Abstract
Hypertension and osteoporosis are two major disorders, which interact with each other. Specific genetic signals involving the fibroblast growth factor receptor-like 1 (FGFRL1) gene are related to high blood pressure and bone growth in giraffes. FGFRL1 is associated with cardiovascular system and bone formation. We performed an association study to investigate the role of FGFRL1 in hypertension, osteoporosis, and height determination in humans. In addition, we identified three kinds of phenotypes in fibroblast growth factor (FGF) genes and examined their association with the FGFRL1 gene. We identified 42 SNPs in the FGFRL1 gene associated with each trait. We then analyzed the potential functional annotation of each SNP. The FGFRL1 gene was found to be associated with height, hypertension, and osteoporosis, consistent with the results of a previous study. In addition, the FGF2, FGF4, FGF10, FGF18, and FGF22 genes were found to interact with the FGFRL1 gene. Our study suggests that both FGFRL1 and FGFRL1-related genes may determine the height and the prevalence of osteoporosis and hypertension in the Korean population.
Introduction
Osteoporosis is characterized by decreased bone mineral density and an increased risk of fractures, and is one of the most common chronic and metabolic bone diseases [1, 2]. Hypertension is the main risk factor for coronary heart disease, stroke, and chronic kidney disease, and is a prominent cause of death worldwide [3]. Both these diseases are metabolic conditions mediated via common pathophysiology, which means potential mechanistic links between osteoporosis and hypertension. Increasing evidence suggests that bone marrow-derived cells play a role in hypertension [4]. Osteoporosis and hypertension appear to be strongly triggered by immune cell activation, including enhanced salt intake, increased sympathetic outflow, and excessive angiotensin II and aldosterone [5]. Besides, various cohort studies reported the interplay between osteoporosis and hypertension [6–8].
A recent study identified specific genetic signals that are associated with adaptation to high blood pressure and bone growth in giraffes, with exceptional anatomy contributing to their unique stature and biological characteristics [9]. Accordingly, the strongest signal was located in the FGFRL1 (fibroblast growth factor receptor-like 1) gene, and genetic experiments were performed in mice for further investigation. Mice carrying the giraffe-type FGFRL1 allele showed resistance to hypertension and high bone mineral density, suggesting that the genetic changes induced pleiotropic effects related to cardiovascular development and bone formation [9].
FGFRL1, the most recently identified member of the FGFR (fibroblast growth factor receptor) family, is related to the cardiovascular system and bone formation [10–12]. Mutant FGFRL1 protein was found in a patient with craniosynostosis syndrome, indicating that the underlying gene was significantly associated with skeletal malformations [13]. Also, deletion of the Fgfrl1 gene in a mouse model resulted in Wolf-Hirschhorn syndrome (WHS), including skeletal anomalies and congenital heart defects [10]. A few genome-wide association studies involving the FGFRL1gene were performed, and most of the studies analyzed bone mineral density [14, 15], while others investigated body height [14] and type 2 diabetes mellitus [16].
The present study is based not only on the correlation between hypertension and osteoporosis but also on previous studies, which elucidated the molecular mechanism of FGFRL1. We focused on the recent study, which demonstrated specific genetic signals related to high blood pressure and bone growth in giraffes [9, 17]. The previous study identified that the FGFRL1 gene contributes to the representative biological characteristics of giraffes. Therefore, we confirmed the replications of the FGFRL1 signals and identified that the FGFRL1 gene is associated with giraffe-related characteristics (height, hypertension, and osteoporosis) in the human population. In addition, we analyzed gene-gene interaction networks. Expression and regulation levels of the FGFRL1 gene were also analyzed in association with the three phenotypes, referring to the Genotype-Tissue Expression (GTEx) project. We identified the genetic variants of the FGFRL1 gene as well as FGFRL1-related genes, which affect the skeletal system and/or hypertension.
