Abstract
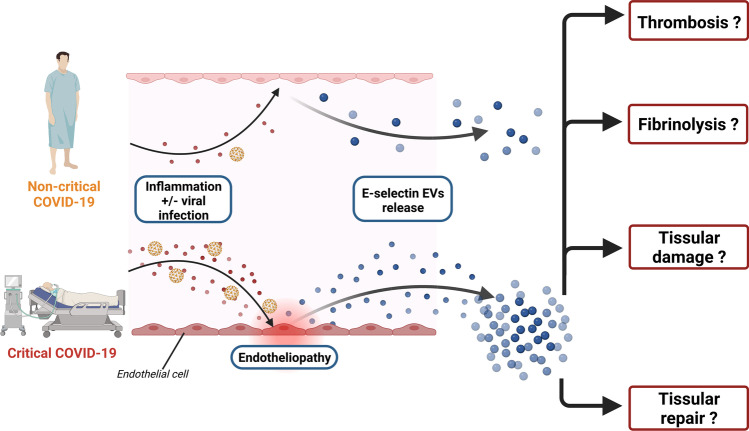
COVID-19 and infectious diseases have been included in strategic development goals (SDG) of United Nations (UN). Severe form of COVID-19 has been described as an endothelial disease. In order to better evaluate Covid-19 endotheliopathy, we characterized several subsets of circulating endothelial extracellular vesicles (EVs) at hospital admission among a cohort of 60 patients whose severity of COVID-19 was classified at the time of inclusion. Degree of COVID-19 severity was determined upon inclusion and categorized as moderate to severe in 40 patients and critical in 20 patients. We measured citrated plasma EVs expressing endothelial membrane markers. Endothelial EVs were defined as harboring VE-cadherin (CD144+), PECAM-1 (CD31 + CD41-) or E-selectin (CD62E+). An increase in CD62E + EV levels on admission to the hospital was significantly associated with critical disease. Moreover, Kaplan-Meier survival curves for CD62E + EV level showed that level ≥ 88,053 EVs/μL at admission was a significant predictor of in hospital mortality (p = 0.004). Moreover, CD62E + EV level ≥ 88,053 EV/μL was significantly associated with higher in-hospital mortality (OR 6.98, 95% CI 2.1–26.4, p = 0.002) in a univariate logistic regression model, while after adjustment to BMI CD62E + EV level ≥ 88,053 EV/μL was always significantly associated with higher in-hospital mortality (OR 5.1, 95% CI 1.4–20.0, p = 0.01). The present findings highlight the potential interest of detecting EVs expressing E-selectin (CD62) to discriminate Covid-19 patients at the time of hospital admission and identify individuals with higher risk of fatal outcome.
Graphical abstract
Keywords: COVID-19, Extracellular vesicles, Endothelial, E-selectin, UN SDG3
Introduction
Infection with SARS-CoV-2 leads in some patients to severe forms described as an endothelial disease [1]. This endotheliopathy is probably at the origin, at least in part, of COVID-19 prothrombotic state [2] giving rise to microthrombosis. Extent of microthrombosis participate to severity of the disease and is a unique characteristic of COVID-19 and in particular of acute respiratory distress syndrome (ARDS) [3]. Despite preventive anticoagulation proposed as soon the COVID-19 outbreak started, D-Dimer levels are associated with severity at admission, with worsening during hospital stay and with mortality independently from venous thrombotic events [4–6]. The SARS-CoV-2 infection of endothelial cells is still a matter of debate but modification of cell morphology, endothelial cells apoptosis or abnormal endothelial organization like intussusceptive angiogenesis is clearly demonstrated [7, 8]. In terms of circulating biomarkers, increase of circulating endothelial cells, angiopoietin-2 or von Willebrand factor have been described as good predictive marker of severity and in-hospital mortality [9–12]. Mechanistically, endothelial activation is probably occurring after combination of hypoxemia and/or inflammatory burst response [2].
