Abstract
Objective
Hepatocellular carcinoma (HCC) is characterized by a high degree of malignancy, rapid proliferation of tumor cells, and early liver metastasis. Resistance to multiple drugs independent of the high expression of secreted protein acidic and rich in cysteine (SPARC) is associated with a high risk of recurrence and mortality. However, the prognostic value of SPARC in patients with HCC remains unclear. Therefore, we performed a meta-analysis to evaluate the relationship between the expression of SPARC and the prognosis of patients with HCC.
Methods
We searched for relevant articles in the CNKI, PubMed, EMBASE, and Web of Science databases. The 95% confidence intervals (CIs) were calculated for combined overall survival (OS) and disease-free survival (DFS) to assess the prognostic value of expression of SPARC in patients with HCC.
Results
In six of the studies, SPARC expression status was significantly associated with OS (combined hazard ratio [HR], 1.38; 95% CI, 1.0–1.82; Z = 2.27, P = 0.02) but not with DFS (combined HR, 0.79; 95% CI, 0.16–4.00, Z = 0.28, P = 0.78). Therefore, it cannot be assumed that upregulated SPARC expression has an effect on DFS in patients with HCC.
Conclusion
Elevated SPARC expression is associated with a low survival rate but not with DFS in patients with HCC. Further studies are needed to confirm our conclusions.
Registration
INPLASY registration number: INPLASY202180115. https://inplasy.com/inplasy-2021-8-0115/.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third leading cause of cancer-related deaths [1,2], with a rapidly increasing incidence and mortality rate [3]. HCC is usually diagnosed at an advanced and unresectable stage when only conventional treatment options can be used and has a median survival after diagnosis of 6–12 months [4,5]. Transarterial chemoembolization is currently the standard of care for HCC [6]. However, there is a significant disparity in the prognoses of individuals with unresectable HCC despite receiving the same treatment. Therefore, it is important to examine the prognostic indicators of HCC in clinical use.
Secreted protein acidic and rich in cysteine (SPARC), also known as osteonectin, was initially identified in bone and endothelial cells [7,8]. It is a 32–35-kDa multifunctional collagen or calcium-binding extracellular matrix glycoprotein belonging to a group of matricellular proteins encoded by genes located at 5q33.1 and consists of a single polypeptide (285 amino acids) comprising the following three biological structural domains: an acidic N-terminal domain, follistatin-like domain, and calcium-binding extracellular domain [9–11]. The human SPARC gene is expressed in numerous tissues and organs, including the bone marrow, whole blood, lymph node, thymus, brain, cerebellum, retina, heart, smooth muscle, skeletal muscle, spinal cord, intestine, colon, adipocytes, kidney, liver, pancreas, thyroid and salivary glands, skin, ovary, uterus, placenta, cervix, and prostate gland [12]. SPARC is a stromal cell glycoprotein that participates in the remodeling of the extracellular matrix and is involved in the development and progression of malignancies [10,13–19]. SPARC has been found to enhance tumorigenesis and metastasis and is associated with a poor prognosis [20–24], especially in pancreatic cancer [25,26], prostate cancer [27], and lung cancer [28]. SPARC is linked to the prognosis of HCC and can increase the proliferation and migration of tumor cells [5,29,30]. Furthermore, it helps HCC cells to acquire a stem cell morphology and promotes epithelial-mesenchymal transition, which is associated with tumor progression and metastasis [31].
Several researchers have evaluated the correlation between SPARC levels in hepatic stellate cells after activation and the prognosis of patients with HCC and found that independent high expression of SPARC can lead to high recurrence and mortality rates [32]. Meanwhile, it has been shown that the incidence of SPARC methylation in HCC tissue is much higher than that in non-tumor tissues and that patients without SPARC methylation have a higher postoperative overall survival (OS) rate than patients with SPARC methylation [33]. However, there are few relevant studies on the prognostic impact of SPARC in patients with HCC, and the predictive significance of SPARC in these patients is unknown. The aim of this meta-analysis was to analyze the prognostic significance of SPARC in patients with HCC to provide an evidence-based platform for future studies.
Materials and methods
We registered this systematic review and meta-analysis with INPLASY (INPLASY202180115) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for meta-analyses [34].
