ N1-ethylspermine > N1,N12-bis(ethyl)spermine, whereas the order of analogue potency regarding the inhibitory effect was inverted, with inhibition constant values of 10, 3.1, 1.5, and 0.98 μM, respectively. Although the above analogues failed to interact with the putrescine-specific uptake system, they exhibited high affinity for the polyamine uptake system encoded by the potABCD operon. Despite this fact, none of the analogues could be internalized by the polyamine transport system, and therefore they could not influence the intracellular polyamine pools and growth of E. coli cells.
The polycationic polyamines putrescine, spermidine, and spermine are present in all living organisms and engage in noncovalent interactions with a wide variety of cellular targets, including nucleic acids, proteins, and phospholipids (26, 29, 39). These interactions affect various processes of cell growth. For instance, preferential stimulation or inhibition of the in vivo synthesis of specific proteins is one of the important functions of polyamines in cell growth and regulation of differentiation (see reference 14 and references therein). Due to its four positive charges at physiological pH, spermine is the most effective of the naturally occurring polyamines both in regulating the in vitro translation process at several levels and in decreasing (but not abolishing) the Mg2+ requirements for protein synthesis (8, 11, 25–27, 39). We have previously demonstrated (3) that in an Escherichia coli cell-free system, spermine at 6 mM Mg2+ displays a concentration-dependent allosteric biphasic activity on ribosomal peptidyltransferase. In agreement with this, accumulation of excess polyamines causes inhibition of cell growth or a decrease in cell viability, primarily through inhibition of protein synthesis (9). On the other hand, blockage of polyamine synthesis by mutations or by inhibitors leads to a virtual cessation of growth, unless exogenous polyamines are provided (29). Accumulating evidence suggests that these inhibitors may be useful therapeutic agents for treatment of a variety of diseases, including cancer (23, 29).
N1,N12-Bis(ethyl)spermine, the most active derivative in depleting intracellular polyamine pools, not only negatively regulates the synthesis of ornithine and S-adenosylmethionine decarboxylases (30, 32), but also induces spermidine/spermine N1-acetyltransferase and substitutes for the functions of spermine in several aspects (4, 6, 10, 12, 23, 29). This analogue, with minor exceptions (24), enters mammalian cells via the polyamine transport system (1). Other analogues, such as dibenzyl polyamine analogues, are substrates for an uptake system distinct from the natural polyamine transport system (2).
The use of specific transport inhibitors is a useful strategy to understand the differential specificity of polyamine transporters. A great deal of effort has been invested in probing the molecular details of polyamine recognition by the transporters and in the isolation and expression of genes encoding the constituents of uptake systems. Although attempts in mammalian systems have not yet been successful, the bacterial polyamine transporters have been cloned and expressed. In E. coli cells, the proteins encoded by potABCD and potFGHI operons constitute the spermidine- or spermine-preferential and the putrescine-specific uptake systems, respectively (13). Another transport system encoded by potE also catalyzes putrescine uptake; however, its ability is significantly lower than those of the potABCD and potFGHI systems. The substrate specificity of the two transport systems is determined by a polyamine-binding protein in the periplasm: PotF for putrescine and PotD for putrescine or spermidine. The amino acid residues in PotD and PotF involved in the interaction with polyamines have been revealed by mutational and x-ray analysis (20, 37, 40). Recently, we have studied in E. coli the interactions of acetyl polyamines with their transporters (17) or peptidyltransferase (18), and we have evaluated the significance of polyamine primary and secondary amino groups, as well as that of chain flexibility, as determinants of these bacterial functions. Since acetyl polyamines per se have no pharmaceutical significance, it was of interest to extend our knowledge by using a series of spermine analogues which are known to have an antiproliferative effect on eukaryotic cells. An investigation of their effects on prokaryotic cells could have an impact not only on basic research, but also on interpretation of potential symbiotic relationships between prokaryotic and eukaryotic cells.
MATERIALS AND METHODS
Materials.
GTP (disodium salt), poly(U), ATP (disodium salt), phenylalanine, puromycin dihydrochloride, heterogeneous tRNA from E. coli W, spermine tetrahydrochloride, N1-acetylspermine, and vitamin B1 were obtained from Sigma Chemical Co. (St. Louis, Mo.). l-Phenyl-[2,3-3H]alanine, [14C]spermine tetrahydrochloride, and [1,4-14C]putrescine dihydrochloride were purchased from Amersham (Arlington Heights, Ill.). Cellulose acetate filters (type Sepraphore III) were obtained from Gelman Sciences (Ann Arbor, Mich.), and cellulose nitrate filters (type HA, 24-nm diameter, 0.45-μm pore size) were obtained from Millipore (Bedford, Mass.). Casamino Acids were obtained from Difco Laboratories (Detroit, Mich.).
Drugs.
N1-Ethylspermine, N1,N12-bis(ethyl)spermine, and N4,N9-bis(ethyl)spermine were synthesized by reduction of the corresponding acetyl analogues of spermine with lithium aluminum hydride for 12 h in refluxing tetrahydrofuran (THF). N1-Acetylspermine, N1,N12-diacetylspermine, N4,N9-diacetylspermine, and N4,N9-dibenzylspermine were synthesized by the application of a general methodology (22), with N-tritylamino acids used as building blocks.
Biochemical preparations.