Extracellular vesicles (EVs) are a heterogeneous group of small-sized vesicles released by most cell types in response to different stimuli [13, 14]. They are composed of a lipid bilayer that encloses a wide range of bioactive material; in particular, they are largely involved in hemostasis since they carry protein involved in thrombosis and fibrinolysis. For that, EVs quantification and characterization has become of great interest in all thromboembolic disease. Thus, in COVID-19 several studies have reported a higher numbers in plasma of EVs derived from platelets, endothelial cells, leukocytes, neutrophils, alveolar-macrophages or alveolar-epithelial cells [15]. Moreover, increased levels of EVs bearing tissue factor (TF) in severe COVID-19 has been found and could directly contribute to thrombosis [16].
The study aimed to better evaluate circulating endothelial EVs with different patterns in confirmed COVID-19 patients at hospital admission. These snapshot indexes of endothelial injury or activation could help to better contribute in COVID-19 severity characterization at entrance in hospital.
Materials and Methods
Study Design and Participants
This observational cohort study was conducted in Georges Pompidou European hospital in Paris, France (2020-A01048–31, March–June 2020 - NCT04624997) and included 60 consecutive hospitalized COVID-19 patients classified according to World Health Organization guidance as moderate/severe (non-critical; n = 40; median oxygen requirement 3 L/min; score 4–7) or critical (n = 20; requiring mechanical ventilation, score 8–9) as previously described [12]. In-hospital mortality was followed up for 90 days for all patients. The two groups were comparable at admission for most clinical and biological characteristics, but critical patients had higher body mass index (BMI = 29.50 kg/m2 [IQR, 25.8–33.6] vs. 26.15 kg/m2 [IQR, 23.6–27.9], p < 0.019), higher white cell and granulocyte counts (respectively p = 0.009 and 0.005) and higher D-dimer, troponin and CRP circulating levels (p < 0.001 for these 3 biomarkers) than non-critical patients.
Plasma Preparation and Extracellular Vesicles (EVs) Isolation
Blood was collected from 60 individuals after admission via venipuncture or pre-existing arterial lines in 3,5 ml 0.109 M citrated plastic tubes (Tube Vacuette, Greiner). Poor platelet plasma (PPP) was prepared from whole blood within 1 hour of sampling by centrifugation twice at 2500 g for 15 min, at 21 °C with the lowest deceleration settings and then stored at −80 °C. Large EVs (lEVs) were then isolated from 500 μl of PFP by one step centrifugation at 20500 x g for 2 h, at 4 °C. lEVs rich-pellet was resuspended in 0.1 μm filtrated PBS 1X and aliquots of 50 μl ×4 have been stored at −80 °C [17, 18].
Large Extracellular Vesicles (lEVs) Characterization by Flow Cytometry
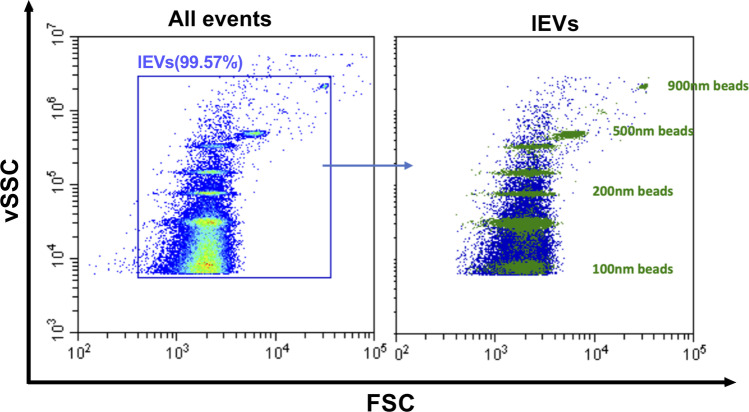
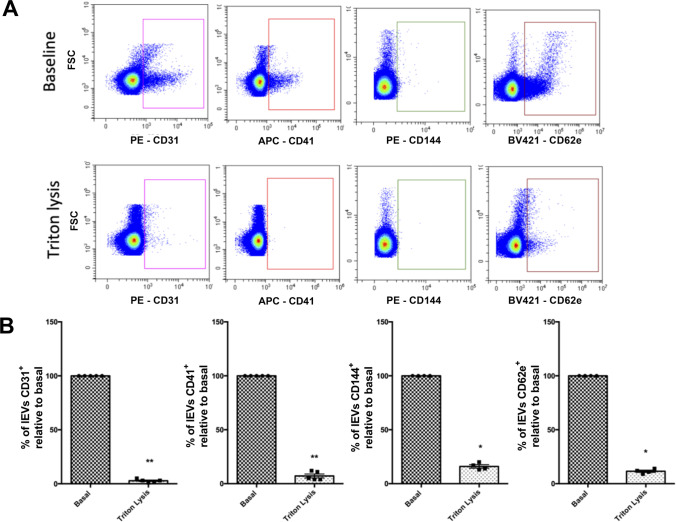
