Abstract
The respiratory symptoms of acute respiratory distress syndrome (ARDS) in the coronavirus disease 2019 (COVID-19) patients is associated with accumulation of pre-inflammatory molecules such as advanced glycation end-products (AGES), calprotectin, high mobility group box family-1 (HMGB1), cytokines, angiotensin converting enzyme 2 (ACE2), and other molecules in the alveolar space of lungs and plasma. The receptor for advanced glycation end products (RAGEs), which is mediated by the mitogen-activated protein kinase (MAPK), plays a critical role in the severity of chronic inflammatory diseases such as diabetes mellitus (DM) and ARDS. The RAGE gene is most expressed in the alveolar epithelial cells (AECs) of the pulmonary system. Several clinical trials are now being conducted to determine the possible association between the levels of soluble isoforms of RAGE (sRAGE and esRAGE) and the severity of the disease in patients with ARDS and acute lung injury (ALI). In the current article, we reviewed the most recent studies on the RAGE/ligands axis and sRAGE/esRAGE levels in acute respiratory illness, with a focus on COVID-19–associated ARDS (CARDS) patients. According to the research conducted so far, sRAGE/esRAGE measurements in patients with CARDS can be used as a powerful chemical indicator among other biomarkers for assessment of early pulmonary involvement. Furthermore, inhibiting RAGE/MAPK and Angiotensin II receptor type 1 (ATR1) in CARDS patients can be a powerful strategy for diminishing cytokine storm and severe respiratory symptoms.
Keywords: Receptor for advanced glycation end products, SARS-CoV- 2, Respiratory distress syndrome, esRAGE, ACE2 protein
Introduction
The largest pandemic of the twenty-first century is undoubtedly the outbreak of coronavirus diseases 2019 (COVID-19) which was initially reported in Wuhan, China, in December 2019 [1]. The pulmonary involvement is found in patients with COVID-19 in the form of severe pneumonia (15%) and acute respiratory distress syndrome (ARDS) (5%) [2]. According to studies, symptoms in infected children and young people are frequently moderate, manifesting in fever and a dry cough. However, in older people or those infected with comorbid conditions such as high blood pressure, diabetes, and cancer, symptoms manifested in lung problems and rushes. The FDA has previously approved several therapies based on the severity of the disease: (1) Monoclonal antibodies are used to treat individuals with mild to moderate illness who do not require hospitalization but do require oxygen treatment [3], (2) To rehabilitate people who need to be hospitalized but don’t require oxygen-therapy, Remdesivir is administered that can inhibit RNA polymerase of the coronavirus [4], (3) Dexamethasone is prescribed to manage patients who need non-invasive oxygen therapy (dexamethasone impedes the development of pulmonary damage). The WHO has also approved some COVID-19 vaccines with distinct immune technological advancement such as Pfizer-BioNTech, Moderna, AstraZeneca, Sinopharm, J&J/Janssen to control the pandemic globally [5].
To date, various clinical investigations on a variety of biomarkers have been conducted to identify the severity of the disease [6]. The receptor for advanced glycation end-products (RAGEs) is a 31 KDa protein, belongs to the immunoglobulins superfamily, and exists in two main types: membrane-bound RAGE (mRAGE) and soluble RAGE (sRAGE). Ligands such as advanced glycation end-products (AGEs) and S100 proteins lead to stimulation of intracellular signaling pathways when binding to RAGE. These signals activate viral inflammatory transcription factors and the nuclear factor kappa B (NF-κB) [7, 8]. After embryonic development, the expression of the RAGE gene decreases in all tissues except for lung tissue. Numerous studies have revealed that the inflammatory pathways associated with RAGE have played an essential role in the pathogenesis of lung tissue, and the regulation of these routes has led to a therapeutic advancement in chronic obstructive pulmonary disease (COPD) and ARDS [9]. RAGE may also play an important role in expediting the physiological age or aging process. The binding of ligands to RAGE, such as AGE, is part of this procedure. Inactivation of RAGE has been proven in multiple trials to be efficacious in lowering symptoms of pulmonary inflammation, particularly ARDS, based on this premise [2].
As a result, lowering the mortality rate of COVID-19 patients by targeting RAGE, particularly in the elderly, could be an effective strategy. The sRAGE is a sort of RAGE competitive inhibitor that comes in two isoforms: a membrane cleaved form (cRAGE) and an endogenous secretory form (esRAGE), both of which inhibit inflammatory responses in cells [10, 11]. The level of sRAGE in the blood depends on the amount of blood sugar and pathogens [12]. In cases of increasing blood glucose levels in diabetic patients, the sRAGE rate increases to prevent potential damage by AGE [13, 14]. According to several researches, the serum levels of sRAGE are also higher in young and healthy individuals [15]. Human angiotensin converting enzyme II (ACE2) is a membrane-bound protein found in a variety of human cells, including the heart, kidney, and lung cells. It has been shown to be one of the main pathways for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry into host cells. As a result, ACE2 receptor inhibitors can be used to minimize the occurrence of acute pulmonary symptoms [16, 17]. sRAGE has been reported to inhibit ACE2 expression in previous investigations. Thus, increasing the presence of sRAGE can contribute to reducing symptoms in SARS-CoV-2 infected patients. Based on a recent study, the serum levels of sRAGE are found to be higher following oxygen therapy in people with ARDS. Therefore, evaluating sRAGE serum levels can be helpful in studying the inflammations engendered by the virus entry into the pulmonary cells, the severity of damage to the lung tissue as well as a diagnostic criteria for hospital admission based on patients' clinical signs, along with measurements of other inflammatory factors such as IL-6, CRP, and D-dimer [6].
RAGE is involved in the SARS-CoV-2-induced acute respiratory distress syndrome. Diabetic individuals infected with SARS-CoV-2 are at a higher risk of developing acute respiratory problems, severe oxygen depletion, and death. In these patients, RAGE activity is a mechanism that raises the probability of a severe form of COVID-19. Higher levels of AGE and other ligands in people with diabetes may produce pro-inflammatory conditions, endothelial cell dysfunction, and vascular leakage, all of which contribute to the destruction caused by the novel coronavirus infection [18]. RAGE is triggered by a variety of ligands and activates several signaling pathways. NF-κB is one of these pathways, and as a consequence, several chemokines and cytokines are elevated. The activation of the angiotensin types I receptor and RAGE-dependent pathways results in a massive cytokine storm [19]. As an example, high mobility group box protein 1 (HMGB1) -a ligand for RAGE- has a variety of roles both inside and outside the cells. The extracellular HMGB1 released from the cells exhibits the potential pathogenic role in viral infectious diseases. Thus, HMGB1 inhibitors or antibodies against HMGB1 can be utilized to protect infected cells from infection-induced probable destruction [20, 21]. SARS-CoV-2 spike glycoprotein may bind to CD147 glycoprotein, a multi-ligand protein that is synthesized in cases of hyperglycemia and RAGE activation, in addition to the AGE primary receptor. CD147 is highly expressed in type II pneumonocytes and in a wide range of other cells, including immune cells, endothelial cells, and platelets, and also plays a significant role in COVID19-related pneumonia. The induction of CD147 glycosylation by AGEs in endothelial cells may increase matrix metalloproteinase (MMPs) activity and destabilize cellular junctions. The modulation of pulmonary inflammation with the help of controlling inflammatory pathways can be a crucial step in the treatment of COVID-19 [22]. Therefore, in the present study, we have reviewed and explored the recent investigations conducted in the field of connections between RAGE and sRAGE with pulmonary inflammation development in patients with COVID-19 to provide a comprehensive response to this possible relationship.