Materials and methods
Study participants
The study data were obtained from the Korean Genome and Epidemiology Study [18] Health Examinees (HEXA) study [18]. The Korean Centers for Disease and Control (KCDC) recruited 173,357 participants for the HEXA study aged 40 to 79 years from 2004 to 2013 who lived in urban (Seoul, Incheon, Daejeon, Daegu, Ulsan, Busan, and Gwangju) and rural (Gyeonggi, Sejong, Gangwon, Chungcheongbuk, Chungcheongnam, Gyeonsangbuk, Gyeonsangnam, Jeollabuk, Jeollanam, and Jeju) areas of Korea [18]. Of these, only 58,698 participants who were available single nucleotide polymorphism (SNP) information and included in the baseline study were selected for the current study. The value of body measurements with each disease are shown in S1 Table. Height [19], weight [20], diastolic blood pressure (DBP), and systolic blood pressure (SBP) were measured. Height and weight were measured using an automated measuring instrument (Dong Sahn Jenix Co., Seoul, Korea) three times for the average values. Each diastolic and systolic blood pressure was measured three times every at intervals of more than 5 minutes by a mercury sphygmomanometer in a seated position, and the average value was used. Body mass index (BMI) was computed in units of kilograms per square meter (kg/m2), using the measured height and weight values. Patients with hypertension (n = 17,086) were defined by SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, or a medical history of hypertension or use of antihypertensive medication. Controls (n = 31,440) were defined by SBP < 120 mmHg and DBP < 80 mmHg and no medical history of hypertension or anti-hypertensive drug use. The diagnosis of osteoporosis was made by a medical doctor based on bone indices measured via whole-body dual-energy X-ray absorptiometry (DXA). The final binary variable, the value we used in this study, was derived from the participants’ medical records through a questionnaire about whether there was a history of the previous diagnosis by a medical doctor. In total, after excluding 89 patients who were either non-respondents or had never been screened for osteoporosis, 55,535 controls and 3,074 cases of osteoporosis were identified.
Genotyping
Genotype data were obtained by the Center for Genome Science, Korea National Institute of Health (KNIH). DNA samples were extracted from peripheral blood and genotyping was done using the Axiom® 2.0 Reagent Kit (Affymetrix Axiom® 2.0 Assay User Guide). Genotype data were generated using the KoreanChip (KCHIP) designed by the Center for Genome Science at the KNIH. Gene location was determined in reference to the National Center for Biotechnology Information (NCBI) Human Genome Build 37 (hg19) assembly. All the gene regions analyzed in this study were expanded by 5 kb at both transcripts ends, and SNPs were selected in this range. The KCHIP has been described comprehensively in previous studies [21]. The only genotypes that satisfied these exclusion criteria: low call rate (< 0.95%), sex inconsistency, cryptic first-degree relatives, and excessive heterozygosity. SNPs with genotype call rates < 95%, Hardy–Weinberg equilibrium (HWE) p-value < 10−6, and minor allele frequency of < 1% were removed. A total of 465,000 variants were included after quality control. A total of 8,056,211 SNPs were used for GWAS after quality control and imputation.
Statistical analysis
PLINK version 1.90 beta (https://www.cog-genomics.org/plink2) was used for most statistical analyses. Imputation for autosomal variants was executed using IMPUTE2 with the reference panel constructed from 1000 phase 3 genomes. A logistic regression, additive genetic model was used after adjustments for age, sex, and body mass index (BMI) to investigate the association between SNPs in the FGFRL1 gene and hypertension and osteoporosis. SNPs associated with height were identified via linear regression additive analysis adjusting for age and sex, with a cut-off p-value of P < 0.05. We sorted out tag SNP, the representative SNP in each genome region with high linkage disequilibrium (LD), from the haplotype block under the condition that the LD measure r2 ≥ 0.8. Regional association plots were generated using LocusZoom (http://locuszoom.org/). The HaploReg database (https://pubs.broadinstitute.org) was used to identify functional effects, such as protein motifs, in the FGFRL1 genetic variants associated with both hypertension and osteoporosis. GTExPortal databases (https://gtexportal.org) were used for expression quantitative trait loci (eQTL) analysis. RegulomeDB (https://regulomedb.org/regulome-search/) was used to rank potential functional roles. We depicted annotated gene networks using STRING database version 11.0 (https://string-db.org/) and selected the genes with direct interactions with the FGFRL1 gene.
Ethical review
This study was approved by the Institutional Review Board of the Korean National Institute of Health (KNIH, KBN-2021-003) and Soonchunhyang University (202012-BR-086-01). Written informed consent was obtained from all participants.
Results
Association of the FGFRL1 gene variants with height, hypertension, and osteoporosis
A total of 44 tag SNPs were identified in the FGFRL1 gene. Logistic regression analysis for hypertension and osteoporosis using the additive genetic model revealed three and six nominally significant SNPs (P < 0.05), respectively (Table 1). Linear regression analysis for height identified nine significant SNPs. Among nine SNPs, 3 and 6 SNPs had positive and negative associations with height, respectively (Table 1). Two SNPs (rs13143527 and rs55639339) were associated with both hypertension and osteoporosis, but none of the height-related SNPs was related to osteoporosis or hypertension. Rs55639339 showed similar patterns of increased risk for hypertension (OR: 1.043) and osteoporosis (OR: 1.103), whereas the rs13143527 variant was associated with a decreased risk of hypertension (OR: 0.967) and osteoporosis (OR: 0.939) (Table 1). A regional plot of the FGFRL1 gene based on height, hypertension, and osteoporosis was drawn using LocusZoom (S1 Fig).