Circulating lEVs were analyzed using Cytoflex flow cytometer (Beckman Coulter, USA) equipped with 3 lasers (405 nm, 488 nm, 638 nm) and 13 band pass filters: 450/45, 525/40 (2), 585/42, 610/20 (2), 660/10 (2), 690/50, 712/25, 780/60 (3). FSC and SSC were resulting from 488 nm laser line excitation while vSSC (violet SSC) was resulting from 405 nm laser line excitation. Before analyzing lEVs, gating strategies were required to define events with a diameter of 0.1 μm to 0.9 μm according to the application note set-up by Spittler, 2015. lEV gating was determined by using a combination of FITC labelled fluorescent Megamix-Plus SSC (Cat# 7803, Biocytex, France) and Megamix-Plus FSC beads (Cat# 7802, Biocytex, France), hereby termed as Gigamix. The Gigamix contains beads of sizes 0.1 μm, 0.16 μm, 0.2 μm, 0.24 μm, 0.3 μm, 0.5 μm and 0.9 μm (Fig. 1). Acquisition settings for lEVs were adjusted as FSC gain at 106, SSC gain 61, vSSC gain at 61 and threshold set on vSSC at 6500 in height. For experiments using fluorescence-conjugated antibodies to stain lEV surface markers, antibodies were directly added to EV-containing sample. All antibodies were centrifuged for 5 min at 13,000×g at 4 °C before they were applied to EV samples. Endothelial EVs were defined as harboring either VE-cadherin (CD144+), PECAM-1 (CD31+CD41−) or E-selectin (CD62E+). All antibodies were from Beckman-Coulter, Villepinte, France) except CD62E, obtained from Becton-Dickinson, Rungis, France. Following MISEV guidelines, prior to EVs staining, the antibodies and their corresponding isotypes were titrated and the appropriate dilution have been determined as summarized in Table 1. All samples were diluted in 0,1 μm filtrated NaCl 0,9% to appropriate dilutions in order to avoid swarm detection. Controls include for all analyses of lEVs negative control with isotype, detergent lysis, buffer-only, buffer with reagent (without EVs) and unstained samples. For the buffer-only control, 0.1 μm-filtered NaCl 0,9% was recorded at the same acquisition settings as all other samples and had a count of less than 100 events s−1. For detergent lysis controls, stained samples were evaluated immediately after addition of Triton 0,05% as described by Gyorgy [17]. Triton detergent exposure decreased over 90% of the positive labeling (Fig. 2). Samples concentrations were measured using the instrument flow rate sensors, resulting in a flow rate of 10 μL min−1. Prior to analysis, a calibration has been performed using weighed volumes of deionized water. After acquisition, data were analyzed using CytExpert software and EVs concentrations were normalized to plasma volume.
Fig. 1.
lEVs gating strategy: gating strategy have been performed using a combination of Megamix-Plus SSC Megamix-Plus FSC fluorescent beads, containing beads of sizes 0.1 μm, 0.16 μm, 0.2 μm, 0.24 μm, 0.3 μm, 0.5 μm and 0.9 μm
Table 1.