The Structure of RAGE and Its Ligands
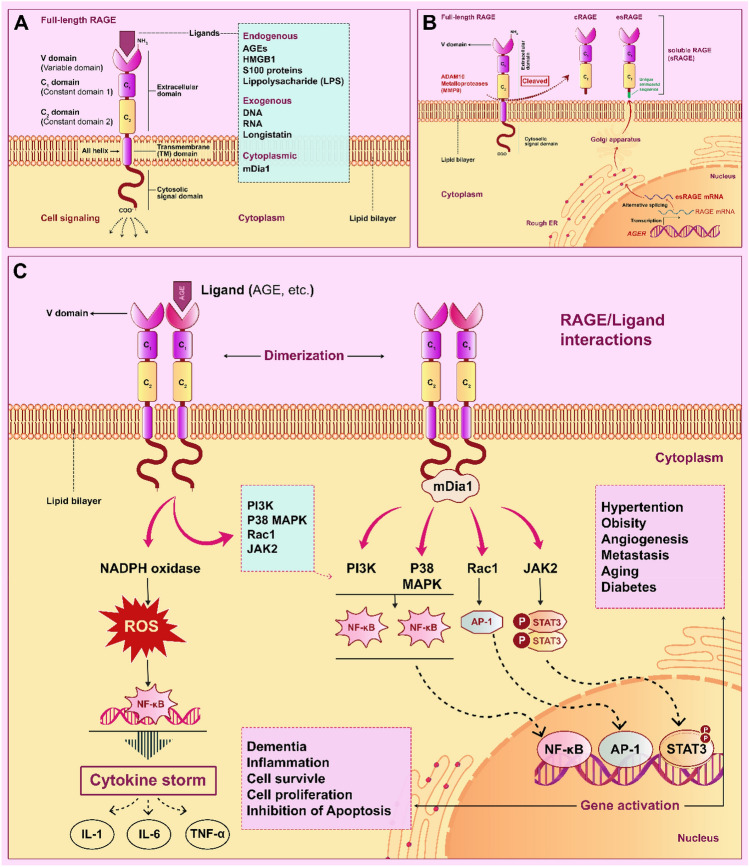
RAGE’s structure is made up of three domains. Its extracellular domain is composed of the constant domain (C1 and C2) and a variable domain (V) linked together by an amino acid sequence. Its structure also includes a transmembrane and a cytoplasmic segment. According to the structural analysis of these regions, C1 and V domains are positively charged, whilst the C2 domain is negatively charged [23]. The charge of these domains is essential for binding a variety of particular ligands to RAGE. The involvement of numerous RAGE ligands activities in initiating inflammatory pathways in cells has been investigated so far [24]. RAGE-specific ligands are classified into three categories: endogenous, cytoplasmic, and exogenous ligands; as examples, AGEs, S100 family, amyloid-β peptide, and HMGB1, which bind to extracellular domains of RAGE (V, C1, and C2) [25] have a critical role in triggering chronic inflammatory conditions such as diabetes [26] and cardiovascular diseases [27], progression of numerous cancer cells [28, 29], pulmonary disorders [30], and neurodegeneration diseases such as Parkinson [31] and Huntington [32]. Of note, the monomeric form of RAGE in the cells’ membrane has a low affinity for interacting with ligands compared to its dimeric form. As a result, RAGE dimerization is an essential phase in activating the cellular response in cells [24]. The mechanism by which RAGE-ligands impact associated signaling pathways in inflammation of cells, particularly lung cells, is described in the following sections (Fig. 1a).
Fig. 1.
Structure of RAGEs and roles of RAGE/ligand axis in pro-inflammatory pathways. A RAGE’s extracellular domain structure is composed of the constant domain (C1 and C2) and a variable domain (V); RAGE ligands (endogenous and exogenous) bind to the extracellular domains of RAGE. Cytoplasmic ligands such as mDia1 bind to the cytoplasmic domain of the RAGE. B Soluble isoforms of RAGE (sRAGE) are included esRAGE and cRAGE; esRAGE is constructed through alternative splicing on the RAGE mRNA, and cRAGE is formed through ADAM10 activity in the extracellular space. C Binding of RAGE ligands (mDia1, AGEs, etc.) to RAGE and its dimerization have significant roles in the inflammatory response in cells through regulation of cellular pathways such as PI3K/Akt/mTOR, MAPK/P38, JAC2/ STAT3, and other pointed pathways in the figure.
Source The Authors
RAGE and Its Isoforms
Regulating the expression of genes such as the RAGE gene (AGER) located on the major histocompatibility complex (MHC) region is a strategy to control inflammation in humans [33]. In human cells, alternative splicing on the RAGE mRNA results in generating different RAGE isoforms, including sRAGE and splice variant esRAGE [34]. These isoforms of RAGE have specific biological functions. Previous studies have suggested that sRAGE and other soluble isoforms of RAGE reduce the damaging effects of RAGE-ligands by inhibiting their binding capability in human cells (Fig. 1b). They have also proposed that measuring the levels of sRAGE and esRAGE in body fluid could be used as a diagnostic for disorders such as diabetes [35], lung cancer [36, 37], cardiometabolic [38], renal disease [18, 39], pulmonary fibrosis [40], sepsis [41], and COVID-19 disease [42]. The breakage of the connection between RAGE’s extracellular and transmembrane domains leads to the formation of cRAGE (causing its extracellular portion to be released) on the cell surface. This mechanism is stimulated via the binding of RAGE-ligands, including TNF-α, lipopolysaccharide (LPS), and HMGB1, to the extracellular domain of RAGE. The generation of cRAGE is related to the overexpression rate of MMP9 and ADAM10 in the stress conditions (increasing reactive oxygen species (ROS) level). Furthermore, by activation of ADAM10, upregulation of TNF- and induction of NF-κB in the RAS/MAPK signaling pathway promotes overproduction of cRAGE in the cells [43]. The serum/plasma levels of soluble isoforms of RAGE, especially sRAGE and esRAGE, significantly correlated with physical exercise [44], and genetic differences in individuals. The genetic variations among people are due to the highly polymorphic in the AGER gene [45].
Roles of RAGE/Ligands in the Inflammatory Disease
Calprotectin/S100 Family
S100 protein families are characterized by the presence of the EF-hand domain in the structure. They can bind to Ca2+ ions with the help of these domains. S100A8 and S100A9 are two main types of the S100 family, both of which can be found in heterodimer form on the cell surface in normal conditions. After neutrophil secretion, S100A9/S8 binds to RAGE and Toll-like receptor 4 (TLR4), causing inflammation and regulating critical processes in the cells such as cell proliferation, angiogenesis, Ca2+ homeostasis, and cellular energy. In individuals with a wide range of diseases, such as vascular inflammation and cardiovascular illnesses, the levels of S100A9/S8 in their blood are much higher than in healthy persons. Zhong et al. discovered that binding of S100A8/9 to RAGE on the surface of vein endothelial cells via the PI3K/Akt/mTOR (mammalian target of rapamycin complex 2) pathway increased angiogenesis in vivo and in vitro in intimal hyperplasia models [46].
Furthermore, the findings of Araki et al. showed that the levels of S100A9/S8 proteins are raised in pulmonary fibrosis in lung fibroblasts cells; they discovered that binding of these heterodimers to RAGEs on the surface of fibroblasts activated the NF-κB pathway, causing inflammation in the cells. According to these findings, inhibiting S100A9/S8 binding to RAGEs can help control and reduce inflammation and inflammatory symptoms in lung complications such as Idiopathic pulmonary fibrosis (IPF), ARDS, and Acute lung injury (ALI) (Fig. 1c) [47].
Advanced Glycation End Products (AGES)
AGEs are one of RAGE's most important ligands; these ligands play an essential role in inflammation in neuron cells by increasing amyloid deposition, diabetes complications in chronic conditions, and cardiovascular disease. AGEs included oxidized compounds with reduced sugars in the blood through nonenzymatic reactions [48, 49]. Ren et al. looked into the possible effects of AGEs/RAGE signaling pathway in hepatic ischemia–reperfusion (HIR) after liver transplantation; various levels of ROS and cytokines are produced in the liver during HIR and released into the bloodstream; the presence of these inflammatory agents in liver cells and other organs, particularly neuron cells, is detrimental to the hippocampus of the brain. Previous findings have shown that levels of AGEs and expression of the RAGE gene are raised in the body under oxidative stress circumstances in various inflammatory conditions. According to the findings of this research, the expression of the RAGE gene was significantly increased in the HIR. As a consequence, overexpression of the RAGE gene can be used as a proper biomarker for stress-induced oxidative damage in cells such as the hippocampus, and targeting RAGE and associated molecules such as NF-κB, ERK1/2, and PI3K in signaling pathways is also a valuable approach for preventing cell damage in stressful situations (Fig. 1c) [50]. Among various glycation end products in the serum, the presence of Nε-(carboxymethyl) lysine or CML in diabetic patients with retinopathy complications, cardiac ischemia, and cardiovascular are increased significantly [51]. The expression of RAGE is reported to be a significant player in the occurrence of nephropathy, neuropathy, and retinopathy problems in diabetes patients with microvascular and macrovasecular issues [52]. Earlier findings have also demonstrated that reduced levels of sRAGE/esRAGE in the blood of obese and diabetic patients enhanced the incidence of steatosis in liver cells and cardiovascular diseases [53, 54]. Wang et al. showed that the concentration of sRAGE/esRAGE in blood was influenced by AGER gene mutations in prediabetes patients; they found that polymorphism in the T429C (T/C allele) hindered the associated mechanism that controls blood glucose concentration by lowering the concentration of soluble forms of RAGE [55]. Additionally, Sebeková K et al. observed that total sRAGE concertation was reduced in non-diabetic patients before the onset of metabolic syndrome symptoms [56].