Database search strategy
We performed a systematic literature search of the CNKI, PubMed, EMBASE, and Web of Science databases from their inception to August 2021. The retrieval strategy was as follows: (1) PubMed: (“Carcinoma, Hepatocellular”[Mesh] OR “Carcinoma, Hepatocellular”[All Fields] OR “HCC”[All Fields] OR “liver cancer”[All Fields]) AND (“Osteonectin”[Mesh] OR “Osteonectin”[All Fields] OR “SPARC”[All Fields] OR “Secreted protein acidic and cysteine rich”[All Fields]) AND (“Prognosis”[Mesh] OR “Prognosis”[All Fields] OR “Prognostic”[All Fields] OR “Survival Analysis”[Mesh] OR “Survival”); (2) EMBASE: (“hepatocellular carcinoma”: ti, ab, kw OR hcc: ti, ab, kw OR “liver cancer”: ti, ab, kw) AND (osteonectin OR sparc OR secreted) AND protein AND acidic AND cysteine AND rich AND (prognosis: ti, ab, kw OR prognostic: ti, ab, kw OR survival: ti, ab, kw); (3) Web of Science: TOPIC: (hepatocellular carcinoma OR HCC OR liver cancer) AND TOPIC: (Osteonectin OR SPARC OR Secreted protein acidic and cysteine rich) AND TOPIC: (Prognosis OR Prognostic OR Survival); (4) CNKI: keywords (hepatocellular carcinoma OR HCC OR liver cancer) and (Prognosis OR Prognostic OR Survival) in Chinese.
Inclusion criteria
To be eligible for inclusion, the following criteria had to be fulfilled: (a) clinical study in patients with HCC; (b) SPARC expression in HCC measured using immunohistochemistry, quantitative real-time polymerase chain reaction, or western blotting; (c) association between SPARC expression and survival outcomes reported; (d) hazard ratio [HR] and 95% confidence interval [CI] for OS according to SPARC status either reported or able to be estimated from the relevant published data.
Exclusion criteria
The following exclusion criteria were applied: (a) publication as a letter, editorial, abstract, review, case report, or expert opinion; (b) an in vitro or in vivo experiment; (c) HRs for OS with 95% CIs not reported and no Kaplan–Meier survival curves available; (d) duplicate article derived from an identical or overlapping patient population (only the most recent and/or complete one used); (e) inclusion of patients with a diagnosis of malignancies other than HCC.
Literature screening
The literature search and screening were performed by two researchers working independently. The titles, abstracts, and keywords were read briefly; the complete text was then reviewed and selected based on the inclusion and exclusion criteria. Finally, inclusion was determined by cross-checking; if the two researchers did not agree on the selection of a particular article, a third qualified researcher was asked to adjudicate.
Extraction of data
Information on the initial author, year of publication, number of study participants, sex ratio, and outcome indicators was collected and summarized using Excel 2019 software.
Quality evaluation of the literature
The quality of the included studies was appraised by two authors using the risk of bias assessment technique in the Cochrane Handbook for Systematic Reviews of Treatments 5.1.0, which is used to assess the risk of publication bias. Investigators and outcome assessors were blinded to all information on random sequence generation and allocation concealment. All articles were evaluated for completeness of outcome data and selective reporting of research outcomes.
Statistical methods
Stata 11.2 and RevMan 5.3 software were used to evaluate the relevant literature. All outcome indicators were continuous variables, and the data are expressed as the odds ratio (OR) with the 95% CI. Heterogeneity between the included studies was estimated. The Q test was performed to determine the heterogeneity of the I2 response. A P-value > 0.1 or an I2 value < 50% indicated statistically significant homogeneity, and a fixed-effects model was used to conduct the meta-analysis. A P-value < 0.1 or an I2 value > 50% indicated statistically significant heterogeneity, and a random-effects model was used with subgroup analysis to investigate the source of heterogeneity. The combined effect size test indicated a statistically significant difference at P ≤ 0.05.
Results
Search results
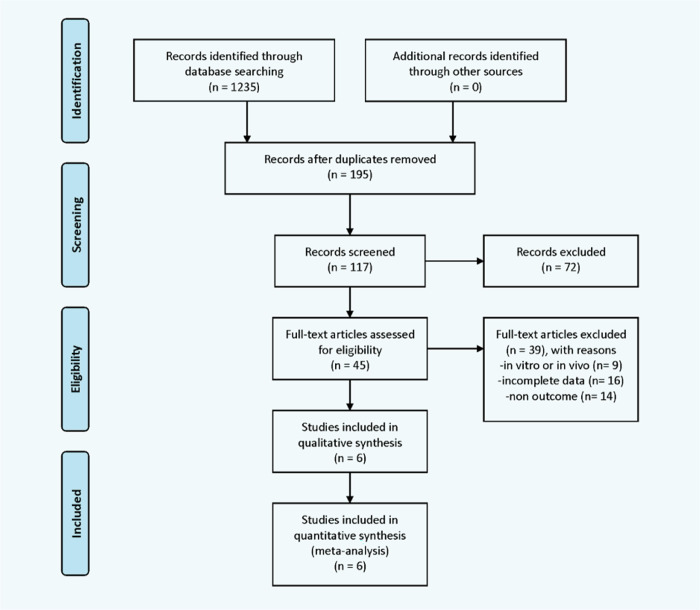
The initial systematic search identified 1235 studies. After eliminating duplicates, 195 articles remained. Based on the title and abstract screening, 72 irrelevant articles were excluded, and 45 were further screened for assessment of eligibility. Thirty-nine studies that did not meet our inclusion criteria were excluded, leaving six [32,33,35–38] for inclusion in the meta-analysis. Fig 1 shows the literature search and article selection processes.