Salt-washed (0.5 M NaCl) and polyamine-depleted ribosomes were obtained from E. coli B cells, as described previously (15). Partially purified translation factors (FWR fraction) and crude acetyl-(Ac)[3H]Phe-tRNA charged with 16.3 pmol of [3H]Phe (86 kcpm total) per A260 unit were prepared as reported previously (15). Complex C, i.e., the Ac[3H]Phe-tRNA-poly(U)-ribosome complex, was prepared and purified through adsorption on cellulose nitrate filters, as described elsewhere (18). The radioactivity trapped on the filters was counted in a liquid scintillation spectrometer. Controls without poly(U) were included in each experiment, and the values obtained were subtracted.
Cell culture.
E. coli B cells were grown aerobically in M9 medium (48 mM Na2HPO4, 22 mM KH2PO4, 9 mM NaCl, 19 mM NH4Cl), supplemented with 0.03 mM FeCl3, 0.1 mM CaCl2, 1 mM MgSO4, 0.01 mM vitamin B1, 0.6% glucose, and 0.1% Casamino Acids, at 37°C in shaking Erlenmeyer flasks. Polyamine analogues (100 μM, total concentration) were added at the time of culture initiation, and growth was followed by measuring the A540. Polyamine pool depletion was tested by treating cells for up to 24 h with analogues. Control cells were washed with ice-cold phosphate-buffered saline prior to the performance of uptake assays with radiolabeled polyamines.
Polyamine pool analysis.
Cells were sampled at the end of each treatment period, washed, and pelleted for extraction with 0.6 N perchloric acid. Each supernatant, after dansylation (28), was analyzed for dansyl polyamines or polyamine analogues by reverse-phase high-performance liquid chromatography (HPLC) by using a Beckman C18-Ultrasphere ODS (5-μm spherical packing, 25 cm by 4.6 mm) column heated at 50°C. For the separation of derivatized polyamines, an initial 3-min isocratic elution with 45% aqueous CH3CN was followed by an 18-min linear gradient to methanol and then by methanol for 9 min. Results were expressed as micromoles of polyamine or polyamine analogue per gram of total protein. The concentration of proteins was determined by the method of Lowry et al. (21).
Kinetic analysis of polyamine uptake.
Polyamine uptake by intact cells was induced and kinetically analyzed as described previously (17), with various concentrations of [14C]putrescine or [14C]spermine in the presence or in the absence of specified concentrations of polyamine analogues.
Peptide bond formation assay and first-order analysis.
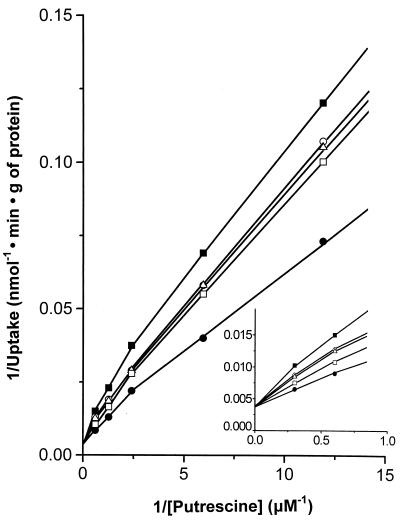
The peptidyltransferase activity of ribosomes was assessed by the puromycin reaction carried out at 25°C in the presence of 6 mM Mg2+ by using complex C adsorbed on a cellulose nitrate filter. Under these conditions, the reaction between complex C and excess puromycin (S) proceeds as an irreversible pseudo-first-order reaction (18):
 |
←
where C′ is a modified species of complex C not participating in reforming complex C and P is the product (AcPhe-puromycin). The relationships
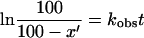 |
1 |
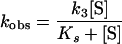 |
2 |
hold, and the values of k3 and Ks could be determined from the double-reciprocal plot of equation 2 by linear regression.
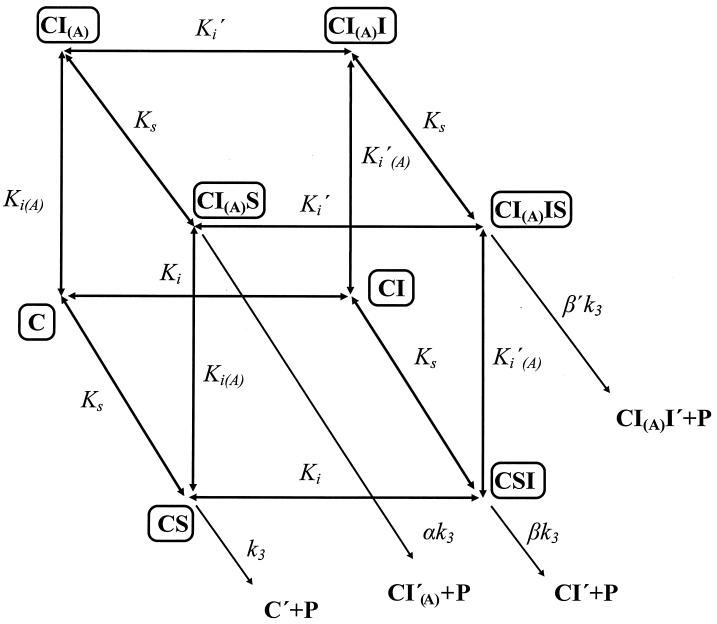
In the presence of spermine analogue, the puromycin reaction follows a complex kinetic scheme illustrated in Fig. 1. In this case, the first-order rate constant (k′obs) obeys the equation
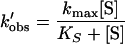 |
3 |
where kmax expresses the apparent catalytic rate constant of peptidyltransferase and is a function of spermine analogue concentration (18). The kinetic parameters involved in the scheme of Fig. 1 were evaluated as reported previously (18). The measurements of the parent compound, spermine, although recently published (3, 17, 18), were repeated and included in the present report for reasons of comparison.