High Mobility Group Box Family (HMGB)
The structure of HMGB1 is consists of a single amphipathic alpha-helix chain with two boxes (A and B), which are joined together by a basic domain. The presence of three cysteine amino acids in its box A (Cys23 and Cys45) and box B (Cys106) play a vital role in cell response to redox condition HMGB1 is involved in the control of gene expression via interacting with chromatin. Moreover, HMGB1 promotes inflammatory signaling pathways in necrotic cells, such as JAK/STAT1 and MAPK, and pathogen-induced immune cells via attaching to its receptors on the cell surface, particularly RAGE and TLRs (Fig. 1c) [57, 58]. Activation of RAGE/ligands and their concentration in the blood have been linked to various inflammatory disorders (see Table 1).
Table 1.
Roles of RAGE/sRAGE/ligands in some inflammatory disease
| Diseases | RAGE/sRAGE/ligands | Definitions | Study type | Refs. |
|---|---|---|---|---|
| T2DM | Increase sRAGE level | Increases sRAGE levels in patients with T2DM after aerobic exercise—Improvement the cardiometabolic risk factor | Randomized controlled trial | [44] |
| T2DM | Increase levels of sRAGE/esRAGE | Increases sRAGE/esRAGE levels in patients with T2DM after supplementation with pioglitazone and glimepiride | Randomized controlled trial | [59] |
| T2DM | Decrease levels of CML and LPS—Increase levels of sRAGE | Positive effects of resistant dextrin (a type of prebiotic supplementation) to decrease the inflammation in T2DM patients and improvement the cardiometabolic risk factor through increase the levels of sRAGE along with decrease the levels of CML and LPS | Randomized controlled trial | [60] |
| Lung cancer | Decrease level of sRAGE during lung cancer | Progression of lung cancer is correlated with level of sRAGE | Case control | [36] |
| BOS | Increase sRAGE level | Early increase of plasma sRAGE levels is proper marker of alveolar cells lung injury | Multi-center cohort study | [61] |
| Tuberculosis and pneumonia | Increase of serum sRAGE level—Increase of pleural sRAGE level | More increase of serum sRAGE level in tuberculosis than pneumonia- More Increase of pleural sRAGE level in pneumonia than tuberculosis | Case control | [62] |
| IPF | Increase of AGE/sRAGE ratio | Increase of sRAGE in the serum of IPF patients is a proper prognostic biomarker | Case control/Observational study | [63] |
| ARDS | Increase plasma sRAGE and S100A12 levels | Increase pulmonary injuries and inflammatory cytokines secretion in sepsis-induced ARDS | In vivo—Case control | [64] |
| ARDS | Increase plasma sRAGE levels | Increase ARDS risk | Cohort/Comparative Study | [65] |
| ALI | Decrease sRAGE level, Increase expression of RAGE/ NF-κB | Induce the lung injury, pulmonary edema, and oxidative stress | In vivo | [66] |
| SSc | Increase levels of sRAGE and HMGB-1 | Association between concentration of sRAGE and HMGB-1 in the severity of SSc | In vivo—Case control | [67] |
Type 2 Diabetes Mellitus; Bronchiolitis obliterans syndrome; Idiopathic pulmonary fibrosis; Acute lung injury; systemic sclerosis
RAGE and Lung Injuries
Pulmonary Fibrosis
Progressive fibrosis in the parenchyma cells and lung alveolar epithelial cells is defined as idiopathic pulmonary fibrosis (IPF). Despite scientific advancements in the treatment of IPF with ‘Nintedanib’, existing medicines are insufficient to prevent mortality in this condition. Therefore, early detection of IPF by using appropriate biomarkers is critical for lowering death rates [68]. Earlier studies have shown that certain polymorphisms in the AGER gene enhance the incidence of progressive lung damage in people with IPF, particularly in individuals with severe IPF, by affecting the levels of sRAGE in the blood [69]. In 2020, Yamaguchi et al. evaluated the concentration of esRAGE in patients with IPF against healthy controls; they found that esRAGE levels in patients' blood and bronchoalveolar lavage (BALF) fluid were dramatically decreased [70]. Furthermore, the findings of Machahua C et al.’s investigations suggest that the accumulation of AGEs (CML) along with decreased expression of RAGE in lung samples from IPF patients (surgical lung biopsy) accelerates the fibrotic process in lung cells as compared to control groups [71].
Interestingly, studies have also demonstrated that the level of soluble forms of RAGE (sRAGE and esRAGE) in patients with COPD and IPF is correlated with single nucleotide polymorphism (SNP) of the AGER gene, which is involved in the severity of lung inflammation. Based on these findings, Kinjo T. proved that SNP (rs2070600) of AGER gene lowered the levels of sRAGE in Japanese patients (minor allele of rs2070600) with combined pulmonary fibrosis and emphysema (CPFE) and IPF [69, 72].
Acute Respiratory Distress Syndrome (ARDS)
In intensive care units (ICUs), the occurrence of severe respiratory problems such as ARDS and ALI in patients with infection is common. The major causes of these difficulties in hospitalized patients are linked to the fundamental functions of pulmonary alveolar AECs, which are the critical cells of the respiratory system responsible for the production and secretion of surfactants liquid. Many researchers have recently focused on detecting inflammatory chemicals such MCP-1, IL-6, and other inflammatory cytokines/chemokines that are significant causes of respiratory failure in ARDS/ALI patients. Based on these findings, Zhou et al. reported that inhibiting long noncoding RNA nuclear paraspeckle assembly transcript 1 (NEAT1) in AECs in vivo (C57BL/6 mice) and in vitro (cell line A549) models can be a promising strategy for reducing acute inflammation of AECs in ARDS and ALI by controlling the production of inflammatory agents such as IL-6 and TNF- and also blocking the activation of the RAGE/HMGB1/NF-κB signaling pathway [12, 73].
Previous research has found that ARDS frequency has increased dramatically in individuals with sepsis who had also had pneumonia. The crucial causes of the lung damages of ARDS-suffered patients are the accumulation of lung fluid (AFC) in the alveolar space, which is secreted from AECs. Based on these findings, Wang H et al. evaluated the mechanism by which amounts of AFC fluid is increased in the alveolar space of lungs in the ARDS /ALI in vivo model (RAGE+/+ and RAGE−/− mice). They discovered that in the presence of RAGE (RAGE +/+ mice), downregulation of some membrane channel proteins in the lungs, such as Na/K-ATPase, ENaC, and ZO-1 increased AFC fluids in the mice lungs [74].
Consequently, modulating the RAGE pathway in ARDS patients might be a promising new treatment option for reducing AFC buildup. In a study accomplished by Audard et al. they explored the anti-RAGE antagonist peptide in an ARDS-induced animal model, which is called RAP; this monoclonal antibody (mAb) reduced the pro-inflammatory activities of these ligands by inhibiting the binding of HMGB1 and S100 proteins to RAGE. The investigation results suggested that suppressing RAGE reduced AFC fluid accumulation and alveolar cell injury in piglet model with ARDS [75].
Acute Lung Injury (ALI)
ALI is a progressive disorder that affects ICU patients who have damaged lungs due to infection, exogenous shock, or trauma. Anti-inflammatory drugs reduce the accumulation of fluid in the airspaces of the lungs, edema, and levels of pro-inflammatory chemicals (such as HMGB1, IL-1, and IL-6) in the body [76]. Numerous research has recently been conducted to investigate novel treatment strategies such as cell and gene therapy in patients with ALI to reduce mortality. Gene therapy has received much attention for treating ALI among the new therapeutic techniques that have been tried so far. As an example, Piao C et al. used polyamidoamine (PAM; a polymeric nanocarrier) that conjugated with Dexamethasone drug (a potent glucocorticoid) (PAM-D)/RAGE-antagonist peptide (RAP) for delivery of adiponectin plasmid (pAPN) to Raw264.7 cells (Mouse Leukemic monocyte macrophage) and the animal model. The results demonstrated that the presence of RAP along with pAPN/PAM-D complex increased the anti-inflammatory effects of Dexamethasone drug and pAPN; they reasoned that RAP’s intrinsic anti-inflammatory properties bolstered the complex’s anti-inflammatory effects. The previous experiment reported that RAP could block the binding of pro-inflammatory ligands including HMGB-1, S100 family, β-amyloid, and LPS to RAGE receptor; thus, using these peptides in gene therapy helps to decrease inflammation [77, 78]. Dexmedetomidine (DEX) was identified as a potential drug for administration in clinical studies in ALI patients by Hu et al. in 2017. DEX decreased the expression of RAGE gene in rats and downregulated HMGB1, NF-κB, and MAPK [76].