Table 1. Genetic variants in FGFRL1 associated with height, hypertension and osteoporosis.
| SNP | Minor allele | MAF | Function | Height | HTN | Osteoporosis | |||
|---|---|---|---|---|---|---|---|---|---|
| β ± s.e | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||||
| rs117864192 | A | 0.031 | intron | -0.249 ± 0.087 | 4.17 × 10 −3 | 1.013 (0.935–1.098) | 0.749 | 0.966 (0.829–1.126) | 0.658 |
| rs73219733 | T | 0.029 | intron | -0.251 ± 0.091 | 5.83 × 10 −3 | 1.008 (0.926–1.097) | 0.855 | 0.999 (0.849–1.175) | 0.987 |
| rs748650 | A | 0.255 | intron | -0.090 ± 0.035 | 9.77 × 10 −3 | 1.017 (0.984–1.050) | 0.315 | 1.047 (0.984–1.113) | 0.146 |
| rs115259783 | G | 0.157 | intron | 0.105 ± 0.041 | 0.011 | 0.979 (0.942–1.018) | 0.287 | 1.031 (0.958–1.109) | 0.421 |
| rs34627176 | A | 0.044 | upstream | -0.177 ± 0.074 | 0.017 | 0.967 (0.903–1.036) | 0.345 | 0.959 (0.839–1.096) | 0.537 |
| rs139932728 | A | 0.021 | upstream | 0.248 ± 0.104 | 0.017 | 1.005 (0.913–1.106) | 0.924 | 1.115 (0.931–1.335) | 0.237 |
| rs3733350 | T | 0.101 | 3’-UTR | 0.113 ± 0.050 | 0.025 | 0.959 (0.915–1.005) | 0.078 | 1.047 (0.959–1.143) | 0.310 |
| rs4647936 | T | 0.030 | 3’-UTR | -0.192 ± 0.089 | 0.030 | 1.053 (0.970–1.143) | 0.215 | 0.928 (0.791–1.088) | 0.358 |
| rs77488513 | T | 0.030 | upstream | -0.180 ± 0.088 | 0.042 | 1.059 (0.976–1.149) | 0.170 | 0.917 (0.781–1.076) | 0.286 |
| rs13143527 | G | 0.291 | intron | -0.063 ± 0.033 | 0.057 | 0.967 (0.937–0.997) | 0.031 | 0.939 (0.885–0.997) | 0.039 |
| rs55639339 | T | 0.140 | intron | -0.015 ± 0.044 | 0.736 | 1.043 (1.002–1.086) | 0.041 | 1.103 (1.023–1.190) | 0.011 |
| rs10010999 | T | 0.348 | upstream | -0.028 ± 0.032 | 0.370 | 0.971 (0.942–0.999) | 0.047 | 0.977 (0.924–1.034) | 0.418 |
| rs74921869 | A | 0.253 | intron | 0.033 ± 0.035 | 0.343 | 1.015 (0.983–1.048) | 0.362 | 1.093 (1.028–1.161) | 4.35 × 10 −3 |
| rs35220088 | C | 0.309 | intron | -0.003 ± 0.033 | 0.924 | 1.001 (0.972–1.032) | 0.926 | 1.084 (1.024–1.148) | 5.86 × 10 −3 |
| rs73070422 | G | 0.140 | intron | -0.015 ± 0.044 | 0.723 | 1.041 (0.999–1.083) | 0.053 | 1.087 (1.008–1.173) | 0.031 |
| rs78590462 | T | 0.066 | intron | -0.065 ± 0.061 | 0.287 | 1.019 (0.963–1.079) | 0.505 | 0.892 (0.798–0.997) | 0.044 |
Age, sex and body mass index (BMI) were included as covariants in all genetic models. SNPs indicated in bold are associated with both hypertension and osteoporosis at P < 0.05. Abbreviations: SNP, single nucleotide polymorphism; Chr, chromosome; MAF, minor allele frequency; HTN, hypertension; β, regression coefficient; s.e, standard error; OR, odds ratio; CI, confidence interval.