Fig 1. Flow chart showing the process used to select articles for meta-analysis.
Study characteristics and quality assessment
This meta-analysis included all studies published in the English language between 2009 and 2020. The studies included a total of 678 patients with HCC. Background data, including sex ratio, mean age, and duration of disease, were comparable between the study and control groups. The patient sample size ranged from 60 to 200 in the six studies. In all studies, SPARC was detected in tumor tissues, mesenchymal cells, or cancer cells by immunohistochemistry or quantitative real-time polymerase chain reaction. Stratification of SPARC expression differed between the studies. HRs for reported or estimated OS and disease-free survival (DFS) with 95% CIs were included. SPARC was mentioned as a predictor of poor prognosis in four studies, and only one [38] found that it had no effect on OS. SPARC was found to be a good predictor of outcomes in one study [35]. The patient characteristics are summarized in Table 1. Huang et al. used quantitative polymerase chain reaction (qPCR) to determine SPARC levels in 120 samples. OS and DFS were recorded [35]. Liu et al. recorded OS and used immunohistochemistry (IHC) to determine SPARC levels in 89 samples [36]. Yang et al. used IHC to determine SPARC levels in 79 samples. OS and DFS were recorded [37]. Ju et al. used qPCR to determine SPARC levels in 130 samples. OS and relapse-free survival (RFS) were recorded [32]. Zhang et al. recorded OS and used methylmion specific polymerase chain reaction (MSP) to determine SPARC levels in 60 samples [33]. Darweesh et al. recorded OS and used qPCR to determine SPARC levels in 200 samples [38].
Table 1. Background characteristics of the study population.
| First author | Country | Method | Subjects | Sex M/F |
Cell type/location | Study group | Control group | SPARC | Outcome | Obtainment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| + | - | ||||||||||
| Huang, et al. 2017 [35] | China | qPCR | 120 | 110/10 | Tumor | HCC tumor tissues | Tumor adjacent liver tissues | 71 | 49 | OS、DFS | Multivariate |
| Liu 2020 [36] |
China | IHC | 89 | 36/7 | Stroma | Patients with HCC | Healthy controls | 11 | 78 | OS | Multivariate |
| Yang 2018 [37] |
China | IHC | 79 | 38/16 | Tumor | Patients with primary liver cancer | Tumor adjacent liver tissues | 35 | 44 | OS、DFS | Multivariate |
| Ju 2009 [32] |
China | qPCR | 130 | 112/18 | Tumor | Patients with HCC | Healthy liver donors | 85 | 45 | OS、RFS | Multivariate |
| Zhang 2012 [33] |
China | MSP | 60 | 9/51 | Tumor | HCC tumor tissues | Nontumorous liver tissues | 21 | 39 | OS | Multivariate |
| Darweesh 2018 [38] |
Egypt | qPCR | 200 | 160/40 | Tumor | Patients with HCC | Healthy controls | NA | NA | OS | Multivariate |
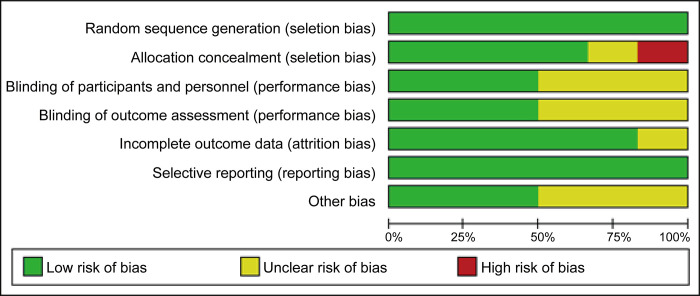