FIG. 1.
Kinetic model for AcPhe-puromycin synthesis carried out in the presence of spermine analogues, with complex C formed in the presence of the FWR fraction. C, complex C; S, puromycin; I and I(A), spermine analogue bound to the inhibition site and activation site of complex C, respectively; C′, CI′, CI′(A), and CI(A)I′, ribosomal complexes after their reaction with puromycin.
RESULTS
Effect of spermine analogues on ribosomal functions. (i) Effect on the stability of complex C.
The stability of complex C, i.e., the AcPhe-tRNA-poly(U)-ribosome complex, was examined at 6 mM Mg2+ by incubation of complex C at 25°C with or without spermine analogues and monitoring the percentage of surviving complex C. In accordance with previous results (16, 18), we observed that 58% of complex C formed in the absence of translation factors (FWR fraction) was inactivated after 20 min of incubation in the absence of spermine or spermine analogues. However, the stability of complex C was greatly enhanced by the presence of spermine analogues, in a dose-dependent manner. Complex C formed in the presence of the FWR fraction exhibited high stability, and therefore the presence of spermine analogues caused only a slight improvement.
(ii) Effect on poly(U)-directed Ac[3H]Phe-tRNA binding to ribosomes.
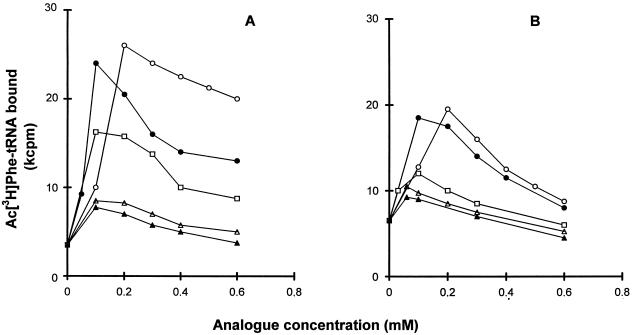
In the absence of spermine or spermine analogues, the optimum concentration of Mg2+ for AcPhe-tRNA binding to poly(U)-programmed ribosomes has been found in our laboratory to equal 10 mM (see reference 3 and references therein). Addition of spermine or spermine analogues at each optimal concentration resulted in a lowering of the Mg2+ optimum to about 6 mM (data not shown). When translation factors were omitted from the standard reaction mixture (Fig. 2A), addition of spermine or spermine analogues at 6 mM Mg2+ caused substantial stimulation of AcPhe-tRNA binding. The stimulatory effect was moderated when the reaction mixture included the FWR fraction (Fig. 2B).
FIG. 2.
Effect of spermine and spermine analogues on poly(U)-directed Ac[3H]Phe-tRNA binding to ribosomes. The binding mixture (25 μl) was prepared in the absence (A) or presence (B) of the FWR fraction (10 μg of protein) and contained 100 mM Tris-HCl (pH 7.2), 100 mM NH4+, 6 mM Mg2+ (acetate), 25.6 A260 units of washed ribosomes per ml, 320 μg of poly(U) per ml, and, finally, spermine (○), N4,N9-dibenzylspermine (●), N1-ethylspermine (□), N4,N9-bis(ethyl)spermine (▵), or N1,N12-bis(ethyl)spermine (▴) at the indicated concentrations. The time course of the reaction was monitored up to 30 min at 25°C. The values of bound Ac[3H]Phe-tRNA were estimated from the maximum level of binding curves.
(iii) Effect on peptide bond formation.
The effects of spermine and spermine analogues on peptide bond formation were compared in an E. coli cell-free system by using the puromycin reaction as a model reaction (38).
The effects of spermine analogues and spermine on the extent of puromycin reaction resembled each other and depended on the experimental conditions under which complex C was formed. When complex C was formed in a complete reaction mixture (containing FWR), all analogues examined appeared to have diminished activity, whereas, when complex C was formed in the absence of the FWR fraction, the extent of peptide bond formation was elevated by spermine analogues and spermine to a comparable degree. For instance, the extent of peptide bond formation was raised from 21% to 60% by increasing the concentration of N1-ethylspermine from zero to 0.2 mM. Based on the concept that the extent of puromycin reaction is a reliable measurement of the AcPhe-tRNA distribution between the ribosomal A site and P site (33), the data support the notion that during complex C formation, the analogues enhance the binding of AcPhe-tRNA at both P and A sites, the former being more stimulated.
In accordance with previous results obtained with spermine (3), polyamine analogues inhibited AcPhe-puromycin synthesis carried out with complex C formed in the absence of translation factors. Detailed kinetic analysis revealed that again the inhibition is of partial noncompetitive type (34), with one molecule of ligand involved in the mechanism of inhibition. Therefore, the CSI complex is catalytically active, but with a lower rate constant (k′3) than CS. The dissociation constant (Ki) and the β (k′3/k3) values obtained from the corresponding 1/Δ intercept replots (not shown) are summarized in Table 1.
TABLE 1.
Kinetic and equilibrium constants of AcPhe-puromycin synthesis carried out in the presence of spermine analogues, with complex C formed in the absence of the FWR fraction
| Effectora | k3b (min−1) | Ksb (μM) | Kic (μM) | βc |
|---|---|---|---|---|
| None (control) | 1.11 | 660 | ||
| SPM | 118 | 0.165 | ||
| N1-MESPM | 138 | 0.138 | ||
| N1,N12-BESPM | 225 | 0.360 | ||
| N4,N9-BESPM | 222 | 0.345 | ||
| N4,N9-DBzSPM | 130 | 0.522 |
SPM, spermine; N1-MESPM, N1-ethylspermine; N1,N12-BESPM, N1,N12-bis(ethyl)spermine; N4,N9-BESPM, N4,N9-bis(ethyl)spermine; N4,N9-DBzSPM, N4,N9-dibenzylspermine.