Association of FGF genes with height, hypertension, and osteoporosis
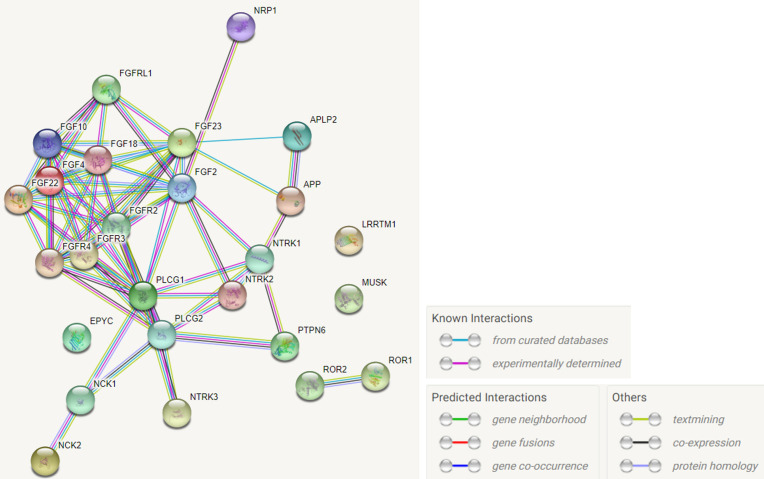
The gene-gene interaction networks generated by STRING revealed connections with high confidence scores (confidence score > 0.7) between FGFRL1 and five fibroblast growth factors (FGF2, FGF4, FGF10, FGF18, and FGF22) (Fig 1). The FGF10, FGF18, FGF2, FGF4, and FGF22 genes, which interact with FGFRL1, were correlated with height, hypertension, and osteoporosis. Overall, SNPs related to height were the most common, and no genetic variant of the FGF4 gene was related to hypertension (S2 Table). Previous studies demonstrated an association between the FGF18 gene and height [14, 24]. Interestingly, rs8109113 in the FGF22 gene was associated with both hypertension and height, and 17 SNPs in FGF10, FGF18, and FGF2 were associated with both osteoporosis and height (Table 2).
Fig 1. Protein-protein interactions with high confidence score (confidence score > 0.7) for FGFRL1.
Proteins in the interaction network are represented as nodes; the line color represents the type of interaction, including known interaction, predicted interaction and other types. These interactions include physical (direct) and functional (indirect) types according to computational predictions and experimental repositories.
Table 2. Genetic variants in the FGF family associated with two of the three phenotypic traits (height, hypertension, and osteoporosis) or below the Bonferroni-corrected significance level.
| Gene | Chr | SNP | Minor allele | MAF | Function | Height | HTN | Osteoporosis | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β ± s.e | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||||||
| FGF2 | 4 | rs167428 | C | 0.087 | intron | 0.112 ± 0.054 | 0.037 | 1.040 (0.99–1.09) | 0.122 | 1.131 (1.03–1.24) | 8.59 × 10 −3 |
| 4 | rs308387 | A | 0.052 | intron | 0.234 ± 0.068 | 5.85 × 10 −4 | 1.023 (0.96–1.09) | 0.487 | 1.062 (0.94–1.20) | 0.317 | |
| FGF4 | 11 | rs58166091 | A | 0.213 | - | 0.114 ± 0.037 | 2.16 × 10 −3 | 0.989 (0.96–1.02) | 0.544 | 1.026 (0.96–1.10) | 0.436 |
| FGF10 | 5 | rs17227836 | C | 0.054 | intron | -0.161 ± 0.067 | 0.016 | 0.993 (0.93–1.06) | 0.816 | 1.144 (1.02–1.28) | 0.021 |
| 5 | rs13154419 | G | 0.412 | intron | 0.112 ± 0.031 | 2.89 × 10 −4 | 0.991 (0.96–1.02) | 0.545 | 0.988 (0.94–1.04) | 0.674 | |
| 5 | rs1448039 | A | 0.500 | intron | -0.094 ± 0.030 | 1.90 × 10 −3 | 1.006 (0.98–1.04) | 0.653 | 1.017 (0.96–1.07) | 0.528 | |
| FGF18 | 5 | rs10463007 | T | 0.404 | - | 0.081 ± 0.031 | 9.26 × 10 −3 | 0.990 (0.96–1.02) | 0.498 | 1.071 (1.01–1.13) | 0.014 |
| FGF22 | 19 | rs8109113 | G | 0.024 | intron | -0.241 ± 0.100 | 0.016 | 0.901(0.82–0.99) | 0.028 | 1.021 (0.85–1.22) | 0.824 |
Age, sex and body mass index (BMI) were included as covariant in all genetic models. Findings with P < 0.05 are indicated in bold. The p-values which satisfied the Bonferroni-corrected significance level regarding each gene are indicated in bold and underlined. Abbreviations: SNP, single nucleotide polymorphism; Chr, chromosome; MAF, minor allele frequency; HTN, hypertension; β, regression coefficient; s.e, standard error; OR, odds ratio; CI, confidence interval.