Five reports clearly included the method of group assignment. Huang et al. used paired paraffin-embedded samples from patients with HCC who had undergone liver resection. Adjacent non-cancerous liver tissue samples were selected as the control group [35]. Liu el al. performed immunohistochemical assays in hepatic tissues collected from patients with HCC (study group) and healthy individuals (control group) [36]. Yang et al. selected patients with primary liver cancer who underwent surgery as the study group and tumor adjacent liver tissues as the control group [37]. Ju et al. selected patients with HCC matching the following criteria to form the study group: (1) pathologically diagnosed HCC; (2) no anticancer treatment or distant metastases before surgery; and (3) history of HCC resection, defined as macroscopically complete removal of the tumors. Meanwhile, a normal liver tissue pool from 10 healthy liver donors was assigned as the control group [32]. Zhang et al. selected HCC tissues as the study group and nontumorous tissues as the control group [33]. Darweesh et al. selected patients with HCC as the study group and healthy volunteers as the control group. Healthy paticipants were those who had normal liver enzyme levels and functions, negative results on viral hepatitis screening, and normal abdominal ultrasound scans [38]. Six did not mention blinding in the outcome analysis. Specific grouping information was not clearly described; only one study was evaluated as having a high risk of bias, and the degree of assignment bias was unknown in one study. Three studies did not specify whether testing was performed using blinded principles, and the amount of missing data was not specified in one article. Testing bias was identified in three studies. Some of the included studies did not describe the staging of HCC. The quality assessment results are shown in Fig 2.
Fig 2. Quality assessment chart.
SPARC and overall survival
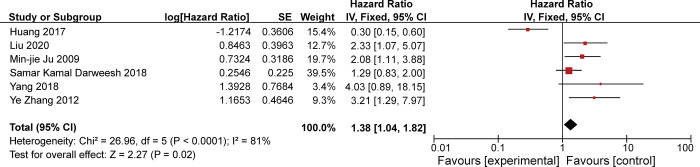
Six studies showed an association between SPARC expression and OS. There was considerable heterogeneity in OS in patients with HCC between the studies (χ2 = 26.96, P<0.001; I2 = 81%). The random-effects model was used for the analysis because the I2 value was >50%, and the combined HR for the six studies was 1.38 (95% CI 1.04–1.82; Z = 2.27, P = 0.02), indicating that upregulated expression of SPARC was significantly associated with OS in patients with HCC (Fig 3).
Fig 3. Forest plot showing the correlation between change in overall survival and secreted protein acidic and rich in cysteine expression in patients with hepatocellular carcinoma.
Two studies revealed the relationship between SPARC expression and DFS, as shown in Fig 4. There was significant heterogeneity in the data (τ2 = 1.23; χ2 = 26.96, P = 0.002; I2 = 90%). Therefore, a random-effects model was used for analysis; the combined HR for the two studies was 0.79 (95% CI, 0.16–4.00; Z = 0.28, P = 0.78), which did not indicate a statistically significant difference. Therefore, upregulated SPARC expression did not appear to affect DFS in patients with HCC.
Fig 4. Forest plot showing the correlation between disease-free survival and secreted protein acidic and rich in cysteine expression in patients with hepatocellular carcinoma.
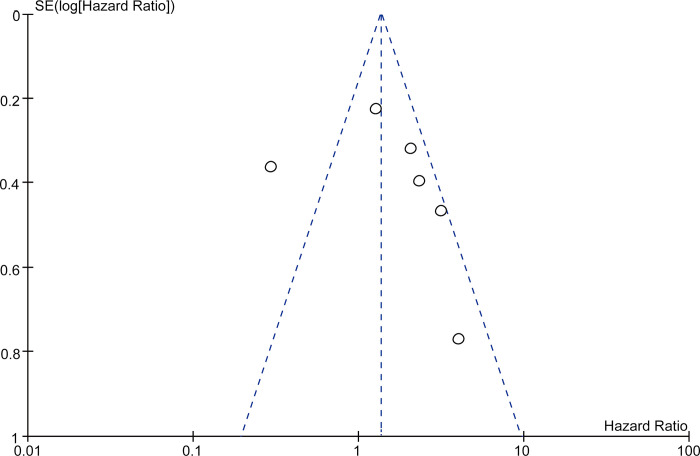
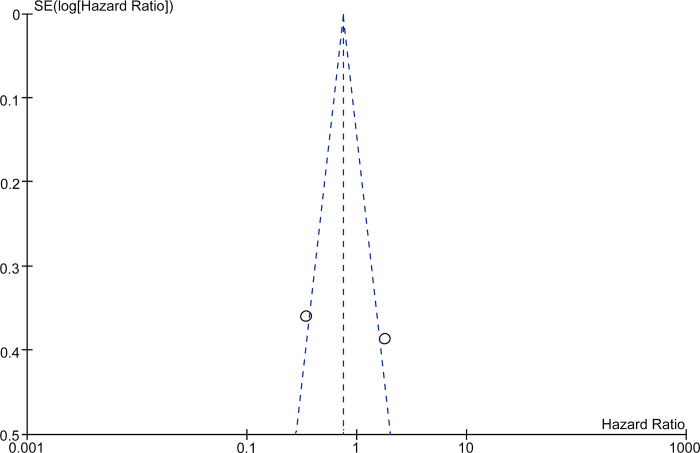
Funnel plots (Figs 5 and 6) constructed to assess publication bias in the studies that included both OS and DFS showed an asymmetric distribution, indicating considerable publication bias.