The k3 and Ks values were calculated by fitting the experimental data to equation 2 by a least-square procedure provided by Microcal Software, Inc.
The Ki and β values were obtained from the corresponding 1/Δ intercept replots.
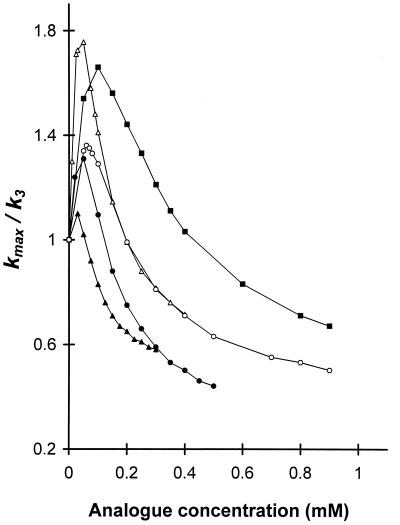
In experiments carried out with complex C formed in the presence of translation factors, the kinetic pattern of spermine analogue impact on peptidyltransferase activity was more complicated, but resembled that of spermine (3). As shown in Fig. 3, there was a biphasic dose response: concentrations up to a certain limit for each analogue stimulated peptidyltransferase activity, with higher concentrations being inhibitory. Among the analogues, N4,N9-dibenzylspermine exhibited the strongest stimulatory effect in a broad concentration range. As shown in Fig. 3, the kmax value increased and peaked at 100 μM with 67% enhancement of its value, approaching an inhibitory phase at around 500 μM. Based on detailed kinetic analysis, similar to that applied for the interpretation of the spermine-bimodal action (3), we concluded that the kinetic scheme of Fig. 1 can adequately explain the present results. It is noteworthy that the resulting fit of theoretical curves to the experimentally measured curves was greatly satisfying. The values of the kinetic parameters, obtained from the best fits, are presented in Table 2.
FIG. 3.
Variation of kmax/k3 as a function of spermine-analogue concentration. Complex C, formed in the presence of the FWR fraction at spermine or spermine analogue concentrations such as those used during the puromycin reaction, was adsorbed on a cellulose nitrate filter, washed, and then reacted with the appropriate concentration of puromycin in reaction buffer containing 6 mM Mg2+ and spermine (▵), N4,N9-dibenzylspermine (■), N4,N9-bis(ethyl)spermine (○), N1-ethylspermine (●), or N1,N12-bis(ethyl)spermine (▴) as indicated. The kmax value was calculated by fitting the experimental data to equation 3 by nonlinear regression. Similarly, the Ks and k3 values for the control experiment (no amine added) were estimated by using equation 2 and were found to be 660 ± 58 μM and 2.10 ± 0.22 min−1, respectively.
TABLE 2.
Kinetic parameters of AcPhe-puromycin synthesis carried out in the presence of spermine analogues, with complex C formed in the presence of the FWR fraction
| Effectora | [I]opt (mM) |
|
β′ | β/Ki (mM−1) | Ki(A) (mM) |
|
K′′i(μM) | % Relative activity of peptidyltransferase | ||
|---|---|---|---|---|---|---|---|---|---|---|
| None (control) | 100 | |||||||||
| SPM | 0.050 | 0.443 | 0.448 | 1.100 | 3.20 | 2.340 | 0.80 | 175 | ||
| N1-MESPM | 0.050 | 0.093 | 0.200 | 0.844 | 3.60 | 1.107 | 1.50 | 131 | ||
| N1,N12-BESPM | 0.025 | 0.082 | 0.450 | 1.510 | 3.47 | 0.277 | 0.98 | 109 | ||
| N4,N9-BESPM | 0.060 | 0.130 | 0.345 | 1.478 | 3.08 | 0.931 | 3.10 | 136 | ||
| N4,N9-DBzSPM | 0.100 | 0.168 | 0.300 | 3.234 | 1.90 | 2.000 | 10.00 | 167 |
Abbreviations of effectors are defined in Table 1.
Polyamine uptake.
According to recent observations in our laboratory (17), spermine transport in E. coli cells obeys simple Michaelis-Menten kinetics (Fig. 4, lower line) with Vmax and Kt values of 83.3 ± 2.4 nmol · min−1 per g of protein and 25.16 ± 1.58 μM, respectively. Competitive inhibition of spermine uptake was observed when E. coli cells were treated with ethyl or benzyl analogues of spermine (Fig. 4). From the Ki values presented in Table 3, it is obvious that the carrier affinity is highest for N4,N9-dibenzylspermine, then lower for N1-ethylspermine and N4,N9-bis(ethyl)-spermine, and lowest for N1,N12-bis(ethyl)spermine.
FIG. 4.
Double-reciprocal plots of [14C]spermine uptake by E. coli B cells, in the presence or absence of various alkyl and benzyl analogues of spermine. E. coli B cells grown in supplemented M9 medium were washed and suspended in buffer A to yield a protein concentration equal to 0.1 mg/ml. The [14C]spermine uptake was determined (●) in the absence of analogues or in the presence of 100 μM N1,N12-bis(ethyl)spermine (■), N4,N9-bis(ethyl)spermine (▴), N1-ethylspermine (○), and N4,N9-dibenzylspermine (□). Each point represents the mean value of four individual measurements.
TABLE 3.