Functional analysis
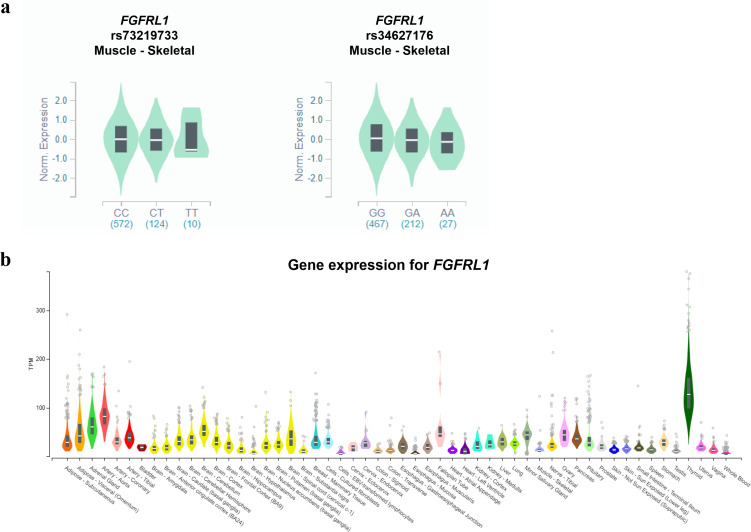
We used the GTEx database and HaploReg to determine the biological functional annotations of the identified genetic variants and genes (Fig 2). The GTEx database was used to obtain tissue expression data for FGFRL1. The FGFRL1 gene is expressed in various tissues, especially in the thyroid gland and arteries (Fig 2B). Rs73219733 and rs34627176, which were associated with height, were significant signals of eQTL for the FGFRL1 gene in skeletal muscle tissue (P = 8.3 × 10−5, 1.7 × 10−12) (Fig 2A). In addition, motif changes were predicted for the two SNPs that were significantly correlated with hypertension and osteoporosis (S3 Table). Based on the results, the FGFRL1 gene expression varies with the SNP genotype.
Fig 2. FGFRL1 gene expression and identification of three SNPs in eQTL.
Each genotype in skeletal muscle is expressed using GTExPortal. (a) Expression of rs73219733 and rs34627176 in FGFRL1. The two SNPs showed lower expression in their minor allele with statistical significance reaching P < 10−4. (b) Expression of the FGFRL1 gene.
Discussion
Our study validated the correlation between FGFRL1 gene and height, hypertension, and osteoporosis. The results are consistent with a previous study that suggested an association of FGFRL1 with the cardiovascular and skeletal systems [10, 12]. We identified 16 SNPs in the FGFRL1 gene that were associated with the traits of interest. We then annotated the potential biological function of these SNPs. Similarly, the FGF2, FGF4, FGF10, FGF18, and FGF22 genes, which showed interactions with the FGFRL1 gene, were associated with height, hypertension, and osteoporosis.
Most previous genetic association studies analyzed the association of FGFRL1 with bone formation or bone density, and seldom with hypertension. In the present study, two SNPs were identified with significant (P < 0.05) association with both osteoporosis and hypertension: the minor allele of rs55639339 was associated with an increased risk of both diseases, while the minor allele of rs10010999 lowered the risk of both diseases, indicating a similar trend in prevalence of both diseases involving each genetic variant. Thus, our results are consistent with previous findings suggesting that osteoporosis is associated with metabolic diseases including hypertension [22]. Meanwhile, FGFRL1 is known to be essential for vasculogenesis and ventricular septation. Indeed, Fgfrl1 -/- embryos manifested defects in ventricular septation and congenital heart defects [10]. Additionally, a functional study in zebrafish revealed a significant role of Fgfrl1 during gill cartilage development and craniofacial skeletogenesis [23–25]. Accordingly, the results of this replication study, which indicate that the genetic differences influence the risk of osteoporosis and hypertension, provide insight into the role of the FGFRL1 gene while supporting previous functional studies.
It is well documented that the FGF/FGFR signaling axis plays an essential role in development, tissue homeostasis, and metabolism [26, 27]. Mutations and SNPs of FGFs are known associated with multiple skeletal disorders [14]. For instance, a constitutionally increased expression of the FGF4 gene is a risk factor for craniosynostosis [27]. Overexpression of FGF2 in mice resulted in shortened long bones along with defective mineralization and osteopenia, suggesting that the gene acts as a negative regulator of bone formation [28, 29]. Also, the genetic knockdown of FGF10 is related to skeletal phenotypes such as craniosynostosis in a mouse model [30], and FGF18 is expressed during osteoblast differentiation [31]. Indeed, the present study revealed significant genetic signals in FGF2, FGF10, and FGF18 associated with height (S4 Table). Furthermore, several SNPs in FGF18 were associated with osteoporosis (S2 Table).
By contrast, genetic variants in FGF2 also showed an association with hypertension. FGF2 is widely expressed in the myocardium, coronary vessels, and smooth muscle cells of the aorta [32, 33]. Besides, FGF2 knock-out mice exhibit reduced vascular tension and decreased arterial blood pressure, suggesting autonomic dysfunction [34, 35]. Interestingly, all of the SNPs in FGF2 that were associated with hypertension suggested that the risk of hypertension was diminished with minor alleles (S2 Table). These results presented that the FGFRL1 signaling pathway could be considered in cardiovascular disease and hypertension in humans, suggesting that the variations in the FGF2 gene implicate the heart or vascular tone.