Fig 5. Funnel plot of studies with overall survival as the evaluation index.
Fig 6. Funnel plot showing disease-free survival as the evaluation index.
Discussion
SPARC is attracting increasing interest as a potential prognostic biomarker in patients with cancer [10,12,39–43]. However, the link between the SPARC expression profile and patient survival is a matter of debate and seems to depend on the type of tumor [44,45]. There have been many studies on SPARC expression in patients with pancreatic [46–48], prostate [27,49], lung [28,50], breast [27,51] and other tumors in terms of carcinogenesis, metastasis, and prognosis [52,53]. However, although upregulated SPARC expression is thought to be associated with a favorable outcome [54], the functional role of SPARC in cancer varies according to tumor type and tissue environment [55]. SPARC expression has been demonstrated to both promote and inhibit various forms of tumor cell activity. Although the sample size in SPARC studies in HCC is very small, several studies have shown that SPARC can boost proliferation and migration of tumor cells and that it may be associated with the prognosis of HCC [5,31,56]. However, until now, there has been no meta-analysis of studies that have suggested the prognostic significance of SPARC in HCC.
Our meta-analysis of six eligible studies including a total of 678 patients is the first to systematically evaluate the role of SPARC in the prognosis of HCC. We found that patients with HCC who had high SPARC expression had a lower OS rate than patients without HCC did. When the HR value for DFS was examined, there was no relationship between expression of SPARC in patients with HCC and DFS. However, only two of the included studies included DFS evaluation and had conflicting results, which is presumably the reason for this negative finding, requiring further study. We also created funnel plots for OS and DFS to analyze bias and found them to be asymmetric, indicating that our results were unstable.
Other researchers have identified abnormal methylation of SPARC in some tumor cell lines and that SPARC methylation induces gene silencing and inhibition of tumor activity [57], whereas demethylating agents can change methylation status and restore gene expression. Therefore, upregulated expression of SPARC in patients with HCC is most likely attributable to methylation. SPARC methylation is more common in HCC tissue [37], and patients with SPARC methylation have a low overall postoperative survival rate. Moreover, multiple significant molecular processes involving SPARC have been found in malignant tumors in both the extracellular matrix and tumor microenvironment, including regulation of modulation, anti-adhesion, apoptosis, growth, migration, and invasion [53]. Furthermore, the tumor suppressor KLF4 decreases tumor invasion by downregulating the expression of SPARC [58]. These findings are consistent with the theory that high SPARC expression is associated with a poor prognosis in patients with HCC.
This research has some limitations. First, the sample size was limited to 678 patients, most of whom were from health centers or hospitals with adequate follow-up, and the quality of the data was variable, which may have affected our results. Second, given that most of the studies were retrospective, selection bias and information bias were inevitable. We identified some risk of bias (Fig 2), especially for allocation concealment. Third, although there is a link between SPARC expression and tumor stage, the TNM (tumor node metastasis) staging system was not used in any of the eligible studies. Therefore, the inclusion of patients with various disease stages may have had an impact on our findings, including for heterogeneity. Finally, we only included studies published in English, which may have resulted in the exclusion of relevant studies published in other languages. Furthermore, the number of relevant studies that have not been published is unknown. Therefore, further research is needed to confirm our present findings regarding the prognostic value of SPARC in patients with HCC.
Conclusion
This comprehensive review and meta-analysis found an association between high SPARC expression and a poor prognosis in patients with HCC. However, this association did not extend to DFS, possibly because DFS was rarely included in the eligible studies. Therefore, more research is needed to confirm our findings and investigate the molecular mechanisms and pathways that influence DFS.