Kinetic parameters of putrescine and spermine transport under the influence of various spermine analoguesa
| Effectorb | Kinetic parameters (μM) for:
|
|||||
|---|---|---|---|---|---|---|
| Spermine transport
|
Putrescine transport
|
|||||
| Kt | Ki | Carrier 1
|
Carrier 2
|
|||
| Kt | Ki | K′t | K′i | |||
| None | 25.16 ± 1.58 | 3.63 ± 0.43 | 0.61 ± 0.10 | |||
| N1-MESPM | 59.66 ± 7.43 | 61.32 ± 2.90 | >103 | |||
| N1,N12-BESPM | 109.92 ± 13.48 | 92.60 ± 6.85 | >103 | |||
| N4,N9-BESPM | 70.86 ± 8.50 | 65.15 ± 3.51 | >103 | |||
| N4,N9-DBzSPM | 23.72 ± 2.30 | 27.21 ± 5.12 | >103 | |||
The values are expressed as means ± standard deviations. In spermine transport, the Vmax value was 83.3 ± 2.4 nmol min−1 g of protein−1. In putrescine transport, the Vmax and V′max values were 220.5 ± 12.1 nmol min−1 g of protein−1 and 63.8 ± 5.1 nmol min−1 g of protein−1, respectively.
Abbreviations of effectors are defined in Table 1.
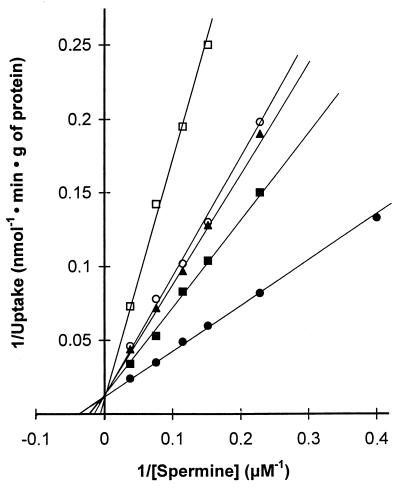
Consistent with previous results (17, 19), the Lineweaver-Burk plot of the initial putrescine uptake was biphasic (Fig. 5, lower line). This suggests that two independent transporters with different affinities for putrescine are involved. The Kt and Vmax values for the first transport system (carrier 1) as well as the K′t and V′max values for the other system (carrier 2) are shown in Table 3. All of the analogues behave as competitive inhibitors. However, as indicated by the Ki values shown in Table 3, they failed to inhibit the high-affinity putrescine uptake system (carrier 2). In contrast, they exhibited significant effectiveness in competing with putrescine for carrier 1, the ranking order once again being N4,N9-dibenzylspermine, followed by N1-ethylspermine and N4,N9-bis(ethyl)spermine, and then N1,N12-bis(ethyl)spermine (Table 3).
FIG. 5.
Double-reciprocal plots of [14C]putrescine uptake by E. coli B cells, in the presence or absence of various ethyl and benzyl analogues of spermine. The incubation mixture of cells was prepared as described in the legend to Fig. 4. The [14C]putrescine uptake was determined in the absence of analogues (●) or in the presence of 100 μM N1,N12-bis(ethyl)spermine (□), N4,N9-bis(ethyl)spermine (▵), N1-ethylspermine (○), and N4,N9-dibenzylspermine (■). Inset, detail of the kinetic plots at high concentrations of [14C]putrescine.
Effect of spermine analogues on E. coli cell growth and polyamine pools.
The effects of spermine analogues on the growth of cultured E. coli cells were screened at 100 μM. Surprisingly, none of the analogues examined showed any measurable effect, either stimulatory or inhibitory, on the growth of this strain. At first sight, these results seem to be in contradiction to the effects of analogues on the polyamine uptake system and peptidyltransferase activity. It is known from several reports (23, 29, 30, 35) that some of the analogues examined exert their action after internalization into the cells. In addition, inhibited uptake of [14C]putrescine or [14C]spermidine is usually taken as a criterion for the ability of the inhibitor to use the polyamine transporters. However, it could not be discounted that one or the other of the spermine analogues mentioned may block the transporters without being efficiently internalized. The ability of E. coli cells to accumulate spermine analogues was thus determined. The results revealed that none of the analogues is able to penetrate the plasma membrane. Only trace amounts of N4,N9-dibenzylspermine could be recovered in E. coli cells after a 24-h incubation with 100 μM, despite the high potency of this analogue as an uptake antagonist. Also, none of the analogues altered the intracellular concentrations of polyamines or the putrescine/spermidine molar ratio. The parent compound, spermine, exhibited the same behavior, although it was accumulated in cells at a concentration of 22 μmol/g of protein.
DISCUSSION
The use of alkyl and arylic derivatives of spermine for the chemotherapy of cancer and several parasitic diseases, including trypanosomiasis and leishmaniasis, has been greatly increased in the past few years (23). N,N′-Bis(ethyl) analogues of spermine have been of particular interest, and some members of this family are now in clinical trials. Given that the fate of certain pathogenic bacteria in animal infection is important in both fundamental and applied microbiology, we decided to explore the ability of a series of spermine analogues to affect the growth of cultured E. coli cells, to inhibit putrescine and spermine transport into cells, and to modulate several ribosomal functions.