Due to the absence of the intracellular tyrosine kinase domain in FGFRL1, which is essential for downstream FGF signaling, FGFRL1 is widely assumed to act as a decoy receptor (competitive inhibitor) that joins and regulates FGF ligands [11, 36, 37]. Interaction between FGF and FGFRs is known to mediate several developmental phenomena, such as differentiation of mesenchymal stromal cells (MSCs) [38–40]. Kahkonen et al. showed that FGFRL1 mRNA expression is remarkably increased during differentiation of MSCs into osteoblasts and adipocyte differentiation, and silencing of FGFR1 and FGFR2 in MSCs decreased the FGFRL1 expression in osteoblasts and adipocytes [20]. The discovery that FGFR1 and FGFR2 modulate FGFRL1 expression in MSCs highlighted the role of FGFRL1 in MSC differentiation into osteoblasts and adipocytes [20]. Thus, consistent with studies associating genetic signals of the FGFRL1 gene, FGFR, and FGF families, our results (gene-gene interaction network (Fig 1) and association test (Tables 1 and 2)) suggested that the interaction between FGFRL1 and FGF family affects the cardiovascular and skeletal systems.
However, our study has several limitations. First, except for hypertension, none of the continuous variables (distal radius speed of sound (DR-SOS), midshaft tibia speed of sound (MT-SOS), and T score) related to osteoporosis were identified only depending on the medical history. Second, although lifestyle and comorbidities definitely influence the risk of hypertension and osteoporosis, we did not evaluate them in the present study. Further studies are needed to evaluate additional factors including gender, lifestyle, and co-morbid conditions.
In conclusion, we have replicated the association between FGFRL1 and height, hypertension, and osteoporosis in the Korean population, and found genetic variants associated with each trait. The genetic variants in the FGF family members that interact with FGFRL1were associated with height, hypertension, and osteoporosis. The findings suggest that the giraffe-specific FGFRL1 gene, which is associated with biological characteristics in giraffes, including tall stature and cardiovascular adaptations, is related to the corresponding phenotype in humans. Thus, our study provides an approach to the genetic basis of the pleiotropic effect of FGF/FGFR signaling.
Supporting information
Signals related to (a) height, (b) hypertension and (c) osteoporosis in the FGFRL1 gene are plotted as -log10 P-values. The color of each SNP plot shows its linkage disequilibrium (LD) (using r2 values) with the novel SNP (purple diamond) within the association locus. The y-axis on the right shows the recombination rate according to the HapMap database. The above image was constructed using the LocusZoom program (http://locuszoom.org/).
(TIF)
(DOCX)
(DOCX)
Abbreviations: A1, minor allele; A2, major allele. The lower the Regulome DB score, the greater the effect on SNP.
(DOCX)
Age, sex and body mass index (BMI) were included as covariant in all genetic models. SNPs associated with both hypertension and osteoporosis in common and had P < 0.05 are indicated in bold. The p-values which are satisfied the Bonferroni-corrected significance level regarding each gene are indicated in bold and underlined. Abbreviations: SNP, single nucleotide polymorphism; Chr, chromosome; MAF, minor allele frequency; HTN, hypertension; β, regression coefficient; s.e, standard error; OR, odd ratio; CI, confidence interval.
(XLSX)
Data Availability
Data cannot be shared publicly because there are ethical or legal restrictions to sharing our data publicly. Data are available from the Korean Centers for Disease and Control (KCDC) Ethics Committee (contact via Institutional Review Board of the Korean National Institute of Health, https://www.nih.go.kr/) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from KCDC (https://kdca.go.kr/), outlined in the Data Availability section of the paper. Furthermore, we supplemented the information of all variants, used in the study, in the S4 Table.