Supporting information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65: 87–108. doi: 10.3322/caac.21262 . [DOI] [PubMed] [Google Scholar]
- 2.Scaggiante B, Kazemi M, Pozzato G, Dapas B, Farra R, Grassi M, et al. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20: 1268–1288. doi: 10.3748/wjg.v20.i5.1268 , PubMed Central PMCID: PMC3921509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136: E359–E386. doi: 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 4.Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Med (Baltim). 2017;96: e5904. doi: 10.1097/MD.0000000000005904 , PubMed Central PMCID: PMC5340426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Liu F, Wang Y, Gao J. Secreted protein acidic and rich in cysteine promotes epithelial–mesenchymal transition of hepatocellular carcinoma cells and acquisition of cancerstem cell phenotypes. J Gastroenterol Hepatol (Australia). 2019;34: 1860–1868. doi: 10.1111/jgh.14692 [DOI] [PubMed] [Google Scholar]
- 6.Mendizabal M, Reddy KR. Current management of hepatocellular carcinoma. Med Clin North Am. 2009;93: 885–900, viii. doi: 10.1016/j.mcna.2009.03.004 . [DOI] [PubMed] [Google Scholar]
- 7.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4 [DOI] [PubMed] [Google Scholar]
- 8.Mason IJ, Murphy D, Münke M, Francke U, Elliott RW, Hogan BL. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986;5: 1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x PubMed Central PMCID: PMC8208664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19: 816–827. doi: 10.1016/s0945-053x(00)00133-5 [DOI] [PubMed] [Google Scholar]
- 10.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14: 608–616. doi: 10.1016/s0955-0674(02)00361-7 [DOI] [PubMed] [Google Scholar]
- 11.Hohenester E, Maurer P, Hohenadl C, Timpl R, Jansonius JN, Engel J PM. Structure of a novel extracellular Ca(2+)-binding module in BM-40. Nat Struct Biol. 1996;3: 67–73. doi: 10.1038/nsb0196-67 [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Chen K, Xu W, Chen D, Tang W, Xia TS. Integrative genomic analyses of secreted protein acidic and rich in cysteine and its role in cancer prediction. Mol Med Rep. 2014;10: 1461–1468. doi: 10.3892/mmr.2014.2339 . [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Jeong D, Ahn TS, Kim HJ, Park DS, Park SY, et al. Expression of secreted protein acidic and rich in cysteine in the stroma of a colorectal carcinoma is associated with patient prognosis. Ann Coloproctol. 2013;29: 93–99. doi: 10.3393/ac.2013.29.3.93 , PubMed Central PMCID: PMC3710779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, et al. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64: 3037–3045. doi: 10.1158/0008-5472.can-03-2028 . [DOI] [PubMed] [Google Scholar]
- 15.Rempel SA, Golembieski WA, Ge S, Lemke N, Elisevich K, Mikkelsen T, et al. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57: 1112–1121. doi: 10.1097/00005072-199812000-00002 [DOI] [PubMed] [Google Scholar]
- 16.Yiu GK, Chan WY, Ng S-W, Chan PS, Cheung KK, Berkowitz RS, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159: 609–622. doi: 10.1016/S0002-9440(10)61732-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2: 215–224. doi: 10.1158/1541-7786.215.2.4 [DOI] [PubMed] [Google Scholar]
- 18.Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-aza-2’deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer. 2008;98: 1810–1819. doi: 10.1038/sj.bjc.6604377 , PubMed Central PMCID: PMC2410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang E, Kang HJ, Koh KH, Rhee H, Kim NK, Kim H. Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer. 2007;121: 567–575. doi: 10.1002/ijc.22706 . [DOI] [PubMed] [Google Scholar]
- 20.Rosenblatt S, Bassuk JA, Alpers CE, Sage EH, Timpl R, Preissner KT. Differential modulation of cell adhesion by interaction between adhesive and counter-adhesive proteins: characterization of the binding of vitronectin to osteonectin (BM40, SPARC). Biochem J. 1997;324: 311–319. doi: 10.1042/bj3240311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie RL, Long GL. Role of N-linked glycosylation in human osteonectin. Effect of carbohydrate removal by N-glycanase and site-directed mutagenesis on structure and binding of type V collagen. J Biol Chem. 1995;270: 23212–23217. doi: 10.1074/jbc.270.39.23212 [DOI] [PubMed] [Google Scholar]