Furthermore, lidocaine (Lido) protects against a variety of lung injuries, including ALI, sepsis-induced lung injury, and ARDS, by inhibiting pro-inflammatory cytokines including IL-1, IL-6, and TNF-α [79–82]. According to Zhang et al. treatment of ALI-rats with ‘Lido’ suppresses acute inflammatory responses in rat lung tissues by lowering RAGE and HMGB1 expression. Altogether, lowering RAGE expression and pro-inflammatory ligands in ALI patients is a potential prospective technique for decreasing mortality due to their multifunctional involvement in pathways including MAPK and NF-κB [83].
LPS is one of the most harmful pro-inflammatory ligand (pathogenic factor) that activates inflammatory pathways in alveolar epithelial cells in individuals with ALI by interacting with the RAGE receptor [84]. Yang et al. employed 'Ketamine' to reduce the pathogenic effects of LPS, based on the mediatory functions of RAGE/ligands in ALI. In their investigation, Ketamine administration dramatically reduced RAGE, HMGB1, and TLR9 expression in ALI-Rats [85].
COVID-19 and RAGE/Ligands Axis
Inflammatory Pathways which are Involved in the Severity of COVID-19 Disease
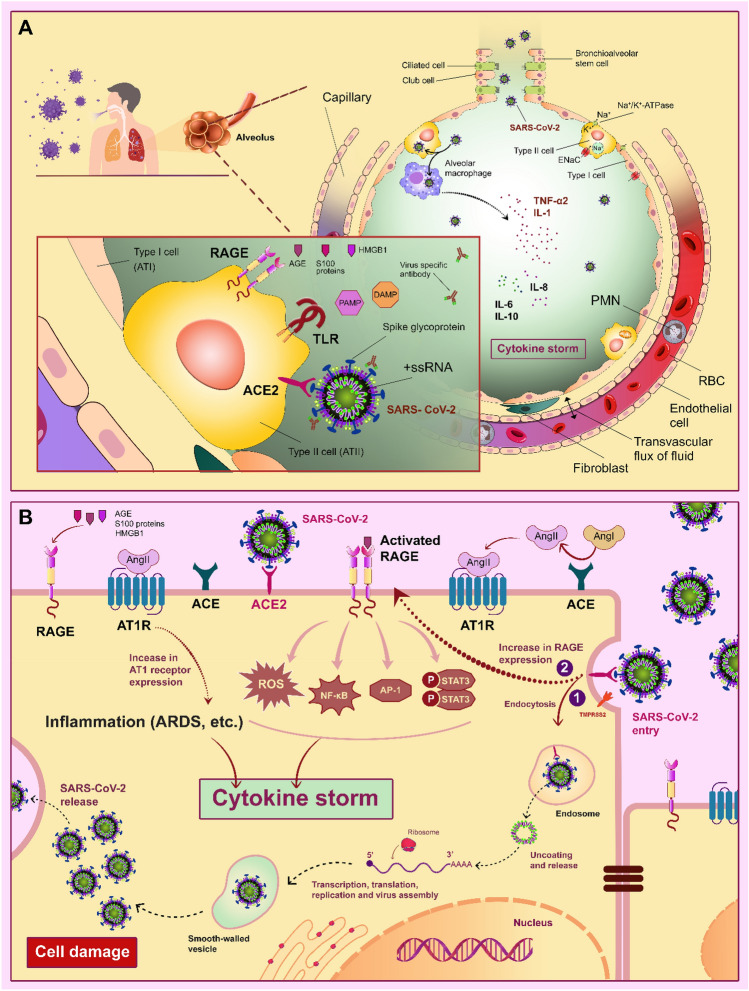
The corona virial family, such as SARS-CoV-2, has four proteins in its structure; among these proteins, spike (S) is a glycoprotein that targets ACE2 on the surfaces of epithelial cells in the respiratory system. The high mortality rate of patients with COVID-19 is related to the increased levels of cytokines (cytokines storm), including IL-10, IL-7, IL-6, MIP-1A, IP-10, and TNF-α in the respiratory system, and as a result, the incidence of acute respiratory symptoms especially ARDS. The activation of cytokines, ROS, and other pro-inflammatory chemicals by LPS attachment to the TLR4 receptor on the surfaces of AECs are one of the significant molecular drivers in ARDS in COVID-19 patients. Petruk et al. investigated the likely mechanism by which LPS affects the COVID-19 based on the association between blood levels of LPS and complications of numerous conditions such as metabolic syndrome, inflammatory bowel syndrome (IBD), Kawasaki disease, COPD, Periodontitis, and obesity. They discovered that the binding of LPS to spikes glycoprotein increased the pro-inflammatory effects in vivo and in vitro models by boosting cytokine levels in peripheral blood mononuclear cells (PBMC) and triggering the NF-κB pathway (see Fig. 2a and b for this section).
Fig. 2.
Contribution of RAGE, AT1R (type 1 angiotensin receptor), ACE2 (angiotensin-converting enzyme 2), and TLR (Toll-like receptor) in alveolar epithelial cells related to COVID-19 pathogenies in a cytokine storm. A SARS-CoV-2-specific receptor (ACE2), RAGE, and TLR in the surface of type 2 alveolar epithelial cells, and related inflammatory ligands including DAMPs (damage-associated molecular patterns), PAMP (pathogen-associated molecular pattern), S100 proteins, and AGE trigger cytokine storm in the respiratory system. In the infected alveolar macrophages with SARS-CoV-2, production and releasing the pro-inflammatory cytokine such as IL-1, IL-6, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α) are stimulated. B-1) Entry of the SARS-CoV-2 is mediated by ACE2/endocytosis in type 2 alveolar epithelial cells. B-2) Binding of S100 proteins, AGE, and HMGB1 to V-domain of RAGE in the surface of type 2 alveolar epithelial cells by regulating the pro-inflammatory transcription factors (Activator protein 1 (AP-1), STAT3, and NF-κB) have key roles in the production of ROS and cytokine storm. Expression of the RAGE gene in alveolar epithelial cells is increased after binding SARS-CoV-2 to ACE2. The binding of angiotensin (Ang) II to AT1R is mediated cell apoptosis, chronic inflammation, pulmonary edema, and cytokine storm in alveolar epithelial cells.
Source The Authors
Consequently, they suggested that measuring LPS levels in patients with severe COVID-19 might be helpful in managing the respiratory system's serious ramifications [86–88]. According to these findings, Teixeira PC et al. demonstrated that in hospitalized COVID-19 patients, increased levels of LPS and pro-inflammatory systemic cytokines and chemokines are associated with the occurrence of severe symptoms in patients with COVID-19 [89]. In 2022, Jose et al. evaluated the anti-inflammatory properties of Kaba Sura kudineer (KSK), a poly-herbal formulation, in LPS-activated RAW 264.7 cells. Its anti-diabetic, anti-viral, and anti-bacterial properties have been discovered in previous studies. Furthermore, this research revealed that KSK, together with Vitamin C and Zinc, lowered the viral load of COVID-19 patients. According to their findings, KSK (25 g/mL) has an anti-inflammatory impact via downregulating TLR4, thereby suppressing the pro-inflammatory effects of LPS [90]. Bindings of multiple ligands, including LPS, bacteria, viruses, and damage-associated molecular patterns (DAMPs) to TLR4, stimulate the production of pro-inflammatory compounds in the cells. Several studies conducted during the COVID-19 pandemic found that targeting TLR4 and RAGE receptors, as well as their ligands, could be beneficial in controlling the severity of COVID-19 patients with ARDS. Considering the importance of genetic heterogeneity of TLR4 and severity of COVID-19, a study was undertaken to investigate the probable associations of TLR4 SNPs in Thr399Ile/Asp299Gly with the severity of COVID-19 patients in the Egyptian population. It is recognized that minor alleles (G and T) of 299Gly and 399Ile of the TLR4 gene are related to the severity symptoms in COVID-19 patients [91]. In addition, Sohn KM et al. found that severe COVID-19 patients have high levels of S100A9 in PBMCs, rather than mild COVID-19 patients, because S100A9 binding to TLR4 promoted the release of different cytokines/chemokines (cytokine storm) during severe COVID-19 conditions [92].