Summary of reagents used for flow cytometry experiments
| Characteristic being measured | Analyte | Analyte detector | Reporter | Isotype | Clone | Dilution | Manufacturer | Cat. Number | Lot Number |
|---|---|---|---|---|---|---|---|---|---|
| Cell surface protein | Human PECAM | Anti-human CD31antibody | PE | Mouse IgG1 | 1F11 | 1/50 | Beckman coulter | IM2409 | 200042 |
| Non-specific binding of antibody | NA | Mouse IgG1 | PE | NA | 679.1Mc7 | 1/50 | Beckman coulter | A07796 | 200053 |
| Cell surface protein | Human GPIIb | Anti-human CD41antibody | APC | Mouse IgG1 | P2 | 1/33 | Beckman coulter | B16894 | 200026 |
| Non-specific binding of antibody | NA | Mouse IgG1 | APC | NA | 679.1Mc7 | 1/67 | Beckman coulter | IM2475 | 200064 |
| Cell surface protein | Human VE-Cadherin | Anti-human CD144 antibody | PE | Mouse IgG1 | TEA 1/31 | 1/20 | Beckman coulter | A07481 | 200031 |
| Non-specific binding of antibody | NA | Mouse IgG1 | PE | NA | 679.1Mc7 | 1/50 | Beckman coulter | A07796 | 200056 |
| Cell surface protein | Human E-Selectin | Anti-human CD62E antibody | BV421 | Mouse IgG1, κ | 68-5H11 | 1/33 | BD | 563360 | 5337980 |
| Non-specific binding of antibody | NA | Mouse IgG1, κ | BV421 | NA | X40 | 1/67 | BD | 562438 | 9301751 |
Fig. 2.
Effect of Triton lysis on lEVs flow cytometry analysis. (A) Representative experiment assessing levels of endothelial-derived lEVs either in absence (top panels; baseline) or presence of Triton (bottom panels). lEVs number dramatically decreases after Triton lysis. (B) Plasma lEVs levels (n = 4) before and after Triton lysis. Data are expressed as mean ± SEM. (*P < 0.05; Mann Withney test)
Results and Discussion
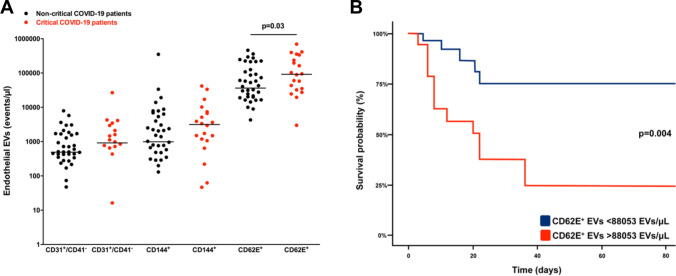
Levels of CD62E+ EVs at admission were significantly higher in critical patients (p = 0.03, Fig. 3A) while other endothelial EVs subtypes (CD31+CD41−: p = 0.26; CD144+: p = 0.10) did not differ between the two groups. We next evaluated the predictive value of CD62E+ EVs on in-hospital mortality. This mortality reached 65% (n = 13) in critical patients, as compared to 10% (n = 4) in the non-critical group (p < 0.01). Time from admission to outcome (death/discharge) was also greater (p < 0.01) in critical patients (20.00 CI 8.00–40.50), when compared to non-critical subjects (10.00 CI 4.00–22.00). The optimum cut-off value for CD62E+ EVs level at admission to predict in-hospital mortality was 88,053 EVs/μL according to the receiver operating characteristic (ROC) curve with a sensitivity of 74.4% (95% CI 44.0–88.0), a specificity of 70.5% (95% CI 58.0–86.0), a positive predictive value of 52.1% (95% CI 31.0–72.0) and a negative predictive value of 86.5% (95% CI 70.0–95.0). Area Under Curve (AUC) for in-hospital mortality was 70.0% (95% CI 54.0–85.0). Therefore, CD62E+ EVs levels ≥88,053 EVs/μL at admission were associated with higher in-hospital mortality (OR 7.0, 95% CI 2.1–26.4, p = 0.002) in a univariate logistic regression model, and remained so after adjustment for BMI (OR 5.1, 95% CI 1.4–20.0, p = 0.01). Kaplan-Meier survival curves confirmed this result (p = 0.004, Fig. 3B), including after adjustment to BMI using a Cox proportional hazard analysis (HR 4.0 95% CI 1.4–11.5, p = 0.009). Plasma levels of endothelial EVs expressing intercellular junctional proteins PECAM-1 (CD31) or VE-Cadherin (CD144) did not discriminate between critical and non-critical COVID-19 patients.
Fig. 3.