- 22.Sosa MS, Girotti MR, Salvatierra E, Prada F, de Olmo JA, Gallango SJ, et al. Proteomic analysis identified N-cadherin, clusterin, and HSP27 as mediators of SPARC (secreted protein, acidic and rich in cysteines) activity in melanoma cells. Proteomics. 2007;7: 4123–4134. doi: 10.1002/pmic.200700255 . [DOI] [PubMed] [Google Scholar]
- 23.Smit DJ, Gardiner BB, Sturm RA. Osteonectin downregulates E-cadherin, induces osteopontin and focal adhesion kinase activity stimulating an invasive melanoma phenotype. Int J Cancer. 2007;121: 2653–2660. doi: 10.1002/ijc.23039 . [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Hao B, Yang Y, Wang R, Li Y, Wu Q. Prognostic role of SPARC expression in gastric cancer: a meta-analysis. Arch Med Sci. 2014;10: 863–869. doi: 10.5114/aoms.2014.46207 , PubMed Central PMCID: PMC4223132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaz J, Ansari D, Sasor A, Andersson R. SPARC: A potential prognostic and therapeutic target in pancreatic cancer. Pancreas. 2015;44: 1024–1035. doi: 10.1097/MPA.0000000000000409 PubMed Central PMCID: PMC4568900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han W, Cao F, Chen MB, Lu RZ, Wang HB, Yu M, et al. Prognostic value of SPARC in patients with pancreatic cancer: A systematic review and meta-analysis. PLOS ONE. 2016;11: e0145803. doi: 10.1371/journal.pone.0145803 , PubMed Central PMCID: PMC4701416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong SY, Crowley D, Bronson RT, Hynes RO. Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin Exp Metastasis. 2008;25: 109–118. doi: 10.1007/s10585-007-9126-2 , PubMed Central PMCID: PMC3252392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabrizio FP, Sparaneo A, Fontana A, Mazza T, Graziano P, Pantalone A, et al. Potential prognostic role of SPARC methylation in non-small-cell lung cancer. Cells. 2020;9. doi: 10.3390/cells9061523 , PubMed Central PMCID: PMC7349117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71: 1247–1261. doi: 10.1002/hep.30889 , PubMed Central PMCID: PMC7000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan S, Meyer AS, Weiler SME, Rupp C, Tóth M, Sticht C, et al. Cytoplasmic localization of the cell polarity factor scribble supports liver tumor formation and tumor cell invasiveness. Hepatology. 2018;67: 1842–1856. doi: 10.1002/hep.29669 . [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Wu H, Liu S, Lei Z, Qin Z, Wen L, et al. SMYD3 controls a Wnt-responsive epigenetic switch for ASCL2 activation and cancer stem cell maintenance. Cancer Lett. 2018;430: 11–24. doi: 10.1016/j.canlet.2018.05.003 . [DOI] [PubMed] [Google Scholar]
- 32.Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131: 498–510. doi: 10.1309/AJCP86PPBNGOHNNL [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Yang B, Du Z, Bai T, Gao YT, Wang YJ, et al. Aberrant methylation of SPARC in human hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2012;18: 2043–2052. doi: 10.3748/wjg.v18.i17.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162: 777–784. doi: 10.7326/M14-2385 . [DOI] [PubMed] [Google Scholar]
- 35.Huang XQ, Zhou ZQ, Zhang XF, Chen CL, Tang Y, Zhu Q, et al. Overexpression of SMOC2 attenuates the tumorigenicity of hepatocellular carcinoma cells and is associated with a positive postoperative prognosis in human hepatocellular carcinoma. J Cancer. 2017;8: 3812–3827. doi: 10.7150/jca.20775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Feng Y, Wang X. SPARC negatively correlates with prognosis after transarterial chemoembolization and facilitates growth and metastasis of hepatocellular carcinoma via ERK/MMP signaling pathways. J Hepatol. 2020;73: S641–S642. doi: 10.1016/S0168-8278(20)31749-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JC. Value of matrix metalloproteinase-9 and secreted protein acidic and rich in cysteine in evaluation of severity and prognosis of primary liver cancer. World Chin J Digestology. 2018;26: 1036–1043. doi: 10.11569/wcjd.v26.i17.1036 [DOI] [Google Scholar]
- 38.Darweesh SK, Abd Alziz RA, Omar H, Sabry D, Fathy W. Secreted protein acidic and rich in cysteine gene variants: impact on susceptibility and survival of hepatocellular carcinoma patients. J Gastroenterol Hepatol (Australia). 2019;34: 1424–1431. doi: 10.1111/jgh.14541 [DOI] [PubMed] [Google Scholar]