Calprotectin (S100A8/A9 heterodimer) is mainly produced by macrophages, platelets, and neutrophils during inflammation in the immune system. Calprotectin expression is frequently elevated in a variety of diseases, including sepsis, lupus, trauma, and COVID-19. Calprotectin levels in the blood, stool and other body fluids have been described in previous research as a useful biologic marker for diagnosing inflammation. The binding of calprotectin to TLR4 and RAGE receptors induced the production of ROS, nitric oxide, and cytokines in the cells. According to a systematic review conducted by Udeh R et al., when compared to other biomarkers such as d-dimer, C-reactive protein, and creatine kinase for diagnosing and predicting the severity of COVID-19 patients, measurements of calprotectin concentration significantly increased in the serum of these patients relatively mild type of COVID-19 patients [93]. In particular, Lee and Shi found that calprotectin concentration was considerably higher in severe COVID-19 patients. Ultimately, their findings showed that elevated levels of calprotectin in the blood of COVID-19 patients with severe conditions function as a pro-inflammatory factor in their respiratory failure [94, 95].
HMGB1 is one of the essential types of DAMP molecules. The concentration of HMGB1 in the plasma of patients with severe inflammation, such as ARDS and pneumonia, was discovered to be higher. Previous research suggested that binding HMGB1 to TLR4 caused the synthesis and release of cytokines into the bloodstream (cytokines storm). HMGB1 also facilitated SARS-CoV-2 entry into the lung cells by increasing the amount of ACE2 receptors on the surface of AECs (Fig. 2a and b). According to the substantial pro-inflammatory effects of HMGB1 in the severity of patients with COVID-19, targeting HMGB1 is a possible point for preventing the highly loaded SARS-CoV-2 in the cells and releasing pro-inflammatory molecules like IL-6 and TNF in AECs [96, 97]. Headache, loss of appetite, diarrhea, anosmia, nausea, weight loss, and pulmonary injury are observed in patients suffering from severe COVID-19. Bolay H et al. demonstrated that elevated levels of various pro-inflammatory molecules such as D- dimer, IL-6, IL-10, ACE2, and HMGB1 in the serum of hospitalized patients with severe COVID-19 likely lead to headache; the results of their study showed that levels of HMGB1 in the serum of hospitalized patients with severe COVID-19 with Pulmonary involvement as a robust biomarker significantly increased [98].
Levels of sRAGE and esRAGE in the Blood of COVID-19 Patients
Despite numerous studies on the pathophysiology of severe COVID-19 patients with respiratory and hypoxemia abnormalities, little attention has been paid to investigating appropriate biomarkers for early detection of respiratory injury, particularly in COVID-19–associated ARDS (CARDS) and death prevention in patients with severe conditions. Among the biomarkers associated with respiratory injury in severe COVID-19, measuring the concentration of sRAGE in the blood is a valuable indicator in these patients. According to the evidence so far, the central cells in the production of sRAGE are alveolar cells in the lungs (type I); thereby, changing the level of them in the plasma is a beneficial marker for detecting the onset of respiratory complications in CARDS patients with severe thromboembolic, capillary leakage in organs, and angiopathy for mechanical ventilation and mortality prevention [99]. Lim et al. evaluated the possible correlation between high serum sRAGE concentration and respiratory failure in COVID-19 patients in the ICU in 2021; they stated that monitoring sRAGE in these patients is worthwhile for coping with mortality and severe pulmonary complications that necessitate mechanical ventilation and high-flow nasal oxygen therapy [6]. Microangiopathy, capillary congestion, and widespread alveolar destruction are the major causes of severe pulmonary injury in CARDS patients. In classical ARDS, evaluation of specific endothelial injury biomarkers such as soluble intercellular adhesion molecules, selectin family (P and E types), soluble vascular cell adhesion molecule, Ang-2, and soluble forms of RAGE in alveolar epithelial injury is considered a predicting marker for reducing respiratory symptoms and death rate. Although the recruitability of CARDS patients is lower than that of classical ARDS patients, a histopathological study revealed that their pathophysiology is identical. The assessments of certain alveolar and endothelia biomarkers in hospitalized severe patients with CARDS and classical ARDS in the University Hospital of Ferrara were utilized to clarify the distinctions between respiratory symptoms of CARDS patients and classical ARDS patients. The levels of soluble intercellular adhesion molecules or ICAM-1 and Ang-2 in the lungs of CARDS patients were higher than in classical ARDS patients due to significant vascular damage in the lungs. However, the levels of RAGE were lower. Furthermore, in CARDS individuals, P-selectin was lower than E-selectin [100].
It is well recognized that measurements of angiotensin II and ACE2 levels are poor biomarkers in severe respiratory dysfunctions such as ALI/ARDS; however, blocking the ACEI and AT1R are robust procedures for alleviating the inflammatory response and mortality in severe COVID-19 patients. As previously stated, NF-κB-related cell signals are activated by Ang II binding to AT1R independent of the presence of RAGE ligands. Altogether, inhibiting the RAGE/NF-B pathway reduced cytokine storms in COVID-19 patients with severe disease and lessened the damaging effects of the AT1R/Ang II in human cells, particularly in respiratory cells [21].
Levels of sRAGE and esRAGE in the Blood of COVID-19 Patients with Diabetes Mellitus
Thrombosis and micro/macrovasecular inflammation are two main complications in DM patients. Through the RAGE signaling pathway, infection with SARS-CoV-2, as well as the creation of AGEs molecules in diabetic patients' blood, exacerbate thrombosis and vasculopathy [101]. The most critical mediator in the morbidities of DM is the hyperactivity of the RAGE receptor. Studies have indicated that the entrance of SARS-CoV-2 to respiratory cells is mediated by ACE2 and AT1R receptors which trigger RAGE receptors in the cells’ surface regardless of the presence of RAGE-related ligands. Recently, Krishnamachary et al. have reported that RAGE-containing vesicles (extracellular vesicles) which are released from damaged cells in patients with COVID-19, can be used as a suitable biomarker in patients with severe COVID-19 with DM [82]. Based on the pro-inflammatory functions of RAGEs in the pathobiology of DM and respiratory involvements, Dozio et al. investigated the probable link between levels of soluble isoforms of RAGE and DM in patients with COVID-19. Overall, they suggested that measurements of cRAGE/esRAGE ratio along with other biomarkers could be as most applicable biomarker of RAGE group in all Covid-19 participants with or without DM, but to confirm these results, more extensive studies are needed [13].
Conclusions
RAGE is a multi-ligands receptor that predominantly is expressed in alveolar epithelial cells of the respiratory system. Measurements of soluble forms of RAGE in our blood, particularly sRAGE and esRAGE, as well as the RAGE gene’s expression rate, are as applicable biomarker in chronic inflammatory diseases such as diabetes, cardiovascular disease, neurodegenerative diseases (e.g., Alzheimer’s disease and Parkinson’s disease), pulmonary disease, classic ARDS, and also for reducing their mortality. Moreover, several studies have shown that targeting the RAGE receptor and its particular ligands, including HMGB1, LPS, AGEs, and the calprotectin family are novel techniques for inhibiting pro-inflammatory pathways in human cells.
Acute respiratory symptoms are the leading cause of mortality with the COVID-19 condition. The AT1R/Ang-2 and RAGE/TLR4/NF-B signaling pathways are activated when pro-inflammatory chemicals such as Ang-2, DAMPs, systemic cytokines, AGEs, and other compounds accumulate in the blood and alveolar spaces of the respiratory system. As a result, the current investigation focused on the involvement of the RAGE/ligands axis and associated pro-inflammatory levels of sRAGE and esRAGE in the severity of COVID-19 patients with ARDS who were hospitalized. Measurements of sRAGE, esRAGE, and sRAGE/esRAGE in patients with CARDS are as powerful biomarkers for alleviating respiratory symptoms and early diagnosis of pulmonary involvements for managing cytokines storms in lung cells, according to clinical research. Due to the multitude roles of RAGE and ligands in the related complications with the severity of COVID-19 such as DM, we suggest that targeting the RAGE/NF-κB signaling pathways along with AT1R/Ang-2 could alleviate pulmonary involvements in the ARDS COVID-19 patients.
Author Contributions
All the authors conceived this study and contributed equally to this work. HP, MS and SA participated in drafting the document and created the figures. HP was mentor and contributed to the supervision of the entire work. All the authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Consent for Publication
All authors agree to publish the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitra Salehi, Email: m.salehi@qums.ac.ir, Email: mitra.salehi1374@gmail.com.
Shahin Amiri, Email: sh_amiri@pasteur.ac.ir, Email: shaahinamiri@gmail.com.