We have previously demonstrated that the effects of spermine on protein synthesis are exerted at both the stages of initiation and elongation and that the charge distribution, as well as the chain flexibility of the ligand, plays a crucial role in its mode of action (3, 16, 18). From the present results, it is evident that the spermine analogues examined not only promote the formation and stabilization of the initiator ribosomal ternary complex, but also have a sparing effect on the Mg2+ requirements; although weaker, these effects are reminiscent of the behavior of spermine (3, 16). We have previously observed (18) that spermine selectively acylated at each amino group shows a much higher Mg2+ optimum, with a plateau at values over 9 mM. It is noteworthy that the analogues’ potential is inversely related to their Ki values, estimated by kinetic analysis (Table 1). This suggests that the ability of analogues to stimulate the binding may be closely related to their affinity for one or more constituents of complex C. The consequence of amino group ethylation to the analogue’s inhibitory activity is quite evident by the Ki and β values shown in Table 1; in going from bis-ethylated compounds to spermine, the ability of each analogue to interact with complex C increases (decrease in Ki values), whereas the reactivity of the CSI complex is concomitantly reduced (decrease in β values). It may be possible that the ethyl substituents at the nitrogens of spermine compromise some electrostatic interactions by increasing the distance between the charged sites of this compound and group of anions fixed to complex C. Thus, one might expect N4,N9-dibenzylspermine to bind more poorly than N4,N9-bis(ethyl)spermine. However, the Ki value of N4,N9-dibenzylspermine was about twofold smaller than that obtained with the second analogue. A reason for such behavior may be the existence of a hydrophobic pocket at the binding site on complex C which helps ligand anchoring. Alternatively, a restricted freedom of rotation around the neighboring carbon and nitrogen atoms, due to the introduction of benzyl groups, may stabilize the positive charges on secondary nitrogens and allow the development of strong hydrogen bond links. The second explanation is consistent with the finding of Frydman et al. (5) that the secondary amines of spermine bind more strongly to tRNA than the more electropositive primary amino groups. Despite the comparable Ki values between N4,N9-dibenzylspermine and spermine, the analogue behaved as a weaker inhibitor, since it is its high β value that increases the reactivity of the corresponding CSI complex.
In experiments carried out in the presence of the FWR fraction, addition of spermine analogue had a dose-dependent biphasic effect on peptide bond formation (Fig. 3). These results suggest that there are two separate binding sites for analogues on complex C, an activation site and an inhibition site. Once again, the polyamine analogues mimic the functions of spermine (3). The potency of each ligand depends on several features. Thus, N1,N12 substitution affords a high affinity to the ligand for the inhibition site (low K′i value), but dramatically lowers the stimulatory effect of ligand on peptidyltransferase activity (low α value). This is in accordance with observations in rabbit reticulocyte translation systems (10, 36). In contrast, derivatization of internal amines by ethyl or benzyl groups has only a little influence on the ability of ligand to stimulate translation. Thus, the degree of peptidyltransferase activation by N4,N9-dibenzylspermine is similar to that obtained by spermine (Table 2), although the optimal concentration of analogue ([I]opt) is twofold greater than that of the parent compound. Taking into account that polyamines with terminal benzyl groups show diminished activity in protein-synthesizing eukaryotic systems (36), our results can be interpreted on the basis of restricted rotation effects described above.
Since all analogues tested behave as competitive inhibitors of polyamine uptake, full characterization of their potency can be made exclusively on the basis of the Ki value, independently of whether putrescine or spermine is used as a substrate. As shown in Table 3, the analogues exhibit similar Ki values for both the carrier of spermine and carrier 1 of putrescine, suggesting the existence, in fact, of a common transporter. Moreover, the Kt value of carrier 1 is in the vicinity of that reported for the putrescine transport system encoded by the potABCD operon (19). These findings, taken together, suggest that all spermine analogues examined compete for the PotD protein. Obviously, ethylation of the terminal amino groups decreases the affinity of ligand for the uptake system, whereas ethyl or benzyl substitution of N4,N9-imino groups can be greatly tolerated. All of the data presented above are consistent with the idea that in addition to the key role of cationic centers, specific hydrophobic interactions must also largely contribute to the affinity of analogues for polyamine transporter. Furthermore, none of the analogues tested is recognized by carrier 2 of putrescine, i.e., by the uptake system encoded by potFGHI. This agrees with a recent interpretation of PotF specificity given by Vassylyev et al. (40), according to which PotF recruits a bulky side chain that protrudes deeply into the binding cavity. In conclusion, the polyamine uptake results in E. coli further support the mimetic character of ethyl and benzyl derivatives of spermine in reference to the parent compound. Finally, it is important to point out that E. coli cells, in contrast to erythrocytes and other mammalian cells (2), do not appear to have a specific uptake system for bis(benzyl)polyamine analogues; both spermine and N4,N9-dibenzylspermine compete for the PotD protein (Table 3).
Surprisingly, none of the analogues added to the medium affected E. coli cell growth. Even prolonged culture in the presence of 100 μM spermine analogue did not show any deviation from the behavior of untreated cells. Also, we failed to detect any effect of the spermine analogues on intracellular polyamine pools. Moreover, no spermine analogue was detected by HPLC analysis in exposed cells, nor did any intermediate product result from stepwise desubstitution and subsequent oxidative degradation (data not shown). Relatively, it has been demonstrated that N1,N12-dialkyl analogues of spermine cannot be used as substrates in spermidine/spermine-N1-acetyltransferase (SSAT)/polyamine oxidase (PAO)-catalyzed oxidative cleavage (30, 31). In addition, SSAT and PAO are not as active in E. coli cells as they are in eukaryotic cells (7). Traces of N4,N9-dibenzylspermine found in E. coli cells after a 24-h incubation with this analogue can be attributed to secondary diffusion effects. In combination, these observations suggest that the spermine analogues examined, although endowed with high affinity for the E. coli polyamine transport system encoded by potABCD, are not efficiently internalized into the cells. Consequently, they should be characterized as pure competitive antagonists of polyamine uptake. Thereby, the failure of SSAT induction in E. coli cells by exogenous N1,N12-bis(ethyl)spermine (7) seems to be reasonable: it is rather the lack of permeation of analogue through the E. coli membrane which explains the above effect, not the inability per se to induce the enzyme.