- 39.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3: 163–165. doi: 10.1007/s12079-009-0069-z , PubMed Central PMCID: PMC2778588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leja J, Essaghir A, Essand M, Wester K, Oberg K, Tötterman TH, et al. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 2009;22: 261–272. doi: 10.1038/modpathol.2008.174 . [DOI] [PubMed] [Google Scholar]
- 41.Thomas SL, Alam R, Lemke N, Schultz LR, Gutiérrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro Oncol. 2010;12: 941–955. doi: 10.1093/neuonc/noq048 , PubMed Central PMCID: PMC2940688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atorrasagasti C, Malvicini M, Aquino JB, Alaniz L, Garcia M, Bolontrade M, et al. Overexpression of SPARC obliterates the in vivo tumorigenicity of human hepatocellular carcinoma cells. Int J Cancer. 2010;126: 2726–2740. doi: 10.1002/ijc.24966 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Hao N, Liu W, Lu M, Sun L, Chen N, et al. In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery. Br J Cancer. 2017;117: 1676–1684. doi: 10.1038/bjc.2017.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, Llera AS. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27: 691–705. doi: 10.1007/s10555-008-9146-7 . [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Jiménez FJ, Caldés T, Iniesta P, Vidart JA, Garcia-Asenjo JL, Benito M. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncol Rep. 2007;17: 1301–1307. doi: 10.3892/or.17.6.1301 [DOI] [PubMed] [Google Scholar]
- 46.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25: 319–325. doi: 10.1200/JCO.2006.07.8824 . [DOI] [PubMed] [Google Scholar]
- 47.Xiao Y, Zhang H, Ma Q, Huang R, Lu J, Liang X, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett. 2019;462: 51–60. doi: 10.1016/j.canlet.2019.07.015 . [DOI] [PubMed] [Google Scholar]
- 48.Prenzel KL, Warnecke-Eberz U, Xi H, Brabender J, Baldus SE, Bollschweiler E, et al. Significant overexpression of SPARC/osteonectin mRNA in pancreatic cancer compared to cancer of the papilla of Vater. Oncol Rep. 2006;15: 1397–1401. [PubMed] [Google Scholar]
- 49.Makridakis M, Vlahou A. Secretome proteomics for discovery of cancer biomarkers. J Proteomics. 2010;73: 2291–2305. doi: 10.1016/j.jprot.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 50.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63: 5376–5380. [PubMed] [Google Scholar]
- 51.Zheng F, Du F, Wang W, Wang Y, Li M, Zhao J, et al. Updated efficacy of adjuvant epirubicin plus cyclophosphamide followed by taxanes versus carboplatin plus taxanes in early triple-negative breast cancer in phase 2 trial: 8.1-year median follow-up. Breast Cancer Res Treat. 2022;191: 97–105. doi: 10.1007/s10549-021-06401-6 . [DOI] [PubMed] [Google Scholar]
- 52.Genasan K, Mehrali M, Veerappan T, Talebian S, Malliga Raman M, Singh S, et al. Calcium-silicate-incorporated gellan-chitosan induced osteogenic differentiation in mesenchymal stromal cells. Polymers (Basel). 2021;13. doi: 10.3390/polym13193211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35: 967–973. doi: 10.1093/carcin/bgu072 . [DOI] [PubMed] [Google Scholar]
- 54.Chew A, Salama P, Robbshaw A, Klopcic B, Zeps N, Platell C, et al. SPARC, FOXP3, CD8 and CD45 correlation with disease recurrence and long-term disease-free survival in colorectal cancer. PLOS ONE. 2011;6: e22047. doi: 10.1371/journal.pone.0022047 , PubMed Central PMCID: PMC3144212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92: 679–690. doi: 10.1002/jcb.20091 . [DOI] [PubMed] [Google Scholar]
- 56.Garg M. Emerging role of microRNAs in cancer stem cells: implications in cancer therapy. World J Stem Cells. 2015;7: 1078–1089. doi: 10.4252/wjsc.v7.i8.1078 , PubMed Central PMCID: PMC4591786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li D, Da L, Tang H, Li T, Zhao M. CpG methylation plays a vital role in determining tissue- and cell-specific expression of the human cell-death-inducing DFF45-like effector A gene through the regulation of Sp1/Sp3 binding. Nucleic Acids Res. 2008;36: 330–341. doi: 10.1093/nar/gkm1028 , PubMed Central PMCID: PMC2248752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Hofstetter WL, He Y, Hu W, Pataer A, Wang L, et al. KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol Ther. 2010;9: 507–513. doi: 10.4161/cbt.9.7.11106 PubMed Central PMCID: PMC2902679. [DOI] [PMC free article] [PubMed] [Google Scholar]