Dariush Ilghari, Email: d.ilghari@gmail.com.
Hossein Piri, Email: hpiri@qums.ac.ir, Email: hosseinpiry@gmail.com.
References
- 1.Amiri S, Haghdoost A, Mostafavi E, Sharifi H, Peykari N, Raeisi A, et al. Iran COVID-19 epidemiology committee: a review of missions, structures, achievements, and challenges. J Res Health Sci. 2021;21(1):e00505-e. doi: 10.34172/jrhs.2021.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yalcin Kehribar D, Cihangiroglu M, Sehmen E, Avci B, Capraz A, Yildirim Bilgin A, et al. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers. 2021;26(2):114–118. doi: 10.1080/1354750X.2020.1861099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiappalupi S, Salvadori L, Vukasinovic A, Donato R, Sorci G, Riuzzi F. Targeting RAGE to prevent SARS-CoV-2-mediated multiple organ failure: hypotheses and perspectives. Life Sci. 2021;272:119251. doi: 10.1016/j.lfs.2021.119251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim A, Radujkovic A, Weigand MA, Merle U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann Intensive Care. 2021;11(1):50. doi: 10.1186/s13613-021-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavakoli A, Salahshourifar I, Hajialilo E, Haghdoost-Yazdi H, Ilghari D, Piri H. Haplotype Analysis of RAGE Gene polymorphisms and association with increased risk of diabetic nephropathy. J Kerman Univ Med Sci. 2022;29(1):50–59. [Google Scholar]
- 8.Beig Parikhani A, Bazaz M, Bamehr H, Fereshteh S, Amiri S, Salehi-Vaziri M, et al. The inclusive review on SARS-CoV-2 biology, epidemiology, diagnosis, and potential management options. Curr Microbiol. 2021;78(4):1099–1114. doi: 10.1007/s00284-021-02396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. (Berl) 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 10.Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Pt 3):1097–1109. doi: 10.1042/bj20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabaudon M, Berthelin P, Pranal T, Roszyk L, Godet T, Faure JS, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep. 2018;8(1):2603. doi: 10.1038/s41598-018-20994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dozio E, Sitzia C, Pistelli L, Cardani R, Rigolini R, Ranucci M, et al. Soluble receptor for advanced glycation end products and its forms in COVID-19 patients with and without diabetes mellitus: a pilot study on their role as disease biomarkers. J Clin Med. 2020;9(11):3785. doi: 10.3390/jcm9113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AC, Lam JK, Shiu SW, Wong Y, Betteridge DJ, Tan KC. Serum level of soluble receptor for advanced glycation end products Is associated with a disintegrin and metalloproteinase 10 in type 1 diabetes. PLoS ONE. 2015;10(9):e0137330. doi: 10.1371/journal.pone.0137330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong LSY, Loo EXL, Kang AYH, Lau HX, Tambyah PA, Tham EH. Age-related differences in immunological responses to SARS-CoV-2. J Allergy Clin Immunol Pract. 2020;8(10):3251–3258. doi: 10.1016/j.jaip.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brojakowska A, Narula J, Shimony R, Bander J. Clinical implications of SARS-CoV-2 interaction with renin angiotensin system: JACC review topic of the week. J Am Coll Cardiol. 2020;75(24):3085–3095. doi: 10.1016/j.jacc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16(8):2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 18.Dozio E, Sitzia C, Pistelli L, Cardani R, Rigolini R, Ranucci M, et al. Soluble receptor for advanced glycation end products and its forms in COVID-19 patients with and without diabetes mellitus: a pilot study on their role as disease biomarkers. J Clin Med. 2020;9(11):3785–3793. doi: 10.3390/jcm9113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerkeni M, Gharbi J. RAGE receptor: may be a potential inflammatory mediator for SARS-COV-2 infection? Med Hypotheses. 2020;144:109950. doi: 10.1016/j.mehy.2020.109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Francesco EM, Vella V, Belfiore A. COVID-19 and diabetes: the importance of controlling RAGE. Front Endocrinol. 2020;11:526. doi: 10.3389/fendo.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas A, Gonzalez I, Morales MA. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE? Inflamm Res. 2020;69(7):641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stilhano RS, Costa AJ, Nishino MS, Shams S, Bartolomeo CS, Breithaupt-Faloppa AC, et al. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: focus on susceptibility factors. FASEB J. 2020;34(11):14103–14119. doi: 10.1096/fj.202001394RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective. J Med Chem. 2017;60(17):7213–7232. doi: 10.1021/acs.jmedchem.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W, Lampe L, Park S, Vangara BS, Waldo GS, Cabantous S, et al. Disulfide bonds within the C2 domain of RAGE play key roles in its dimerization and biogenesis. PLoS ONE. 2012;7(12):e50736. doi: 10.1371/journal.pone.0050736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, Jeong MS, Jang SB. Molecular characteristics of RAGE and advances in small-molecule inhibitors. Int J Mol Sci. 2021;22(13):6904–6926. doi: 10.3390/ijms22136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarneri F, Custurone P, Papaianni V, Gangemi S. Involvement of RAGE and oxidative stress in inflammatory and infectious skin diseases. Antioxidants (Basel, Switzerland) 2021;10(1):82–96. doi: 10.3390/antiox10010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad MN, Farah AI, Al-Qirim TM. The cardiovascular complications of diabetes: a striking link through protein glycation. Roman J. Internal Med. = Revue roumaine de medecine interne. 2020;58(4):188–98. doi: 10.2478/rjim-2020-0021. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Cai L, Guo X, Li Z, Liao X, Zhang X, et al. HMGB1-activated fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma. 2021;68(1):71–78. doi: 10.4149/neo_2020_200610N620. [DOI] [PubMed] [Google Scholar]
- 29.Okui T, Hiasa M, Ryumon S, Ono K, Kunisada Y, Ibaragi S, et al. The HMGB1/RAGE axis induces bone pain associated with colonization of 4T1 mouse breast cancer in bone. J Bone Oncol. 2021;26:100330. doi: 10.1016/j.jbo.2020.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A, Kaur S, Sarkar M, Sarin BC, Changotra H. The AGE-RAGE axis and RAGE genetics in chronic obstructive pulmonary disease. Clin Rev Allergy Immunol. 2021;60(2):244–258. doi: 10.1007/s12016-020-08815-4. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Wang X, Tuo M, Ma J, Xie A. RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett. 2018;672:65–69. doi: 10.1016/j.neulet.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 32.Shi D, Chang JW, Choi J, Connor B, O'Carroll SJ, Nicholson LFB, et al. Receptor for advanced glycation end products (RAGE) is expressed predominantly in medium spiny neurons of tgHD rat striatum. Neuroscience. 2018;380:146–151. doi: 10.1016/j.neuroscience.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Olaoba OT, Kadasah S, Vetter SW, Leclerc E. RAGE signaling in melanoma tumors. Int J Mol Sci. 2020;21(23):8989–9021. doi: 10.3390/ijms21238989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23(6):1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52(11):2251–2263. doi: 10.1007/s00125-009-1458-9. [DOI] [PubMed] [Google Scholar]
- 36.Jing R, Cui M, Wang J, Wang H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma. 2010;57(1):55–61. doi: 10.4149/neo_2010_01_055. [DOI] [PubMed] [Google Scholar]
- 37.Faiz A, van den Berge M, Vermeulen CJ, Ten Hacken NH, Guryev V, Pouwels SD. AGER expression and alternative splicing in bronchial biopsies of smokers and never smokers. Respir Res. 2019;20(1):1–4. doi: 10.1186/s12931-019-1038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomis SJ, Chen Y, Sacks DB, Christenson ES, Christenson RH, Rebholz CM, et al. Cross-sectional analysis of AGE-CML, sRAGE, and esRAGE with diabetes and cardiometabolic risk factors in a community-based cohort. Clin Chem. 2017;63(5):980–989. doi: 10.1373/clinchem.2016.264135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyagawa T, Iwata Y, Oshima M, Ogura H, Sato K, Nakagawa S, et al. Soluble receptor for advanced glycation end products protects from ischemia-and reperfusion-induced acute kidney injury. Biology Open. 2021;11(1):1–8. doi: 10.1242/bio.058852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi K, Iwamoto H, Mazur W, Miura S, Sakamoto S, Horimasu Y, et al. Reduced endogenous secretory RAGE in blood and bronchoalveolar lavage fluid is associated with poor prognosis in idiopathic pulmonary fibrosis. Respir Res. 2020;21(1):1–8. doi: 10.1186/s12931-020-01410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones T, Feng R, Reilly J, Johansson E, Dunn T, Riley T, et al. Cleaved RAGE is the predominant early-evoked sRAGE isoform and confers risk for sepsis-associated ARDS. D105 critical care: ventilator induced lung injury and ARDS-from mice to biomarkers in ARDS: American Thoracic Society; 2018. p. A7534-A.