Distinct structure-activity relationships relevant to the spermine molecule have become apparent during this study. Our findings also reinforce the notion that high heterogeneity of polyamine transport system specificity exists among living cells. This heterogeneity may be a useful feature that can be exploited in investigating potential symbiotic relationships between prokaryotic and eukaryotic cells and in orienting chemical-synthetic work to the creation of drugs which can achieve selective uptake into the desired target cells.
ACKNOWLEDGMENTS
We thank D. Drainas and D. Synetos for critical reading of the manuscript. We also thank D. Vynios for valuable advice regarding HPLC analysis.
REFERENCES
- 1.Bergeron R J, Hawthorne T R, Vinson J R T, Beck D E, Ingeno M J. Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res. 1989;49:2959–2964. [PubMed] [Google Scholar]
- 2.Byers T L, Bitonti A J, McCann P P. Bis(benzyl)-polyamine analogues are substrates for a mammalian cell-transport system which is distinct from the polyamine-transport system. Biochem J. 1990;269:35–40. doi: 10.1042/bj2690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drainas D, Kalpaxis D L. Bimodal action of spermine on ribosomal peptidyltransferase at low concentrations of magnesium ions. Biochim Biophys Acta. 1993;1208:55–64. doi: 10.1016/0167-4838(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 4.Fogel-Petrovic M, Kramer D L, Vujcic S, Miller J, McManis J S, Bergeron R J, Porter C W. Structural basis for differential induction of spermidine/spermine N1-acetyltransferase activity by novel spermine analogs. Mol Pharmacol. 1997;52:69–74. doi: 10.1124/mol.52.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Frydman L, Rossomando P C, Frydman V, Fernandez C O, Frydman B, Samegima K. Interactions between natural polyamines and tRNA: an 15N-NMR analysis. Proc Natl Acad Sci USA. 1992;89:9186–9190. doi: 10.1073/pnas.89.19.9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuchi J, Kashiwagi K, Kusama-Eguchi K, Terao K, Shirahata A, Igarashi K. Mechanism of the inhibition of cell growth by N1,N12-bis(ethyl)spermine. Eur J Biochem. 1992;209:689–696. doi: 10.1111/j.1432-1033.1992.tb17337.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuchi J, Kashiwagi K, Takio K, Igarashi K. Properties and structure of spermidine acetyltransferase in Escherichia coli. J Biol Chem. 1994;269:22581–22585. [PubMed] [Google Scholar]
- 8.Gross M, Rubino M S. Regulation of eukaryotic initiator factor-2B activity by polyamines and amino acid starvation in rabbit reticulocyte lysate. J Biol Chem. 1989;264:21879–21884. [PubMed] [Google Scholar]
- 9.He Y, Kashiwagi K, Fukuchi J, Terao K, Shirahata A, Igarashi K. Correlation between the inhibition of cell growth by accumulated polyamines and the decrease of magnesium and ATP. Eur J Biochem. 1993;217:89–96. doi: 10.1111/j.1432-1033.1993.tb18222.x. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Suzuki T, Kashiwagi K, Kusama-Eguchi K, Shirahata A, Igarashi K. Correlation between the inhibition of cell growth by bis(ethyl) polyamine analogues and the decrease in the function of mitochondria. Eur J Biochem. 1994;221:391–398. doi: 10.1111/j.1432-1033.1994.tb18751.x. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi K, Matsuo Y, Mitsui K, Hirose S. Effect of polypeptide initiation factors on the spermidine stimulation of initiation complex formation. Biochem Biophys Res Commun. 1980;93:360–368. doi: 10.1016/0006-291x(80)91085-2. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi K, Kashiwagi K, Fukuchi J, Isobe Y, Otomo S, Shirahata A. Spermine-like functions of N1,N12-bis(ethyl)spermine: stimulation of protein synthesis and cell growth and inhibition of gastric ulceration. Biochem Biophys Res Commun. 1990;172:715–720. doi: 10.1016/0006-291x(90)90733-4. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi K, Kashiwagi K. Polyamine transport in Escherichia coli. Amino Acids. 1996;10:83–97. doi: 10.1007/BF00806095. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi K, Saisho T, Yuguchi M, Kashiwagi K. Molecular mechanism of polyamine stimulation of the synthesis of oligopeptide-binding protein. J Biol Chem. 1997;272:4058–4064. doi: 10.1074/jbc.272.7.4058. [DOI] [PubMed] [Google Scholar]
- 15.Kalpaxis D L, Theocharis D, Coutsogeorgopoulos C. Kinetic studies on ribosomal peptidyltransferase. The behaviour of the inhibitor blasticidin S. Eur J Biochem. 1986;154:267–271. doi: 10.1111/j.1432-1033.1986.tb09392.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalpaxis D L, Drainas D. Effect of spermine on peptide-bond formation, catalyzed by ribosomal peptidyltransferase. Mol Cell Biochem. 1992;115:19–26. doi: 10.1007/BF00229091. [DOI] [PubMed] [Google Scholar]