Funding Statement
This study was supported by the Soonchunhyang University Research Fund [SCH-20211020] and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [NRF-2020R1F1A1071977]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhou J, Zhang Q, Yuan X, Wang J, Li C, Sheng H, et al. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013; 57: 30–35. doi: 10.1016/j.bone.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 2.Al-Hariri M, Aldhafery B. Association of Hypertension and Lipid Profile with Osteoporosis. Scientifica. 2020; 2020. doi: 10.1155/2020/7075815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnier M, Egan BM. Adherence in hypertension: a review of prevalence, risk factors, impact, and management. Circ Res. 2019; 124: 1124–1140. doi: 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 4.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. Exp Med. 2007; 204: 2449–2460. doi: 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do Carmo L, Harrison DG. Hypertension and Osteoporosis: Common Pathophysiological Mechanisms. Medicine in Novel Technology and Devices. 2020: 100047. doi: 10.1016/j.medntd.2020.100047 [DOI] [Google Scholar]
- 6.Chai H, Ge J, Li L, Li J, Ye Y. Hypertension is associated with osteoporosis: a case-control study in Chinese postmenopausal women. BMC Musculoskelet Disord. 2021; 22: 1–7. doi: 10.1186/s12891-021-04124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HH, Huang CY, Hwang LC. Association between metabolic syndrome and osteoporosis in Taiwanese middle-aged and elderly participants. Arch Osteoporos. 2018; 13: 1–7. doi: 10.1007/s11657-018-0467-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Zeng Y, Tao L, Liu S, Ni Z, Huang Q, et al. Meta-analysis of hypertension and osteoporotic fracture risk in women and men. Osteoporos Int. 2017; 28: 2309–2318. doi: 10.1007/s00198-017-4050-z [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Gao J, Cui X, Li Z, Chen L, Yuan Y, et al. A towering genome: Experimentally validated adaptations to high blood pressure and extreme stature in the giraffe. Sci Adv. 2021; 7: eabe9459. doi: 10.1126/sciadv.abe9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catela C, Bilbao-Cortes D, Slonimsky E, Kratsios P, Rosenthal N, Te Welscher P. Multiple congenital malformations of Wolf-Hirschhorn syndrome are recapitulated in Fgfrl1 null mice. Dis Model Mech. 2009; 2: 283–294. doi: 10.1242/dmm.002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trueb B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell Mol Life Sci. 2011; 68: 951–964. doi: 10.1007/s00018-010-0576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019; 51: 258–266. doi: 10.1038/s41588-018-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieckmann T, Zhuang L, Flück CE, Trueb B. Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biophys Acta Mol Basis. 2009; 1792: 112–121. doi: 10.1016/j.bbadis.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Kichaev G, Bhatia G, Loh PR, Gazal S, Burch K, Freund MK, et al. Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet. 2019; 104: 65–75. doi: 10.1016/j.ajhg.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SK. Identification of 613 new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture. PLoS One. 2018; 13: e0200785. doi: 10.1371/journal.pone.0200785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018; 50: 1505–1513. doi: 10.1038/s41588-018-0241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agaba M, Ishengoma E, Miller WC, McGrath BC, Hudson CN, Reina OCB, et al. Giraffe genome sequence reveals clues to its unique morphology and physiology. Nat Commun. 2016; 7: 1–8. doi: 10.1038/ncomms11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Han BG, group tK. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. International Journal of Epidemiology. 2016; 46: e20–e. doi: 10.1093/ije/dyv316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubakov D, Liu F, Van Zelm M, Vermeulen J, Oostra B, Van Duijn C, et al. Estimating human age from T-cell DNA rearrangements. Curr Biol. 2010; 20: 970–971. doi: 10.1016/j.cub.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 20.Kähkönen T, Ivaska K, Jiang M, Büki K, Väänänen H, Härkönen P. Role of fibroblast growth factor receptors (FGFR) and FGFR like-1 (FGFRL1) in mesenchymal stromal cell differentiation to osteoblasts and adipocytes. Mol Cell Endocrinol. 2018; 461: 194–204. doi: 10.1016/j.mce.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 21.Moon S, Kim YJ, Han S, Hwang MY, Shin DM, Park MY, et al. The Korea biobank array: design and identification of coding variants associated with blood biochemical traits. Sci Rep. 2019; 9: 1–11. doi: 10.1038/s41598-018-37832-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin KY, Chan CY, Subramaniam S, Muhammad N, Fairus A, Ng PY, et al. Positive association between metabolic syndrome and bone mineral density among Malaysians. Int J Med Sci. 2020; 17: 2585. doi: 10.7150/ijms.49030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C, Flores MV, Murison G, Crosier K, Crosier P. An essential role for zebrafish Fgfrl1 during gill cartilage development. Mech Dev. 2006; 123: 925–940. doi: 10.1016/j.mod.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 24.Hogan BM, Hunter MP, Oates AC, Crowhurst MO, Hall NE, Heath JK, et al. Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm. Dev Biol. 2004; 276: 508–522. doi: 10.1016/j.ydbio.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 25.Hanaoka R, Ohmori Y, Uyemura K, Hosoya T, Hotta Y, Shirao T, et al. Zebrafish gcmb is required for pharyngeal cartilage formation. Mech Dev. 2004; 121: 1235–1247. doi: 10.1016/j.mod.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 26.Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020; 5: 1–38. doi: 10.1038/s41392-020-00222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grillo L, Greco D, Pettinato R, Avola E, Potenza N, Castiglia L, et al. Increased FGF3 and FGF4 gene dosage is a risk factor for craniosynostosis. Gene. 2014; 534: 435–439. doi: 10.1016/j.gene.2013.09.120 [DOI] [PubMed] [Google Scholar]