- 42.Sellegounder D, Zafari P, Rajabinejad M, Taghadosi M, Kapahi P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int Immunopharmacol. 2021;98:107806. doi: 10.1016/j.intimp.2021.107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erusalimsky JD. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021;42:101958. doi: 10.1016/j.redox.2021.101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi KM, Han KA, Ahn HJ, Hwang SY, Hong HC, Choi HY, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97(10):3751–3758. doi: 10.1210/jc.2012-1951. [DOI] [PubMed] [Google Scholar]
- 45.Aglago EK, Rinaldi S, Freisling H, Jiao L, Hughes DJ, Fedirko V, et al. Soluble receptor for advanced glycation end-products (sRAGE) and colorectal cancer risk: a case-control study nested within a European prospective cohort. Cancer Epidemiol Biomark Prevent. 2021;30(1):182–192. doi: 10.1158/1055-9965.EPI-20-0855. [DOI] [PubMed] [Google Scholar]
- 46.Zhong X, Xie F, Chen L, Liu Z, Wang Q. S100A8 and S100A9 promote endothelial cell activation through the RAGE-mediated mammalian target of rapamycin complex 2 pathway. Mol Med Rep. 2020;22(6):5293–5303. doi: 10.3892/mmr.2020.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araki K, Kinoshita R, Tomonobu N, Gohara Y, Tomida S, Takahashi Y, et al. The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis. J Mol Med. 2021;99(1):131–145. doi: 10.1007/s00109-020-02001-x. [DOI] [PubMed] [Google Scholar]
- 48.de la Cruz-Ares S, Cardelo MP, Gutiérrez-Mariscal FM, Torres-Peña JD, García-Rios A, Katsiki N, et al. Endothelial dysfunction and advanced glycation end products in patients with newly diagnosed Versus established diabetes: from the CORDIOPREV study. Nutrients. 2020;12(1):238. doi: 10.3390/nu12010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutierrez-Mariscal FM, Cardelo MP, de la Cruz S, Alcala-Diaz JF, Roncero-Ramos I, Guler I, et al. Reduction in circulating advanced glycation end products by mediterranean diet is associated with increased likelihood of type 2 diabetes remission in patients with coronary heart disease: from the cordioprev study. Mol Nutr Food Res. 2021;65(1):e1901290. doi: 10.1002/mnfr.201901290. [DOI] [PubMed] [Google Scholar]
- 50.Ren L, Yan H. Targeting AGEs-RAGE pathway inhibits inflammation and presents neuroprotective effect against hepatic ischemia-reperfusion induced hippocampus damage. Clin Res Hepatol Gastroenterol. 2021;46:101792. doi: 10.1016/j.clinre.2021.101792. [DOI] [PubMed] [Google Scholar]
- 51.Brinkley T, Semba R, Kritchevsky S, Houston D. Dietary protein intake and serum age/rage levels in the health ABC study. Innov Aging. 2018;2(1):707. doi: 10.1093/geroni/igy023.2622. [DOI] [Google Scholar]
- 52.Ramya R, Coral K, Bharathidevi SR. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp Eye Res. 2021;206:108519. doi: 10.1016/j.exer.2021.108519. [DOI] [PubMed] [Google Scholar]
- 53.Gupta A, Uribarri J. Dietary advanced glycation end products and their potential role in cardiometabolic disease in children. Horm Res Paediatr. 2016;85(5):291–300. doi: 10.1159/000444053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiavaroli V, D’Adamo E, Giannini C, de Giorgis T, De Marco S, Chiarelli F, et al. Serum levels of receptors for advanced glycation end products in normal-weight and obese children born small and large for gestational age. Diabetes Care. 2012;35(6):1361–1363. doi: 10.2337/dc11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Zhang W, Zhao H, Wang Y, Lu C, Li X, et al. Fasting blood soluble RAGE may be causally implicated in impaired glucose metabolism in Chinese patients with primary hypertension. Gene. 2018;639:11–17. doi: 10.1016/j.gene.2017.09.066. [DOI] [PubMed] [Google Scholar]
- 56.Sebeková K, Krivošíková Z, Gajdoš M. Total plasma Nε-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin Chem Lab Med. 2014;52(1):139–149. doi: 10.1515/cclm-2012-0879. [DOI] [PubMed] [Google Scholar]
- 57.Anggayasti WL, Mancera RL, Bottomley S, Helmerhorst E. The self-association of HMGB1 and its possible role in the binding to DNA and cell membrane receptors. FEBS Lett. 2017;591(2):282–294. doi: 10.1002/1873-3468.12545. [DOI] [PubMed] [Google Scholar]
- 58.Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol. 2013;56(4):739–744. doi: 10.1016/j.molimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Koyama H, Tanaka S, Monden M, Shoji T, Morioka T, Fukumoto S, et al. Comparison of effects of pioglitazone and glimepiride on plasma soluble RAGE and RAGE expression in peripheral mononuclear cells in type 2 diabetes: randomized controlled trial (PioRAGE) Atherosclerosis. 2014;234(2):329–334. doi: 10.1016/j.atherosclerosis.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Farhangi MA, Dehghan P, Namazi N. Prebiotic supplementation modulates advanced glycation end-products (AGEs), soluble receptor for AGEs (sRAGE), and cardiometabolic risk factors through improving metabolic endotoxemia: a randomized-controlled clinical trial. Eur J Nutr. 2020;59(7):3009–3021. doi: 10.1007/s00394-019-02140-z. [DOI] [PubMed] [Google Scholar]
- 61.Shah RJ, Bellamy SL, Lee JC, Cantu E, Diamond JM, Mangalmurti N, et al. Early plasma soluble receptor for advanced glycation end-product levels are associated with bronchiolitis obliterans syndrome. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2013;13(3):754–759. doi: 10.1111/ajt.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sim YS, Kim DG, Shin TR. The diagnostic utility and tendency of the soluble receptor for advanced glycation end products (sRAGE) in exudative pleural effusion. J Thorac Dis. 2016;8(7):1731–1737. doi: 10.21037/jtd.2016.05.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machahua C, Montes-Worboys A, Planas-Cerezales L, Buendia-Flores R, Molina-Molina M, Vicens-Zygmunt V. Serum AGE/RAGEs as potential biomarker in idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):215. doi: 10.1186/s12931-018-0924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Han N, Shen Y. S100A12 promotes inflammation and cell apoptosis in sepsis-induced ARDS via activation of NLRP3 inflammasome signaling. Mol Immunol. 2020;122:38–48. doi: 10.1016/j.molimm.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 65.Jones TK, Feng R, Kerchberger VE, Reilly JP, Anderson BJ, Shashaty MGS, et al. Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(1):47–56. doi: 10.1164/rccm.201810-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Mao YF, Zhao Y, Xu DF, Wang Y, Xu CF, et al. Upregulation of matrix metalloproteinase-9 protects against sepsis-induced acute lung injury via promoting the release of soluble receptor for advanced glycation end products. Oxid Med Cell Longev. 2021;2021:8889313. doi: 10.1155/2021/8889313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshizaki A, Komura K, Iwata Y, Ogawa F, Hara T, Muroi E, et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol. 2009;29(2):180–189. doi: 10.1007/s10875-008-9252-x. [DOI] [PubMed] [Google Scholar]