- 17.Karahalios P, Mamos P, Vynios D H, Papaioannou D, Kalpaxis D L. The effect of acylated polyamine derivatives on polyamine uptake mechanism, cell growth, and polyamine pools in Escherichia coli, and the pursuit of structure/activity relationships. Eur J Biochem. 1998;251:998–1004. doi: 10.1046/j.1432-1327.1998.2510998.x. [DOI] [PubMed] [Google Scholar]
- 18.Karahalios P, Mamos P, Karigiannis G, Kalpaxis D L. Structure/function correlation of spermine-analogue-induced modulation of peptidyltransferase activity. Eur J Biochem. 1998;258:437–444. doi: 10.1046/j.1432-1327.1998.2580437.x. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwagi K, Hosokawa N, Furuchi T, Kobayashi H, Sasakawa C, Yoshikawa M, Igarashi K. Isolation of polyamine transport-deficient mutants of Escherichia coli and cloning of the genes for polyamine transport proteins. J Biol Chem. 1990;265:20893–20897. [PubMed] [Google Scholar]
- 20.Kashiwagi K, Pistocchi R, Shibuya S, Sugiyama S, Morikawa K, Igarashi K. Spermidine-preferential uptake system in Escherichia coli. J Biol Chem. 1996;271:12205–12208. doi: 10.1074/jbc.271.21.12205. [DOI] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Mamos P, Karigiannis G, Athanassopoulos C, Bichta S, Kalpaxis D L, Papaioannou D. Simple total synthesis of N-substituted polyamine derivatives using N-tritylamino acids. Tetrahedron Lett. 1995;36:5187–5190. [Google Scholar]
- 23.Marton L J, Pegg A E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 24.Mi Z, Kramer D L, Miller J T, Bergeron R J, Bernacki R, Porter C W. Human prostatic carcinoma cell lines display altered regulation of polyamine transport in response to polyamine analogs and inhibitors. Prostate. 1998;34:51–60. doi: 10.1002/(sici)1097-0045(19980101)34:1<51::aid-pros7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Mikulik K, Anderova M. Role of polyamines in the binding of initiator tRNA to the 70S ribosomes of extreme thermophilic bacterium Calderobacterium hydrogenophilum. Arch Microbiol. 1994;161:508–513. [Google Scholar]
- 26.Miyamoto S, Kashiwagi K, Ito K, Watanabe S, Igarashi K. Estimation of polyamine distribution and polyamine stimulation of protein synthesis in Escherichia coli. Arch Biochem Biophys. 1993;300:63–68. doi: 10.1006/abbi.1993.1009. [DOI] [PubMed] [Google Scholar]
- 27.Naranda T, Kucan Z. Effect of spermine on the efficiency and fidelity of the codon-specific binding of tRNA to the ribosomes. Eur J Biochem. 1989;182:291–297. doi: 10.1111/j.1432-1033.1989.tb14829.x. [DOI] [PubMed] [Google Scholar]
- 28.Outinen K, Vuoreta P, Hinkkanen R, Hiltunen R, Vuorela H. Optimization of the HPLC analysis of biogenic amines in Peucedanum palustre plants and cell culture lines. Planta Med. 1995;61:259–263. doi: 10.1055/s-2006-958068. [DOI] [PubMed] [Google Scholar]
- 29.Pegg A E. Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 30.Pegg A E, Poulin R, Coward J K. Use of aminopropyl-transferase inhibitors and of non-metabolizable analogs to study polyamine regulation and function. Int J Biochem Cell Biol. 1995;27:425–442. doi: 10.1016/1357-2725(95)00007-c. [DOI] [PubMed] [Google Scholar]
- 31.Porter C W, McManis J S, Casero R A, Bergeron R J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987;47:2821–2825. [PubMed] [Google Scholar]
- 32.Porter C W, Pegg A E, Ganis B, Madhabala R, Bergeron R J. Combined regulation of ornithine and S-adenosylmethionine decarboxylases by spermine and the spermine analogue N1,N12-bis(ethyl)spermine. Biochem J. 1990;268:207–212. doi: 10.1042/bj2680207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rheinberger H-J, Sternback H, Nierhaus K H. Three tRNA binding sites on Escherichia coli ribosomes. Proc Natl Acad Sci USA. 1981;78:5310–5314. doi: 10.1073/pnas.78.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segel I H. Enzyme kinetics. New York, N.Y: John Wiley & Sons; 1993. pp. 166–169. [Google Scholar]
- 35.Seiler N, Delcros J G, Moulinoux J P. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 36.Synder R D, Edwards M L. Effects of polyamine analogs on the extent and fidelity of in vitro polypeptide synthesis. Biochim Biophys Res Commun. 1991;176:1383–1392. doi: 10.1016/0006-291x(91)90440-i. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama S, Matsuo Y, Maenaka K, Vassylyev D G, Matsusima M, Kashiwagi K, Igarashi K, Morikawa K. The 1.8-Å x-ray structure of the Escherichia coli PotD protein complexed with spermidine and the mechanism of polyamine binding. Protein Sci. 1996;5:1984–1990. doi: 10.1002/pro.5560051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Synetos D, Coutsogeorgopoulos C. Studies on the catalytic rate constant of ribosomal peptidyltransferase. Biochim Biophys Acta. 1987;923:275–285. doi: 10.1016/0304-4165(87)90014-6. [DOI] [PubMed] [Google Scholar]
- 39.Tabor C W, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 40.Vassylyev D G, Tomitori H, Kashiwagi K, Morikawa K, Igarashi K. Crystal structure and mutational analysis of the Escherichia coli putrescine receptor. J Biol Chem. 1998;273:17604–17609. doi: 10.1074/jbc.273.28.17604. [DOI] [PubMed] [Google Scholar]