- 28.Coffin JD, Florkiewicz RZ, Neumann J, Mort-Hopkins T, Dorn 2nd G, Lightfoot P, et al. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol Biol Cell. 1995; 6: 1861–1873. doi: 10.1091/mbc.6.12.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobue T, Naganawa T, Xiao L, Okada Y, Tanaka Y, Ito M, et al. Over‐expression of fibroblast growth factor‐2 causes defective bone mineralization and osteopenia in transgenic mice. J Cell Biochem. 2005; 95: 83–94. doi: 10.1002/jcb.20389 [DOI] [PubMed] [Google Scholar]
- 30.Hajihosseini MK, Duarte R, Pegrum J, Donjacour A, Lana‐Elola E, Rice DP, et al. Evidence that Fgf10 contributes to the skeletal and visceral defects of an Apert syndrome mouse model. Dev Dyn. 2009; 238: 376–385. doi: 10.1002/dvdy.21648 [DOI] [PubMed] [Google Scholar]
- 31.Ornitz DM, Marie PJ. Fibroblast growth factors in skeletal development. Curr Top Dev Biol. 2019; 133: 195–234. doi: 10.1016/bs.ctdb.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 32.Liao S, Bodmer J, Pietras D, Azhar M, Doetschman T, Schultz JEJ. Biological functions of the low and high molecular weight protein isoforms of fibroblast growth factor‐2 in cardiovascular development and disease. Dev Dyn. 2009; 238: 249–264. doi: 10.1002/dvdy.21677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House SL, House BE, Glascock B, Kimball T, Nusayr E, Schultz JEJ, et al. Fibroblast growth factor 2 mediates isoproterenol-induced cardiac hypertrophy through activation of the extracellular regulated kinase. Mol Cell Pharmacol. 2010; 2: 143. doi: 10.4255/mcpharmacol.10.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, et al. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998; 4: 201–207. doi: 10.1038/nm0298-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dono R, Texido G, Dussel R, Ehmke H, Zeller R. Impaired cerebral cortex development and blood pressure regulation in FGF‐2‐deficient mice. EMBO J. 1998; 17: 4213–4225. doi: 10.1093/emboj/17.15.4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P, et al. Identification of a new fibroblast growth factor receptor, FGFR5. Gene. 2001; 271: 171–182. doi: 10.1016/s0378-1119(01)00518-2 [DOI] [PubMed] [Google Scholar]
- 37.Trueb B, Zhuang L, Taeschler S, Wiedemann M. Characterization of FGFRL1, a novel fibroblast growth factor (FGF) receptor preferentially expressed in skeletal tissues. J Biol Chem. 2003; 278: 33857–33865. doi: 10.1074/jbc.M300281200 [DOI] [PubMed] [Google Scholar]
- 38.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009; 8: 235–253. doi: 10.1038/nrd2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015; 4: 215–266. doi: 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du X, Xie Y, Xian CJ, Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012; 227: 3731–3743. doi: 10.1002/jcp.24083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Signals related to (a) height, (b) hypertension and (c) osteoporosis in the FGFRL1 gene are plotted as -log10 P-values. The color of each SNP plot shows its linkage disequilibrium (LD) (using r2 values) with the novel SNP (purple diamond) within the association locus. The y-axis on the right shows the recombination rate according to the HapMap database. The above image was constructed using the LocusZoom program (http://locuszoom.org/).
(TIF)
(DOCX)
(DOCX)
Abbreviations: A1, minor allele; A2, major allele. The lower the Regulome DB score, the greater the effect on SNP.
(DOCX)
Age, sex and body mass index (BMI) were included as covariant in all genetic models. SNPs associated with both hypertension and osteoporosis in common and had P < 0.05 are indicated in bold. The p-values which are satisfied the Bonferroni-corrected significance level regarding each gene are indicated in bold and underlined. Abbreviations: SNP, single nucleotide polymorphism; Chr, chromosome; MAF, minor allele frequency; HTN, hypertension; β, regression coefficient; s.e, standard error; OR, odd ratio; CI, confidence interval.
(XLSX)
Data Availability Statement
Data cannot be shared publicly because there are ethical or legal restrictions to sharing our data publicly. Data are available from the Korean Centers for Disease and Control (KCDC) Ethics Committee (contact via Institutional Review Board of the Korean National Institute of Health, https://www.nih.go.kr/) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from KCDC (https://kdca.go.kr/), outlined in the Data Availability section of the paper. Furthermore, we supplemented the information of all variants, used in the study, in the S4 Table.