- 68.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi K, Iwamoto H, Horimasu Y, Ohshimo S, Fujitaka K, Hamada H, et al. AGER gene polymorphisms and soluble receptor for advanced glycation end product in patients with idiopathic pulmonary fibrosis. Respirology (Carlton, Vic) 2017;22(5):965–971. doi: 10.1111/resp.12995. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi K, Iwamoto H, Mazur W, Miura S, Sakamoto S, Horimasu Y, et al. Reduced endogenous secretory RAGE in blood and bronchoalveolar lavage fluid is associated with poor prognosis in idiopathic pulmonary fibrosis. Respir Res. 2020;21(1):145. doi: 10.1186/s12931-020-01410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machahua C, Montes-Worboys A, Llatjos R, Escobar I, Dorca J, Molina-Molina M, et al. Increased AGE-RAGE ratio in idiopathic pulmonary fibrosis. Respir Res. 2016;17(1):144. doi: 10.1186/s12931-016-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kinjo T, Kitaguchi Y, Droma Y, Yasuo M, Wada Y, Ueno F, et al. The Gly82Ser mutation in AGER contributes to pathogenesis of pulmonary fibrosis in combined pulmonary fibrosis and emphysema (CPFE) in Japanese patients. Sci Rep. 2020;10(1):12811. doi: 10.1038/s41598-020-69184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou H, Wang X, Zhang B. Depression of lncRNA NEAT1 antagonizes LPS-evoked acute injury and inflammatory response in alveolar epithelial cells via HMGB1-RAGE signaling. Mediators Inflamm. 2020;2020:8019467. doi: 10.1155/2020/8019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Wang T, Yuan Z, Cao Y, Zhou Y, He J, et al. Role of receptor for advanced glycation end products in regulating lung fluid balance in lipopolysaccharide-induced acute lung injury and infection-related acute respiratory distress syndrome. Shock (Augusta, Ga) 2018;50(4):472–482. doi: 10.1097/SHK.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 75.Audard J, Godet T, Blondonnet R, Joffredo JB, Paquette B, Belville C, et al. Inhibition of the receptor for advanced glycation End-products in acute respiratory distress syndrome: a randomised laboratory trial in piglets. Sci Rep. 2019;9(1):9227. doi: 10.1038/s41598-019-45798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu H, Shi D, Hu C, Yuan X, Zhang J, Sun H. Dexmedetomidine mitigates CLP-stimulated acute lung injury via restraining the RAGE pathway. Am J Transl Res. 2017;9(12):5245–5258. [PMC free article] [PubMed] [Google Scholar]
- 77.Piao C, Zhuang C, Choi M, Ha J, Lee M. A RAGE-antagonist peptide potentiates polymeric micelle-mediated intracellular delivery of plasmid DNA for acute lung injury gene therapy. Nanoscale. 2020;12(25):13606–13617. doi: 10.1039/D0NR01367F. [DOI] [PubMed] [Google Scholar]
- 78.Weber DJ, Allette YM, Wilkes DS, White FA. The HMGB1-RAGE inflammatory pathway: implications for brain injury-induced pulmonary dysfunction. Antioxid Redox Signal. 2015;23(17):1316–1328. doi: 10.1089/ars.2015.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng G, Liu S, Wang GL, Liu GJ. Lidocaine attenuates lipopolysaccharide-induced acute lung injury through inhibiting NF-kappaB activation. Pharmacology. 2008;81(1):32–40. doi: 10.1159/000107792. [DOI] [PubMed] [Google Scholar]
- 80.Lee PY, Tsai PS, Huang YH, Huang CJ. Inhibition of toll-like receptor-4, nuclear factor-kappaB and mitogen-activated protein kinase by lignocaine may involve voltage-sensitive sodium channels. Clin Exp Pharmacol Physiol. 2008;35(9):1052–1058. doi: 10.1111/j.1440-1681.2008.04962.x. [DOI] [PubMed] [Google Scholar]
- 81.Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ, Li AZ, et al. The protective effect of lidocaine on lipopolysaccharide-induced acute lung injury in rats through NF-κB and p38 MAPK signaling pathway and excessive inflammatory responses. Eur Rev Med Pharmacol Sci. 2018;22(7):2099–2108. doi: 10.26355/eurrev_201804_14743. [DOI] [PubMed] [Google Scholar]
- 82.Krishnamachary B, Cook C, Spikes L, Chalise P, Dhillon NK. The Potential Role of Extracellular Vesicles in COVID-19 Associated Endothelial injury and Pro-inflammation. medRxiv. 2020:2020.08.27.20182808.
- 83.Zhang Z, Zhou J, Liao C, Li X, Liu M, Song D, et al. RAGE deficiency attenuates the protective effect of Lidocaine against sepsis-induced acute lung injury. Inflammation. 2017;40(2):601–611. doi: 10.1007/s10753-016-0507-z. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang M, Wang CY, Shen A. Ketamine alleviates LPS induced lung injury by inhibiting HMGB1-RAGE level. Eur Rev Med Pharmacol Sci. 2018;22(6):1830–1836. doi: 10.26355/eurrev_201803_14603. [DOI] [PubMed] [Google Scholar]
- 85.Yang C, Song Y, Wang H. Suppression of RAGE and TLR9 by ketamine contributes to attenuation of lipopolysaccharide-induced acute lung injury. J Investig Surg. 2017;30(3):177–186. doi: 10.1080/08941939.2016.1232448. [DOI] [PubMed] [Google Scholar]
- 86.Petruk G, Puthia M, Petrlova J, Samsudin F, Strömdahl AC, Cerps S, et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Mol Cell Biol. 2020;12(12):916–932. doi: 10.1093/jmcb/mjaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niu Y, Chen Y, Sun P, Wang Y, Luo J, Ding Y, et al. Intragastric and atomized administration of canagliflozin inhibit inflammatory cytokine storm in lipopolysaccharide-treated sepsis in mice: a potential COVID-19 treatment. Int Immunopharmacol. 2021;96:107773. doi: 10.1016/j.intimp.2021.107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7(2):e06187. doi: 10.1016/j.heliyon.2021.e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teixeira PC, Dorneles GP, Filho PCS, Silva IMd, Schipper LL, Postiga IA, et al. Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. medRxiv. 2021:2021.06.24.21259468. [DOI] [PMC free article] [PubMed]
- 90.Jose SP, M R, S S, Rajan S, Saji S, Narayanan V, et al. Anti-inflammatory effect of Kaba Sura Kudineer (AYUSH approved COVID-19 drug)-A Siddha poly-herbal formulation against lipopolysaccharide induced inflammatory response in RAW-264.7 macrophages cells. J Ethnopharmacol. 2022;283:114738 [DOI] [PMC free article] [PubMed]
- 91.Taha SI, Shata AK, Baioumy SA, Fouad SH, Anis SG, Mossad IM, et al. Toll-like receptor 4 polymorphisms (896A/G and 1196C/T) as an indicator of COVID-19 severity in a convenience sample of Egyptian patients. J Inflamm Res. 2021;14:6293–6303. doi: 10.2147/JIR.S343246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sohn KM, Lee SG, Kim HJ, Cheon S, Jeong H, Lee J, et al. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J Korean Med Sci. 2020;35(38):e343. doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Udeh R, Advani S, de Guadiana Romualdo LG, Dolja-Gore X. Calprotectin, an emerging biomarker of interest in COVID-19: a systematic review and meta-analysis. J Clin Med. 2021;10(4):775–789. doi: 10.3390/jcm10040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee A, Nahm CH, Lee JS, Lee MK, Lee KR. Assessment of antiphospholipid antibodies and calprotectin as biomarkers for discriminating mild from severe COVID-19. J Clin Lab Anal. 2021;35(11):e24004. doi: 10.1002/jcla.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi H, Zuo Y, Yalavarthi S, Gockman K, Zuo M, Madison JA, et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J Leukoc Biol. 2021;109(1):67–72. doi: 10.1002/JLB.3COVCRA0720-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen R, Huang Y, Quan J, Liu J, Wang H, Billiar TR, et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6(12):e05672. doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sivakorn C, Dechsanga J, Jamjumrus L, Boonnak K, Schultz MJ, Dorndorp AM, et al. High mobility group box 1 and interleukin 6 at intensive care unit admission as biomarkers in critically Ill COVID-19 patients. Am J Trop Med Hyg. 2021;105(1):73–80. doi: 10.4269/ajtmh.21-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bolay H, Karadas Ö, Oztürk B, Sonkaya R, Tasdelen B, Bulut TDS, et al. HMGB1, NLRP3, IL-6 and ACE2 levels are elevated in COVID-19 with headache: a window to the infection-related headache mechanism. J Headache Pain. 2021;22(1):94. doi: 10.1186/s10194-021-01306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kapandji N, Yvin E, Devriese M, de Margerie-Mellon C, Moratelli G, Lemiale V, et al. Importance of lung epithelial injury in COVID-19-associated acute respiratory distress syndrome: value of plasma soluble receptor for advanced glycation end-products. Am J Respir Crit Care Med. 2021;204(3):359–362. doi: 10.1164/rccm.202104-1070LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care. 2021;25(1):74. doi: 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freda CT, Yin W, Ghebrehiwet B, Rubenstein DA. SARS-CoV-2 proteins regulate inflammatory, thrombotic and diabetic responses in human arterial fibroblasts. Clinical Immunol. (Orlando, Fla) 2021;227:108733. doi: 10.1016/j.clim.2021.108733. [DOI] [PMC free article] [PubMed] [Google Scholar]