Endothelial circulating EVs in COVID-19 patients. 3A: CD31 + CD41-, CD144+ and CD62E+ EVs levels at admission (Mann-Whitney and Fisher’s tests) 3B: Kaplan–Meier survival curves, illustrating prognostic impact of CD62E+ EVs
In this study, we demonstrated that lEVs expressing CD62 (E-selectin) in COVID-19 patients were related to severity of patients at admission and to in-hospital mortality. To our knowledge, this study is the first one to examine different panel of endothelial circulating EVs in different range of COVID-19 severities, from symptomatic to critically ill patients. On the contrary, plasma levels of endothelial EVs expressing the intercellular junctional proteins PECAM-1 (CD31) or VE-Cadherin (CD144) do not discriminate patients with COVID-19. Endothelial EVs, more than just a biomarker, are active partners in coagulation, fibrinolysis, inflammation, cell survival, endothelial regeneration and angiogenesis and have been described to actively contributing to vascular diseases progression [19]. EVs have been described to regulate cellular components of innate immunity, including macrophages, monocytes, granulocytes, NK cells, and dendritic cells as well as soluble components of the innate immunity system, including the ComC [20]. In COVID-19, several stimuli leading to endothelial cell activation could explain EVs increase including hypoxic conditions [21], and inflammation [22]. Interestingly, increased interleukin-6 levels in late complicated COVID-19 stages have been shown to stimulate CD62E+ EVs release in vitro [23]. Moreover, in COVID-19, increased EVs could clearly be active partners in cross talk between cells by participating to complications of inflammation and coagulopathy but also could have beneficial effect as cardioprotection or reducing thromboinflammatory process by decreasing endothelial ICAM-1 expression on endothelial cells [24]. According to current COVID-19 outbreak, there are several limitations in our study. First, we did not performed an iterative biomarker measurement over time to provide a more accurate picture of endothelial activation during COVID-19 evolution. Second limitation is the absence of comparison of this increased EVs levels in different wave of COVID-19 considering new variants, new treatments and vaccination effect on COVID-19 severity. Indeed, dexamethasone has now been incorporated into the standard of care for COVID-19 hospital patients [25]. Lower mortality after dexamethasone incorporation into standard of care have been observed: relationship between endothelial EVs regarding anti-inflammatory drugs should be of interest.
All in all, our results highlight the potential for EVs expressing E-selectin (CD62E) to discriminate COVID-19 patients at admission and identify higher risk of in-hospital mortality. Understanding contribution of endothelial EVs biological effects on thrombosis, thromboinflammation or tissular regeneration is still to determine. Plasma levels of CD62E+ EVs at admission may help to identify patients for anti-inflammatory therapies.
Data availability
Raw Data are available upon request to corresponding author.
Authors Contributions
All the undersigning authors have substantially contributed to the paper. DMS and CMB designed the present study and wrote the manuscript. RC performed statistical analyses. FM, CLG and AP performed and/or analyzed the data, NG, LS, OS, TM and JLD included patients and edited the manuscript. All authors declare that the submitted work is original and has not been published before (neither in English nor in any other language) and that the work is not under consideration for publication elsewhere.
Funding
This work has been funded with grants from French national agency for research ANR SARCODO (Fondation de France), ANR-17-ECVD-0002-01 and Crédit Agricole d’Ile-de-France Mécénat.
Declarations
Ethical Approval for SARCODO Study
Authorization number 2020–04-048/ 2020-A01048–31/ 20.04.21.49318) - NCT: NCT04624997.
Consent to Participate
The study SARCODO was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Consent to Publish
All patients provided written informed consent to have their data published.
Competing Interests
All authors declare no conflict.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chantal M. Boulanger, Email: Chantal.boulanger@inserm.fr
David M. Smadja, Email: david.smadja@aphp.fr
References
- 1.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. European Heart Journal. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smadja DM, Mentzer SJ, Fontenay M, et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis. 2021;24(4):755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl J-L, Peron N, Chocron R, et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: A hypothesis-generating study. Annals of Intensive Care. 2020;10(1):95. doi: 10.1186/s13613-020-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goudot, G., Chocron, R., Augy, J.-L., et al. (2020). Predictive factor for COVID-19 worsening: Insights for high-sensitivity troponin and D-dimer and correlation with right ventricular afterload. Frontiers in Medicine;7586307. [DOI] [PMC free article] [PubMed]
- 5.Chocron R, Duceau B, Gendron N, et al. D-dimer at hospital admission for COVID-19 are associated with in-hospital mortality, independent of venous thromboembolism: Insights from a French multicenter cohort study. Archives of Cardiovascular Diseases. 2021;114(5):381–393. doi: 10.1016/j.acvd.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smadja DM, Bory OM, Diehl J-L, et al. Daily monitoring of D-dimer allows outcomes prediction in COVID-19. TH Open Companion J Thromb Haemost. 2022;6(1):e21–e25. doi: 10.1055/a-1709-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond Engl. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. The New England Journal of Medicine. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guervilly C, Burtey S, Sabatier F, et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. The Journal of Infectious Diseases. 2020;222(11):1789–1793. doi: 10.1093/infdis/jiaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khider L, Gendron N, Goudot G, et al. Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost JTH. 2020;18(9):2391–2399. doi: 10.1111/jth.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippe A, Chocron R, Gendron N, et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021;24(3):505–517. doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulanger CM, Loyer X, Rautou P-E, Amabile N. Extracellular vesicles in coronary artery disease. Nature Reviews. Cardiology. 2017;14(5):259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 14.Ridger VC, Boulanger CM, Angelillo-Scherrer A, et al. Microvesicles in vascular homeostasis and diseases. Position paper of the European Society of Cardiology (ESC) working group on atherosclerosis and vascular biology. Thrombosis and Haemostasis. 2017;117(7):1296–1316. doi: 10.1160/TH16-12-0943. [DOI] [PubMed] [Google Scholar]
- 15.Traby L, Kollars M, Kussmann M, et al. Extracellular vesicles and Citrullinated histone H3 in coronavirus disease 2019 patients. Thrombosis and Haemostasis. 2022;122(1):113–122. doi: 10.1055/a-1522-4131. [DOI] [PubMed] [Google Scholar]
- 16.Guervilly C, Bonifay A, Burtey S, et al. Dissemination of extreme levels of extracellular vesicles: Tissue factor activity in patients with severe COVID-19. Blood Advances. 2021;5(3):628–634. doi: 10.1182/bloodadvances.2020003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.György B, Módos K, Pállinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 18.Welsh JA, Van Der Pol E, Arkesteijn GJA, et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J Extracell Vesicles. 2020;9(1):1713526. doi: 10.1080/20013078.2020.1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathiesen A, Hamilton T, Carter N, Brown M, McPheat W, Dobrian A. Endothelial extracellular vesicles: From keepers of health to messengers of disease. International Journal of Molecular Sciences. 2021;22(9):4640. doi: 10.3390/ijms22094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak MZ, Ratajczak J. Innate immunity communicates using the language of extracellular microvesicles. Stem Cell Reviews and Reports. 2021;17(2):502–510. doi: 10.1007/s12015-021-10138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichler Hefti J, Leichtle A, Stutz M, et al. Increased endothelial microparticles and oxidative stress at extreme altitude. European Journal of Applied Physiology. 2016;116(4):739–748. doi: 10.1007/s00421-015-3309-3. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinkhani, B., Kuypers, S., van den Akker, N. M. S., Molin, D. G. M., Michiels L. (2018). Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Frontiers in Immunology;91789. [DOI] [PMC free article] [PubMed]
- 23.Curtis AM, Wilkinson PF, Gui M, Gales TL, Hu E, Edelberg JM. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J Thromb Haemost JTH. 2009;7(4):701–709. doi: 10.1111/j.1538-7836.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Jiang Z, Webster KA, et al. Enhanced Cardioprotection by human endometrium mesenchymal stem cells driven by Exosomal MicroRNA-21. Stem Cells Translational Medicine. 2017;6(1):209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta, J., Rolta, R., Mehta, B. B., Kaushik, N., Choi, E. H., Kaushik, N. K. (2022). Role of Dexamethasone and Methylprednisolone Corticosteroids in Coronavirus Disease 2019 Hospitalized patients: A review. Frontiers in Microbiology;13813358. [DOI] [PMC free article] [PubMed]